Abstract
The COVID-19 pandemic posed unprecedented challenges to healthcare systems worldwide, particularly in managing critically ill patients requiring mechanical ventilation early in the pandemic. Surging patient volumes strained hospital resources and complicated the implementation of standard-of-care intensive care unit (ICU) practices, including sedation management. The objective of this study was to evaluate the impact of an evidence-based ICU sedation bundle during the early COVID-19 pandemic. The bundle was designed by a multi-disciplinary collaborative to reinforce best clinical practices related to ICU sedation. The bundle was implemented prospectively with retrospective analysis of electronic medical record data. The setting was the ICUs of a single-center tertiary hospital. The patients were the ICU patients requiring mechanical ventilation for confirmed COVID-19 between March and June 2020. A learning health collaborative developed a sedation bundle encouraging goal-directed sedation and use of adjunctive strategies to avoid excessive sedative administration. Implementation strategies included structured in-service training, audit and feedback, and continuous improvement. Sedative utilization and clinical outcomes were compared between patients admitted before and after the sedation bundle implementation. Quasi-experimental interrupted time-series analyses of pre and post intervention sedative utilization, hospital length of stay, and number of days free of delirium, coma, or death in 21 days (as a quantitative measure of encephalopathy burden). The analysis used the time duration between start of the COVID-19 wave and ICU admission to identify a “breakpoint” indicating a change in observed trends. A total of 183 patients (age 59.0 ± 15.9 years) were included, with 83 (45%) admitted before the intervention began. Benzodiazepine utilization increased for patients admitted after the bundle implementation, while agents intended to reduce benzodiazepine use showed no greater utilization. No “breakpoint” was identified to suggest the bundle impacted any endpoint measure. However, increasing time between COVID-19 wave start and ICU admission was associated with fewer delirium, coma, and death-free days (β = − 0.044 [95% CI − 0.085, − 0.003] days/wave day); more days of benzodiazepine infusion (β = 0.056 [95% CI 0.025, 0.088] days/wave day); and a higher maximum benzodiazepine infusion rate (β = 0.079 [95% CI 0.037, 0.120] mg/h/wave day). The evidence-based practice bundle did not significantly alter sedation utilization patterns during the first COVID-19 wave. Sedation practices deteriorated and encephalopathy burden increased over time, highlighting that strategies to reinforce clinical practices may be hindered under conditions of extreme healthcare system strain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronavirus Disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, is a multi-organ system disease that may lead to critical illness primarily through effects on the respiratory system resulting in severe pneumonia, respiratory failure, and acute respiratory distress syndrome (ARDS). Early in the COVID-19 pandemic, hospitals around the world faced unprecedented challenges as they grappled with a sudden and massive influx of critically ill patients, many requiring mechanical ventilation. For instance, in the early months of the pandemic, some of the most severely affected hospitals experienced intensive care unit utilization (ICU) rates approaching 300% of pre-pandemic capacity and daily utilization rates exceeding 100% capacity for months on end [1]. Overall, in the early months of the pandemic, 40% of ICU patients were admitted to surge capacity beds, representing the conversion of other hospital areas into makeshift ICUs [2].
This sudden increase in critically ill patients placed immense strain on healthcare systems, leading to shortages of ICU beds, ventilators, and essential medications [2]. In the US, over 70% of hospitals had to buy or borrow additional mechanical ventilators while 30% used noninvasive ventilators or anesthesia machines to provide mechanical ventilation [3]. Healthcare system strain was further compounded by staffing shortages, supply chain disruptions, and the need to rapidly adapt to evolving treatment protocols [4]. In an international survey, 85% of ICUs employed non-ICU nurses and 58% employed non-ICU physicians to address staffing shortages [2].
In the ICU, sedation is a critical component of care for patients receiving mechanical ventilation. Adequate sedation is essential for facilitating the use of mechanical ventilation and other life-saving interventions. Sedation helps ensure patient comfort, reduces patient anxiety and agitation, prevents self-harm by reducing the risk of self-extubating during invasive mechanical ventilation, and promotes ventilatory synchrony to reduce the risk of pressure- and volume-induced lung injury [5,6,7]. However, achieving the right balance of sedation is complex. Too little sedation can lead to agitation, anxiety, and discomfort while excessive sedation may contribute to longer mechanical ventilation, prolonged ICU stays, and increased delirium and encephalopathy (the spectrum of global brain dysfunction from subsyndromal delirium to delirium to coma) [5].
Early in the COVID-19 pandemic, there were numerous reports of critically ill COVID-19 patients requiring unusually high doses of sedatives to achieve adequate sedation [8]. This was partly due to the severe and often refractory agitation observed in these patients, which standard ICU sedation approaches struggled to manage. Our hospital experienced similar challenges, with many patients demonstrating severe agitation refractory to our standard ICU sedation protocols. This situation raised significant concerns because of the increased morbidity associated with excessive sedation. In addition to compounding institutional strain through longer hospital stays and increased resource utilization, excessive sedation is strongly associated with increased occurrence of delirium and encephalopathy [9]. Delirium is associated with increased mortality and greater risk for subsequent institutionalization and progression to dementia in the elderly [10]. ICU delirium is also strongly associated with clinically significant long-term global cognitive dysfunction, irrespective of patient age [11]. Moreover, sedative-associated ICU delirium appears to be as detrimental to cognition as hypoxia- or sepsis-associated delirium and more detrimental than delirium associated with metabolic causes [12].
