Abstract
Hypogonadism is a risk factor for cardiovascular disease (CVD) in men related, in part, to increased oxidative stress. Elevated large artery stiffness and central pulsatile hemodynamics (e.g., pulse pressure and wave reflection magnitude) are independent risk factors for CVD. However, whether large artery stiffness and central pulsatile hemodynamics are (1) elevated in hypogonadal men independent of traditional CVD risk factors and (2) related to increased oxidative stress is unknown. Young men (N = 23; 30 ± 4 years) and middle-aged/older (MA/O) men with normal (> 400–1000 ng/dL; n = 57; 59 ± 7 years) or low testosterone (< 300 ng/dL; n = 21; 59 ± 7 years) underwent assessments of large artery stiffness (carotid ß-stiffness via ultrasonography) and central pulsatile hemodynamics (pulse wave analysis; SphygmoCor XCEL) following an infusion of saline or vitamin C to test the tonic suppression of vascular function by oxidative stress. Carotid stiffness differed by age (p < 0.001) and gonadal status within MA/O men (low testosterone vs. normal testosterone: 9.3 ± 0.7 vs. 8.0 ± 0.3U, p = 0.036). Central pulsatile hemodynamics did not differ by age or gonadal status (p > 0.119). Vitamin C did not alter carotid stiffness in any group (p > 0.171). There was a significant group × infusion interaction on aortic reflection magnitude (p = 0.015). Vitamin C treatment reduced aortic reflection magnitude in young and MA/O men with normal testosterone (both p < 0.001) but not MA/O men with low testosterone (p = 0.891). Collectively, hypogonadism may accelerate age-related large artery stiffening in MA/O men with low testosterone, independent of CVD risk factors; however, this is not related to increased reactive oxygen species sensitive to an acute vitamin C infusion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypogonadism, defined by low endogenous serum testosterone, is a risk factor for cardiovascular disease (CVD) in men [1,2,3]. Testosterone levels peak around age 30 in men and decline progressively (1–2% annually) across the life span. Vascular aging, characterized by increased large elastic artery stiffness, is an independent risk factor for the development of CVD [4]. Large artery stiffness is increased in men with hypogonadism [1, 5, 6] and is improved following testosterone administration [6]. However, the controversy surrounding the potentially greater CVD risk in older men treated with testosterone [7, 8] has prompted concern regarding testosterone safety [9]. These concerns highlight the need to better understand the mechanisms by which low testosterone may lead to increased CVD risk to inform novel sex-specific therapeutic strategies to reduce CVD risk in hypogonadal men.
Stiffening of the large elastic arteries in the central circulation (e.g., aorta and common carotid arteries) precedes and contributes to the development of CVD related to the loss of the buffering capacity of the central arteries to protect the downstream microvasculature from excessive pulsatile pressure [10]. Large artery stiffening contributes to widened pulse pressure by altering the timing of the hemodynamic components of the pressure waveform (e.g., forward and backward pressure wave amplitude) [11] that are associated with vascular remodeling to increase intimal-medial thickness [12] and target end organ damage [10]. Forward pressure wave amplitude improves CVD event risk prediction in older adults beyond aortic stiffness and mean arterial pressure alone [13], suggesting that consideration of pulse pressure hemodynamic components may provide additional insight into mechanisms of CVD risk in aging adults.
Oxidative stress, which represents the imbalance between the production and destruction of reactive oxygen species, is a key mechanism contributing to vascular dysfunction in aging adults [14,15,16,17,18,19]. Increased oxidative stress can induce both acute and chronic increases in central artery stiffness and pulsatile hemodynamics [20,21,22] via reductions in nitric oxide bioavailability and increased generation of reactive oxygen species [23]. Chronic elevations in oxidative stress degrade elastin and increase collagen deposition through greater crosslinking of advanced glycation end-products to augment large artery stiffness and pulsatile pressure hemodynamics [21]. Testosterone has important antioxidant [24, 25] and anti-inflammatory properties that are reduced in hypogonadal men [26, 27]. However, whether oxidative stress and inflammation are mechanistically linked to greater age-associated large elastic artery stiffening and pulsatile pressure hemodynamics in middle-aged/older men with low testosterone is unknown.
Accordingly, this study investigated whether low testosterone was associated with greater vascular dysfunction and underlying mechanisms. We hypothesized that large artery stiffness and pulsatile hemodynamics (e.g., central forward and backward pressure wave amplitude, reflection coefficient) would be greater in middle-aged/older men compared with young men and that this effect would be greater in middle-aged/older men with low testosterone (total testosterone < 300 ng/dL) compared with normal testosterone (total testosterone ≥ 400 ng/dL). Furthermore, to evaluate underlying mechanisms related to oxidative stress, we measured vascular function at baseline during an acute saline infusion and following a supraphysiological dose of vitamin C to test the tonic suppression of vascular function by reactive oxygen species. We hypothesized that group differences in large artery stiffness and pulsatile hemodynamics between middle-aged/older men with low versus normal testosterone would be related to increased oxidative stress and abolished following the supraphysiological dose of vitamin C.
