Abstract
Purpose
Mining activities generate large amounts of wastes that may contain potentially toxic elements (PTE), which, if released into the environment, may cause air, water and soil pollution long after mining operations have ceased. This paper describes the environmental relevance of efflorescences, topsoils and surface waters from the abandoned mine of Sierra Minera of Cartagena-La Unión, SE Spain. The exposure of the population to arsenic and the associated risk were also assessed.
Materials and methods
A total of 10 topsoils and surface waters and 11 efflorescences affected at varying degrees by mining activities were studied. The total potentially toxic element content (As, Cd, Cu, Fe, Pb and Zn) was determined in all samples. In addition, the mineralogical composition of solid samples was determined by X-ray diffraction and some efflorescences were also analysed using a scanning electron microscopy-energy dispersive X-ray spectrometer. Finally, an arsenic-intake risk assessment was carried out, both as regards carcinogenic and non-carcinogenic effects and considering the total and the bioaccesible As content.
Results and discussion
The study area is heavily polluted as a result of historical mining and processing activities, during which time great amounts of wastes were produced, characterised by a high PTE content, acidic pH and minerals resulting from supergene alteration. The supergene mineralogical assemblages include soluble metal salts, mainly sulphates, iron hydroxysulphates and iron oxyhydroxides, all of which form ochreous precipitates. Topsoil samples showed risk values and hazard quotients higher than the reference levels, particularly for children. In the efflorescences, these values were lower but still unacceptable.
Conclusions
The efflorescences are of significance for monitoring purposes because they are involved in cycles of retention release of hydrogen ions, sulphate and potentially toxic elements. In addition, in a semi-arid climate, such as the study area, these minerals contribute to our understanding of the response of the system to episodic rainfall events. In general, it was observed that the arsenic in collected samples represent a potential risk for human health through ingestion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Sites where metal ores are mined or processed are potential sources of environmental pollution. In ancient mining districts, with long histories of mining and metallurgical activity, mine wastes represent one of the main sources of pollution for soils, water, air and biota (Castillo et al. 2013). Acid mine drainage (AMD) from sulphide-rich waste dumps exposed to weathering processes is an important focus of environmental impact (Valente and Leal Gomes 2009; Valente et al. 2011), generally due to their low pH, high salinity and high potentially toxic element (PTE) content.
The general process of AMD formation results from a series of interconnected steps that primarily involve the oxidation of sulphides, such as pyrite (FeS2), as a result of exposure to oxygen, water and microorganisms. The process of AMD generation is very complex since it involves chemical and biological reactions, and the overall process is accompanied by the development of newly formed iron-rich solid phases generically represented by the ferric hydroxide (Fe(OH)3(s)) (Hammarstrom et al. 2005; Valente et al. 2013; Simate and Ndlovu 2014). These ferric hydroxides occur as ochre mixtures that are responsible for the strong visual impact of the watercourses.
Another important consequence of AMD is the precipitation of secondary minerals, which may involve intermediary species (Carbone et al. 2013). These species are mainly represented by efflorescent sulphate minerals, but also clay minerals, hydrated salts, metallic oxides, arsenates, carbonates, phosphates and native elements. Such secondary materials are of great relevance in environmental studies, for their role in monitoring pollutants in contaminated environments: efflorescent salts are often very soluble and represent a temporary host for many potentially toxic elements (PTEs) (Buckby et al. 2003; Valente et al. 2013) whilst ochreous precipitates represent a more stable sink for metals (Lottermoser 2007; Nordstrom 2011).
Although efflorescent salts are ephemeral, they have a strong influence on surface environments (Dold and Fontboté 2001; Harris et al. 2003) and could provide valuable information concerning primary minerals and the composition of the solutions from which they formed, as well as about the equilibrium conditions prevailing in waste dumps (Valente and Leal Gomes 2009).
The present study represents a contribution to the knowledge of the mineralogy and geochemistry of the solid phases and surface waters formed in the AMD setting of the Sierra Minera (SE, Spain) and their environmental significance. In addition, the exposure of the population to arsenic and the associated risk were assessed.
2 Environmental setting
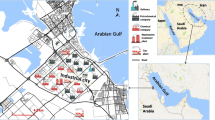
Sierra Minera is a belt approximately 25 km long of low rising hills (maximum height, 431 masl) in the SE of Murcia Region (Spain), in the Cartagena, La Unión area. The average annual temperature in the area is 17 °C, and precipitation does not exceed 300 mm, with occasional torrential rainfall, which frequently occurs between the end of summer and autumn (García-Lorenzo et al. 2014a). Perennial streams do not exist, and surface water consists of ephemeral watercourses, which only flow during rainfall events. Stream flow is flashy and a significant amount of sediment is transported after sustained rainfall (Robles-Arenas and Candela 2010). Water bodies in the studied area are not used for human water supply, due to the seasonal nature and the reduced flow.
The soils of the study zone show the typical characteristics of a semi-arid climatic region where mining activities have been carried out for many years. They have been formed from carbonate (limestones, dolomites, sandy marls and conglomerates) and volcanic rocks. The soils of the Sierra Minera, according to FAO-UNESCO (1990) classification, are Lithosols, Calcic and Luvic Xerosols and Urbic Anthrosols. In the zone influenced by the mining area, they are Calcic, Petrocalcic and Luvic Xerosols, Calcic Luvisols and Calcareous Fluvisols (Pérez-Sirvent et al. 2012).
