Abstract
Purpose
Sequential nutrient regeneration and organic matter (OM) degradation were studied in surface coastal sediments of the Gulf of Trieste (northern Adriatic Sea).
Materials and methods
In situ benthic chambers were used under normoxic, anoxic and reoxic conditions. Diffusive benthic fluxes were calculated from pore water modelling using a diffusion-advection-reaction model.
Results and discussion
Intensive NH4 + and PO4 3− anoxic regeneration was subsequently slower in prolonged anoxia. NH4 + production was probably also a consequence of dissimilatory nitrate reduction to NH4 +. The presence of laterally pumping of oxygenated water by benthic infauna can explain the presence of NO3 − in anoxia. Anoxic phases were characterized by enhanced dissolution of biogenic Si. Reoxygenation was characterized by enhanced bioturbation. Nitrification caused NH4 + decrease. P precipitated quickly as carbonate fluorapatite and FePO4 and adsorption of P onto Fe-hydroxides could also occur. Diffusive fluxes at the sediment-water interface (SWI) revealed high anoxic NH4 + effluxes, while PO4 3− fluxes were very low. High NH4 +/PO4 3− flux ratios in anoxic and reoxic phases suggested an excess of benthic inorganic N.
Conclusions
Nutrient budgets at the sediment-water interface of this sandy coastal sediment showed intensive anoxic recycling of inorganic N, but low P and Si cycling in all redox phases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The Gulf of Trieste is an example of shallow coastal marine environments that are characterized by relatively low organic matter (OM) contents in sediments (Ogrinc et al. 2005). The largest fraction of sedimentary OM originates from the pelagic production, contributing about 2/3 of the total gross primary production in the gulf. It escapes degradation in the water column and settles on the sediment bed (Kemp et al. 1999). It was estimated that about half of the autochthonous pelagic OM is deposited onto sediments (Faganeli et al. 1995). The remaining OM originates from benthic primary production, which is a significant source of easily degradable OM in this environment due to the euphotic zone reaching the bottom (Kemp et al. 1999; Welker et al. 2002).
Vertical stratification of water masses in the gulf leads to O2 depletion in the bottom water layers causing hypoxia and even anoxia (Kemp et al. 1999). Coastal hypoxia and anoxia are widespread and increasingly common events that can result in seasonal or even persistent “dead zones“(Diaz and Rosenberg 2008) that are also known in the Gulf of Trieste (Faganeli et al. 1985; Stachowitsch 1991). Hypoxia and anoxia can also influence activities of benthic infauna and the biogeochemical processes that occur in sediment by marine microorganisms. Changes in sediment burrowing and reworking by benthic invertebrates influence nutrient release and the redox cycling of elements at the sediment-water interface (SWI) (Hunter et al. 2012). With the occurrence of hypoxia or even anoxia, the activity of sediment infauna decreases, limiting the sediment reworking and the reoxidation of reduced chemical species originating from the deeper sediment layers where anoxic OM mineralization takes place (Hines et al. 1997; Middelburg and Levin 2009). This leads to upward migration of reductive layers and enhances the importance of sulphate reduction in the topmost sediment layers (Metzger et al. 2014). The mortality of benthic macrofauna increases, due to the accumulation of toxic hydrogen sulphide, and the decaying biomass acts as an additional source of “fresh”, easily degradable OM.
The aim of this study was to simulate anoxia and reoxidation for various periods of time in order to investigate the consequences of shifts in benthic nutrient biogeochemical processes in coastal areas. Since the composition of pore water serves as a sensitive indicator of early diagenetic reactions (Berner 1980), it was the primary focus of this research. Because it is still great uncertainty in the magnitude of benthic nutrient fluxes under various oxygen levels, it is important to assess its significance in internal nutrient recycling. These results also enable discussion of the consequences of advanced remineralization in a relatively organic poor sedimentary environment.
2 Materials and methods
2.1 Study area
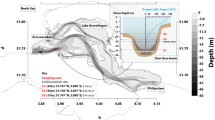
The study site, 21 m deep, was located 2.3 km off Piran (Slovenia) in the southern part of the Gulf of Trieste (northern Adriatic Sea) at the Marine Biological Station hydrographic buoy Vida (45° 32.300′ N, 13° 33.000′ E; Fig. 1). This northernmost part of the Adriatic Sea covers an area of 500 km2, with a maximal depth of 25 m, and is isolated from the rest of northern Adriatic by a shoal. The main circulation is cyclonic, and the outflow of seawater mostly occurs along the shallow northern coast after mixing with river waters. The salinity of the bottom waters ranges from 36.0 to 38.5 and bottom water temperatures range from 8 °C in winter to 20 °C in summer. A density gradient in late summer can result in bottom water layer hypoxia and even anoxia (Faganeli et al. 1985). Sediment in the southern part of the gulf is composed mainly of silty sand that is high in biogenic carbonate, populated mostly by brittle stars, sponges and tunicates (Ogorelec et al. 1991; Stachowitsch et al. 2007). It is actively bioturbated by polychaetes and bivalves down to a depth of 10 cm (Cermelj et al. 1997). The sediment surface is populated by microalgae composed mostly of diatoms (Welker et al. 2002). The sedimentation rate, determined using 210Pb, is approximately 1.2 mm year−1 in the central part of the gulf increasing up to 3 mm year−1 towards the shore (Ogorelec et al. 1991).
