Abstract
Purpose
Both overharvesting and climate changes have greatly altered forest composition in northeastern China; however, forest-specific effects on soil organic carbon (SOC), N, and compositional features in different soil fractions have not yet been defined.
Materials and methods
By sampling from broad-leaved Korean pine forest (the climax vegetation) and aspen–birch forest (the secondary forest), five soil fractions were separated by a physicochemical soil fractionation method, and Fourier transform infrared spectroscopy, X-ray diffraction analysis, and X-ray photoelectron spectrometry were used for functional groups, mineral diffraction, and elemental composition determination together with SOC and N measurements.
Results and discussion
Aspen–birch forests tended to sequestrate more SOC in the slow fractions (sand and aggregate [SA] and easily oxidized fractions) and more N in the sensitive fractions (particulate and soluble fractions), indicating that in aspen–birch forests, high SOC sequestration (1.26-fold) coincided with the active and rapid N supply. Much higher percentages (13.1–40.5 %) of O–H and N–H stretching, O–H bending, and C=O, COO–, and C–H stretching, and also the much lower quartz grain size and mineral diffraction peaks in SA and acid-insoluble fraction (over 85 % of total soil mass), in aspen–birch forests were possibly associated with the 1.17- to 1.53-fold higher SOC compared to broad-leaved Korean pine forest. However, elemental composition on soil particles might marginally contribute to the SOC and N forest-dependent differences.
Conclusions
Considering the increase of aspen–birch forests and the decrease of broad-leaved Korean pine forests in historical and future scenarios in northeastern China, more SOC has been and also will sequestrate in intact soils and stable soil fractions, with more N in sensitive fractions, and these should be highlighted in evaluating forest C and N dynamics during forest successions in this region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Broad-leaved Korean pine forests and aspen–birch forests are two typical forests in northeastern China (Zhou 1997). The former is a late-successional natural forest mixed by various broad-leaved trees (Populus, Quercus, Acer and ash sepcies, etc.) and Korean pine (Pinus koraiensis), recognized as a local climatic climax community (Wang 1957; Li 1984), while the latter is a relatively new secondary forest type that replaced broad-leaved Korean pine forest when it was destroyed by clear cutting and burning (Zhou 1997). Timber overharvesting has sharply decreased the broad-leaved Korean pine forest both in area and timber volume and increased the aspen–birch secondary forest (Yao and Man 2004), which currently is the main timber resource in northeastern China (Yu et al. 2015). Due to global warming and altered precipitation patterns, broadleaf forests, including aspen–birch forest, will be doubled at the expense of coniferous forest area, including broad-leaved Korean pine forest (Cheng and Yan 2008). Multiple studies have investigated vegetation composition (Makohonienko et al. 2008), litter quality and decomposition (Jiang et al. 2013), biomass C sequestration capacity (Zhu et al. 2010; Dai et al. 2013), biomass productivity (Li 1984; Tang et al. 2009; Yan and Feng 2006), and tree growth differences (Li 1983; Gong et al. 2009) in broad-leaved Korean pine forests and aspen–birch forests. Compared to broadleaf forests, pine species are considered to have a limited capacity to increase soil C stocks after planting as it was found that soil organic carbon (SOC) in pine-afforested soils is lower than that in agricultural soils (Giddens et al. 1997; Guo and Gifford 2002; Markewitz et al. 2002; Guo et al. 2007). Soil C and N variations between aspen–birch forests and broad-leaved Korean pine forests and the underlying mechanisms play a key role in long-term soil C sequestration and N supply (Yan and Feng 2006), and this information is important for developing forest management plans in national-scale forest programs, such as the Natural Forest Protection Program in which over 200 billion RMB has been invested by the central government of China since 1998 (Zu et al. 2009; Yu et al. 2011).
Soil fractionation via physicochemical methods is an effective way to reveal the underlying mechanisms of soil C sequestration and N supply (Six et al. 2000a, b; Zimmermann et al. 2007; Ji et al. 2014). Traditional fractionation methods (e.g., wet sieving method) can distinguish different soil fractions according to different particle sizes (Arnarson and Keil 2001; Karhu et al. 2010; Baah-Acheamfour et al. 2014). In combination with chemical oxidants, such as hypochlorous and hydrofluoric acids, fractionation methods can also distinguish the acid-insoluble (AI) and easily oxidizable (EO) fractions (Zimmermann et al. 2007), while in combination with different density solvents can distinguish light (e.g., particulate [PTO] fraction) and heavy fractions (e.g. silt and clay fraction or sand and aggregate [SA] fraction; Six et al. 2000b; Zimmermann et al. 2007; Plante et al. 2010). Soil fractions are usually associated with SOC protection from microbial decomposition and physical degradation (Rumpel et al. 2004; Zimmermann et al. 2007; Stockmann et al. 2013) and soil management indicators (Denef et al. 2007; Wong et al. 2016) and can provide information on SOC sequestration capacity and nutrient sustainability in the long term (Doetterl et al. 2012; Feng et al. 2013; Stockmann et al. 2013). Therefore, proper fractionation methods need to be used in order to reveal which soil fractions are responsible for the observed soil C and N differences between broad-leaved Korean pine forests and aspen–birch forests in northeastern China.
New technologies in soil science allow the study of SOC sequestration and N supply from functional groups (Vohland et al. 2011; Bernier et al. 2013; Li et al. 2013b; Wang et al. 2014a, b), soil mineral composition (Arnarson and Keil 2001; Feng et al. 2006), and elemental composition (Yuan et al. 1998; Arnarson and Keil 2001) in different soil fractions. Using Fourier transform infrared spectroscopy (FTIR), different stretching and bending vibration bands (i.e., O–H, N, N–H, C, C–H, C, C=O, C, C–O, Si–O–Si) in the soil and organic matter can be analyzed qualitatively and semi-quantitatively (Li et al. 2013b; Wang et al. 2014a) for studying the effects of soil fertilization, water irrigation, and soil fungi–soil colloid interactions (Vohland et al. 2011; Bernier et al. 2013; Li et al. 2013b; Wang et al. 2014a, b). X-ray diffraction (XRD) analysis is used for identifying soil mineral composition, crystallinity, mineral diffraction peaks, and grain size (Arnarson and Keil 2001; Feng et al. 2006; Li et al. 2013b), while X-ray photoelectron spectrometry (XPS) is applied to analyze the elemental composition on the surface of soil particles and electron binding energy and determine the variation in elemental composition (C1s, Ca2p, N1s, O1s, Si2p, Al2p, Al2p, and Fe2p) on the surface of soil particles (Yuan et al. 1998; Arnarson and Keil 2001; Wang et al. 2014b; Feng et al. 2015). These data, in combination with SOC and N concentrations in different soil fractions, provide new insights on C sequestration and N supply and can reveal the underlying mechanisms in different forest types (Arnarson and Keil 2001; Wang et al. 2014b; Ji et al. 2015).