Sierra Minera consists of Paleozoic (mostly micaschists) and overthrusted Triassic rocks (metamorphic and sedimentary rocks, mainly carbonate), emplaced during the Alpine orogeny as part of the Betic chain of southern and southeastern Spain (Ovejero et al. 1976). Miocene volcanism triggered hydrothermal activity, which resulted in the formation of one of the most important polymetallic mining districts of the SE Iberian Peninsula, known to ancient Mediterranean cultures some 3000 years ago (Manteca and Ovejero 1992). The mining district of La Unión was one of the most important mining sites of Spain and is a perfect example of the many relationships among the Miocene magmatism, tectonics, and metallogenic processes in SE Spain.
The ore deposits in Sierra Minera have iron, lead and zinc as the main metal components (Oen et al. 1975) and appeared as massive strata-bound (locally named “mantos”). Two types of mineralogical association are observed in the mantos: (1) greenalite-magnetite-siderite-sulphide-silica and (2) sulphide-siderite-silica. The sulphides in (1) are pyrite, sphalerite, galena, marcasite, tetrahedrite and stannite disseminated in a matrix of greenalite. Assemblage (2) contains pyrite, pyrrhotite, marcasite, galena and sphalerite as the principal sulphides (López-García et al. 1988).
Mining activities originally (from the seventh century bc) focused on Ag and Pb. Following many centuries of intermittent activity, mining resumed in the XIX century, with a renewed interest in Zn and Fe. From the mid-twentieth century, large open pits dominated mining works, until 1990, when mining ceased altogether.
The area of the mining includes five population nuclei with around 20,000 inhabitants (Conesa et al. 2008). The surroundings of the mining area have a population of more than 200,000 inhabitants, including the city of Cartagena. During summer the population noticeably increases because of tourists who come to the Mar Menor lagoon and Mediterranean Sea beaches.
3 Sampling sites, sampling design and collection of samples
To carry out the study, the topographical situation and the mining environment were taken into account, and three different situations were considered, namely the R33 area, El Gorguel and Portman bay (Fig. 1).
A selective sampling was carried out in the three zones, trying to include different material morphologies. At each sampling point, topsoil was collected at a depth of 0–20 cm. Water samples were collected on temporary streams or puddles formed after a rain episode. Efflorescences were located in the soil surface, covering 80 % of the surface. They were collected at a depth between 0.5 and 10 cm.
3.1 R33
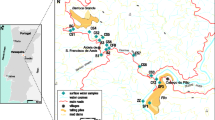
R33 is the highest zone (Fig. 2), which corresponds to the Gossan area and showed little influence of recent mining operations. Tailing dumps are not present in this area. This area corresponds to the highest part of the Sierra Minera and shows abundant archaeological remains, such as Roman smelter ores. The geological characteristics pointed to the existence of a large volcanic dome (postmiocenic dacite and trachiandesites), accompanied by volcanic dikes. In this zone, intense hydrothermal alteration, accompanied by mineralisation (mainly Fe, but also Pb, Ag, Cu, Sn) has taken place. One consequence of the hydrothermal activity is the pyritization-silicification of the host rocks of the volcanic dome, followed by an oxidation process, which has resulted in a goethite-rich Gossan formed by a ferruginous-siliceous crust.
The supergene alteration of assemblages (1) and (2) results in the formation of Gossan. The most important ore are lead and silver, which could leach and precipitate as sulphates (jarosite and anglesite), carbonates (cerusite) and oxides (goethite and Pb coronadite), native silver and halides (cerargyrite) (López-García et al. 1988).
In the R33 area a total of three topsoils, two efflorescences and three surface water samples were taken. Two types of surface waters (R33-3W, an acid mine drainage water, collected from the tailing dump, and R33-2W, a runoff water sample collected from near R33-1W), were collected.
3.2 El Gorguel
El Gorguel area is traversed by the Avenque gully watercourse. In this zone, old mining activities were developed until the 1970s, and nine tailing dumps have been identified, some of them with more than 100 years. Some tailing dumps are located at the head of the ravine, and soils showed high trace elements content (Martínez-Sánchez and Pérez-Sirvent 2013), representing an important contamination source. The slope is important and erosion processes are intense, with high pollutant dispersion risk, both soluble and particulate, which could reach the Mediterranean Sea. In the middle and steepest part of the ravine, PTEs contamination is low and only focuses on the narrow channel. Taking into account the carbonate nature of surrounding areas, mixing of acid waters with non-affected waters produces the precipitation of iron carbonates, retaining high amounts of this element. The whole process involves a natural attenuation of pollution.
The mouth of the ravine is filled with mining wastes, and the formed alluvial fan showed high concentrations of PTES.
In this area, four sampling points, in which it was thought there might be a relationship between sediment, water and efflorescence, were selected. A total of four topsoils, six efflorescences and four waters were collected.