2.2 Experimental design and sampling procedure
In situ benthic experiments, conducted in 2010 and 2011, included a simulation of different episodes of anoxia using Plexiglas 50 × 50 × 50 cm benthic chambers. More detailed experimental designed is presented in Metzger et al. (2014). Each simulation experiment consisted of three separate phases. The first phase, called normoxia, represented conditions that governed the environment at the time of sampling. This phase was followed by the anoxic phase, in which the system inside the benthic chamber slowly became anoxic due to benthic respiration and limited contact with the outer environment. Durations of anoxia were simulated throughout the project lasting 9 days, 1 month, 2 months and 10 months. The anoxic phase was followed by a recovery phase, in which the benthic chamber was removed and the system was left to recover for at least 9 days. This experimental set-up, despite certain constrains, mimicked the course of events in the natural ecosystem occurring in this sandy coastal sediment of the Gulf of Trieste.
In an experiment conducted in 2010, with anoxia onset lasting 9 days, a benthic chamber called an Experimental Anoxia Generating Unit (EAGU) was equipped with sensors for dissolved oxygen (O2) and sulphide (H2S) and with cameras (Stachowitsch et al. 2007) used primarily for studying the behaviour and survival/mortality of benthic infauna (Riedel et al. 2014). The other chambers used were simpler without sensors and cameras. They were however designed with special rubber-closed openings used for sample manipulations inside the chamber.
All benthic chamber deployments and sediment core samplings were performed by scuba divers. Firstly, before the deployment of chambers, four cores (normoxia) were sampled using Plexiglas tubes (6 cm i.d., 12-cm length) and sealed with rubber stoppers underwater. The chambers were pushed approximately 2 cm into the sediment and carefully sealed to avoid any leakage of ambient water into or experimental water out of the chambers. In the experiment using EAGU, O2 and H2S sensors were used to monitor the evolution of anoxia inside the chamber. Temporal O2 variations (Fig. 2) revealed the decrease in O2 concentrations from initial values of 190 μmol l−1 to values close to zero after 7 days. At the end of the experiment, core samples were again obtained by scuba divers. After sampling, the benthic chamber was carefully removed to avoid any further disturbance of the sediment. The area previously covered by the benthic chamber and anoxic water column became exposed to ambient seawater, and the recovery period began. The recovery experiment was not performed following the 2-month anoxia experiment. After 9-day and 1-month anoxia experiments, the recovery sediment cores were sampled after 7-day and 1-month periods, respectively, whereas in the 10-month experiment, they were collected after a 3-month period.
After each campaign, all samples were carefully transported to the laboratory, where the cores were extruded and sectioned into 0.5-cm-thick slices for the upper 2 cm and 1-cm slices for the next 5 cm. All core sample manipulations, including pore water, were performed in an N2-filled glove bag or box. Core slices were placed into sterile 50-ml centrifuge tubes (Corning Inc.) to allow pore water extraction. This was performed by centrifugation at 3500 rpm for 20 min at the temperature measured at the sea bottom at the time of sampling. Pore waters were filtered through 0.2-μm Millipore filters in an inert atmosphere and three or four cores combined to obtain enough sample for all required analyses. Sediments devoid of pore waters (solid phase) were kept frozen until analysed.
2.3 Analyses
2.3.1 Solid phase
Sediment samples for Corg and Ntot analyses were freeze-dried, ground in an agate mortar and sieved through a 200-μm mesh to remove coarse shell debris. Corg was determined in sediments acidified with 1 M HCl to remove carbonates quantitatively (Hedges and Stern 1984), whereas Ntot was determined in non-acidified samples using a CHN analyser (Elementar vario MICRO CUBE; Elementar Analysensysteme GmbH, Hanau, Germany). Total P was analysed by extraction with 1 M HCl after ignition, and Porg was calculated from the difference in P contents of 1 M HCl extracts before and after ignition of the samples (Aspila et al. 1976). Biogenic Si (Sibiog) was extracted with Na2CO3 (DeMaster 1981) and the dissolved Si(OH)4 (dSi) determined colorimetrically (Grasshoff et al. 1999). The precision of Corg, Ntot, Ptot and Pinorg and Sibiog analyses was ±3 %. A small sediment core was also sampled to determine the porosity (ϕ) of sediments at the experimental site according to Cermelj et al. (1997).