The hypothesis of this study was that broad-leaved Korean pine forests and aspen–birch forests differed in SOC sequestration and N supply due to differences in soil fractions, functional groups, mineral diffraction, and elemental composition. Based on this hypothesis, we aimed to answer the following questions:
-
1.
How much different are these two forests in SOC sequestration and N supply, and which soil fraction mainly contributes to these differences? What are the possible reasons?
-
2.
What are the differences in functional groups, mineral diffraction, and elemental composition between the soil fractions and how do these differences contribute to SOC sequestration and N supply in broad-leaved Korean pine forests and aspen–birch forests?
-
3.
On the basis of historical changes and future climate, what implications can be drawn for local afforestation practices and natural forest management?
2 Materials and methods
2.1 Study sites, basic information on the studied forests, and soil sample collection
The study sites are about 720–1100 m above sea level located at the Changbai Mountains in southeastern Jilin Province (42°21′–42°24′N, 127°59′–128°05′E), close to the research station of the Chinese Academy of Sciences. This region has a warm temperate continental monsoon climate with a typical long cold winter and short warm summer, an average annual temperature of 3.6 °C, and precipitation of 700–1400 mm. The coldest month is January with an average temperature of −15.6 °C, while the hottest month is July with an average temperature of 19.7 °C.
The broad-leaved Korean pine natural forest included in this study is mainly composed of P. koraiensis (average age, 300 years) and other accompanying species, including Populus cathayana, Quercus mongolica, Acer triflorum, Ulmus japonica, and Fraxinus mandschurica. Tree density (over 10 m in height) is 998 trees per hectare. The tree composition ratio of P. koraiensis:Q. mongolica:A. triflorium:P. cathayana is 73:13:13:1 in tree density, and the corresponding ratio in the cross-section area of diameter at breast height (DBH) is 63:21:1:15. These composition ratios indicate that broad-leaved Korean pine forest is a kind of conifer forest dominated by pine trees (63–73 %).
The aspen–birch forest included in this study is mainly composed of Betula platyphylla, Populus davidiana, Salix matsudana, Larix gmelinii, and P. cathayana. Tree density (over 9 m in height) is 2000 trees per hectare. The tree composition ratio of B. platyphylla:Populus sp. (both P. davidiana and P. cathayana):S. matsudana:L. gmelinii is 78:9:5:9 in tree density, and the corresponding ratio in the cross-section area of DBH is 75:16:5:4. These composition ratios indicate that aspen–birch forest is a kind of broad-leaved forest dominated by birch (75–78 %) and aspen (9–16 %). Except for tree species differences, the study sites were selected based on their similarities in topography, slope direction and position, and soil parent material. In both sites, the soil is typical dark brown forest soil.
In October 2011, 15 composited soil samples (each composited sample was a mix of at least three subsamples) were collected in the 0- to 30-cm soil layer from aspen–birch forests, and six composited soil samples were collected in broad-leaved Korean pine forests using the quadrat method with a similar sampling protocol. Six quadrats (three sampling point in a 20 × 20-m quadrat for soil sampling) were surveyed in the broad-leaved Korean pine forests, while 15 quadrats of the aspen–birch forests were surveyed and sampled in the same way. The age of dominant species of Korean pine in broad-leaved Korean pine forests were averaged around 200 years, while the aspen–birch forests used in this paper were about 40 years old after cutting of the natural forests, on average. Soil samples were fully air-dried and cleaned of small stones, distinguishable plant roots, and other debris. A part of the soil samples was passed through a 2-mm sieve for soil fractionation analysis. Surplus samples were smashed and passed through a 0.25-mm sieve for analyzing intact soil SOC and N.
2.2 Soil fractionation by a physicochemical method
Soil fractions with different physicochemical stabilities were separated by a physicochemical method as described by Zimmermann et al. (2007). Soil samples (<2 mm) were added to water (1:5) and dispersed for 1 min using a probe-type ultrasonic vibrator (SCIENTZ-IID, China). The dispersed suspension was wet-sieved through a 63-μm sieve until the rinsing water was clear. The soil fraction >63 μm was left on the sieve and that <63 μm was filtered. The soil was separated by stirring the >63-μm fraction with 1.8 g cm−3 NaI solution. Floatage was the particulate organic fraction (PTO) and precipitate was the sand and aggregate fraction. The soil fraction <63 μm was centrifuged and the supernatant was collected by suction filtration (filter membrane, 0.45 μm). The soluble organic fraction (SBO) was frozen-dried from the filtrated solution (Scientz-10N, Ningbo Scientz Biotechnology Co., Ltd., China) and the silt and clay solid particles (>0.45 μm) were oxidized by sodium hypochlorite (6 % NaClO) and the residues recognized as the AI fraction. The EO fraction was calculated as the difference between silt and clay and AI. According to Zimmermann et al. (2007), AI and EO are fractions related to silt and clay, while SA is the fraction related to aggregates. Moreover, AI is the passive and refractory fraction, EO and SA are slow fractions, and PTO and SBO are sensitive fractions to land use and vegetation changes.
2.3 Determination of soil C and N
SOC and N contents in the SBO fraction (SBO solution before freeze drying) were measured with multi N/C3100 (Analytik Jena AG, Germany), while in PTO, SA, AI, and intact soil, the SOC content was measured by the soil-heated dichromate/titration method and the N content determined by the Semimicro-Kjeldahl method (Bao 2000) as described by (Wang et al. 2014c). SOC and N contents in the EO fraction were calculated as the difference between silt and clay and AI (Fig. 1). According to the pre-experiment, the ratio between data from N/C3100 and from the heated dichromate/titration method is 1.45; this factor was used to change the traditional data to N/C3100 data.