3.3 Portman
Portman Bay is the reception area of the mining materials (Fig. 2), located close to the La Unión mining region. From 1957 to 1990, it was the site of the ore concentration plant known as “Lavadero Roberto”. In this plant, the ore was ground and passed through flotation circuits to separate the sulphides (galena, sphalerite and pyrite). Once the ores had been separated, the waste materials were discharged directly into the sea, originally in the inner part of the bay, but later on, wastes were discharged farther offshore (Martínez-Sánchez et al. 2008; García-Lorenzo et al. 2014a). The mine waste composition is related to the original mineral paragenesis that was mined: paragenesis 1 and paragenesis 2 (Peña et al. 2013). Up to 1967, magnetite was also recovered, but later this practice ceased, in order to prioritise the recovery of sulphides, so that magnetite became a waste. In addition, the wastes showed a significantly high content of PTEs and substantial proportions of iron oxides, including magnetite, goethite and hematite. As a result of dumping, the whole bay filled up with wastes, which also extended into the Mediterranean Sea. Consequently the coast line advanced about 800 m, gaining 70 ha of land from the sea. At sea, coastal dynamics transported fine particles offshore while the sand fraction remained in filling the bay. In addition, discharges were also made directly into the bay’s surface. These wastes had a high content of fine particulates and have been affected by weathering processes. However, materials in depressions experienced reducing conditions. In summary, the bay contains largely unaltered mine wastes, but a small proportion of the infilling materials have experienced weathering and, locally, reducing conditions (García-Lorenzo et al. 2014b).
Three representative sampling points were selected (Martínez-Sánchez et al. 2014), all of them directly affected by discharges from Lavadero Roberto. In these sampling points, three topsoils, surface waters and efflorescences were collected.
4 Methodology
4.1 Topsoil/efflorescence samples
4.1.1 General analytical determinations
Topsoils and efflorescences were air-dried, and in the case of topsoils, the samples were sieved through a 2-mm mesh screen. The pH and the electrical conductivity (EC) were determined in a 1:5 (weight/volume) suspension for topsoils and in a 1:1 (weight/volume) suspension for efflorescences. In all cases, pure, deionised water (resistivity ≥18 MOhm/cm; Milli-Q) was used.
4.1.2 Mineralogical composition and SEM-EDX analysis
For the mineralogical composition analysis, solid samples were finely ground (<50 μm) and sieved. The mineralogical composition was studied by X-ray diffraction (XRD), utilising a Philips PW3040 diffractometer, equipped with an automatic window and Ni filter, using the Cu-Kα line. The experimental conditions were: angular range (3.02–69.98 2θ), step size (0.04 2θ), intensity range (2.42–206.04 counts s−1). The powder diffraction file (PDF2) database was used for peak identification, taking into account that the determination of minerals from soils using this procedure is not accurate below 5 % of the total weight in a sample (depending on the crystallinity of individual minerals).
As a complementary technique, the samples were also observed by using a scanning electron microscope (SEM) JEOL-6100, together with an X-ray energy-dispersion spectrometer (Oxford ISIS).
4.1.3 Total and bioaccesible PTEs content
To determine the total PTE content, topsoils and efflorescences were first ground to a fine powder using a zirconium ball mill. Aliquots (0.1 g) of solid samples were placed in Teflon vessels, and a mixture of 5 ml concentrated HF, 200 μl concentrated HNO3 and 5 ml water was added (Matusiewicz 2003).
An ETHOS laboratory microwave system (Milestone, Sorisole (BG), Italy) equipped with temperature and pressure feedback controls, magnetic stirring capability and ten high-pressure vessels of 100 ml inner volume, operating at a maximum exit power of 1500 W, was employed for the digestion processes. When digestion in the microwave system was complete, the samples were transferred to a volumetric flask and brought to 50 ml before measurement. Teflon or other suitable plasticware was used for handling these liquids. All the experiments were conducted in triplicate.
The diagnosis and cleanup of contaminated soils is based on risk assessment. It is necessary to evaluate the adverse effects that dangerous substances in the soil may have on human health or the harm they may cause to ecosystems as a function of the use to which they are put (US EPA 1989). The evaluation of the risks to human health must cover pathways of direct exposure and ingestion of soil and is carried out as a function of the oral toxicity reference values of the contaminant in question (González-Ciudad 2014). However, one problem that may arise in calculating risk is over-conservatism because a perception of risk may not be realistic or may involve over estimation (Meunier et al. 2011). This happens, for example, when the risk is calculated considering the total concentration of the contaminant or if it is assumed that 100 % of the ingested dose is bioavailable (Martínez-Sánchez et al. 2013).
Contaminant bioaccesibility, rather than total content, is used as a key indicator of the potential risk that contaminants pose to the environment and human health (Navarro et al. 2006). Oral bioaccesibility refers to the fraction that is soluble in the gastrointestinal environment and is available for absorption by blood. To assess the bioaccesible fraction, the gastric solution was prepared according to the standard operating procedure (SOP) developed by the Solubility/Bioavailability Research Consortium (SBRC) (Ruby et al. 1999; Kelley et al. 2002).