The strain experienced by healthcare systems during the COVID-19 pandemic highlights a need for greater system resiliency in preparation for potential future crises. At individual healthcare institutions, this preparation may include identifying means to reinforce best clinical practices when confronted with constraints on institutional resources. In intensive care, the ICU liberation ABCDEF Bundle is one of the core evidence-based best practices. The ABCDEF Bundle addresses key aspects of care, including sedation hold (awakening) trials, spontaneous breathing trials, choice of analgesic and sedative agents, delirium monitoring and management, early mobilization, and family engagement in care. Greater compliance with the ABCDEF bundle has been demonstrated to reduce hospital mortality and days of delirium or coma [13]. However, during the COVID-19 pandemic, the implementation of the ABCDEF Bundle faced significant challenges from overwhelming numbers of critically ill patients, severe levels of patient agitation, staffing shortages, and resource limitations. Data suggest that adherence to best practices such as the ABCDEF bundle suffered during the COVID-19 pandemic and remained suppressed even after the height of the crisis [14, 15]. This sustained decline potentially stalled years of efforts to improve healthcare quality; therefore, proactively developing robust strategies to enhancing adherence to best practices in the face of public health crises might have long-lasting implications. To mitigate the negative impacts of institutional strain on patient outcomes during the early pandemic, our institution sought to reinforce ABCDEF bundle adherence by protecting previous high-fidelity goal-directed sedation administration practices with an emphasis on avoiding excessive sedation and mitigating sedation-related delirium and encephalopathy, which could compound institutional strain by lengthening ICU stays while worsening patient outcomes [5, 16]. Committed to a learning health system, we convened a learning health collaborative to develop, implement, and assess an evidence-based COVID-19 ICU sedation bundle [17, 18]. Based on early observations of critically ill COVID-19 patients requiring extreme medication dosages to achieve adequate sedation and the challenges posed by severe agitation, we hypothesized that the implementation of an evidence-based ICU sedation bundle would improve sedation practices and clinical outcomes during the first wave of the COVID-19 pandemic. Specifically, we hypothesized that the sedation bundle implementation would reduce excess sedative use, shorten ICU stays, and decrease the incidence of delirium and encephalopathy. To evaluate this hypothesis, we employed a prospective implementation of the sedation bundle with a retrospective analysis of electronic medical record data from ICU patients requiring mechanical ventilation for confirmed COVID-19 between March and June 2020. The methods included structured in-service training, audit and feedback, and continuous improvement strategies to support the bundle’s implementation. The study compared sedative utilization and clinical outcomes, such as hospital length of stay and the number of days free of delirium, coma, or death, between patients admitted before and after the bundle implementation. Additionally, quasi-experimental interrupted time-series analyses were conducted to assess the impact of the sedation bundle and examine the relationship between the duration from the COVID-19 wave onset to ICU admission (as an indicator of accumulating institutional strain) and sedation practices and clinical outcomes. Moreover, we investigated whether these relationships differed between older adults (65 years and older) and younger patients. Using these observations, we provide insights into the impact of institutional strain on sedation practices and patient outcomes during a public health crisis.
Materials and methods
We designed a COVID-19 sedation bundle through a learning health collaborative, implemented the bundle through structured in-service training, and conducted an interrupted time-series analysis. Key bundle objectives were [1] consistent utilization of Richmond Agitation and Sedation Scale (RASS) goals to titrate sedation, [2] a preference for providing as-needed RASS-directed opioid or benzodiazepine boluses over increasing continuous infusion rates, and [3] minimization of benzodiazepine infusions in favor of alternative sedative agents and adjunctive strategies (for example, propofol, ketamine, dexmedetomidine, and anti-agitation agents such as antipsychotics). Key clinical outcomes included days of and maximum rate of benzodiazepine infusion, hospital length of stay, and days free of coma, delirium, and death within 21 days. Days free of delirium, coma, and death were used as a quantitative measure of encephalopathy burden with more free days representing a lower encephalopathy burden.
Patients and data collection
We included all patients ≥ 18 years of age with admission date to our institution between March 5th 2020 and June 10th 2020 with a laboratory-confirmed diagnosis of COVID-19 undergoing invasive mechanical ventilation. These dates encompass the first wave of the COVID-19 pandemic at our institution in Cook County, IL [19]. We excluded patients who received mechanical ventilation only for surgical procedures or were intubated for another indication and subsequently developed COVID-19. Patients transferred from outside facilities were included unless they received ≥ 48 h of intensive care at the outside facility, including mechanical ventilation in emergency departments. Patients were divided into before and after sedation bundle implementation (April 12th 2020). Demographic, comorbidity, medication administration, and clinical endpoint data were obtained from the electronic medical record or by manual chart review when variables were not amenable to electronic query.
We collected data on sedative, analgesic, and anti-agitation medication administration during the initial period of mechanical ventilation up to day 21. As-needed (pro re nata, “PRN”) administrations of medications were quantified as total dose administered during the mechanical ventilation period. Since infusion rates could be adjusted frequently, we quantified infusion medication administration as number of days of infusion, the maximum infusion rate, and the sum of daily peak infusion rates over the course of mechanical ventilation up to 21 days. Opioid medication doses were converted to morphine equivalents and benzodiazepine medication doses were converted to lorazepam equivalents [20, 21]. We quantified days with delirium or coma up to 21 days with serial, protocolized evaluations of Confusion Assessment Method-ICU (CAM-ICU) and Richmond Agitation and Sedation Scale (RASS) scores of − 4 or − 5, respectively [12, 22, 23]. We assumed patients discharged before 21 days remained alive and free of delirium and coma.
This retrospective study was approved by the Northwestern University institutional review board with waiver of patient consent (STU00212627: “COVID-19 neuro manifestations” approved 5/15/2020) and was conduct in accordance with institutional ethical standards for human experimentation and the Helsinki Declaration of 1975.
Bundle development and implementation
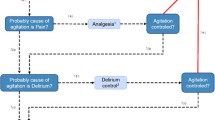
A learning health collaborative consisting of pulmonary intensivists, neurologic intensivists, psychiatrists, intensive care pharmacists, and intensive care nurses developed a sedation management protocol. Protocol details are available in Appendix 1 and summarized in Fig. 1. The protocol was developed by Delphi methodology with committee facilitators (EML, KG) developing the initial protocol draft based on Society of Critical Care Medicine clinical practice guidelines [5] supplemented with additional references, as noted in Appendix 1 [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. Overall, there was a preference for sedatives with short durations of action and low tendency for accumulation to facilitate shorter mechanical ventilation times in the face of high ICU bed demand. Committee consensus was reached after four rounds of revision.