Flowchart of soil fractions based on particle components as described by Zimmermann et al. (2007)
2.4 FTIR analysis
Potassium bromide was oven-dried for 4 h at 120 °C. Before measurement, soil samples and potassium bromide were kept in a vacuum dryer for at least 48 h and then 1.6 mg of soil and 200 mg of potassium bromide were carefully mixed and ground and then pressed into a sample disk for FTIR analysis (IRAffinity-1, Shimadzu, Japan) at a wave spectrum of 4000–500 cm−1. As shown in Fig. 2a, the functional traits were classified into five groups (Johnson and Aochi 1996). Functional group Ι included O–H stretching of carboxylic acids, phenols, and alcohols, N–H stretching of amines and amides, and stretching of structural OH (O–H and N–H stretching); functional group II included aliphatic C–H stretching (C–H stretching); functional group III included O–H bending and C=O stretching of carboxylic acids, amides, and ketones (O–H bending and C=O stretching); functional group IV included symmetric COO– stretching (COO– stretching); and functional group V included C–O stretching, O–H bending of –COOH, C–O stretching of polysaccharides, and Si–O–Si stretching (Si–O–Si stretching, C–O stretching, and –O–H bending of COOH). All the images were adjusted to the same size, and ImageJ (http://imagej.nih.gov/ij/) was used to compute the peak area of each functional group. The area reflected the relative content of each functional group (Wang et al. 2014a).
2.5 XRD analysis
An XRD meter (D/Max 2200, Rigaku, Japan) equipped with a rotating anode (Philips, the Netherlands) was used for XRD analysis. CuK α1 radiation was generated at 30 mA and 40 kV. The range of 2θ diffraction angles was 10–35°, with steps of 0.02° and a measuring time of 0.3 s per step. XRD data were analyzed using Jade 5 (http://mdi-jade.software.informer.com/5.0/) to calculate grain size and the peak number of different soil fractions (Wang et al. 2014a). The sketch map of soil mineral identification shown in Fig. 2b was developed as described by Feng et al. (2006).
2.6 XPS analysis
A K-Alpha X-ray photoelectron spectrometer equipped with a concentric hemispherical analyzer in the standard configuration (Thermo Scientific, Waltham, MA) was used for chemical analysis. Soil samples were carefully placed on highly oriented pyrolytic graphite before examination. The vacuum system consisted of a turbomolecular pump and a titanium sublimation pump. The residual pressure before analysis was lower than 10−7 Pa. The X-ray source was AlKα and run at 30 mA and 80 kV. The incident angle was 49.1° and the emission angle was 0° with respect to each sample’s normal surface. All spectra were obtained in digital mode. The wide-scan spectra were acquired from 1000 to 0 eV. Detailed spectra were processed using Casa XPS 2.3.12 (Casa Software Ltd., UK). An iterated Shirley–Sherwood background subtraction was applied before peak fitting using a nonlinear least squares algorithm. The atomic concentration of the elements was calculated using Casa XPS (Li et al. 2013b). As shown in Fig. 2c, ten elements in total were measured from the XPS spectrum.
2.7 Underlying reason clarification and implications of importance from referenced data
The underlying reasons for the observed differences between the two forests were clarified by a review of previous studies on litter quantity, litter quality, and microbial activities between aspen–birch forests and broad-leaved Korean pine forests (Li 1984, 2003; Guo et al. 2006; Li et al. 2007, 2013a; Luan et al. 2012; Xia et al. 2012; Hu et al. 2013; Zhang and Cui 2016).
The importance of these soil changes in SOC and N in intact soil and different fractions was also highlighted by the forest changes in history and possible changes under future climates. Historical changes of these two forests from 1949 to 1997 were referenced from Yao and Man (2004), and their changes in future climates in the next 100 years were referenced from Cheng and Yan (2008).
2.8 Data analysis
Analysis of variance, in conjunction with Student’s t test (p < 0.05), was performed to identify any differences in SOC, N, fraction percentage, functional groups, mineral diffraction peaks, soil mineral grain size, and elemental composition on the surface of soil particles between aspen–birch forests and broad-leaved Korean pine forests. In Figs. 1, 2, 3, 4, 5, 6, 7, and 8, the significant differences (p < 0.05) are expressed as percentages using aspen–birch forest as a reference. Pooled data were used for regression analysis between SOC, N, and C/N ratio and functional groups, mineral diffraction peaks, soil mineral grain size, and elemental composition in order to check the possible contribution of these parameters to SOC sequestration and N supply. We assumed that high correlation coefficients along with significant forest-related differences would indicate that these parameters were possibly responsible for the observed differences between the broad-leaved Korean pine forest and aspen–birch forest and were related to any underlying mechanisms of C and N dynamics in the soil. SPSS 17.0 was used for these statistical analyses.
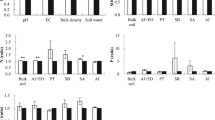
Comparison of functional groups in different soil fractions between aspen–birch forest (ABF) and broad-leaved Korean pine forest (KF). Arrows and percentages indicate the significant differences (p < 0.05) of the aspen–birch forest compared to broad-leaved Korean pine forest. Functional group Ι, O–H and N–H stretching; functional group II, C–H stretching; functional group III, O–H bending and C=O stretching; functional group IV, COO– stretching; and functional group V, Si–O–Si and C–O stretching and –O–H bending of COOH. Soil fractions: AI acid-insoluble, SA sand and aggregate, EO easily oxidized, PTO particulate organic, SBO soluble organic, AI+EO combined AI and EO
Comparison of the diffraction peaks and grain size of three soil minerals (montmorillonite, quartz, and plagioclase) in different soil fractions between the aspen–birch forest (ABF) and broad-leaved Korean pine forest (KF). Arrows and percentages indicate the significant differences (p < 0.05) of the aspen–birch forest compared to broad-leaved Korean pine forest. Soil fractions: AI acid-insoluble, SA sand and aggregate, EO easily oxidized, PTO particulate organic, SBO soluble organic, AI+EO combined AI and EO
Comparison of element composition (C1s, Ca2p, N1s, O1s, Si2p, Al2p, Al2p, and Fe2p) in different soil fractions between the aspen–birch forest (ABF) and broad-leaved Korean pine forest (KF). Arrows and percentages indicate the significant differences (p < 0.05) of the aspen–birch forest compared to broad-leaved Korean pine forest. Soil fractions: AI acid-insoluble, SA sand and aggregate, EO easily oxidized, PTO particulate organic, SBO soluble organic, AI+EO combined AI and EO
3 Results
3.1 SOC, N, and C/N ratio in intact soil and different soil fractions of the two forests
The percentage of each fraction (weight of each fraction to total soil mass, in percent) was similar between the aspen–birch forest and broad-leaved Korean pine forest. Therefore, forest type did not affect the fraction percentage significantly (p > 0.05; Table 1). The maximum percentage was that of AI (about 66 % of total soil mass), followed by SA (20 % of total soil mass), EO (10 % of total soil mass), and PTO (4 % of total soil mass), while the minimum percentage was that of SBO (0.1 % of total soil mass). Thus, AI and SA (over 85 % of total soil mass) greatly controlled soil C and N in both forests.