Materials and methods
Study design
This cross-sectional study was part of a registered clinical trial (ClincialTrials.gov Identifier NCT02758431) conducted between 2016 and 2023. This study was approved by the Colorado Multiple Institutional Review Board, and procedures were conducted according to The Declaration of Helsinki. All participants provided written informed consent prior to participating. All study visits and measurements were performed at the Colorado Clinical and Translational Sciences Institute (CCTSI) Clinical and Translational Research Center (CTRC).
Study participants
Healthy young (18–40 years) and middle-aged/older (50–75 years) men of all racial and ethnic backgrounds were recruited from the Denver Metropolitan Area. Young men had clinically normal testosterone levels (serum testosterone ≥ 13.9 nmol/L (> 400 ng/dL); n = 23). Middle-aged/older men were categorized into two groups: normal testosterone (serum testosterone ≥ 13.9 nmol/L (> 400 ng/dL; n = 57)) and low testosterone (serum testosterone < 10.4 nmol/L (< 300 ng/dL), n = 21) at screening. Serum testosterone levels were measured in the morning under fasted conditions and were confirmed on the day of the vascular testing (assay described below). Middle-aged/older men with low and normal testosterone were matched by age to evaluate the effects of low testosterone on vascular function, independent of age.
Eligibility criteria have been described previously [20]. Briefly, men were included if they met the following criteria: (1) no use of sex hormones for at least 1 year; (2) BMI < 40 kg/m2; (3) nonsmokers; (4) resting blood pressure < 160/90 mmHg; (5) non-diabetic and fasted plasma glucose < 7.0 mmol/L (< 126 mg/dL); (6) healthy and free from cardiovascular, cancer, renal, liver, or respiratory disease as assessed by medical history, physical exam, standard blood chemistries (comprehensive metabolic panel, complete blood count and thyroid stimulating hormone), and electrocardiogram (ECG) at rest and during a clinician-supervised graded exercise treadmill test to fatigue; (7) sedentary or recreationally active (< 3 days/week of vigorous aerobic exercise); (8) no use of medications that influence cardiovascular function including antihypertensive and lipid-lowering medications; and (9) no use of vitamins, herbal supplements, minerals, or anti-inflammatory medications, or willing to stop 1 month prior to and throughout the study.
Participant characteristics
Seated blood pressure was measured in the morning under fasted conditions with adequate hydration (no caffeine, water encouraged) and abstinence from exercise following ≥ 10 min of seated rest in triplicate in both arms using an oscillometric blood pressure cuff (Carescape V100, GE medical systems). The average of the higher arm is reported in participant characteristics. Total body fat was determined using dual x-ray absorptiometry (Hologic Horizon). Hip and waist circumference were measured by a trained technician in triplicate and averaged. Peak oxygen consumption (VO2peak) testing with 12-lead ECG (Quinton Q4500; Quinton Instruments, Seattle, WA) in response to a maximal graded treadmill exercise test was used to calculate cardiorespiratory fitness as described [20] with the highest 30-s average oxygen uptake (V̇o2) recorded as V̇o2peak.
Vascular testing
Vascular testing was conducted in the supine position following an overnight fast with proper hydration (water only, no caffeine). Participants abstained from exercise for ≥ 20 h prior to the study visit. Vascular testing was measured following 10 min of quiet rest. Central blood pressure and components of central pulse pressure (e.g., forward and backward pressure wave amplitude, reflection coefficient) were measured by trained researched personnel using the standard pulse wave analysis software (PWA, SphygmoCor XCEL, AtCor Medical, Sydney, Australia) as previously described [28]. Briefly, brachial blood pressure was measured from an automated oscillometric upper-arm cuff device (SphygmoCor XCEL, AtCor Medical, Sydney, Australia). Following the initial blood pressure measurement, a volumetric displacement waveform was acquired from the same upper-arm cuff inflated to a sub-diastolic level of pressure. The brachial volumetric blood pressure waveform was ensembled and averaged, and its peak and nadir were calibrated to systolic and diastolic blood pressure, respectively. The brachial waveform was then reconstructed into an estimated aortic blood pressure waveform via a generalized transfer function. Forward and backward traveling blood pressure waves were automatically derived by the SphygmoCor software, which applies a pressure-only version of wave separation analysis to the estimated aortic blood pressure waveform. Regarding the wave separation method, an aortic flow waveform approximating a triangle is constructed from the morphology of the aortic blood pressure waveform. Wave separation analysis can then be performed to derive aortic forward and backward pressure wave amplitude and reflection magnitude coefficient (backward wave amplitude/forward wave amplitude expressed as a percent). Large elastic artery stiffness was measured by common carotid artery stiffness using high-resolution carotid ultrasonography (GE Vivid I) with a 12-MHz linear transducer. All images were coded by number, blinded to the study group, and analyzed by two trained investigators (K.L.M. and L.E.D.). Briefly, 15-s 2D carotid images were acquired 1–2 cm proximal to the carotid bulb and analyzed using semiautomated edge-detection software (Vascular Analysis Tools v. 5.5; MIA LLC, Coralville, IA) to calculate the maximum (systolic) and minimum (diastolic) diameters and IMT [12] as previously described [29]. Carotid stiffness was measured as carotid β-stiffness index [30] and distensibility coefficient [32] calculated from carotid ultrasonography and central blood pressure derived from PWA. The carotid β-stiffness index was the primary outcome because it provides an index of arterial stiffness adjusted for distending pressure. The carotid distensibility coefficient was calculated as an alternative measure of arterial stiffness to confirm findings with the primary outcome. Carotid IMT was measured on the far wall during end diastole and averaged across cardiac cycles as a measure of wall thickness.
