Abstract
Purpose
To reveal if the input of pyrogenic organic matter (PyOM) affects the nitrogen (N) cycling in soil and its N can be used for the synthesis of microbial biomass, we investigated the incorporation of 15N from 15N-enriched grass residues (OM) or their PyOM into extractable amino acids (AAs) of soil organic matter (SOM) from an unburnt and a burnt soil amended with those residues.
Materials and methods
Pots seeded with Lolium perenne and filled with soil from a burnt and an unburnt Cambisol from southern Spain were topped either with 15N-enriched grass residues (15N-OM), its 15N-PyOM, mixtures of KNO3 (Ni) and 15N-OM or 15N-PyOM, as well as K15NO3 mixed with non-enriched OM or PyOM. After incubation of the pots for up to 16 months under controlled conditions, the AAs, extracted from the litter-free soil, were quantified by gas chromatography mass spectrometry (GC/MS). The fate of the added 15N (15Nadd) was followed by isotopic ratio mass spectrometry (IRMS) and analyzed by statistical means.
Results and discussion
The contribution of extractable AAs to SOM of the non-amended burnt soil was twice of that for the unburnt soil. After amendment, their yields and the percentage of 15Nadd recovered in AAs were always higher for the burnt soil. Stabilization of proteinaceous residues during the incubation was indicated. Already after 2 weeks, 15Nadd from 15N-PyOM was recovered within the AAs.
Conclusions
Our experiment confirmed that N from PyOM is incorporated into the peptidic fraction of SOM of post-fire soils. The efficiency of this incorporation is not altered by the presence of Ni and vice versa. We demonstrated further a short-term and medium-term impact of fire on N cycling in soils, expressed by alteration of the contribution of acid-extractable AAs to the soil organic N (SON) pool.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
It is widely known that N is a limiting nutrient for plants and microorganisms. As such, it represents an important entity not only in the overall N cycle but also for C sequestration in soils. Aside from inorganic N sources provided by biotic and abiotic N fixation, N precipitation, and N mineral fertilizers, the N supply in soils is refilled by organic N of decaying biomass. Here, it is mainly bound in peptides and AAs (Friedel and Scheller 2002). Only small amounts of N can be assigned to amino sugars, nucleic acids, alkaloids, or tetrapyrroles. Most of these compounds enter the soil during litter fall. Applying common extraction approaches using acid hydrolysis between 7 and 50% of the total soil organic N (SON) of fire-unaffected soils can be assigned to AAs in peptides and proteins (Stevenson 1982). According to the non-destructive method of solid-state 15N NMR spectroscopy, more than 80% of SON in fire-unaffected soils is attributable to peptide-like compounds (Knicker et al. 1993). They may be part of decaying microbial residues, plant debris, or exo-enzymes but are also constituents of non-hydrolyzable residues and the slow turning SON pool (Knicker 2011b). The mechanisms responsible for the high chemical and biochemical recalcitrance of the latter, however, are still to be revealed.
It is widely accepted that a change of N availability for soil organisms, i.e., by alteration of input amounts or the kind of source quality, can have a major impact on the biological productivity of a soil (Gärdenäs et al. 2011). In particular, in fire-prone regions, such alterations are commonly induced by vegetation fire. High-intensity fires can increase the inorganic N content of soils but reduce considerably the input of organic N due to the complete destruction of the organic layer (Rovira et al. 2012; Varela et al. 2015) and underwood vegetation. Moderate and prescribed wildfires, on the other hand, can increase the organic carbon (Corg) and total nitrogen (Nt) contents (Scharenbroch et al. 2012) by the incorporation of partly charred residues and production of fresh litter from regrown plants after release of nutrients from the ash. As demonstrated by solid-state 13C and 15N NMR spectroscopy, partial combustion of leaves, litter, and humified material in the O horizons leads to a transformation of the proteinaceous material into so-called black nitrogen (BN) (Knicker 2007). This fraction is composed of heterocyclic aromatic structures such as pyrroles, imidazoles, and indoles (Almendros et al. 2003; Knicker 2010) which represent an integrated part of the polyaromatic network of pyrogenic organic matter (PyOM) (Knicker et al. 2008). As such, they were considered to contribute to the stable SOM pool, once they have been incorporated into the soil (Knicker and Skjemstad 2000). Indeed, earlier studies reported that the mean residence time of PyOM in soil is in the range of millennia or even more (DeLuca and Aplet 2008) although others detected mean residence time of 300 years (Lehndorff et al. 2014). Thus, the replacement of biogenic organic N sources by BN shortly after the fire may markedly decrease the fraction of bioavailable SON. However, previous experiments have demonstrated that the presence of BN can reduce the chemical recalcitrance of charred residues (Knicker 2010). It was shown that it is attacked by microorganisms, already after a few weeks after its addition to soils (Hilscher and Knicker 2011). It was further reported that the N mobilized during the degradation of BN can be taken up by plants (de la Rosa and Knicker 2011). These findings cast doubts on the role of BN as efficient N sink and raise the questions on how it is involved in the N cycling in soils. In order to fill this knowledge gap, we were interested if and to which extend N from BN is recycled by soil microorganisms for the built-up of their biomass. Therefore, we performed pot experiments in which the incorporation of 15N from 15N-enriched charred grass residues amended to a soil matrix into soil AAs was monitored for a period of 16 months. This design simulated the fate of BN during the time directly after the fire. Amino acids were chosen as markers for biological turnover of BN, since they are almost absent in BN but represent as a building block of peptides and proteins a major fraction of the soil biomass and its organic residues. In order to take the competition between plants and microorganisms for bioavailable N into account, Lolium perenne was grown on the amended soils. However, possible incorporation of 15N into the plants was determined but not considered in the presence study since its focus was the inclusion of BN-derived nitrogen into the biogenic proteinaceous fraction of SON. By including experiments with amendments composed of either only 15N-enriched plant residues or 15N-enriched plant residues mixed with unenriched PyOM or Ni and vice versa, we hoped to identify potential preferential use of one or the other N source. Whereas the amendment of the fresh PyOM provided information on the short-term impacts of BN addition on the N cycling, the medium-term effects were approached by comparing the 15N incorporation into AAs of soil materials derived from the same region but either from an unburnt site or from a location having experienced an intense fire 7 years before sampling.