In both forests, SOC had the maximum value in SBO (330–400 mg g−1) and PTO (300–350 mg g−1), followed by SA and EO, while the lowest value was observed in AI (Table 1). SOC in the slow fractions (SA and EO) of the aspen–birch forest was significantly higher than that of the broad-leaved Korean pine forest by 50–53 % (p < 0.05). However, SOC in the sensitive (1.14-fold higher in SBO and 0.89-fold higher in PTO) and passive (1.17-fold higher in AI) fractions of aspen–birch forest was not significantly higher compared to that of broad-leaved Korean pine forest (p > 0.05). SOC in intact soil of the aspen–birch forest was 1.26-fold higher than that in broad-leaved Korean pine forest (p < 0.05; Table 1).
The N concentration in SA of the aspen–birch forest was higher than that of broad-leaved Korean pine forest by 13 %, while N in AI, the largest fraction of intact soil (66 % of total soil mass), was lower by 17 %. However, these differences were not statistically significant (p > 0.05). N concentration was 2.11- to 2.13-fold higher in the PTO and SBO of the aspen–birch forest compared to broad-leaved Korean pine forest (p < 0.05); however, these fractions were less than 5 % of the total soil mass (Table 1). SOC and N allocations into different fractions resulted in 26 % higher SOC sequestration in the aspen–birch forest compared to broad-leaved Korean pine forest, with only slight differences in N concentration (2 %; Table 1).
The intact soil of the aspen–birch forest had 1.08-fold higher C/N ratio compared to broad-leaved Korean pine forest (p > 0.05), probably because of the 1.36- to 1.47-fold higher C/N ratio in the SA, EO, and PTO of the aspen–birch forest (p < 0.05; Table 1).
3.2 Functional group patterns in different soil fractions of the two forests
In SA, O–H and N–H stretching (functional group I), O–H bending and C=O stretching (functional group III), and COO– stretching (functional group IV) were significantly increased by 13.1–40.5 % in the aspen–birch forest compared to broad-leaved Korean pine forest, while other functional groups showed no differences between the two forests (Fig. 3). In AI, the most significant changes were found in C–H stretching (functional group II), which was increased by 38.3 % in the aspen–birch forest compared to broad-leaved Korean pine forest (p < 0.05), and Si–O–Si and C–O stretching and –O–H bending of COOH (functional group V), which were increased by 8.4 % (Fig. 3). Owing to the over 88 % of total soil mass in these two fractions (SA and AI), their higher functional groups in the aspen–birch forest are related to the 1.26-fold higher SOC in the aspen–birch forest compared with that in broad-leaved Korean pine forest (Table 1). When EO was included into AI (silt and clay, AI+EO), O–H bending and C=O stretching (functional group III) and COO– stretching (functional group IV) were decreased by 22.4 and 27.3 %, respectively, in the aspen–birch forest compared to broad-leaved Korean pine forest, and Si–O–Si and C–O stretching and –O–H bending of COOH (functional group V) were increased by 8.2 % (Fig. 3). In the PTO fraction, C–H stretching (functional group II) was increased by 28.9 % in the aspen–birch forest compared to broad-leaved Korean pine forest, while O–H and N–H stretching (functional group I) was reduced by 8.8 % (Fig. 3). In the SBO fraction, COO– stretching (functional group IV) was significantly reduced by 26.2 % in the aspen–birch forest compared to that in broad-leaved Korean pine forest, while C–H stretching (functional group II) was increased by 32.3 % (Fig. 3).
3.3 Mineral diffraction peaks and mineral grain size in different soil fractions of the two forests
Based on the X-ray diffraction pattern, three minerals were identified: montmorillonite (4.45–4.52 Å), quartz (3.35–3.37 and 4.26–4.28 Å), and plagioclase (3.2–3.22, 3.75–3.79, and 4.03–4.07 Å). Their average grain size and diffraction peaks are shown in Fig. 4.
Compared to the broad-leaved Korean pine forest, the grain size of montmorillonite in the SA of the aspen–birch forest was reduced by 32.3 %, while that of plagioclase was increased by 16.1 % (p < 0.05; Fig. 4). In the SA fraction, the diffraction peaks in the aspen–birch forest were reduced by 32.5 % compared to broad-leaved Korean pine forest (Fig. 4). In AI, the grain size of quartz and plagioclase was reduced by 8.6–9.2 % in the aspen–birch forest compared to that in broad-leaved Korean pine forest. In AI+EO, the grain size of montmorillonite was increased by 28.1 % in the aspen–birch forest compared to that in broad-leaved Korean pine forest, while that of plagioclase was increased by 9.2 %. Diffraction peaks in the AI fractions did not differ between the aspen–birch forest and broad-leaved Korean pine forest (Fig. 4). In the PTO fraction, the grain size of montmorillonite was increased by 102.2 %, with no obvious changes in that of quartz or plagioclase. In the SBO fraction, the size grain of montmorillonite was increased by 31.9 % in the aspen–birch forest compared to broad-leaved Korean pine forest, without any obvious changes in that of plagioclase (Fig. 4). Diffraction peaks in the PTO and SBO fractions in the aspen–birch forest were reduced by 19.1–20 % compared to those in broad-leaved Korean pine forest (Fig. 4).
3.4 Differences in the elemental composition of soil fractions between the two forests
In general, inter-forest data showed that there were no evident differences in O (30–50 %), C (30–50 %), Al (about 6 %), Fe (about 1 %), N (about 2 %), and Si (about 7 %) between the aspen–birch forest and broad-leaved Korean pine forest at different soil fractions, while significant differences were observed in Na (about 1 %), Mg (<1 %), Ca (<1 %), and K (<1 %; Fig. 5).