Zinc and iron were determined by flame atomic absorption spectrometry (FAAS) using a CONTRA AA700 High-Resolution Continuum Source Atomic Absorption Spectrophotometer. Lead, cadmium and copper were determined by electrothermal atomic absorption spectrometry (ETAAS). Arsenic was measured by atomic fluorescence spectrometry (AFS) using an automated continuous flow hydride generation spectrometer (PSA Millenium Merlin 10055). The reliability of the results was verified by means of certified reference materials (NIST SRM 2711 Montana Soil, NIST SRM 2709 San Joaquin Soil, NCS DC 73319, NCS DC 73320, NCS DC 73321, NCS DC 73323, NCS DC 73324, NCS DC 73325, NRC BCSS-1, NRC PACS-1), and the results are shown in Table 1.
4.2 Water samples
Surface waters were filtered through 0.45 μm pore size filters and properly stored including refrigeration at 4 °C for analytical determinations. The pH and EC were determined by the usual procedure and PTE content by FAAS, ETAAS or AFS.
4.3 Exposure assessment and risk characterization for arsenic
The concentrations of PTEs in soils were used to make an exposure assessment and risk characterisation for the local population according to the methodology described by the US Environmental Protection Agency (US EPA 1989) and applied in the study area in the framework of the remediation Pilot Project of Portman Bay (Martínez-Sánchez et al. 2013; González-Ciudad 2014; Pérez-Sirvent et al. 2014).
A full risk assessment was not carried out. In order to perform a comparative study between the two types of solid sample (efflorescences and topsoils), only one route (intake) and one element (arsenic) was considered. Arsenic is considered as a type A contaminant (US EPA 1998), and the risk assessment procedure was applied in two population groups: children (6 years old) and adults. A residential use with the maximum occupation (300 days) was considered.
4.3.1 Dose–response assessment
For non-carcinogenic effects, the US EPA has developed the oral reference dose (RfD), whilst oral slope factors (SF) have been developed for cancer risk assessment. RfD is an estimate of daily exposure that will probably have no adverse effect on the human population (Smith 1996). SF relates exposure to the probability of having a carcinogenic effect (Peña-Fernández et al. 2014). Both RfD and SF are expressed in milligrams per kilogram per day.
4.3.2 Exposure assessment
For exposure assessment, the chronic daily intake (CDI) of the selected contaminant through the ingestion pathway was calculated, averaged over the period of exposure, considering both the total content and the bioaccesible fraction of arsenic.
where Cs is the concentration at the point of exposure (mg/kg); IR is the soil ingestion rate (mg/day); EF the exposure frequency (day/year); ED the exposure duration (year); CF the unit conversion factor of 10−6 (dimensionless); FI the ingest factor (dimensionless); BW the body weight (kg); and AT the average time (days).
4.3.3 Risk characterisation
Once the CDI values were obtained, the dimensionless carcinogenic risk (Risk), that is, the incremental probability of developing cancer during a lifetime was calculated by means of the equation (Basta et al. 2001):
where SF is the cancer slope factor whose units are milligrams per kilogram per day. A risk value under 10−5 is assumed to be safe.
The non-carcinogenic risks were characterised by calculating the hazard quotient (HQ), which represents the relation between CDI and RfD, where RfD is the reference dose (units in mg kg−1 day−1). A hazard quotient under 1 is assumed to be safe.
4.4 Statistical analyses
A correlation matrix analysis and a principal component analysis (PCA) were carried out for the selected variables. Values of p < 0.05 were considered to be significant. SPSS version 19 software was used for the statistical analysis of the data.
5 Results and discussion
5.1 Geochemistry of solid samples (topsoil/efflorescences) and surface waters
The characteristics of the surface waters affected by mining activities are highly variable, depending on the proximity to the contamination sources (tailing dumps), the material origin, weathering, rainfall intensity and the time elapsed between the sample collection and the rain episode. However, all waters had a pH lower than 4, were red or orange in colour (associated with ferric iron mobilisation) and had a high PTEs content.
When evaporation processes had occurred, both in surface puddles and in pore waters, hydrated salts, mainly sulphates, were crystallised. Even when most of the salts formed were iron, magnesium and calcium sulphates, they cannot be considered pure phases and isomorphic replacements are important. Taking into account that PTEs could be stored in these phases and that are present in a soluble form, the toxicity of these materials needs to be studied.
5.1.1 Gossan area (R33)
Topsoils collected in this area show high iron, lead and arsenic concentrations (Table 2). On the other hand, the zinc content is lower than in other topsoils in the same area (Navarro-Hervás et al. 2012).
The mineralogical composition (Table 3) of R33-3 and R33-4 is typical of Gossan samples: R33-4 is enriched in goethite (59 %) and R33-3 in kaolinite (6 %). No soluble salts are found in these topsoils, since this area has been in contact with the atmospheric agents for a long period of time. Any soluble salts formed in the past could have been washed away, leaving only stable minerals, whether inherited from the parent material or newly formed, such as iron oxides and oxyhydroxides. The mineralogical composition of R33-5 is similar to that of other samples collected from the Sierra Minera (García-Lorenzo et al. 2014c), containing phyllosilicates (20 %), quartz (15 %), iron oxides (15 % goethite and 10 % hematite) and jarosite (23 %). The presence of jarosite is compatible with the presence of efflorescences and acidic waters.
