Abstract
Virtual error amplification (VEA) in visual feedback enhances attentive control over postural stability, although the neural mechanisms are still debated. This study investigated the distinct cortical control of unsteady stance in older adults using VEA through cross-frequency modulation of postural fluctuations and scalp EEG. Thirty-seven community-dwelling older adults (68.1 ± 3.6 years) maintained an upright stance on a stabilometer while receiving either VEA or real error feedback. Along with postural fluctuation dynamics, phase–amplitude coupling (PAC) and amplitude–amplitude coupling (AAC) were analyzed for postural fluctuations under 2 Hz and EEG sub-bands (theta, alpha, and beta). The results revealed a higher mean frequency of the postural fluctuation phase (p = .005) and a greater root mean square of the postural fluctuation amplitude (p = .003) with VEA compared to the control condition. VEA also reduced PAC between the postural fluctuation phase and beta-band EEG in the left frontal (p = .009), sensorimotor (p = .002), and occipital (p = .018) areas. Conversely, VEA increased the AAC of posture fluctuation amplitude and beta-band EEG in FP2 (p = .027). Neither theta nor alpha band PAC or AAC were affected by VEA. VEA optimizes postural strategies in older adults during stabilometer stance by enhancing visuospatial attentive control of postural responses and facilitating the transition of motor states against postural perturbations through a disinhibitory process. Incorporating VEA into virtual reality technology is advocated as a valuable strategy for optimizing therapeutic interventions in postural therapy, particularly to mitigate the risk of falls among older adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Navigating uneven surfaces requires agility and adaptability, thus posing a greater challenge for older adults vulnerable to falls. A recommended strategy involves providing visual feedback that displays real-time task performance to train older adults who are prone to falls. Recent research suggests that virtual error amplification (VEA) in visual feedback is more effective for postural training than traditional feedback displaying real errors in both young [1,2,3] and older adults [4]. In VEA, computer technology artificially magnifies the size of execution errors deviating from the desired posture. The magnified visual errors outside the expected state could direct focus attention to the deviant events [4], according to the cue utilization hypothesis [5]. Incorporating VEA to reallocate cognitive resources has the potential to promote engagement in postural training [6] and better prepare older adults for a wider array of real-world challenges [3,4,5,6,7]. Postural training utilizing VEA has led to distinct plasticity changes for maintaining stance stability in both young [3] and older adults [4]. Nevertheless, the neuro-behavioral understanding of how VEA optimizes postural control remains limited, especially with regard to cortical reorganization within the context of postural dynamics.
Brain oscillations are sensitive to postural training, and they reflect the context-dependent integration of visual information and motor command execution [8, 9]. Increased theta power in front-central areas during challenging postural tasks suggests heightened cognitive demands for resolving sensorimotor conflicts and motor planning to maintain balance [10, 11]. Virtual error amplification (VEA) may potentiate mid-frontal theta activity for enhanced error monitoring and response selection [3]. Alpha power indexes the degree of relaxed wakefulness and cortical inhibition [12], with reduced visual reliance correlating with increased alpha power in central, parietal, and occipital regions [13]. Conversely, alpha power diminishes with increasing cognitive load during dual-task standing [14]. Beta-band activity maintains “the status quo” during voluntary movements, with alternations between synchronization and desynchronization in the frontal and parietal cortex during fall-recovery cycles [15]. In destabilizing posture situations, older adults exhibit higher beta power in postural control-related cortical areas to meet increased balance demands [16, 17]. In young adults, the immediate effect of VEA is a reduced visual load, leading to decreased prefrontal–parietal and prefrontal–occipital connectivity in the beta band during unsteady stance [3].
Postural fluctuations occur in a state-dependent manner, the result of intermittent control mechanisms to correct deviations from the desired stance pattern for postural stabilization [18,19,20]. The magnitude and regularity of postural fluctuations increase with age [21, 22], leading to coarser and simpler postural adjustments in older adults [23]. While postural fluctuations reflect strategies of postural correction, little attention has been given to the phase aspect of postural fluctuations, which denotes the organized timing relationship between different spectral components of postural sway with respect to perturbations. This aspect provides an alternative dimension for understanding the coordination and sequencing of movements in maintaining balance. This study investigated the neuro-behavioral aspects of postural control in older adults using VEA through phase–amplitude coupling (PAC) and amplitude–amplitude coupling (AAC) between low-frequency postural fluctuations and high-frequency scalp EEG. Cross-frequency modulation, encompassing PAC and AAC, traditionally used to characterize coordination among band-specific cortical activity across distributed cortical areas [24, 25], was employed to elucidate implicit coupling between low-frequency postural fluctuations and high-frequency cortical oscillations during postural regulation with VEA. The hypothesis suggests a feedback-dependent topological organization of cross-frequency modulation between postural fluctuations and EEG in the theta, alpha, and beta bands. Within the context of brain-behavioral coupling, the findings offer a novel insight into neural mechanisms for enhancing attentive control and postural flexibility in older adults using VEA.