In the SA fraction, Na was decreased by 18.8 % in the SA of the aspen–birch forest compared to broad-leaved Korean pine forest, while the differences in all other elements (C, O, Si, Al, Fe, N, Mg, K, and Ca) in the same soil fraction were not significant (p > 0.05; Fig. 5). In the AI fraction, K and Mg were increased by 12.3 and 11 %, respectively, in the aspen–birch forest compared to broad-leaved Korean pine forest, while Na and Ca were decreased by about 13 % (Fig. 5). In the AI+EO of the aspen–birch forest, Na, K, and Mg were increased by 20.9–82 % compared to those in broad-leaved Korean pine forest (Fig. 5). In the PTO fraction of the aspen–birch forest, Mg was increased by 76.3 % compared to that in broad-leaved Korean pine forest, while Na was reduced by 24.7 %. In the SBO fraction of the aspen–birch forest, Ca, Na, and Mg were increased by 36.5–87.7 % compared to those in broad-leaved Korean pine forest (Fig. 5).
3.5 Relationships among functional groups, soil minerals, elemental composition, and soil C and N
As presented in Fig. 6, the relative contents of O–H bending and C=O stretching (functional group III) and C–H stretching (functional group II) were positively correlated with SOC (R 2 = 0.77–0.79, p < 0.0001), and their relationships with N were similar to those with SOC (R 2 = 0.75–0.82, p < 0.0001). Of all five functional groups, only functional group V (Si–O–Si stretching, C–O stretching, and –O–H bending of COOH) was significantly correlated with the C/N ratio (R 2 = 0.464, p < 0.0001; Fig. 8). Quartz grain size and mineral diffraction peaks were negatively correlated with SOC (R 2 = 0.43–0.51, p < 0.0001), and much weaker negative relationships were also observed with N (R 2 = 0.08–0.46; Fig. 6). XPS data were significantly correlated with SOC and soil N (Fig. 7). Positive relationships were found in C1s and Ca2p, while negative relationships were found in O1s, Si2p, and Al2p. Much weaker relationships were found between SOC, N, and N1s (data not shown here).
4 Discussion
4.1 SOC sequestration is higher and N supply is faster in aspen–birch forest compared to broad-leaved Korean pine forest: soil fraction analysis and possible underlying reason
Many previous studies showed that pine forests have a lower ability to accumulate SOC in mineral soils compared to broadleaf forests. For example, Giddens et al. (1997) reported that total N and SOC were often lower in soil samples from Pinus radiata forests (p < 0.05). Turner and Lambert (2000) found that soil C continued to decline within the first 24 years of a P. radiata plantation (a decrease of about 65 t carbon ha−1). Guo and Gifford (2002) found that broadleaf tree plantations placed onto prior native forests or pastures did not affect soil C stocks, while pine plantations reduced soil C stocks by 12–15 %. Laganiere et al. (2010) suggested that broadleaf species had a greater capacity to accumulate SOC than do conifer species when the surface organic layer was not included. Guo et al. (2007) found that soil C and N stocks at 100 cm under a pine plantation were significantly lower by 20 and 15 %, respectively, compared to those under a pasture. Ten years after afforestation of grassland in New Zealand, Huang et al. (2011) found a 33 % increase in the light fraction of SOC in broadleaf trees (Eucalyptus nitens), while no changes or a 33 % decrease was found in conifer species (P. radiata and Cupressus macrocarpa). In this study, we confirmed that SOC was 1.26-fold higher and the C/N ratio was 1.08-fold higher in the aspen–birch forest compared to broad-leaved Korean pine forest, on average (Table 1), which supports the conclusion that pine forests could sequestrate lower C in mineral soils compared with broadleaf forests (Guo and Gifford 2002).
Different soil fractions had different stabilities and turnover times (Huang et al. 2011; Six et al. 2000a). The fractions in this paper can be grouped into slow fractions (SA and EO), sensitive fractions (PTO and SBO), and passive fraction (AI; Six et al. 2000b; Zimmermann et al. 2007). The turnover of the sensitive fractions is usually 10–50 years, while the non-sensitive fractions have a nominal radiocarbon age of 50,000 years (Six et al. 2000b; Zimmermann et al. 2007). Our results indicated that SOC in the aspen–birch forest was more stable (1.5-fold higher in the slow fractions of SA and EO with 1.3-fold higher C/N) and more N was stored in sensitive fractions (2.1-fold higher PTO and SBO) compared to those in broad-leaved Korean pine forest (Table 1). This kind of fraction-related alteration of SOC and N dynamics was also reported in previous studies. For example, SOC sequestration was positively affected by microaggregate protection in afforested soil (Cong et al. 2015) and by the association between organic material and mineral composition in degraded farmland, and also SOC was found decreased in the heavy fraction, but not in the light fraction, in urban environments (Chen et al. 2013). Comparing afforestation to grassland established on croplands, Poeplau and Don (2013) found similar SOC gains, while afforestation tended to sequestrate more SOC in the light fraction. Our data showed that the higher amount of SOC in the stable fractions (SA, EO, and AI) and of N in the sensitive fractions (PTO and SBO) favored SOC sequestration and nutrient supply in the aspen–birch forest compared to broad-leaved Korean pine forest (Table 1).
The underlying reason for this type of differentiation of SOC and N dynamics between the two forests should be related to litter quality, litter quantity, and soil microbial activity. The net primary productivity of these two forests was quite similar (<3 % differences), while annual litterfall from the aspen–birch forests was 1.6-fold higher than that from broad-leaved Korean pine forests (Li 1984; Table 2), with a 1.4-fold decomposition rate in aspen–birch litters (Guo et al. 2006; Table 2). On average, aspen–birch litter had 1.12-fold litter C and 66 % litter N, which resulted in a 1.7-fold higher litter C/N compared with Korean pine litters (Table 2). Moreover, the much abundant plant species in the aspen–birch forest compared with broad-leaved Korean pine forest (Xia et al. 2012) may induce composition-richer litter returning to the soil and fertilize it in the long run (Wang et al. 2014d; Table 2). In the case of microbial activity, the aspen–birch forest soil had higher microbial C, N, P, and microbial quotients and various enzymatic activities (polyphenol oxidase, peroxidase, cellulose, acid phosphatase, and cellobiase) for C, N, and P metabolism (Table 2), probably indicating the faster turnover of SOM in aspen–birch forests compared with old-growth broad-leaved Korean pine forests (Hu et al. 2013).
4.2 Functional groups and soil mineral composition possibly contribute to inter-forest variations in C and N dynamics: more complex than expected
At the moment, limited studies have used FTIR, XRD, and XPS techniques in soil fractionation and the identification of forest-dependent differences in C and N dynamics. This study showed the complex relationships among soil fractions, tree species, and soil C and N dynamics (Figs. 4, 5, 6, and 9).