The collected efflorescences are formed of iron and zinc salts, with high cadmium content. R33-1E is composed of zincocopiapite (ZnFe3+ 4(SO4)6(OH)2·18H2O) together with bilinite (Fe2+Fe3+ 2(SO4)4·22H2O) and amorphous phases (Table 3). R33-2E is composed of copiapite (Fe2+Fe3+ 2(SO4)6(OH)2·20H2O), alunogen (Al2(SO4)3·17H2O), slavikite ((H3O+)Mg6Fe15(SO4)21(OH)18·98H2O) and minerals from the halotrichite group, apjohnite (Mn2+Al2(SO4)4·22H2O) and bilinite. Both efflorescences showed a high amorphous percentage, because secondary minerals from AMD do not usually form crystalline structures.
All waters had an acid pH and high electrical conductivity and a medium content of dissolved PTEs (Table 4).
5.1.2 El Gorguel
As regards the PTE contents, topsoil samples had very high concentrations, except for cadmium (Table 2). In addition, all samples showed an acid pH and high EC values, particularly CP7 and CP9. Efflorescences showed an acid pH and very high EC values, together with a very high PTE content (Table 2).
The mineralogical composition of topsoil samples (Table 3) included phyllosilicates from host rocks and gangue accompanying the mineralisation (such as chlorite and biotite) and kaolinite, formed by hydrothermal alteration. In addition, these samples are formed by jarosite, particularly natrojarosite, because seawater was used for the flotation processes, gypsum and iron oxides and oxyhydroxides as alteration products of mining wastes. Soluble salts are present in some samples, particularly parabutlerite (Fe(SO4)(OH)·2H2O) in CP9.
The efflorescences had a very variable mineralogical composition (Table 3). CP7J and CP9J are not exactly efflorescences, because they showed jarosite, mica (phologopite) and minerals inherited from the parent rock. These samples also showed parabutlerite in their mineralogical composition, 21 % in CP7J and 19 % in CP9J, and copiapite in CP7J. In addition, both samples showed gypsum in their mineralogical composition. These efflorescences are examples of supergene alteration crusts, as a consequence of the water erosion that has affected the materials of the tailing dumps located upstream.
CP1EA and CP1EB are formed of minerals from the halotrichite group: dietrichite ((Zn2+, Fe2+, Mn2+)Al2(SO4)4·22H2O) in CP1EA and apjohnite and dietrichite in CP1EB. In addition, rozenite group minerals are present in both samples: rozenite (FeSO4·4H2O) in CP1EA and starkeyite (MgSO4·9H2O) in CP1EB. Paracoquimbite (Fe2(SO4)3·9H2O) was also quantified while copiapite is only present in CP1EA. Both samples showed a high amorphous percentage. Efflorescences CP3EA and CP3EB are mainly composed of fibroferrite (Fe3+(SO4)(OH)·4H2O) and copiapite.
When CP1E was analysed by SEM-EDX to characterise the chemical composition of individual particles, three different morphologies were identified, corresponding to stoichiometric compositions similar to paracoquimbite, dietrichite and apjohnite. The complete spectrum is shown in Fig. 3.
In water samples, the PTE concentrations (Table 4) agree with the results obtained for topsoil samples, indicating that zinc and cadmium have a high solubility. Even in samples with a low content, the mobilisation of cadmium exceed the 50 %.
5.1.3 Portman Bay
Topsoil samples showed pH values ranging from 1.66 to 4.66 (Table 2), indicating the acidic nature of the topsoils. The concentrations of PTEs are also summarised in Table 2, proving the strong influence of mining activities. The efflorescences also showed acid pH, reaching 0.8 in C18E. These efflorescences showed very high electrical conductivity values and very high PTE content. The surface waters collected after a rain episode had an acid pH and very high soluble PTE contents (Table 4).
The mineralogical composition of topsoils (Table 3) showed a high percentage of weathered minerals, such as jarosite or iron oxides and oxyhydroxides, together with minerals more associated with primary mineralisation, such as phyllosilicates, pyrite or magnetite.
Efflorescence C15E corresponds to the most recent topsoil sample, and is composed of hexahydrite (MgSO4·6H2O) and copiapite (Table 3). C18E efflorescence also presents hexahydrite, particularly ferrohexahydrite (FeSO4·6H2O) and the halotrichite group mineral: pickeringite (MgAl2(SO4)4·22H2O) and bilinite. Finally, in C17E aluminium has been released and alunogen is present (17 %), together with 67 % of copiapite. The amorphous percentage is high, ranging from 16 to 31 %, confirming the great alteration of these elements.
The SEM results for the Portman efflorescences indicated fibres and irregular and complex aggregates of copiapite, together with more spheroidal and symmetrical forms of alunogen, in C17E (Fig. 4), while C18E presents a more dense appearance, reflecting its high percentage of amorphous phases. EDS data showed that some of the compositions suggest the presence of ferrohexahydrite and minerals from the halotrichite group, particularly pickeringite and bilinite (Fig. 5).
5.2 Multivariate statistics
A statistical analysis was carried out for the analysed variables in order to determine the tendency of the elements in each type of sample and to study the relationships between surface waters and solid samples (both topsoils and efflorescences).