2 Materials and methods
2.1 Sample material
The soil matrix for the incubation experiments derived from the first 5 cm of a burnt (N 37° 30′ W 6° 19′ AZQB2-1) and an unburnt Cambisol (IUSS Working Group WRB 2014) (N 37° 32′ W 6° 15′, AZQU2-3) from the Sierra de Aznalcóllar, southern Spain. The soils were sampled 7 years after an intense fire which occurred in this region in 2004. A more detailed description of the fire history of this region and the medium-term impact of the fire on soil parameters and organic matter composition can be obtained in López-Martín et al. (2016).
After removing visible root and plant residues, the soil was sieved through a 2-mm sieve, oven-dried at 40 °C, and stored at a dark and dry place before use. The respective soil parameters (pH, electrical conductivity, Corg, Nt, and nitrate) were previously determined (López-Martín et al. 2016). Briefly, the pH values of the sandy-loamy soils are 5.7 and 4.2 for the unburnt and burnt soil. The Corg and Nt content of the unburnt soil are 69.2 ± 1.4 mg Corg g soil−1 and 4.1 ± 0.3 mg N g soil−1, whereas concentrations of 57.6 ± 1.9 mg Corg g soil−1 and 2.6 ± 0.0 mg N g soil−1 were determined for the material from the burnt area.
The plant residues (OM) were produced by growing rye grass (L. perenne) on material from the unburnt soil under greenhouse conditions at 24 ± 2/17 ± 2 °C (16-h day/8-h night) and a relative humidity of 60 ± 10%. The only N source of the non-enriched plants was the N available in the soil, whereas the 15N enrichment of 15N-OM was achieved by a weekly watering with a K15NO3 − solution (99 atom%, 0.5 g l−1) to avoid access of N fertilization. In both experiments, the grass was harvested every second week by cutting the aboveground biomass with scissors. After drying it in paper bags, in an oven at 40 °C, the material was stored on a dark and dry place for further use. Since the N content of plants depends not only on the N availability during growth but also on the age of the plants, the yields after each cut were combined and homogenized before storing. The non-enriched (PyOM) and the 15N-enriched residues (15N-PyOM) were obtained after cutting the grass into pieces of approximately 10 cm and charring them in preheated ceramic trays (Ø = 15.5 cm) in a Muffler furnace at 350 °C, during 8 min and under oxic conditions. Whereas enough raw material for the production of non-enriched PyOM was yielded from the first growing experiment, a second sowing but with the same soil material was performed to obtain sufficient 15N-OM for the production of 15N-PyOM. The harvested grass of the respective cutting events was combined and homogenized before charring to overcome fluctuations in the efficiency of 15N incorporation during plant growth and to yield in homogeneously enriched PyOM. In order to ensure homogenous charring conditions, the grass layer in the trays did not exceed 0.5 cm. At the end of the heating period, the charred organic matter was cooled down staying in the switched-off furnace of which the door was kept open. For the 15N-PyOM, the charred material of both sowings was mixed thoroughly.
2.2 Incubation experiment
Sixteen plastic pots were filled with 100 g of sieved burnt or unburnt soil. Subsequently, 0.25 g of L. perenne seeds was planted and covered with the following amendments: (1) 30 mg of K15NO3 (15Ni), (2) 600 mg of 15N-OM, (3) 300 mg of non-enriched plant residues and 30 mg of K15NO3 (OM + 15Ni), (4) 300 mg of 15N-OM plus 30 mg of KNO3 (15N-OM + Ni), (5) 600 mg of 15N-PyOM, (6) 300 mg of PyOM plus 30 mg K15NO3 (PyOM + 15Ni), and (7) 300 mg of 15N-PyOM plus KNO3 (15N-PyOM + Ni). The amounts of total N (Nadd), 15N (15Nadd), and organic C (Corg/add) to each pot are summarized in Table 1. Note that the amount of amendments was adjusted to ensure comparable C/N ratios of material (soil + amendment) in the pots and to avoid excess of N, both of which could have resulted in alterations of the N cycling. Additionally, control pots filled with soil matrix and planted with seeds but without any amendment were prepared. All pots were incubated in a greenhouse operating at the same conditions as used for the production of the grass residues. The pots were watered with 30 ml of deionized water every 3 days. The pots were perforated at the bottom in order to remove excess of water. Every month, we simulated a bigger rain event by adding 100 ml of water. After 0.5, 1, 8, and 16 months, two pots per amendment were sampled and treated as duplicates. Except for the 2-week experiment, the above-ground biomass was removed by cutting monthly until the fourth month of incubation. Thereafter, the cutting was every 4 months, due to slower plant growth. At the end of the experiment, after removing the grass, the remaining litter layer, which was mainly composed of residual amendment, and the roots were separated from the mineral soil. All materials were dried at 40 °C in an oven and stored for further analysis.
