Abstract
Purpose
The impact of biochar on subsoil organic carbon mineralisation has never been assessed despite its susceptibility to downward transport after soil amendment. In this study, we analysed the potential mineralisation of biochar and plant material as well as their effect on native soil organic matter (SOM) decomposition in subsoil horizons.
Materials and methods
We used 13C-labelled biochar and plant material to allow disentangling substrate mineralisation and priming effects. The substrates were added to two mountain subsoils under different land use and incubated for 1 year under optimum conditions. We analysed for physical parameters and C mineralisation in the two soils. Moreover, microbial communities were assessed by phospholipid fatty acid (PLFA) analyses.
Results and discussion
Our results indicated contrasting potential C mineralisation of subsoils under different land use probably related to sampling depth, contribution of stabilised organic matter compounds, carbon content as well as quality. In general, very low proportions of biochar were mineralised in the two soils as compared to plant material. The mineralisation of each of the added substrates (biochar and plant material) was slightly, but significantly different in the two soils. Native C mineralisation was much higher after plant material addition than after biochar addition. Subsoil type influenced the kind and magnitude of priming effects for both added substrates.
Conclusions
Biochar mineralisation and its priming effects in subsoil are small as compared to uncharred plant litter. We suggest that substrate mineralization and priming effects induced on subsoil organic matter are dependent on the composition of the added substrate, as well as soil parameters rather than microbial community characteristics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pyrogenic C produced by pyrolysis with the purpose of soil amendment (biochar) is characterised by a highly recalcitrant aromatic structure. After its addition to soil, very low degradation rates of less than 1 % of added fresh biochar have been reported (Kuzyakov et al. 2009; Jones et al. 2011). Most C release from biochar in laboratory incubation experiments occurs in the first few days after its addition to soil (Smith et al. 2010; Farrell et al. 2013). The mineralisation rate and amount of C mineralised from biochar depend on its feedstock as well as pyrolysis temperature (Singh et al. 2012). It has been suggested that abiotic release of inorganic C (Jones et al. 2011) and residual uncharred material is accounting for the mineralisable biochar fraction (Fabbri et al. 2012). Recently, it was shown that labile biochar compounds were rapidly incorporated into Gram-positive bacteria (Farrell et al. 2013) and may also influence native soil C mineralisation through positive or negative priming effects (Zimmerman et al. 2011; Cely et al. 2014). Priming effects induced by biochar may be caused by its content of dissolved or volatile organic carbon (Luo et al. 2011; Spokas et al. 2011). They were found to be a short-term phenomenon (Zimmerman et al. 2011; Singh and Cowie 2014; Naisse et al. 2014).
Up to now, most studies were concerned with pyrogenic C degradation and its priming effects after addition to surface soil horizons, where the amendment generally occurs (e.g. Hamer et al. 2004; Nocentini et al. 2010). However, biochar deposited on the soils’ surface may be subject to rapid vertical transport to lower soil depths in solid (Rumpel et al. 2009) and/or dissolved form (Jaffé et al. 2013). The fate and impact of biochar transported into subsoil horizons are unresolved issues. While several authors reported accumulation of pyrogenic C in subsoil horizons (Brodowski et al. 2007; Knicker 2011 and references therein), others did not (Alexis et al. 2012). Its effect on microbial communities and their functioning in subsoil horizons has never been assessed. Below the A horizon where fresh litter input is reduced, microbial communities are found to be different from those in topsoil (Eilers et al. 2012). They may be much better adapted to degradation of stable organic matter and hence probably also the degradation of pyrogenic C including biochar.
In this study, we assessed the impact of biochar on the microbiological functioning of subsoil horizons and compared it to addition of plant material. We evaluated the short-term mineralisation potential of the added substrates and their capacity to induce priming effects when added to soil below the A horizon. We used 13C-labelled biochar to increase sensitivity, in order to quantify more accurately the mineralisation of the labile part of biochar and to investigate in detail its effects on native soil organic matter (SOM). As soil parameters were found to have an influence on biochar mineralisation (Fang et al. 2014), we incubated the 13C-labelled char produced at bench scale and the original 13C-labelled plant material in two subsoils under different land use. The objectives of this study were (1) to assess biochar mineralisation in soil as compared to uncharred biomass and (2) to evaluate the priming effects induced by both materials on stabilised organic matter of subsoil horizons.