Methods
Participants
This study employed a single-session cross-sectional experimental design, involving 37 retired older adults (22 females, 15 males; age, 68.1 ± 2.6 years; height, 159.6 ± 8.4 cm; weight, 57.4 ± 10.0 kg; BMI, 22.41 ± 10.0 kg/m2) with no neurological, cognitive, or degenerative conditions such as Parkinson’s disease, dementia, or stroke, and no severe cardiovascular conditions. They were functionally independent, physically active, and capable of walking. Approval was obtained from the institutional human research review board at the University Hospital (No. B-ER-105–032), and participants provided informed consent according to the principles of the Declaration of Helsinki.
Experimental procedures
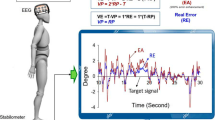
The experimental procedures closely followed our prior work [3]. In brief, participants maintained stability on a stabilometer (dimensions: 50 cm × 58 cm area, 25 cm radius, 18.5 cm height) for 60 s while receiving visual guidance. The movements of the stabilometer plate and a horizontal target line representing the ground level were shown simultaneously on a computer monitor (Fig. 1A). Two types of visual feedback were provided: real error (RE) and virtual error amplification (VEA) [3, 4, 26]. Participants aimed to align the plate’s movement with the target line. In the RE condition, visualized postural sway (VP) matched the plate’s movement, with visualized error (VE) equal to real error (VE = RE). In contrast, in the VEA condition, VP underwent a mathematical transformation (VP = 2RP-T, where RP is real postural sway and T is the target signal), resulting in a doubled visualized error (VE = 2RE). Essentially, participants perceived doubled execution errors in the VEA condition. Participants were unaware of the feedback type. Three 60-s experimental trials for both the RE and VEA conditions were assigned in a balanced order (participants with odd numbers: RE-VEA-RE-VEA-RE-VEA; participants with even numbers: VEA-RE-VEA-RE-VEA-RE) (Fig. 1B). A 3-min break was provided between every two trials to prevent fatigue.
A System setup and manipulation of error feedback for stabilometer stance. With mathematical transformation, the visualized postural sway in the error amplification (VEA) condition virtually doubled the size of the real execution error displayed in the control condition. The mathematical transformation is displayed in the formula boxes. In contrast, the subjects in the control condition received on-line error feedback without any manipulation. The subjects could see either real error feedback or error amplification feedback, depending on the order of the experimental trials. (VP, visualized postural sway; RP, real postural sway; VE, visualized error; RE, real error; T, target signal). B The flowchart of experiment. The subjects were divided into two groups. Each group conducted six experimental trials of stabilometer stance, following either the RE-first feedback protocol or the VEA-first feedback protocol, assigned in a balanced order. Below the flowchart, postural sway data from the six experimental trials of a typical subject in the odd-number group is displayed
Instrumentation
Continuous EEG data were recorded with a NuAmps amplifier (NeuroScan Inc., EI Paso, USA) and Ag–AgCl scalp electrodes following the International 10–20 system. Scalp EEG signals were localized at various cortical areas (Fp1/2, Fz, F3/4, F7/8, FT7/8, FCz, FC3/4, Cz, C3/4, CPz, CP3/4, Pz, P3/4, T3/4, T5/6, TP7/8, Oz, and O1/2). Reference electrodes were placed on each side of the mastoid process (A1/A2), and the ground electrode was placed on the forehead. To mitigate eye movement and blink artifacts, horizontal electrooculography (EOG) data were collected with electrodes at the outer canthus of the left and right eyes. For vertical EOG assessment, two electrodes were placed infra- and supra-orbitally at the right eye. Electrode impedances were maintained below 5 kΩ. The EEG data were recorded with a band-pass filter (cut-off frequencies, 0.1–70 Hz) and a 60 Hz notch filter. The tilting angle of the stabilometer surface was recorded with an inclinometer (Model FAS-A, LORD MicroStrain, USA) mounted by the side of the stabilometer. EEG data and angular plate movement were synchronized with the AD controller of the LabView platform (Labview v.8.5, National Instruments, USA), with a sampling rate set at 1 kHz.
Data analysis
Characterization of postural fluctuations
Angular plate movements (in degrees) underwent initial processing with a zero-phase low-pass filter (cut-off frequency, 4 Hz). Postural fluctuations were derived from the angular plate movements after the removal of linear trends. The initial and final 2 s of postural fluctuations in each trial were excluded from further analysis. Time series of postural fluctuations, totaling 56 s, were decomposed into the amplitude and phase components using the Hilbert transform (Fig. 2A). Postural stability during the stabilometer stance was indexed with root mean square (RMS). For the phase of postural fluctuations, which has been overlooked in the literature, additional characterization was conducted using RMS, sample entropy (SampEn), and mean frequency (MF). SampEn, ranging from 0 to 2, reflects the complexity of a biosignal, with higher values indicating greater complexity. The spectral profiles of the postural fluctuation phase were estimated using a fast Fourier transform and the Welch method (Hanning window, window length, 20 s, 20% overlapping segment). Mean frequency (MF) was determined from the spectra of postural fluctuations (Fig. 2A).
