Abstract
Purpose
There is a paucity of data regarding the multiple timescale variations of heterotrophic respiration (R H) and autotrophic respiration (R A) as well as the primary controlling factors. The objective of this study is to find the temporal variations of total soil respiration (R S) and its components, revealing the driving factors at different timescales.
Materials and methods
A trenching method was used to distinguish R S, R H, and R A in a spruce-fir valley forest in northeastern China. We used the closed dynamic chamber method to measure the soil respiration rate. Analyses of R S, R H, and R A in relation to biotic and abiotic factors were conducted to realize the temporal variations at different timescales.
Results and discussion
Only R S and R H showed a distinct diurnal variation and soil temperature (T S) can explain 68 and 59 % of the daily variation, respectively. R S, R H, and R A showed a pronounced, single peak curve seasonally, and T S can explain 11–95 % of the seasonal variation. Soil moisture (W S) maintained at a relatively high level and was not related to R S, R H, or R A on a seasonal scale, and there was no significant relationship between the seasonal R S, R A, and root biomass. However, for 5 years, only the mean R A of the growing season was significantly related to the mean W S, which can explain 39 % of the inter-annual variation of R A. The annual variations of litterfall and the relative growth rate of stems were not related to R S, R H, or R A. The contribution of R H to R S was larger, and the temperature sensitivity was 2.01–3.71 for R S, 1.90–3.08 for R H, and 2.20–5.65 for R A.
Conclusions
R S, R H, and R A show different temporal variations at multiple timescales. When W S is not restricted, T S is the primary driving factor of daily and seasonal variation of R S and R H. In this site, R H accounts for a large proportion of R S and plays a crucial role in determining the magnitude and temporal variation of R S.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soil respiration (R S) is the second largest carbon efflux between terrestrial ecosystems and the atmosphere (78–98 Gt C yr−1) (Raich et al. 2002; Bond-Lamberty and Thomson 2010). The annual emission of R S is 10 times greater than the combustion of fossil fuels (Schlesinger 1997). R S plays a crucial role in regulating soil carbon dynamics and global climate change. Generally, R S is considered to be composed of heterotrophic (R H, respiration by soil microbes and fauna) and autotrophic components (R A, respiration by root and rhizosphere microorganisms) (Scott-Denton et al. 2006). Different responses of these two components determine the important role of R S in regulating the global carbon balance (Schuur and Trumbore 2005).
Some studies have shown that R S exhibits variations at different timescales (Savage and Davidson 2001; Ohashi et al. 2008; Hanpattanakit et al. 2015). Generally, R S changes along with soil temperature (T S) throughout the day (Shi et al. 2006; Hanpattanakit et al. 2015), whereas Yuste et al. (2004) found the short-term variation of R S to be related to the diurnal pattern of plant phenology and productivity. In temperate and boreal forests, T S is the primary driving factor that controls the seasonal change of R S (Vargas et al. 2010; Pang et al. 2013), whereas soil moisture (W S) is the restricting factor in tropical or semi-arid areas (Ohashi et al. 2008; Moyes and Bowling 2012). At large timescales, such as inter-annual, decadal, or even larger, R S may be controlled by abiotic factors such as soil texture, T S, W S, and annual precipitation (Bonal et al. 2008; Moyes and Bowling 2012; You et al. 2013). The long-term change of the soil carbon pool could be the result of strong feedback between climate and the ecosystem carbon balance, which depends on the cumulative effect of litterfall products and its decomposition in R S (Schmidt et al. 2011). Previous studies on the temporal dynamics of R S have primarily concentrated on the single timescale, and overlooking the components of R S. Our understanding of the multiple timescale variations and controlling factors of R H and R A are still limited.
Multiple variations of R H and R A are influenced by different biotic and abiotic factors. The short-term variation of R H is primarily controlled by T S and W S (Li et al. 2011; Savage et al. 2013). Moreover, the variations of substrate availability, and microbial composition and its quantity, also have a large impact (Hopkins et al. 2014; Whitaker et al. 2014). Aside from T S and W S, photosynthesis, transportation of photosynthates from canopy to roots, and phenological characteristics also have significant effects on the short-term variation of R A (Kodama et al. 2008; Savage et al. 2013). Li et al. (2011) found the decomposition of soil organic carbon was positive related to T S, whereas the variation of R A was the opposite. Savage et al. (2013) showed a positive relationship between photosynthetically active radiation and R A. The R S increased linearly with an increase in gross primary productivity (GPP); GPP and T S could explain 53 % of the daily variation of R S (Han et al. 2014). Comparatively, T S is a variable that changed drastically by season in temperate forests, primarily restricting the seasonal pattern of R H. However, R A is affected by plant physiological changes (e.g., root growth and turnover, change of leaf area) (Luo and Zhou 2006; Prolingheuer et al. 2014). Although some studies have indicated that the changes of a series of physiological and ecological processes due to climate change would cause inter-annual variations of R S (King et al. 2004; Luo and Zhou 2006), there is still a paucity of knowledge about the inter-annual variations of R H and R A, especially the relationships between multiple temporal variations, and the annual litterfall (LF), root biomass (RB), and relative growth rate of stems (RGR) are not recognized adequately. Because the multiple timescale variations of R H and R A are driven by different environmental factors, the sensitivity of R S to T S and W S depends on the ratios of these two components (Butler et al. 2012). A wide range of the contribution of R A to R S (RC) has been reported (33–89 %) (Raich and Tufekciogul 2000; Subke et al. 2006; Wang and Yang 2007). It is vital to improve our understanding of how R H and R A respond to different environmental factors to accurately estimate the regional soil carbon dynamics, as well as the response to global climate change.