Firstly, increases in SOC in the aspen–birch forest usually accompany higher functional groups I, II, III, and IV (8.8–40.5 %), but lower quartz grain size and diffraction peaks (8.6–32.5 %) in two main soil fractions (AI and SA, 88 % of total soil mass; Figs. 3, 4, and 9). Owing to the close correlations between SOC (N) and functional groups (II and III), quartz grain size, or mineral diffraction peaks (Figs. 6 and 7), thus, functional groups and soil mineral composition possibly contributed to the forest-dependent variations in soil C and N dynamics. Previous studies revealed that the functional groups can predict the variation in soil fertility (Vohland et al. 2011) and any changes in aliphatic C–H and hydrophilic components due to irrigation (Bernier et al. 2013), while soil mineral composition contributes to soil C stabilization (Cong et al. 2015). The interaction of glomalin-related organic materials and fungi–soil colloids affects SOC sequestration through the alteration of soil mineral crystallinity and functional groups (Li et al. 2013b; Wang et al. 2014b). Our results demonstrated that SOC differences between the aspen–birch forest and broad-leaved Korean pine forest were related to the soil mineral composition and functional group variation in different soil fractions, particularly in AI and SA (over 85 % of total soil mass).
Secondly, elemental composition at the soil particle surface showed much marginal contribution to the SOC and N differences among the two forests (Figs. 5, 7, and 9). XPS data showed that the elemental ratios on the surface of soil colloids were close to loam during soil fungi-aided improvement (Li et al. 2013b), and the dependence of organic–mineral interaction on the density fractions was related to their elemental composition (Arnarson and Keil 2001). Similar to previous reports (Arnarson and Keil 2001; Li et al. 2013b), we confirmed that about 80 % of the elements on the surface of soil particles were C and O, followed by Si (about 10 %) and Al (about 6 %). Inter-forest differences in SOC are hardly explained by the elemental differences in the different soil fractions (Figs. 5 and 7). Since XPS measurements were performed at the top 0–10 nm of soil particles (Yuan et al. 1998; Arnarson and Keil 2001), our data indicated that elemental composition on the surface of soil particles did not explain any differences in SOC sequestration between the aspen–birch forest and broad-leaved Korean pine forest.
Thirdly, soil C/N ratio may be related to Si–O–Si and C–O stretching and –O–H bending of COOH (Fig. 8). There was a 1.3- to 1.5-fold higher C/N ratio in the SA, EO, and PTO fractions (Table 1). Functional group V (Si–O–Si and C-O stretching and –O–H bending of COOH) in the AI of the aspen–birch forest was over 8 % higher than that in broad-leaved Korean pine forest (Fig. 3), indicating that this functional group possibly contributes to the increase of higher C/N in the aspen–birch forest.
4.3 Importance of aspen–birch forest for SOC sequestration: historical and future perspectives
Both historical data and future climate modeling indicate that broadleaf forests, including aspen–birch forest, will become predominant in the regions of broad-leaved Korean pine forest. From 1949 to 1997, broad-leaved Korean pine forest was reduced by 55 %, while aspen–birch forest increased by 45 % (Fig. 10), along with the sharp decline in timber quantity and quality as well as ecological services (Yao and Man 2004). In northeastern China, both global warming and altered precipitation patterns have sharply decreased coniferous forests by 20–30 %, but sharply increased the percentage of broadleaf forests by 70–80 % (Cheng and Yan 2008; Fig. 10). As shown in this paper, this trend greatly affected soil processes (Table 1), i.e., compared to broad-leaved Korean pine forest, there is higher SOC sequestration in the slow fractions (SA and EO) of the aspen–birch forest, while N supply was 2.1-fold higher in the sensitive fractions (SBO and PTO). Biomass C sequestration in the aspen–birch forest and broad-leaved Korean pine forest of northeastern China has been studied previously. In northeastern China, biomass C sequestration in broad-leaved Korean pine forest is 55–102 ton ha−1, with an annual accumulation rate of 2.7–3.61 ton ha−1 year−1 (Yan and Feng 2006; Tang et al. 2009), much lower than that of aspen–birch forest (10.049 ton ha−1 year−1; Li 1984). Our finding in this paper indicates that the aspen–birch forest is also more robust in SOC sequestration and more active in N supply for biomass growth compared to broad-leaved Korean pine forest (Table 1 and Fig. 9). Future studies need to pay more attention to the changes in SOC allocation in different soil fractions, particularly using the RothC model (Zimmermann et al. 2007) for scaling up from site measurement to the regional level. Few studies on global and local carbon modeling have taken SOC sequestration changes into account (Piao et al. 2009).
Historical (left) and future (right) changes in the aspen–birch forest (ABF) and broad-leaved Korean pine forest (KF) of northeastern China. Left, Historical changes of broad-leaved Korean pine and aspen–birch forests from 1949 to 1997 (Yao and Man 2004). Right, Future changes in broadleaf forests, including aspen–birch forest, and coniferous forests, including broad-leaved Korean pine forest, in the next 100 years due to global warming and alteration in precipitation patterns (Cheng and Yan 2008). Broadleaf forests include Populus sp., Betula platyphylla, Quercus mongolica, Tilia, Fraxinus mandschurica, and Ulmus japonica, while coniferous forests include Abies, Picea, Pinus sp., and Larix gmelinii
5 Conclusions
Aspen–birch forests tend to sequestrate more SOC with slight changes in total N concentration compared to broad-leaved Korean pine forests. Moreover, aspen–birch forests have significantly higher SOC in the slow fractions (SA and EO) and higher N in the sensitive fractions (PTO and SBO) compared to broad-leaved Korean pine forests. The underlying reason should be related to the forest-specific differences in returning litter quantity, litter quality, and microbial activity. Functional group traits and soil mineral features, rather than elemental position on the surface of soil particles, possibly contribute to forest-dependent variations in soil C and N dynamics. Both historical data and future climate modeling indicate that broadleaf forests, including aspen–birch forests, will become predominant in northeastern China. Similar to the much higher biomass C sequestration in aspen–birch forests, our findings indicate that future studies need to pay more attention to SOC sequestration.
