Abstract
Purpose
Concentrations and transformations of mercury were measured in river, estuarine, and marine sediments to determine factors affecting the fate of mercury entering the northern Adriatic Sea.
Materials and methods
Radiotracer methodology was used to compare rates of mercury methylation (203Hg), MeHg demethylation (14C), and sulfate reduction (35S) in sediment depth profiles to concentrations of total and dissolved mercury species in the lower freshwater region of the Isonzo River, the coastal lagoons, and in the Gulf of Trieste, northern Adriatic Sea.
Results and discussion
Mercury was readily methylated and demethylated in all sediments, but the relative activity of these processes varied greatly with location. Methylation activity increased greatly from freshwater to the marine regions; however, demethylation was extremely high in the estuarine and lagoon sites. Ratios of methylation to demethylation were low in these coastal sites but increased further offshore in the gulf, which agreed with increased ratios of MeHg to total Hg (%MeHg) in gulf sediments. Comparisons of microbial activities indicated that sulfate reduction strongly controlled both methylation and demethylation. However, Hg methylation in coastal lagoon sediments was controlled by rapid demethylation and the bioavailability of Hg that was affected by Hg adsorption and precipitation. Methylation in offshore marine sites correlated with sulfate reduction but not the partitioning of Hg between pore water and solid phases. The decrease in sulfide production offshore exacerbated Hg methylation.
Conclusions
The freshwater to marine gradient in the Idrija/Soča/Isonzo/Adriatic region is dynamic, exhibiting horizontally variable rates of microbial activities and Hg transformations that create “hot spots” of MeHg accumulation that are controlled differently in each region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Methylmercury (MeHg), a potent neurotoxin, has become a global pollutant that enters the environment from a variety of dispersed sources such as the atmosphere or from point sources (Clarkson 2002). Much of the consumption of MeHg by humans is via fish, which bioaccumulate MeHg from the food web (Fitzgerald and Clarkson 1991; Bloom 1992). Even though the global use of mercury (Hg) has decreased recently, it is still released significantly from energy generation, incineration, and mobilization from mining activities (Fitzgerald 1993). Mercury can also be transported hundreds of kilometers from point sources, such as Hg mines, and this Hg can become bioavailable and methylated to MeHg, thus posing a threat to wildlife and humans (Hines et al. 2000).
MeHg is produced anaerobically by sulfate-reducing bacteria (Compeau and Bartha 1985; Gilmour et al. 1992; Choi et al. 1994; Lin et al. 2011), iron-reducing bacteria (Fleming et al. 2006; Kerin et al. 2006; Lu et al. 2016), methanogenic bacteria (Yu et al. 2013), and fermenting bacteria (Gilmour et al. 2013), and it has been shown that sulfur cycling is important in controlling the bioavailability and transformation of Hg species (Benoit et al. 1999; Hammerschmidt and Fitzgerald 2004; Schartup et al. 2014). Hg and MeHg are particle reactive, and the partitioning between solid and dissolved phases and binding to organic matter have been shown to be strongly related to the rate of Hg methylation and the fraction of total Hg present as MeHg (Mason and Lawrence 1999; Varekamp et al. 2000; Hammerschmidt and Fitzgerald 2004; Han et al. 2007).
The demethylation of MeHg is also microbially mediated, and it can cause a decrease in the quantity of MeHg available for bioaccumulation (Marvin-DiPasquale et al. 2000; Barkay and Wagner-Dobler 2005). Bacterially mediated MeHg demethylation occurs via either a reductive process that produces Hg0 and CH4 or by an oxidative process that produces Hg2+ and CO2 (Barkay et al. 2003).
The Idrija Hg mine in west-central Slovenia, which is the second largest mine in the world, opened in the late fifteenth century, and was mined continually until recently. Of the five million metric tons of Hg ore mined, over 25% of the Hg is thought to have dissipated into the environment and the mining operation severely enhanced the mobilization of Hg through smelting activities and the deposition of mine tailings in Idrija (Palinkas et al. 1995). Even though the mine was recently closed, the system still delivers about 1.5 t of Hg to the sea annually, which is about 60 km away from Idrija, and the MeHg is elevated in the marine environment (Sirca and Rajar 1997; Horvat et al. 1999; Faganeli et al. 2003).
Hines et al. (2000) reported data on the concentrations of total Hg and MeHg in unfiltered water samples collected throughout the Idrija system from relatively pristine sites upstream of the Hg mine into the Gulf of Trieste over 60 km away. It was shown that although total Hg and MeHg concentrations decreased greatly in samples collected in the Idrija River moving downstream from the mine, both of these parameters increased again several fold in the Soča River despite the fact that the Soča system diluted the Hg greatly. This mobilization of Hg species was believed to be due to processes occurring in fine sediments deposited behind dams, and it has been shown that organic matter accumulation within reservoir sediments enhances Hg methylation therein (Meng et al. 2016). In addition, both species were further mobilized, i.e., they increased in concentration even more as the river system mixed with seawater in the estuarine portion of the Soča/Isonzo River in Italy, presumably as a result of the mobilization of Hg from fine cinnabar particles as dissolved sulfide increased in the sediments (Paquette and Helz 1995). The ratio of MeHg to total Hg in these unfiltered water samples increased greatly in the impoundment freshwater region and again in the coastal marine sites indicating that the remobilized Hg was available for methylating bacteria to produce MeHg at great distances from the Hg source at the upstream mine. Hence, changing biogeochemical conditions throughout the system appears to control a very dynamic Hg cycle that could pose a threat to biota several tens of kilometers from a point source of Hg.
The study presented here is a compilation of data from other studies that focused on specific regions of the system such as the freshwater impoundments, the brackish region in the upper estuarine area of the Idrija River, the mouth of the Idrija River, the coastal lagoons, and a gradient of sites from the coast into the center of the Gulf of Trieste in the northern Adriatic Sea (Hines et al. 2000, 2006, and 2012). Here, we compare processes affecting Hg biogeochemistry at these locations and demonstrate how differently these areas may transform Hg as it moves into the marine environment, and how much of these differences are due to horizontal changes in sulfate reduction.