The Asian temperate mixed forest, one of the three largest temperate mixed forests in the world, is predominantly distributed in northeastern China, accounting for one third of the nation’s total forests (Department of Forestry of PR China 1994). As an important component of dark coniferous forests, which are widely distributed in north temperate zones, spruce-fir forests typically distribute in subalpine regions; however, due to the existence of an inversion layer, they are generally distributed in narrow valleys and local gullies in the eastern mountainous region of northeastern China. Therefore, we refer to them as spruce-fir valley forests. Spruce-fir valley forests are characterized by relatively high soil moisture and low soil temperatures. As a consequence of global warming, island permafrost is melting and the natural southern boundary of the spruce-fir valley forest will move northward. All of this will result in great changes to the habitat of the spruce-fir valley forest, which greatly influences the regional carbon cycle. However, the studies regarding the multiple timescale variations of R S, R H, and R A in this region have not been conducted; it is crucial to study how R S and its components vary at different timescales for this site, to accurately estimate the soil carbon dynamics of boreal forests in the context of global warming. In this study, we conducted continuous measurements of R S, R H, and R A in a spruce-fir valley forest during the growing season from 2010 to 2014. Our main objectives were to analyze the temporal variations of R S, R H, and R A at different timescales with a specific emphasis on the roles of biotic (LF, RGR, and RB) and abiotic factors (T S and W S).
2 Materials and methods
2.1 Site description and experimental design
This study was conducted in a spruce-fir valley forest in the Liangshui National Reserve (128° 53′ 20″ E, 47° 10′ 50″ N) in northeastern China. The elevation is 280–707 m, and the climate is classified as continental monsoon. The mean annual temperature is −0.3 °C, with a frost-free period of 100–120 days and snow period of 130–150 days. The mean annual precipitation is 676 mm and occurs during the summer, and the annual average evaporation is 805 mm. The soil is dark-brown forest soil (by Chinese classification), which is equivalent to Humaquepts or Cryoboralfs, based on the American Soil Taxonomy (Soil Survey Staff 1999), with 60–80 cm of soil thickness. The soil properties are as follows: 90.9 g kg−1 soil organic carbon (C), 7.5 g kg−1 total nitrogen (N), C: N 11.5, pH 4.8, soil bulk density 0.47 g cm−3, and see details in Shi and Jin (2016). The spruce-fir valley forest is the non-zonal climax vegetation, which is greater than 300 years, and belongs to the evergreen coniferous forest. The forest is primarily composed of Abies nephrolepis, Picea koraiensis, Acer ukurunduense, Pinus koraiensis, Betula costata, and Larix gmelinii.
We established three 20 m × 30 m replicate plots in the spruce-fir valley forest. Eight polyvinyl chloride (PVC) collars (10.4-cm inside diameter, 6-cm height) were randomly inserted into the soil in each plot to a depth of 4 cm (including the litter layer) to measure the R S, which was considered to be the total soil respiration rate. Four subplots (2 m × 2 m) were randomly established in each plot in October 2009. On the outside boundaries of the subplots, we dug a trench to the bedrock or below where few roots existed. To prevent root growth into the trenched plots, and avoiding the blockage of air circulation and water, we used a double-layer of nylon mesh to line the trenches and then refilled the trenches with the same excavated soil. Additionally, we removed all of the living plants and kept the surface free of seedlings and herbaceous vegetation throughout the study. We installed three PVC collars in each subplot. To minimize the effects of soil disturbance and fine root decomposition following trenching, we began measuring the soil respiration rate of the trenched subplots and untrenched plots starting in the growing season of 2010 and ending in 2014. The soil respiration rate of the trenched plots represents measured R H.
2.2 Soil respiration measurement
We used an LI-6400 portable CO2 infrared gas analyzer (IRGA) (LI-COR Inc., Lincoln, NE, USA) to measure the soil respiration rate approximately every 2 weeks during the growing season from 2010 to 2014. The soil respiration rate was measured on rainless days a total of 49 times. Due to the analyzer cannot run at low temperatures, we did not conduct measurements during non-growing seasons (T S lower than 0 °C). The T S at a depth of 5 cm was concurrently measured with the soil respiration rate next to each collar using a portable temperature probe provided with the LI-6400. Simultaneously, W S at a depth of 5 cm was measured using a time-domain reflectometry (TDR). Additionally, we measured the R S and R H every 2 h during the daytime from 0600 to 1800 hours and every 3 h at night from 1800 to 0300 hours on rainless days in August 2012, as well as the T S, a total of 10 times.