2 Material and methods
2.1 Soil sampling
Soils were sampled in the Vicdessos valley in the French Pyrenees (42° 42′ 34 N; 1° 25′ 543 E) at 1200-m altitude under forest and grassland. Rainfall in the region amounts to 1200 mm, and mean annual temperature is 9.5 °C. Soils are Cambisols. Samples were taken on landscape plateaus avoiding steep slopes. We sampled subsoils below the A horizon. Sampling depth was 7–35 cm for the grassland soil and 35–45 cm for the forest soil. Soil samples were sieved to pass a 2-mm sieve, and an aliquot was ground for chemical analyses.
Clay content, pH and CEC were determined at the French National Analytical Laboratory at Arras. Carbon and nitrogen content of bulk soil was measured with an elemental analyser (CHN NA 1500, Carlo Elba). The 14C activity of bulk soil was determined after decarbonisation by accelerator mass spectrometry (AMS) in the 14C Poznan Radiocarbon Laboratory, Poland. For these measurements, CO2 was produced from soil samples by combustion at 900 °C and reduced to graphite. The measured 14C activity was corrected for isotope fractionation (Stuiver and Polach 1977), and it was expressed in percent modern carbon (pMC). Radiocarbon age was expressed in years before present (years BP). General soil parameters are presented in Table 1.
2.2 Microbial biomass and phospholipid fatty acid (PLFA) extraction
Microbial biomass C was determined by the chloroform fumigation–extraction method (Vance et al. 1987). PLFAs were extracted from 5 g of the initial soil to study the relative abundances of specific microbial groups and differences in their community structure between the two subsoils. Briefly, PLFA fractions were obtained by one-phase chloroform–methanol–citrate buffer extraction and subsequent separation on silicic acid columns (Frostegård et al. 1993). After transesterification, PLFAs were taken up in dichloromethane and fatty acid methyl esters were separated by gas chromatography on a nonpolar BPX-5 column. Identification of PLFAs was based on comparison with methyl esters of the bacterial acid methyl esters (BAME) mix (Supelco, Sigma-Aldrich, USA). The concentration of individual PLFAs was determined relatively based on their contribution to the total BAME content.
2.3 Production of 13C-labelled plant material and biochar
13C-labelled plant material was rye grass (Dactylus glomerata) produced under an atmosphere containing 13C-labelled CO2 (2 %). Isotopic labelling of plants was carried out continuously during 3 months at the labelling facility of Lans. The resulting isotopic label gave a δ 13C ratio of 3700 ‰, which did not change upon biochar production. 13C-labelled biochar was produced from the labelled plant material by pyrolysis at bench scale during 1.5 h at 550 °C. Chemical parameters of the 13C-enriched plant material and biochar used for the experiment are presented in Table 2.
2.4 Laboratory incubation
We used 31 g of each soil and added 0.3 and 0.4 g respectively of 13C-labelled plant material and biochar for an input of 5.6 mg C g soil−1 corresponding to an agronomic amendment of 30 t ha−1. Such a high amount of labelled material was used in order to be in the detectable range of any biochar effects on soil carbon. Triplicates of each sample were incubated in closed vials. In addition, three replicates of soils without plant or biochar addition were incubated as control. The vials were put into an incubation chamber under controlled temperature and moisture conditions for up to 1 year. Temperature was set at 20 °C, and water content was adjusted to pF 2.5 and maintained during the incubation period through water addition after gravimetric assessment (see below). Carbon mineralisation was monitored gas chromatographically. CO2 concentration and its isotopic composition were measured in the headspace of the incubation vials at days 1, 3, 8, 16, 28, 37, 63, 108, 162, 223, and 336 after the start of the incubation. To do so, we used a syringe and removed air from the headspace twice. The first aliquot was injected into a micro GC (Agilent 490) equipped with a heat conductivity detector to determine its CO2 concentration. The second gas sample was injected into a gas chromatograph (Hewlett Packard 5890) coupled to an isotope ratio mass spectrometer (Micromass-GVI Optima) to determine its δ 13C signature. After these measurements, samples were flushed with synthetic air (19 % O2, 81 % N2) to avoid an internal CO2 concentration of more than 2 %, which could have affected microbial activity. At the same time, water content of the samples was controlled and adjusted with de-ionised water if necessary.