2 Materials and methods
2.1 Site description
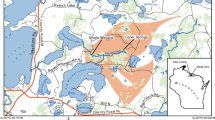
The river Idrijča (Idrija River) flows through the mercury-mining town of Idrija in western Slovenia and merges with the Soča River about 24 km downstream from Idrija (Fig. 1). The river Soča has three dams associated with hydroelectric power plants. The bed load is nearly eliminated in the impoundments behind these dams, while transport of suspended sediment remains high, especially during high water discharge. After crossing the Slovene–Italian border, the river changes its name from the Soča to the Isonzo and flows over low plains to the Gulf of Trieste at a mean discharge of ~170 m3 s−1 (Mosetti 1983).
The Gulf of Trieste is a 500-km2, shallow (20–25 m) basin in the northernmost part of the Adriatic Sea. The gulf is bounded by Slovenia to the southeast and Italy to the north and west. The salinity of gulf waters ranges between 33 and 38.5‰, and bottom water temperatures from 8 (February) to 22 °C (September). Vertical temperature and salinity gradients in late summer result in bottom water oxygen depletion and occasionally hypoxia and anoxia. Sediments in the northern part of the gulf primarily originate from the Isonzo River and are mostly composed of carbonates. Sedimentation rates (210Pb) are ~1 mm year−1 in the central part of the gulf to ~3–5 mm year−1 near the shore (Faganeli 1989; Ogorelec et al. 1991). Surface sediments are bioturbated primarily by polychaetes and bivalves down to the depth of 10–15 cm (Avcin and Vriser 1983; Hines et al. 1997).
The Grado and Marano lagoon system (45° 40′–45° 45′ N, 13° 05′–13° 35′ E) (160 km2) is composed of two lagoons, with an average width of 5 km extending for about 35 km, along the northern Adriatic coast (Fig. 1). The primary source of suspended sediments arrives from the sea as the contribution of river deltas and from erosion of the barrier islands (Brambati 1970). The Grado Lagoon (76 km2) is located in the eastern sector of the system, is very shallow (<1 m, on average), and consists of tidal flats and salt marshes. The Marano Lagoon (84 km2) to the west is slightly deeper than the Grado Lagoon and also contains a larger surface area that is covered with salt marshes. Sediment supply in the lagoons has been historically provided by the Isonzo River located east of the lagoon system (Covelli et al. 2001). Mercury concentrations show a progressive westward decrease from about 10 μg g−1 (Grado Lagoon) to 1 μg g−1 (Marano Lagoon) (Brambati 2001).
2.2 Sample handling
Six-centimeter diameter sediment cores were collected by hand at each site. After transport to the laboratory and removal of the overlying water by siphon, cores were placed into an N2-filled glove bag and six individual horizontal sections from the upper 10–12 cm from each core were removed, placed into jars, and homogenized. Sample aliquots were added to N2-flushed serum vials for Hg transformation measurements and 5-cc syringes for SO4 2−-reduction measurements.
Separate cores were used for Hg analysis, which were also extruded, sectioned, centrifuged, and filtered under N2. Pore waters were collected in pre-acid-cleaned containers and stored frozen until analysis. Solid phase samples for Hg analyses were freeze-dried, homogenized with a mortar and pestle, and sieved through a 420-μm screen to remove coarse shell debris.
2.3 Hg transformation analyses
Sample aliquots (3 mL sediment) for Hg methylation and MeHg demethylation assays were placed in duplicate into 13-mL serum vials, which were injected with 3.0 mL of anoxic seawater to prepare slurries. All reaction vessels were sealed with butyl rubber septa and crimped with aluminum seals. Septa for methylation assays were lined with Teflon. Vials for Hg methylation were injected with 2.0 μL of a solution containing 0.5 μCi of 203HgCl2, which amounted to ~170 ng Hg(II) per vial, and which was less than the ambient Hg concentrations by ~5–90-fold. Vials for MeHg demethylation were injected with 2.0 μL of a solution containing 0.1 μCi of 14CH3HgCl, which amounted to ~334 ng Hg mL−1 sediment, and which exceeded ambient MeHg concentrations by ~60–370-fold. The 14CH3HgCl was purified before use by extraction into methylene chloride (CH2Cl2) followed by back extraction into distilled water. The latter extraction included the removal of CH2Cl2 by bubbling with N2. Purified 14C–MeHg was assayed and determined to be 100% MeHg. Vials were incubated for ~18 h at in situ temperature in the dark. After incubation, vials for methylation assays received multiple injections of small volumes of 6 N HCI to stop the reaction, to dissolve carbonate material, and to store samples at low pH; 4–6 mL of 6 N HCI was required to dissolve carbonates. Alkali (1.0 mL NaOH, 3 N) was added following incubation to stop the demethylation reaction and to sequester 14CO2 in the liquid phase. Killed controls for both methylation and demethylation consisted of identical subsamples treated with acid or alkali, respectively, prior to adding radioisotopes.
For methylation assays, MeHg was extracted twice into toluene following treatment with CuSO4 and KCl in H2SO4. Pooled toluene extracts were dehydrated using anhydrous NaSO4 and radioactivity determined by scintillation counting. Methylation activity, determined from conversion of 203HgCl2 to methyl-203Hg, yielded a first-order rate constant that is a measure of the fraction of inorganic 203Hg converted to methyl-203Hg per time. Demethylation was determined by measuring 14C in CO2 and CH4 using a gas-stripping and trapping system similar to that described by Gray and Hines (2009). Briefly, CH4 was flushed from the vial with air (30 mL/min for 15 min) and combusted to CO2 in a CuO-packed quartz column at 850 °C. The resulting CO2 was trapped in phenethylamine, methanol, and a toluene-based scintillation fluid. One milliliter of CH4 was injected into each vial to facilitate removal of CH4. Following 14CH4 measurements, samples were transferred to 120-mL vials and were slowly acidified with 6.0 N HCl to convert 14C to 14CO2 that was stripped via a stream of N2 for and trapped as described previously. Radioactivity was determined by scintillation counting. Demethylation activity, determined from the conversion of 14C–MeHg to 14CO2 and/or 14CH4, also yielded a first-order rate constant.
