Abstract
It is well established from previous cross-sectional studies that telomeres shorten with age. However, due to a considerable inter-individual variation in telomere length (TL), its relationship with biological aging is difficult to unpick. Longitudinal repeated assessments of TL changes within individuals should augment our understanding of TL dynamics in aging. This study disentangles within- and inter-individual effects of age on leukocyte telomere length (LTL) dynamics in a large population-based cohort of older adults. A total of 4053 subjects aged 50 and older from the WHO Study on global AGEing and adult health (SAGE) in Shanghai were studied. Relative LTL (T/S ratio) was measured at baseline (2009–2010) and follow-up (2017–2018) by quantitative real-time polymerase chain reaction. We used linear random slope models to analyze LTL dynamics in relation to age and sex and within-subject centering method to distinguish within- versus between-subject effects. We observed LTL shortening in 66.32%, maintenance in 11.23%, and elongation in 22.45% of the study participants. LTL declined significantly with age both cross-sectionally and longitudinally. More importantly, the longitudinal decline in LTL was much greater than the cross-sectional decline (− 0.017 (p < 0.001) versus − 0.002 (p < 0.001) per year). Furthermore, women had a lower within-subject LTL shortening rate than men (− 0.014 versus − 0.020 per year, p < 0.001). The within-individual longitudinal decline in LTL was much greater than the inter-individual cross-sectional decline, indicating that chronological age might impose a greater impact on LTL shortening than other influencing factors combined. Moreover, women showed a lower within-individual LTL shortening rate than men.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Telomeres are nucleoprotein complexes at the ends of eukaryotic chromosomes, comprised of repetitive DNA sequences (5′-TTAGGG-3′) and its binding proteins [1]. Telomeres maintain genome stability by protecting the ends of chromosomes from damage and degradation [1]. Due to the “end replication problem,” telomeres cannot be fully replicated by DNA polymerases during somatic cell division [2, 3]. As a result, telomeres typically shorten by 50–200 base pairs with each somatic cell division [4]. When telomere length (TL) reaches a critical level, cells cannot divide anymore and undergo senescence or apoptosis [5,6,7].
It is well established from previous studies that telomeres shorten with age. A considerable amount of cross-sectional studies have shown an age-dependent shortening of leukocyte telomere length (LTL) in humans of 20–40 base pairs per year [8]. However, the cross-sectional design can only infer inter-individual variation in TL at one time point. There is a large inter-individual difference in TL at any age cross-sectionally, commencing at birth, and approximately 36–82% is explained by heritability [9,10,11,12,13,14,15]. Besides heritability, sex and ethnicity have also been associated to TL. Females and African and Hispanic origin tend to have longer telomeres compared to males and European origin [16,17,18]. TL shortening rate is also highly variable between individuals [19, 20]. Previous research has suggested that, for a given age, approximately 30% of the difference in longitudinal age-dependent TL attrition rate between individuals is heritable [12, 14, 15]. Given the considerable inter-individual variation in TL, its relationship with the process of biological aging is difficult to unpick. For instance, the difference in TL between individuals at the same age might be due to different cross-sectional TL at birth, different longitudinal change rate in TL in biological aging afterward, or both. Hence, longitudinal repeated assessments of TL which can reflect within-individual change should augment our understanding of TL dynamics in human aging. However, such studies remain scarce, and the results are inconsistent [8, 21]. Moreover, in contrast with prior cross-sectional studies, some longitudinal studies on TL found no significant associations of telomere trajectory with sex or ethnicity [8, 22, 23].
In the present study, we sought to evaluate LTL dynamics in a large longitudinal population-based cohort of Chinese adults aged 50 and older. In particular, we aimed to disentangle within- and inter-individual effects of age on LTL change over time. Furthermore, we tested for sex differences in LTL dynamics throughout the study.