2.3 Elemental composition and determination of 15N content
The Ct and Nt contents and the 15N isotopic signatures of the soils and the soil amendments were determined using a Flash 2000 HT combustion elemental microanalyzer and Flash HT Plus combustion elemental analyzer via a ConFlo IV unit to a continuous flow Delta V Advantage isotope ratio mass spectrometer (IRMS) (Thermo Scientific, Bremen, Germany). Since the pH of both soils was below 7 (6 for the unburnt, 5 for the burnt), the determined Ct corresponds to Corg.
2.4 Extraction and analysis of amino acids yielded after hydrolysis
Approximately 1 g of incubated soil or 0.2 g of fresh and charred organic amendments was weighted into a glass bottle (15 ml) and mixed with 5 ml of 6 M HCl and 0.05 mg of L-norleucine as an internal standard. L-norleucine was used for quantification of the AA loss during the purification steps. The soils and the organic amendments were hydrolyzed at 110 °C, for 22 h and under N2 atmosphere. After the hydrolysis, the samples were filtered through glass fiber membrane filters (0.07 μm, Wicom Perfect Flow, Germany) and the hydrolysate was dried under a flow of N2 to remove the HCl. The dried hydrolysates were redissolved in 4 ml of 0.1 M HCl, and 0.05 mg of trans-4-(aminomethyl)cyclohexane carboxylic acid was added as a second internal standard (Nowak et al. 2011). The solution containing the hydrolyzed and free AAs was purified by passage over H+ exchanged DOWEX 50W X8 resin. Prior to elution of the AAs with 2.5 M ammonium hydroxide, the impurities were washed out with 25 ml 0.1 M oxalic acid and then with 5 ml 0.01 M HCl and 5 ml of distilled water which were used to eliminate residues of oxalic acid. The carboxylic groups of AAs were esterified with isopropanol/acetylchloride (1:4 v/v; 1 h, 110 °C), and the amino groups were trifluoroacetylated with 1 ml of trifluoroacetic anhydride/dichlormethane (1:1 v/v; 1 h, 60 °C) (Miltner et al. 2009). After derivatization, the impurities were extracted into the aquatic phase of a mixture of chloroform/phosphate buffers, and the chloroform phase was dried under N2 (Ueda et al. 1989) and stored at −4 °C for subsequent analysis.
Samples were reconstituted in 100 μl dichloromethane, and the derivatized AAs were identified and quantified in duplicates by means of gas chromatography-mass spectrometry (GC-MS). A BPX5 column (30 m × 250 μm × 0.25 μm; SGE, TOWN, COUNTRY) and 7890A GC System (Agilent Technologies, Waldbronn, Germany) with a detector 5975C inert XL MSD (Agilent Technologies) were used. The initial temperature of 50 °C was kept for 5 min. Then, 100 °C was reached with 30 °C min−1 and held for 5 min. After that, the temperature increased at 10 °C min−1 to 175 °C where it remained constant for 5 min, then further heated to 250 °C at 10 °C min−1, held for 5 min, and to 325 °C at 30 °C min−1, held for 5 min. The injection was performed at a split ratio of 1:20 and at an injector temperature of 280 °C. For identification and quantification of the individual AAs, an external standard (200 μl) containing alanine, glycine, threonine, serine, valine, leucine, isoleucine, cysteine, proline, aspartic acid, methionine, glutamic acid, phenylalanine, tyrosine, lysine, histidine, and cysteine at a concentration of 2.5 μmol ml−1 for all AAs except for cysteine which had a concentration of 1.25 μmol ml−1 was used. For the calculation of the contribution of AAs, the recovery of individual AAs summed up after they have been identified by comparing the retention time and the mass spectra with the external standard, using the MSD ChemStation software (Agilent Technologies). In order to determine the content of 15N of the total AAs (15NAAs), the 15N of the individual derivatized AAs was measured by GC-combustion-isotope ratio-MS (GC-C-irMS) and summed up. Therefore, the AAs were separated with a 7890 A GC System (Agilent Technologies) equipped with a BPX5 column (50 m × 0.32 mm × 0.5 μm) using the following temperature program: increase of the temperature with 10 °C min−1 from 50 to 80 °C, which was kept for 7 min before 120 °C was reached with 3 °C min−1 and held for 5 min; heating to 210 °C at 3 °C min−1 After 5 min, the temperature was increased at 20 °C min−1 to 300 °C for 5 min. The samples were injected in the splitless mode at an injector temperature of 250 °C. The eluting compounds were combusted, and the resulting N2 was analyzed for its isotopic composition by means of a Finnigan MAT 253 IRMS (Thermo Finnigan, Bremen, Germany). In order to determine the amount of 15N recovered from 15Nadd of each treatment in the respective AA fractions, the 15NAAs content of the control soil with natural 15N abundance was subtracted from the 15NAA contents of the amended soils.