2.4 Sulfate reduction activity
Sulfate reduction rates were determined using 35S and the chromium reduction assay as described previously (Hines et al. 1997, 2008). Briefly, sample aliquots from each vertical subsection of sediment were placed anaerobically into 5.0-cc syringes (distal end removed) and sealed with rubber stoppers. 35SO4 was injected into syringes that were then incubated overnight at in situ temperature in an N2-filled jar. The reaction was stopped by freezing, and reduced S species were stripped by an N2 gas stream while refluxing in a mixture of HCl, reduced Cr, and ethanol (Hines et al. 2008). The resulting H2S was trapped as ZnS in a Zn acetate solution, and radioactivity of half of the material was determined by scintillation counting.
2.5 Mercury speciation
Mercury speciation was determined as described by Horvat et al. (2002). Total Hg in sediment samples was determined using cold vapor atomic absorption (CVAAS) after acid digestion (Horvat 1991). Accuracy was determined using certified reference material (PACS-2 marine sediment, NRCC) and the relative standard deviation of at least three determinations was <5%. Samples for total dissolved (pore water) Hg were acidified and oxidized by exposure to ultraviolet light in the presence of BrCl. After a pre-reduction using NH2OH· HCl, Hg was determined by CVAAS after reduction by SnCl2 and gold trapping (Horvat et al. 1987; Horvat 1991). The reproducibility was 4% and BCR 579 was used as a certified reference material.
Total MeHg in sediment samples and dissolved MeHg in pore water were determined using solvent extraction, aqueous phase ethylation, gas chromatographic separation, pyrolysis, and cold vapor atomic fluorescence (CVAFS) detection (Horvat et al. 1993). Accuracy for total MeHg analyses was determined using two certified reference materials (BCR 580 estuarine sediment and IAEA-433 marine sediment). Recovery of MeHg was estimated by spiking samples with a known quantity of MeHg prior to analysis. The limit of detection was 50 pg MeHg L−1 for water samples and 50 pg MeHg g−1 for sediments.
3 Results
The majority of the data used for the present analysis were reported previously in publications that focused on specific regions of the Idrija, Soča, and Isonzo Rivers, the coastal lagoons of northwest Italy, and the Gulf of Trieste in the northern Adriatic Sea (Hines et al. 2000, 2006, 2012). The goal here is to combine aspects of those data to obtain a more cohesive understanding of how Hg behavior changes along the freshwater to marine gradient.
3.1 Site I6
Site I6, which is located at the mouth of the Isonzo River (Fig. 1), is the only site along the freshwater–marine gradient for which sedimentary data have not been published previously. Figure 2 depicts depth profiles of sedimentary Hg transformation activities (rate constants) and the concentrations of dissolved Hg and MeHg over the upper 10 cm for summer (top) and winter (bottom). These profiles are similar and have vertical structure to many of the profiles for the other sites throughout the system. Both methylation and demethylation were quite active near the surface and decreased over fourfold with depth. The subsurface maximum at ~7 cm in sulfate reduction rate and concentration of dissolved Hg species is typical of coastal and offshore sites in this region during summer and is due to bioturbation activities by burrowing infauna (Hines et al. 1997, 2006). In both seasons, the correspondence between sulfate reduction and Hg transformation rates supports the premise that S cycling is important for controlling Hg cycling.
Depth profiles at site I6 of sulfate reduction rates, methylation potential (K meth), demethylation potential (K deg), and dissolved concentrations of total Hg (Hgtot) and methylmercury (MeHg) for summer (top) and winter (bottom). Hg species were not determined in winter. The bars for the K meth data represent the sum of the demethylation products 14CO2 (white bars) and 14CH4 (black bars)
Demethylation was primarily oxidative, i.e., the methyl group of MeHg was converted mostly to CO2. Demethylation rate constants were ~15 times higher than those for methylation. Dissolved Hgtot concentrations were over 100 ng L−1 while MeHg was nearly 3 ng L−1. Concentrations of both species decreased with depth.
3.2 Changes in Hg transformations and Hg speciation along the freshwater–marine gradient
Summer depth profile data from the five sites within the Soča and Isonzo Rivers and the Gulf of Trieste were averaged and plotted as a downstream gradient (Fig. 3). Sulfate reduction rates were not determined in sediments from the impoundment and the brackish site, but data for marine sites from the mouth of the Isonzo River and into the center of the Gulf of Trieste show a rather steep decrease in sulfate reduction activity moving offshore into the gulf, with a high of over 350 nmol mL−1 day−1 at site I6 to a low of ~40 nmol mL−1 day−1 at site CZ which is located near the center of the Gulf of Trieste approximately 15–20 km from the coasts of Italy and Slovenia (Fig. 3). Although sulfate reduction was not determined at the impoundment and estuary sites (brackish), it is assumed that these sites support much lower rates of sulfate reduction since they are often freshwater (the impoundment site is always fresh) and therefore contain low concentrations of sulfate. The estuary site experiences seawater influence during dry periods at high tide, and we determined concentrations of dissolved sulfide over 75 μM in sedimentary pore waters at this site (Hines et al. 2006).