2.3.2 Pore waters
Concentrations of NH4 +, NO3 −, PO4 3− and dSi in pore waters were determined using standard colorimetric methods (Grasshoff et al. 1999). Detection limits for nutrients were 0.5 μmol l−1 for NO3 −, 0.4 μmol l−1 for NH4 + and 0.01 μmol l−1 for PO4 3− and for dSi. Precision based on replicate analyses was ±3 % for nutrients.
2.4 Pore water and benthic flux modelling
Fluxes of PO4 3−, NH4 + and dSi across the sediment-water interface (SWI) were determined using Fick’s first law of diffusion including diffusion-advection model (Lerman 1979):
where F is the rate of flux [mol m−2 s−1], ϕ is sediment porosity, D s is the effective diffusion coefficient [m−2 year−l], ∂C/∂z is the concentration gradient across the SWI [mol dm−4], ω is the sedimentation rate [cm s-l] and C is the concentration in solution [mol dm−3]. The measured ϕ was 0.55, while values of D s were corrected for temperature and average porosity (Lerman 1979) and are collected in Table 2 for different species. ∂C/∂z is the pore water solute gradient, which is simulated using the diffusion-advection-reaction model for PO4 3−, NH4 + and dSi. The model describes measured nutrient distributions in pore waters.
The diffusion-advection-reaction model included the degradation of OM with the first-order kinetics, equilibrium adsorption from pore water onto sediment particles and dissolution precipitation reactions using the following equation (Lerman 1979):
where k is the reaction rate constant for a first-order removal process, J is the rate of dissolution, R 0 is the production rate at the SWI [mol dm−3 s−1] and β is the depth attenuation constant [cm−1]. The change of PO4 3−, NH4 + and dSi with depth and time in the model was described for every species separately, depending on the processes influencing their distribution (Liu et al. 2003): (1) decomposition of OM to NH4 + and PO4 3−; (2) equilibrium adsorption for NH4 + and PO4 3−; (3) precipitation of PO4 3− to form authigenic minerals and (4) dissolution of biogenic silica, assuming first-order reaction. The change of nutrient concentrations with depth (1-D) can thus be described by steady-state differential equations collected in Table 1. These equations assume a constant ϕ, which is justified based on previous measurements. Uni-dimensionality (Berner 1980), i.e. changes in sediment are predominantly vertical, as well as a series of quasi steady states (Boudreau 1997) was assumed due to continuous forcing (OM mineralization) exhibiting rather long relative time scales (Cermelj et al. 2001). Parameters used in the diffusion-advection-reaction model together with the model output data are collected in Table 2. Based on the diagenetic models, the diffusive fluxes were calculated and the data are presented in Table 3.
3 Results and discussion
3.1 Normoxic conditions
Results of Corg, Ntot, Ptot, Pinorg, Porg and Sibiog (Fig. 3) from the normoxic phase agreed with previously reported values from surficial sediment at this study site (Cermelj et al. 1997; Ogrinc et al. 2005). Little vertical variation of concentration in all measured parameters was observed except in Ptot and Sibiog, which decreased with depth. The Corg/Ntot ratios (molar) ranged from 6 to 12 and Corg/Porg ratios (molar) averaged 155 indicating similar Corg/Ntot ratios and higher Corg/Porg ratios comparing to our previous investigation with an average Corg:Ntot:Porg ratio of 100:18:1 (Ogrinc and Faganeli 2006).
Normoxic pore water profiles were comparable with those reported previously for the same sampling point (Ogrinc and Faganeli 2006; Faganeli and Ogrinc 2009). NH4 + and PO4 3− concentrations in pore water (Figs. 4 and 5) were low between 20 and 115 μmol l−1 for NH4 + and 2 and 40 μmol l−1 for PO4 3−. Concentrations in overlaying water were lower at around 1 μmol l−1 for NH4 + and 0.1 μmol l−1 for PO4 3−. NO3 − concentrations in pore water (Fig. 4) in the normoxic phase ranged from 5 to 35 μmol l−1, whereas the concentration in overlaying water was around 0.1 μmol l−1. The profile of dSi showed lower concentrations at SWI and uniform levels at about 100 μmol l−1 at all depths analysed.