As regards the total contents, lead was correlated with iron, copper and arsenic, and cadmium was correlated with zinc and copper content, indicating that the elements that characterise these materials have a common origin in these mineralisations (pyrite, sphalerite and galena (PSG)). Iron was the most abundant element in the efflorescences and topsoil samples, but in surface waters, the iron content was lower than the zinc content. In addition, the iron content was negatively correlated with the pH. These results agree with the fact that pyrite is the most abundant mineral in the ore but has not been fully exploited; instead, it has become a residue, supplying metals to the alteration products in topsoil, efflorescence and waters (Robles-Arenas et al. 2006). The existing association between iron and lead is explained by the genetic relationship between galena and iron sulphides, and also because these elements do not show significant mobility in supergene alteration process (García-Lorenzo et al. 2014c).
The sphalerite accompanying the other sulphides showed a very different behaviour, with a high degree of zinc and cadmium mobility. Copper could be present as chalcopyrite accompanied by iron or as oxidised phases, carbonates or silicates (crysocolla) (Sainz de Baranda et al. 2003), like arsenic as arsenopyrite (Navarro-Hervás et al. 2012), but always is a minority. When these elements are released during weathering, they follow different paths depending on the physicochemical conditions of the medium.
The materials forming the topsoils are very heterogeneous, consisting of altered mineral processing wastes or highly altered mine tailings. The correlation analysis only suggested a correlation between cadmium and zinc.
Surface waters also showed a high degree of heterogeneity, the samples having similar characteristics to these of topsoils and efflorescences. Except for the waters collected in R33, the rest were rainwaters that have passed in different extent through the sampling point.
In surface waters, the target elements appear closely related. It is of note the negative correlation between pH, arsenic and iron, and the positive correlation between the electrical conductivity and the lead content, probably due to its possible transport in the form of lead chloride (Fernández-Ortiz de Vallejuelo et al. 2014).
Finally, in the efflorescences the correlations found between the studied elements differed from those found in the water samples, with arsenic, lead, copper, iron and cadmium being closely correlated. Zinc was not related with the other elements because of its greater abundance and mobility.
These results were confirmed by the PCA. In this case, three components explained 73 % of the total variance. The pH had a high score on the first factor (Table 5), followed by arsenic and copper in the positive side, and electrical conductivity on the negative side. Factor 2 represents the lead sulphides, together high iron, and the third factor includes the more mobile elements and less influenced by pH, zinc and cadmium. The PCA score plot (Fig. 6) showed that almost all samples had similar behaviour, regardless of their composition. Only some samples appeared to deviate, such as these with a high pH (R33-4 and R33-5) or those with very acid values and a high iron and lead content. Samples with high cadmium values such as R331E and CP1E were also separate.
When the relations between efflorescences and topsoil and water samples were studied, the results suggested that the electrical conductivity and the lead and iron content are correlated in the efflorescences and the pH and the arsenic, lead, iron and zinc contents in the waters and topsoils. It cannot therefore be affirmed that there is a clear genetic association between water and efflorescences or between efflorescences and topsoils. However, the hydrated surface crusts result from the evaporation of the soluble materials in topsoils and the soluble content of waters.
The formation of the different mineral phases depends on the relative concentration of the elements present and the solubility product of the phases formed. The first sulphates to crystallise were those of the divalent elements (Zn, Mg and Fe2+), since their solubility product is lower, followed by sulphates of trivalent metals (Al and Fe3+) which formed minerals from copiapite or halotrichite group depending on the M3+/M2+ molar ratio: four in the copiapite group and two in the halotrichite group (Pérez-Sirvent et al. 1998; García-Rizo et al. 1999).
5.3 Human health risk
Environmental exposure to arsenic was calculated from the total concentration and also from the bioaccesible content of the solid samples (Martínez-Sánchez et al. 2013).
The selected samples showed risk values higher than the reference levels, particularly for children (Figs. 7 and 8). In topsoil samples, the risk and HQ values were lower when the bioaccesible content was considered (Fig. 7). In the efflorescences (Fig. 8) these values were lower but still unacceptable, because arsenic was related to the amorphous and soluble phases. The results obtained considering only the bioaccesible content are very similar to those obtained considering the total content
In light of the pH and the powdery appearance of the efflorescences, possible inhalation and dermal exposure must be considered. In addition, other elements should be assessed for a complete risk assessment. In sites where the presence of efflorescences is significant, two different behaviours have to be considered (topsoil and efflorescence), efflorescences being an additional risk source for human health, through oral intake.
6 Conclusions
As regards the results obtained, we conclude that mining activities in the studied area have produced many wastes with high concentration of PTEs, representing a serious problem. These materials are suffering weathering processes (supergene alteration). Due to the absence of control measures, the production of acid mine waters and soluble efflorescences could cause significant mobilisation of PTEs into the environment.
According to the results reported in this study, the human health risks from arsenic intake are unacceptable. Efflorescences represent a greater risk than topsoils, even if they have a lower PTE content. These secondary materials are of great relevance in risk assessment procedures, especially as regard their role in monitoring pollutants in contaminated environments.
Finally, the work illustrates the value of combining DRX, SEM-EDX and chemical data to increase our understanding of potential environmental threats.