Blood sampling
Blood sampling occurred on the day of vascular testing, and all assays were performed at the CCTSI CTRC core laboratory as described previously [20, 33]. An intravenous catheter was placed into an antecubital vein for the saline and vitamin C infusions (described below) and blood sampling (described below) prior to the start of vascular testing. Fasted plasma concentrations of glucose, insulin, total cholesterol (Roche Diagnostic systems, Indianapolis, IN), and high-density lipoprotein cholesterol (Diagnostic Chemical Ltd, Oxford CT) were determined using enzymatic/colorimetric methods. Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation [34]. Oxidized LDL was measured using an enzyme-linked immunosorbent plate assay (Alpco Diagnostics, Windham, NH). Serum total antioxidant status (TAS), a measure of overall antioxidant capacity, was measured using an enzymatic kit (Randox Laboratories, Oceanside, CA). Interleukin-6 (IL-6) was measured using an enzyme-linked immunoassay, and high-sensitivity C-reactive protein (CRP) was measured using an immunoturbidimetric method. Total serum testosterone, estradiol, and sex hormone binding globulin (SHBG) were measured via chemiluminescence using a Beckman Coulter Access II analyzer. Free testosterone was calculated for each participant from concentrations of serum testosterone, SHBG, and albumin using an online algorithm (www.issam.ch) using the Vermeulen equation [35].
Vitamin C infusion protocol
A well-described experimental model using vitamin C to temporarily reduce ROS was utilized to investigate the role of systemic oxidative stress as a possible mechanism for large elastic artery stiffening as described previously [16, 36,37,38]. Vitamin C is a potent water-soluble antioxidant that can be infused at rates that attain supraphysiological plasma vitamin C concentrations known to reduce the bioavailability of superoxide anions [39]. Briefly, vascular testing was obtained after a 20-min bolus of normal isotonic saline, followed by a “drip” infusion (control condition). Vascular testing was repeated after a bolus of 100-mL vitamin C (i.e., ascorbic acid) solution dosed at 0.06 g vitamin C per kg fat-free mass per 100 mL of normal saline (prepared by a research pharmacist at the University of Colorado Hospital). The vitamin C solution was infused 5 mL/min over 20 min followed by a “drip” infusion at a rate of 1.7 mL/min. The saline infusion always preceded the vitamin C infusion to avoid any persistent effects of vitamin C. The time between saline and vitamin C measurements was approximately 60–90 min. The dose of vitamin C has been previously shown to temporarily improve large artery stiffness, femoral artery blood flow, endothelial function, and left ventricular diastolic function in older adults [16, 36,37,38, 40, 41]. The difference in vascular function during vitamin C versus saline infusion was taken as a measure of the modulation of large artery stiffness and pulsatile hemodynamics by tonic reactive oxygen species.
Data and statistical analysis
Data are reported as mean ± SD unless otherwise noted. Data were tested for normality using the Shapiro–Wilk normality test and log transformed when data were not normally distributed. Participant characteristics, sex hormones, blood markers of vascular function, and arterial stiffness parameters were analyzed using a one-way ANOVA with Tukey HSD post hoc testing and Bonferroni corrected for multiple comparisons. Changes in measures of large artery stiffness in response to the vitamin C infusion were analyzed using a two-way ANOVA with group and infusion (i.e., saline or vitamin C) as factors. In the case of significant interaction effects, post hoc testing was performed with Tukey’s HSD and paired t-tests to determine the main effects. Statistical significance was set at p < 0.05. Data analysis was performed with IBM SPSS Statistics version 27.0 and GraphPad Prism.