The final sedation bundle was implemented using previously successful strategies used in acute stroke quality metrics at our institution and other implementation strategies, including audit and feedback [17, 18, 40]. Bedside nurses were trained in the protocol and underlying evidence over a 10-day period using a series of “in-service education sessions” facilitated by nursing education coordinators. Educational materials and laminated copies of the full protocol were available for reference in each patient room. Laminated summary pocket cards were provided to each nursing, clinician, and pharmacist team member. Laminated protocol summaries were posted to both sides of patients’ ICU room doors (Fig. 1). Sedation practices and protocol adherence were evaluated daily on multi-disciplinary ICU rounds using a rounding checklist with ICU team pharmacists functioning as quality improvement leaders. Feedback on sedation utilization was provided to staff during daily ICU and change of shift rounds.
The learning health collaborative solicitated feedback from attending intensivists and intensive care pharmacists twice after bundle implementation to consider whether modifications was needed, and none was identified.
Statistical analysis
We used descriptive statistics and t-test, chi square test, and Wilcoxon rank sum test, as appropriate, to compare characteristics, sedative/analgesic utilization patterns, and clinical outcomes between patients admitted to the ICU before versus after implementation of the bundle. Next, we performed interrupted time-series analyses of dependent variables sedative/analgesic utilization, hospital length of stay, and number of days free of delirium, coma, or death in 21 days against the independent variable time duration between start of the COVID-19 wave (March 5th 2020 00:00:01) and ICU admission. We prespecified adjustment for ICU admission acute physiology score (APS) and need for therapeutic paralysis during hospitalization (other than for intubation) as indicators of initial and subsequent COVID-19 disease severity and included adjustment for patient sex. Of note, male sex has been associated with greater risk for hospital admission and critical illness from COVID-19 during the first wave of the COVID-19 pandemic [41]. Interrupted time-series analysis is the strongest, quasi-experimental design to evaluate the effects of time-delimited interventions on level (model intercept) and trend (model slope) of an outcome [42]. Impactful interventions yield a statistically significant change in level or trend, representing a “breakpoint” in the observed outcome. We used the R “segmented” package (R Foundation for Statistical Computing, version 4.2.1) to identify any breakpoints and to develop corresponding segmented linear regression models. To account for the possibility that factors other than our intervention could impact outcomes, we allowed the package to identify breakpoints at any time during the study. If no significant breakpoint was identified, then the data was modeled using linear regression without segmentation. Davies test was used to identify any statistically significant breakpoints. In instances where models suggested a relationship between time from wave onset and sedation practices or clinical outcome in the entire cohort, we repeated model development dichotomized by patient age as 65 years and older versus younger than 65 years. We considered p ≤ 0.05 to be statistically significant.
Results
We included 183 patients (age 59.0 ± 15.9 years, 62.8% male) admitted to ICU during the study period with 83 (45%) admitted before the sedation bundle implementation. Patient characteristics are summarized in Supplemental eTable 1. Before and After Bundle patients were similar. Before patients were less often Hispanic, utilized prone positioning less often, and tended towards less comorbid diabetes mellitus. Mean daily ventilator utilization from March through June 2020 was 63.1 ± 8.4 ventilators/day (minimum 43, maximum 79). While our institution did not track ventilator utilization prior to March 2020, the study period utilization was significantly increased (p < 0.001) compared to the non-wave period of March through June 2022 (mean 41.4 ± 3.8 ventilators/day, minimum 34, maximum 45).
Group comparison
Supplemental eTable 2 summarizes the utilization of sedative, analgesic, and anti-agitation medications dichotomized relative to bundle initiation date. Generally, sedative utilization was greater for patients admitted after bundle initiation, and benzodiazepine and opioid-sparing agents, such as ketamine and neuroleptics, showed no greater utilization. In fact, dexmedetomidine and quetiapine, as benzodiazepine-sparing agents, were less frequently used in patients admitted after bundle initiation. Of note, the amount of infused and as-needed “PRN” opioids and benzodiazepines and the number of days of benzodiazepine infusion were greater in the period after bundle implementation.
Table 1 summarizes key clinical outcomes dichotomized relative to bundle initiation date. Patients admitted after bundle initiation experienced greater hospital mortality, longer hospital length of stay, and tended towards longer ICU length of stay. While days of delirium did not differ, there was a non-significant tendency towards more days in coma and fewer delirium, coma, and death-free days in those admitted after bundle initiation.
Interrupted time-series analysis
We examined delirium, coma, and death-free days, hospital length of stay, opioid infusion measurements, as-needed “PRN” opioid utilization, benzodiazepine infusion measurements, and as-needed “PRN” benzodiazepine utilization for evidence of a “breakpoint” in the duration between the start of the COVID-19 wave and the date of ICU admission. Identification of a significant breakpoint would suggest our bundle (or concurrent change in another factor) affected a change in the trend of observed data. In no instance was a significant breakpoint identified (all Davies test > 0.30). Lack of a breakpoint suggests the duration passed within the COVID-19 wave could be associated with clinical outcome or medication utilization patterns, which could contribute to outcome differences between After and Before Bundle groups. In fact, linear regression models adjusted for APS, need for paralysis, and patient sex (Table 2) showed increasing time between COVID-19 wave start and ICU admission was associated with more days in coma (β = 0.042 [95% CI 0.008, 0.075] comatose days/wave day, p = 0.015); fewer delirium, coma, and death-free days (i.e., greater encephalopathy burden, β = − 0.044 [95% CI − 0.085, − 0.003] free days/wave day, p = 0.036); more days of benzodiazepine infusion (β = 0.056 [95% CI 0.025, 0.088] benzodiazepine days/wave day, p < 0.001), greater maximum benzodiazepine infusion rate (β = 0.079 [95% CI 0.037, 0.120] mg/h/wave day, p < 0.001), and greater benzodiazepine sum of daily peak infusion rates (β = 0.446 [95% CI 0.085, 0.807] mg/h/wave day, p = 0.016). Both paralytic use and higher APS were associated with more days in coma and fewer delirium, coma, and death-free days (paralytic: β = − 5.44 95% CI [− 7.29, − 3.59] free days, APS: β = − 0.068 95% CI [− 0.105, − 0.030] free days/APS point); however, paralytic use, but not APS score, was associated with more days of benzodiazepine and opioid infusion and greater benzodiazepine and opioid maximum infusion rate and sum of daily peak infusion rates. Male sex was associated with higher maximum opioid infusion rates (β = 5.73 95% CI [0.280, 11.2] mg morphine equivalents/h) and a non-significant tendency (0.05 < p < 0.1) towards more coma days, more benzodiazepine infusion days, higher maximum benzodiazepine infusion rate, and higher opioid sum of daily peak infusion rates.