Material and methods
Study population
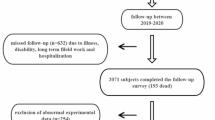
Participants were drawn from a large ongoing population-based cohort study, the WHO Study on global AGEing and adult health (SAGE) in Shanghai. Detailed descriptions of SAGE have been previously described [24]. Briefly, the SAGE is a population representative household cohort study in China, Ghana, India, Mexico, Russian Federation, and South Africa, which measured health status and well-being, health systems coverage and responsiveness, and health care expenditures. SAGE focuses on adults aged 50 and older with a smaller cohort of respondents aged 18–49 years included for comparison purposes. Of the 15,050 individuals in SAGE China sample, 1932 were from Shanghai. We then enlarged the sample size of SAGE in Shanghai to 9524, to obtain a sub-state representative sample using the same multistage clustered sampling method and survey instrument. 99.4% of the SAGE Shanghai sample are Han Chinese, 0.3% are other races, and 0.3% have missing information on race. At baseline (wave 1) from 2009 to 2010, 8629 community dwellers aged 50 and older were recruited from five districts of Shanghai, China. Of subjects recruited, 8154 (94.5%) had LTL measured at wave 1. Among these, 4053 (49.7%) subjects had LTL measured again at wave 3 (2017–2018) and were included in this study. This study was approved by the Shanghai Center for Disease Control and Prevention Ethical Review Committee. All participants provided informed written consent.
Leukocyte telomere length measurements
Relative LTL was measured at both wave 1 and wave 3. Relative LTL in the DNA extracted from peripheral blood leukocytes were determined by quantitative real-time polymerase chain reaction (Q-PCR) [25], with β-globin as a single copy reference gene. In short, two Q-PCRs were undertaken for each sample, a telomere-specific Q-PCR and a single-copy gene-specific Q-PCR (β-globin). Telomere (T) PCR and single copy gene (S) PCR were always performed in separate 384-well plates. Two master mixes of PCR reagents were prepared, one with the T primer pair, and the other one with the S primer pair. The primer sequences were as follows: telomere-F: 5′-ACACTAAGGTTTGGGTTTGGGTTTGGGTTTGGGTTAGTGT-3′; telomere-R: 5′-TGTTAGGTATCCCTATCCCTATCCCTATCCCTATCCCTAACA-3′; β-globin-F: 5′-GCTTCTGACACAACTGTGTTCACTAGC-3′; and β-globin-R: 5-CACCAACTT CATCCACGTTCACC-3′. Both T and S had a final reaction volume of 10 μl per well. The composition shared by the T PCR mix is as follows: SYBR® Green master mix 5 μl, telomere-F 400 nM 1 μl, telomere-R 400 nM 1 μl, and H2O 2 μl. The composition shared by the S PCR mix is as follows: SYBR® Green master mix 5 μl, β-globin-F 500 nM 1 μl, β-globin-R 500 nM 1 μl, and H2O 2 μl. A high-precision automatic pipette workstation of TEasy AP 400 Tiangen biotech (Beijing) Co. Ltd was used for transferring in a 384-well format plate a volume of 9 μl reaction mix and 1 μl DNA (10 ng/μl). All PCRs were performed on a 7900HT Fast Real-Time PCR System (Applied Biosystems). The thermal cycling profile for both amplicons began with a 95 °C incubation for 15 min; for telomere PCR, there followed 40 cycles of 95 °C for 15 s, 56 °C for 1 min; and for β-globin PCR, there followed 40 cycles of 95 °C for 15 s, 58 °C for 1 min. At the end of each real-time PCR reaction to verify the specificity of amplified, a melting curve was added for both T and S PCR. A fresh standard curve, from a pooled control samples, ranging from 60 to 0.74 ng/μl (serial dilutions 1:2), was included in every T and S PCR runs. Each sample was run in duplicate. Quality control parameters used for the amplifications comprised a cut-off of 0.25 for the standard deviation (SD) of the threshold cycle (Ct) for sample replicates. The standard deviation of cycle threshold (Ct) value for duplicate sample was smaller than 0.25. At an SD above 0.25, the sample was reanalyzed. The correlation coefficient (R2) for the standard curve in each run was larger than 0.98, and the real-time PCR amplification efficiencies were between 90 and 110%. The average of the two T measurements was divided by the average of the two S measurements to calculate the average relative telomere length (T/S ratio). All tests were performed by the same technician in the same laboratory of the Shanghai Municipal Center for Disease Control and Prevention. To get a relatively moderate cut point and allow for possible fluctuations, subjects with LTL change more than − 5% were grouped as shortened group, between − 5 and 5% as stable group, and more than 5% as elongated group.