2.5 Isotopic composition of bulk material
Carbon and nitrogen content as well as 13C content of bulk soil, biochar and plant material were measured with an elemental analyser (CHN NA 1500, Carlo Elba) coupled to an isotopic ratio mass spectrometer (VG Sira 10).
2.6 Calculations
For calculation of C stable isotope ratios, we used a laboratory reference calibrated against the Vienna Pee Dee Belemnite (VPDB) international standard. Results were expressed as δ 13C defined as the molar isotopic ratio (13C/12C) relative to the VPDB standard.
Mineralisation of 13C-labelled biochar and biomass as well as priming effects (PE) induced by both materials on native SOM were monitored by recording δ 13C of CO2 evolved from the incubated soils. The proportion of CO2 evolved from 13C-labelled biochar and biomass was calculated by isotopic mass balance using Eq. (2):
where δ 13Ctreatment is the δ 13C (‰) of evolved CO2 from soil–biochar or soil–biomass mixture, δ 13Ccontrol is the δ 13C (‰) of CO2 evolved from soil without addition and δ 13Cadded material is the δ 13C (‰) of labelled biomass or biochar. Thanks to the isotopic labelling, it was possible to determine the amount of native SOM subjected to priming after addition of labelled material. The priming effect (PE) was calculated as the difference between the total amount of soil organic C mineralized at different time intervals during incubation in treatments with labelled biomass or biochar addition (Ctreatment) and the control soil without addition (Ccontrol):
2.7 Statistical analyses
Data obtained after the incubation experiments were expressed as the means of three replicate incubations ± standard error. Differences between soil treatments were determined using one-way analysis of variance (ANOVA) and Tukey’s multiple comparison tests.
Mineralisation of SOM and labelled biomass or biochar was modelled by a two-component first-order exponential model using the following equation:
Curve fitting was carried out using a Bayesian curve fitting method and the R software for statistical computing version 2.15.1.
3 Results and discussion
3.1 General soil parameters
Both soils were characterised by an acidic pH, a sandy texture and high organic carbon content (Table 1). These features are common to subsoil horizons of mountain regions (Budge et al. 2011). However, the subsoil under grassland was distinguished from the subsoil under forest by higher contents of C and N and cation exchange capacity (CEC). This soil also showed higher contents of microbial biomass and a higher 14C activity (Table 1). The 14C activity was 107 pmC in grassland soil versus 96 pmC in forest soil, corresponding to a radiocarbon age of 250 years BP in the latter. The 14C age of the grassland soil is modern, indicating high contribution of carbon aged less than 50 years. This may be related to the lower sampling depth and/or higher input of recent organic matter through higher root biomass contribution and activity, which may have led to higher C turnover under grassland as compared to forest (Rumpel 2011).
3.2 Microbial biomass content and composition
Microbial biomass ranged between 12 and 43 mg kg−1 soil (Table 1). It was higher in grassland subsoil compared to subsoil under forest even when normalised to the contrasting C contents of both soils. Despite the differences in microbial biomass contents, PLFA analyses did not allow for the observation of significant differences in microbial community composition between the two subsoils (Table 3). The higher microbial biomass contents in subsoil under grassland as compared to forest may be explained by the lower sampling depth. At lower depths, generally organic matter presents a lower degree of stabilisation and may thus favour microbial biomass development as compared to deeper subsoil horizons (Blume et al. 2002). However, it is interesting to note that the composition of the microbial biomass was not significantly different between the two soils (Table 3). Our results are in contrast to the general observation of microbial community shift with depth (Eilers et al. 2012) but in line with observations of Stone and Plante (2014), showing that two soils with contrasting C content were characterised by similar microbial community structures. Our results may be explained by the small difference in sampling depth between the two soils as compared to other studies. We suggest that in our case, the depth difference between the two samples was insufficient to induce shifts in microbial biomass composition.