Models dichotomized by patient age
There were 81 (44.3%) patients 65 years of age or older and 102 (55.7%) younger than 65 years of age. Hospital mortality was numerically greater in older (21%) than younger (17.6%) patients but not statistically different (p = 0.70), and there was no significant difference in the number of death days in the first 21 days of hospitalization (p = 0.32). In the entire cohort, younger patients had numerically more days free of delirium, coma, or death (median [IQR]: 9.5 [2, 16] versus 7 [2, 13], p = 0.16) and similar number of days in coma (median [IQR]: 8.5 [3, 13] versus 7 [4, 14], p = 0.69) as older patients. However, in the entire cohort, younger patients had more days of benzodiazepine infusion (median [IQR]: 5 [0, 11] versus 0 [0, 5], p < 0.001), greater maximum benzodiazepine infusion rate (median [IQR]: 0 [0, 10.75] versus 0 [0, 5], p < 0.001), and greater benzodiazepine sum of daily peak infusion rate (median [IQR]: 22.63 [0, 74.0] versus 0 [0, 16.5], p < 0.001). Models dichotomized by age (Fig. 2) demonstrated that, with increasing time between COVID-19 wave start and ICU admission, younger patients had a significant decrease in delirium, coma, and death-free days (i.e., increasing encephalopathy burden, β = − 0.065 [95% CI − 0.121, − 0.009] free days/wave day, p = 0.029) driven by increase in coma days (β = 0.062 [95% CI 0.018, 0.105] coma days/wave day, p = 0.006). While older patients had numerically fewer delirium, coma, and death-free days and more coma days, the association with increasing time between COVID-19 wave start and ICU admission was not statistically significant in older patients (p > 0.3). Both older and younger patients had an increase in benzodiazepine exposure with increasing time between COVID-19 wave start and ICU admission, but younger patients had a greater increase in maximum benzodiazepine infusion rate than older patients during the COVID-19 wave (β = 0.102 [95% CI 0.034, 0.170] versus β = 0.030 [95% CI − 0.002, 0.063] mg/h/wave day).
Discussion
In our quasi-experimental study of sedation bundle implementation to minimize benzodiazepine use, mitigate sedation practice deterioration, and optimize sedation-related clinical outcomes during the first wave of COVID-19, we unexpectedly found patients admitted after bundle implementation experienced greater benzodiazepine utilization and no change in benzodiazepine-sparing adjunct utilization. Clinical outcomes showed longer hospital length of stay, and a tendency towards fewer delirium, coma, and death-free days (driven primarily by coma days) after implementation. There are multiple hypotheses for these findings including ineffective bundle implementation related to resource constraints, task shifting (i.e., inclusion of less specialized workers in the ICU workflow due to staffing shortages), or poor fit to the specific clinical scenario (e.g., benzodiazepine-sparing adjuncts may be less effective in COVID-19).
We utilized strategies of structured in-service education and continuous practice evaluation and audit and feedback to encourage adherence and improvement in sedation practices. Strategies were similar to those successfully implemented at our institution to improve acute stroke practices and by others to improve ICU practices before the COVID-19 pandemic [17, 18, 40, 43, 44]. Importantly, interrupted time-series analysis showed no evidence of a breakpoint in benzodiazepine or opioid utilization measurements, hospital length of stay, or delirium, coma, and death-free days suggesting the bundle did not impact the key outcomes targeted. Rather, models showed time from the start of the COVID-19 wave was significantly associated with more days of benzodiazepine infusion, higher benzodiazepine infusion rates, more days in coma, and fewer days free of delirium, coma, and death (i.e., greater encephalopathy burden). Over time these changes were substantial. For example, a patient admitted on the last day of the wave would be expected to experience 4 days more coma and 5.4 more benzodiazepine infusion days than a patient admitted on the first day. Similarly, ICU admission on the last day rather than first day of the wave impacted days free of delirium, coma, and death nearly as much as developing COVID-19 requiring mechanical ventilation with therapeutic paralysis (4.3 and 5.4 fewer days free, respectively). Moreover, baseline sedation requirements may have been compounded by the positive association between time of admission in the COVID-19 wave and benzodiazepine utilization.
Perhaps unexpectedly, the decrease in delirium, coma, and death-free days and increase in coma days over the course of the COVID-19 wave appeared to be driven primarily by younger patients rather than those 65 years of age and older. This finding may be related to the corresponding greater increase in maximum benzodiazepine infusion rate in younger patients through the course of the COVID-19 wave. Why younger patients might have been more impacted is unclear; possibilities include greater severity of agitation in younger patients driven partly by a robust inflammatory response unique to COVID-19 [45]. This effect may have been less prominent in older patients secondary to impaired early inflammatory responses and immunosenescence, with less severe physiologic derangements that were subsequently targeted by sedation for ventilator or nursing care compliance [46].
The increase in encephalopathy burden that we observed over the course of the first COVID-19 wave likely translated to worse patient outcomes. Mediation analysis from a propensity matched cohort study of mechanically ventilated COVID-19 acute respiratory distress syndrome (ARDS) patients versus non-COVID-19 mechanically ventilated ARDS patients suggested that 58% of the effect of COVID-19 on in-hospital mortality was mediated through the indirect effect of coma, and 52% of the effect of COVID-19 diagnosis on coma was mediated through the indirect effect of sedative dose [41]. Therefore, coma is likely a major contributor to COVID-19 patient mortality, but while important, sedative agent doses alone do not completely explain the association. In addition, we previously demonstrated that hospitalized COVID-19 patients who experienced encephalopathy had worse functional outcome at discharge [23]. Moreover, pre-pandemic data demonstrates that an increasing burden of ICU delirium is associated with greater long-term global cognitive dysfunction, irrespective of patient age, and that the phenotype of sedation-associated delirium contributes to cognitive dysfunction as much as sepsis or hypoxia-associated delirium [11, 12]. In the case of benzodiazepine-related encephalopathy in the ICU, multiple factors may interact to contribute to brain injury and subsequent cognitive dysfunction. Benzodiazepine administration may lead to reduction in cerebral blood flow and increased cerebrovascular resistance [47, 48]. Critically ill COVID-19 patients may already have compromised cerebral perfusion and impaired cerebral oxygenation due to underlying systemic conditions including pneumonia, systemic hypotension, and cardiac dysfunction as well as possible concurrent impairment of cerebral autoregulation [49, 50]. Positive pressure mechanical ventilation, prone ventilation position, and extracorporeal membrane oxygenation also have the potential to adversely impact cerebral perfusion and cerebral autoregulation [51,52,53,54,55]. Therefore, excessive benzodiazepine usage may exacerbate factors contributing to the risk of brain injury from cerebral hypoperfusion and hypoxia. Additionally, ICU patients often receive multiple medications that could have additive or synergistic effects with benzodiazepines to alter neurotransmitter levels and depress CNS functions. Metabolic disturbances, particularly from renal or hepatic dysfunction, the robust systemic inflammation seen in COVID-19, or interactions between the host and the SARS-CoV-2 virus may further exacerbate encephalopathy [22, 54, 56]. Therefore, benzodiazepine-related encephalopathy in COVID-19 patients is likely part of a multifactorial pathophysiologic process influenced by critical illness and the intensive care environment.