2.5 Solid-state 15N NMR spectroscopy
Prior to NMR analysis, the soil samples were demineralized with 10% (v/v) hydrofluoric acid (HF) (Gonçalves et al. 2003) in order to remove paramagnetic ions and to concentrate the OM. Briefly, 10 g of dried soil sample was weighed into a polyethylene bottle, and 40 ml of HF was added. The closed bottles were shaken for 2 h. After centrifugation, the supernatant was removed and discarded. The same procedure was repeated four times. The concentrated OM was washed with deionized water and freeze-dried.
The solid-state cross-polarization (CP) magic angle spinning (MAS) 15N-NMR spectra of the HF-treated soils were acquired with a Varian 7.05 T Unity Inova (15N resonance frequency 60.8 MHz), and the fresh and charred OM were obtained with a Bruker DMX 400 (15N resonance frequency 40.6 MHz) Bruker Avance III 600 (15N resonance frequency 60.8 MHz) using a spinning speed of 8, 4, and 15 kHz, respectively. A ramped 1H pulse was applied during the contact time of 0.7 ms. Using a pulse delay time of 200 ms, 7500 scans were accumulated for the spectra of the labeled fresh and charred OM. For the 15N NMR spectra of the soil samples, 1,000,000 scans were acquired with a contact time of 1 ms and a delay time 0.4 s.
3 Statistical analysis
The statistical analyses were accomplished using the software SPSS Statistic 17.0. Differences between the results obtained from different sampling events were evaluated using Mann-Whitney test. The impact of soil type and the effect of the substrate amendment on the change of the amount of newly synthesized AAs were analyzed using the Wilcoxon test. In order to reveal the impact of the fire history on NAAs extractability and 15N incorporation into NAAs, we statistically combined the results of all variations (both with amendment and with incubation time) of each soil. For more detailed information about the impact of the source material on the incorporation of 15Nadd in AAs of soils, the treatments were grouped into the following four sets: (I) control (C), (II) inorganic source (15Ni, OM + 15Ni, PyOM + 15Ni), (III) organic litter (15N-OM + Ni, 15N-OM), and (IV) charred organic matter (15N-PyOM + Ni, 15N-PyOM). Finally, with the aim to study the effect of the presence of inorganic nitrogen on the use and degradation of organic N, we compared the relative contribution of 15NAAs to NAAs in the experiments with the addition of 15Ni, 15N-OM, or 15N-PyOM from burnt and unburnt soil. A p value ≤0.05 was considered as statistically significant.
4 Results and discussion
4.1 Elemental composition of organic C, Nt, and 15N enrichment in the soils, OM, and the PyOM
Table 2 lists the organic C, Nt, and 15N contents of PyOM, OM, and the burnt and unburnt soils. Recent statistical evaluation of SOM alterations due to fire in the probed area indicated that 7 years after the event, the Corg and Nt values are only slightly higher in the unburnt area. A comparable observation is reported by Alcañiz et al. (2016) for soils recovered for 9 years after a prescribe fire in a Mediterranean area.
The non-enriched and 15N-enriched L. perenne showed C/N values between 6 and 13, which is in the range found in other studies (Knicker and Lüdemann 1995; de la Rosa and Knicker 2011; Hilscher and Knicker 2011). Note that the respective values for the PyOM and 15N-PyOM are within this range, which indicates that in spite of N losses during heating, a considerable fraction of the organic N was incorporated as BN into the charred material. For both unburnt and charred plant residues, the 15N content is 0.373 and 0.401 atom%, which agrees with the natural 15N abundance. The slightly higher value found for PyOM may be caused by the loss of N volatile compounds during combustion leading to a depletion of the lighter isotope (Fraser et al. 2013). The atom% 15N for unburnt and burnt soil ranges between 0.355 and 0.377 which also corresponds with the natural 15N abundance in organic material (Robinson 2001). The 15N abundance in 15N-OM is with 54.495 ± 0.332 atom% considerably higher than in 15N-PyOM (20.641 ± 0.261 atom%), which is best explained with the fact that the source material of those residues derived from two different growing experiments.
4.2 Distribution of N forms in the starting materials
The 15N NMR spectra (Fig. 1) of the burnt and unburnt soils are dominated by the signal between −240 and −285 ppm which is assigned to amide N (Knicker and Lüdemann 1995). Note that indole-type N and proline N may also contribute to the region between −240 and −250 ppm. A small signal appears at −346 ppm which is most likely caused by N bound to Cε in lysine and by N in other free amino groups of amino acids and amino sugars (Witanowski et al. 1993; Knicker 2011a). The solid-state 15N NMR spectrum of the burnt soil shows no major intensity in the BN-typical region of pyrrole-type and indole-type N between −145 and −250 ppm (Knicker 2010). Since in other studies of the same sampling area, signals of BN were clearly dominating the solid-state 15N spectrum of a burnt soil collected 4 weeks after an intense fire (Knicker 2011c), the low intensity in the chemical shift region of heterocyclic N may indicate that BN has been partially removed either by degradation, erosion, or leaching. The dominance of amide N in this spectrum, most tentatively of biogenic origin, is in line with recent results obtained by 13C NMR spectroscopy, revealing a fast recovery of the SOM to its pre-fire composition (López-Martín et al. 2016).
In contrast to the solid-state 15N NMR spectrum of the fresh grass material, which is dominated by signals of peptides (Knicker and Lüdemann 1995; de la Rosa and Knicker 2011), the 15N NMR spectrum of PyOM confirms the presence of BN by clear signals peaking at −235 and −245 ppm which are typical for pyrrole N and indole N. However, some intensity is still recovered between −250 and −285 ppm which may indicate that not all peptides were transformed into heterocyclic N.