Depth-averaged values of sulfate reduction rates (a), Hg transformation rates (b), and concentrations of dissolved (c) and total (d) Hg species from the freshwater site in the Soča River into the center of the Gulf of Trieste. Data represent averages of six vertical sections within the upper 10–12 cm of sediment from each site in summer. Panels b are methylation (b1), demethylation (b2), and the ratio of methylation to demethylation (b3). Panels c are dissolved (filtered pore water) concentrations of total Hg (c1), MeHg (c2), and the percent of the total attributed to MeHg (c3). Panel d is similar to c, but for total Hg and MeHg (solid phase). Data are from Hines et al. (2006) and Fig. 3 (top). ND no data collected
The potential to methylate Hg (K meth) increased exponentially over 60-fold from the freshwater site into the gulf sediments (Fig. 3 (b1)). There was a 10-fold increase in activity between the impoundment and site I6 at the Isonzo mouth. The degradation of MeHg (K deg) by demethylating bacteria increased from ~3 to ~17% per day between the impoundment and site I6, but decreased greatly again in the center of the gulf to ~4% per day (Fig. 3 (b2)). These data are averages over the upper 10 cm of sediment, and rates of MeHg demethylation up to 60% per day were noted in the upper 1.0 cm of lagoon sediments located west of the Isonzo mouth (Hines et al. 2012), so it appears that shallow water coastal sites in general actively demethylate MeHg. When depth average ratios of methylation to demethylation rate constants (K meth/K deg) were compared, this ratio increased greatly from the mouth of the Isonzo River (site I6) into the Gulf of Trieste sediments with the highest ratio occurring at site CZ (Fig. 3 (b3)). This ratio can be considered as a relative measure of the potential for the occurrence of net methylation, i.e., a high ratio indicates high likelihood that MeHg will be formed since the ability of demethylating bacteria to degrade MeHg is lower, and vice versa. However, since these data are potential rates based on the ability of the sample to degrade a tracer that may not be in the same chemical state as the ambient Hg species, these rates and ratios should be interpreted relative to each other and not as a direct measure of activity. Therefore, a ratio of 1.0 does not necessarily indicate that MeHg is being created and degraded at the same rate. However, if a site that exhibits a high ratio compared to another site, it is more likely to accumulate MeHg. It was interesting that despite the very high rates of demethylation encountered at site D6, the rate of methylation was so high that the net methylation potential was high compared to the more coastal sites. The concentration of the radio-MeHg utilized was quite high relative to ambient MeHg, and it was likely that MeHg degradation was stimulated in incubations. Therefore, here we are emphasizing the comparison between sites rather than absolute rates, and the differences between sites were often large.
Figure 3 also displays gradients in depth-averaged concentrations of dissolved and total (solid) Hgtot and MeHg and the %MeHg in both phases in summer (Fig. 3 (c and d)). There was a trend in which %MeHg was relatively high in the freshwater impoundment sediments, decreased in the estuarine region, and then increased again in the gulf. Dissolved %MeHg increased over 20-fold from site I6 to CZ (Fig. 3 (c3)). We did not measure solid phase Hg species at site I6, but the solid %MeHg increased 3-fold from site D6 to CZ.
Multiple cores were combined and sectioned for all rate measurements, and incubations of depth sections were conducted in triplicate. The standard deviations of rate measurements of these triplicates were usually less than 15% of the mean, and differences in average rates among sites were often quite large, over 10 to even 100-fold in some cases (Fig. 3). Two major patterns emerged: (1) several-fold differences between freshwater and brackish sites (impoundment, estuary, and I6) compared to marine sites (D6, AA, and CZ) and (2) rather large gradients within each of these two combined categories. These differences were much larger than the variabilities within sites, indicating that the major horizontal changes highlighted in Fig. 3 were truly different.
3.3 Comparison of coastal lagoons and Gulf of Trieste sediments
To investigate the effects of bacterial sulfate reduction on Hg methylation in the Gulf of Trieste and the coastal estuarine and lagoon environments, we plotted all depth profile methylation rate constant (K meth) data (upper 10–12 cm of sediment) as a function of rates of sulfate reduction for the three Gulf of Trieste sites (D6, AA, CZ) (Hines et al. 2000, 2006) and four sites in the nearby Marano and Grado Lagoons (Hines et al. 2012) for three seasons (Fig. 4a). We also included data from site I6 for warm (October) and cold (March) seasons (triangles, Fig. 4a, b). The Gulf of Trieste data exhibited a regression line that is 35 times steeper than for the lagoon sites. Data from site I6 was similar to the lagoon data with a much lower slope than the gulf data. Although site I6 is in the heart of the Isonzo River, it is only slightly deeper than the lagoon sites and supports similar rates of microbial activities including relatively high rates of MeHg demethylation (Fig. 2; Hines et al. 2012). A comparison of MeHg demethylation activities (K deg) as a function of sulfate reduction (Fig. 4b) revealed that unlike methylation data, regression lines were similar for both the lagoon and Gulf of Trieste data.
Regression analysis of (a) potential methylation (K meth) and (b) demethylation (K deg) as a function of rates of sulfate reduction in Marano and Grado Lagoons (coastal lagoons) (squares) and the Gulf of Trieste (circles). Data for site I6 (Fig. 3) are added (triangles) but are not included in statistical analysis. The inset in a is an expansion of the lower value data. Lagoon data are from Hines et al. (2012), and Gulf of Trieste data are from Hines et al. (2000, 2006)
Sediment–water partition coefficients (K D; L kg−1) were calculated as
where [Hg]S is the solid phase concentration of the Hg species in nanograms per kilogram, and [Hg]PW is the pore water concentration of the Hg species in nanograms per liter. Increases in K D occur when dissolved Hg species decrease in concentration relative to solid phase concentrations, and vice versa. Table 1 shows the significance of relationships between K meth values and Log K D, dissolved Hgtot, %dissolved MeHg, and sulfate reduction rates at the lagoon and Gulf of Trieste sites. All parameters were significantly correlated with K meth at the lagoon sites, but only sulfate reduction correlated (p < 0.05) at the gulf sites.