The sedation protocol we implemented in this study was based on pre-pandemic literature and guideline recommendations intended to inform the general management of the critically ill patient population. The pre-pandemic literature suggests that propofol and opioid infusions for procedural moderate-to-deep sedation and propofol or dexmedetomidine with opioid infusions for ICU sedation are associated with a low risk of harm or serious adverse events [5, 57]. Moreover, there is evidence in the COVID-19 literature that our general practice of preferring agents including propofol, dexmedetomidine, and ketamine over benzodiazepines for sedation is justified. Analysis of data from 35 hospitals in the Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study (VIRUS) COVID-19 Registry found that ICU sedation practices for COVID-19 patients are characterized by two clusters [58]. The first cluster resembles our practice of generally administering opioids and propofol with less frequent use of additional sedatives (including benzodiazepines, alpha-agonists like dexmedetomidine, and ketamine) while the second cluster predominantly uses opioids and benzodiazepines without other sedatives. Admission to a hospital following the first sedation cluster practice was associated with shorter duration of mechanical ventilation, shorter ICU and hospital length of stay, and lower mortality compared to the benzodiazepine predominant cluster [58]. However, therapies in the ICU, including sedation, should account for individual patient comorbidities and risks. For example, propofol and dexmedetomidine, as well as larger doses of benzodiazepines, can depress cardiac output and cause or contribute to hypotension [59]. Such adverse effects might be poorly tolerated by COVID-19 patients with pre-existing cardiac or vascular disease or in the setting of frailty [60], and in these patients, the clinician might consider the use of sedative agents and adjuncts, such as ketamine, characterized by more favorable hemodynamic profiles [59]. Since most studies comparing ICU sedation strategies have focused on delirium rates, duration of intubation, ICU or hospital length of stay, and mortality with little data on functional or cognitive outcome or comorbidity-relevant adverse effects [5], the intensivist needs to apply clinical judgement when individualizing sedation strategies in an attempt to minimize morbidity.
In addition, our sedation protocol was developed and implemented early in the pandemic and was not tailored to our current knowledge of COVID-19 or COVID-19-specific therapies. The multi-system nature of COVID-19 and the predominant role of inflammation has since become apparent [60,61,62]. It appears likely that SARS-CoV-2 has tropism for a wide variety of cells beyond the lung, including the endothelium and cells of the central nervous system [60,61,62]. Beyond the potential for direct viral infection and subsequent dysfunction of multiple organs, the robust systemic inflammation seen in critically ill COVID-19 patients can lead to cytokine and free radical–mediated cellular dysfunction and apoptosis as well as organ injury through macro- and microvascular thromboses [61, 62]. Dysregulation of the bidirectional interactions between the nervous system and cardiovascular system as a result of viral infection and/or inflammation-mediated injury may be of particular relevance to sedation management strategies and critical illness encephalopathy. Disruption of sympathetic and parasympathetic outflow, as a consequence of nervous system dysfunction, could contribute to cardiovascular injury that in turn exacerbates neuronal injury through hypoperfusion and thrombosis, perpetuating a vicious cycle [62]. It is possible that maladaptive sedation strategies could compound such a vicious cycle both through adverse hemodynamic effects and by exacerbating nervous system dysfunction in the encephalopathic patient. Moreover, one might expect patients with chronic cardiovascular or neurologic diseases to be more susceptible to this vicious cycle, particularly if advanced age or frailty has contributed to vascular dysfunction through premorbid endothelial cell senescence [60, 62]. Since implementing our sedation protocol, specific therapeutic strategies have become available for the treatment of COVID-19 in the ICU. It is possible that therapies including the anti-viral remdesivir or immunotherapies such as dexamethasone, baricitinib, and tocilizumab might modulate the underlying processes contributing to severe agitation and play a role in addressing the high sedation needs seen in the early COVID-19 waves; however, the possible impact of these treatments on sedation requirements and neurologic morbidity is unproven since these endpoints were not investigated in clinical trials [61].
Other than assessing for a breakpoint corresponding to protocol initiation, our study was not designed to identify specific factors responsible for observed patterns. Nonetheless, an appealing explanation for our observations is that time from start of the COVID-19 wave represents accumulating strain on the healthcare system which negatively impacted effective implementation of the sedation bundle. For example, the increase in coma days over time could reflect increasing difficulty in implementing RASS goal-directed sedation administration. This hypothesis is consistent with our ventilator utilization during the first COVID-19 wave being over 50% greater than the seasonal non-wave pandemic utilization. This hypothesis is also consistent with data from the Centers for Disease Control showing increased excess deaths in the weeks following higher ICU bed occupancy during the COVID-19 pandemic [63]. Furthermore, Castagna et al. showed that overall hospital occupancy rate was an independent risk factor for COVID-19 inpatient mortality, specifically an increase of 0.7% in 30-day in-hospital mortality for each 1% increase in bed occupancy [64]. Bravata et al. found a hazard ratio for death of 1.94 for COVID-19 ICU patients when ICU demand was over 75–100% of peak caseload [65]. Similarly, Kadri et al. analyzed data from 558 U.S. hospitals and showed that nearly 1 in 4 COVID-19 deaths may have been due to caseload-related hospital strain [66]. Pre-pandemic data also suggests that hospital capacity strain is associated with increased mortality and worsened health outcomes [67]. Our system also experienced stressors during the first COVID-19 wave that are more difficult to quantify than ventilator utilization, including redeployment of surgical and outpatient providers to inpatient COVID-19 care, utilization of contract nurse staffing, enhanced infection control protocols, supply chain disruptions, exposure of staff to COVID-19, and the physical and emotional toll of long hours in a stressful work environment. The mechanisms leading to worsened clinical endpoints in the setting of hospital strain are likely multifactorial. Hospital capacity during the pandemic was frequently increased disproportionately to staffing increases, resulting in higher patient to nurse ratios and reallocation of non-ICU clinicians to ICU care roles [2, 68].