Results
Participant characteristics
Participant clinical characteristics are reported in Table 1. Total and free testosterone were lower in middle-aged/older men with low testosterone (p < 0.001) but did not differ between young and middle-aged/older men with normal testosterone (p > 0.845). There were differences between young and MA/O men in BMI, total body fat, seated blood pressure, total cholesterol, and LDL cholesterol, but these characteristics did not differ by gonadal status. Waist-to-hip ratio differed by age (p < 0.001) and gonadal status in middle-aged/older men (p = 0.007). Fasted insulin was higher in middle-aged/older men with low testosterone compared with young men (p = 0.003) but not middle-aged/older adults (p = 0.143). Insulin did not differ between young and middle-aged/older men with normal testosterone (p = 0.159). IL-6 and CRP were higher in middle-aged/older men (both p < 0.001) but did not differ by gonadal status (p > 0.174) in middle-aged/older men. OxLDL did not differ by age (p > 0.173) or gonadal status (p = 0.985). TAS was lower in middle-aged/older men with normal testosterone compared with young men (p = 0.006) but was not different than middle-aged/older men with normal testosterone (p = 0.203).
Large artery stiffness and pressure hemodynamics
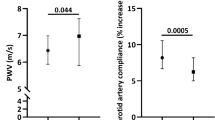
Vascular data are presented in Table 2 and Fig. 1. As expected, there was an effect of age on carotid stiffness and IMT (p < 0.001). Carotid stiffness (carotid β-stiffness, p = 0.036; distensibility coefficient, p = 0.028) and IMT (p = 0.021) were greater in middle-aged/older men with low testosterone compared with normal testosterone (Fig. 1) despite being similar in age, BMI, and blood pressure. There was an effect of age on central blood pressure, backward pressure wave amplitude, and reflection magnitude (all p < 0.004); however, these variables did not differ by gonadal status (all p > 0.119). Forward pressure wave amplitude did not differ across groups (p = 0.497).
Group differences in A carotid beta stiffness, B carotid distensibility coefficient, and C carotid intima-medial thickness between young men, middle-aged/older (MA/O) men with normal testosterone (T), and low testosterone. Group differences were tested using one-way ANOVA with Tukey’s post hoc testing. p-values reflect group differences. Carotid beta stiffness, distensibility coefficient, and intima-medial thickness were elevated with age and the presence of hypogonadism amongst MA/O men. Figures were created using GraphPad Prism
Effects of vitamin C infusion on arterial stiffness and pressure hemodynamics
Changes in large artery stiffness and pulsatile pressure hemodynamics during the vitamin C infusion are shown in Table 3. There was no effect of the vitamin C infusion on carotid stiffness, and this effect did not differ by group (p = 0.834). There was a significant group × infusion interaction on central reflection magnitude (p = 0.015) only. Central reflection magnitude was reduced in young men and middle-aged/older men with normal testosterone only. The effect of the vitamin C infusion on central pulse pressure (p = 0.094) and backward wave pulse amplitude (p = 0.088) marginally differed by group. No other indices of pulsatile hemodynamics were altered by the vitamin C infusion (all p > 0.328).
Discussion
This study determined if the presence of hypogonadism augmented age-associated differences in large artery stiffness and pulsatile hemodynamics (e.g., central pulse pressure and wave reflection magnitude) due to an exacerbation of oxidative stress. Consistent with our hypothesis, large artery stiffness and carotid artery IMT were greater in middle-aged/older men with low testosterone compared with normal testosterone despite being matched for age and having similar BMI and blood pressure levels. Contrary to our hypothesis, acute vitamin C treatment did not alter large artery stiffness in any group, nor did biomarkers of oxidative stress and inflammation differ by gonadal status. Large artery stiffness remained greater in middle-aged/older men with low compared with normal testosterone. Acute vitamin C treatment improved central reflection magnitude in young and middle-aged/older men with normal testosterone only. Collectively, these data suggest for the first time that age-associated large artery stiffening may be accelerated in hypogonadal men related to changes in vascular structure that are likely not acutely modifiable by a vitamin C intervention and do not appear to be related to reactive oxygen species that are sensitive to a vitamin C infusion [42]. Additionally, these data highlight the importance of considering gonadal status in men in cardiovascular aging research.
Large artery stiffness and components of central pulse pressure with aging and hypogonadism
Large artery stiffness describes the inter-relation between vessel wall stiffness, wall thickness, and lumen diameter in the large central elastic arteries [43] The pressure generated during cardiac contraction interacts with vessel wall diameter and wall stiffness of the proximal aorta to produce an incident forward pressure wave [44]. The forward pressure wave typically travels at a relatively low velocity in young adults and is reflected at distal sites of an impedance mismatch to return to the heart to boost coronary artery perfusion during diastole [44]. However, with aging, the loss of the buffering capacity of the central arteries increases forward pressure wave amplitude that travels at a greater velocity through the central arteries [45]. The backward reflected pressure wave returns to the proximal aorta earlier in systole to augment central systolic BP and reduce diastolic perfusion pressure, resulting in a widened pulse pressure [10]. Chronic elevations in central pulse pressure promote vascular remodeling to limit the exposure of the target end organs to excessive pulsatile energy, highlighting its importance in studies of CVD risk [10].
