Abstract
Purpose
The objectives of this study were to investigate the abundance and composition of the superficial biofilm on the bed sediments of the Anllóns River (NW Spain), to evaluate the relationships between biochemical parameters and biological methods based on identification and counting, and to explore the relationships between biofilm growth and the properties of the sedimentary habitat, mainly the trophic state.
Materials and methods
Bed sediment samples (0–5 cm) were collected in two different seasons (winter and summer) at four sampling sites along the river course. Physicochemical properties of pore waters and sediments were determined. Biological properties included the determination of dehydrogenase activity (DHA) and phytopigment (Chl a Chl b and total carotenoids) concentrations, as well as taxonomic identification. For taxonomic identification, two sampling methods were compared: the Pasteur pipette method and a mini-corer method. Total and relative algal abundances (TA and RA, respectively) and genus richness were calculated. The relationships between the different variables were examined using Pearson correlations and principal component analysis.
Results and discussion
The main taxa belonged to Chlorophyta, Cyanophyta, Euglenophyta, and Heterokontophyta. The most abundant class was Bacillariophyceae, which represents >86 % of the total abundances in the superficial sediments. The highest total algal abundance and genus richness were observed in summer at the river mouth, where DHA and phytopigment concentrations were also the highest. The statistical analysis revealed positive correlations between TA and the biochemical parameters (DHA and phytopigments) as well as positive relationships of these three parameters with the physicochemical properties of the sediments, such as electrical conductivity, and the concentrations of fine particles, C, N, S, and total P.
Conclusions
The results of this study reveal the positive relationships between the biochemical properties (phytopigments and respiratory activity) and total algal abundances determined by taxonomic identification and counting. All of these properties presented evidence of a clear influence of the nutrients and organic matter contents of the sediments, pointing to the importance of the site conditions, particularly the trophic state, in the development of benthic microflora.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biofilms covering the surfaces of rocks, mineral grains, and plant debris are common in aquatic environments. Biofilms are composed of heterotrophic and autotrophic microorganisms, immersed in a complex matrix of extracellular polymeric substances (EPS). The primary role of biofilms is the protection of microbial communities in conditions of environmental stress (Decho 2000; Flemming and Wingender 2001).
Epipsammic biofilms represent the interface between water and granular sediments, affecting the exchange of solutes among these environmental compartments and the physical stability of the sediment. It has been demonstrated that biofilms affect the role of sediments as potential sources or sinks for contaminants (Devesa-Rey et al. 2009; Gerbersdorf et al. 2011) and that the presence of biofilms alters the adsorption and release of pollutants by bed sediments (Leadbeater and Callow 1992; Gainswin et al. 2006; Prieto et al. 2013). EPS form adhesive coatings on sediment particles, increasing their erosion threshold, and consequently increasing sediment stability and limiting their resuspension (Sutherland et al. 1998; Stal 2003; De Brouwer et al. 2005; Flemming 2006; Tolhurst et al. 2006; Ziervogel and Forster 2006; Gerbersdorf and Wieprecht 2015). It has been observed that the stabilization of the sediment matrix by biofilms reduces the potential release of pollutants due to resuspension (León-Morales et al. 2006; Gerbersdorf et al. 2007; Lubarsky et al. 2010). Despite this evidence regarding the influence of biofilms on the physical and chemical properties of the granular bed sediments, their role is often ignored when the geochemical processes (i.e., retention, mobility, bioavailability, and speciation of pollutants) occurring at the sediment–water interfaces are studied.
Previous studies of diatom benthic populations indicated that the Anllóns River (NW Spain) was moderately to heavily contaminated (Ector 1992). Research into the chemical composition of the bed sediments of the Anllóns River confirmed that the contributing basin is affected by diffuse pollution due to agricultural activities and urban and industrial discharges into the water course (Devesa-Rey et al. 2008a; 2012). Ecotoxicity was observed at some points along the river course, which in several cases was attributed to high arsenic (As) concentrations (Devesa-Rey et al. 2008a), related to past mining activities in gold-rich areas containing arsenopyrites (Devesa-Rey et al. 2008a, 2010). In fact, no evidence for other potentially toxic elements could be found in the Anllóns sediments (Devesa-Rey et al. 2011). Arsenic is of particular concern in this basin; for example, Rubinos et al. (2010, 2011) have shown that As can be mobilized in the Anllóns riverbed sediments under conditions of high salinity, alkaline pH, or high phosphorus (P) concentrations, as well as during high-flow resuspension events. Once mobilized, As may interact with the biofilm. Arsenate—the most common form of As in natural waters—could affect periphyton communities, inhibiting algal growth and photosynthetic capacity, changing community composition and diatom sizes, and reducing the ability of the community to retain P (Blanck and Wangberg 1988, 1991; Wangberg et al. 1991; Rodríguez Castro et al. 2015). Furthermore, biofilms may affect the fate of As in the sediments, as demonstrated by Prieto et al. (2013), who observed that epipsammic biofilms increased AsV sorption by the Anllóns River sediments, particularly in the presence of phosphate. Moreover, the presence of biofilms strongly affected the speciation of As in the water column by decreasing the occurrence of aqueous available AsIII (Prieto et al. 2014).
So far, there have been no studies into the abundance and composition of epipsammic biofilms in river sediments of northwestern Spain, and the limited available data on algae in the region’s freshwaters refer to epilithic or epiphytic phytobenthos (Margalef 1955, 1956; Ector 1992; Noguerol Seoane 1993; López Rodríguez and Penalta Rodríguez 2004). Nevertheless, the study of biofilms deserves special attention because some species of benthic microalgae are indicative of contamination. In particular, benthic diatoms have characteristics that make them well suited for the bio-indication of the quality of inland waters, namely, (i) their abundance and great taxonomic diversity, (ii) their ability to colonize different environments, and (iii) the preservation of frustules in sediments (López Rodríguez 2005).
In this work, we aim to assess the relationships between biological methods for the evaluation of epipsammic biofilm growth, based on taxonomic identification and counting, and biochemical methods. Respiration, as an index of the overall biological activity in the sediment, and phytopigments, as a measure of the abundance of photosynthetic organisms in biofilms, were selected as the biochemical properties to be tested.
The activity of the enzyme dehydrogenase (DHA) can be used to determine respiratory activity, as it provides a measure of the activity of oxidation–reduction enzymes responsible for dissociating H+ from H2O, thus diverting 2e− into the electron transport process. Dehydrogenase activity is present in all active microorganisms and can be used in both aerobic and anaerobic environments. The DHA has been used to measure the activity of stream and river microbial communities and their responses to disturbances (Trevors et al. 1982; Blenkinsopp and Lock 1990; Ponsati et al. 2015).