2.3 Relative growth rate of stems measurement
The diameter at breast height (DBH) was measured for trees with a DBH greater than 10 cm that were within 3–8 m of each collar in the untrenched plots. We numbered and positioned each of the trees and installed self-made tree measuring devices to monitor the DBH growth. If some tree measuring devices were damaged or the DBH of some trees grew to be 10 cm during the measurement period, then those devices were replaced or the trees were installed with new devices, recording their number and position. If some large trees fell, those were recorded as well. Then, the relative growth rate of the stem diameter (RGR/cm cm−1 year−1) was calculated as follows (Poorter et al. 2008):
where DBH0 and DBH t represent the DBH early and late in the growing season, respectively, and t represents the time.
2.4 Litterfall measurement
To measure the litterfall (LF), six square litter traps (area 1 m2) composed of wire (diameter 8 mm) and nylon mesh (bore diameter 1 mm, depth 0.5–0.6 m) were randomly placed in each plot. The distance from the bottom of the litter traps to the forest floor was 0.5 m. We collected LF once a month from 1 May to November (a period within which most LF occurs) between 2009 and 2014. Each sample was weighed after being oven dried at 65 °C. We summed up the LF of each litter trap from May to November and then averaged to get the mean LF of each year.
2.5 Root biomass measurement
Ten soil cores were randomly taken to estimate the root biomass (RB) using a 5-cm diameter corer at each plot, once at the end of each month from May to September 2012. The soil cores were taken from the forest floor surface down to 40 cm. Next, small roots (<5 mm in diameter) in the samples were collected, dried at 60 °C to a constant mass, and weighed.
2.6 Data analysis
The following exponential function (Luo et al. 2001) was used to describe the temperature dependence of soil respiration:
where R is the measured soil respiration (R S, R H, and R A), R 0 is the basal respiration at 0 °C, T is the soil temperature at 5 cm (°C) and k is the temperature coefficient, which is related to Q 10 (the increasing multiples of the soil respiration rate when the temperature increases by 10 °C). Q 10 is calculated as follows:
Due to the T S of trenched plots was larger than the T S of untrenched plots during the measurements (Fig. 2), which could make biases for the estimation of R H and R A. To ensure the comparability of soil respiration and eliminate the effects of trenching on the T S, measured R H was corrected where T S diverged on trenched and untrenched plots throughout the measurements. We used a simple model to correct the measured R H (Schindlbacher et al. 2009). The model is derived from measured R H of trenched plots from 2010 to 2014 (Fig. 5) and the models information see detail in Table 1. Hence, we used the Model R H to represent R H in this paper, and R A was calculated by the difference between R S and R H. The contribution of R A (RC) was calculated by ratio of R A to R S. The relationship between measured R H and Model R H see Fig. S1 (Electronic Supplementary Material). In addition, though there were differences of W S between trenched and untrenched plots, it was not significant. We found W S was not the restricted variable and was not related to R S or R H in this site. Mean T S and W S of trenched and untrenched plots for 5 years see Table S1 (Electronic Supplementary Material).
We used one-way ANOVA and LSD tests to compare the differences in the mean soil respiration rate of the growing season and related factors. A regression analysis was performed to test the relationships between soil respiration and the LF, RGR, and RB. The significance level was set as 0.05. All statistical analyses were performed using SPSS 19.0 (SPSS Inc., USA). Graphs were generated using Origin Pro 9.1 (Origin Lab Inc., USA).
3 Results
3.1 The diurnal variation of soil respiration
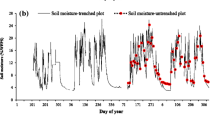
In this study, R S and R H all showed a pronounced diurnal variation with a single peak curve (Fig. 1), and the diurnal coefficients of variation (CV) was 12.97 and 8.40 %, respectively. The minimum and maximum values occurred at 0600 and 1600 hours, which coincided with the T S. The regression analysis (Eq. (2)) indicated that T S could explain 52.3 % of the diurnal variation of R S and 58.5 % for the measured R H, respectively, although the diurnal difference of T S was close to 2.5 °C, and the CV was below 10 %. The diurnal change of R A was not coincided with T S, and there was no significant relationship between them. In addition, the CV of R A was 22 % and the contribution of R A to R S was 36–50 %.
3.2 The seasonal variation of soil respiration
The seasonal ranges of R S, R H, and R A were 1.17–9.51, 0.69–3.5, and 0.19–6.45 μmol m−2 s−1, respectively (Fig. 2). R A had a higher CV of 44.74–95.15 %, whereas the CV of R S and R H were close to each other (Fig. 3). It is worth mentioning that R H was larger than R A during the growing season throughout the measurement and the large portion of R S was held by R H apart from 2014 (Fig. 4). In general, RC showed a pronounced decreasing trend from the end of July to October, while the contribution of R H was on the contrary.