While overall hospital mortality was greater after bundle initiation, mortality before 21 days of ICU care was no different between groups. This mortality pattern may suggest contributors unrelated to the sedation bundle or institutional strain. For example, patients admitted later in the COVID-19 wave may have been more subject to the effects of healthcare disparities [69,70,71]. Alternatively, while we accounted for disease severity, changes in patients’ perceptions of the pandemic may have contributed to apprehension of coming to the hospital, leading to delay in admission that resulted in more advanced disease on presentation as the wave progressed [72, 73]. There may also have been a cumulative effect from factors that did not individually achieve significant differences, such as more external transfers and greater extracorporeal membrane oxygenation utilization.
Our study, combined with literature on the ramifications of hospital strain, underscores the importance of anticipating and preparing for the impact of hospital strain during future healthcare crises. The strategies leveraged in our bundle have previously effectuated clinical care improvements, even within our own institution [17, 18, 43]. However, the critical difference may be that prior studies did not attempt implementation during a pandemic prompting a healthcare crisis. Our study suggests that once a system reaches a critical level of strain, initiatives to reinforce best clinical practices as a response to healthcare crisis may be extremely challenging. Protecting the integrity of best clinical practices may instead require enhancing system resiliency to avoid excess strain once a crisis occurs. This impression is consistent with qualitative interviews of hospital leaders that suggest the most effective way to address hospital strain is typically felt to be through ensuring sufficient resources, in particular staffing. Nevertheless, it is common for hospitals to first attempt cost-neutral, though ineffective interventions [74].
Deterioration in best clinical practices might also have long-lasting implications for the cognitive outcomes and healthy aging of the affected patients, particularly in relation to ICU care practices. In a pre-COVID-19 pandemic cohort of ICU patients, the duration of sedative-associated delirium was found to be a potent predictor of worse global cognitive function at 3 and 12 months [12]. Moreover, pre-COVID-19 pandemic literature suggests that ICU delirium is strongly associated with clinically significant cognitive dysfunction even 12 months after critical illness and that this cognitive dysfunction manifests in patients younger than 50 years of age to a similar degree as patients older than 65 years of age [11]. Our study suggests that accumulating healthcare system stress during the first COVID-19 wave may have impacted benzodiazepine dosing and encephalopathy burden to a greater degree in younger patients; as such, if this greater ICU encephalopathy burden translates to long-term cognitive morbidity, these younger patients might experience many years of lost quality of life and a long trajectory of less healthy aging.
There are other limitations of our study to consider. While the bundle was implemented prospectively, data collection was performed retrospectively. Due to health system strain, resources were not available to develop a real-time output of adherence, sedation utilization, and other clinical measures in the ICU. Similarly, provider feedback on protocol utilization came in the form of adherence and appropriateness assessments made on daily ICU rounds without access to computer-generated summary reports on sedation practices. While we leveraged strategies that were previously successful at effectuating clinical improvement in our institution [17, 18], resource demands limited the duration of in-service training to 10 days. This highlights that institutional strain may also challenge the implementation of strategies that were successful in non-strained times. While we used a quasi-experimental interrupted time-series analysis to strengthen our level of evidence, we did not have another set of ICUs that could function as a control where no attempt at reinforcing sedation practices was made.
Conclusion
We found no indication that a bundle initiative to improve sedation administration practices implemented within the first COVID-19 wave impacted practice patterns. Rather, sedation administration practices deteriorated further, and encephalopathy burden increased as a function of the time passed in the COVID-19 wave. Previously successful strategies to reinforce best clinical practices may be hindered when healthcare systems are under increased strain during public health crises.
References
Douin DJ, Ward MJ, Lindsell CJ, et al. ICU Bed utilization during the coronavirus disease 2019 pandemic in a multistate analysis-March to June 2020. Crit Care Explor. 2021;3(3):e0361.
Greco M, De Corte T, Ercole A, et al. Clinical and organizational factors associated with mortality during the peak of first COVID-19 wave: the global UNITE-COVID study. Intensive Care Med. 2022;48(6):690–705.
Kerlin MP, Costa DK, Davis BS, et al. Actions taken by US hospitals to prepare for increased demand for intensive care during the first wave of COVID-19: a national survey. Chest. 2021;160(2):519–28.
Arabi YM, Myatra SN, Lobo SM. Surging ICU during COVID-19 pandemic: an overview. Curr Opin Crit Care. 2022;28(6):638–44.
Devlin JW, Skrobik Y, Gelinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–73.
Vinayak AG, Gehlbach B, Pohlman AS, et al. The relationship between sedative infusion requirements and permissive hypercapnia in critically ill, mechanically ventilated patients. Crit Care Med. 2006;34(6):1668–73.
Yoshida T, Fujino Y, Amato MB, et al. Fifty years of research in ARDS. Spontaneous breathing during mechanical ventilation. Risks, mechanisms, and management. Am J Respir Crit Care Med. 2017;195(8):985–992.
Flinspach AN, Booke H, Zacharowski K, et al. High sedation needs of critically ill COVID-19 ARDS patients-a monocentric observational study. PLoS ONE. 2021;16(7):e0253778.
Rasulo FA, Badenes R, Longhitano Y, et al. Excessive sedation as a risk factor for delirium: a comparison between two cohorts of ARDS critically ill patients with and without COVID-19. Life (Basel). 2022;12(12).
