Abstract
Purpose
Surface sediments contaminated with high levels of multiple heavy metal(loid) species are very common environmental problems. Especially, the labile and bioaccessible fractions of heavy metal(loid)s in the sediments are posing serious risks to the biota and the overlaying water quality. This study aimed at developing a potential method to manage the activity of the labile fractions of heavy metal(loid)s in surface sediments.
Materials and methods
This study assessed the feasibility of adding iron powder, a low-cost industrial by-product, to sediments containing high levels of Pb, As, and Cd to adsorb labile fractions of heavy metal(loid)s onto the sorbent surfaces and to retrieve the heavy metal(loid) laden powders by applying external magnetic field. In addition, the redistribution of Pb, Cd, and As in different sediment fractions, the dissolved fraction and the sorbent-adsorbed fraction, was also investigated and characterized.
Results and discussion
The results indicate that the bioactive labile fractions (exchangeable and carbonate-bound fractions) of heavy metal(loid)s are prone to concentrating onto iron powders and can be selectively removed from the sediments by magnetic retrieval. In addition, iron addition induces conversion of labile fractions of heavy metal(loid)s into more stabilized fractions.
Conclusions
Overall, the process can effectively minimize the activity of labile fractions of heavy metal(loid)s in surface sediments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy metal(loid) accumulation in sediments (Wisseman and Cook 1977; Jiang et al. 2013; Haydar et al. 2014; Tang et al. 2014) is a global scale environmental problem among the most common and challenging sites that require remediation. After heavy metal(loid)s are released to the aquatic environment, they are generally enriched and deposited in the sediments. Especially, industrial activities and mining activities have resulted in substantial accumulation of heavy metal(loid)s in sediments worldwide (Teng et al. 2003; Miller et al. 2014). Very often, natural dissolution and desorption of heavy metal(loid) species from sediments, variation of sediment physical and chemical properties, and resuspension due to natural impact and human activities (e.g. sediment dredging) may result in release of heavy metal(loid)s back to the upper water column (Cantwell et al. 2002; Eggleton and Thomas 2004; Kalnejais et al. 2007). Consequently, sediments can act as an important long-term point source of heavy metal(loid) pollution. Even worse, heavy metal(loid)s are toxic and cannot be degraded like organic contaminants. As a result, high levels of heavy metal(loid)s in sediments can harm benthic organisms, enter aquatic food chain, and continuously contaminate upper water column, thereby endangering the safety of drinking water, irrigation water, human food chain, etc. (Bryan and Langston 1992; Tabari et al. 2010; Tchounwou et al. 2012). Consequently, proper management and remediation of sediments contaminated with high levels of heavy metal(loid)s are of great importance in protecting public health and environmental safety.
Dredging and excavation (Francingues et al. 2008; Akcil et al. 2015) are traditional methods frequently applied to deal with contaminated sediments. Both treatment methods require follow-up treatment on land, which can be costly and complex (Mulligan et al. 2001). As for dredging, potential loss and dispersion of contaminated sediments through resuspension is another key limitation (Bridges et al. 2008). To minimize potential resuspension and dispersion of sediments due to natural impact or dredging, in situ capping with sand, gravel, bentonite, and other reactive materials has been tested at field scales to some extent (Peng et al. 2009). In addition, in situ solidification/stabilization of sediments by injecting or mixing of various amendments, such as lime and phosphate, to reduce the bioavailability of heavy metal(loid)s, has also been tried at field scales (Renholds 1998). Though in situ treatment may reduce the availability and activity of heavy metal(loid)s to some extent, it is not able to remove heavy metal(loid)s from the sites. As a result, heavy metal(loid)s may be remobilized or resuspended due to natural impact or change of physical and chemical conditions.
An attractive and alternative approach, using magnetically retrievable sorbents to adsorb and concentrate heavy metal(loid)s, followed by magnetic retrieval of the heavy metal(loid) laden sorbents with external magnetic field, may overcome the above mentioned issues with traditional sediment treatment technologies, as outlined in Fig. 1. Though similar approaches have been frequently studied and applied to remove heavy metal(loid)s in aqueous solutions (Giakisikli and Anthemidis 2013; Dave and Chopda 2014), treatment of sediments with this approach has not been studied in detail yet.