In the present study, aging was associated with greater carotid stiffness and carotid IMT in middle-aged/older men when compared with young men at baseline (saline condition), consistent with prior studies [31, 45]. Notably, middle-aged/older men with low testosterone exhibited higher large artery stiffness and carotid IMT compared with middle-aged/older men with normal testosterone. The magnitude of difference in carotid ß-stiffness (> + 1U) between gonadal groups has previously been shown to be clinically predictive of CVD event risk [46]. These data are consistent with a prior cross-sectional study in middle-aged/older men with and without CVD [47] demonstrating that hypogonadism may exacerbate the effects of aging on large artery stiffness, particularly in middle-aged men [1]. Moreover, a prior interventional study showed large artery stiffness was improved following 12 weeks of testosterone supplementation in hypogonadal middle-aged/older men [6]. Vlachopoulos et al. previously demonstrated that aortic stiffness, measured via carotid-femoral pulse wave velocity (cfPWV), was significantly greater in men with low compared to normal testosterone and that men with low testosterone exhibited cfPWV values comparable to men with normal testosterone that were one decade older [1]. Collectively, these data suggest that maintaining testosterone levels within clinically normal limits may be beneficial for vascular health in middle-aged/older men.
Despite differences in large artery stiffness between gonadal groups, central (i.e., aortic) blood pressure and components of central pulse pressure (e.g., forward and backward wave amplitude, central wave reflection magnitude) differed only by age but not gonadal status. Aging is associated with adverse alterations in central pulsatile hemodynamics that are augmented with both local increased vessel wall stiffness and remodeling [10, 48]. Explanations for the lack of gonadal status group differences in central blood pressure and components of central pulse pressure are unclear but may be due to the lack of difference in seated (brachial) blood pressure in middle-aged/older men with low and normal testosterone, compensatory changes in blood pressure regulation, or vascular remodeling or be related to techniques used to derive components of pulse pressure. For example, adverse effects of hypogonadism on large artery stiffness were greater in men with higher mean arterial pressure (MAP, > 102 mmHg) compared with lower (< 101 mmHg), suggesting an interactive effect between testosterone and brachial blood pressure [1]; however, this previous study did not measure central pulsatile hemodynamics and MAP and central pulse pressure did not differ by gonadal status in the present study. Alternatively, data from our group suggests hypogonadism accelerates age-related declines in cardiovagal baroreflex sensitivity [49], indicating differences in blood pressure regulation could also contribute. Further, the large arteries will remodel in response to chronic exposure to elevated central pulse pressure in an attempt to normalize wall circumferential stress [50]. Finally, the lack of observed differences in components of pulse pressure may be related to methods used to calculate components of pulse pressure by the SphygmoCor XCEL. Prior studies suggest that pressure-only wave separation techniques, which use a more physiologically representative synthetic aortic flow, provide better estimates of forward and backward wave amplitudes [51, 52].
Role of oxidative stress in large artery stiffness and components of pulse pressure
Elevated oxidative stress and inflammation have been proposed to mediate vascular dysfunction in hypogonadal men because of the loss of antioxidant and anti-inflammatory effects of testosterone [3]. Large artery stiffening adversely alters the amplitude and timing of the forward and backward pressure waves contributing to increased central pulse pressure and oxidative stress in a cyclical manner [10, 53]. However, in the present study, the acute vitamin C infusion did not improve large artery stiffness in either middle-aged/older men with low or normal testosterone and large artery stiffness remained greater in middle-aged/older men with low compared to normal testosterone. Although we did not observe significant differences in circulating oxidized LDL, TAS, IL-6, or CRP between the middle-aged/older groups, plasma biomarkers may not accurately reflect levels of oxidative stress or inflammation observed at the local vascular level. Additionally, it is possible that other sources of reactive oxygen species and/or inflammation that we did not measure (e.g., nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, xanthine oxidase, catalase, tumor necrosis factor α) may also contribute [54].
The lack of the vitamin C effect on large artery stiffness and components of pulse pressure is consistent with prior studies of healthy middle-aged/older men that showed intravenous vitamin C did not significantly alter large artery stiffness [55] or central blood pressure [56] in young or middle-aged/older men; however, importantly, gonadal status was not considered in these studies. In the present study, central reflection magnitude was reduced following the vitamin C infusion in young and middle-aged/older men with normal testosterone but not in middle-aged/older men with low testosterone. The interactive group × time effect of the vitamin C intervention on central pulse pressure and backward pressure wave amplitude approached significance. Because large artery stiffness was not altered following the vitamin C infusion, the reduction in the reflection magnitude could be explained by downstream increased peripheral vasodilation. Downstream peripheral vasodilation reduces peripheral vascular resistance which in turn alters the magnitude and timing of the backward reflected wave back to the heart [10]. Peripheral vasodilation reduces the relative amount of energy reflected back to the aorta with the arrival of the reflected wave occurring relatively later in the cardiac cycle because of downstream peripheral vascular resistance and increased impedance matching. This decreases the magnitude of the aortic backward pressure wave amplitude and reflection coefficient. Peripheral vascular resistance is increased with aging [57] but is improved following vitamin C infusion in aging men [58]. Although testosterone administration reduced peripheral vascular resistance in middle-aged/older men with heart failure [59], whether central hemodynamics are influenced by changes in peripheral vascular resistance and/or gonadal status remains unclear. The lack of an effect on the central reflection coefficient in middle-aged/older men with low testosterone in the present study may further support the idea that hypogonadal men have greater chronic systemic vascular remodeling that is less modifiable by acute treatments because we did not observe an effect of the vitamin C infusion on any indices of wave reflection. Collectively, these data suggest that age-associated large artery stiffening in hypogonadal men may be driven by structural changes (e.g., increased collagen deposition, intimal-medial thickening) that are not acutely modifiable by a vitamin C intervention. Alternatively, other sources of reactive oxygen species that are weakly scavenged by vitamin C (e.g., peroxynitrite) [42] could be involved.