R S, R H, and R A showed a similar tendency with T S (Fig. 2), and there was a significant exponential relationship between T S and soil respiration (Fig. 5), with R 2 ranging from 0.451 to 0.950 for R S, from 0.608 to 0.945 for measured R H, and from 0.114 to 0.843 for R A. However, W S maintained at a high level (Fig. 2), and we found that W S could not explain the seasonal variations of R S and its components by multivariate regression analyses (Wang et al. 2006) (Table S2, Electronic Supplementary Material). The CV of T S and W S are 32–51 and 26–43 %, respectively (data not shown). In addition, we found the RB was significantly different between different months, except the fourth soil layer (30–40 cm) (Table 2). The total RB ranged from 390.14 to 824.71 g m−2, whereas we found no significant relationship between the seasonal R S, R A, and RB (Fig. S2, Electronic Supplementary Material).
3.3 The inter-annual variation of soil respiration
The mean R S, R H, R A, and RC of the growing season exhibited pronounced inter-annual variation, and the maximum values occurred in 2012 or 2014 (Table 3); the annual CV for each was 20.31, 8.19, and 41.53 %, respectively, which are all less than the corresponding seasonal CV. Although there were significant differences in the annual mean T S and W S, the CV for both was low (10–17 %). We found only the annual mean W S to be significantly related to the annual mean R A, and the W S could explain 38.7 % of its inter-annual variation (Fig. 6). The mean value of the contribution of R A ranged from 32.23 to 47.84 %. We found the Q 10 of R A to have the highest maximum annual fluctuation, whereas the Q 10 of R S and R H were similar (Table 3).
We measured the RGR and LF continuously, and the results are shown in Table 4. The RGR ranged from 1.11 to 3.34 % and the LF ranged from 318.92 g m−2 a−1 to 450.72 g m−2 year−1. However, we found no significant difference of LF between years, and the regression analysis showed that both RGR and LF had no discernible relationship with the annual mean R S, R H, or R A (P > 0.05) (Fig. S2, Electronic Supplementary Material).
4 Discussion
4.1 The diurnal variation of soil respiration
T S is a variable that changed strongly at the diurnal scale and is the primary driving factor for the diurnal variation of R S and R H, which can explain 52 and 59 % of the daily variation. This is consistent with the results of other terrestrial ecosystems (Tang et al. 2005; Vargas et al. 2010). Li et al. (2011) found that T S could explain more than 60 % of the diurnal variation of R S and R H, which is similar to our study. Jensen et al. (1996) conducted a two-day measurement in a Pinus radiata forest in New Zealand and found no obvious diurnal variation of R S, which may be caused by the little fluctuation in T S. However, we found the diurnal pattern of R A to not be related to T S, which may be a result of R A being more affected by other biotic factors, in addition to T S. The photosynthesis intensity and the transportation of photosynthates from the canopy to roots have pronounced effects on the diurnal variation of R A (Davidson and Holbrook 2009; Kuzyakov and Gavrichkova 2010; Vargas et al. 2010). A high-frequency measurement of the roots and microbial respiration in Harvard forest showed that the R A was closely related to canopy photosynthesis, and that T S could not explain the amplitude of R A on a day scale (Savage et al. 2013). Additionally, the direct connection between solar radiation and T S typically obscures the relativity between the R A and these two factors (Kuzyakov and Gavrichkova 2010). The diurnal patterns of R H and R A were controlled by different environmental factors, and the ratios of R H and R A to R S may determine the diurnal variation of R S.
4.2 The seasonal change of soil respiration
The seasonal patterns of R S, R H, and R A exhibited a single peak curve. The CV of the seasonal R S is 31–60 %, which is higher than the results of Wang et al. (2006) (25 %). This difference may be caused by the composition of tree species and the soil microclimate. The spruce-fir valley forest is sensitive to hydrothermal conditions, and the local permafrost is melting as a result of global warming. All of these changes may result in a relatively high seasonal variation of soil respiration along with the seasonal changes of T S and W S. In addition, we found the CV of R A to be higher than the R H (Fig. 3), perhaps due to the mixed effects of the seasonal change of W S and the plant phenology on roots. Changes in T S, W S, and their interaction could cause soil respiration to have a corresponding variation trend (Saiz et al. 2006; Kukumägi et al. 2014; Shi et al. 2015). In this study, T S can explain 80–95 % of the variation of the seasonal R S and R H in most cases, whereas the explanation for R A is very different throughout the 5-year study (Fig. 5). Shi et al. (2015) obtained similar results in three coniferous forests in the same area, with an R 2 ranging from 0.343 to 0.580 for R A. In general, R H is regulated by soil microorganisms, which are primarily controlled by T S and the substrate availability (Han et al. 2007). Hopkins et al. (2014) found that warming increased the turnover rate of soil organic carbon, through an incubation experiment. The results of Billings et al. (1998) also indicated that the temporal variation of soil respiration was consistent with the changes of T S when W S was not limited. Furthermore, the contribution of R H to R S was 33–86 % (Fig. 4), and W S maintained at a relatively high level (20–60 %) (Fig. 2) and was not the restricted variable during the measurement. Thus, R H exerts a crucial role in determining the temporal variation of R S in this site, and we speculate that T S is the primary driving factor of the seasonal R S and R H in temperate forests when the W S is adequate. We only measured the RB from May to September 2012, and we found there was no significant relationship between the seasonal R A and RB (including the first soil layer and the total). Generally, R A consists of the actual root respiration and rhizosphere microbial respiration (Scott-Denton et al. 2006); in addition to RB, the unit root respiration rate and rhizosphere microbial activity are important factors controlling the variation of R A (Luo and Zhou 2006). The respiration by rhizosphere organisms and ectomycorrhizae contributed approximately 50 % to the R A (Subke et al. 2011). Additionally, other than RB, the metabolic activity of root systems also has a pronounced effect on the temporal variation of R A (Vargas and Allen 2008). R A may be a result of complicated interactions among environmental factors. Thus, it is necessary to conduct additional controlled experiments and analyzing methods to distinguish impacts of environmental variables on R A and to identify which processes driving the seasonal variation of R A (Zhang et al. 2013).