A Flowchart illustrating the signal processing steps to obtain phase–amplitude and amplitude–amplitude couplings between postural fluctuations and scalp EEG. The postural fluctuations were decomposed into amplitude and phase components using the Hilbert transform. The scalp EEG was conditioned with band-pass filter to sub-band EEG (theta, 4–7 Hz; alpha, 8–12 Hz; and beta, 13–35 Hz). B Schematic illustration depicting the quantification of phase–amplitude coupling (PAC) from a typical dataset. PAC was calculated between the phase components of postural fluctuations and the amplitudes of different sub-band EEG signals. The permutation test was employed to examine the statistical significance of PAC. The Z-value of PAC was used to compare the observed PAC with the shuffled coupling values through the permutation of the phase component of postural fluctuations
Phase–amplitude coupling (PAC) and amplitude–amplitude coupling (AAC) of postural fluctuations and sub-band EEG
All EEG data underwent initial conditioning with a band-pass filter (cut-off frequencies, 1 and 60 Hz) using a zero-phase finite impulse response (FIR) filter (60 dB/octave). Blinks were identified by creating a bipolar vertical electrooculography (EOG) channel, obtained by subtracting activity from the infraorbitally placed electrode from that in the superorbitally placed electrode. Eye movement or blink artifacts were then removed from the EEG using linear regression analysis based on bipolar vertical and horizontal electrooculogram channels [27, 28] in the NeuroScan 4.3 software program (NeuroScan Inc., El Paso, TX, USA). As with the stabilometer plate data, the first and last 2 s of EEG signals were excluded from analysis. For all EEG channels, band-specific EEG signals (theta, 4–7 Hz; alpha, 8–12 Hz; and beta, 13–35 Hz) were filtered using a zero-phase finite impulse response (FIR) band-pass filter (60 dB/octave) (Fig. 2A). EEG components potentially contaminated by low-frequency movement artifacts (< 4 Hz) and muscle activity (> 35 Hz) of the head and neck were not included in the analysis.
Phase–amplitude analysis of the postural fluctuation phases and amplitudes of sub-band EEG of all recording electrodes was performed using the modulation index (MI) (29). The force fluctuation phases were binned by the phases (18 bins, Δϕ = π/9), and the amplitudes of each sub-band EEG (theta, alpha, and beta) were averaged within each phase bin (Fig. 2B). Next, the mean amplitude was calculated for each of the 18 phase bins across all sub-band oscillations, producing a band-specific phase–amplitude plot for the experimental trial. Given its resilience to confounding factors such as signal-to-noise ratio and data length (Hülsemann et al., 2019), this study employed a modulation index (MI) utilizing the Kullback–Leibler distance and Shannon entropy to measure the divergence of a phase–amplitude plot. It is formulated as follows:
where P(j) is the amplitude for a given bin j, N is the number of bins (N = 18), and log(N) represents the entropy of a uniform distribution.
The observed modulation index (MI) values underwent a permutation test comparing them to a distribution of shuffled coupling values, following the approach outlined by Hülsemann et al. (2019) [30]. Shuffled coupling values were determined by calculating the MI values between the permuted time series of the phase components of the postural fluctuations and the amplitude time series of sub-band EEG in each EEG channel. This shuffling process was repeated 250 times in this study. To standardize the observed MI, it was normalized to the distribution of shuffled coupling values using the following formula:
where MI denotes the PAC coupling value, µ denotes the mean, and σ denotes the standard deviation (SD).
Next, amplitude–amplitude analysis was conducted by computing correlation coefficients between the amplitudes of postural fluctuations and the amplitudes of EEG signals within each channel across various frequency bands. Similarly, the observed AAC values underwent a permutation test (shuffling process 250 times) comparing them to shuffled coupling values. This process involved calculating AAC values between the permuted time series of postural fluctuation amplitudes and the amplitude time series of EEG in each channel. Finally, the observed AAC values were standardized using a specific formula:
where R denotes the AAC coupling value, µ denotes the mean, and σ denotes the standard deviation (SD). Data analysis was conducted offline in Matlab R2019a (The Mathworks Inc., Natick, USA).