Statistical analyses
Since repeated LTL measurements on individuals are two-level hierarchical data, with LTL measurement occasions at level 1 (j) and individuals at level 2 (i), we analyzed LTL variation using linear random slope models with maximum-likelihood estimates. By modeling individuals and their longitudinal measurements as nested random effects, random effects model splits total variance into between-individual and within-individual components. A random effect assumes different random intercepts and slopes for each subject, and this characterizes idiosyncratic variation that is due to individual differences. In the case of our model here, regression models were fitted to each individual’s longitudinal LTL trajectory, resulting in an average model for the sample (fixed effects) plus individual deviations from the average model (random effects). Covariates included age as the time scale and sex. We formalized our approach in the following standard random slope model:
The random intercept term u0i, random slope term u1i, and residual error term e0ij are assumed to be drawn from a Gaussian distribution with mean 0 and variance \( {\sigma}_{\mu 0}^2 \), \( {\sigma}_{\mu 1}^2 \), and \( {\sigma}_e^2, \)respectively. Subscripts refer to the level at which parameters are variable; age is variable within and between individuals.
To distinguish within- versus between-subject effects, we used within-subject centering method which involves subtracting subject’s mean age from each observation age (ageij-\( \overline{ag{e}_i} \)) [26]. Two new predictor variables were derived to express the within- and between-subject variation components. The new model with these two new fixed effects is only slightly different from the standard random slope model in Eq. (1):
and it allows us to test whether either the within-subject effect (βW) or the between-subject effect (βB) is significant. The mean age estimate (βB) in the model represents between-subject differences, the cross-sectional slope of the relationship between LTL and age. The delta age estimate (βW) represents the within-subject effect of LTL change with age, the longitudinal slope of LTL change within individuals between wave 1 and wave 3.
To test for potential sex difference in the cross-sectional (mean age) and longitudinal (delta age) associations of LTL with age, we added the interaction terms sexi×\( \overline{ag{e}_i} \) and sexi×(ageij-\( \overline{ag{e}_i} \)) in model (2):
Based on these findings, we then conduct our analyses separately by sex:
Subject ID and age (for model 1)/delta age (for models 2–4) were included as random effects. All models were adjusted for sampling weights. Individual weights at person level were calculated based on the selection probability at each stage of selection. Individual weights were post-stratified by district, sex, and age groups according to the 2010 population projections of Shanghai and weight up to the total number of persons aged 50 and older. Moreover, we used Student’s t test and one-way ANOVA for continuous variables and Chi-square test for categorical variables to compare LTL and LTL changes in different groups. Pearson’s correlation analysis was used to evaluate the correlations of LTL change and LTL at wave 3 with baseline LTL. All analyses were performed in Stata 16.0.
Results
Of 4053 subjects who had LTL measured at both wave 1 and wave 3 with an interval between 7 and 8 years, 1859 (45.87%) were men and 2194 (54.13%) were women. The subjects ranged in age from 50 to 91 at baseline, with a mean age of 61. As shown in Table 1, subjects had a mean relative LTL of 0.80 and 0.67 at wave 1 and wave 3, respectively. On average, relative LTL shortened by 0.13 from wave 1 to wave 3, at a rate of − 0.02 per year. Older age groups had a significantly shorter LTL at both wave 1 and wave 3 compared with their younger counterparts (p < 0.01); however, no significant differences were found in the relative LTL changes among different age groups (p = 0.32). Compared with men, women had a significantly shorter LTL at wave 1 (p < 0.01); but due to a lower LTL shortening rate in women (relative LTL change per year: − 0.02 (men) versus − 0.01 (women), p<0.01), the difference in LTL at wave 3 was no longer significant (p = 0.22). Women’s mean relative LTL is significantly shorter than men’s at wave 1 in younger age groups (50–59 (p < 0.01), 60–69 (p < 0.01), and 70–79 (p = 0.04)), but not in the oldest age group (> = 80 (p = 0.14)). Women’s yearly relative LTL shortening rate is significantly lower than men’s (− 0.01 versus − 0.02) in younger age groups (50–59 (p < 0.01) and 60–69 (p < 0.01)), but not in older age groups (70–79 (p = 0.06) and > = 80 (p = 0.17)).