4.3 The inter-annual variability of soil respiration
Many forest ecosystems have been observed for the inter-annual variation of R S, but there has been little observation of the R H and R A (Moyes and Bowling 2012; You et al. 2013). In this study, R H exhibited a relatively low inter-annual variation, which may be due to the small fluctuation of T S (approximately 10 %). In addition, the spruce-fir valley forest is an evergreen coniferous forest, where the annual litterfall input is relatively stable (Table 4). We found no pronounced difference in the annual litterfall during the 5-year study, and any difference was not related to the annual R S, R H, and R A. However, Zimmermann et al. (2009) found that litterfall could account for 37 % of the inter-annual variation of R S in a tropical montane cloud forest in Peru. This difference could be caused by the quantity and quality of the annual litterfall of different forest types. In tropical forests, the different decomposition rates of litterfall between dry and wet seasons in different years may result in a high annual variation of soil respiration, whereas the effect may be different in a coniferous forest. The similar values of T S and RGR for the last 4 years of the study may cause these two factors are both not related to soil respiration. However, the mean R A of the growing season was negatively related to the mean W S (Fig. 6), which could explain 38.7 % of the inter-annual variation of R A. Spruce-fir valley forests typically distribute in areas where there are rivers or streams and the W S is relatively high (34.13–44.95 %) (Table 3). However, the normal development of roots will be restricted due to oxygen deficit with high soil-water content (Greenway and Gibbs 2003), and the R A may decrease. In contrast, soil microorganisms can adapt to a wide variety of soil-water conditions (Luo and Zhou 2006). The negative relationship between R A and W S may explain why the R A was larger than R H in 2014 which is the year that had the lowest W S (Table 3). Moreover, Fig. 2 also showed the W S throughout 2014 was lower relative to the other years which could enhance R A, whereas the R H was not related to W S. R A was more sensitive to W S which could be due to the root and rhizosphere activity associated with phenology was restricted by W S (Curiel yuste et al. 2004). Hence, in the case of less fluctuation of R H (Fig. 3), the increasing of R A in 2014 due to low W S may lead to R A being larger, correspondingly. Although some studies have demonstrated that W S could account for the inter-annual variation of R S (Martin and Bolstad 2005; Kishimoto-Mo et al. 2015), it was not the case at this site. This may be related to the large portion of R S held by R H. Additionally, the annual CV of T S and W S ranged from 10 to 17 %, which was far less than the annual CV of R A. Therefore, only T S and W S could not fully explain the inter-annual variations of R A and R H. Changes in plant phenology and precipitation throughout the 5-year study may also be responsible for the inter-annual variation in soil respiration.
4.4 The contributions of R H and R A to R S
The RC decreased starting approximately in the mid-growing season which coincided with T S (Fig. 4), and it may be due to R A was more sensitive to temperature (Table 3). Hence, the change of R A was larger than R H with the decreasing of T S. RC ranged from 20 to 50 % in most cases (Fig. 4), which is similar to the result of Shi et al. (2015) (27–34 %) for three coniferous forests in the same reserve. However, the contribution of R H to R S was at a higher level (33–86 %). This phenomenon may be caused by the large amount of coarse woody debris in the site and the high soil-water content (Jin et al. 2009). The coarse woody debris contributes to the nutrient cycling of the forest site and has a pronounced influence on the transportation and storage of soil sediments (Jomura et al. 2008), and the accumulation of soil organic matter in the spruce-fir valley forest is high (Liu et al. 2014). In addition, the input of litterfall occurs throughout the year, which maintains a stable amount of soil microbial biomass (Liu et al. 2014) and the high W S (Fig. 2) may restrict R A.