4.3 Total amino acids and total N of the extracted AAs in the starting materials
The contents of extracted AAs in the control soil of the unburnt area and in that of the nearby burnt region were 2.9 ± 0.2 and 4.0 ± 0.6 mg AAs g soil−1, respectively (Table 2). These values are in the range of those found for soils studied by Amelung and Zhang (2001) where the total AAs varied between 0.5 and 16.0 mg AAs g soil−1. In general, only a small proportion of Nt of soils is hydrolyzed and recovered as NAAs by the used method. Friedel and Scheller (2002) or Amelung et al. (2006) recovered 28 to 50 and 22 to 46% of Nt as NAAs. In our approach, the recovery of Nt as NAAs was between 9 and 19% for the soils (Table 2). Higher AA contents and NAA recoveries were obtained for OM and 15N-OM. Here, the contribution of NAAs to Nt ranged from 31 to 53%. It seems that due to the higher humification degree of SOM, their peptides are better protected from hydrolysis than those in fresh litter. Considerably low amounts of AAs were obtained for PyOM (4.9 ± 1.1 mg g dry material−1). In PyOM and 15N-PyOM, 2.51 ± 0.62 and 1.93 ± 0.02% of Nt accounted for NAAs. Comparably, only 2% of the total 15Nadd in 15N-PyOM was amended as 15NAAs to the soil before starting the experiment.
Statistical analysis of the extractable NAA contents normalized to Nt (Table 2) for both soils confirmed that in the fire-affected area, the percentage of Nt attributable to AAs is twice the amount determined for the unburnt soil (p = 0.01). Thus, although the N content and the dominance of peptide N in the soils of the burnt region recovered to the status of the unburnt soil, (López-Martín et al. 2016), the quality of the present peptides seems to be still affected by the former fire. Possibly, the fire history resulted in the production of fresh peptides which are more accessible to hydrolysis than those commonly accumulated in soils during humification.
4.4 Extractability of total AAs and NAAs as a function of incubation time
During the incubation, the mean concentration of extracted AAs in the soils treated with the different amendments varied between 2.4 and 13.2 mg g soil−1 (Table 3). In order to assess the relationship of the AAs extractability with either incubation time, we statistically compared the combined treatments of the unburnt with those of the burnt applying the Wilconxon test. As it can be revealed from Table 4, this analysis confirms decreasing recovery of AAs with incubation time. Comparing the results between burnt and unburnt soils showed further that the yields were always higher for the latter. A comparable approach was used for the statistical analysis of the relationship between NAAs extractability with either incubation time or soil type (Fig. 2). Here, it should be noted that with ongoing incubation, the Nt content of the soils did not change significantly, neither between unburnt and burnt material nor between the different amendments, indicating that no major N loss by volatilization occurred. NMR spectroscopic data (data not shown) confirmed that no heterocyclic N was formed during the incubation, although for both soils, the amount of extractable NAAs decreased from the beginning until the end of the experiment (Fig. 2). Thus, the decline of extractable AAs with incubation time points toward ongoing AAs sequestration and transformation as it was suggested by Miltner et al. (2009) and also agrees with findings by Creamer et al. (2012) and Nowak et al. (2013). Comparing the experiments with burnt and unburnt soils, we observed a general lower extractability of the NAAs in the burnt soils. However, this difference is only statistically confirmed for the samples analyzed after 8 (p = 0.000) and 16 (p = 0.013) months of incubation. These observations may evidence that in our soils, the fire history can indeed affect the N cycling, although the impact was only clearly discernable at the end of the experiment.
Effect of time on the percentage of Nt which is extractable with the AAs (NAAs) from burnt and unburnt soils amended with N-rich burnt and unburnt organic matter or inorganic N. For statistical reason, the impact of the kind of the amendment was not considered. Median values ± interquartile range (Mann-Whitney test, p < 0.005, n = 16)
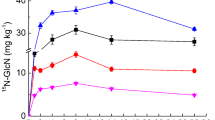
4.5 Incorporation of 15Nadd into NAAs
Figure 3 shows that already 2 weeks after addition of the amendment, 15Nadd is incorporated into the NAAs. Its amount varied between 2 and 4% of 15Nadd. The low values may be explained by the facts that (1) part of 15Nadd was also incorporated into the growing plant residues and that (2) not all AAs of the soil were in an extractable form. For the experiment with 15N-PyOM, the recovery of 15NAAs derived from 15Nadd varied between 0.67 and 12.62% of 15Nadd which in average is slightly higher than the amount of 15NAAs added at the beginning of the experiment. This may allow the conclusion that BN underwent a microbial transformation into non-heterocyclic residues. This is supported by the observation that already after 4 months, approximately 2% of 15Nadd was recovered in the leaves of the freshly grown grass (data not shown). The 15Nadd incorporated into NAAs decreases with time. The assimilation of 15Nadd into NAAs was a bit higher in burnt than in unburnt soil with statistical differences at month 8 (p = 0.039) suggesting that the fire history after 7 years did not affect the incorporation of organic N derived from fresh litter into peptideous material of SOM and microbial biomass.