Other mechanisms
Hypogonadism is associated with major comorbidities including an increased risk for type 2 diabetes and obesity [60]. Accordingly, men were excluded for the presence of type 2 diabetes or fasted glucose > 126 mg/dL and had similar BMIs and total body fat between the gonadal groups. Nevertheless, middle-aged older men with low testosterone had higher fasted triglycerides and a greater waist-to-hip ratio compared with middle-aged/older men with normal testosterone and fasted insulin was greater in middle-aged/older men with low testosterone compared with young men. Large artery stiffness is also influenced by the increased crosslinking of elastin and collagen by advanced glycation end-products (AGEs) [61]. Physiological levels of testosterone protect human umbilical endothelial cells from AGE-induced apoptosis, inflammation, and oxidative stress [62]. Greater AGEs are associated with carotid IMT in patients with type 1 diabetes [63] and type 2 diabetes [64]. Whether AGEs contribute to greater large artery stiffness and carotid IMT in hypogonadal men is unknown. Alternatively, prior studies demonstrate impaired autonomic control of cardiovascular function [49] and greater peripheral endothelial dysfunction [65] in middle-aged/older men with low compared with normal testosterone associated with higher inflammation. Our group has previously shown that middle-aged/older men with low testosterone exhibit lower cardiovagal baroreflex sensitivity and heart rate variability in the high-frequency domain (reflecting parasympathetic tone) compared with middle-aged/older men with normal testosterone [49]. Prior work demonstrates that the large elastic arteries are sympathetically innervated and that greater sympathetic nerve activity is associated with carotid IMT in middle-aged/older men [66, 67].
Limitations
First, only healthy men without clinical or subclinical CVD or diabetes and who were sedentary-to-recreationally active were enrolled. Second, we excluded middle-aged/older men with testosterone levels between 10.4 and 13.8 nmol/L (300–399 ng/dL) to evaluate cross-sectional differences in testosterone, limiting our ability to generalize findings to middle-aged/older men with testosterone levels between 10.4 and 13.8 nmol/L (300–399 ng/dL) or young men with low testosterone. Future studies should examine the impact of chronically low testosterone on large artery stiffness and whether there is a “threshold” level of testosterone at which large artery stiffening and thickening occur. The onset of hypogonadism in men is challenging to identify because symptoms of hypogonadism overlap with those of chronological aging and testosterone is not routinely measured [68]. Therefore, we are unable to evaluate the relations between the duration of hypogonadism and vascular function in the present study.
Conclusions
Hypogonadism is a risk factor for increased CVD risk in men [69]; however, the precise mechanisms by which this occurs remain unclear, limiting the ability to develop efficacious treatments to reduce CVD risk in hypogonadal men. The present study suggests that hypogonadism accelerates age-related large artery stiffening and thickening in middle-aged/older men with low testosterone compared with normal testosterone, independent of CVD risk factors. This apparent acceleration in stiffening may be driven by structural changes (e.g., increased collagen deposition, intimal-medial thickening) in the large central arteries that were not acutely modifiable by a vitamin C intervention, suggesting that mechanisms other than increased reactive oxygen species, at least those sensitive to an acute vitamin C infusion, contribute. Future studies are needed to identify efficacious treatments for reducing CVD risk in hypogonadism by attenuating or reversing the adverse changes in the structural components of large artery stiffness in hypogonadism.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Vlachopoulos C, et al. Testosterone deficiency: a determinant of aortic stiffness in men. Atherosclerosis. 2014;233(1):278–83.
Hougaku H, et al. Relationship between androgenic hormones and arterial stiffness, based on longitudinal hormone measurements. Am J Physiol Endocrinol Metab. 2006;290(2):E234-242.
Moreau KL. Modulatory influence of sex hormones on vascular aging. Am J Physiol-Heart Circ Physiol. 2019;316(3):H522–6.
Mitchell GF, et al. Arterial Stiffness and Cardiovascular Events. Circulation. 2010;121(4):505–11.
Canpolat U, et al. Impaired aortic elastic properties in patients with adult-onset hypogonadism. Blood Press. 2013;22(2):114–9.