References
Basta NT, Rodriguez RR, Casteel SW (2001) Bioavailability and risk of arsenic exposure by the soil ingestion pathway. In: Frankenberger WT (ed) Environmental chemistry of arsenic. Marcel Dekker, New York, p 117
Buckby T, Black S, Coleman ML, Hodson ME (2003) Fe-sulphate-rich evaporative mineral precipitates from the Rio Tinto, southwest Spain. Mineral Mag 67:263–278
Carbone C, Dinelli E, Marescotti P, Gasparotto G, Lucchetti G (2013) The role of AMD secondary minerals in controlling environmental pollution: Indications from bulk leaching tests. J Geochem Explor 132:188–200
Castillo S, de la Rosa J, Sánchez de la Campa JD, González-Castanedo Y, Fernández-Caliani JC, González I, Romero A (2013) Contribution of mine wastes to atmospheric metal deposition in the surrounding area of an abandoned heavily polluted mining district (Rio Tinto mines, Spain). Sci Total Environ 449:363–372
Conesa HM, Schulina R, Nowasck B (2008) Mining landscape: a cultural tourist opportunity or an environmental problem? The study case of the Cartagena–La Unión Mining District (SE Spain). Ecol Econ 64:690–700
Dold B, Fontboté L (2001) Element cycling and secondary mineralogy in porphyry copper tailings as a function of climate, primary mineralogy and mineral processing. J Geochem Explor 74:3–55
FAO-UNESCO (1990) Soil map of the world—revised legend. FAO, Rome
Fernández-Ortiz de Vallejuelo S, Gredilla A, de Diego A, Arana G, Madariaga JM (2014) Methodology to assess the mobility of trace elements between water and contaminated estuarine sediments as a function of the site physico-chemical characteristics. Sci Total Environ 473–474:359–371
García-Lorenzo ML, Martínez-Sánchez MJ, Pérez-Sirvent C (2014a) Application of a plant bioassay for the evaluation of ecotoxicological risks of heavy metals in sediments affected by mining activities. J Soils Sediments 14:1753–1765
García-Lorenzo ML, Martínez-Sánchez MJ, Pérez-Sirvent C, Agudo I, Recio C (2014b) Isotope geochemistry of waters affected by mining activities in Sierra Minera and Portman bay (SE, Spain). Appl Geochem 51:139–147
García-Lorenzo ML, Pérez-Sirvent C, Molina-Ruiz J, Martínez-Sánchez MJ (2014c) Mobility indices for the assessment of metal contamination in soils affected by old mining activities. J Geochem Explor 147(Part B):117–129
García-Rizo C, Martínez-Sánchez MJ, Pérez-Sirvent C (1999) Environmental transfer of zinc in calcareous soils in zones near old mining sites with semi-aridic climate. Chemosphere 39(2):209–227
González-Ciudad E (2014) Evaluación en nave cerrada de los riesgos para la salud en tecnosoles procedentes de residuos de minería polimetálica. Doctoral thesis, University of Murcia
Hammarstrom JM, Seal RR II, Meier AL, Kornfeld JM (2005) Secondary sulphate minerals associated with acid drainage in the eastern US: recycling of metals and acidity in surficial environments. Chem Geol 215:407–431
Harris DL, Lottermoser BG, Duchesne J (2003) Ephemeral acid mine drainage at the Montalbion silver mine, North Queensland. Aust J Earth Sci 50:797–809
Kelley ME, Brauning SE, Schoof RA, Ruby MV (2002) Assessing oral bioavailability of metals in soil. Battelle, Columbia
López-García JA, Lunar R, Oyarzun R (1988) Silver and lead mineralogy in Gossan-type deposits of Sierra de Cartagena, southeast Spain. Trans Inst Min Metall, Sect B 97:82–88
Lottermoser B (2007) Mine wastes- characterization, treatment and environmental impacts. Springer, Berlin
Manteca JI, Ovejero G (1992) Los yacimientos Zn, Pb, Ag-Fe del distrito minero de La Unión-Cartagena, Bética Oriental. CSIC Textos Universitarios 15:1085–1110
Martínez-Sánchez MJ, Pérez-Sirvent C (2013) Diagnóstico y Recuperación de la Contaminación del Suelo en Portmán-Sierra Minera. In: Baños González I, Baños Paéz P (eds) Portmán: de el “Portus Magnus” del Mediterráneo Occidental a la Bahía Aterrada. Editum, Murcia, pp 313–344
Martínez-Sánchez MJ, Navarro MC, Pérez-Sirvent C, Marimón J, Vidal J, García-Lorenzo ML, Bech J (2008) Assessment of the mobility of metals in a mining-impacted coastal area (Spain, Western Mediterranean). J Geochem Explor 86:171–182
Martínez-Sánchez MJ, Martínez-López S, Martínez-Martínez LB, Pérez-Sirvent C (2013) Importance of the oral arsenic bioaccessibility factor for characterising the risk associated with soil ingestion in a mining-influenced zone. J Environ Manag 116:10–17
Martínez-Sánchez MJ, García-Lorenzo ML, Pérez-Sirvent C, González E, Pérez V, Martínez S, Martínez L, Molina J (2014) Heavy metal immobilisation by limestone filler in soils contaminated by mining activities: effects on metal leaching and ecotoxicity. Int J Min Reclam Environ 28(6):414–425