References
Arnarson TS, Keil RG (2001) Organic–mineral interactions in marine sediments studied using density fractionation and X-ray photoelectron spectroscopy. Org Geochem 32:1401–1415
Baah-Acheamfour M, Carlyle CN, Bork EW, Chang SX (2014) Trees increase soil carbon and its stability in three agroforestry systems in Central Alberta, Canada. Forest Ecol Manage 328:131–139
Bao S (2000) Soil agricultural chemistry analysis. Chinese Agriculture Press, Beijing
Bernier M-H, Levy GJ, Fine P, Borisover M (2013) Organic matter composition in soils irrigated with treated wastewater: FT-IR spectroscopic analysis of bulk soil samples. Geoderma 209–210:233–240
Chen H, Zhang W, Gilliam F, Liu L, Huang J, Zhang T, Wang W, Mo J (2013) Changes in soil carbon sequestration in Pinus massoniana forests along an urban-to-rural gradient of southern China. Biogeosciences 10:6609–6616
Cheng X, Yan X (2008) Effects of climate change on typical forest in the northeast of China. Acta Ecol Sin 28:534–543
Cong W, Ren T, Li B (2015) Assessing the impact of afforestation on soil organic C sequestration by means of sequential density fractionation. PLoS One 10:e0117897
Dai L, Jia J, Yu D, Lewis BJ, Zhou L, Zhou W, Zhao W, Jiang L (2013) Effects of climate change on biomass carbon sequestration in old-growth forest ecosystems on Changbai Mountain in northeast China. Forest Ecol Manage 300:106–116
Denef K, Zotarelli L, Boddey RM, Six J (2007) Microaggregate-associated carbon as a diagnostic fraction for management-induced changes in soil organic carbon in two Oxisols. Soil Biol Biochem 39:1165–1172
Doetterl S, Six J, Van Wesemael B, Van Oost K (2012) Carbon cycling in eroding landscapes: geomorphic controls on soil organic C pool composition and C stabilization. Glob Chang Biol 18:2218–2232
Feng J, L-x Z, Yang Z-c, L-p Z (2006) Analysis on compositions of clay minerals in black soil of Central Jilin, China. Global Geol 25:380–384
Feng W, Plante AF, Six J (2013) Improving estimates of maximal organic carbon stabilization by fine soil particles. Biogeochemistry 112:81–93
Feng W, Boily JF, Erhagen B, Nilsson MB, Klaminder J (2015) Millennia-old organic carbon in a boreal paleosol: chemical properties and their link to mineralizable carbon fraction. J Soils Sediments 16:1–10
Giddens KM, Parfitt RL, Percival HJ (1997) Comparison of some soil properties under Pinus radiata and improved pasture. New Zeal J Agric Res 40:409–416
Gong Z, Kang X, Gu L, Yang H (2009) Growth process and succession trend of poplar–birch secondary forests in Changbai Mts, Northeast China. Forest Res 22:379–384
Guo L, Wang M, Gifford R (2007) The change of soil carbon stocks and fine root dynamics after land use change from a native pasture to a pine plantation. Plant Soil 299:251–262
Guo LB, Gifford RM (2002) Soil carbon stocks and land use change: a meta analysis. Glob Chang Biol 8:345–360
Guo Z, Zheng J, Ma Y, Li Q, Yu G, Han S, Fan C, Liu W (2006) Researches on litterfall decomposition rates and model simulating of main species in various forest vegetations of Changbai Mountains, China. Acta Ecol Sin 26:1037–1046
Hu S, Zhang Y, Shi R, Han S, Li H, Xu H (2013) Temporal variations of soil microbial biomass and enzyme activities during the secondary succession of primary broadleaved Pinus koraiensis forests in Changbai Mountains of northeast China. Chinese J Appl Ecol 24:366–372
Huang Z, Davis MR, Condron LM, Clinton PW (2011) Soil carbon pools, plant biomarkers and mean carbon residence time after afforestation of grassland with three tree species. Soil Biol Biochem 43:1341–1349
Ji C, Wang H, Wang W, Han S (2014) Organic C and N in various surface mineral soil fractions in the broadleaved Korean pine mixed forests in Changbai Mt. Bull Bot Res 34:372–379
Ji C, Wang W, Han S, Zu Y (2015) Features of soil C and N dynamics in a typical secondary poplar–birch forest in northeast China. Acta Ecol Sin 35:5675–5685
Jiang Y, Yin X, Wang F (2013) The influence of litter mixing on decomposition and soil fauna assemblages in a Pinus koraiensis mixed broad-leaved forest of the Changbai Mountains, China. Eur J Soil Biol 55:28–39
Johnson CT, Aochi YO (1996) Fourier transform infrared and Raman spectroscopy. In: Sparks DL et al. (eds) Methods of soil analysis part 3:269–321. Soil Science Society of America, Inc., American Society of Agronomy, Inc., Madison, Wisconsin
Karhu K, Fritze H, Hämäläinen K, Vanhala P, Jungner H, Oinonen M, Sonninen E, Tuomi M, Spetz P, Kitunen V, Liski J (2010) Temperature sensitivity of soil carbon fractions in boreal forest soil. Ecology 91:370–376
Laganiere J, Angers DA, Pare D (2010) Carbon accumulation in agricultural soils after afforestation: a meta-analysis. Glob Chang Biol 16:439–453
Li F (1984) A research of the bioproductivity of Korean pine broadleaf forest and its secondary forest of poplar–birch. Chinese J Ecol 2:8–12
Li H, Li Z, Guo S, Li J (2013a) Effect of different nitrogen and phosphorus supply levels on C, N accumulations and partitioning in Pinus koraiensis seedlings. J Northwest For Univ 28:24–29
Li K (1983) Studies on the process of growth of Korean pine (Pinus koraiensis). Scientia Silvae Sinicae 19:126–136
Li X (2003) Ecosystem carbon processes of forest in Changbaishan Mts. Doctoral thesis, Northeast Forestry University, Harbin, 65 pp
Li X, Zhang Y, Niu L, Han S (2007) Litter decomposition processes in the pure birch (Betula platyphlla) and birch–poplar mixed forest. Acta Ecol Sin 27:1782–1790
Li Y, Wang H, Wang W, Yang L, Zu Y (2013b) Ectomycorrhizal influence on particle size, surface structure, mineral crystallinity, functional groups, and elemental composition of soil colloids from different soil origins. Sci World J 2013:698752
Luan K, Fan X, Wang W (2012) Difference in leaf-fall phenology between Betula platyphylla and Populus davidiana and its ecological significance. J Northeast For Univ 40:20–22
Makohonienko M, Kitagawa H, Fujiki T, Liu X, Yasuda Y, Yin H (2008) Late Holocene vegetation changes and human impact in the Changbai Mountains area, northeast China. Quat Int 184:94–108