Statistical analysis
The postural variables from the three experimental trials in both the VEA and control conditions were averaged for each subject. Paired t-statistics were used to contrast the RMS of postural fluctuation amplitude between the VEA and control conditions. Multivariate Hotelling’s T-squared statistics were applied to assess the visual feedback effect (VEA vs. control) on the variables (RMS, MF, and SampEn) of postural fluctuation phase. The post hoc test comprised paired t-tests, with significance determined using the Simes test to ascertain the level of significant difference. The Simes test, unlike the Bonferroni test, avoids over-correction. For all post hoc hypotheses (\(H={\cap }_{i=1}^{m}\)), the Simes test did not reject elementary Hi if pi ≤ i*0.05/m for ordered unadjusted p values (p1 ≤ … ≤ pm). The type 1 error rate using the Simes test was exactly 0.05. Also, Pearson’s correlation was conducted to examine the significance of the correlation between the normalized variables ((VEA-control)/control) of amplitude and the phase components of the postural fluctuations significantly modifiable by VEA. Next, standardized MI (ZMI) and AAC (ZR) of all EEG electrodes in both the VEA and control conditions were evaluated using paired t-tests to pinpoint feedback-dependent regions of interest. In this study, ZMI in the electrodes of the left frontal (F3 and FC3); sensorimotor (C3, C4, CP3, CPz, CP4, P3, Pz, and P4); and occipital (O2 and Oz) areas of ZMI were significantly different between the VEA and control conditions (p < 0.05). Hence, ZMI within the left frontal, sensorimotor, and occipital areas were pooled. Hotelling’s T-squared statistics were applied to assess the feedback effect (VEA vs. control) on the pooled ZMI in the three regions of interest. For AAC, only ZR in the FP2 was significantly different in the VEA and control conditions (p < 0.05). All statistical analyses were performed in IBM SPSS Statistics (v19). The level of significance was 0.05.
Results
The results of paired t-statistics showed that the RMS of postural fluctuation amplitude was smaller in the VEA condition than in the control condition (p = 0.003) (Table 1(A)). The results of Hotelling’s T-squared statistics indicated a significant visual feedback effect on postural fluctuation phase (Wilks’ Λ = 0.681, p = 0.004) (Table 1(B)). For postural fluctuation phase, VEA exhibited greater MF (p = 0.005) than the control condition, while the RMS and SampEn of the postural fluctuation phase were not significantly dependent on feedback mode (p > 0.05). On the other hand, post hoc analysis for postural fluctuation amplitude revealed that VEA resulted in a smaller RMS (p = 0.003) and higher MF (p = 0.025) compared to the control condition. Pearson’s correlation analysis revealed a significant correlation between the normalized decrease in the RMS of the postural fluctuation amplitude and the normalized increase in the MF of postural fluctuation phase with the use of VEA (r = 0.418, p = 0.010) (Table 1(C)).
Figure 3 illustrates the topological distribution of phase–amplitude coupling (PAC) between postural fluctuation phase and the EEG of various bands during stabilometer stance with real and VEA feedback. Paired t-statistics results showed no significant differences in PAC between the phase of postural fluctuations and the amplitude of EEG in the theta and alpha rhythms (the two upper rows) (p > 0.05). However, VEA significantly altered the topological distribution of PAC for beta rhythms in the left frontal, sensorimotor, and occipital regions (p < 0.05) (the bottom row), with a decreasing trend of beta PAC observed in the regions of interest. Figure 4 illustrates the topological distribution of amplitude–amplitude coupling (AAC) for the theta, alpha, and beta rhythms during stabilometer stance with real and VEA feedback. Paired t-statistics results indicated that AAC between the amplitude of postural fluctuations and the amplitude of EEG in the theta and alpha rhythms did not significantly vary with the visual feedback mode (the two upper rows) (p > 0.05). Conversely, AAC in Fp2 of the beta band was significantly influenced by VEA (p < 0.05) (the bottom row), revealing an increase in AAC in the VEA condition.
Scalp maps contrasting the topological distribution of phase–amplitude coupling (PAC) involving the phase components of postural fluctuations and sub-band EEG between the control and virtual error amplification (VEA) conditions. The scalp maps in the right column depict the topological distribution of P values based on paired t-statistics of PAC in the control and VEA conditions
Scalp maps contrasting the topological distribution of amplitude–amplitude coupling (AAC) involving the amplitude components of postural fluctuations and sub-band EEG amplitudes between the control and virtual error amplification (VEA) conditions. The scalp maps in the right column depict the topological distribution of P values based on paired t-statistics of AAC in the control and VEA conditions
Figure 5A contrasts the mean PAC values in the beta band for the regions of interest (left frontal (LF), sensorimotor (SM), and occipital (O) areas) between the control and VEA conditions. The results of Hotelling’s T-squared statistics indicated that the mean PAC values of the regions of interest were different for the two feedback modes (Wilks’ Λ = 0.744, p = 0.017). Post hoc analysis revealed that the mean PAC values in the LF (t36 = 2.747, p = 0.009), SM (t36 = 3.268, p = 0.002), and O (t36 = 2.474, p = 0.018) areas were consistently smaller in the VEA condition than in the control condition. Figure 5B contrasts the mean AAC values in the beta band in FP2 between the control and VEA conditions. The paired t-test results showed a significant increase in AAC value in FP2 with VEA compared to the control condition (t36 = − 2.299, p = 0.027).