Matusiewicz H (2003) Wet digestion methods. In: Mester Z, Sturgeon R (eds) Sample preparation for trace element analysis. Elsevier, Amsterdam, pp 195–235
Meunier L, Koch I, Reimer KJ (2011) Effect of particle size on arsenic bioaccessibility in gold mine tailings of Nova Scotia. Sci Total Environ 409:2233–2243
Navarro MC, Pérez-Sirvent C, Martínez-Sánchez MJ, Vidal J, Marimón J (2006) Lead, cadmium and arsenic bioavailability in the abandoned mine site of Cabezo Rajao (Murcia, SE Spain). Chemosphere 63:484–489
Navarro-Hervás MC, Pérez-Sirvent C, Martínez-Sánchez MJ, García-Lorenzo ML, Molina J (2012) Weathering processes in waste materials from a mining area in a semiarid zone. Appl Geochem 27:1991–2000
Nordstrom DK (2011) Hydrogeochemical processes governing the origin, transport and fate of major and trace elements from mine wastes and mineralized rock to surface waters. Appl Geochem 26:1777–1791
Oen IS, Fernandez JC, Manteca JI (1975) The lead-zinc and associated ores of La Union, Sierra de Cartagena, Spain. Econ Geol 70:1259–1278
Ovejero G, Jacquin JP, Servajean G (1976) Les minéralisations et leur contexte géologique dans la Sierra de Cartagena (sud-est de léspagne). Bull Soc Geol Fr 7:619–633
Peña JA, Manteca JI, Martínez-Pagán P, Teixidó T (2013) Magnetic gradient map of the mine tailings in Portman Bay (Murcia, Spain) and its contribution to the understanding of the bay infilling process. J Appl Geophys 95:115–120
Peña-Fernández A, González-Muñoz MJ, Lobo-Bedmar MC (2014) Establishing the importance of human health risk assessment for metals and metalloids in urban environments. Environ Int 72:176–185
Pérez-Sirvent C, Martínez-Sánchez J, García-Rizo C (1998) Lead mobilization in calcareous agricultural soils. In: Iskandar IK, Magdi Selim H (eds) Fate and transport of heavy metals in the vadose zone. CRC Press, Boca Raton, pp 177–199
Pérez-Sirvent C, Martínez-Sánchez MJ, Martínez-López S, Bech J, Bolan N (2012) Distribution and bioaccumulation of arsenic and antimony in Dittrichia viscose growing in mining-affected semiarid soils in southeast Spain. J Geochem Explor 123:128–135
Pérez-Sirvent C, Martínez-Sánchez MJ, García-Lorenzo ML, Hernández-Córdoba M, Molina J, Martínez S, González E, Pérez-Espinosa V (2014) A preliminary zonation to support the remediation and the risk assessment of an area contaminated by potential toxic elements in Murcia Region (SE, Spain). Procedia Earth Plan Sci 10:388–391
Robles-Arenas VM, Candela L (2010) Hydrological conceptual model characterisation of an abandoned mine site in semiarid climate. The Sierra de Cartagena-La Unión (SE Spain). Geol Acta 8(3):235–248
Robles-Arenas VM, Rodríguez R, García C, Manteca JI, Candela L (2006) Sulphide-mining impacts in the physical environment: Sierra de Cartagena–La Unión (SE Spain) case study. Environ Geol 51:47–64
Ruby MV, Schoof R, Brattin W, Goldade M, Post G, Harnois M, Mosby DE, Casteel SW, Berti W, Carpenter M, Edwards D, Cragin D, Chappell W (1999) Advances in evaluating the oral bioavailability of inorganics in soil for use in human health risk assessment. Environ Sci Technol 33:3697–3705
Sainz de Baranda B, González de Tánago J, Viñals J (2003) Secondary minerals of the Mazarrón-Águilas mining district, Murcia province, Spain. Mineral Rec 34:315–334
Simate GS, Ndlovu S (2014) Acid mine drainage: challenges and opportunities. J Environ Chem Eng 2:1785–1803
Smith RL (1996) Risk-based concentrations: prioritising environmental problems using limited data. Toxicology 106:243–266
US EPA (1989) Risk assessment guidance for superfund. Volume 1: human health evaluation manual (part A). Office of Emergency and Remedial Response
US EPA (1998) Risk assessment guidance for superfund volume 1: human health evaluation manual (Part D)
Valente TM, Leal Gomes CL (2009) Occurrence, properties and pollution potential of environmental minerals in acid mine drainage. Sci Total Environ 407:1135–1152
Valente T, Antunes M, Braga MA, Pamplona J (2011) Geochemistry and mineralogy of ochre-precipitates formed as waste products of passive mine water treatment. Geochem Explor Environ Anal 11:103–106
Valente TM, Grande JA, de la Torre ML, Santiesteban M, Cerón JC (2013) Mineralogy and environmental relevance of AMD-precipitates from the Tharsis mines, Iberian Pyrite Belt (SW, Spain). Appl Geochem 39:11–25
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Claudio Bini
Rights and permissions
About this article
Cite this article
Pérez-Sirvent, C., Hernández-Pérez, C., Martínez-Sánchez, M.J. et al. Geochemical characterisation of surface waters, topsoils and efflorescences in a historic metal-mining area in Spain. J Soils Sediments 16, 1238–1252 (2016). https://doi.org/10.1007/s11368-015-1141-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1141-3