4 Discussion
4.1 Bacterial activity and chemical gradients
Hines et al. (2000) reported large changes in the concentrations of Hgtot and MeHg in unfiltered water samples in the Idrija/Soča/Isonzo river system from upstream of the Hg mine in the town of Idrija to the Gulf of Trieste several tens of kilometers away. Of primary importance were the findings shown in Figs. 2 and 3 of Hines et al. (2000). Hgtot and MeHg in unfiltered water increased over 100-fold from just above to just downstream of the mining region and remained relatively high throughout the 24 km of the Idrija River. After the confluence of the Idrija and the Soča Rivers, both Hg species decreased greatly due to the dilution by the much larger Soča River. However, Hgtot, and especially MeHg, increased several fold as waters flowed downstream in the Soča River and this was interpreted as a consequence of anaerobic conditions created in sediments trapped behind hydropower dams. This mobilization and methylation of Hg resulted in a marked increase in the %MeHg in the water column from ~0.4% at the end of the Idrija River to ~4.5% in the Soča (Fig. 3 in Hines et al. 2000). The concentrations of Hg species increased markedly in the estuarine and near-shore marine waters as well, but the %MeHg decreased relative to values in the freshwater Soča River. Therefore, those data revealed the dissolution of inorganic Hg in the Soča River impoundments and especially in near-shore marine sediments with a concomitant enhancement of Hg methylation. Impoundments and riverbanks can be hot spots for Hg methylation (Meng et al. 2016; Singer et al. 2016).
The sedimentary data from the present study demonstrated the role of sedimentary biogeochemical and chemical processes in controlling the movement and transformation of Hg species into the overlying water and the fact that the importance of specific processes changed along the gradient from the freshwater to the marine environment. In general, this gradient can be separated into three primary geographic regions that experience differences in the relative importance of microbial activities and the role of the sulfur cycle in affecting Hg mobility and methylation: (1) the Soča River region that contains dams and is represented here as the “impoundment” site; (2) the transition from freshwater to marine represented here as the “estuary” site that is considered a brackish site and the higher salinity I6 site at the mouth of the Isonzo River. The sites in Grado and Marano Lagoons can also be considered in this grouping; and (3) the Gulf of Trieste sites D6, AA, and CZ.
Comparing data from these groupings lead to the following overarching conclusions:
-
1.
The ability to methylate Hg increases greatly from freshwater to marine sites (Fig. 3 (b1)). However, since the main source of Hg is from upstream in the river system and the concentration of total Hg generally decreases into the gulf (Hines et al. 2000; Horvat et al. 2002), the amount of actual Hg and MeHg that accumulates is greater in the freshwater region. D6 is exceptional since it is a sink for Hg-laden particles leaving the mouth of the Isonzo River (Covelli et al. 2001).
-
2.
Demethylation of MeHg is high in shallow water sediments near the coast such as site I6 (Figs. 2 and 3 (b2)) and in the nearby Grado and Marano Lagoons (Hines et al. 2012). This demethylation activity may be an important sink for MeHg leading to the relatively low dissolved and solid phase %MeHg levels in the estuarine and near-shore sites (estuary, I6, and D6). The decrease in demethylation activity from the coast into the gulf resulted in a significant increase in the methylation–demethylation ratio in the gulf (Fig. 3 (b3)), which may also have contributed to the increase in the %MeHg of both the dissolved and solid phase Hg in the gulf.
-
3.
Hg dissolution increased in the estuarine regions as sedimentary sulfide production increased during sulfate reduction (Fig. 3 (c1)). It has been shown that dissolved sulfide can lead to the dissolution of cinnabar (Paquette and Helz 1995), which is the primary mineral mined in Idrija and transported to the gulf. This mobilization of inorganic Hg in the sediments in the estuarine region was responsible for the decrease in the %MeHg of the dissolved phases in the pore waters, and these pore waters were the likely source of Hg species in the overly waters that also exhibited a decrease in %MeHg (Hines et al. 2000). This remobilized Hg appeared to be bioavailable since MeHg concentrations also increased (Fig. 3 (c2)), but the dissolution of Hg exceeded the methylation increase, which led to a decrease in the %MeHg at the estuarine sites (Fig. 3 (c3)). It has also been shown that solid phase cinnabar may serve as a substrate for Hg methylation in the presence of organic matter (Graham et al. 2012; Zhang et al. 2012), so it is likely that the transported cinnabar was a source of dissolved inorganic Hg and MeHg. Other studies have shown a correlation between K meth and %MeHg in sediments (Hammerschmidt and Fitzgerald 2004, 2006; Drott et al. 2008), but this did not occur in the gradient data presented here. We found a significant statistical relationship between K meth and %MeHg when lagoon data alone were analyzed (Hines et al. 2000), which shows that methylation potential can be an important controlling factor when regions of our gradient are isolated. However, the lack of any such relationship for the gradient data is probably due to the widely varying biogeochemical conditions encountered along the gradient in which inorganic Hg is mobilized in some regions, like the estuary, and immobilized in others, like the near-shore site D6. Hence in our case, changes in %MeHg are more a function of relative changes in Hg dissolution and the decreasing concentration of Hgtot from freshwater to offshore, than to changes in Hg methylation activities.
-
4.
The ability of sediments to produce and accumulate MeHg increases moving offshore in the gulf. Even though the total Hg is diluted moving offshore, the sediments tend to accumulate an increasing fraction of the Hg as MeHg and this is seen in both the dissolved and solid phases (Fig. 3 (d1 and d2)). These data are for summer, and the pore water concentrations of Hg species vary seasonally (Hines et al. 2000, 2006). However, the solid phase concentrations vary little throughout the year, so these data are a good indication that the offshore increase in %MeHg is robust. Of course, the relative distribution of Hg and MeHg is also affected strongly by organic content (Lindberg and Harriss 1974; Hammerschmidt and Fitzgerald 2004, 2006) and grain size with MeHg in the gulf being associated more with fine-grained, organic-rich sediments than is the inorganic Hg (Covelli et al. 2001).
-
5.
Sulfate reduction has a strong effect on Hg transformations and this influence varies along the freshwater–marine gradient. Sulfate-reducing bacteria are important methylators of Hg (Compeau and Bartha 1985; Gilmour et al. 1992; Choi et al. 1994) and the sulfide they produce plays a significant role in the solubility and bioavailability of Hg (Benoit et al. 1999; Hammerschmidt and Fitzgerald 2004). At low concentrations, zero-valent Hg-S complexes are thought to serve as a primary vector for movement of Hg into cells capable of methylating Hg to MeHg (Benoit et al. 1999, 2003). However, at higher concentrations, dissolved sulfide leads to the precipitation of Hg as HgS minerals that are not available for bacterially-mediated methylation and the ability of bacteria to methylate can be highly curtailed (Merritt and Amirbahman 2009).