Discussion
By examining postural fluctuation dynamics and cross-frequency modulations of postural fluctuations and EEG, this study showed that virtual error amplification (VEA) amended postural strategies with improved stability for older adults on a stabilometer. With VEA, PAC analysis revealed decoupling of postural fluctuation phase from beta oscillations in the fronto-centro-occipital regions. AAC analysis showed enhanced coupling between postural fluctuation amplitude and beta oscillations in the right prefrontal area. VEA did not affect cross-frequency coupling between low-frequency postural fluctuations and theta/alpha bands in older adults.
Characteristic changes in postural fluctuations with VEA for older adults
Older adults using virtual error amplification (VEA) demonstrated improved stability during stabilometer stance, as indicated by the smaller RMS of postural fluctuation amplitude (Table 1(A)). The postural benefit was associated with a higher MF in the phase components of the force fluctuations (Table 1(B)). Within the intermittent control framework [18,19,20], postural sway represents periodic corrective actions aimed at countering excessive deviations from the upright position. In the VEA condition, the reduced amplitude of the postural fluctuations suggests finer postural scaling to the target position of the visual feedback for enhancing stability. An increase in the MF of the postural fluctuation phase induced by VEA suggests more frequent corrective attempts, which is functionally correlated with the reduced postural fluctuation amplitude (r = 0.418, p = 0.010). The present findings align with previous research in younger adults [3] and other timing-based motor tasks [26, 31, 32]. However, in contrast to the VEA effects in young adults [3], there was no significant increase in the entropy measure of postural fluctuations in older adults, possibly due to a loss of complexity modulation in posture in this population [33]. VEA did not effectively enhance postural regulation in a manner as intricate as that observed in young adults, likely due to degenerative structural changes in postural networks. Overall, integrating VEA into virtual reality technology is still recommended to enhance therapeutic processes in postural therapy, particularly for fall prevention in older adults.
VEA-related decrease in phase–amplitude coupling between postural fluctuation phase and beta oscillations
The improvement in postural stability with VEA is associated with the reorganization of descending commands, as indicated by the phase–amplitude coupling (PAC) between postural fluctuation phase and beta oscillations (Fig. 3). PAC decoupling in the beta band occurs within posture-related cortical networks in the left frontal, sensorimotor, and occipital regions (Figs. 3, 5A). These networks, encompassing the parietal-occipital region, frontal-central region, and occipital lobe, orchestrate muscle activity to manage visual challenges during standing [9, 34, 35]. Beta cortical activity, commonly observed during routine motor/cognitive tasks, contributes to maintaining a stationary postural position (36,37). Beta synchronization aids in executing minute responsive postural adjustments based on reliable sensory feedback. However, its functional advantages in preserving the “status quo” are contextually dependent [38]. Remaining inflexibly in the “status quo” can hinder the update of appropriate postural responses against drastic perturbation. Elevated relative beta power in the parietal area during sensory manipulation with postural perturbations has been linked to deteriorated postural control performance and longer movement latency [17, 39]. Therefore, a transient decrease in beta synchronization reflects functionally necessary adaptations to postural threats or escalating balance demands [40].
In the VEA condition, the reduction in PAC between the force fluctuation phase and beta oscillations within the posture-related networks (Figs. 3C and 5A) mirrors beta desynchronization, a response to heightened postural demands. This decoupling of PAC in the beta band reflects a departure from the “status quo” to facilitate a transition in the motor state concerning the phasing of postural fluctuations. This argument gains empirical support from the simultaneous increase in the mean frequency of the postural fluctuation phase in the VEA condition (Table 1(A)). Therefore, our findings suggest that phase information indeed plays an influential role in cortical control for stance stability when VEA is applied. In previous work with younger adults [3, 41], VEA was shown to enhance the attentive focus on action monitoring with the mid-frontal area [3]. Consistent with previous observations, this study further demonstrates that focusing on phase variations from amplified visualized errors facilitates the updating of postural strategies, enabling swift and adaptable responses to dynamic environments rather than rigid adherence to established routines. This aids in coordinating motor responses from the sensorimotor network to cope with postural threats for older adults during stabilometer stance.
VEA-related increase in amplitude–amplitude coupling between postural fluctuation phase and beta oscillations
Interestingly, AAC between force fluctuation amplitude and EEG Fp2 in the beta band of the older adults was significantly enhanced with VEA during stabilometer stance (Figs. 4 and 5B). In fact, local EEG activity in the beta band in Fp2 was also observed to be enhanced in young adults using VEA during stabilometer stance, though the functional significance of right prefrontal activation remains unclear [3]. Interconnected with other frontoparietal cortical regions, the right prefrontal cortex is implicated in motor-related cognitive responses [42], including visuospatial attention [43] and action plan preparation [44], especially in early motor learning [45] and during complex visuomotor tasks [46]. Therefore, increased activation in the right prefrontal area is considered indicative of motor planning in alignment with visuomotor rules [47]. In this study, the rise in AAC in the right prefrontal cortex may reflect an awareness of the current postural state being uncertain in the VEA condition. This occurred when older adults directed their attention to evaluate spatial cues on the computer monitor during trajectory-path processing and interpreted the exaggerated mismatch in amplitude as indicative of postural destabilization. The increase in AAC between postural fluctuations and activation of the right prefrontal cortex in the beta band may relate to heightened visuospatial attention, which is functionally crucial for resolving conflicts between motor intentions and augmented visualized errors [48]. The application of VEA could be an example scenario of the cue utilization hypothesis [5]. The attention of participants could be optimally tuned to relevant performance cues (error information) with VEA, leading to superior postural performance for avoidance of occasional distractions [4, 6, 7]. Attentional narrowing to environmental cues relevant to postural stability is critical for fall prevention in older adults.