We further grouped the subjects into three groups in Table 2 according to their relative LTL change within individuals from wave 1 to wave 3. The majority of our study subjects (66.32%) had shortened LTL, 11.23% had stable LTL, while 22.45% had elongated LTL from wave 1 to wave 3. No significant difference was observed in the patterns of relative LTL change among different age groups (p = 0.67). Compared with men, women had a significantly lower percentage of shortened LTL and a higher percentage of elongated LTL (p < 0.01). Compared with the shortened group, the elongated group had a shorter LTL at wave 1 (p < 0.01) but a longer LTL at wave 3 (p < 0.01). LTL change and LTL at wave 3 were both significantly correlated with baseline LTL (Supplementary Figure 1). Individuals with the longest baseline LTL tended to shorten their LTL the most and vice versa (r = − 0.61, p < 0.01). The positive correlation between LTL at wave 1 and LTL at wave 3 was much weaker (r = 0.12, p < 0.01).
As shown in Table 3, relative LTL declined significantly with age (model 1), at a rate of − 0.006 (p < 0.001) per year. The sex effect is strongly significant; women’s LTL was 0.015 (p = 0.006) shorter than men’s on average. The subject-specific slopes did not vary much between individuals (random slope SD = 0.001), compared to the variation in intercepts between individuals (random intercept SD = 0.164). Model 2 reveals that relative LTL significantly declined with age both cross-sectionally, comparing individuals that differ in age (mean age), and longitudinally, within individuals at different ages (delta age). The longitudinal decline rate (within-subject slope) was much larger than cross-sectional decline rate (between-subject slope). Older individuals had shorter LTL, and the slope of this cross-sectional decline (β for mean age) was − 0.002 (p < 0.001) per year. Longitudinal LTL shortening rate (β for delta age) was much larger, at − 0.017 (p < 0.001) per year. We next allowed the effect of age on LTL change to differ for men and women by adding interaction terms sexi×\( \overline{ag{e}_i} \) (cross-sectionally) and sexi×(ageij-\( \overline{ag{e}_i} \)) (longitudinally) in model 3. The interaction between sex and mean age was insignificant (β = 0.000, p = 0.670), indicating that the cross-sectional effect of age on LTL decline between individuals did not differ by sex. In contrast, the interaction between sex and delta age was highly significant (β=0.007, p < 0.001), which means the longitudinal effect of age on LTL decline within individuals differed by sex. We therefore repeated the analysis for men and women separately (model 4). Compared with men, women had a lower longitudinal LTL shortening rate within individuals (− 0.020 (men) versus − 0.014 (women)). Finally, we visualized the sex difference in model predicted mean LTL in relation to age in Supplementary Figure 2. Men had longer LTL than women from age 50 to late 70s, whereas due to a lower TL attrition rate in women, the opposite was observed after late 70s.
We also stratified our analysis by age group at wave 1 (Table 4). Significant within-subject effects were observed for all age groups (p < 0.001), and the longitudinal LTL decline rates (within-subject slopes) were similar across all age groups. However, the between-subject effect was only significant for age group 60–69 (p = 0.015). The longitudinal decline rate (within-subject slope) was much larger than cross-sectional decline rate (between-subject slope) for all age groups.
Discussion
To the best of our knowledge, this is the largest cohort study so far reporting the longitudinal LTL change in relation to age and sex and one of few such studies among Asians. More importantly, in contrast to prior studies, our study was able to disentangle the within-individual effect from inter-individual effect of age on TL dynamics over time, which provides an important additional dimension to our understanding of TL dynamics and aging process. We found that LTL declined significantly with age both cross-sectionally and longitudinally. The within-individual longitudinal decline was much greater than the inter-individual cross-sectional decline. Furthermore, women showed a lower within-individual LTL shortening rate than men.