Yaron M, et al. Effect of testosterone replacement therapy on arterial stiffness in older hypogonadal men. Eur J Endocrinol. 2009;160(5):839–46.
Vigen R, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829–36.
Basaria S, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109–22.
Carson Iii CC, Rosano G. Exogenous testosterone, cardiovascular events, and cardiovascular risk factors in elderly men: a review of trial data. J Sex Med. 2012;9(1):54–67.
Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol (1985). 2008;105(5):1652–60.
Cooper LL, et al. Components of hemodynamic load and cardiovascular events the Framingham Heart Study. Circulation. 2015;131(4):354–61.
Dubey RK, et al. Vascular consequences of menopause and hormone therapy: Importance of timing of treatment and type of estrogen. Cardiovasc Res. 2005;66(2):295–306.
Cooper LL, et al. Components of hemodynamic load and cardiovascular events. Circulation. 2015;131(4):354–61.
Eskurza I, et al. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556(Pt 1):315–24.
Taddei S, et al. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38(2):274–9.
Moreau KL, et al. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension. 2005;45(6):1107–12.
Brinkley TE, et al. Plasma oxidized low-density lipoprotein levels and arterial stiffness in older adults: the health, aging, and body composition study. Hypertension. 2009;53(5):846–52.
Li S-Y, et al. Aging induces cardiac diastolic dysfunction, oxidative stress, accumulation of advanced glycation endproducts and protein modification. Aging Cell. 2005;4(2):57–64.
Dai D-F, et al. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119(21):2789–97.
Babcock MC, et al. Oxidative stress and inflammation are associated with age-related endothelial dysfunction in men with low testosterone. J Clin Endocrinol Metab. 2022;107(2):e500–14.
Pierce GL, et al. Is it good to have a stiff aorta with aging? Causes and consequences. Physiology. 2022;37(3):154–73.
Pierce GL. Mechanisms and subclinical consequences of aortic stiffness. Hypertension. 2017;70(5):848–53.
Donato AJ, et al. Cellular and molecular biology of aging endothelial cells. J Mol Cell Cardiol. 2015;89:122–35.
Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 2001;892(2):255–62.
Mancini A, et al. Effects of testosterone on antioxidant systems in male secondary hypogonadism. J Androl. 2008;29(6):622–9.
Mohamad NV, et al. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male. 2019;22(2):129–40.
Maggio M, et al. The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest. 2005;28(11 Suppl Proceedings):116–9.
Butlin M, Qasem A. Large artery stiffness assessment using SphygmoCor technology. Pulse. 2017;4(4):180–92.
Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol. 1998;18(1):127–32.
DuBose LE, et al. Carotid β-stiffness index is associated with slower processing speed but not working memory or white matter integrity in healthy middle-aged/older adults. J Appl Physiol. 2017;122(4):868–76.
Vermeersch SJ, et al. Age and gender related patterns in carotid-femoral PWV and carotid and femoral stiffness in a large healthy, middle-aged population. J Hypertens. 2008;26(7):1411–9.
Gamble G, et al. Estimation of arterial stiffness, compliance, and distensibility from M-mode ultrasound measurements of the common carotid artery. Stroke. 1994;25(1):11–6.
Moreau KL, et al. Decline in endothelial function across the menopause transition in healthy women is related to decreased estradiol and increased oxidative stress. GeroScience. 2020;42(6):1699–714.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502.
Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–72.
Moreau KL, et al. Oxidative stress contributes to chronic leg vasoconstriction in estrogen-deficient postmenopausal women. J Appl Physiol (1985). 2007;102(3):890–5.
Moreau KL, et al. Oxidative stress explains differences in large elastic artery compliance between sedentary and habitually exercising postmenopausal women. Menopause. 2006;13(6):951–8.
Hildreth KL, Kohrt WM, Moreau KL. Oxidative stress contributes to large elastic arterial stiffening across the stages of the menopausal transition. Menopause. 2014;21(6):624–32.
Jackson TS, et al. Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res. 1998;83(9):916–22.
Ozemek C, et al. Acute ascorbic acid infusion increases left ventricular diastolic function in postmenopausal women. Maturitas. 2016;92:154–61.
Moreau KL, et al. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab. 2013;98(11):4507–15.
Kuzkaya N, et al. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 2003;278(25):22546–54.
Mitchell GF. Arterial stiffness and wave reflection in hypertension: pathophysiologic and therapeutic implications. J Curr Hypertens Rep. 2004;6(6):436–41.
Mitchell GF, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility – Reykjavik Study. Brain. 2011;134(11):3398–407.
Mitchell GF, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: The Framingham Heart Study. Hypertension. 2004;43(6):1239–45.
van Sloten TT, Stehouwer CDA. Carotid stiffness: a novel cerebrovascular disease risk factor. Pulse. 2016;4(1):24–7.
Hougaku H, et al. Relationship between androgenic hormones and arterial stiffness, based on longitudinal hormone measurements. Am J Physiol-Endocrinol Metab. 2006;290(2):E234–42.