The determination of phytopigment concentrations by spectrophotometry is commonly used to estimate biofilm growth over various surfaces (Ortega-Calvo et al. 1995; Tomaselli et al. 2002; Guasch et al. 2004; Serra et al. 2009; Vázquez-Nion et al. 2013). It has been applied extensively to the measurement of microphytobenthos in sediments. Phytopigments determined by this method were highly correlated with instrumental color measurements performed with the Anllóns sediments (Sanmartín et al. 2011).
The present study attempts to assess the overall biological activity in the surface sediment by means of the determination of the DHA activity and that of the autotrophic biomass by means of phytopigment analysis. The statistical relationships between these biochemical measurements and the parameters derived from the taxonomic identification, such as algal abundance and genus richness, are tested, and the effect of sediment properties (particle size, nutrients, and the presence of toxicants) on biofilm growth is also examined. This information will be useful for the evaluation of biofilm abundance and composition on riverbed sediments and for the assessment of its effects on the biogeochemical cycles of the elements, particularly those which, like As, have significant effects on the environment and public health.
2 Materials and methods
2.1 Study area
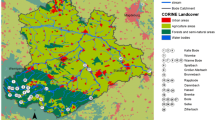
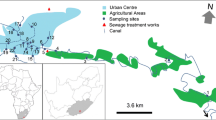
The Anllóns River is located in the NW of Spain (Fig. 1 ). The basin drains a rural catchment of 516 km2 dedicated to agricultural, forestry, and cattle raising activities. The two main human settlements located in the basin are Carballo, with a population of >31,000, and Ponteceso, with a population of ∼7000. The river runs over schists in the upper area, basic rocks (gabbros and amphibolites) in the middle area, granite of two micas in the lower stretch of the river, and biotitic gneiss at the mouth. Gold mining activities were carried out in the area since the Roman Empire and were intermittently in operation until the nineteenth century. In the mineralized areas, Au is associated with As sulfides, which are probably the origin of the high As concentrations detected in the surface sediments downstream from the mineralized area (Rubinos et al. 2003; Devesa-Rey et al. 2008b).
Surficial riverbed sediment samples were taken at four sites, distributed along the watercourse between the locality of A Ponte and the river mouth at Ponteceso (Fig. 1 ). The sites were identified with a number and two letters. The number indicates the position from the locality of A Ponte along the watercourse and the letters are abbreviations of the location. The sites were selected taking into account the contamination detected in previous studies (Table 1). Thus, site 1AP is influenced by diffuse pollution whereas sites 2CA and 4PO are located downstream from the biggest towns in the area (Carballo and Ponteceso) and are influenced by wastewater treatment plants. Site 3XA is located in the area of Au–As mineralization. Sampling sites 1AP, 2CA, and 3XA were mostly shaded by riparian vegetation with Alnus glutinosa and Fraxinus excelsior, whereas site 4PO was situated in the estuary, without riparian vegetation, and received more sunlight.
Sampling was carried out in February and July 2012 between 10 a.m. and 4 p.m. Sediments were collected with a small plastic shovel from the top 4 cm at various points of the same site, mixed, and homogenized to form a composite sample. Sediment samples were stored in thermally insulated airtight plastic boxes and transported to the laboratory, where pore water was removed by centrifugation at 2000 rpm. Subsequently, a portion of the solid samples was stored at 4 °C until biochemical analyses, and the remainder was immediately freeze-dried and later sieved by 2 mm for the determination of the general properties of the sediment.
2.2 Analyses
The Eh and pH in the surface of the sediment were measured in situ with a portable device (Hanna HI 9025 microcomputer, Padova, Italy) at the same time as sampling. Eh values obtained with the Pt-Ag/AgCl electrodes were corrected to refer them to the H2 by adding 245 mV.
Analyses of the pore water included total P (PT-PW), which was determined after acid digestion (APHA 2005) with 1 mL H2SO4 31 % and 0.1 g (NH4)2S2O8 at 120 °C for 30 min by colorimetric determination using the phosphomolybdenum blue method (Murphy and Riley 1962). Soluble P (PSOL-PW) was measured by colorimetric determination in pore water samples filtered at 0.45 μm. pH and electrical conductivity (EC) were measured with a Hanna HI 9025 microcomputer and HI 9033 device (Padova, Italy), respectively.
With regard to sediment analyses, total P (PT) was determined by means of an acid digestion (HF, H2SO4, HCl, 10:1:10) followed by colorimetric determination, as mentioned above. Bioavailable P (PBIO) was estimated by extracting the sediment with NaOH 0.1 M using a 1:100 sediment/solution ratio (Wolf et al. 1985). Total carbon (C), nitrogen (N), and sulfur (S) were determined by elemental analysis using a macrosample Leco TruSpec CHNS instrument (St. Joseph, Michigan, USA) based on the total combustion of the sample and subsequent determination of the combustion gases using a thermal conductivity detector. Grain size distribution was determined by wet sieving and the pipette method as per Guitián and Carballas (1976). Total As content of the sediment samples was determined by microwave-assisted acid digestion (150 °C), employing Teflon™ microwave digestion vessels containing 0.2 g sediment and 9 mL HNO3(conc) + 1 mL HF(conc), followed by As analysis using hydride generation atomic absorption spectrometry (HGAAS, Perkin-Elmer M2100 coupled with an MHS-10 hydride generation unit, Waltham, MA, USA), by reaction of acidified samples with 3 % NaBH4/1 % NaOH as reductant to generate arsine gas (AsH3).
Measurement of respiratory activity by means of DHA is based on intercepting e− flow through mitochondrial and microsomal electron transport systems using a surrogate electron acceptor, 2,3,5-triphenyltetrazolium chloride (TTC), which has a slightly higher redox potential than that of the coenzyme UQ –cytochrome b complex, one of several cytochrome compounds used by eukaryotic organisms as terminal electron acceptors, and similar cytochrome electron acceptors are used by prokaryotic organisms (Packard 1971; Broberg 1985). The DHA was measured in the fresh samples by the reduction of TTC to triphenylformazan (TPF), following the method described by Tabatabai (1982). The DHA was measured at 485 and 520 mm to avoid possible interferences with photosynthetic pigments (Patil et al. 2000).