Concept: in situ iron treatment of heavy metal(loid)s contaminated sediments and subsequent magnetic retrieval of laden heavy metal(loid)s ( :overlaying and pore waters of contaminated sediments;
:overlaying and pore waters of contaminated sediments;  : contaminated sediments;
: contaminated sediments;  :heavy metal(loid)s in the sediments;
:heavy metal(loid)s in the sediments;  :dissolved heavy metal(loid)s in overlaying and pore waters;
:dissolved heavy metal(loid)s in overlaying and pore waters;  :iron powders as heavy metal(loid) sorbents;
:iron powders as heavy metal(loid) sorbents;  :heavy metal(loid) laden iron powders)
:heavy metal(loid) laden iron powders)
This study applied iron powders, a common industrial by-product of low cost, as a magnetically retrievable heavy metal(loid) sorbent for sediment treatment. Iron powder has several characteristics that make it a good candidate for sediment remediation, including its proven adsorption potential toward heavy metal(loid)s (Pb, Cd, Hg, Cr, As, Zn, Cu, etc.) in aqueous solutions (Xu and Dong 2008; Hashim et al. 2011; Gasparatos 2013; Komarek et al. 2013; O’Carroll et al. 2013; Fu et al. 2014; Ghasemzadeh et al. 2014; Guo et al. 2015), capability to immobilize heavy metal(loid)s in soil and sediments (Schulz 1989; Feng et al. 2007), and high magnetic susceptibility. In addition, the sorbents are in powder forms and may be applied conveniently through surface spreading, while the high density of the sorbents will facilitate settling and anchoring of the sorbents onto the surface sediments.
Batch studies with real river sediments heavily contaminated with Pb, Cd, and As were carried out to assess the feasibility of the proposed approach for sediment remediation. It is worth noting that real river sediments are often contaminated with multiple heavy metal(loid) species that exist in multiple fractions, frequently categorized into F1 (exchangeable form), F2 (bound to carbonates), F3 (bound to Fe-Mn oxides), F4 (bound to organic matters), and F5 (residual form) (Tack and Verloo 1995). Due to the complexity of the issue, this study focused on stabilization and magnetic retrieval of the labile and bioaccessible fractions of heavy metal(loid)s. In addition, the redistribution of Pb, Cd, and As in different sediment fractions, the dissolved fraction and the sorbent-adsorbed fraction, were investigated and characterized.
2 Materials and methods
2.1 Materials
Iron powder was analytical grade (100 mesh, 98%). Nitric acid (68–70%), hydrochloric acid (36%), acetic acid (99.7%), magnesium chloride hexahydrate (99%), anhydrous sodium acetate (99%), hydroxylamine hydrochloride (99%), hydrogen peroxide (30%), and ammonium acetate (98%) are all guaranteed grade. All reagents were from Aladdin Reagent, China and used as received. Milli-Q ultrapure water was used to prepare all solutions.
Surface sediment samples at 0~10 cm depth from the estuary of Maba River, a main tributary of North Pearl River in Guangdong Province, China, were collected with a grab sampler in March 2014. The surface sediments were heavily contaminated with a variety of heavy metal(loid) species due to historical mining activities in the area. The collected sediment samples were thoroughly dried at room temperature and ground to pass a 100-mesh sieve. The organic carbon content of the sediment was ~4.34%, determined by the hydrated heat potassium dichromate oxidation-colorimetry method, and pH of the sediment was ~7.2, analyzed according to US EPA Method 9045D.
2.2 Experimental procedures
Dried sediment samples (50 g) were weighed into 250-mL depolished open-mouth bottles. Designated amounts of iron powders (0, 0.5, 2, or 5 wt% of sediment samples) were added to the sediment and thoroughly mixed with glass stirring rods. Thereafter, Milli-Q ultra pure water (70 mL) was added into the bottle to submerge the sediment samples. Then, the bottle was capped and put on a temperature control shaker (HZQ-X100, Suzhou Pei Ying Experimental Equipment, Suzhou, China) maintained at 25 °C with a shaking speed of ~180 rpm. The experiments were run in duplicates.
At designated intervals (2 h, 7, 14, 21, and 42 days), samples were taken out and rested on the bench to let the solid particles settle down to the bottom. An NdFeB round base magnet (diameter ~3.5 cm; thickness ~2.0 cm; 380–400 mT; manufactured by XHC Magnets Inc., Shenzhen, China) wrapped with a plastic bag to avoid contact with water was lowered into the bottle. The magnet was gently moved around right above the sediment surface, and magnetically retrievable material was recovered through a non-contact approach. The recovered material was put into a beaker, while residual sediment and water were transferred to a centrifuge tube and the sediment solid was separated from the water by centrifugation. The water was used to wash the magnetically retrieved material and the original bottle to transfer residual sediments into the centrifuge tube, followed by centrifugation again to separate water from the sediment solids. The above washing and cleaning steps were repeated three times. Thereafter, the separated aqueous solution was filtered through 0.45-μm membrane syringe filters, and the filtrate was collected for heavy metal(loid) analysis. The residual sediment and the magnetically recovered material were air-dried at room temperature in the fume hood, ground in a mortal to fine powders, and weighed.
2.3 Analytical procedures
Tessier sequential extraction procedures (Abollino et al. 2011) were applied to evaluate the fractionation of heavy metal(loid)s in the sediments. The concentrations of Pb, As, Cd, and Cr were analyzed by an inductively coupled plasma mass spectrometer (ICP-MS, Agilent 7700×), carried out by Kingmed Diagnostics (Guangzhou, China), a certified third-party analytical company. The digestion and extraction procedures for the sediment and magnetically retrieved samples, optimized for ICP-MS analysis (Agilent 7700x) by collaboration with professionals from Kingmed Diagnostics, are provided in Text S1 in Electronic Supplementary Material.