Conclusions
The study demonstrated improved postural balance in older adults with VEA and described the underlying cross-frequency brain-behavioral modulations. Compared to traditional visual feedback, VEA not only reduced the amplitude of postural fluctuations but also enhanced the mean frequencies of both phase and amplitude in older adults during stabilometer stance. In particular, the VEA-induced spectral increase in the postural fluctuation phase was significantly related to enhanced postural stability during stabilometer stance. These observed balance benefits at the behavioral level were accompanied by (1) a global decoupling of postural fluctuation phase and beta oscillations in the fronto-centro-occipital regions and (2) enhanced amplitude–amplitude coupling between postural fluctuations and beta oscillations in the right prefrontal lobe. These neural scenarios suggest that VEA could facilitate visuospatial processing of postural cues and flexible shifts in postural strategies that enable rapid adaptation to varying stance constraints in older adults.
Data availability
The data sets generated and analyzed in this study are available from the corresponding author upon reasonable request. Sample data are available under reasonable request for replication purposes.
Code availability
The scripts for analysis are available from the corresponding author upon reasonable request.
References
Ferrari M, Bisconti S, Spezialetti M, Basso Moro S, Di Palo C, Placidi G, Quaresima V. Prefrontal cortex activated bilaterally by a tilt board balance task: a functional near-infrared spectroscopy study in a semi-immersive virtual reality environment. Brain Topogr. 2014;27(3):353–65. https://doi.org/10.1007/s10548-013-0320-z.
O’Brien K, Crowell CR, Schmiedeler J. Error augmentation feedback for lateral weight shifting. Gait Posture. 2017;54:178–82. https://doi.org/10.1016/j.gaitpost.2017.03.003.
Chen YC, Tsai YY, Chang GC, Hwang IS. Cortical reorganization to improve dynamic balance control with error amplification feedback. J Neuroeng Rehabil. 2022;19(1):3. https://doi.org/10.1186/s12984-022-00980-1.
Chen YC, Chang GC, Huang WM, Hwang IS. Quick balance skill improvement after short-term training with error amplification feedback for older adults. NPJ Sci Learn. 2023;8(1):3. https://doi.org/10.1038/s41539-022-00151-w.
Easterbrook JA. The effect of emotion on cue utilization and the organization of behavior. Psychol Rev. 1959;66(3):183–201. https://doi.org/10.1037/h0047707.
Krause D, Koers T, Maurer LK. Valence-dependent brain potentials of processing augmented feedback in learning a complex arm movement sequence. Psychophysiology. 2020;57(3):e13508. https://doi.org/10.1016/j.neuroscience.2021.04.016.
Abdollahi F, Case Lazarro ED, Listenberger M, Kenyon RV, Kovic M, Bogey RA, Hedeker D, Jovanovic BD, Patton JL. Error augmentation enhancing arm recovery in individuals with chronic stroke: a randomized crossover design. Neurorehabil Neural Repair. 2014;28(2):120–8. https://doi.org/10.1177/1545968313498649.
Chen YC, Huang CC, Zhao CG, Hwang IS. Visual effect on brain connectome that scales feedforward and feedback processes of aged postural system during unstable stance. Front Aging Neurosci. 2021;13:679412. https://doi.org/10.3389/fnagi.2021.679412.
Tsai YY, Chen YC, Zhao CG, Hwang IS. Adaptations of postural sway dynamics and cortical response to unstable stance with stroboscopic vision in older adults. Front Physiol. 2022;13:919184. https://doi.org/10.3389/fphys.2022.919184.
Peterson SM, Ferris DP. Differentiation in theta and beta electrocortical activity between visual and physical perturbations to walking and standing balance. ENeuro. 2018;5(4):ENEURO.0207-18.2018. https://doi.org/10.1523/ENEURO.0207-18.2018.
Stokkermans M, Solis-Escalante T, Cohen MX, Weerdesteyn V. Midfrontal theta dynamics index the monitoring of postural stability. Cereb Cortex. 2023;33(7):3454–66. https://doi.org/10.1093/cercor/bhac283.
Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev. 2007;53(1):63–88. https://doi.org/10.1016/j.brainresrev.2006.06.003.