3.2 Anoxic conditions
During 1-month and 2-month anoxia, the surface Corg/Ntot ratios rose to values from 12 to 15 and 11 to 21, respectively, due to the lowering of Ntot levels. The lowest Corg and Ntot levels in anoxia were observed in surface layer, and Corg/Ntot ratios were approximately constant, averaging 10 (molar), except in 2-month anoxia experiment. The results of 10-month anoxia resulted in relatively high values of Ntot, comparable to those in normoxia, with Corg values being higher than those in all short-term experiments. Only changes in the pool of Porg were observed; the degradation of Porg was most evident in the 2-month anoxia experiment, reflected in higher Corg/Porg ratios. Sibiog levels increased in the 9-day anoxia experiment but were somewhat lower under the longer anoxic conditions (Fig. 3).
Pore water concentrations of NH4 + in the 9-day anoxia experiment were much higher than normoxic concentrations (Fig. 4). They ranged from 100 to 200 μmol l−1. During 1 month of anoxia, the concentrations of NH4 + were even higher, reaching maximum values of 700 μmol l−1. There was a noticeable increase in concentrations at depths between 0.5 and 4 cm. In the 2- and 10-month anoxia experiments, the concentrations of NH4 + fell and stabilized at around 300 μmol l−1 and 200 μmol l−1, respectively. Concentrations of NO3 − (Fig. 4) during the 9-day and 1-month anoxia experiments did not change much and were comparable to those under normoxic conditions, ranging from 10 to 40 μmol l−1, with peaks overlapping those of NH4 + profiles. In the 2- and 10-month anoxia experiments, NO3 −concentration increase, reaching values up to 100 μmol l−1. The 9-day anoxia PO4 3− profile was similar to that of NH4 + (Fig. 4). Concentrations increase in only two active layers, reaching values of 20 and 40 μmol l−1. Concentrations in the PO4 3− depth profiles in the 1-, 2- and 10-month anoxia experiments remained nearly constant throughout the sediment cores (Fig. 5). They ranged from 0.5 to 9 μmol l−1 and, therefore, did not exceed the values observed in the normoxia or 9-day anoxia experiments.
It is apparent that the longer the span of anoxia, the less pronounced is the rise of concentrations of NH4 + and PO4 3− in pore waters. The greater part of the regeneration of NH4 + and PO4 3− in anoxic marine environments probably results from hydrolytic and/or fermentative processes that produce low molecular weight molecules from complex sedimentary OM (Burdige 2002). The utilization of these compounds by sulphate-reducing bacteria then results in the mineralization of sedimentary OM (Burdige 2002). Sulphate reduction was demonstrated as a major anaerobic path in OM mineralization in the surface sediment of the Gulf of Trieste (Hines et al. 1997). DET results of SO4 2− concentrations during anoxia experiments (Metzger et al. 2014) clearly showed the migration of redox layers towards the SWI as well as the increasing importance of sulphate reduction over prolonged anoxia spans. Consequently, the sedimentary Corg/Norg and Corg/Porg ratios generally increase with depth in surface anoxic coastal marine and estuarine sediment (Blackburn and Henriksen 1983; Ingall and Jahnke 1997). This is usually considered as an indication of preferential mineralization of organic nitrogen (Macko et al. 1993) and phosphorus (Ingall and Jahnke 1997) as well as a consequence of the limited microbial ability to accumulate phosphorus under anoxic conditions, slow microbial degradation and enhanced preservation of Corg in anoxic sediments (Ingall et al. 1993; Steenbergh et al. 2011). Under anoxic conditions, higher pore water NH4 + and PO4 3− levels can also be related to the higher density of decaying infaunal organisms that provides additional easily degradable “fresh” OM to microbes (Riedel et al. 2014). A build-up of NH4 + in pore waters can also be a consequence of other important processes in the N cycle in anoxic environments. Dissimilatory nitrate reduction to ammonium (DNRA) (Gardner et al. 2006) can supersede respiratory denitrification as a process of NO3 − removal from the system, when labile OM is more available. Redox potential and ambient NO3 − concentrations are low, and H2S production is high (Canfield et al. 2005; Burgin and Hamilton 2007). The NO3 − behaviour in sediments of the Gulf of Trieste was somewhat unusual, since it was expected to decrease in anoxic pore waters due to denitrification that rapidly removes NO3 −. The results of the 2-month anoxia experiment indicate a possible breach in anoxia, since increased concentrations of NO3 − were seen at two separate depths, suggesting that there was active sediment reworking (outside chamber) (Cermelj et al. 1997). Anammox (anaerobic ammonium oxidation) might be running simultaneously (Dalsgaard et al. 2005; Engstrom et al. 2005) producing small amounts of NO3 − under anoxic conditions that is probably formed as a byproduct in CO2 reduction (van de Graaf et al. 1997), especially in the 2- and 10-month anoxia experiments.