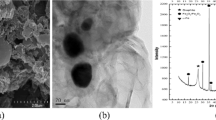
High-resolution X-ray photoelectron spectroscopy (HR-XPS) was also applied to characterize the distribution and valence of Fe, Pb, Cd, and As on the surface of the original iron powders and magnetically retrieved iron powders. The HR-XPS analysis was carried out by XPS technicians of Tianhe Science and Research (a professional analytical service company from Shandong, China), using a Thermo ESCALAB 250XI (Thermo Fisher, UK) with a monochromatic Al X-ray source (hv = 1486.6 eV, 150 W). High-resolution scans were carried out for regions of Fe 2p, Pb 4f, Cd 3d, and As 3d, with a passage energy of 30 eV.
2.4 Potential ecological risk assessment of heavy metal(loid)s in sediment
First, potential ecological risk index (RI) assessment developed by Håkanson (Hakanson 1980) was applied to monitor sediment potential ecological risks during the treatment, where RI was calculated as the sum of individual risk factors (E i r ) in the sediments. The assessment procedures are provided in Text S2, Electronic Supplementary Material. For individual element, an E i r value less than 40 is considered low potential ecological risk; 40 ≤ E i r < 80, moderate risk; 80 ≤ E i r < 160, high risk; 160 ≤ E i r < 320, very high risk; and E i r ≥ 320, extremely high risk.
Secondly, risk assessment code (RAC) classification (Perin et al. 1985) based on the percentage of F1 and F2 fractions of each heavy metal(loid) species was calculated during the remediation process to assess the mobility and bioavailability profiles of respectively concerned heavy metal(loid) species in the sediment. According to RACs, a value less than 1% is considered no risk to the aquatic environment; 1–10%, low risk; 11–30%, medium risk; 31–50%, high risk; and >50%, very high risk.
2.5 Statistical analysis
Bivariate correlation analysis using the SPSS statistical package (Window version 16) was carried out for each heavy metal(loid) species. For the designated heavy metal(loid) species, the treatment time; iron dosage; concentrations of F1, F2, F3, F4, and F5; the amount of magnetically recovered heavy metal(loid)s; and RACs of the residual sediments were designated as the variables.
3 Results and discussion
3.1 Fraction distribution and risk assessment of heavy metal(loid)s in the original sediment
Preliminary analysis of the original sediment samples with ICP-MS indicated that the original sediment was mainly contaminated with Pb, As, Cd, and Cr. Distribution and fractions of Pb, As, Cd, and Cr in the untreated sediments as well as their individual potential ecological risk factors (E i r ) and RACs are summarized in Table S1, Electronic Supplementary Material. The concentration of Pb in the sediment is ~1537 mg kg−1, ~42.7 times of the average soil background level in Guangdong Province (CNEMC 1990), with an E i r of ~213 and an RAC of ~31.3%; As, ~1032 mg kg−1 and ~116 times of the average background level (CNEMC 1990), with an E i r of ~1160 and an RAC of 0.5%; Cd, ~40.2 mg kg−1 and ~718 times of the average background level (CNEMC 1990), with an E i r of ~21,550 and an RAC of ~67.0%; and Cr, ~43.9 mg kg−1, slightly lower than the average background level (CNEMC 1990), with an E i r of ~1.7 and an RAC of ~4.0%. Apparently, historical mining activities in this area have caused serious deposition and enrichment of Pb, As, and Cd in the sediments. According to individual E i r , Cd and As were characterized as extremely high ecological risk; Pb, high risk; and Cr, negligible ecological risk.
According to RACs (Table S1, Electronic Supplementary Material), Cd is classified as very high risk with an RAC of ~67.0%; Pb, high risk with an RAC of ~31.3%; Cr, low risk with an RAC of ~4.0%; and As, no risk with an RAC of ~0.5%. The results indicate that Cd and Pb in the sediment, especially Cd, have high mobility and expose very serious risk to the aquatic biota health. Though the concentration of As in the sediment is high, it mainly exists in the residual fraction. Even so, As is a highly toxic element, and even very low concentration of As in the pore water may present serious hazard to the aquatic biota (Smedley and Kinniburgh 2005).
3.2 Recovery of iron sorbents from the sediment
The results (Table S2, Electronic Supplementary Material) indicate that iron powders, the sorbents, can be effectively magnetically retrieved from the sediment. The magnetic retrieval rate was very high, and at the beginning, greater amounts of materials than the added iron powders were retrieved. For example, magnetic separation recovered ~1.14 g of dried material with 0.5 wt% iron addition (0.25 g) from the sediment (a dried weight of 50 g), ~2.20 g with 2 wt% iron addition (1.00 g), and 3.37 g with 5 wt% iron addition (2.50 g). Under magnetic field, iron powders can act as small magnets that can attract fine magnetically susceptible minerals, such as magnetite and hematite, which can be retrieved along with the iron powders. In contrast, there was negligible recovery of material from the sediment in the absence of iron powders through the non-contact magnetic recovery approach used in this study, while ~1.0 g of dried material could be retrieved when the original dried sediment (50 g) was tumble-mixed with the magnet. As iron powders aged in the sediment, the recovered weight was reduced. This was potentially due to iron rust flaking off from the iron surface, as implied by observation of the behavior of the iron powders in water without sediment present.