Contribution of 15N from 15N amendments (15Nadd) recovered as 15N of AAs (15NAAs) after incubation of soil material from an unburnt and burnt Cambisol amended with 15N-enriched burnt and unburnt organic matter and inorganic 15N. For statistical reason, the impact of the kind of amendment was not considered. Median values ± interquartile range (Mann-Whitney test, p < 0.005, n = 16)
4.6 Impact of the N sources on the 15N content of NAAs
In order to obtain more detailed information about the impact of the source material on the incorporation of 15Nadd in AAs of soils, the contribution of 15N to the total N of the amino acids (NAAs) was determined and the results were statistically analyzed after grouping the treatments according to their 15Nadd source into the following four sets: (I) control, (II) inorganic source (15Ni, OM + 15Ni, PyOM + 15Ni), (III) organic litter (15N-OM + Ni, 15N-OM), and (IV) charred organic matter (15N-PyOM + Ni, 15N-PyOM). Note that the value of 0.366% corresponds to the natural 15N abundance and was found for the control set I (Fig. 4). The fact that relative to the control set I, all other sets had higher 15NAA recoveries indicates that 15Nadd has been incorporated into the extractable AAs fraction of the soil. However, since in average, the amount of 15Nadd in NAAs in the soil is only slightly higher than the percentage of NAAs in PyOM, we cannot unbiasedly differentiate if the recovered 15NAAs originate from the accumulation of PyOM-derived AAs or from AAs which were newly synthesized by microorganisms from BN.
Contribution of 15N (15NAAs) to N of AAs (NAAs) after incubation of soil material from an unburnt and burnt Cambisol amended with 15N-enriched burnt and unburnt organic matter and inorganic 15N. In order to evaluate the impact of the source material, the impact of the fire history of the soil was not considered and the results were grouped depending on the N source: control (C), inorganic N (15Ni), fresh organic N (15N-OM), and charred organic N (15N-PyOM) Median values ± interquartile range (Wilcoxon test, p < 0.005, n = 8)
In general, the contribution of 15NAAs to the total NAAs increased continuously until the eighth month (Fig. 4) although the recovery of the latter decreased with incubation time (Fig. 2). At the end of the experiment time, the contribution of 15NAAs to total NAAs is approximately 46% higher than that obtained after 2 weeks. From this, it can be concluded that peptidic pool suffered a fast turnover in which the original AAs were mobilized and replaced by new peptides with AAs containing 15Nadd. Whereas the first was most likely used for the synthesis of new biomass, the latter may have been released from biomass which already had been incorporated 15N from the amendment. Possible sources of the released material are decaying residue, exudates, or as exo-enzymes. Statistical analysis confirmed that differences also can be discerned between samples analyzed after the same incubation time but with different amendments (p < 0.05) (Table 4). Compared with the experiments amended with 15N-PyOM, those with the addition of 15N-OM always showed higher 15Nadd contribution to NAAs (Fig. 4). However, here, one has to bear in mind that we cannot discriminate if the extracted AAs derived from the 15N-enriched plant residues which were incorporated into SOM or from newly synthesized biomass.
The 15Nadd contribution to NAAs increases for the Ni and OM treatments only until the eighth month. After that, we could not reveal any significant statistical difference. However, the 15NAA recovery from the PyOM source augments slower but continuously until the end of the experiment (p = 0.002; 0.0050 ± 0.0004 mg 15NAAs mg NAAs −1). This value is slightly but significantly higher than the natural abundance of 15N. Note that the incorporation of 15Nadd from Ni was only little bit higher than from PyOM which is interesting considering that inorganic N forms are commonly highly bioavailable (Hu et al. 2016), whereas BN is commonly assumed to be biochemically more recalcitrant.
4.7 Competition between Ni and Norg
In order to investigate how the presence of inorganic nitrogen affects the turnover and degradation of organic N, we statistically compared the relative contribution of 15NAAs to NAAs in the experiments with the addition of 15Ni, 15N-OM, or 15N-PyOM, respectively (Fig. 5). Note that if two different N sources are used and one is 15N-enriched and the other not, the increase of the relative contribution of 15NAAs to NAAs indicates the use of the 15N source and vice versa. The immobilization of 15Ni increases until the eighth month which is confirmed by the statistical difference between control and the combination of Ni with fresh or charred material at each month (p < 0.05) (Fig. 5a). However, statistically, additional amendment of unenriched OM and PyOM did not affect the contribution of AAs formed after immobilization of 15Ni. Concomitantly, no significant impact of alternative organic N sources on the percentage of added 15Ni which is incorporated into AAs (Fig. 6a) was revealed.
Contribution of 15N to the N of AAs (NAAs) after incubation of soil material (both from an unburnt and burnt Cambisol) amended with a inorganic 15N (15Ni) and with 15Ni together with unburnt (OM) or burnt organic matter (PyOM), b 15N-OM and 15N-OM together with Ni, and c 15N-PyOM and 15N-PyOM together with Ni. Median values ± interquartile range (Wilcoxon test, p < 0.005, n = 4)
Recovery of 15N from 15N-enriched amendments (15Nadd) in AAs (15NAAs) after incubation of soil material from an unburnt and burnt Cambisol amended with a 15Ni and with 15Ni together with unburnt (OM) or burnt organic matter (PyOM), b 15N-OM and 15N-OM together with Ni, and c 15N-PyOM and 15N-PyOM together with Ni. Median values ± interquartile range (Mann-Whitney test, p < 0.005, n = 4)
With respect to the incorporation of 15N from 15N-OM (Fig. 5b), there is a tendency that more 15N is incorporated into NAAs if no Ni is present. This trend would suggest that if both inorganic and organic N are present, both sources are used at the same time. However, according to Fig. 6b, the efficiency of the use of 15N derived from 15N-OM (expressed as the percentage of 15N derived from 15Nadd which was recovered in AAs) was statistically not affected by an additional Ni source which is in line with Zhang et al. (2015) reporting that there, they observed no preferential incorporation of Ni over plant residue N into microbial amino sugars.