A significant amount of P is released from Fe-(oxy)hydroxides upon transition from oxic to anoxic conditions during bottom water hypoxia and anoxia leading to increased regeneration of P from sediments (Ogrinc and Faganeli 2006; Faganeli and Ogrinc 2009) and thus to increase recycling of P in the system (Conley et al. 2009). This is supported by the low NH4 +/PO4 3− flux ratio of 11 in the 9-day anoxia experiment. Conversely, a high NH4 +/PO4 3− flux ratio of 540 was observed after 10 months of anoxia, indicating the possible retention of P under anaerobic conditions.
During all anoxia experiments, concentrations of dSi increased to levels of >200 μmol l−1 (Fig. 5) due to enhanced microbial and chemical dissolution of Sibiog (opal) (Canfield et al. 2005; Bellias et al. 2007).
3.3 Reoxygenation
In the recovery phases, the Corg and Ptot and Porg levels remained approximately unchanged during 9 days of recovery but were higher in a 1-month recovery. Conversely, Ntot values in both phases were much lower than those in normoxia (Fig. 3).
High dSi levels in pore water, around 150 μmol l−1, also persisted during reoxidation phases. The irregular (“disrupted”) depth profiles of NH4 + observed after 1-month recovery and of PO4 3− and dSi observed after 1 year of recovery indicate the importance of enhanced bioturbation. Reoxygenation of previously anoxic sediments promotes OM remineralization (Hulthe et al. 1998; Bastviken et al. 2004). Unfortunately, data on pore water nutrient concentrations in the 7-day recovery period during O2 penetration are lacking. We observed, however, a peak of NO3 − at a depth of 4–5 cm in the 7-day reoxygenation phase, which may be a consequence of the enhanced bioturbation clearly evident in NH4 + and dSi pore water profiles, especially in the 1-year recovery experiment. Nitrification was the probable cause of relative NH4 + depletion. When oxic conditions reappear, P rapidly precipitates, probably as authigenic carbonate fluorapatite, FePO4 or is adsorbed onto Fe-hydroxides (Ogrinc and Faganeli 2006), most probably onto goethite that has a large sorption capacity for phosphate (Arčon et al. 1999).
3.4 Model calculation and fluxes
The depth profiles of model calculated and measured NH4 +, PO4 3− and dSi concentrations are presented in Figs. 4 and 5. The diagenetic model gave a reasonably good description of the distribution for nutrients in pore water in the 1- and 10-months anoxia and 1-month recovery experiments. The 1-year recovery experiment conditions, however, were not reproduced well, since no clear pattern appeared in the measured distribution of nutrient concentrations, probably due to enhanced bioturbation (Cermelj et al. 1997). No evidence of bioturbation was observed in the 2- and 10-month anoxic chambers. In diagenetic models, bioturbation could be treated as a diffusion-like process. The biodiffusional coefficient (D b), depending on the infauna size and the distance between burrows (Aller 1982), could thus be used in diagenetic model. Since we have no quantitative information about the recolonization by bioturbating organisms during the recovery intervals, it was not possible to determine D b and include it into the diagenetic model. Another process that could influence the PO4 3− distribution in pore water and is not included in the model is the redox sensitivity of the interaction between P and Fe-oxides. Higher PO4 3− peaks were observed in all profiles except for normoxia and 10-month anoxia indicating dissolution of P-bearing Fe-oxyhydroxides at various depths as the redox zonation changes in response to imposed anoxia. Furthermore, it was observed that the depth attenuation coefficient β increased with the increasing time of the anoxic condition present for both NH4 + and PO4 3− from 2.0 to 6.3 and from 1.6 to 3.8, respectively. These data show a more pronounced release of both nutrients due to more intense remineralization processes.
The calculated NH4 + benthic fluxes ranged from −34 to 513 μmol m−2 day−1 and those of PO4 3− from 1.3 to 13.8 μmol m−2 day−1 (Table 3). The highest NH4 + efflux was observed after 1 month of anoxia. On the other hand, the influx of NH4 + into sediments was observed after 1 year of recovery. Higher PO4 3− fluxes were observed during anoxic conditions, especially in the 9-day anoxia experiment. The dSi fluxes ranged from −140 to 286 μmol m−2 day−1, the highest being recorded after 10 months of anoxia. A large NH4 +/PO4 3− difference in flux ratio was observed between different anoxic experiments. The highest ratio of 540 was observed in the 10-month anoxic experiment, followed by those in the 1-month and 9-day anoxic experiments (ratios of 44 and 11, respectively). The lowest NH4 +/PO4 3− flux ratio was observed in the 2-month anoxic experiment in parallel to the observed influx of Fe in sediments (Metzger et al. 2014). These results confirmed the importance of simultaneous release of PO4 3− (and dissolved Fe) at the transition from oxic to anoxic conditions observed in the first two anoxic experiments (9-day and 1-month experiments). They suggest an important association of PO4 3− with Fe-oxyhydroxide in sediments in the Gulf of Trieste.