3.3 Removal and stabilization of labile Pb in the sediment
Once iron powders were added to the sediment, aqueous Pb concentrations were reduced right away, compared to that in the blank (Fig. 2a). Especially, 2 and 5 wt% iron addition could reduce aqueous Pb concentration to below the detection limit in less than 7 days. Therefore, iron addition is effective in minimizing the leaching of Pb2+ from sediments to the water column. Previous literature studies also show that iron or iron rust is highly effective in removing dissolved Pb2+ through zero valent iron reduction to Pb (Ponder et al. 2000), adsorption, co-precipitation, or oxidation by iron(hydr)oxides (Arancibia-Miranda et al. 2014; Tomasevic et al. 2014).
After iron addition to the sediments, magnetic separation also successfully retrieved a portion of Pb from the sediments, which was confirmed both by HR-XPS survey for Pb 4f region on the surface of the retrieved materials and ICP-MS analysis of the digested samples. As illustrated in Fig. 3b, the retrieved Pb showed a single Pb 4f photoelectron peak at ~138.7 eV, corresponding to the characteristic peak position of Pb2+ (Li and Zhang 2007), which implies that Pb was mainly immobilized on the surface of iron powders by sorption but not by reduction. Furthermore, HR-XPS survey of surface Fe 2p region of both the surface of original iron powders and retrieved ones (Fig. 3a) only showed characteristic Fe3+ photoelectron peaks (Li and Zhang 2007). This indicates extensive oxidation of surface iron, while iron(hydr)oxides are known for their ability to adsorb Pb2+ (Nelson et al. 2002).
From the beginning to 14 days, Pb removal percentage increased as the treatment time increased, while Pb removal percentage also increased as iron dosage rose, as illustrated in Fig. 2b. Maximum Pb removal reached ~0.6% with 0.5 wt% iron addition, 2.2% with 2 wt% iron addition, and 6.1% with 5 wt% iron addition. Further increasing the contact time over 14 days yielded lower Pb removal percentage with 5 wt% iron addition, which coincided with reduced amounts of magnetically retrieved materials. This implies that loss of a portion of Pb with increasing treatment time is potentially due to Pb-laden rust flaking off from the iron powders. Therefore, retrieval time should be optimized to achieve maximum removal of heavy metal(loid)s from the sediments.
Bivariate correlation analysis (Table S3, Electronic Supplementary Material) indicates that Pb removal is highly positively correlated to iron dosage (r = 0.925). On the other hand, Pb removal was significantly negatively correlated with F1 (r = −0.753) and F2 (r = −0.917) fractions of Pb. The results imply that magnetically retrieved Pb is mainly derived from F1 and F2. This is consistent with the fact that F1 and F2 are more prone to being remobilized than F3, F4, and F5.
Iron treatment is also effective in stabilizing Pb in the residual sediment. As illustrated in Pb fraction profiles (Fig. 2c), iron treatment substantially reduced the concentrations of F1 and F2. The higher the iron dosage, the less F1 and F2 fractions remained. On the contrary, iron treatment increased the concentrations of F3 and F5, whereas insignificant impact on F4 was observed. The observation is consistent with previous observation (Gil-Diaz et al. 2014) that zero valent iron can accelerate the transformation of F1 and F2 fractions of Pb into more stable fractions, especially into F3 and F5. First, Pb-laden rust flaking off may contribute to increased amount of F3. In addition, aging can induce labile fractions of heavy metal(loid)s transformation into more stable fractions (Ma and Uren 1998; Huang et al. 2015), as heavy metal(loid) species on the mineral surfaces can diffuse into the micropores or be entrapped by microporous solids (Ma and Uren 1998).
The bivariate analysis also implies potential redistribution of Pb among various fractions. The results indicate that F1 is highly positively correlated to F2, which is consistent with the fact that both fractions are remobilized and concentrated onto the iron powders. On the other hand, F1 is significantly negatively correlated to F3 and F5, implying that F1 could be converted to F3 and F5. In addition, F2 is significantly negatively correlated to F3, F4, and F5, indicating that F2 could be converted into F3, F4, and F5. The results are consistent with the observation that iron addition results in stabilization of Pb in sediments.
In accordance with removal and stabilization of labile Pb fractions in the residual sediments, RACs (Fig. S1a, Electronic Supplementary Material) indicate that 5 wt% iron addition reduced RACs of Pb from 23.4% (medium risk) in the original sediment to 2.7% (low risk) after 42 days of treatment. Therefore, iron treatment is effective in reducing the mobility of Pb in the sediment and minimizing its risk to the aquatic biota. Furthermore, correlation analysis (Table S3, Electronic Supplementary Material) indicates that the RACs of Pb are significantly and negatively correlated to iron dosage, the magnetically retrieved amount of Pb and F3, F4, and F5, whereas it is significantly and positively related to F1 and F2. The results confirm that iron dosage is the most important factors regulating RACs of Pb, while adsorption of F1 and F2 onto the iron powders and consequent removal of Pb through magnetic retrieval, conversion of F1 and F2 to F3, F4, or F5, all contribute to effective reduction of RACs and the bioaccessibility of Pb in the sediments.