With respect to the control, addition of 15N-PyOM with and without Ni does not significantly alter the abundance of 15N in NAAs until 2 weeks after starting the experiment (Fig. 5c), but incorporation of 15N from 15N-PyOM starts to be statistically relevant after 1 month and continues until the end of the incubation (p < 0.05) (Table 5). The contribution of 15N from 15N-PyOM in NAAs is significantly higher in the experiments without additional Ni amendment, confirming that in the presence of both, both N sources are used simultaneously. Comparable to the unburnt N source, we have no statistical proof for a decrease of the efficiency of incorporating 15Nadd from PyOM if additional Ni is present (Fig. 6c).
5 Summary and conclusions
The performed pot experiment carried out for 16 months, using burnt and unburnt soil, clearly demonstrated that former fire events can have an impact on N cycling in soils. Although the extraction of AAs from both soils resulted in comparable yields, the relative contribution of extractable NAAs to Nt is significantly higher in the fire-affected soils. Considering that the NMR spectra attributed almost all of the organic N to peptide, these results allow the conclusion that after fire events, the newly synthesized peptides have a lower resistance against acid hydrolysis than peptides immobilized in soil organic matter formed without fire impact. Addition of fresh litter seems to shift the higher extractability of AAs toward the unburnt soil, possibly because the first provides additional labile AAs and allows for a higher microbial activity leading to new biomolecules with low resistance against acid hydrolysis. The fact that in several pots, more 15N from 15N-PyOM was recovered in AAs than added as 15NAAs suggests that aside from incorporation of PyOM-derived AAs into the soil AA pool, at least some 15N of BN was recycled for the built-up of peptides in newly synthesized microbial biomass. With this step, the BN-derived nitrogen has transformed into a biogenic N source and is expected to behave as such during N cycling within the SON pool. Statistical analysis of our data did not reveal a significant impact of the presence of organic N on the percentage of added inorganic N which was incorporated into the peptidic N pool and vice versa. Although we could not prove unbiasedly a preferential immobilization of the N of any of the tested sources into AAs, our studies indicate that both inorganic N and organic N are simultaneously used as N supply. Thus, the presence of easily bioavailable N is not hindering the synthesis of new soil peptides from N in bound charred organic residues.
References
Alcañiz M, Outeiro L, Francos M, Farguell J, Úbeda X (2016) Long-term dynamics of soil chemical properties after a prescribed fire in a Mediterranean forest (Montgrí Massif, Catalonia, Spain). Sci Total Environ 572:1329–1335
Almendros G, Knicker H, González-Vila FJ (2003) Rearrangement of carbon and nitrogen forms in peat after progressive thermal oxidation as determined by solid-state 13C- and 15N-NMR spectroscopy. Org Geochem 34:1559–1568
Amelung W, Zhang X (2001) Determination of amino acid enantiomers in soils. Soil Biol Biochem 33:553–562
Amelung W, Zhang X, Flach KW (2006) Amino acids in grassland soils: climatic effects on concentrations and chirality. Geoderma 130:207–217
Creamer CA, Filley TR, Olk DC, Plante A, Peltre C, Top SM, Boutton TW (2012) Degree of woody encroachment into grasslands controls soil carbohydrate and amino compound changes during long term laboratory incubation. Org Geochem 52:23–31
de la Rosa JM, Knicker H (2011) Bioavailability of N released from N-rich pyrogenic organic matter: an incubation study. Soil Biol Biochem 43:2368–2373
DeLuca TH, Aplet GH (2008) Charcoal and carbon storage in forest soils of the Rocky Mountain West. Fron Ecol Environt 6:18–24
Fraser RA, Bogaard A, Charles M, Styring A, Wallace M, Jones G, Ditchfield P, Heaton THE (2013) Assessing natural variation and the effects of charring, burial and pre-treatment on the stable carbon and nitrogen isotope values of archaeobotanical cereals and pulses. J Archaeol Sci 40:4754–4766
Friedel JK, Scheller E (2002) Composition of hydrolysable amino acids in soil organic matter and soil microbial biomass. Soil Biol Biochem 34:315–325
Gärdenäs AI, Ågren GI, Bird JA, Clarhlom M, Hallin S, Ineson P, Kätterer T, Knicker H, Nilsson SI, Näsholm T, Ogle S, Paustian K, Persson T, Stendahl J (2011) Knowledge gaps in soil carbon and nitrogen interactions—from molecular to global scale. Soil Biol Biochem 43:702–717
Gonçalves CN, Dalmolin RSD, Dick DP, Knicker H, Klamt E, Kögel-Knabner I (2003) The effect of 10% HF treatment on the resolution of CPMAS 13C NMR spectra and on the quality of organic matter in Ferralsols. Geoderma 116:373–392
Hilscher A, Knicker H (2011) Degradation of grass-derived pyrogenic organic material, transport of the residues within a soil column and distribution in soil organic matter fractions during a 28 month microcosm experiment. Org Geochem 42:42–54
Hu G, He H, Zhang W, Zhao J, Cui J, Li B, Zhang X (2016) The transformation and renewal of soil amino acids induced by the availability of extraneous C and N. Soil Biol Biochem 96:86–96