Phytopigments (chlorophyll-a, chlorophyll-b, and carotenoids) were solubilized by extraction with dimethyl sulfoxide for 46 min, using an extractant/sediment ratio of 3.6 ml g−1 at 57 °C, following the method optimized by Devesa et al. (2007) for sediment analysis. In the extracts, chlorophyll-a, chlorophyll-b, and total carotenoids were determined spectrophotometrically (Cary 100 Conc., Varian, Santa Clara, CA, USA). Equations (1) to (3) were employed to determine the concentrations of chlorophyll-a (Chl a) and b (Chl b), as well as total carotenoids (Cx+c), in micrograms per milliliter, following Wellburn (1994):
where Chl a and Chl b represent the concentration of chlorophyll-a and chlorophyll-b, respectively, and Cx+c represents the concentration of total carotenoids, comprising the oxidized forms (xantophylls) and the reduced forms (carotenes). A665.1, A649.1, and A480 represent the absorbance of the extracts at 665.1, 649.1, and 480 nm, respectively. To estimate the phaeopigments, 0.5 % of 1 M HCl was added to the extracts and, after 10 min, the absorbance at 665.1 and 649 nm was measured again. The difference of absorbance between the nonacidified and acidified samples corresponds to the absorbance of the chlorophyll. Each sample was submitted to several extractions (between two and three) until no phytopigments were quantified in the extracts, and the concentrations obtained were added to obtain the total concentration.
The taxonomic identification required specific sampling, which were carried out simultaneously to the sampling of the sediments subjected to characterization. There is no standard method for biofilm sampling in unconsolidated granular materials such as riverbed sediments. Therefore, in this study, two sampling techniques on sediment surfaces were tested and compared: (1) the Pasteur pipette method, whereby samples were collected from a known area of 1 cm2 at five points in the area, transferred to glass tubes, and made up to a known volume, and (2) a novel corer method, in which sampling was done by introducing in the sediment surface a plastic corer of 2.4 cm diameter × 3.0 cm height, sealed at the top with Prolene® film to preserve biofilm integrity (Penalta-Rodríguez et al. 2008). After collection, the samples were cooled and transported to the laboratory where they were preserved with a solution of 4 % formaldehyde. Algae were identified at the lowest taxonomic level (individuals/cm2) under an optical microscope (Olympus BX61 with a Nomarski interference contrast, Tokyo, Japan). Algal abundance was calculated, including five replicates of each sample. For the pipette method, an aliquot of 0.1 mL was taken from the glass tubes. For the corer method, 2-mm-diameter areas were sampled from each core. In both cases, samples were placed on slides and observed at ×20 magnification. Additionally, selected unaltered sediment samples taken with the corer were observed by scanning electron microscopy (SEM) (Zeiss EVO LS 15, Jena, Germany) to study the association of microalgae populations with mineral phases.
2.3 Statistical analysis
Student’s t test was carried out to analyze the differences between sampling methods for taxonomic identification. As a first step, data were checked for normal distribution. Single Pearson correlations and principal component analysis (PCA) were calculated with the statistic software package SPSS v20.0 to analyze the possible relationships between the biological and chemical parameters.
3 Results and discussion
3.1 Physicochemical properties of the bed sediments
In Table 2, it can be seen that the sediments had neutral to slightly acidic pH (6.1–7.0). The oxidation/reduction potential, measured as Eh, ranged between −21.5 and 59.0 mV and was indicative of a moderately anoxic state of the surface layer of the sediment. The pore waters extracted from the sediments showed pH values slightly higher than those measured in situ. EC values fell within the range 0.17–0.47 mS cm−1 from samples 3XA to 2CA, whereas sample 4PO showed a higher value (9.46 and 14.25 mS cm−1 in winter and summer, respectively) that evidenced the marine influence in the estuary. Total phosphorus (PT-PW) in the pore water varied from 0.21 to 0.48 mg L−1 and exceeded 0.1 mg L−1, which is the maximum acceptable concentration to avoid accelerated eutrophication or to promote algal blooms (USEPA 1986). Soluble phosphorus (PSOL-PW) fell within the range 0.003–0.13 mg L−1. These values would classify the pore water quality as Good or High ecological status according to Recommendations on Phosphorus Standards for Rivers derived from the Water Framework Directive 2000/60/EC (WFD UK 2008). No significant differences in P concentrations were observed among sites or sampling season. Although P concentrations could exhibit seasonal variations due to (i) remineralization of organic matter in the water column and sediment, (ii) uptake for phytoplankton growth, and (iii) excretion by zooplankton (Laurent et al. 2012), in the Anllóns catchment these effects can be counteracted by P inputs from wastewaters, which are more pronounced in low flow periods, and from agricultural activities which are more intense in spring and summer in this area.
The sediments showed a predominance of the sandy fraction, which always exceed 60 %. Site 4PO exhibited the finest texture in both samplings. This fact may be attributed to the decrease in the river slope, and the mixing of freshwaters and saline waters, favoring the deposition of fine materials in the proximity of the estuary.
Carbon (C) and nitrogen (N) also showed the highest values at site 4PO (∼6 and 0.5 %, respectively) and the lowest at site 3XA (∼0.6 and 0.04 %, respectively) at both sampling times. This may be related to more favorable deposition conditions, and to the highest clay and silt contents—and consequently to the highest surface area—at site 4PO, favoring organic matter (OM) and N retention. This behavior has been previously described by Bergamaschi et al. (1997), who reported that C and N were strongly related to sediment surface area, and by Xiaoxia et al. (2005), who reported that total N presented positive correlations with the fine particle content in the surface sediments of the southern Yellow Sea, China. As C/N ratios can be used as an indication of OM origin in riverbed sediments; C/N ratios <12 are typical for OM associated to algal biomass and therefore from autochthonous origin (Müller 1977), whereas C/N ratios >12 are indicative of OM rich in lignin and cellulose as well as poor in N and are attributable to terrestrial origin (Lamb et al. 2006). Sediment samples analyzed in this study presented C/N ratios from 12 to 16, indicative of the predominance of OM from terrestrial origin. These values are in the range of those reported by Devesa-Rey et al. (2009) for riverbed sediments from 14 sampling sites in the Anllóns River, with values varying from 5 to 36 (mean of 13.4), as well as of those reported by Barral et al. (2012) who found C/N values from 13 to 35 (mean of 18.1), for 10 sediments from the same river. Moreover, C/N ratios for soils and suspended sediments in the Anllóns River catchment, analyzed by Iglesias et al. (2011), were also in the same range, with mean values of 14.6 and 10.7, respectively.
Sulfur (S) in the sediments may be present both in inorganic and organic form. In the samples analyzed, S contents ranged between 0.02 and 0.78 %, with the highest value at site 4PO, where the estuary conditions favor S reduction and precipitation. Sulfur contents were lower in summer, pointing to a biological oxidation of the organic S and its transformation into soluble sulfates.