3.4 Removal and stabilization of labile Cd in the sediment
Once iron powders were added to the sediment, the aqueous concentrations of Cd dropped immediately (Fig. 4a). Especially, 2 and 5 wt% iron additions were very effective in minimizing the leaching of Cd to the upper water column, and the aqueous concentrations of Cd were about one half and one third of those in the blank control. Compared with the results of Pb, iron treatment was not able to fully block the leaching of Cd to the water column, potentially due to the much more active nature of Cd (Lu et al. 2005) and its higher RACs in the sediment. Due to the differences of cation exchange capacity and affinity for sediment between Cd2+ and Pb2+, Cd2+ is normally more mobile than Pb2+ (Christophi and Axe 2000; Oh et al. 2009). In addition, previous studies indicate that the sorption of Pb2+ onto iron(hydr)oxides species may adversely affect the sorption of Cd2+, whereas the sorption of Cd2+ would impact the sorption of Pb2+ to a lower extent (Mohapatra et al. 2009; Oh et al. 2009). Consequently, Cd2+ is more difficult to immobilize than Pb2+ in the sediment.
After iron addition to the sediments, magnetic retrieval was effective in retrieving Cd from the sediment, which was revealed by HR-XPS analysis of the retrieved iron powders and ICP-MS analysis of the digested samples. HR-XPS showed a Cd 3d photoelectron peak at ~400 eV (Fig. 3c), corresponding to the characteristic peak position of Cd2+. The results show that Cd was mainly immobilized on the surface of iron powders by sorption. Previous literature also shows that zero valent iron mainly removes Cd2+ through sorption by surface iron(hydr)oxides but not by reduction (Li and Zhang 2007).
Magnetic separation achieved ~10.5% Cd removal with 2 wt% iron addition and ~19.3% Cd removal with 5 wt% iron addition (Fig. 4b). Due to effective Cd removal, E i r of Cd could be reduced by a value of ~4159 with 5 wt% iron addition. Similar to the observation in Pb removal, there was a tendency of Cd loss from the iron powders as the treatment time increased, due to heavy metal(loid) laden iron rust flaking off. Maximum Cd removal was achieved in 7 days. Compared with Pb, Cd seemed to reach maximum removal capacity in less time and showed a greater tendency to remobilize. As previously discussed, the more active nature of Cd and its higher original RACs is a double-edged sword. On one hand, Cd can be easily remobilized from the sediments and adsorbed by iron powders. On the other hand, Cd on the sorbent is prone to remobilization, while the adsorption of Pb onto the sorbents can facilitate the desorption of Cd as well (Mohapatra et al. 2009; Oh et al. 2009). Therefore, optimization of iron dosage and treatment time is highly crucial to achieve maximum removal of Cd.
Correlation analysis (Table S4, Electronic Supplementary Material ) indicates that Cd removal is highly positively correlated to the iron dosage (r = 0.871). On the other hand, Cd removal is significantly negatively correlated with F1 (r = −0.904) and F2 (r = −0.808) concentrations of Cd. The results imply that the magnetically retrieved Cd is mainly derived from F1 and F2 fractions, similar to the observation for Pb removal.
Iron treatment also results in stabilizing Cd in the residual sediment. As illustrated in Cd fraction time profiles (Fig. 4c), iron treatment results in substantial reduction of F1, slight reduction of F2, and obvious increase of F3. The greater the iron dosage, the less F1 and F2 remained. The results imply that F1 and F2 can be remobilized and adsorbed onto the iron powder surface, while portions of F3 may be derived from Cd-laden rust flaking off the iron powders. The observation is also confirmed by the correlation analysis (Table S4, Electronic Supplementary Material ). F1 is significantly positively correlated to F2, consistent with the fact that both fractions are remobilized and concentrated on the iron powders. On the other hand, both F1 and F2 are highly negatively correlated to F3, implying that portions of F1 and F2 may be converted to F3.
In accordance with effective removal and stabilization of labile fractions of Cd in the sediment, RACs of Cd (Fig. S1b, Electronic Supplementary Material) indicate that 5 wt% iron addition could reduce RAC from ~49% in the original sediment to 36.6% after 42 days. Compared with the results of Pb, it is harder to control the RACs of Cd due to Cd’s more active nature. Consequently, higher iron dosage is required to control the bioactivity of Cd to the aquatic environment.
In addition, the correlation analysis indicates that RACs are highly negatively correlated to iron dosage, the magnetically retrieved amount of Cd and F3 concentration, whereas it is significantly positively related to F1 and F2. The results reconfirm that iron dosage is the most important factor determining RACs as previously discussed, while F1 and F2 concentrating on the iron surface and the subsequent magnetic removal of Cd; the conversion of F1 and F2 to F3 are main pathways responsible for the reduction of RACs and the bioaccessibility of Cd.