Ma L, Marshall PJ, Wright WG. The impact of external and internal focus of attention on visual dependence and EEG alpha oscillations during postural control. J Neuroeng Rehabil. 2022;19(1):81. https://doi.org/10.1186/s12984-022-01059-7.
Kahya M, Gouskova NA, Lo OY, Zhou J, Cappon D, Finnerty E, Pascual-Leone A, Lipsitz LA, Hausdorff JM, Manor B. Brain activity during dual-task standing in older adults. J Neuroeng Rehabil. 2022;19(1):123. https://doi.org/10.1186/s12984-022-01095-3.
Nakamura A, Miura R, Suzuki Y, Morasso P, Nomura T. Discrete cortical control during quiet stance revealed by desynchronization and rebound of beta oscillations. Neurosci Lett. 2023;814:137443. https://doi.org/10.1016/j.neulet.2023.137443.
Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34(5):721–33. https://doi.org/10.1016/j.neubiorev.2009.10.005.
Hu Y, Petruzzello SJ, Hernandez ME. Beta cortical oscillatory activities and their relationship to postural control in a standing balance demanding test: influence of aging. Front Aging Neurosci. 2023;15:1126002. https://doi.org/10.3389/fnagi.2023.1126002.
Asai Y, Tasaka Y, Nomura K, Nomura T, Casadio M, Morasso P. A model of postural control in quiet standing: robust compensation of delay-induced instability using intermittent activation of feedback control. PLoS One. 2009;4(7):e6169. https://doi.org/10.1371/journal.pone.0006169.
Gawthrop P, Loram I, Lakie M, Gollee H. Intermittent control: a computational theory of human control. Biol Cybern. 2011;104(1–2):31–51. https://doi.org/10.1007/s00422-010-0416-4.
Stins JF, Roerdink M. Unveiling intermittency in the control of quiet upright standing: beyond automatic behavior. Front Neurol. 2018;9:850. https://doi.org/10.3389/fneur.2018.00850.
Kouzaki M, Masani K. Postural sway during quiet standing is related to physiological tremor and muscle volume in young and elderly adults. Gait Posture. 2012;35(1):11–7. https://doi.org/10.1016/j.gaitpost.2011.03.028.
Błaszczyk JW. The use of force-plate posturography in the assessment of postural instability. Gait Posture. 2016;44:1–6. https://doi.org/10.1016/j.gaitpost.2015.10.014.
Rasman BG, van der Zalm C, Forbes PA. Age-related impairments and influence of visual feedback when learning to stand with unexpected sensorimotor delays. Front Aging Neurosci. 2023;15:1325012. https://doi.org/10.3389/fnagi.2023.
Young CK, Eggermont JJ. Coupling of mesoscopic brain oscillations: recent advances in analytical and theoretical perspectives. Prog Neurobiol. 2009;89(1):61–78. https://doi.org/10.1016/j.pneurobio.2009.06.002.
Hyafil A, Giraud AL, Fontolan L, Gutkin B. Neural cross-frequency coupling: connecting architectures, mechanisms, and functions. Trends Neurosci. 2015;38(11):725–40. https://doi.org/10.1016/j.tins.2015.09.001.
Hwang IS, Lin YT, Huang WM, Yang ZR, Hu CL, Chen YC. Alterations in neural control of constant isometric contraction with the size of error feedback. PLoS One. 2017;12(1):e0170824. https://doi.org/10.1371/journal.pone.0170824.
Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. https://doi.org/10.1111/j.1469-8986.1986.tb00696.x.
Croft RJ, Barry RJ. Removal of ocular artifact from the EEG: a review. Neurophysiol Clin. 2000;30:5–19. https://doi.org/10.1016/S0987-7053(00)00055-1.
Tort AB, Komorowski R, Eichenbaum H, Kopell N. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol. 2010;104(2):1195–2101. https://doi.org/10.1152/jn.00106.2010.
Hülsemann MJ, Naumann E, Rasch B. Quantification of phase-amplitude coupling in neuronal oscillations: comparison of phase-locking value, mean vector length, modulation index, and generalized-linear-modeling-cross-frequency-coupling. Front Neurosci. 2019;13:573. https://doi.org/10.3389/fnins.2019.00573.
Milanese C, Corte S, Salvetti L, Cavedon V, Agostini T. Correction of a technical error in the golf swing: error amplification versus direct instruction. J Mot Behav. 2016;48(4):365–76. https://doi.org/10.1080/00222895.2015.1102699.
Milot MH, Marchal-Crespo L, Beaulieu LD, Reinkensmeyer DJ, Cramer SC. Neural circuits activated by error amplification and haptic guidance training techniques during performance of a timing-based motor task by healthy individuals. Exp Brain Res. 2018;236(11):3085–99. https://doi.org/10.1007/s00221-018-5365-5.
Ko JH, Newell KM. Aging and the complexity of center of pressure in static and dynamic postural tasks. Neurosci Lett. 2016;610:104–9. https://doi.org/10.1016/j.neulet.2015.10.069.