TL has been increasingly attracting attention as a potential biomarker of aging. A body of research has found that TL is generally inversely correlated with chronological age [21]. TL shortening rate is considerably faster during early stages of life following birth and development than throughout adulthood [27], which could be explained by the expansion of the hematopoietic system through replications in tandem with the growing soma [28]. The majority of prior studies in telomere biology and aging are cross-sectional in nature, which makes the interpretation of the data complicated due to the high inter-individual variability in TL. TL has been shown to be highly variable among individuals of the same age in all age ranges assessed [9,10,11,12,13,14,15]. At birth, inter-individual variations in LTL amount to as much as 4 kb; during adulthood the inter-individual variations might be as wide as or wider than those at birth [29]. The high inter-individual variability in TL at birth and afterward suggests that there might be other influencing factors besides chronological age, such as genetic, epigenetic, environmental, and lifestyle factors [27, 30]. Hence, the cross-sectional TL at a single time point not only reflects the aging effect but also integrates the cumulative lifetime burdens of genetic, epigenetic, environmental, and lifestyle exposures. The considerable inter-individual variation in TL makes it difficult to fully appreciate TL dynamics in relation to age without knowing other unmeasured confounding factors. Accordingly, longitudinal evaluation of TL that can access TL change rate and disentangle the longitudinal within-individual effect from the cross-sectional inter-individual effect of age on TL should provide a better understanding of TL dynamics in biological aging. The present longitudinal study examined the cross-sectional and longitudinal associations of LTL with age across a wide range of age in a large cohort. In accordance with a previous host of cross-sectional studies and a few cohort studies, we found that LTL declined significantly with age both cross-sectionally and longitudinally. More importantly, our study showed that the within-individual longitudinal LTL decline with age was much greater than the inter-individual cross-sectional decline, indicating that chronological age might impose a greater impact on LTL shortening than other influencing factors combined. Although chronological age is the most broadly used indicator of aging, it is only an indicator of time level in the aging process. In addition to time, the complex process of biological aging is the result of genetic and environmental factors. As the rate of aging is not uniform in humans, biological age may vary from chronological age, due to genetic heterogeneity and the influence of environmental factors. Biological age is based on a setting of biological markers, which is a parameter for evaluating the functional status of individuals. Therefore, biological age might be a more optimal indicator in evaluating biological aging than chronological age. Future studies using biological age instead of chronological age in evaluating the association between individual aging and TL shorting are therefore recommended.
Longitudinal studies on TL change within individuals are only now emerging. Most of these longitudinal studies unexpectedly found that 1.5–25% of individuals exhibited telomere elongation over time, which is fundamentally distinct from the findings in cross-sectional studies [8, 23, 31,32,33]. Our study also observed telomere elongation in 22.45% of our participants aged 50 and older during 7–8 years of follow-up, which is consistent with other longitudinal cohorts. The differences in the percentages of people who exhibited elongated LTL might be due to different definitions of LTL elongation and follow-up periods in longitudinal studies. The definition of LTL elongation varies from greater than 0 to 10% increase in LTL change. Our study defines LTL elongation as more than 5% increase in LTL change. If we change this definition to 10%, the proportion of elongated LTL would decrease from 22.45 to 18.58% in our sample. Nevertheless, both percentages are within the range of elongated LTL observed in longitudinal studies in the literature. It still remains unclear what causes telomere elongation in longitudinal studies. Several biological explanations for this phenomenon have been raised, including telomerase-mediated reverse transcription, subtelomeric DNA amplification, and telomeric DNA homologous recombination [34]. An alternative explanation for telomere elongation is measurement errors of TL [31, 35]. Namely, the TL measurement methods used in epidemiological studies may not be sufficiently reliable in precision. If the coefficient of variation (CV) for TL measurement methods exceeds the actual telomere attrition rate, a statistical artifact caused by measurement errors might arise [29]. Theoretical considerations suggest that prolonged follow-up periods and large cohorts would attain credible inter-individual variations in telomere attrition rates [29]. This notion was supported by a longitudinal study which found telomere elongation in 14.4% of participants at 5.8-year follow-up, 10.7% at 6.6-year follow-up, and only 1.5% at 12.4-year follow-up in a young cohort (aged between 19.9 and 41.5 at baseline) [31]. In contrast, another longitudinal study which reported elongated LTL in 46% of their sample at old age across 20 years claimed that, with a coefficient of variation from the Q-PCR analyses ~ 7%, the LTL elongation was likely to be a biological phenomenon rather than measurement error [22]. What is more, as with any repeated measurement of a continuous variable, the possibility of regression to the mean should be considered, which might contribute to the correlation between LTL change and baseline LTL observed in our study. Despite the possible measurement errors, our random slope models did find a significant within-individual decline in LTL on average. It is important to bear in mind that our data are limited to leukocyte samples only and the measurements are relative values normalized to β-globin DNA, not actual telomere lengths. Further research that takes both biological causes and measurement errors into account is warranted, to explore why telomere elongation has been observed in our study and in other longitudinal studies.