Sulfate reduction rates decreased exponentially from the mouth of the Isonzo River into the center of the Gulf of Trieste (Fig. 3 (a)), but methylation potential and %MeHg increased (Fig. 3 (b1 and c3)). When sulfate reduction rates are high, such as what occurred at I6, ambient Hg and Hg injected as a tracer should be immobilized as HgS minerals that are not readily methylated (Gilmour and Riedel 1995). As sulfide production decreased offshore, conditions became more conducive to methylation, which resulted in higher methylation/demethylation ratios (Fig. 3 (b3)) and increased %MeHg values (Fig. 3 (c3)). Therefore, even though the Gulf of Trieste sediments contain less total Hg than their near-shore counterparts, biogeochemical conditions in gulf sediments favor the production of MeHg and pose a threat to marine biota. The offshore sites also exhibited much less active demethylation potential compared to methylation, which exacerbated the potential for MeHg accumulation in gulf sediments, and as will be discussed in more detail in the following section, sulfate-reducing bacteria are important demethylators in the marine sediments studied. However, unlike methylation, demethylation was probably not as significantly affected by the speciation of the sulfide product, so a simple decrease in sulfate reduction (or by proxy, bacterial activity in general) was sufficient to quantitatively decrease demethylation. Therefore, the decrease in bacterial activity (i.e., sulfate reduction) in the gulf, and the concomitant decrease in sulfide production, led to an enhancement in net Hg methylation.
The freshwater portion of the system also exhibited relatively high concentrations of Hg species and %MeHg values (Fig. 3 (d1, d2, and d3)). The only site that was truly freshwater all-year was the impoundment site, which had the highest total MeHg concentrations of all sites investigated. Sediments at this site were fine grained, organic rich, and low in sulfide (Hines et al. 2006), which are conditions conductive to MeHg formation. K meth rates are indictors of the fraction of Hg that is methylated. The low K meth rates at the impoundment site are in part a function of the high total Hg present at that site, which tended to dilute the radio-Hg amendment. These low rates are not a direct indication that methylation is slow. Unlike the marine sites where we have sulfate reduction data, we cannot assess the role of the sulfur cycle in affecting the Hg cycle in the impoundment site. However, the freshwater nature of the impoundment probably supported active methanogenesis, and methanogens have been shown to methylate Hg (Yu et al. 2013).
4.2 Sulfate reduction and Hg transformations in lagoons and the Gulf of Trieste
Methylation rate constants correlated significantly with rates of sulfate reduction in sediments from both the lagoon sites and in the sites in the Gulf of Trieste (Fig. 4a). However, the slope of the regression line was much steeper in the gulf sediments, indicating that even low rates of sulfate reduction could lead to significant methylation activity. In fact, even at quite low rates of sulfate reduction, K meth values were substantially higher in the gulf sediments than in the lagoons. This difference underscores the possible importance of sulfide as a controller of Hg methylation. In the lagoons and site I6 where sulfate reduction was much more rapid, it was likely that the higher sulfide production rates and associated higher concentrations of solid phase-reduced sulfur compounds led to Hg adsorption and precipitation that lowered the availability of Hg for methylation (Hollweg et al. 2009; Liu et al. 2009). The role of adsorption of Hg to solid phases could be seen by the significant correlation between Log K D and K meth (Table 1) that shows that methylation in the lagoons is affected strongly by the ability of Hg to bind to particles and that the solubility of Hg is an important factor in controlling Hg bioavailability. Other studies have noted a relationship between Log K D and K meth in marine sediments (Hammerschmidt and Fitzgerald 2004, 2006; Han et al. 2007). In the gulf, adsorption of Hg to particles is less important and the direct role of sulfate reduction activity increases in importance. In fact, the correlation coefficients (r 2) displayed in Fig. 4a show that half of the variability of Hg methylation in the gulf could be explained by rates of sulfate reduction, while only 24% could in the lagoons.
Demethylation rate constants also correlated with rates of sulfate reduction in the lagoons and in the Gulf of Trieste (Fig. 4b). However, unlike the methylation data, the slopes of the regression lines were nearly identical between the two regions, indicating that the effect of sulfate reduction on demethylation was similar in both regions. Demethylation decreased offshore similarly to sulfate reduction, while methylation increased offshore leading to a large increase in the K meth/K deg ratio in gulf sediments (Fig. 3 (b3)). Therefore, the rapid demethylation activity in the lagoons was probably also partly responsible for the fact the methylation activity increased little as rates of sulfate reduction in lagoons increased. Sulfate-reducing bacteria in lagoon sediments were much more active as MeHg demethylators, which degraded MeHg more readily, leading to low %MeHg values and lower K meth activities. Hence, it is likely that both demethylation and Hg precipitation and adsorption prevented MeHg accumulation in the lagoons and that the lack of significant importance of these processes in the gulf allowed MeHg production to proliferate.
These results underscore the complexity of Hg transformations and potential Hg transport within a system subject to large Hg concentration gradients (mining source) and a freshwater–marine gradient. The movement of riverine Hg downstream, its diversion into either coastal lagoons or deeper offshore environments, and variations in organic composition and rates of degradation of these organics (and concomitant S cycling) have created a highly active and dynamic system in which controls on methylation and demethylation vary greatly.