IUSS Working Group WRB (2014) World reference base for soil resources 2014. International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports No. 106. FAO, Rome
Knicker H (2007) Vegetation fires and burnings, how does char input affect the nature and stability of soil organic nitrogen and carbon?—a review. Biogeochemistry 85:91–118
Knicker H (2010) “Black nitrogen”—an important fraction in determining the recalcitrance of charcoal. Org Geochem 41:947–950
Knicker H (2011a) Pyrogenic organic matter in soil: its origin and occurrence, its chemistry and survival in soil environments. Quatern Int 243:251–263
Knicker H (2011b) Soil organic N—an under-rated player for C sequestration in soils? Soil Biol Biochem 43:1118–1129
Knicker H (2011c) Solid state CPMAS 13C and 15N NMR spectroscopy in organic geochemistry and how spin dynamics can either aggravate or improve spectra interpretation. Org Geochem 42:867–890
Knicker H, Fründ R, Lüdemann H-D (1993) The chemical nature of nitrogen in soil organic matter. Naturwissenschaften 80:219–221
Knicker H, Hilscher A, González-Vila FJ, Almendros G (2008) A new conceptual model for the structural properties of char produced during vegetation fires. Org Geochem 39:935–939
Knicker H, Lüdemann HD (1995) N-15 and C-13 CPMAS and solution NMR studies of N-15 enriched plant material during 600 days of microbial degradation. Org Geochem 23:329–341
Knicker H, Skjemstad JO (2000) Nature of organic carbon and nitrogen in physically protected organic matter of some Australian soils as revealed by solid-state 13C and 15N NMR spectroscopy. Aust J Soil Res 38:113–127
Lehndorff E, Roth PJ, Cao ZH, Amelung W (2014) Black carbon accrual during 2000 years of paddy-rice and non-paddy cropping in the Yangtze River Delta, China. Glol Change Bio 20:1968–1978l
López-Martín M, Velasco-Molina M, Knicker H (2016) Variability of the quality and quantity of organic matter in soil affected by multiple wildfires. J Soils Sediments 16:360–370
Miltner A, Kindler R, Knicker H, Richnow H-H, Kästner M (2009) Fate of microbial biomass-derived amino acids in soil and their contribution to soil organic matter. Org Geochem 40:978–985
Nowak KM, Girardi C, Miltner A, Gehre M, Schäffer A, Kästner M (2013) Contribution of microorganisms to non-extractable residue formation during biodegradation of ibuprofen in soil. SciTotal Environ 445–446:377–384
Nowak KM, Miltner A, Gehre M, Schäffer A, Kästner M (2011) Formation and fate of bound residues from microbial biomass during 2,4-D degradation in soil. Environ Sci Technol 45:999–1006
Robinson D (2001) δ15N as an integrator of the nitrogen cycle. Trends Ecol Evol 16:153–162
Rovira P, Romanyà J, Duguy B (2012) Long-term effects of wildfires on the biochemical quality of soil organic matter: a study on Mediterranean shrublands. Geoderma 179–180:9–19
Scharenbroch BC, Nix B, Jacobs KA, Bowles ML (2012) Two decades of low-severity prescribed fire increases soil nutrient availability in a Midwestern, USA oak (Quercus) forest. Geoderma 183–184:80–91
Stevenson FJ (1982) Organic forms of soil nitrogen. In: Stevenson FJ (ed) Nitrogen in Agricultural Soils. ASA-CSSA-SSSA, Madison, pp. 67–122
Ueda K, Morgan SL, Fox A, Gilbart J, Sonesson A, Larsson L, Odham G (1989) D-Alanine as a chemical marker for the determination of streptococcal cell wall levels in mammalian tissues by gas chromatography/negative ion chemical ionization mass spectrometry. Anal Chem 61:265–270
Varela ME, Benito E, Keizer JJ (2015) Influence of wildfire severity on soil physical degradation in two pine forest stands of NW Spain. Catena 133:342–348
Witanowski M, Stefaniak L, Webb GA (1993) Nitrogen NMR spectroscopy. Annual reports on NMR spectroscopy. Academic Press, London
Zhang H, Ding W, Luo J, Bolan N, Yu H (2015) The dynamics of glucose-derived 13C incorporation into aggregates of a sandy loam soil following two-decade compost or inorganic fertilizer amendments. Soil Till Res 148:14–19
Acknowledgements
The Ministerio de Economía y Competitividad (MINECO) de España and the European Regional Development Fund (ERDF) are acknowledged for financial support of the project (CGL2009-10557). The MINECO is also gratefully acknowledged for providing the Formación de Professional Investigator (FPI) grant (BES-2010-42581) and the International Humic Substance Society for donating the IHSS Training Award to the senior author. The Helmholtz Centre for Environmental Research UFZ is thanked for hosting the awardee. We also thank Ursula Günther and Matthias Gehre (UFZ, Department of Isotope Biogeochemistry) for their help with the compound specific isotope analysis of the amino acids.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zucong Cai
Rights and permissions
About this article
Cite this article
López-Martín, M., Nowak, K.M., Milter, A. et al. Incorporation of N from burnt and unburnt 15N grass residues into the peptidic fraction of fire affected and unaffected soils. J Soils Sediments 17, 1554–1564 (2017). https://doi.org/10.1007/s11368-016-1624-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-016-1624-x