3.5 Tentative benthic nutrient budgets
The differences between N, P and Si cycling in oxic, anoxic and reoxic phases can be estimated by comparing their benthic diffusive fluxes and their burial and by assuming steady-state conditions. The amounts of Ntot, Ptot. and Sibiog buried in sediment were calculated from the sedimentation rate (ω) and the concentration (C) of sedimentary Ntot, Ptot and Sibiog in the deepest measured sediment layer (approximately 7 cm), since they are nearly constant and less affected by bioturbation. The following equation is used:
where ρ is the dry density (2.5 g cm−3; Ogorelec et al. 1991), ϕ the porosity (0.55) and ω the sedimentation rate (1.2 mm year−1) estimated from 210Pb measurements. Burial fluxes of Ntot, Ptot and Sibiog amounted to 170, 30 and 6800 μmol m−2 day−1, respectively. Comparison between benthic fluxes (Table 3) and burial revealed that the intensive recycling of dissolved inorganic N at the SWI is enhanced in anoxic conditions, while P benthic fluxes appear extremely low even in anoxic phases (Table 3). This suggests an important role for P precipitation, probably as authigenic carbonate fluorapatite (Ruttenberg and Berner 1993; Ingall and Jahnke 1997). Si recycling appears to be much less intensive, as compared to the burial flux in all redox phases indicating rather low Sibiog solubility. The comparison between diffusive and laboratory incubated benthic fluxes under oxic and anoxic conditions, reported previously for the same sampling point (Faganeli and Ogrinc 2009), reveals lower values of NH4 + and PO4 3− diffusive fluxes for both conditions. This can be attributed to the presence of bioturbation (Cermelj et al. 1997), not included in the modelling, and to differences in experimental approach. This may include a coarse (insufficient) resolution of pore water concentration gradients near the SWI and the fact that the benthic fluxes integrate the processes in the whole sedimentary column, while diffusive fluxes consider only changes at SWI. Also, the use of one-dimensional models may underestimate the real fluxes in areas of high spatial biological variability. Nutrient availability in overlying and pore waters in the gulf is also controlled by the metabolic activity of microphytobenthos, mostly composed of diatoms, particularly in the normoxic and reoxygenation phases (Welker et al. 2002).
4 Conclusions
-
1.
The sequential biogeochemical changes occurring during the OM degradation and nutrient regeneration in relatively OM poor coastal surface sediments of the Gulf of Trieste under various redox conditions, normoxic, anoxic and reoxic, are documented. The studies of sequential nutrient regeneration and OM degradation were primarily focused on variations of pore water composition. NH4 + and PO4 3− regeneration was more intensive in anoxic phases, but the prolonged span of anoxia reduced their increase. Consequently, the solid phase Ntot and Porg contents decreased, increasing the Corg/Ntot and Corg/Porg ratios. Higher pore water NH4 + levels were presumably also a consequence of the dissimilatory nitrate reduction to NH4 + that is a concurrent process of denitrification in removing NO3 −. The presence of NO3 − in anoxic phases can be explained by the laterally pumping of oxygenated water by benthic infauna. Enhanced dissolution of Sibiog was also observed in the anoxic phase.
-
2.
Reoxygenation was characterized by enhanced bioturbation. Nitrification caused decreased in NH4 + concentrations, while P can be adsorbed onto Fe-hydroxides or precipitated quickly as carbonate fluorapatite and/or FePO4.
-
3.
Diffusive benthic fluxes at the SWI, calculated from pore water modelling using the diffusion-advection-reaction model, showed high anoxic NH4 + effluxes, while PO4 3− fluxes were very low. The high NH4 +/PO4 3− flux ratios in the anoxic and reoxic phases suggest a P retention or an excess of N in benthos.
-
4.
Nutrient budgets at the SWI of this sandy sediment, i.e. comparison between diffusive benthic and burial fluxes, show intensive anoxic recycling of inorganic N and low P and Si recycling in all redox phases.