Markewitz D, Sartori F, Craft C (2002) Soil change and carbon storage in longleaf pine stands planted on marginal agricultural lands. Ecol Appl 12:1276–1285
Piao S, Fang J, Ciais P, Peylin P, Huang Y, Sitch S, Wang T (2009) The carbon balance of terrestrial ecosystems in China. Nature 458:1009–1013
Plante AF, Conant RT, Carlson J, Greenwood R, Shulman JM, Haddix ML, Paul EA (2010) Decomposition temperature sensitivity of isolated soil organic matter fractions. Soil Biol Biochem 42:1991–1996
Poeplau C, Don A (2013) Sensitivity of soil organic carbon stocks and fractions to different land-use changes across Europe. Geoderma 192:189–201
Rumpel C, Eusterhues K, Kögel-Knabner I (2004) Location and chemical composition of stabilized organic carbon in topsoil and subsoil horizons of two acid forest soils. Soil Biol Biochem 36:177–190
Six J, Elliott E, Paustian K (2000a) Soil macroaggregate turnover and microaggregate formation: a mechanism for C sequestration under no-tillage agriculture. Soil Biol Biochem 32:2099–2103
Six J, Merckx R, Kimpe K, Paustian K, Elliott ET (2000b) A re-evaluation of the enriched labile soil organic matter fraction. Eur J Soil Sci 51:283–293
Stockmann U et al (2013) The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agr Ecosyst Environ 164:80–99
Tang F, Han S, Zhang J (2009) Carbon dynamics of broad-leaved Korean pine forest ecosystem in Changbai Mountains and its responses to climate change. Chin J Appl Ecol 20:1285–1292
Turner J, Lambert M (2000) Change in organic carbon in forest plantation soils in eastern Australia. For Ecol Manag 133:231–247
Vohland M, Besold J, Hill J, Fründ H-C (2011) Comparing different multivariate calibration methods for the determination of soil organic carbon pools with visible to near infrared spectroscopy. Geoderma 166:198–205
Wang Q, Wu Y, Wang W, Zhong Z, Pei Z, Ren J, Wang H, Zu Y (2014a) Spatial variations in concentration, compositions of glomalin related soil protein in poplar plantations in northeastern China, and possible relations with soil physicochemical properties. Sci World J 2014(1):160403. doi:10.1155/2014/160403
Wang W, Li Y, Wang H, Zu Y (2014b) Differences in the activities of eight enzymes from ten soil fungi and their possible influences on the surface structure, functional groups, and element composition of soil colloids. PLoS One 9:e111740
Wang W, Wang H, Zu Y (2014c) Temporal changes in SOM, N, P, K, and their stoichiometric ratios during reforestation in China and interactions with soil depths: importance of deep-layer soil and management implications. For Ecol Manag 325:8–17
Wang Y, Chang SX, Fang S, Tian Y (2014d) Contrasting decomposition rates and nutrient release patterns in mixed vs singular species litter in agroforestry systems. J Soils Sediments 14:1071–1081
Wang Z (1957) Advice for the harvesting methods of Korean pine forest in Xiaoxinganling Mts region. Scientia Silvae Sinicae 3:281–288
Wong VNL, McNaughton C, Pearson A (2016) Changes in soil organic carbon fractions after remediation of a coastal floodplain soil. J Environ Manag 168:280–287
Xia F, Zaho X, Pan C, Fan J, Wang J, Ni R, He H, Chen B, Ding S, Jiang J (2012) Seasonal variation of understory herbs in primary broad-leaved Korean pine forest and secondary aspen-birch forest in Changbai Mountains and its environmental interpretation. J Northeast For Univ 40:83–89
Yan P, Feng X (2006) Spatial distribution and carbon storage in primitive boradleaved Korean pine forest. J Northeast For Univ 34:23–25
Yao C, Man X (2004) Historical evolvement and dynamic swing of forest resources in Heilongjiang. J Northeast For Univ 32:8–11
Yu D, Zhou L, Zhou W, Ding H, Wang Q, Wang Y, Wu X, Dai L (2011) Forest management in northeast China: history, problems, and challenges. Environ Manag 48:1122–1135
Yu D, Zhou W, Bao Y, Qi L, Zhou L, Dai L (2015) Forest management of Korean pine and broadleaf mixed forest in northeastern China since the implementation of Natural Forest Protection Project. Acta Ecol Sin 35:10–17
Yuan G, Soma M, Seyama H, Theng BKG, Lavkulich LM, Takamatsu T (1998) Assessing the surface composition of soil particles from some Podzolic soils by X-ray photoelectron spectroscopy. Geoderma 86:169–181
Zhang Y, Cui X (2016) Effects of higher CO2 concentration on carbon and nitrogen characteristics of Pinus koraiensis seedling and its soil in an experimental environment. J Najing For Univ (natural science version), pp 27–32
Zhou Y (1997) Vegetation geography of northeastern China. Science Press, Beijing
Zhu B, Wang X, Fang J, Piao S, Shen H, Zhao S, Peng C (2010) Altitudinal changes in carbon storage of temperate forests on Mt Changbai, northeast China. J Plant Res 123:439–452
Zimmermann M, Leifeld J, Schmidt MWI, Smith P, Fuhrer J (2007) Measured soil organic matter fractions can be related to pools in the RothC model. Eur J Soil Sci 58:658–667
Zu Y-G, Wang W-J, Wang H-M, Liu W, Cui S, Koike T (2009) Soil CO2 efflux, carbon dynamics, and change in thermal conditions from contrasting clear-cut sites during natural restoration and uncut larch forests in northeastern China. Clim Chang 96:137–159
Acknowledgments
This study was supported financially by China’s National Foundation of Natural Sciences (31170575 and 41373075), Outstanding Youth Fund from Heilongjiang Province (JC201401), and basic research fund for national universities from the Ministry of Education of China (2572014EA01). We would like to thank Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhiqun Huang
Rights and permissions
About this article
Cite this article
Wang, W., Zhou, W., Wang, H. et al. Organic carbon and nitrogen dynamics in different soil fractions between broad-leaved Korean pine forests and aspen–birch forests in northeastern China. J Soils Sediments 17, 2257–2273 (2017). https://doi.org/10.1007/s11368-016-1438-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-016-1438-x