3.5 Removal and stabilization of As in the sediment
Once iron powders were added to the sediment, aqueous As concentrations were reduced substantially (Fig. 5a), compared with results in the blank. Especially, 2 and 5 wt% iron additions were very effective in minimizing the release of As to the water column, and 5 wt% iron additions could reduce the dissolved As concentrations to ~1 ppb in 42 days. Therefore, sediment treatment with iron powders is effective in minimizing the leaching of As from the sediment.
In addition, magnetic retrieval was also able to remove a portion of As from the sediment, which was illustrated both by HR-XPS survey for As 3d region on the surface of the retrieved powders (Fig. 3c) and ICP-MS of the digested samples. The removal percentage of As (Fig. 5b) was relatively lower than that of Pb and Cd under the same conditions. This is consistent with the predominant existence of As in the residual fraction. Even so, the treatment could still achieve ~1.5% removal of As with 2 wt% iron addition and ~4% removal of As with 5 wt% iron.
Though As mainly exists as stable F5, iron treatment is still important in controlling the bioaccessibility of As in the sediments. As illustrated in As fraction time profiles (Fig. 5c), iron treatment resulted in substantial reduction of F1, F2, and even F3. The higher the iron dosage, the lower F1, F2, and F3 fractions remained. In accordance, iron treatment is extremely effective in minimizing the RACs of As in the sediment (Fig. S1c, Electronic Supplementary Material). Iron addition (0.5 wt%) could reduce the RACs from ~0.6% to ~0.05% in 42 days, whereas the RACs were negligible with 5 wt% iron addition. The results indicate that iron is very effective in controlling the mobility and bioavailability of As in the sediment.
Bivariate correlation analysis (Table S5, Electronic Supplementary Material) indicates that As removal is highly positively correlated to the iron dosage (r = 0.964). On the other hand, As removal is significantly negatively correlated with F1 (r = −0.658), F2 (r = −0.743), and F3 (r = −0.835). In accordance with this, F1, F2, and F3 are mutually, significantly and positively correlated with each other, as all three fractions are remobilized and concentrated on the iron sorbents.
In addition, the correlation indicates that RACs of As are significantly and negatively correlated to iron dosage, the magnetically retrieved amount of As, but significantly and positively related to F1, F2, and F3. The results indicate that iron dosage is the most important factor controlling the RACs of As, while magnetic removal of As (mainly derived from F1, F2, and F3) mainly contributes to the reduction of RACs and minimizing the bioaccessibility of As in the sediment.
The proposed approach is also effective in reducing Cr concentration in the sediment and retrieving a portion of Cr from the sediment (Fig. S2, Electronic Supplementary Material), but the remediation of Cr would not be discussed in detail as the concentration of Cr in the sediment was similar to the soil background level.
4 Conclusions
The results indicate that in situ treatment with iron powders and subsequent magnetic retrieval may be a promising approach for the management of surface sediments contaminated with multiple heavy metal(loid) species. The addition of iron powders, an industrial low-cost by-product, to the sediment can immediately reduce and even block the leaching of heavy metal(loid)s to the upper water column, adsorb a portion of labile heavy metal(loid)s onto the iron powder surface, and stabilize labile heavy metal(loid)s remained in the sediment. The subsequent magnetic retrieval can effectively recover the heavy metal(loid) laden iron powders, where heavy metal(loid)s retrieved are mainly from the most mobile and bioaccessible F1 and F2. The retrieved amount of heavy metal(loid)s is closely correlated to iron dosage and treatment time. Especially, cautions should be paid to potential loss or remobilization of heavy metal(loid)s from the sorbent as treatment time increases, and sorbent retrieval time should be optimized to achieve maximum heavy metal(loid) retrieval.
Overall, the coupling process shows good potential in minimizing potential ecological risks of sediments, the RACs of heavy metal(loid)s, and bioaccessibility of heavy metal(loid)s to the aquatic biota, e.g., E i r of Cd could be reduced by a value of ~4159 with 5 wt% iron addition due to effective magnetic retrieval/removal of Cd from the sediment, while RACs of Pb was reduced from 23.4% (medium risk) in the original sediment to 2.7% (low risk) after 42 days of treatment. The approach is effective for multiple heavy metal(loid) species, thereby presenting a general treatment option for surface sediments contaminated with heavy metal(loid)s. Though this lab study indicates the potential of the proposed approach, further research is necessary to evaluate its field applicability, and results from this study will provide valuable guidance for future studies.