Previous studies on sex difference in TL have revealed conflicting results. A vast number of cross-sectional studies reported that women have longer TL than men [16, 36, 37]; and most cohort studies reported a slower rate of TL shortening in women than men [22, 23, 32, 38, 39], with some exceptions that found longer TL in men than women or no significant difference across sexes [8, 40]. The biological basis of the sex difference in TL is not fully understood. It has been proposed that the longer TL in women compared with men stems from a slower TL attrition rate, presumably attributed to higher levels of estrogen [41]. Estrogen activates telomerase via direct and indirect effects on the reverse transcriptase subunit (hTERT) promoter, which is known to be sufficient to reconstitute telomerase activity in vitro [42]. Our study confirms a slower LTL attrition rate in women than men. However, the sex differences in LTL at wave 1 and wave 3 contradict most cross-sectional studies and some cohort studies that showed longer TL in women than men but are in line with the Lothian Birth Cohort study and the Newcastle study using Q-PCR assay which also found longer TL in men than women [40, 43]. Some researchers proposed that the association between gender and TL might vary by age [16]. The subjects in our study (50–91 years with a mean age of 61 at baseline) were younger than those in the Lothian Birth Cohort study (70–92 years) [40] but older than those in the Newcastle study (50 years) [43]. Subjects in other cohort studies using Q-PCR assay that reported no significant difference in TL between men and women, such as the BHS study (19–37 years) [44] and the pSoBid study (35–64 years) [45], were younger than our subjects. A meta-analysis from 36 cohorts (36,230 participants) with a wide range of mean ages from 37 to 90 years showed longer telomeres in women than men [16]. However, the strength of these associations varied by measurement method: the summary estimates of effect showed longer telomeres in females than males only for the TRF Southern Blot method, but not for the Q-PCR nor the Flow-FISH method [16]. This difference cannot be explained by differential random measurement error but might be due to method-specific bias [16]. Our model predicted mean LTL in relation to age for men and women (Supplementary Figure 2) suggesting a more complex scenario for the sex difference in TL dynamics. Men had longer LTL than women from age 50 to late 70s, whereas due to a slower TL attrition rate in women, the opposite was observed after late 70s. Hence, we propose that the sex difference in TL might depend on the age of study participants if evaluated cross-sectionally. Further research covering a wide range of age, especially large-scale longitudinal studies, with direct comparison of Southern blot results with Q-PCR or other methods in the same participants may help to clarify the sex differences in TL.
Strengths of this study include the longitudinal cohort design, a large sample size consisting of 4053 subjects, a wide range of age from 50 to 91 at baseline, and a relatively long follow-up period between 7 and 8 years with repeated assessments of LTL. Furthermore, our study was able to disentangle the within-individual effect from the inter-individual effect of age on TL dynamics over time, which provides an important additional dimension to our understanding of TL dynamics and aging process. A potential limitation of our study is the loss to follow-up bias in longitudinal studies. Compared to subjects with LTL data at both time points (n = 4053), those who dropped out at wave 3 (n = 4101) were 3.7 years younger at baseline (p < 0.01), with higher rates of loss to follow-up in age group 70–79 and over 80. However, they did not have a significant difference in sex distribution (p = 0.24). Since the relative TLT change did not differ in different age groups in our study, we believe it is unlikely that the loss to follow-up bias would alter our results.
In summary, our data show that LTL declined significantly with age both cross-sectionally and longitudinally. More importantly, the within-individual longitudinal decline was much greater than the inter-individual cross-sectional decline, indicating that chronological age might impose a greater impact on LTL shortening than other influencing factors combined. Furthermore, women showed a slower within-individual LTL shortening rate than men.
References
Blackburn EH, Epel ES, Lin J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350(6265):1193–8.
Gilson E, Géli V. How telomeres are replicated. Nat Rev Mol Cell Biol. 2007;8(10):825–38.
Lingner J, Cooper JP, Cech TR. Telomerase and DNA end replication: no longer a lagging strand problem? Science. 1995;269(5230):1533–4.
Herrmann M, Pusceddu I, März W, Herrmann W. Telomere biology and age-related diseases. Clin Chem Lab Med. 2018;56(8):1210–22.
Hayflick L. Mortality and immortality at the cellular level. A review. Biochemistry (Mosc). 1997;62(11):1180–90.
Wong JMY, Collins K. Telomere maintenance and disease. Lancet. 2003;362(9388):983–8.
Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464(7288):520–8.