4.5 The temperature sensitivity of soil respiration
The Q 10 of R S ranged from 2.01 to 3.71, which is similar to the results of Wang et al. (2006) and You et al. (2013) for temperate forests in northeastern China. Ma et al. (2014) found that the range of Q 10 for R H and R A was 2.69–3.03 and 3.06–4.39, respectively, in four larch plantations in northern China. However, the fluctuation of Q 10 for R A in this site is higher (Table 3). This indicates that the Q 10 of R S and R H may be similar in temperate forests in northern China. However, the Q 10 of R A is different due to the tree species composition and local soil microclimate, especially the soil-water conditions, which may stimulate the sensitivity of roots to temperature. The results of Laganière et al. (2012) also indicated that the influence of boreal forest composition on soil respiration is mediated through the soil microclimate. Furthermore, the superposition effect of photosynthesis, T S, and W S will result in a higher Q 10 of R A (Subke and Bahn 2010; Jiang et al., 2013), as observed in our site. However, for R H, the Q 10 depends on the soil microclimate, the utilization of substrate, and the activity of soil microorganisms (Erhagen et al. 2015; Song et al. 2015). Thus, these biotic and abiotic factors should be considered simultaneously when studying the temperature sensitivity of R S and its components.
5 Conclusions
Our results indicate that R S, R H, and R A exhibit different temporal variations at multiple timescales. T S is the fundamental driving factor of the diurnal and seasonal variation of R S and R H when W S is not limited, whereas R A may be affected by the interaction of biotic and abiotic factors, such as ectomycorrhizae, rhizosphere microorganisms, photosynthesis, and soil microclimate; this needs to be further researched. Additionally, the annual variation of W S is an important factor that regulates the inter-annual variation of R A in this site. The larger ratio of R H to R S demonstrates that R H plays a crucial role in determining the magnitude and temporal variation of R S. In this site, R A is more sensitive to T S and has a larger inter-annual fluctuation. The variability of R H and R A in multiple timescales is different and controlled by different environmental factors. It is necessary to consider these two components separately in carbon cycle simulations of regional ecosystems and in predicting global climate change.
References
Billings SA, Richter DD, Yarie J (1998) Soil carbon dioxide fluxes and profile concentrations in two boreal forests. Can J Forest Res 28:1773–1783
Bonal D, Bosc A, Ponton S et al (2008) Impact of severe dry season on net ecosystem exchange in the neotropical rainforest of French Guiana. Global Change Biol 14:1917–1933
Bond-Lamberty B, Thomson A (2010) Temperature-associated increases in the global soil respiration record. Nature 464:579–582
Butler A, Meir P, Saiz G, Maracahipes L, Marimon BS, Grace J (2012) Annual variation in soil respiration and its component parts in two structurally contrasting woody savannas in Central Brazil. Plant Soil 352:129–142
Curiel Yuste J, Janssens IA, Carrara A, Ceulemans R (2004) Annual Q10 of soil respiration reflects plant phenological patterns as well as temperature sensitivity. Global Change Biol 10:161–169
Davidson EA, Holbrook NM (2009) Is temporal variation of soil respiration linked to the phenology of photosynthesis? In: Phenology of ecosystem processes: applications in global change research. Springer, New York
Department of Forestry of PR China (1994) Statistics of China’s forest resources (1989 – 1993). Beijing
Erhagen B, Ilstedt U, Nilsson MB (2015) Temperature sensitivity of heterotrophic soil CO2 production increases with increasing carbon substrate uptake rate. Soil Biol Biochem 80:45–52
Greenway H, Gibbs J (2003) Review: mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to essential processes. Funct Plant Biol 30:999–1036
Han G, Zhou G, Xu Z, Yang Y, Liu J, Shi K (2007) Biotic and abiotic factors controlling the spatial and temporal variation of soil respiration in an agricultural ecosystem. Soil Biol Biochem 39:418–425
Han G, Luo Y, Li D, Xia J, Xing Q, Yu J (2014) Ecosystem photosynthesis regulates soil respiration on a diurnal scale with a short-term time lag in a coastal wetland. Soil Biol Biochem 68:85–94
Hanpattanakit P, Leclerc MY, Mcmillan AMS et al (2015) Multiple timescale variations and controls of soil respiration in a tropical dry dipterocarp forest, western Thailand. Plant Soil 390:167–181
Hopkins FM, Filley TR, Gleixner G, Lange M, Top SM, Trumbore SE (2014) Increased belowground carbon inputs and warming promote loss of soil organic carbon through complementary microbial responses. Soil Biol Biochem 76:57–69