References
Avcin A, Vriser B (1983) The northern Istrian soft bottom communities: the example of Piran Bay (north Adriatic). Bioloski Vestnik 31:129–160
Barkay T, Miller SM, Summers AO (2003) Bacterial mercury resistance from atoms to ecosystems. FEMS Microbiol Rev 27:355–384
Barkay T, Wagner-Dobler I (2005) Microbial transformations of mercury: potentials, challenges, and achievements in controlling mercury toxicity in the environment. Adv Appl Microbiol 57:1–52
Benoit JM, Gilmour CC, Mason RP, Heyes A (1999) Sulfide controls on mercury speciation and bioavailability to methylating bacteria in sediment pore waters. Environ Sci Technol 33:951–957
Benoit JM, Gilmour CC, Heyes A, Mason RP, Miller CL (2003) Geochemical and biological controls over methylmercury production and degradation in aquatic ecosystems. ACS Symp Ser 835:262–297
Bloom NS (1992) On the chemical form of mercury in edible fish and marine invertebrate tissue. Can J Fish Aquatic Sci 49:1010–1017
Brambati A (1970) Provenienza, trasporto e accumulo dei sedimenti recenti nelle lagune di Marano e di Grado e nei litorali tra i fiumi Isonzo e Tagliamento. Memorie della Società Geologica Italiana 9:281–329
Brambati A (2001) Coastal sediments and biota as indicators of Hg contamination in the Marano and Grado Lagoons. RMZ – Materials and Geoenvironment 48:165–171
Choi S-C, Chase T Jr, Bartha R (1994) Enzymatic catalysis of mercury methylation by Desulfovibrio desulfuricans LS. Appl Environ Microbiol 60:1342–1346
Clarkson TW (2002) The three modern faces of mercury. Environ Health Perspect 110:11–23
Compeau GC, Bartha R (1985) Sulfate-reducing bacteria: principal methylators of mercury in anoxic estuarine sediment. Appl Environ Microbiol 50:498–502
Covelli S, Faganeli J, Horvat M, Brambati A (2001) Mercury contamination of coastal sediments as the result of long-term cinnabar mining activity (Gulf of Trieste, northern Adriatic sea). Appl Geochem 16:541–558
Drott A, Lambertsson L, Bjoern E, Skyllberg U (2008) Do potential methylation rates reflect accumulated methyl mercury in contaminated sediments? Environ Sci Technol 42:153–158
Faganeli J (1989) Sedimentation of particulate nitrogen and amino acids in shallow coastal waters (Gulf of Trieste, northern Adriatic). Mar Chem 26:67–80
Faganeli J, Horvat M, Covelli S, Fajon V, Logar M, Lipej L, Cermelj B (2003) Mercury and methylmercury in the Gulf of Trieste (northern Adriatic Sea). Sci Tot Environ 304:315–326
Fitzgerald WF, Clarkson TW (1991) Mercury and monomethylmercury—present and future concerns. Environ Health Perspect 96:159–166
Fitzgerald WF (1993) Mercury as a global pollutant. The World & I 10:192–199
Fleming EJ, Mack EE, Green PG, Nelson DC (2006) Mercury methylation from unexpected sources: molybdate-inhibited freshwater sediments and an iron-reducing bacterium. Appl Environ Microbiol 72:457–464
Gilmour CC, Henry EA, Mitchell R (1992) Sulfate stimulation of mercury methylation in freshwater sediments. Environ Sci Technol 26:2281–2287
Gilmour CC, Riedel GS (1995) Measurements of Hg methylation in sediments using high specific activity 203Hg and ambient incubation. Water Air Soil Poll 80:747–756
Gilmour CC, Podar M, Bullock AL, Graham AM, Brown SD, Somenahally AC, Johs A, Hurt RA, Bailey KL, Elias DA (2013) Mercury methylation by novel microorganisms from new environments. Environ Sci Technol 47:11810–11820
Graham AM, Aiken GR, Gilmour CC (2012) Dissolved organic matter enhances microbial mercury methylation under sulfidic conditions. Environ Sci Technol 46:2715–2723
Gray JE, Hines ME (2009) Biogeochemical mercury methylation influenced by reservoir eutrophication, Salmon Falls Creek Reservoir, Idaho, USA. Chem Geol 258:157–167
Hammerschmidt CR, Fitzgerald WF (2004) Geochemical controls on the production and distribution of methylmercury in near-shore marine sediments. Environ Sci Technol 38:1487–1495
Hammerschmidt CR, Fitzgerald WF (2006) Methylmercury cycling in sediments on the continental shelf of southern New England. Geochim Cosmochim Acta 70:918–930
Han S, Obraztsova A, Pretto P, Choe K-Y, Gieskes J, Deheyn DD, Tebo BM (2007) Biogeochemical factors affecting mercury methylation in sediments of the Venice Lagoon, Italy. Environ Toxicol Chem 26:655–663
Hines ME, Faganeli J, Planinc R (1997) Sedimentary anaerobic microbial biogeochemistry in the Gulf of Trieste, northern Adriatic Sea: influences of bottom water oxygen depletion. Biogeochemisty 39:65–86
Hines ME, Horvat M, Faganeli J, Bonzongo JCJ, Barkay T, Major EB, Scott KJ, Bailey EA, Warwick JJ, Lyons WB (2000) Mercury biogeochemistry in the Idrija River, Slovenia, from above the mine into the Gulf of Trieste. Environ Res 83:129–139
Hines ME, Faganeli J, Adatto I, Horvat M (2006) Microbial mercury transformations in marine, estuarine and freshwater sediment downstream of the Idrija mercury mine, Slovenia. Appl Geochem 21:1924–1939
Hines ME, Visscher PT, Teske A, Devereux R (2008) Sulfur cycling. In: Hurst CJ et al (eds) Manual for environmental microbiology. American Society for Microbiology Press, Washington, DC, pp. 497–510
Hines ME, Poitras EN, Covelli S, Faganeli J, Emili A, Zizek S, Horvat M (2012) Mercury methylation and demethylation in Hg-contaminated lagoon sediments (Marano and Grado Lagoon, Italy). Estuar Coast Shelf Sci 113:85–95