In the sediments, PT varied from 205 mg kg−1 (1AP) to 1130 mg kg−1 (4PO). On average, 71 % of the P (51–90 %) in the sediments is in bioavailable form, with the highest PBIO corresponding to 4PO. This high bioavailability may have a direct effect on the primary productivity and, thus, on biofilm formation (Sterling et al. 2000). Phosphorus concentrations are slightly lower than those determined by Devesa-Rey et al. (2008a) but similar to those previously obtained by Barral et al. (2012) for the sediments of the Anllóns River. At two sites, total P concentrations exceed the lowest effect level (LEL) established by the Ontario Sediment Quality Guidelines (Persaud et al. 1993), which is set at 600 mg kg−1, although they did not exceed the severe effect level (SEL), set at 2000 mg kg−1. Below SEL, the sediment is considered clean to marginally polluted, and it is expected that this level of contamination will have no effect on the majority of sediment-dwelling organisms.
Arsenic in the sediments ranged between 7.3 and 43.3 mg kg−1 and was higher in samples 3XA and 4PO, downstream from the Au–As mineralization area. These values were lower than the maximum values detected in the sampling campaign performed by Devesa-Rey et al. (2008b), which reached 264 mg kg−1. The As concentrations found in the bed sediments were also lower than reference As levels for soils in Galicia (50 or 140 mg kg−1 in soils over slates with arsenopyrite; Macías Vázquez and Calvo de Anta R 2009) and were lower or close to the thresholds of the European WFD for suspended matter and sediment (40 mg kg−1; EU-WFD 2000). The values were also lower than the effects range median (ERM; the level at which half of the studies reported harmful effects) set at 70 mg kg−1 by Long et al. (1995). Nevertheless, with the exception of site 1AP, these values exceeded in all cases the effects range low (ERL; the lowest concentration of a metal that produced adverse effects in 10 % of the data reviewed) set at 8.2 mg kg−1 by the same authors. Arsenic mobility is a key factor in the toxicity of this element. In the sediments of the Anllóns River, As exhibited low mobility because it was found to be mainly associated to the least mobile fractions—bound to Fe–Al oxides and in the residual phase (Devesa-Rey et al. 2008b; Rubinos et al. 2011)—although it can increase in conditions of increased pH, higher salinity, higher P concentrations, or higher liquid/solid ratios (Rubinos et al. 2010, 2011). Devesa-Rey et al. (2008b), applying the toxicity characteristic leaching procedure (TCLP) to sediments of the Anllóns River, determined As concentrations in extracts lower than Criteria Continuous Concentration (CCC) for As set at 150 μg L−1 by USEPA (1996).
3.2 Biological characterization of the bed sediments
Dehydrogenase activity showed the maximum value at site 4PO in both seasons (1457 and 3075 mg kg−1 day−1 in winter and summer, respectively), followed by site 2CA (Table 3). Both sites are characterized by their nutrient richness, which could justify their greater biological activity. Dehydrogenase activity values were in the range of activity values reported by Filimon et al. (2013) for sediments in Serbian water streams with varying levels of metal pollution and were slightly higher than those reported for a wide range of soils in the world (Dick et al. 1996). Filimon et al. (2013) identified the difficulty in classifying polluted sites from the assessment of enzymatic activities, which depend on season and location, as well as the difficulty to extrapolate to field surveys the linear relationship found in lab experiments between microbial enzymatic activities and levels of trace elements in soils and sediments. Thus, although it is possible to make comparisons between sites with similar characteristics over time or after the impact of a potential contaminant, a particular DHA value does not classify a site as contaminated.
All the evaluated phytopigments (Chl a, Chl b, and total carotenoids) presented a similar behavior (Table 3). Phytopigment concentrations were similar to those previously found by Devesa-Rey et al. (2009) and Sanmartín et al. (2011) for the same river, which ranged between 3.4 and 83.8 and 2.2 and 60.1 mg kg−1, respectively. The concentrations were slightly lower than those obtained by Gerbersdorf et al. (2007) in the sediments of the Neckar River (Germany), where Chl a varied from 35 to 197 mg kg−1.
The highest phytopigment values corresponded to site 1AP in winter and particularly to 4PO in summer, when the phytopigment concentrations at this site reached values up to five times higher than at the other sites. Sanmartín et al. (2011) also reported the highest phytopigment contents at 4PO. It seems that the brackish water in the estuary favors the development of the autotrophic population at this site. In fact, most estuaries worldwide are turbid and highly productive due to constant allochthonous nutrient inputs (Pinckney et al. 2001). The abundance of nutrients at 1AP and 4PO and the greater light availability due to the absence of riparian vegetation at 4PO may explain the higher growth of autotrophic populations at these sites. Positive relationships between N and P and algal biomass have already been demonstrated at different studies (Biggs and Close 1989; Dodds et al. 1997, 2002; Chételat et al. 1999, 2006; Biggs 2000), and positive correlations between nutrients and phytopigments were previously reported for the Anllóns riverbed sediments (Devesa-Rey et al. 2009, 2010). Phytopigments were also positively correlated with oxalate-extractable Cu and Zn, which are essential elements for the growth and metabolism of the benthic microflora (Devesa-Rey et al. 2009). Nevertheless, at high concentrations, these trace metals could also become toxic (Soldo and Behra 2000) and could induce a shift in the community composition with the dominance of green algae in Cu- or Zn-exposed phototrophic biofilms (Genter et al. 1987; Serra et al. 2009; Tlili et al. 2010, 2011).
The taxonomic identification of algae found in the surface sediments employing the Pasteur pipette and the corer method is summarized in Table 4. The main taxa identified belonged to the divisions Cyanophyta, Heterokontophyta (Bacillariophyceae), Euglenophyta, and Clorophyta. Total algal abundances (TA) and genus richness (GR) were calculated for both sampling methods (Fig. 2 ). The TA and GR could only be determined for two sites in winter (1AP and 4PO) due to the low amount of microalgae found in 2CA and 3XA, whereas in summer these parameters could be determined for the four sites. The TA was similar using both sampling methods, being only higher (p < 0.05) with the Pasteur pipette method for 1AP in winter and summer and for 3XA in summer, when algal abundances were lower. Genus richness was similar using both sampling techniques except for site 1AP in winter, which showed higher GR (p < 0.05) with the pipette method. In comparison, the corer method generally exhibited higher precision for TA (lower values of relative standard deviation) and enabled the direct observation of unaltered sediment surfaces by SEM, for the study of the distribution of microalgae populations and their close association with mineral phases (Fig. 3). Site 4PO showed the highest TA and GR values in both seasons, although algal growth was remarkably higher in summer, which is in accordance with the highest phytopigment and nutrient concentrations and light availability at this site. These results were also in accordance with the data reported by Aguilera et al. (2007) for the benthic eukaryotic community of the Río Tinto (Huelva), who stated that total cell abundances were generally highest in September, decreasing dramatically in January, whereas diversity remained fairly constant during the year at most sampling stations.