Bendix L, Thinggaard M, Fenger M, Kolvraa S, Avlund K, Linneberg A, et al. Longitudinal changes in leukocyte telomere length and mortality in humans. J Gerontol A Biol Sci Med Sci. 2014;69(2):231–9.
Andrew T, Aviv A, Falchi M, Surdulescu GL, Gardner JP, Lu X, et al. Mapping genetic loci that determine leukocyte telomere length in a large sample of unselected female sibling pairs. Am J Hum Genet. 2006;78(3):480–6.
Vasa-Nicotera M, Brouilette S, Mangino M, Thompson JR, Braund P, Clemitson J-R, et al. Mapping of a major locus that determines telomere length in humans. Am J Hum Genet. 2005;76(1):147–51.
Bischoff C, Graakjaer J, Petersen HC, Hjelmborg JVB, Vaupel JW, Bohr V, et al. The heritability of telomere length among the elderly and oldest-old. Twin Res Hum Genet. 2005;8(5):433–9.
Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55(5):876–82.
Njajou OT, Cawthon RM, Damcott CM, Wu S-H, Ott S, Garant MJ, et al. Telomere length is paternally inherited and is associated with parental lifespan. Proc Natl Acad Sci U S A. 2007;104(29):12135–9.
Broer L, Codd V, Nyholt DR, Deelen J, Mangino M, Willemsen G, et al. Meta-analysis of telomere length in 19,713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur J Hum Genet. 2013;21(10):1163–8.
Hjelmborg JB, Dalgård C, Möller S, Steenstrup T, Kimura M, Christensen K, et al. The heritability of leucocyte telomere length dynamics. J Med Genet. 2015;52(5):297–302.
Gardner M, Bann D, Wiley L, Cooper R, Hardy R, Nitsch D, et al. Gender and telomere length: systematic review and meta-analysis. Exp Gerontol. 2014;51:15–27.
Hansen MEB, Hunt SC, Stone RC, Horvath K, Herbig U, Ranciaro A, et al. Shorter telomere length in Europeans than in Africans due to polygenetic adaptation. Hum Mol Genet. 2016;25(11):2324–30.
Lynch SM, Peek MK, Mitra N, Ravichandran K, Branas C, Spangler E, et al. Race, ethnicity, psychosocial factors, and telomere length in a multicenter setting. PLoS One. 2016;11(1):e0146723.
Aviv A, Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, et al. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am J Epidemiol. 2009;169(3):323–9.
Nordfjäll K, Svenson U, Norrback K-F, Adolfsson R, Lenner P, Roos G. The individual blood cell telomere attrition rate is telomere length dependent. PLoS Genet. 2009;5(2):e1000375.
Müezzinler A, Zaineddin AK, Brenner H. A systematic review of leukocyte telomere length and age in adults. Ageing Res Rev. 2013;12(2):509–19.
Berglund K, Reynolds CA, Ploner A, Gerritsen L, Hovatta I, Pedersen NL, et al. Longitudinal decline of leukocyte telomere length in old age and the association with sex and genetic risk. Aging (Albany NY). 2016;8(7):1398–415.
Farzaneh-Far R, Lin J, Epel E, Lapham K, Blackburn E, Whooley MA. Telomere length trajectory and its determinants in persons with coronary artery disease: longitudinal findings from the heart and soul study. PLoS One. 2010;5(1):e8612.
Kowal P, Chatterji S, Naidoo N, Biritwum R, Fan W, Lopez Ridaura R, et al. Data resource profile: the World Health Organization Study on global AGEing and adult health (SAGE). Int J Epidemiol. 2012;41(6):1639–49.
Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47.
van de Pol M, Wright J. A simple method for distinguishing within- versus between-subject effects using mixed models. Anim Behav. 2009;77(3):753–8.
Turner KJ, Vasu V, Griffin DK. Telomere biology and human phenotype. Cells. 2019;8(1):73. https://doi.org/10.3390/cells8010073.
Sidorov I, Kimura M, Yashin A, Aviv A. Leukocyte telomere dynamics and human hematopoietic stem cell kinetics during somatic growth. Exp Hematol. 2009 Apr;37(4):514–24.
Aviv A. The epidemiology of human telomeres: faults and promises. J Gerontol A Biol Sci Med Sci. 2008;63(9):979–83.