Jensen LS, Mueller T, Tate KR, Ross DJ, Magid J, Nielsen NE (1996) Soil surface CO2 flux as an index of soil respiration in situ: a comparison of two chamber methods. Soil Biol Biochem 28:1297–1306
Jiang H, Deng Q, Zhou G et al (2013) Responses of soil respiration and its temperature/moisture sensitivity to precipitation in three subtropical forests in southern China. Biogeosciences 10:3963–3982
Jin G, Liu Z, Cai H, Tai B, Jiang X, Liu Y (2009) Coarse woody debris (CWD) in a spruce-fir valley forest in Xiao xingan mountains, China (in Chinese with English abstract). J Nat Res 24:1256–1266
Jomura M, Kominami Y, Dannoura M, Kanazawa Y (2008) Spatial variation in respiration from coarse woody debris in a temperate secondary broad-leaved forest in Japan. Forest Ecol Manag 255:149–155
King JS, Hanson PJ, Bernhardt E, Deangelis P, Norby RJ, Pregitzer KS (2004) A multiyear synthesis of soil respiration responses to elevated atmospheric CO2 from four forest FACE experiments. Global Change Biol 10:1027–1042
Kishimoto-Mo AW, Yonemura S, Uchida M, Kondo M, Murayama S, Koizumi H (2015) Contribution of soil moisture to seasonal and annual variations of soil CO2 efflux in a humid cool-temperate oak-birch forest in central Japan. Ecol Res 30:311–325
Kodama N, Barnard RL, Salmon Y et al (2008) Temporal dynamics of the carbon isotope composition in a Pinus sylvestris stand: from newly assimilated organic carbon to respired carbon dioxide. Oecologia 156:737–750
Kukumägi M, Ostonen I, Kupper P et al (2014) The effects of elevated atmospheric humidity on soil respiration components in a young silver birch forest. Agr Forest Meteorol 194:167–174
Kuzyakov Y, Gavrichkova O (2010) REVIEW: time lag between photosynthesis and carbon dioxide efflux from soil: a review of mechanisms and controls. Global Change Biol 16:3386–3406
Laganière J, Paré D, Bergeron Y, Chen HYH (2012) The effect of boreal forest composition on soil respiration is mediated through variations in soil temperature and C quality. Soil Biol Biochem 53:18–27
Li Z, Wang X, Zhang R, Zhang J, Tian C (2011) Contrasting diurnal variations in soil organic carbon decomposition and root respiration due to a hysteresis effect with soil temperature in a Gossypium s. (cotton) plantation. Plant Soil 343:347–355
Liu C, Liu Y, Jin G (2014) Seasonal dynamics of soil microbial biomass in six forest types in Xiaoxing’an Mountains, China (in Chinese with English abstract). Acta Ecol Sin 34:1–9
Luo Y, Zhou X (2006) Soil respiration and the environment. Academic Press, An imprint of Elsevier Science, London
Luo Y, Wan S, Hui D, Wallace LL (2001) Acclimatization of soil respiration to warming in a tall grass prairie. Nature 413:622–625
Ma Y, Piao S, Sun Z, Lin X, Wang T, Yue C, Yang Y (2014) Stand ages regulate the response of soil respiration to temperature in a Larix principis-rupprechtii plantation. Agr Forest Meteorol 184:179–187
Martin JG, Bolstad PV (2005) Annual soil respiration in broadleaf forests of northern Wisconsin: influence of moisture and site biological, chemical, and physical characteristics. Biogeochemistry 73:149–182
Moyes AB, Bowling DR (2012) Interannual variation in seasonal drivers of soil respiration in a semi-arid Rocky Mountain meadow. Biogeochemistry 113:683–697
Ohashi M, Kumagai TO, Kume T, Gyokusen K, Saitoh TM, Suzuki M (2008) Characteristics of soil CO2 efflux variability in an aseasonal tropical rainforest in Borneo Island. Biogeochemistry 90:275–289
Pang X, Bao W, Zhu B, Cheng W (2013) Responses of soil respiration and its temperature sensitivity to thinning in a pine plantation. Agr Forest Meteorol 171:57–64
Poorter L, Wright SJ, Paz H et al (2008) Are functional traits good predictors of demographic rates? Evidence from five neotropical forests. Ecology 89:1908–1920
Prolingheuer N, Scharnagl B, Graf A, Vereecken H, Herbst M (2014) On the spatial variation of soil rhizospheric and heterotrophic respiration in a winter wheat stand. Agr Forest Meteorol 195:24–31
Raich JW, Tufekciogul A (2000) Vegetation and soil respiration: correlations and controls. Biogeochemistry 48:71–90
Raich JW, Potter CS, Bhagawati D (2002) Interannual variability in global soil respiration, 1980-94. Global Change Biol 8:800–812
Saiz G, Green C, Butterbach-Bahl K, Kiese R, Avitabile V, Farrell EP (2006) Seasonal and spatial variability of soil respiration in four Sitka spruce stands. Plant Soil 287:161–176
Savage KE, Davidson EA (2001) Interannual variation of soil respiration in two New England forests. Global Biogeochem Cy 15:337–350
Savage K, Davidson EA, Tang J (2013) Diel patterns of autotrophic and heterotrophic respiration among phenological stages. Global Change Biol 19:1151–1159