Witlox J, Eurelings LS, de Jonghe JF, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443–51.
Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–16.
Girard TD, Thompson JL, Pandharipande PP, et al. Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. Lancet Respir Med. 2018;6(3):213–22.
Barnes-Daly MA, Phillips G, Ely EW. Improving hospital survival and reducing brain dysfunction at seven california community hospitals: implementing PAD guidelines via the ABCDEF bundle in 6,064 patients. Crit Care Med. 2017;45(2):171–8.
Braun A, Garner O, Staggers K, et al. Effects of the COVID-19 pandemic on sedation practices and ABCDEF bundle compliance: a national survey of intensivists in the United States. Chest. 2022;161(6 supplement):A201.
Liu K, Nakamura K, Katsukawa H, et al. Implementation of the ABCDEF bundle for critically ill ICU patients during the COVID-19 pandemic: a multi-national 1-day point prevalence study. Front Med (Lausanne). 2021;8:735860.
Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med. 2019;47(1):3–14.
Prabhakaran S, Lee J, O’Neill K. Regional learning collaboratives produce rapid and sustainable improvements in stroke thrombolysis times. Circ Cardiovasc Qual Outcomes. 2016;9(5):585–92.
Ruff I, Liberman A, Caprio F, et al. A resident boot camp for reducing door-to-needle times at academic medical centers. Neurol Clin Pract. 2017;7:237–45.
COVID-19 Surveillance Data. [cited 3/24/2023]Available from: https://ccdphcd.shinyapps.io/covid19/
Swarm RA, Paice JA, Anghelescu DL, et al. Adult cancer pain, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(8):977–1007.
Barr J, Zomorodi K, Bertaccini EJ, et al. A double-blind, randomized comparison of i.v. lorazepam versus midazolam for sedation of ICU patients via a pharmacologic model. Anesthesiology. 2001;95(2):286–298.
Batra A, Clark JR, Kang AK, Ali S, Patel TR, Shlobin NA, Hoffman SC, Lim PH, Orban ZS, Visvabharathy L, Graham EL, Sullivan DP, Muller WA, Chou SH, Ungvári Z, Koralnik IJ, Liotta EM. Persistent viral RNA shedding of SARS-CoV-2 is associated with delirium incidence and six-month mortality in hospitalized COVID-19 patients. Geroscience. 2022;44(3):1241–1254. https://doi.org/10.1007/s11357-022-00561-z.
Liotta EM, Batra A, Clark JR, Shlobin NA, Hoffman SC, Orban ZS, Koralnik IJ. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clin Transl Neurol. 2020;7(11):2221–2230. https://doi.org/10.1002/acn3.51210.
Mirrakhimov AE, Voore P, Halytskyy O, et al. Propofol infusion syndrome in adults: a clinical update. Crit Care Res Pract. 2015;2015:260385.
Patanwala AE, Duby J, Waters D, et al. Opioid conversions in acute care. Ann Pharmacother. 2007;41(2):255–66.
Baldo BA, Rose MA. The anaesthetist, opioid analgesic drugs, and serotonin toxicity: a mechanistic and clinical review. Br J Anaesth. 2020;124(1):44–62.
Fernandez A, Lantigua H, Lesch C, et al. High-dose midazolam infusion for refractory status epilepticus. Neurology. 2014;82(4):359–65.
Sener S, Eken C, Schultz CH, et al. Ketamine with and without midazolam for emergency department sedation in adults: a randomized controlled trial. Ann Emerg Med. 2011;57(2):109–114 e102.
Green SM, Roback MG, Kennedy RM, et al. Clinical practice guideline for emergency department ketamine dissociative sedation: 2011 update. Ann Emerg Med. 2011;57(5):449–61.
Fang Y, Wang X. Ketamine for the treatment of refractory status epilepticus. Seizure. 2015;30:14–20.
Fraser GLRR. Phenobarbital provides effective sedation for a select cohort of adult ICU patients intolerant of standard treatment: a brief report. Hosp Pharm. 2006;41(1):17–23.
Srour H, Pandya K, Flannery A, et al. Enteral guanfacine to treat severe anxiety and agitation complicating critical care after cardiac surgery. Semin Cardiothorac Vasc Anesth. 2018;22(4):403–6.
Davies SJ, Burhan AM, Kim D, et al. Sequential drug treatment algorithm for agitation and aggression in Alzheimer’s and mixed dementia. J Psychopharmacol. 2018;32(5):509–23.
Ringman JM, Schneider L. Treatment options for agitation in dementia. Curr Treat Options Neurol. 2019;21(7):30.
Levine AR, Carrasquillo L, Mueller J, et al. High-dose gabapentin for the treatment of severe alcohol withdrawal syndrome: a retrospective cohort analysis. Pharmacotherapy. 2019;39(9):881–8.
O’Croinin D, Ni Chonghaile M, Higgins B, et al. Bench-to-bedside review: permissive hypercapnia. Crit Care. 2005;9(1):51–9.
Maccioli GA. Dexmedetomidine to facilitate drug withdrawal. Anesthesiology. 2003;98(2):575–7.
Schweickert WD, Kress JP. Strategies to optimize analgesia and sedation. Crit Care. 2008;12 Suppl 3(Suppl 3):S6.
Clark JR, Liotta EM, Reish NJ, et al. Abnormal movements in hospitalized COVID-19 patients: a case series. J Neurol Sci. 2021;423:117377.
Trogrlic Z, van der Jagt M, Bakker J, et al. A systematic review of implementation strategies for assessment, prevention, and management of ICU delirium and their effect on clinical outcomes. Crit Care. 2015;19(1):157.
Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966.
Wagner AK, Soumerai SB, Zhang F, et al. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309.
Brown JC, Querubin JA, Ding L, et al. Improving ABCDEF bundle compliance and clinical outcomes in the ICU: randomized control trial to assess the impact of performance measurement, feedback, and data literacy training. Crit Care Explor. 2022;4(4):e0679.
Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725–32.
McElvaney OJ, McEvoy NL, McElvaney OF, et al. Characterization of the inflammatory response to severe COVID-19 illness. Am J Respir Crit Care Med. 2020;202(6):812–21.