References
Abollino O, Malandrino M, Giacomino A, Mentasti E (2011) The role of chemometrics in single and sequential extraction assays: a review: part I. Extraction procedures, uni- and bivariate techniques and multivariate variable reduction techniques for pattern recognition. Anal Chim Acta 688:104–121
Akcil A, Erust C, Ozdemiroglu S, Fonti V, Beolchini F (2015) A review of approaches and techniques used in aquatic contaminated sediments: metal removal and stabilization by chemical and biotechnological processes. J Clean Prod 86:24–36
Arancibia-Miranda N, Baltazar SE, García A, Romero AH, Rubio MA, Altbir D (2014) Lead removal by nano-scale zero valent iron: surface analysis and pH effect. Mater Res Bull 59:341–348
Bridges TS, Ells S, Hayes D, Mount D, Nadeau SC, Palermo MR, Patmont C, Schroeder P (2008b2008a) ERDC/EL TR-08-4. The Four Rs of environmental dredging: resuspension, release, residual, and risk. http://el.erdc.usace.army.mil/elpubs/pdf/trel08-4.pdf. Accessed 28 March 2016
Bryan GW, Langston WJ (1992) Bioavailability, accumulation and effects of heavy metals in sediments with special reference to United Kingdom estuaries: a review. Environ Pollut 76:89–131
Cantwell MG, Burgess RM, Kester DR (2002) Release and phase partitioning of metals from anoxic estuarine sediments during periods of simulated resuspension. Environ Sci Technol 36:5328–5334
Christophi C, Axe L (2000) Competition of Cd, Cu, and Pb adsorption on goethite. J Environ Eng 126:66–74
CNEMC (China National Environmental Monitoring Center) (1990) Background values of soil elements in China. Environmental Science Press of China, Beijing
Dave PN, Chopda LV (2014) Application of iron oxide nanomaterials for the removal of heavy metals. J Nanotechnol 2014:1–14
Eggleton J, Thomas KV (2004) A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events. Environ Int 30:973–980
Feng N, Bitton G, Yeager P, Bonzongo JC, Boularbah A (2007) Heavy metal removal from soils using magnetic separation: 1. Laboratory experiments. Clean 35:362–369
Francingues KEG, Burton GA, Norman R, Wolfe J, Danny DR, Donna JV, John R (2008) Evaluating the effectiveness of contaminated-sediment dredging. Environ Sci Technol 42:5042–5047
Fu F, Dionysiou DD, Liu H (2014) The use of zero-valent iron for groundwater remediation and wastewater treatment: a review. J Hazard Mater 267:194–205
Gasparatos D (2013) Sequestration of heavy metals from soil with Fe-Mn concretions and nodules. Environ Chem Lett 11:1–9
Ghasemzadeh G, Momenpour M, Omidi F, Hosseini MR, Ahani M, Barzegari A (2014) Applications of nanomaterials in water treatment and environmental remediation. Front Env Sci Eng 8:471–482
Giakisikli G, Anthemidis AN (2013) Magnetic materials as sorbents for metal/metalloid preconcentration and/or separation. A review. Anal Chim Acta 789:1–16
Gil-Diaz MM, Perez-Sanz A, Vicente MA, Lobo MC (2014) Immobilisation of Pb and Zn in soils using stabilised zero-valent iron nanoparticles: effects on soil properties. Clean 42:1776–1784
Guo X, Zhao Y, Qiu Y, Shi X (2015) Zero-valent iron nanoparticle-supported composite materials for environmental remediation applications. Curr Nanosci 11:748–759
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14:975–1001
Hashim MA, Mukhopadhyay S, Sahu JN, Sengupta B (2011) Remediation technologies for heavy metal contaminated groundwater. J Environ Manag 92:2355–2388
Haydar CM, Nehme N, Awad S, Koubaissy B, Fakih M, Yaacoub A, Toufaily J, Villeras F, Hamieh T (2014) Assessing contamination level of heavy metals in the Lake of Qaraaoun. Lebanon. Phys Procedia 55:285–290
Huang B, Li ZW, Huang JQ, Chen GQ, Nie XD, Ma WM, Yao HB, Zhen JM, Zeng GM (2015) Aging effect on the leaching behavior of heavy metals (Cu, Zn, and Cd) in red paddy soil. Environ Sci Pollut Res 22:11467–11477
Jiang M, Zeng G, Zhang C, Ma X, Chen M, Zhang J, Lu L, Yu Q, Hu L, Liu L (2013) Assessment of heavy metal contamination in the surrounding soils and surface sediments in Xiawangang River, Qingshuitang District. PLoS One 8:e71176
Kalnejais LH, Martin WR, Signell RP, Bothner MH (2007) Role of sediment resuspension in the remobilization of particulate-phase metals from coastal sediments. Environ Sci Technol 41:2282–2288
Komarek M, Vanek A, Ettler V (2013) Chemical stabilization of metals and arsenic in contaminated soils using oxides—a review. Environ Pollut 172:9–22
Li X-q, Zhang W-x (2007) Sequestration of metal cations with zerovalent iron nanoparticles—a study with high resolution X-ray photoelectron spectroscopy (HR-XPS). J Phys Chem C 111:6939–6946
Lu A, Zhang S, X-q S (2005) Time effect on the fractionation of heavy metals in soils. Geoderma 125:225–234