The relative algal abundances (RA) were determined for all the sites in summer but only for 1AP and 4PO in winter, due to the low amount of microalgae found in 2CA and 3XA in this season (Fig. 4 ). The RA varied with the sampling site and time, as well as with the sampling technique. The most abundant division was Heterokontophyta, and, specifically, the most abundant class was Bacillariophyceae, which in general showed RA > 98 %. Exceptions were site 1AP in winter (employing the pipette method) and site 2CA in summer, where between 8 and 14 % belonged to other divisions (Cyanophyta, Chlorophyta, and Euglenophyta). As revealed in this study, benthic microalgae are often dominated by diatoms on sandy sediments (Hickman and Round 1970; Colijn and De Jonge 1984; Aberle and Wiltshire 2006) whereas green algae and cyanobacteria occur rarely or only at some seasonal stages (Hillebrand and Kahlert 2001; Aberle and Wiltshire 2006).
Among Bacillariophyceae, Navicula was usually the predominant genus using the corer method. The only exception was at site 1AP in the winter sampling, for which benthopelagic Melosira was the most abundant genus. More variable results were found using the pipette method. In this case, Navicula only predominated in 3XA and 4PO (Fig. 4 ), whereas Melosira, Achnantes, and Ulnaria (U. ulna) prevailed in the other cases. The predominance of Navicula in our study, particularly at site 4PO, where it showed the highest RA (66–97 %), can be explained by the tolerance of the Navicula species to organic pollution and to eutrophic conditions in water (Lange-Bertalot 2001; Segura-García et al. 2010), as well as to brackish and electrolyte-rich waters (Ehrlich 1995; Cox 1996; Lange-Bertalot 2001). Studying diatom populations, Ector (1992) indicated that the Anllóns River is moderately contaminated in spring, to heavily contaminated in summer, due to urban and industrial effluents. Furthermore, De la Peña (2003) observed contamination downstream from the town of Carballo and attributed the predominance of tolerant species such as Navicula minima and Gomphonema parvulum to the influence of eutrophication and organic pollution.
The influence of seasonality could be observed in the general increase of Navicula in the summer sampling, as well as of Achnantes and Cocconeis at 1AP. The increase of Navicula could be explained by its tolerance to contamination, which increases in summer in the Anllóns River (Ector 1992). The dominance of species susceptible to contamination, such as Achnantes minutissima, was reported by De la Peña (2003) for unpolluted locations in the Anllóns River, such as 1AP. Typical brackish species were observed at site 4PO (at the river mouth), such as Achnanthes brevipes, Entomoneis sp., Diploneis sp, Melosira nummuloides, Pleurosigma sp, Surirella striatula, and Tryblionella sp., which suggests the adaptation to osmotic stress and the predominance of the saline-resistant species.
Among the freshwater microalgae groups, Chl a is present in all photosynthetic algae whereas Chl b is present in the Chlorophyta (green algae) and Euglenophyta divisions (Leavitt and Hodgson 2001). Bacillariophyceae (diatoms), the predominant class in this study, presents, as major photosynthetic pigments, chlorophyll a and c, along with fucoxanthin (carotenoid) as an accessory pigment (Gómez et al. 2009). Despite the predominance of diatoms in the biofilms of the Anllóns River, significant concentrations of Chl b were found in this study, which cannot be attributed to these microalgae class. Moreover, the ratios Chl b/a ranged between 0.22 and 1.71, which are similar to those published by Devesa-Rey et al. (2009) and Sanmartín et al. (2011) (0–1.79 and 0.62–1.23, respectively) for bed sediments from the Anllóns River. Nevertheless, these ratios were much higher than those reported by Schlüter et al. (2006), who quantified phytoplankton groups in lakes, analyzing 20 different algal cultures, obtaining Chl b/a ratios of 0.23–0.41 and 0.20–0.23 for Chlorophytes and Euglenophytes, respectively. Hence, the higher ratios found in this study could suggest the contribution of aquatic and terrestrial plants to Chl b concentrations. This is in agreement with the C/N ratios obtained in this study, which were indicative of the allochthonous origin of the OM.
3.3 Relationships between variables
Pearson correlations were calculated for the data obtained for the summer sampling, which was the most complete (Table 5). Total and soluble P, pH of the pore water, and total As in the sediments are not shown in the table as they did not present significant correlations (p < 0.05) with the other parameters. There was a negative correlation between Eh and pH. Electrical conductivity showed positive correlations with the content of fine particles, and with nutrient content (C, N, P, and S) which was also positively correlated with the content of fine particles, which may promote OM and nutrient deposition. Among the biological parameters, the concentrations of phytopigments and DHA and TA values showed positive correlations between them as well as with EC, fine particle content, and the nutrient content.
PCA was initially carried out for all the variables analyzed in the summer sampling. Two principal components (PC) with an eigenvalue >1 were extracted (Fig. 5), which explained 95.6 % of the total variance. PCA corroborated the importance of site conditions on the benthic microflora, given that DHA, phytopigments, TA, and GR were placed in the same quadrant as nutrients, OM, and fine particle classes of the sediments. This association can be explained because site conditions favorable for fine particle deposition are also prone to the accumulation of OM and nutrients, due to low energy and sorption phenomena, and these conditions seem to be favorable for the development of biofilms. Sand content and Eh are in the opposite quadrant (Fig. 5), indicating that those sites with a coarser texture—which also have less favorable OM accumulation and, therefore, lower O2 consumption due to OM decomposition, and higher Eh—are less suitable for biofilm growth.
In conclusion, the results corroborate the control of biological activity by site conditions, mainly by the trophic state (N, P, and OM), and suggest that a comprehensive study of nutrients, phytopigments, and benthic microflora could contribute to a better understanding of the ecological status in riverbed sediments.
4 Conclusions
The results of this study reveal the positive relationships between the biological properties (phytopigments and respiration) and total algal abundances determined by taxonomic identification. Similar results were obtained when sampling with the Pasteur pipette method and the corer method, but the latter enables the observation of microalgae distribution over mineral surfaces by SEM. Bacillariophiceae, namely, the genus Navicula, are the most abundant algae in the analyzed sediments. A clear influence of the nutrient (N, P) and OM contents of the sediments is observed in the development of benthic microflora, pointing to the importance of the site conditions, particularly of the trophic state.