Mitchell GF, et al. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation. 2010;122(14):1379–86.
Babcock MC, et al. Age-associated reductions in cardiovagal baroreflex sensitivity are exaggerated in middle-aged and older men with low testosterone. J Appl Physiol. 2022;133(2):403–15.
Nguyen PH, Tuzun E, Quick CM. Aortic pulse pressure homeostasis emerges from physiological adaptation of systemic arteries to local mechanical stresses. Am J Physiol Regul Integr Comp Physiol. 2016;311(3):R522–31.
Shenouda N, et al. Personalized physiologic flow waveforms improve wave reflection estimates compared to triangular flow waveforms in adults. Am J Physiol-Heart Circ Physiol. 2021;320(5):H1802–12.
Armstrong MK, et al. Aortic pressure-only wave separation analysis in adolescents: accuracy and associations with left ventricular mass index. J Hum Hypertens. 2023;37(12):1119–25.
Ryan SM, et al. Increases in pulse pressure impair acetylcholine-induced vascular relaxation. Am J Physiol-Heart Circ Physiol. 1995;268(1):H359–63.
Schulz E, Anter E, Keaney JF Jr. Oxidative stress, antioxidants, and endothelial function. Curr Med Chem. 2004;11(9):1093–104.
Eskurza I, et al. Ascorbic acid does not affect large elastic artery compliance or central blood pressure in young and older men. Am J Physiol-Heart Circ Physiol. 2004;286(4):H1528–34.
Eskurza I, et al. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556(1):315–24.
Dinenno FA, et al. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation. 1999;100(2):164–70.
Jablonski KL, et al. High-dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. J Appl Physiol. 2007;103(5):1715–21.
Pugh PJ, Jones TH, Channer KS. Acute haemodynamic effects of testosterone in men with chronic heart failure. Eur Heart J. 2003;24(10):909–15.
Yeo S, et al. Burden of male hypogonadism and major comorbidities, and the clinical, economic, and humanistic benefits of testosterone therapy: a narrative review. ClinEcon Outcomes Res. 2021;31–38.
Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(5):932–43.
Xie Y, et al. Protective effects of physiological testosterone on advanced glycation end product-induced injury in human endothelial cells. Mol Med Rep. 2017;15(3):1165–71.
Lopes-Virella MF, et al. Levels of Oxidized LDL and advanced glycation end products–modified LDL in circulating immune complexes are strongly associated with increased levels of carotid intima-media thickness and its progression in type 1 diabetes. Diabetes. 2011;60(2):582–9.
Li J, et al. Advanced glycation end product levels were correlated with inflammation and carotid atherosclerosis in type 2 diabetes patients. Open Life Sci. 2020;15(1):364–72.
Babcock MC, et al. Assessment of macro and microvascular function in aging males. J Appl Physiol. 2021;130(1):96–103.
Holwerda SW, et al. Sex and age differences in the association between sympathetic outflow and central elastic artery wall thickness in humans. Am J Physiol-Heart Circ Physiol. 2019;317(3):H552–60.
Holwerda SW, et al. Elevated muscle sympathetic nerve activity contributes to central artery stiffness in young and middle-age/older adults. J Hypertens. 2019;73(5):1025–35.
Moreau KL, Babcock MC, Hildreth KL. Sex differences in vascular aging in response to testosterone. Biol Sex Differ. 2020;11(1):1–14.
Tajar A, et al. Characteristics of androgen deficiency in late-onset hypogonadism: results from the European Male Aging Study (EMAS). J Clin Endocrinol Metab. 2012;97(5):1508–16.
Nardin C, et al. Cardiovascular phenotype of elevated blood pressure differs markedly between young males and females. Hypertension. 2018;72(6):1277–84.
Acknowledgements
We wish to acknowledge Teresa Witten and Ashley Brubaker for their contributions to this research.
Funding
NIH R01AG049762, T32AG000279, F32AG071273, K12HD057022 Colorado Clinical and Translational Sciences Institute UM1 TR004399, Colorado Nutrition and Obesity Research Center P30 DK048520 and Eastern Colorado GRECC.
Author information
Authors and Affiliations
Contributions
K.L.M. conceived and designed the research. K.L.H. and B.L.S. provided medical oversight of the study participants, evaluated inclusion and exclusion criteria, and reviewed adverse events. L.E.D. and K.L.M. analyzed data. L.E.D. and K.L.M. performed the statistical analyses. All authors helped in the interpretation of the data, drafting of the manuscript, and approving the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures were reviewed and approved by the Colorado Multiple Institutional Review Board (COMIRB).
Consent to participate
All participants gave their written informed consent to participate.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
DuBose, L.E., Babcock, M.C., Kohrt, W.M. et al. Gonadal status modulates large elastic artery stiffness in healthy middle-aged and older men. GeroScience (2024). https://doi.org/10.1007/s11357-024-01293-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11357-024-01293-y