3.3 Carbon mineralisation
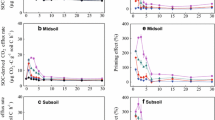
Mineralisation rates of the two soils with and without substrate addition are depicted in Fig. 1. Without substrate addition, carbon was mineralised at a higher rate in subsoil under grassland as compared to subsoil under forest (p < 0.01). Addition of 13C-labelled plant material as well as biochar increased microbial respiration in both soils (p < 0.01, Fig. 1). Cumulative C mineralisation at the end of incubation ranged between 2 and 3 mg C g−1 when plant biomass was added and was around 0.5 mg C g−1 for soil with added biochar (Fig. 1). When plant material was added, a much higher increase in respiration was noted for grassland compared to forest soil, whereas after biochar addition, cumulative mineralisation rates were in similar range for both soils (Fig. 1). The mineralisation of both types of material was determined, thanks to stable isotope labelling. Cumulative mineralisation showed that after 336 days of incubation, between 0.4 and 0.6 % of initial biochar was mineralised, whereas the mineralisation of plant material was around 25 % of initial C added (Fig. 2). With both materials, slightly but significantly higher mineralisation was achieved in the grassland compared to the forest subsoil (Fig. 2). However, despite the strong difference in respiration rates (Fig. 1), both substrates degraded similarly in the two soils (Fig. 2). This suggests that the ability of microorganisms to degrade labile as well as stable substrates did not differ in the two subsoils. Quantitative information on the amount of C mineralised in the different treatments for both substrates as well as their priming effects on native SOM are presented in Table 4.
Total C mineralised ranged between 120 and 506 mg C kg−1 (Table 4). In accordance with the high respiration rates, around four times more C was released from subsoil under grassland compared to subsoil under forest. This may be partly explained not only by its higher C content (Don et al. 2013) but also by the potentially better degradability of SOM in grassland soil as indicated by its lower C/N ratio and higher 14C activity (Table 1). Indeed, it is likely that organic matter from needle litter under conifer forest has a slower mineralization rate at 20 °C than organic material from grass litter under grassland (Wetterstedt et al. 2010). It is interesting to note that C mineralised from the two substrates was significantly different but in a similar range. Only 10 to 20 % more plant residue and biochar was mineralised in grassland soil compared to subsoil under forest, despite a much lower activity in the latter without substrate addition (Fig. 1). Differences in microbial activity were once again evident when comparing native SOM mineralisation after substrate addition, which was determined thanks to the 13C label. Native SOM mineralisation induced by addition of plant litter to grassland soil led to about four times higher C mineralisation compared to the forest soil. This is a very interesting result because the difference in the release of native C induced by priming between the two sites was in a similar range as the difference in C mineralisation without substrate addition.
It is interesting to note that the difference in C mineralisation between the two subsoils was evident even after normalisation for the soils’ contrasting C contents (Table 1). This result might be explained by the lower depth from which the forest soil was taken. Usually, general soil parameters, such as carbon and nitrogen content, radiocarbon age decrease gradually with increasing soil depths (Rumpel and Kögel-Knabner 2011; Table 1). Therefore, a higher concentration of stabilised SOM compounds may be present in deeper subsoil horizons. Moreover, the microbial communities may be more and more limited by energy due to the declining input of fresh plant material with increasing depth (Fontaine et al. 2007). However, our data only partly sustain this hypothesis, as priming of native SOM (Table 4) was higher under grassland as compared to forest despite the potentially greater effect on microbial communities under forest, where samples were taken at a lower depth.
For biochar, a very small positive priming effect was noted for the forest soil, whereas the grassland soil showed no priming effect to counterbalance biochar mineralisation (Table 4). This is in contrast with many other studies and the results of laboratory as well as field experiments with surface soil, showing that pyrogenic C generally has some effect on SOM decomposition. Fire-derived pyrogenic C was found to increase SOM decomposition (Wardle et al. 2008; Hamer et al. 2004; Nocentini et al. 2010) whereas biochar induced a negative priming effect (Naisse et al. 2014; Ventura et al. 2014). Biochar priming effects induced on SOM were found to be dependent on pyrolysis temperature (Luo et al. 2011), which could explain these contrasting results. Indeed, the 13C-labelled char produced at 550 °C may be richer in soluble compounds compared to gasification char produced at 1200 °C used in the other experiments. Moreover, in the present experiment, we used soil below the surface horizon with lower contribution of fresh particulate organic matter. The microbial community structure present in these horizons may be very different from those found in topsoil horizons (Eilers et al. 2012) and better adapted to the degradation of rather stable organic matter compounds. Therefore, the observed response to biochar addition could also be explained by specific characteristics of subsoil communities. However, PLFA analyses did not sustain this hypothesis as the contribution of fungi and Gram+ as well as Gram− bacteria was similar for the two soils (Table 2), and also principal component analyses with single PLFA (data not shown) did not show significant differences between the two soils. Our results are in agreement with a study by Bruun and EL-Zehery (2012), who found no effect of biochar produced at 450 °C on SOM mineralisation in the top 10 cm of a Luvisol. Therefore, the response of SOM mineralisation on biochar addition may depend not only on biochar characteristics but also on soil parameters.