References
Aberle N, Wiltshire KH (2006) Seasonality and diversity patterns of microphytobenthos in a mesotrophic lake. Arch Hydrobiol 167:447–465
Aguilera A, Zettler E, Gómez F, Amaral-Zettler L, Rodríguez N, Amils R (2007) Distribution and seasonal variability in the benthic eukaryotic community of Río Tinto (SW, Spain), an acidic, high metal extreme environment. Syst Appl Microbiol 30:531–546
American Public Health Association (APHA) (2005) Standard methods for the examination of water and wastewater. 21st ed, American Water Works Association; Water Pollution Control Federation. Washington, USA
Barral MT, Devesa-Rey R, Ruiz B, Díaz-Fierros F (2012) Evaluation of phosphorous species in the bed sediments of an Atlantic Basin: bioavailability and relation with surface active components of the sediment. Soil Sed Contam 21:1–18
Bergamaschi BA, Tsamakis E, CEIL RG, Eglinton TI, Montlucon DB, Hedges JI (1997) The effect of grain size and surface area on organic matter, lignin and carbohydrate concentration, and molecular compositions in Peru Margin sediments. Geochim Cosmochim Acta 61:1247–1260
Biggs BJF (2000) Eutrophication of streams and rivers: dissolved nutrient–chlorophyll relationships for benthic algae. J N Am Benthol Soc 19:17–31
Biggs B, Close M (1989) Periphyton biomass dynamics in gravel bed rivers: the relative effects of flows and nutrients. Freshwater Biol 22:209–231
Blanck H, Wangberg S-A (1988) Validity of an ecotoxicological test system: short-term and long-term effects of arsenate on marine periphyton communities in laboratory systems. Can J Fish Aquat Sci 45:1807–1815
Blanck H, Wangberg S-A (1991) Pattern of cotolerance in marine periphyton communities established under arsenate stress. Aquat Toxicol 218:1–14
Blenkinsopp SA, Lock MA (1990) The measurement of electron transport system activity in river biofilms. Water Res 24:441–445
Broberg A (1985) A modified method for studies of electron transport system activity in freshwater sediments. Hydrobiologia 120:181–187
Chételat J, Pick FR, Morin A (1999) Periphyton biomass and community composition in rivers of different nutrient status. Can J Fish Aquat Sci 56:560–569
Chételat J, Pick FR, Hamilton PB (2006) Potamoplankton size structure and taxonomic composition: influence of river size and nutrient concentrations. Limnol Oceanogr 51:681–689
Colijn F, De Jonge VN (1984) Primary production of microphytobenthos in the Ems-Dollard Estuary. Mar Ecol Prog Ser 14:185–196
Cox EJ (1996) Identifications of freshwaters diatoms from live material. Chapman & Hall, Oxford, 158 pp
De Brouwer JFC, Wolfstein K, Ruddy GK, Jones TER, Stal LJ (2005) Biogenic stabilization of intertidal sediments: the importance of extracellular polymeric substances produced by benthic diatoms. Microb Ecol 49:501–512
De la Peña S (2003) Biomonitorización de la calidad del agua de ríos de la provincia de A Coruña usando diatomeas. PhD Thesis, University of A Coruña, Spain
Decho AW (2000) Microbial biofilms in intertidal systems: an overview. Cont Shelf Res 20:1257–1273
Devesa R, Moldes AB, Díaz-Fierros F, Barral MT (2007) Extraction study of algal pigments in river bed sediments by applying factorial designs. Talanta 72:1546–1551
Devesa-Rey R, Barral MT (2012) Allochthonous versus autochthonous naturally occurring organic matter in the Anllóns river bed sediments (Spain). Environ Earth Sci 66:773–782
Devesa-Rey R, Moldes AB, Díaz-Fierros F, Barral MT (2008a) Toxicity of Anllóns River sediment extracts using microtox and the zucconi phytotoxicity test. B Environ Contam Tox 80:225–230
Devesa-Rey R, Paradelo R, Díaz-Fierros F, Barral MT (2008b) Fractionation and bioavailability of arsenic in the bed sediments of the Anllóns River (NW Spain). Water Air Soil Pollut 195:189–199
Devesa-Rey R, Moldes AB, Díaz-Fierros F, Barral MT (2009) Study of phytopigments in river bed sediments: effects of the organic matter, nutrients and metal composition. Environ Monit Assess 153:147–159
Devesa-Rey R, Moldes AB, Sanmartín P, Prieto-Fernández A, Barral MT (2010) Application of an incomplete factorial design for the formation of an autotrophic biofilm on river bed sediments at a microcosms scale. J Soils Sediments 10:1623–1632
Devesa-Rey R, Díaz-Fierros F, Barral MT (2011) Assessment of enrichment factors and grain size influence on the metal distribution in riverbed sediments (Anllóns River, NW Spain). Environ Monit Assess 179:371–388
Dick RP, Breakwell DP, Turco RF (1996) Soil enzyme activities and biodiversity measurements as integrative microbiological indicators. chapter 15. In: Doran JW, Jones AJ (eds) Methods for assessing soil quality. SSSA special publication number 49. Soil Science Society of America Inc, Madison
Dodds WK, Smith VH, Zander B (1997) Developing nutrient targets to control benthic chlorophyll levels in streams: a case study of the Clark Fork River. Water Res 31:1738–1750
Dodds WK, Smith VH, Lohman K (2002) Nitrogen and phosphorus relationships to benthic algal biomass in temperate streams. Can J Fish Aquat Sci 59:865–874
Ector L (1992) Control de la calidad biológica de las aguas superficiales en la red de aforos de Galicia-costa mediante diatomeas bénticas. In: Antelo-Cortizas JM (ed) Calidad del agua en las estaciones de aforo de los ríos de Galicia. Años hidrológicos 1989–90, 1990–91. Fundación Empresa Universidad Gallega (FEUGA). Consellería de Ordenación do Territorio e Obras Públicas, Xunta de Galicia, Spain
Ehrlich A (1995) Atlas of the inland-water diatom flora of Israel. Flora Palestina. 166 pp. the Geological-Survey of Israel. The Israel Academy of Sciences and Humanities, Israel
EU-WFD (2000) Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for community action in the field of water policy. Off J Europ Comm L 327/1
Filimon MN, Nica DV, Ostafe V, Bordean DM, Borozan AB, Vlad DC, Popescu R (2013) Use of enzymatic tools for biomonitoring inorganic pollution in aquatic sediments: a case study (Bor, Serbia). Chem Cent J 7:59
Flemming HC (2006) Biological and chemical processes: their role in sediment dynamics and pollutant mobility. Sedymo 2006 Symposium, Book of Abstracts. Hamburg University of Technology, Germany, p 69
Flemming HC, Wingender J (2001) Relevance of microbial extracellular polymeric substances (EPs)—part I: structural and ecological aspects. Water Sci Technol 43:1–8