References
Aller RC (1982) Topics in geobiology: the effects of macrobenthos on chemical properties of marine sediment and overlying water. In: McCall PL, Tevesz MJS (eds) Animal-sediment relations: the biogenic alteration of sediments. Plenum Press, New York, USA, pp 53–102
Arčon I, Ogrinc N, Kodre A, Faganeli J (1999) EXAFS and XANES characterization of sedimentary iron in the Gulf of Trieste (N Adriatic). J Synchrot Rad 6:659–660
Aspila KI, Agemian H, Chau HSY (1976) A semi-automated method for the determination of inorganic, organic and total phosphate in sediments. Analyst 101:187–197
Bastviken D, Persson L, Odham G, Tranvik L (2004) Degradation of dissolved organic matter in oxic and anoxic lake water. Limnol Oceanogr 49:109–116
Bellias C, Dassenakis M, Scoullos M (2007) Study of N, P and Si fluxes between fish farm and sediment and seawater. Results of simulation experiments employing a benthic chamber under various redox conditions. Mar Chem 103:266–275
Berner RA (1980) Early diagenesis. Princeton University Press, Princeton, USA
Blackburn TH, Henriksen K (1983) Nitrogen cycling in different types of sediments from Danish waters. Limnol Oceanogr 28:477–493
Boudreau BP (1997) Diagenetic models and their implementation: modelling transport and reactions in aquatic sediments. Springer, Berlin, Germany
Burdige DJ (2002) Sediment pore waters. In: Hansell C, Carlson C (eds) Biogeochemistry of marine dissolved organic matter. Academic, New York, USA, pp 611–664
Burgin AJ, Hamilton SK (2007) Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front Ecol Environ 5:89–96
Canfield DE, Thamdrup B, Krstensen E (2005) Aquatic geomicrobiology, advances in marine biology, vol 48. Elsevier, Amsterdam, Netherlands
Cermelj B, Bertuzzi A, Faganeli J (1997) Modelling of pore water nutrient distribution and benthic fluxes in shallow coastal waters (Gulf of Trieste, Northern Adriatic). Water Air Soil Pollut 99:435–444
Cermelj B, Ogrinc N, Faganeli J (2001) Anoxic mineralization of biogenic debris in near-shore marine sediments (Gulf of Trieste, northern Adriatic). Sci Tot Environ 266:143–152
Conley DJ, Bjorck S, Destouni G, Gustafsson BG, Hietanen S, Kortekaas M, Kuosa H, Markus Meier HE, Mulle Karulis B, Nordberg K, Norkko A, Nurmberd G, Pitkanen H, Rabalais NN, Rosenberg R, Savchuk OP, Slomp CP, Voss M, Wulff F, Zillen L (2009) Hypoxia-related processes in the Baltic Sea. Environ Sci Technol 43:3412–3420
Dalsgaard T, Thamdrup B, Canfield DE (2005) Anaerobic ammonium oxidation (anammox) in the marine environment. Res Microbiol 156:457–464
DeMaster DJ (1981) The supply and accumulation of silica in the marine environment. Geochim Cosmochim Acta 45:1715–1732
Diaz RJ, Rosenberg R (2008) Spreading dead zones and consequences for marine ecosystems. Science 321:926–929
Engstrom P, Dalsgaard T, Hulth S, Aller RC (2005) Anaerobic ammonium oxidation by nitrite (anammox): implications for N2 production in coastal marine sediments. Geochim Cosmochim Acta 69:2057–2065
Faganeli J, Ogrinc N (2009) Oxic-anoxic transition of benthic fluxes from the coastal marine environment (Gulf of Trieste, northern Adriatic Sea). Mar Freshwat Res 60:700–711
Faganeli J, Avčin A, Fanuko N, Malej A, Turk V, Tusnik P, Vriser B, Vukovič A (1985) Bottom layer anoxia in the central part of the Gulf of Trieste in the late summer of 1983. Mar Pollut Bull 16:75–78
Faganeli J, Kovač N, Leskovšek H, Pezdič J (1995) Sources and fluxes of particulate organic matter in shall coastal waters characterized by summer macroaggregate formation. Biogeochem 29:71–88
Gardner WS, McCarthy MJ, An S, Sobolev D, Sell KS, Brock D (2006) Nitrogen fixation and dissimilatory nitrate reduction to ammonium (DNRA) support nitrogen dynamics in Texas estuaries. Limnol Oceanogr 51:558–568
Grasshoff K, Kremling K, Ehrhardt M (1999) Methods of seawater analysis, 3rd edn. Wiley-VCH, Weinheim, Germany
Hedges JI, Stern JH (1984) Carbon and nitrogen determinations of carbonate-containing solids. Limnol Oceanogr 29:195–212
Hines ME, Faganeli J, Planinc R (1997) Sedimentary anaerobic microbial biogeochemistry in the Gulf of Trieste, northern Adriatic Sea: Influences of bottom water oxygen depletion. Biogeochem 39:65–86
Hulthe G, Hulth S, Hall POJ (1998) Effect of oxygen on degradation rates of refractory and labile organic matter in continental margin sediments. Geochim Cosmoschim Acta 62:1319–1328
Hunter WR, Levin L, Kitazato H, Witte U (2012) Macrobenthic assemblage structure and organismal stoichiometry control faunal processing of particulate organic carbon and nitrogen in oxygen minimum zone sediments. Biogeosciences 9:993–1006