Ma YB, Uren NC (1998) Transformations of heavy metals added to soil—application of a new sequential extraction procedure. Geoderma 84:157–168
Miller H, Croudace IW, Bull JM, Cotterill CJ, Dix JK, Taylor RN (2014) A 500 year sediment lake record of anthropogenic and natural inputs to Windermere (English Lake District) using double-spike lead isotopes, radiochronology, and sediment microanalysis. Environ Sci Technol 48:7254–7263
Mohapatra M, Rout K, Mohapatra BK, Anand S (2009) Sorption behavior of Pb(II) and Cd(II) on iron ore slime and characterization of metal ion loaded sorbent. J Hazard Mater 166:1506–1513
Mulligan CN, Yong RN, Gibbs BF (2001) An evaluation of technologies for the heavy metal remediation of dredged sediments. J Hazard Mater 85:145–163
Nelson YM, Lion LW, Shuler ML, Ghiorse WC (2002) Effect of oxide formation mechanisms on lead adsorption by biogenic manganese (hydr)oxides, iron (hydr)oxides, and their mixtures. Environ Sci Technol 36:421–425
O'Carroll D, Sleep B, Krol M, Boparai H, Kocur C (2013) Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv Water Resour 51:104–122
Oh S, Kwak MY, Shin WS (2009) Competitive sorption of lead and cadmium onto sediments. Chem Eng J 152:376–388
Peng J-f, Song Y-h, Yuan P, Cui X-y, Qiu G-l (2009) The remediation of heavy metals contaminated sediment. J Hazard Mater 161:633–640
Perin GCL, Lucchese M, Cirillo R, Dotta L, Zanetta ML, Oro AA (1985) Heavy metal speciation in the sediments of northern Adriatic Sea. A new approach for environmental toxicity determination. In: Lakkas TD (ed) Heavy metals in the environment. CEP Consultants, Edinburg, pp 454–456
Ponder SM, Darab JG, Mallouk TE (2000) Remediation of Cr(VI) and Pb(II) aqueous solutions using supported, nanoscale zero-valent iron. Environ Sci Technol 34:2564–2569
Renholds J (1998) In situ treatment of contaminated sediments. Technology status report prepared for the U.S. EPA Technology Innovation Office under a National Network of Environmental Management Studies Fellowship. https://clu-in.org/products/intern/renhold.htm. Accessed 28 March 2016.
Schulz R (1989) Mercury fixation in contaminated sediments as a management option at Albany, Western Australia. Water Sci Technol 21(2):45–51
Smedley PL, Kinniburgh DG (2005) Chapter 1. Source and behaviour of arsenic in natural waters, British Geological Survey, Wallingford, Oxon OX10 8BB, UK
Tabari S, Saravi SSS, Bandany GA, Dehghan A, Shokrzadeh M (2010) Heavy metals (Zn, Pb, Cd and Cr) in fish, water and sediments sampled form Southern Caspian Sea. Iran Toxicol Ind Health 26:649–656
Tack FMG, Verloo MG (1995) Chemical speciation and fractionation in soil and sediment heavy-metal analysis—a review. Int J Environ An Ch 59:225–238
Tang W, Shan B, Zhang H, Zhang W, Zhao Y, Ding Y, Rong N, Zhu X (2014) Heavy metal contamination in the surface sediments of representative limnetic ecosystems in Eastern China. Sci Rep 4:7152
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metals toxicity and the environment. EXS 101:133–164
Teng Y, Tuo X, Ni S, Zhang C, Xu Z (2003) Environmental geochemistry of heavy metal contaminants in soil and stream sediment in Panzhihua mining and smelting area, Southwestern China. Chin J Geochem 22:253–262
Tomasevic DD, Kozma G, Kerkez DV, Dalmacija BD, Dalmacija MB, Becelic-Tomin MR, Kukovecz A, Konya Z, Roncevic S (2014) Toxic metal immobilization in contaminated sediment using bentonite- and kaolinite-supported nano zero-valent iron. J Nanopart Res 16:15
Wisseman RW, Cook SF Jr (1977) Heavy metal accumulation in the sediments of a Washington lake. Bull Environ Contam Toxicol 18:77–82
Xu Z, Dong M (2008) Synthesis, characterization, and application of magnetic nanocomposites for the removal of heavy metals from industrial effluents. In: Shah V (ed) Emerging environmental technologies. Springer, Dordrecht, pp 105–148
Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China-Guangdong Joint Fund (No. U1201234) and the National Natural Science Foundation of China (No. 41201304).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Karl J. Rockne
Electronic supplementary material
ESM 1
Additional information or data on sample work-up and analytical procedures, bivariate analysis results, RACs data and remediation results about Cr. This material is available online free of charge. (DOCX 55.6 kb)
Rights and permissions
About this article
Cite this article
Ou, T., Guan, M., Mai, Y. et al. Remediation of heavy metal(loid)s contaminated sediments—in situ iron treatment and subsequent magnetic extraction. J Soils Sediments 17, 2202–2213 (2017). https://doi.org/10.1007/s11368-017-1670-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-017-1670-z