4 Conclusions
We assessed the effect of biochar versus plant material addition below the A horizon (subsoil) on C mineralisation potential of two mountain soils under different land use. Our results indicated differences in native C mineralisation between the two subsoils with and without substrate addition. Much higher mineralisation of plant material as compared to biochar indicated that subsoil microbial biomass is not particularly adapted to the degradation of stable aromatic material. Mineralisation of added substrates was slightly but significantly different in the two subsoils. The priming effect on native SOM was dependent on the type of material added and also subsoil type specific. Biochar addition induced a small positive priming effects or a negative priming effect that was outbalanced by biochar degradation. Priming effects could depend on subsoils’ C content, sampling depth and/or contribution of stabilised SOM compounds more than microbial biomass characteristics. Therefore, our results suggest that soil parameters need to be taken into account when evaluating the impact of biochar addition on subsoil carbon.
References
Alexis MS, Rasse DP, Knicker H, Anquetil C, Rumpel C (2012) Evolution of soil organic matter after prescribed fire: a 20-year chronosequence. Geoderma 189–190:98–107
Blume E, Bischoff M, Reichert JM, Moorman T, Konopka A, and Turco RF (2002) Surface and subsurface microbial biomass, community structure and metabolic activity as a function of soil depth and season. Appl Soil Ecol 20:171–181
Brodowski S, Amelung W, Haumaier L, Zech W (2007) Black carbon contribution to stable humus in German arable soils. Geoderma 139:220–228
Bruun S EL-Zehery T (2012) Biochar effect on the mineralization of soil organic matter. Pesq Agrop Brasileira 47:665–671
Budge K, Leifeld J, Hiltbrunner E, Fuhrer J (2011) Alpine grassland soils contain large proportion of labile carbon but indicate long turnover times. Biogeosci 8:1911–1923
Cely P, Tarquis AM, Paz-Ferreiro J, Méndez A, Gascó G (2014) Factors driving the carbon mineralization priming effect in a sandy loam soil amended with different types of biochar. Solid Earth 5:585–594
Don A, Rodenbeck C, Gleixner G (2013) Unexpected control of soil carbon turnover by soil carbon concentration. Environ Chem Lett 11:407–413
Eilers KG, Debenport S, Anderson S, Fierer N (2012) Digging deeper to find unique microbial communities: the strong effect of depth on the structure of bacterial and archaeal communities in soil. Soil Biol Biochem 50:58–65
Fabbri D, Torri C, Spokas KA (2012) Analytical pyrolysis of synthetic chars derived from biomass with potential agronomic application (biochar). Relationships with impacts on microbial carbon dioxide production. J Anal Appl Pyrol 93:77–84
Fang Y, Singh B, Singh BP, Krull E, (2014) Biochar carbon stability in four contrasting soils. Eur Soil Sci 65:60–71
Farrell M, Kuhn TK, Macdonald LM, Maddern TM, Murphy DV, Hall PA, Singh BP, Baumann K, Krull ES, Baldock JA (2013) Microbial utilisation of biochar-derived carbon. Sci Tot Environ 465:288–297
Fontaine S, Barot S, Barré P, Bdioui N, Mary B, Rumpel C (2007) Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450:277–281
Frostegard A, Baath E, Tunlid A (1993) Shifts in the structure of soil microbial communities in limed forest as revealed by phospholipid fatty-acid analysis. Soil Biol Biochem 25:723–730
Hamer U, Marschner B, Brodowski S, Amelung W (2004) Interactive priming of black carbon and glucose mineralisation. Org Geochem 35:823–830
Jaffé R, Ding Y, Niggemann J, Vähätalo AV, Stubbins A, Spencer RG, Campbell J, Dittmar T (2013) Global charcoal mobilization from soils via dissolution and riverine transport to the oceans. Science 340:345–347
Jones DL, Murphy DV, Khalid M, Ahmad W, Edwards-Jones G, DeLuca TH (2011) Short-term biochar-induced increase in soil CO2 release is both biotically and abiotically mediated. Soil Biol Biochem 43:1723–1731