Ingall E, Jahnke R (1997) Influence of water-column anoxia the elemental fractionation of carbon and phosphorus during sediment diagenesis. Mar Geol 139:219–229
Ingall ED, Bustin RM, van Cappellen P (1993) Influence of water column anoxia on the burial and preservation carbon and phosphorus in marine shales. Geochim Cosmochim Acta 57:303–316
Kemp M, Faganeli J, Puškaric S, Smith EM, Boynton WR (1999) Pelagic-benthic coupling and nutrient cycling. In: Malone TC et al (eds) Ecosystems at the land-sea margin: drainage basin to coastal sea. American Geophysical Union, Washington DC, USA, pp 295–339
Lerman A (1979) Geochemical processes: water and sediment environments. Wiley, New York, USA
Liu SM, Zhang J, Jiang WS (2003) Pore water nutrient regeneration in shallow coastal Bohai Sea, China. J Oceanogr 59:377–385
Macko SA, Engel MH, Parker PL (1993) Early diagenesis of organic matter in sediments. In: Engel MH, Macko SA (eds) Organic geochemistry. Plenum, New York, USA, pp 211–224
Metzger E, Langlet D, Viollier E, Koron N, Riedel B, Faganeli J, Tharaud M, Geslin E, Jorissen F, Stachowitsch M (2014) Artificially induced migration of redox layers in a coastal sediment from the Northern Adriatic. Biogeosciences 11:2211–2224
Middelburg J, Levin LA (2009) Coastal hypoxia and sediment biogeochemistry. Biogeosciences 6:1273–1293
Ogorelec B, Mišič M, Faganeli J (1991) Marine geology of the Gulf of Trieste (northern Adriatic): sedimentological aspects. Mar Geol 99:79–92
Ogrinc N, Faganeli J (2006) Phosphorus regeneration and burial in near-shore marine sediments (the Gulf of Trieste, northern Adriatic Sea). Estuar Coast Shelf Sci 67:579–588
Ogrinc N, Fontolan G, Faganeli J, Covelli S (2005) Carbon and nitrogen isotope compositions of organic matter in coastal marine sediments (the Gulf of Trieste, N Adriatic Sea): indicators of sources and preservation. Mar Chem 95:163–181
Riedel B, Pados T, Pretterebner K, Schiemer L, Steckbauer A, Haselmair A, Zuschin M, Stachowitsch M (2014) Effect of hypoxia and anoxia on invertebrate behaviour: ecological perspectives from species to community level. Biogeosciences 11:1491–1518
Ruttenberg KC, Berner RA (1993) Authigenic apatite formation and burial in sediments from non-upwelling, continental margin environments. Geochim Cosmochim Acta 57:991–1007
Stachowitsch M (1991) Anoxia in the Northern Adriatic Sea: rapid death, slow recovery. In: Tyson RV, Pearson TH (eds) Modern and ancient continental shelf anoxia. Geol. Soc., London, UK, Spec. Publ. 58., pp 119–129
Stachowitsch M, Riedel B, Zuschin M, Machan R (2007) Oxygen depletion and benthic mortalities: the first in situ experimental approach to documenting an elusive phenomenon. Limnol Oceanogr Methods 5:344–352
Steenbergh AK, Bodelier PLE, Hoogveld HL, Slomp CP, Laanbroek HJ (2011) Phosphatase relieve carbon limitation of microbial activity in Baltic Sea sediments along a redox-gradient. Limnol Oceanogr 56:2018–2026
Van de Graaf AA, de Brujin P, Robertson LA, Jetten MSM, Kuenen JG (1997) Metabolic pathway of anaerobic ammonium oxidation on the basis of N-15 studies in a fluidized bed reactor. Microbiology-UK 143:2415–2421
Welker C, Sdrigotti E, Covelli S, Faganeli J (2002) Microphytobenthos in the Gulf of Trieste (Northern Adriatic Sea): relationship with labile sedimentary organic matter and nutrients. Estuar Coast Shelf Sci 55:259–273
Acknowledgments
This research was conducted in the framework of the Austrian Science Fund (FWF) project P21542-B17 entitled “Low dissolved oxygen events in the Northern Adriatic: in situ experimental insights into benthic responses before, during and post-anoxia”. This part of the research was also supported financially by the Research Programme P1-0143 and the Young Researcher Programme of the Slovenian Research Agency (ARRS). We thank M. Stachowitsch, M. Zuschin, I. Gallmetzer and A. Haselmair for the technical and diving support. Authors also thank R. Pain for the linguistic corrections.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Carolyn Oldham
Rights and permissions
About this article
Cite this article
Koron, N., Ogrinc, N., Metzger, E. et al. The impact of induced redox transitions on nutrient diagenesis in coastal marine sediments (Gulf of Trieste, northern Adriatic Sea). J Soils Sediments 15, 2443–2452 (2015). https://doi.org/10.1007/s11368-015-1215-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1215-2