Marlin A, Mochizuki G, Staines WR, McIlroy WE. Localizing evoked cortical activity associated with balance reactions: does the anterior cingulate play a role? J Neurophysiol. 2014;111(12):2634–43. https://doi.org/10.1152/jn.00511.2013.
Saradjian AH. Sensory modulation of movement, posture and locomotion. Neurophysiol Clin. 2015;45(4–5):255–67. https://doi.org/10.1016/j.neucli.2015.09.004.
Engel AK, Fries P. Beta-band oscillations–signalling the status quo? Curr Opin Neurobiol. 2010;20(2):156–65. https://doi.org/10.1016/j.conb.2010.02.015.
Echeverria-Altuna I, Quinn AJ, Zokaei N, Woolrich MW, Nobre AC, van Ede F. Transient beta activity and cortico-muscular connectivity during sustained motor behaviour. Prog Neurobiol. 2022;214:102281. https://doi.org/10.1016/j.pneurobio.2022.102281.
Spitzer B, Haegens S. Beyond the status quo: a role for beta oscillations in endogenous content (re)activation. eNeuro. 2017;4(4):ENEURO.0170-17.2017. https://doi.org/10.1523/ENEURO.0170-17.2017.
Ghosn NJ, Palmer JA, Borich MR, Ting LH, Payne AM. Cortical beta oscillatory activity evoked during reactive balance recovery scales with perturbation difficulty and individual balance ability. Brain Sci. 2020;10(11):860. https://doi.org/10.3390/brainsci10110860.
Malcolm BR, Foxe JJ, Joshi S, Verghese J, Mahoney JR, Molholm S. De Sanctis P Aging-related changes in cortical mechanisms supporting postural control during base of support and optic flow manipulations. Eur J Neurosci. 2021;54(12):8139–57. https://doi.org/10.1111/ejn.15004.
Marchal-Crespo L, Rappo N, Riener R. The effectiveness of robotic training depends on motor task characteristics. Exp Brain Res. 2017;235(12):3799–816. https://doi.org/10.1007/s00221-017-5099-9.
Silveira R, Prado RCR, Brietzke C, Coelho-Júnior HJ, Santos TM, Pires FO, Asano RY. Prefrontal cortex asymmetry and psychological responses to exercise: a systematic review. Physiol Behav. 2019;208:112580. https://doi.org/10.1016/j.physbeh.2019.112580.
Pollmann S, von Cramon DY. Object working memory and visuospatial processing: functional neuroanatomy analyzed by event-related fMRI. Exp Brain Res. 2000;133(1):12–22. https://doi.org/10.1007/s002210000396.
Hoshi E. Cortico-basal ganglia networks subserving goal-directed behavior mediated by conditional visuo-goal association. Front Neural Circ. 2013;7:158. https://doi.org/10.3389/fncir.2013.00158.
Halsband U, Lange RK. Motor learning in man: a review of functional and clinical studies. J Physiol Paris. 2006;99(4–6):414–24. https://doi.org/10.1016/j.jphysparis.2006.03.007.
Le DT, Ogawa H, Tsuyuhara M, Watanabe K, Watanabe T, Ochi R, Nishijo H, Mihara M, Fujita N, Urakawa S. Coupled versus decoupled visuomotor feedback: differential frontoparietal activity during curved reach planning on simultaneous functional near-infrared spectroscopy and electroencephalography. Brain Behav. 2022;12(7):e268. https://doi.org/10.1002/brb3.2681.
Tanji J, Shima K, Mushiake H. Concept-based behavioral planning and the lateral prefrontal cortex. Trends Cogn Sci. 2007;11(12):528–34. https://doi.org/10.1016/j.tics.2007.09.007.
Rinaldi MC, Piras F, Pizzamiglio L. Lack of awareness for spatial and verbal constructive apraxia. Neuropsychologia. 2010;48(6):1574–82. https://doi.org/10.1016/j.neuropsychologia.2010.01.026.
Funding
This work was supported by grants from the Ministry of Science and Technology, Taiwan, R.O.C., under grant numbers MOST 105–2410-H-040–009 and 107–2314-B-006–017-MY3.
Author information
Authors and Affiliations
Contributions
Y.C.C. and I.S.H. designed the experiments. Y.Y.T. and C.G.Z. conducted the experiments. Y.C.C., W.M.H., and I.S.H. analyzed the data. Y.C.C., Y.Y.T., and I.S.H. wrote the paper. Y.C.C. and I.S.H. acquired funding.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in this study were in accordance with the ethical standards of the Helsinki Declaration and approved by the National Cheng Kung University Hospital Research Ethics Committee.
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Consent for publication
The participant has provided consent for medical photography and consented for the photographs to be used in the publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Chen, YC., Tsai, YY., Huang, WM. et al. Cross-frequency modulation of postural fluctuations and scalp EEG in older adults: error amplification feedback for rapid balance adjustments. GeroScience (2024). https://doi.org/10.1007/s11357-024-01258-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11357-024-01258-1