Gainswin BE, House WA, Leadbeater BSC, Armitage PD, Patten J (2006) The effects of sediment size fraction and associated algal biofilms on the kinetics of phosphorus release. Sci Total Environ 360:142–157
Genter RB, Cherry DS, Smith EP, Cairns JJ (1987) Algal-periphyton population and community changes from zinc stress in stream mesocosms. Hydrobiologia 153:87–92
Gerbersdorf SU, Wieprecht S (2015) Biostabilization of cohesive sediments: revisiting the role of abiotic conditions, physiology and diversity of microbes, polymeric secretion, and biofilm architecture. Geobiology 13:68–97
Gerbersdorf SU, Jancke T, Westrich B (2007) Sediment properties for assessing the erosion risk of contaminated riverine sites. a comprehensive approach to evaluate sediment properties and their covariance patterns over depth in relation to erosion resistance—first investigations in natural sediments at three contaminated reservoirs. J Soils Sediments 7:25–35
Gerbersdorf SU, Hollert H, Brinkmann M, Wieprecht S, Schüttrumpf H, Manz W (2011) Anthropogenic pollutants affect ecosystem services of freshwater sediments: the need for a “triad plus x” approach. J Soils Sediments 11:1099–1114
Gómez N, Donato JC, Giorgi A, Guasch H, Mateo P, Sabater S (2009) La biota de los ríos: los microorganismos autótrofos. Chap. 12. In: Elosegui A, Sabater S (eds) Conceptos y técnicas en ecología fluvial. Fundación BBVA, Bilbao
Guasch H, Navarro E, Serra A, Sabater S (2004) Phosphate limitation influences the sensitivity to copper in periphytic algae. Freshwater Biol 49:463–473
Guitián F, Carballas T (eds) (1976) Técnicas de Análisis de Suelos. Ed. Pico Sacro, Santiago de Compostela, 288 pp
Hickman M, Round FE (1970) Primary production and standing crops of epipsammic and epipelic algae. Br Phycol J 5:247–255
Hillebrand H, Kahlert M (2001) Effect of grazing and nutrient supply on periphyton biomass and nutrient stoichiometry in habitats of different productivity. Limnol Oceanogr 46:1881–1898
Iglesias ML, Devesa-Rey R, Pérez-Moreira R, Díaz-Fierros F, Barral MT (2011) Phosphorus transfer across boundaries: from basin soils to river bed sediments. J Soils Sediments 11:1125–1134
Lamb AL, Wilson GP, Leng MJ (2006) A review of coastal palaeoclimate and relative sea-level reconstructions using δ13C and C/N ratios in organic material. Earth-Sci Rev 75:29–57
Lange-Bertalot H (2001) Navicula sensu stricto. 10 genera separated from Navicula sensu lato. Frustulia. In: Lange-bertalot H (ed) Diatoms of Europe 2. Diatoms of the European inland waters and comparable habitats. ARG Gantner Verlag KG, Vaduz, pp 1–526
Laurent A, Fennel K, Hu J, Hetland R (2012) Simulating the effects of phosphorus limitation in the Mississippi and Atchafalaya River plumes. Biogeosciences 9:4707–4723
Leadbeater BSC, Callow ME (1992) Formation, composition and physiology of algal biofilm. In: Mello LF, Bott TR, Fletcher M, Caldeville B (eds) Biofilms—science and technology. Kluwer Academic Publishing, Dordrecht, pp 113–124
Leavitt PR, Hodgson DA (2001) Sedimentary pigments. In: Smol JP, Birks HJB, Last WM (eds) Tracking environmental change using lake sediments, vol 3, Terrestrial, algal and siliceous indicators. Kluwer Academic Publishers, Dordrecht, pp 295–325
León-Morales CF, Strathman M, Flemming HC (2006) Role of biofilms on the mobility of pollutants in rivers. Sedymo 2006 Symposium, Book of Abstracts. Hamburg University of Technology, Marzo, p 48
Long E, MacDonald D, Smith S, Calder F (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19:81–97
López Rodríguez MC (2005) Algas doceacuícolas nos ríos de galiza. Adega (Ed) caderno n° 15. Os ríos galegos (II): Calidade e biodiversidade 7–16. ISSN 1137–0262
López Rodríguez MC, Penalta Rodríguez M (2004) Aportación al conocimiento de la flora ficológica del Macizo Central Gallego (N.O. España). Anales de Biología de Murcia 46:79–91
Lubarsky HV, Hubas C, Chocholek M, Larson F, Manz W, Paterson DM, Gerbersdorf SU (2010) The stabilisation potential of individual and mixed assemblages of natural bacteria and microalgae. PLoS ONE 5(11):e13794
Macías Vázquez F, Calvo de Anta R (2009) Niveles genéricos de referencia de metales pesados y otros elementos traza en suelos de Galicia. Consellería de Medio Ambiente e Desenvolvemento Sostible (Xunta de Galicia). Galicia, Spain
Margalef R (1955) Comunidades bióticas de las aguas dulces del noroeste de España. Publicaciones del Instituto de Biología Aplicada 21:5–85
Margalef R (1956) Algas de agua dulce del norte de España. Publicaciones del Instituto de Biología Aplicada 22:5–47
Müller PJ (1977) C/N ratios in Pacific deep sea sediment: effect of inorganic ammonium and organic nitrogen compound sorbed by clays. Geochim Cosmochim Acta 41:765–776
Murphy J, Riley J (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Noguerol Seoane A (1993) Algas dulceacuícolas de la Sierra de Invernadeiro (Orense, N.O.España). Nova Acta Científica Compostelana (Bioloxía) 4:5–13
Ortega-Calvo JJ, Arino X, Hernandez-Marine M, Saiz-Jimenez C (1995) Factors affecting the weathering and colonization of monuments by phototrophic microorganisms. Sci Total Environ 167:329–341
Packard TT (1971) The measurement of respiratory electron transport activity in marine phytoplankton. J Mar Res 29:235–244
Patil RD, Dalev PG, Mark JE, Vassileva E, Fakirov S (2000) Biodegradation of chemically modified gelatin films in lake and river waters. J Appl Polym Sci 76:29–37
Penalta-Rodríguez M, López-Rodríguez MC, Devesa-Rey R, Iglesias ML, Paradelo R, Diaz-Fierros F, Barral MT (2008) Composición algal del biofilm en sedimentos de fondo del Río Anllóns. XIV Congreso de la Asociación Ibérica de Limnología. Asociación Ibérica de Limnología, Spain
Persaud D, Jaagumagui R, Hayton A (1993) Guidelines for the protection and management on aquatic sediment quality in Ontario. Ontario Ministry of the Environment and Energy. Ontario, Canada
Pinckney JL, Paerl HW, Tester P, Richardson TL (2001) The role of nutrient loading and eutrophication in Estuarine ecology a definition of eutrophication. Environ Health Perspect 109(5):699–706
Ponsati L, Acuña V, Aristi I, Arroita M, García-Berthou E, von Schiller D, Elosegi A, Sabater (2015) Biofilm responses to flow regulation by dams in Mediterranean Rivers. River Res Applic 31(8):1003–1016