Knicker H (2011) Pyrogenic organic matter in soil: its origin and occurrence, its chemistry and survival in soil environments. Q Int 243:251–263
Kuzyakov Y, Subbotina I, Bogomolova I, Xu XL (2009) Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol Biochem 41:210–219
Luo Y, Durenkamp M, De Nobili M, Lin Q, Brookes PC (2011) Short term soil priming effects and the mineralisation of biochar following its incorporation to soils of different pH. Soil Biol Biochem 43:2304–2314
Naisse C, Girardin C, Lefèvre R, Pozzi A, Maas R, Stark A, Rumpel C (2014) Effect of physical weathering on the carbon sequestration potential of biochars and hydrochars in soil. Glob Chang Biol Bioeng. doi:10.1111/gcbb.12158
Nocentini C, Guenet B, Di Mattia E, Certini G, Bardoux G, Rumpel C (2010) Charcoal mineralisation potential of microbial inocula from burned and unburned forest soil with and without substrate addition. Soil Biol Biochem 42:1472–1478
Rumpel C (2011) Carbon storage and organic matter dynamics in grassland soils. In: Lemaire G, Hodgson J, Chabbi A (eds) Grassland Productivity and Ecosystem Services. CAB International, pp 65–72
Rumpel C, Kögel-Knabner I (2011) Deep soil organic matter—a key but poorly understood component of terrestrial C cycle. Plant Soil 338:143–158
Rumpel C, Ba A, Darboux F, Chaplot V, Planchon O (2009) Erosion budget of pyrogenic carbon at meter scale and process selectivity. Geoderma 154:131–137
Singh BP, Cowie AL (2014) Long-term influence of biochar on native organic carbon mineralisation in a low-carbon clayey soil. Sci Rep 4:3687. doi:10.1038/srep03687
Singh BP, Cowie AL, Smernik RJ (2012) Biochar Carbon Stability in a Clayey Soil As a Function of Feedstock and Pyrolysis Temperature. Environ Sci Technol 46:11770–11778
Smith JL, Collins HP, Bailey VL (2010) The effect of young biochar on soil respiration. Soil Biol Biochem 42:2345–2347
Spokas KA, Novak JM, Stewart CE, Cantrell KB, Uchimiya M, DuSaire MG, Ro KS (2011) Qualitative analysis of volatile organic compounds on biochar. Chemosphere 85:869–882
Stone MM, Plante AF, (2014) Changes in phosphatase kinetics with soil depth across a variable tropical landscape. Soil Biology and Biochemistry 71:61–67
Stuiver M, Polach HA (1977) Discussion: reporting of 14C data. Radiocarbon 19:355–363
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass-C. Soil Biol Biochem 19:703–707.
Ventura M, Alberti G, Viger M, Jenkins J, Girardin C, Baronti S, Zaldei A, Taylor G, Rumpel C, Miglietta F, Tonon G (2014) Biochar mineralization and priming effect on SOM decomposition in two European short rotation coppices. Glob Chang Biol Bioeng. doi:10.1111/gcbb.12219
Wardle DA, Nilsson MC, Zackerisson O (2008) Fire-derived charcoal causes loss of forest humus. Science 320:629
Wetterstedt MJA, Persson T, Agren GI (2010) Temperature sensitivity and substrate quality in soil organic decomposition: results of an incubation study with three substrates. Glob Chang Biol 16:1806–1819
Zimmerman AR, Gao B, Ahn MY (2011) Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol Biochem 43:1169–1179
Acknowledgments
Our research was funded by the European Community under the framework of the EuroChar project (FP7-ENV-478 2010ID-265179). We thank the Observatoire Homme-Milieux Pyrenees Haut-Vicdessos CNRS-INEE for access to the sampling sites and the Poznan radiocarbon laboratory for the 14C activity analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Frank G. A. Verheijen
Rights and permissions
About this article
Cite this article
Naisse, C., Girardin, C., Davasse, B. et al. Effect of biochar addition on C mineralisation and soil organic matter priming in two subsoil horizons. J Soils Sediments 15, 825–832 (2015). https://doi.org/10.1007/s11368-014-1002-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-014-1002-5