Hollweg TA, Gilmour CC, Mason RP (2009) Methylmercury production in sediments of Chesapeake Bay and the mid-Atlantic continental margin. Mar Chem 114:86–101
Horvat M, Zvonaric T, Stegnar P (1987) Determination of mercury in seawater by cold vapour atomic absorption spectrometry. Acta Adriat 28:59–63
Horvat M (1991) Determination of methylmercury in biological certified reference materials. Water, Air, Soil Poll 56:95–102
Horvat M, Liang L, Bloom N (1993) Comparison of distillation with other current isolation methods for the determination of methyl mercury compounds in low level environmental samples, part 2: water. Anal Chim Acta 282:153–168
Horvat M, Covelli S, Faganeli J, Logar M, Mandic V, Rajar R, Sirca A, Zagar D (1999) Mercury in contaminated coastal environments, a case study: the Gulf of Trieste. Sci Total Environ 238:43–56
Horvat M, Jereb V, Fajon V, Logar M, Kotnik M, Faganeli J, Hines ME, Bonzongo J-C (2002) Mercury distribution in water, sediment and soil in the Idrijca and Soca river systems. Geochem Explor Environ Analysis 2:287–296
Kerin EJ, Gilmour CC, Roden E, Suzuki MT, Coates JD, Mason RP (2006) Mercury methylation by dissimilatory iron-reducing bacteria. Appl Environ Microbiol 72:7919–7921
Lin C-C, Yee N, Barkay T (2011) Microbial transformation in the mercury cycle. In: Liu L, Cai Y, O’Driscoll N (eds) Environmental chemistry and toxicology of mercury. John Wiley & Sons, Inc, USA In print, pp. 155–191
Lindberg SE, Harriss RC (1974) Mercury-organic matter associations in estuarine sediments and interstitial water. Environ Sci Technol 8:459–462
Liu J, Valsaraj KT, Delaune RD (2009) Inhibition of mercury methylation by iron sulfides in an anoxic sediment. Environ Eng Sci 26:833–840
Lu X, Liu Y, Johs A, Zhao L, Wang T, Yang Z, Lin H, Elias DA, Pierce EM, Liang L, Barkay T, Gu B (2016) Anaerobic mercury methylation and demethylation by Geobacter bemidjiensis Bem. Environ Sci Technol 50:4366–4373
Marvin-DiPasquale MC, Agee J, McGowan C, Oremland RS, Thomas M, Krabbenhoft D, Gilmour CC (2000) Methyl-mercury degradation pathways: a comparison among three mercury-impacted ecosystems. Environ Sci Technol 34:4908–4917
Mason RP, Lawrence AL (1999) Concentration, distribution, and bioavailability of mercury and methylmercury in sediments of Baltimore Harbor and Chesapeake Bay, Maryland, USA. Environ Toxicol Chem 18:2438–2447
Meng B, Feng X, Qiu G, Li Z, Yao H, Shang L, Yan H (2016) The impacts of organic matter on the distribution and methylation of mercury in a hydroelectric reservoir in Wujiang River, Southwest China. Environ Toxicol Chem 35:191–199
Merritt KA, Amirbahman A (2009) Mercury methylation dynamics in estuarine and coastal marine environments—a critical review. Earth Sci Rev 96:54–66
Mosetti F (1983) Sintesi sull’idrologia del Friuli-Venezia–Gulia. Quaderni dell’Ente Tutela Pesca 6:1–295
Ogorelec B, Misic M, Faganeli J (1991) Marine geology of the Gulf of Trieste (northern Adriatic): sedimentological aspects. Mar Geol 99:79–92
Palinkas LA, Pirc S, Miko SF, Durn G, Namjesnik K, Kapelj S (1995) The Idrija mercury mine, Slovenia, a semi-millenium of continuous operation: an ecological impact. In: Richardson M (ed) Environmental toxicology assessment. Taylor and Francis, United Kingdom, pp. 317–339
Paquette KE, Helz GR (1995) Solubility of cinnabar (red HgS) and implications for mercury speciation in sulfidic waters. Water Air Soil Pollut 80:1053–1056
Schartup AT, Balcom PH, Mason RP (2014) Sediment–porewater partitioning, total sulfur, and methylmercury production in estuaries. Environ Sci Technol 48:954–960
Singer MB, Harrison LR, Donovan PM, Blum JD, Marvin-DiPasquale M (2016) Hydrologic indicators of hot spots and hot moments of mercury methylation potential along river corridors. Sci Total Environ 568:697–711
Sirca A, Rajar R (1997) Calibration of a 2D mercury transport and fate model of the Gulf of Trieste. In: Rajar R, Brebbia M (eds) Proc. 4th Internat Conf Water Pollut. Computational mechanics publication, Southampton, pp 503–512
Varekamp JC, Buchholz tB MR, Mecray EL, Kreulen B (2000) Mercury in Long Island Sound sediments. J Coast Res 16:613–626
Yu R-Q, Reinfelder JR, Hines ME, Barkay T (2013) Mercury methylation by the methanogen Methanospirillum hungatei. Appl Environ Micorbiol 79:6325–6330
Zhang T, Kim B, Levard C, Reinsch BC, Lowry GV, Deshusses MA, Hsu-Kim H (2012) Methylation of mercury by bacteria exposed to dissolved, nanoparticulate, and microparticulate mercuric sulfides. Environ Sci Technol 46:6950–6958
Acknowledgements
We appreciated the technical assistance of E. Poitras, D. Warden, T Koprivnjak, and I. Skuk. Financial support was provided by the US National Science Foundation; the Slovene Ministry of Education, Science and Sport; and the Commissario Delegato for the Marano and Grado Lagoons. We appreciated comments on the manuscript by Tamar Barkay.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Nives Ogrinc
Rights and permissions
About this article
Cite this article
Hines, M.E., Covelli, S., Faganeli, J. et al. Controls on microbial mercury transformations in contaminated sediments downstream of the Idrija mercury mine (West Slovenia) to the Gulf of Trieste (northern Adriatic). J Soils Sediments 17, 1961–1971 (2017). https://doi.org/10.1007/s11368-016-1616-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-016-1616-x