Dugdale HL, Richardson DS. Heritability of telomere variation: it is all about the environment! Philos Trans R Soc Lond Ser B Biol Sci. 2018;373(1741):20160450. https://doi.org/10.1098/rstb.2016.0450.
Chen W, Kimura M, Kim S, Cao X, Srinivasan SR, Berenson GS, et al. Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: age-dependent telomere shortening is the rule. J Gerontol A Biol Sci Med Sci. 2011;66(3):312–9.
Guzzardi MA, Iozzo P, Salonen M, Kajantie E, Eriksson JG. Rate of telomere shortening and metabolic and cardiovascular risk factors: a longitudinal study in the 1934-44 Helsinki Birth Cohort Study. Ann Med. 2015;47(6):499–505.
Simons MJP, Stulp G, Nakagawa S. A statistical approach to distinguish telomere elongation from error in longitudinal datasets. Biogerontology. 2014;15(1):99–103.
Liu J, Wang L, Wang Z, Liu J-P. Roles of telomere biology in cell senescence, replicative and chronological ageing. Cells. 2019;8(1):54. https://doi.org/10.3390/cells8010054.
Steenstrup T, Hjelmborg JVB, Kark JD, Christensen K, Aviv A. The telomere lengthening conundrum--artifact or biology? Nucleic Acids Res. 2013 Jul;41(13):e131.
Cherif H, Tarry JL, Ozanne SE, Hales CN. Ageing and telomeres: a study into organ- and gender-specific telomere shortening. Nucleic Acids Res. 2003;31(5):1576–83.
Sanders JL, Newman AB. Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiol Rev. 2013;35:112–31.
Bekaert S, De Meyer T, Rietzschel ER, De Buyzere ML, De Bacquer D, Langlois M, et al. Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell. 2007;6(5):639–47.
Dalgård C, Benetos A, Verhulst S, Labat C, Kark JD, Christensen K, et al. Leukocyte telomere length dynamics in women and men: menopause vs age effects. Int J Epidemiol. 2015;44(5):1688–95.
Harris SE, Marioni RE, Martin-Ruiz C, Pattie A, Gow AJ, Cox SR, et al. Longitudinal telomere length shortening and cognitive and physical decline in later life: the Lothian Birth Cohorts 1936 and 1921. Mech Ageing Dev. 2016;154:43–8.
Aviv A, Shay JW. Reflections on telomere dynamics and ageing-related diseases in humans. Philos Trans R Soc Lond Ser B Biol Sci. 2018;373(1741):20160436. https://doi.org/10.1098/rstb.2016.0436.
Kyo S, Takakura M, Kanaya T, Zhuo W, Fujimoto K, Nishio Y, et al. Estrogen activates telomerase. Cancer Res. 1999;59(23):5917–21.
Adams J, Martin-Ruiz C, Pearce MS, White M, Parker L, Zglinicki TV. No association between socio-economic status and white blood cell telomere length. Aging Cell. 2007;6(1):125–8.
Hunt SC, Chen W, Gardner JP, Kimura M, Srinivasan SR, Eckfeldt JH, et al. Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell. 2008;7(4):451–8.
Shiels PG, McGlynn LM, MacIntyre A, Johnson PCD, Batty GD, Burns H, et al. Accelerated telomere attrition is associated with relative household income, diet and inflammation in the pSoBid cohort. PLoS One. 2011;6(7):e22521.
Acknowledgments
The authors thank the participants and the investigators who participated in this study.
Funding
This work was supported by the WHO and the US National Institute on Aging through Interagency Agreements (OGHA 04034785, YA1323-08-CN-0020, Y1-AG-1005-01) and through a research grant (R01-AG034479); the Program for Outstanding Medical Academic Leader, Shanghai, China (2019LJ24); and Shanghai Municipal Health Commission (20204Y0196, 2020YJZX0113, 201840118).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Figure 1
Correlations of LTL change and LTL at Wave 3 with baseline LTL (PNG 5883 kb)
Supplementary Figure 2
Predicted mean LTL in relation to age (by sex) from standard random slope model including interaction between age and sex (PNG 6131 kb)
About this article
Cite this article
Huang, Z., Liu, C., Ruan, Y. et al. Dynamics of leukocyte telomere length in adults aged 50 and older: a longitudinal population-based cohort study. GeroScience 43, 645–654 (2021). https://doi.org/10.1007/s11357-020-00320-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-020-00320-y