Schindlbacher A, Zechmeister‐Boltenstern S, Jandl R (2009) Carbon losses due to soil warming: do autotrophic and heterotrophic soil respiration respond equally? Global Change Biol 15:901–913
Schlesinger WH (1997) Biogeochemistry: an analysis of global change, Academic Press
Schmidt MWI, Torn MS, Samuel A et al (2011) Persistence of soil organic matter as an ecosystem property. Nature Nature 478:49–56
Schuur EG, Trumbore SE (2005) Partitioning sources of soil respiration in boreal spruce forest using radiocarbon. Global Change Biol 12:165–176
Scott-Denton LE, Rosenstiel TN, Monson RK (2006) Differential controls by climate and substrate over the heterotrophic and rhizospheric components of soil respiration. Global Change Biol 12:205–216
Shi B, Jin G (2016) Variability of soil respiration at different spatial scales in temperate forests. Biol Fertil Soils. doi:10.1007/s00374-016-1100-1
Shi P-L, Zhang X-Z, Zhong Z-M, Ouyang H (2006) Diurnal and seasonal variability of soil CO2 efflux in a cropland ecosystem on the Tibetan Plateau. Agr Forest Meteorol 137:220–233
Shi B, Gao W, Jin G (2015) Effects on rhizospheric and heterotrophic respiration of conversion from primary forest to secondary forest and plantations in northeast China. Eur J of Soil Biol 66:11–18
Soil survey staff (1999) soil taxonomy: a basic system of soil classification for making and interpreting soil surveys. USDA Natural Resources Conservation Service, Washington, D.C
Song W, Chen S, Wu B, Zhu Y, Zhou Y, Lu Q, Lin G (2015) Simulated rain addition modifies diurnal patterns and temperature sensitivities of autotrophic and heterotrophic soil respiration in an arid desert ecosystem. Soil Biol Biochem 82:143–152
Subke JA, Bahn M (2010) On the ‘temperature sensitivity’ of soil respiration: can we use the immeasurable to predict the unknown? Soil Biol Biochem 42:1653–1656
Subke JA, Inglima I, Francesca Cotrufo M (2006) Trends and methodological impacts in soil CO2 efflux partitioning: a metaanalytical review. Global Change Biol 12:921–943
Subke JA, Voke NR, Leronni V, Garnett MH, Ineson P (2011) Dynamics and pathways of autotrophic and heterotrophic soil CO2 efflux revealed by forest girdling. J Ecol 99:186–193
Tang J, Baldocchi DD, Xu L (2005) Tree photosynthesis modulates soil respiration on a diurnal time scale. Global Change Biol 11:1298–1304
Vargas R, Allen MF (2008) Dynamics of fine root, fungal rhizomorphs, and soil respiration in a mixed temperate forest: integrating sensors and observations. Vadose Zone J 7:1055–1064
Vargas R, Detto M, Baldocchi DD, Allen MF (2010) Multiscale analysis of temporal variability of soil CO2 production as influenced by weather and vegetation. Global Change Biol 16:1589–1605
Wang C, Yang J (2007) Rhizospheric and heterotrophic components of soil respiration in six Chinese temperate forests. Global Change Biol 13:123–131
Wang C, Yang J, Zhang Q (2006) Soil respiration in six temperate forests in China. Global Change Biol 12:2103–2114
Whitaker J, Ostle N, Nottingham AT et al (2014) Microbial community composition explains soil respiration responses to changing carbon inputs along an Andes-to-Amazon elevation gradient. J Ecol 102:1058–1071
You W, Wei W, Zhang H, Yan T, Xing Z (2013) Temporal patterns of soil CO2 efflux in a temperate Korean Larch (Larix olgensis Herry.) plantation, Northeast China. Trees Struct Funct 27:1417–1428
Yuste JC, Janssens IA, Carrara AR (2004) Annual Q10 of soil respiration reflects plant phenological patterns as well as temperature sensitivity. Global Change Biol 10:161–169
Zhang Q, Lei HM, Yang DW (2013) Seasonal variations in soil respiration, heterotrophic respiration and autotrophic respiration of a wheat and maize rotation cropland in the North China Plain. Agr Forest Meteorol 180:34–43
Zimmermann M, Meir P, Bird M, Malhi Y, Ccahuana A (2009) Litter contribution to diurnal and annual soil respiration in a tropical montane cloud forest. Soil Biol Biochem 41:1338–1340
Acknowledgments
We are grateful to the two anonymous reviewers for their constructive suggestions and comments. We also would like to thank the editor for grammatical editing of the manuscript. This work was financially supported by the Fundamental Research Funds for Central Universities (DL13EA05), and the Program for Changjiang Scholars and Innovative Research Team in Universities (IRT_15R09).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Juxiu Liu
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 59 kb)
Rights and permissions
About this article
Cite this article
Han, MG., Shi, BK. & Jin, GZ. Temporal variations of soil respiration at multiple timescales in a spruce-fir valley forest, northeastern China. J Soils Sediments 16, 2385–2394 (2016). https://doi.org/10.1007/s11368-016-1440-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-016-1440-3