Xie C, Li Q, Li L, et al. Association of early inflammation with age and asymptomatic disease in COVID-19. J Inflamm Res. 2021;14:1207–16.
Rockoff MA, Naughton KV, Shapiro HM, et al. Cerebral circulatory and metabolic responses to intravenously administered lorazepam. Anesthesiology. 1980;53(3):215–8.
Matthew E, Andreason P, Pettigrew K, et al. Benzodiazepine receptors mediate regional blood flow changes in the living human brain. Proc Natl Acad Sci U S A. 1995;92(7):2775–9.
Caldas J, Passos R, Sancho L, et al. Monitoring cerebral hemodynamics in COVID-19 patients in the prone position. J Crit Care. 2022;70:154055.
Ziai WC, Cho SM, Johansen MC, et al. Transcranial Doppler in acute COVID-19 infection: unexpected associations. Stroke. 2021;52(7):2422–6.
Kazmi SO, Sivakumar S, Karakitsos D, et al. Cerebral pathophysiology in extracorporeal membrane oxygenation: pitfalls in daily clinical management. Crit Care Res Pract. 2018;2018:3237810.
Zunino G, Battaglini D, Godoy DA. Effects of positive end-expiratory pressure on intracranial pressure, cerebral perfusion pressure, and brain oxygenation in acute brain injury: friend or foe? A scoping review. J Intensive Med. 2024;4(2):247–60.
Chen H, Zhou XF, Zhou DW, et al. Effect of increased positive end-expiratory pressure on intracranial pressure and cerebral oxygenation: impact of respiratory mechanics and hypovolemia. BMC Neurosci. 2021;22(1):72.
Bassi T, Taran S, Girard TD, et al. Ventilator-associated brain injury: a new priority for research in mechanical ventilation. Am J Respir Crit Care Med. 2024;209(10):1186–8.
Hojlund J, Sandmand M, Sonne M, et al. Effect of head rotation on cerebral blood velocity in the prone position. Anesthesiol Res Pract. 2012;2012:647258.
Clark JR, Batra A, Shlobin NA, et al. Acute-care hospital reencounters in COVID-19 patients. Geroscience. 2021;43(4):2041–53.
Barends CRM, Driesens MK, van Amsterdam K, et al. Moderate-to-deep sedation using target-controlled infusions of propofol and remifentanil: adverse events and risk factors: a retrospective cohort study of 2937 procedures. Anesth Analg. 2020;131(4):1173–83.
Rucci JM, Law AC, Bolesta S, Quinn EK, Garcia MA, Gajic O, Boman K, Yus S, Goodspeed VM, Kumar V, Kashyap R, Walkey AJ. Society of critical care medicine discovery viral infection and respiratory illness universal study COVID-19 registry investigator group. Variation in sedative and analgesic use during the COVID-19 pandemic and associated outcomes. Chest Crit Care. 2024;2(1):100047. https://doi.org/10.1016/j.chstcc.2024.100047.
Keswani M, Mehta N, Mazer-Amirshahi M, et al. Sedation in mechanically ventilated covid-19 patients: a narrative review for emergency medicine providers. Am J Emerg Med. 2022;54:309–11.
Moccia F, Gerbino A, Lionetti V, et al. COVID-19-associated cardiovascular morbidity in older adults: a position paper from the Italian Society of Cardiovascular Researches. Geroscience. 2020;42(4):1021–49.
Graham EL, Koralnik IJ, Liotta EM. Therapeutic approaches to the neurologic manifestations of COVID-19. Neurotherapeutics. 2022;19(5):1435–66.
Lionetti V, Bollini S, Coppini R, et al. Understanding the heart-brain axis response in COVID-19 patients: a suggestive perspective for therapeutic development. Pharmacol Res. 2021;168:105581.
French G, Hulse M, Nguyen D, et al. Impact of hospital strain on excess deaths during the COVID-19 pandemic - United States, July 2020-July 2021. MMWR Morb Mortal Wkly Rep. 2021;70(46):1613–6.
Castagna F, Xue X, Saeed O, et al. Hospital bed occupancy rate is an independent risk factor for COVID-19 inpatient mortality: a pandemic epicentre cohort study. BMJ Open. 2022;12(2):e058171.
Bravata DM, Perkins AJ, Myers LJ, et al. Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021;4(1):e2034266.
Kadri SS, Sun J, Lawandi A, et al. Association between caseload surge and COVID-19 survival in 558 U.S. hospitals, March to August 2020. Ann Intern Med. 2021;174(9):1240–1251.
Eriksson CO, Stoner RC, Eden KB, et al. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686–96.
Hoogendoorn ME, Brinkman S, Bosman RJ, et al. The impact of COVID-19 on nursing workload and planning of nursing staff on the intensive care: a prospective descriptive multicenter study. Int J Nurs Stud. 2021;121:104005.
Magesh S, John D, Li WT, et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic-review and meta-analysis. JAMA Netw Open. 2021;4(11):e2134147.
Soto GJ, Martin GS, Gong MN. Healthcare disparities in critical illness. Crit Care Med. 2013;41(12):2784–93.
Stamm B, Royan R, Trifan G, et al. Household income is associated with functional outcomes in a multi-institutional cohort of patients with ischemic stroke and COVID-19. J Stroke Cerebrovasc Dis. 2023;32(5):107059.
Myers LC, Liu VX. The COVID-19 pandemic strikes again and again and again. JAMA Netw Open. 2022;5(3):e221760.
Prodan CI, Batra A, Ungvari Z, et al. Stringent public health measures during COVID-19 across ischemic stroke care systems: the potential impact of patient perceptions on health care-seeking behaviors. Geroscience. 2022;44(3):1255–62.
Arogyaswamy S, Vukovic N, Keniston A, et al. The impact of hospital capacity strain: a qualitative analysis of experience and solutions at 13 academic medical centers. J Gen Intern Med. 2022;37(6):1463–74.
Funding
Dr. Liotta is supported by NIH/NIA grant K23AG078705.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Clark, J.R., Batra, A., Tessier, R.A. et al. Impact of healthcare system strain on the implementation of ICU sedation practices and encephalopathy burden during the early COVID-19 pandemic. GeroScience (2024). https://doi.org/10.1007/s11357-024-01336-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11357-024-01336-4