Prieto DM, Devesa-Rey R, Rubinos DA et al. (2013) Arsenate retention by epipsammic biofilms developed on streambed sediments. Influence of phosphate. BioMed Res Inter Volume 2013, Article ID 591634
Prieto DM, Rubinos DA, Devesa-Rey R et al. (2014) Influence of epipsammic biofilms on the retention and speciation of arsenic in freshwater environments. Science across bridges, borders and boundaries. Abstract Book of SETAC Europe 24th Annual Meeting Basel, Switzerland, 11–15 May 2014
Rodríguez Castro MC, Urrea G, Guasch H (2015) Influence of the interaction between phosphate and arsenate on periphyton’s growth and its nutrient uptake capacity. Sci Total Environ 503–504:122–132
Rubinos D, Barral MT, Ruíz B, Ruíz M, Rial ME, Alvarez, Díaz-Fierros F (2003) Phosphate and arsenate retention in sediments of the Anllóns river (northwest Spain). Water Sci Technol 48:159–166
Rubinos D, Iglesias L, Devesa-Rey R, Díaz-Fierros F, Barral MT (2010) Arsenic release from river sediments in a gold-mining area (Anllóns River basin, Spain): effect of time, pH and phosphorous concentration. Eur J Mineral 22:665–678
Rubinos D, Iglesias L, Díaz-Fierros F, Barral MT (2011) Interacting effect of pH, phosphate and time on the release of arsenic from polluted river sediments (Anllóns River, Spain). Aquat Geochem 17:281–306
Sanmartín P, Devesa-Rey R, Prieto B, Barral MT (2011) Nondestructive assessment of phytopigments in riverbed sediments by the use of instrumental color measurements. J Soils Sediments 11:841–851
Schlüter L, Lauridsen TL, Krogh G, Jorgensen T (2006) Identification and quantification of phytoplankton groups in lakes using new pigment ratios – a comparison between pigment analysis by HPLC and microscopy. Freshwater Biol 51:1474–1485
Segura-García V, Israde-Alcántara I, Maidana NI (2010) The genus Navicula sensu stricto in the upper Lerma Basin, Mexico. I. Diatom Res 25:367–383
Serra A, Corcoll N, Guasch H (2009) Cu accumulation and toxicity in fluvial periphyton: the influence of exposure history. Chemosphere 74:633–641
Soldo D, Behra R (2000) Long-term effects of Cu on the structure of freshwater periphyton communities and their tolerance to Cu, Zn, nickel and silver. Aquat Toxicol 47:181–189
Stal LJ (2003) Microphytobenthos, their extracellular polymeric substances, and the morphogenesis of intertidal sediments. Geomicrobiol J 20:463–478
Sterling MS, Ashley KJ, Bautista AB (2000) Slow release fertilizer for rehabilitating oligotrophic streams: a physical characterization. Water Qual Res J Canada 35:73–94
Sutherland TF, Amos CL, Grant J (1998) The erosion threshold of biotic sediments: a comparison of methods. Geol Soc Special Publ 139:295–307
Tabatabai MA (1982) Soil enzymes. In: Page AL (ed) Methods of soil analysis. part 2. chemical and microbiological properties. American Society of Agronomy, Madison, pp 903–948
Tlili A, Bérard A, Roulier JL, Volat B, Montuelle B (2010) PO4 3− dependence of the tolerance of autotrophic and heterotrophic biofilm communities to copper and diuron. Aquat Toxicol 98:165–177
Tlili A, Maréchal M, Bérard A, Volat B, Montuelle B (2011) Enhanced co-tolerance and co-sensitivity from long-term metal exposures of heterotrophic and autotrophic components of fluvial biofilms. Sci Total Enviorn 409:4335–4343
Tolhurst TJ, Defew EC, De Brouwer JFC, Wolfstein K, Stal LJ, Paterson DM (2006) Small-scale temporal and spatial variability in the erosion threshold and properties of cohesive intertidal sediments. Cont Shelf Res 26:351–362
Tomaselli L, Lamenti G, Tiano P (2002) Chlorophyll fluorescence for evaluating biocide treatments against phototrophic biodeteriogens. Ann Microbiol 52:197–206
Trevors JT, Mayfield CI, Inniss WE (1982) Measurement of electron transport system (ETS) activity in soil. Microb Ecol 8:163–168
USEPA (1986) Quality criteria for water. Technical report 440/5-86-001. Office of Water Regulations and Standards, Washington, DC
USEPA, Office of Water Regulations and Standards (1996) Water quality criteria documents for the protection of aquatic life in ambient water: 1995 updates. United States Environmental Protection Agency, EPA 820-B-96-001, United States Environmental Protection Agency, Washington DC
Vázquez-Nion D, Sanmartín P, Silva B, Prieto B (2013) Reliability of color measurements for monitoring pigment content in a biofilm forming cyanobacterium. Int Biodeterior Biodegrad 84:220–226
Wangberg S-A, Heyman U, Blanck H (1991) Long-term and short-term arsenate toxicity to fresh-water phytoplankton and periphyton in limnocorrals. Can J Fish Aquat Sci 48:173–182
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolutions. J Plant Physiol 144:307–313
WFD UK TAG (2008) UK Environmental Standards and Conditions (Phase 1). UK Technical Advisory Group on the WFD. 73 pp, [http://www.wfduk.org/UK_Environmental_Standards/]
Wolf A, Baker D, Pionke H, Kunishi H (1985) Soil tests for estimating labile, soluble, and algae-available phosphorus in agricultural soils. J Environ Qual 14:341–404
Xiaoxia L, Jinming S, Huamao Y, Xuegang L, Tianrong Z, Ning L, Xuelu G, Xuefa S (2005) Grain-size related nitrogen distribution in southern Yellow Sea surface sediments. Chin J Oceanol Limnol 23:306–316
Ziervogel K, Forster S (2006) Do benthic diatoms influence erosion thresholds of coastal subtidal sediments? J Sea Res 55:43–53
Acknowledgments
The authors wish to thank the Spanish Ministry of Economy and Competitiveness for (MINECO-FEDER) for financial support (project ref. CGL2010-22059 and CGL2013-46003-P). Rosa Devesa-Rey gratefully acknowledges the financial support of the Ángeles Alvariño Programme of the Xunta de Galicia. Diego Martiñá Prieto wishes to acknowledge the financial support of the Spanish Ministry of Economy and Competitiveness for his FPI Fellowship (BES-2011-044514).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Sabine Ulrike Gerbersdorf
Rights and permissions
About this article
Cite this article
Prieto, D.M., Devesa-Rey, R., Paradelo, R. et al. Monitoring benthic microflora in river bed sediments: a case study in the Anllóns River (Spain). J Soils Sediments 16, 1825–1839 (2016). https://doi.org/10.1007/s11368-016-1395-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-016-1395-4