Abstract
Aging alters bladder functions where a decrease in filling, storage and emptying is observed. These changes cause urinary incontinence, especially in women. The aim of this study is to examine how aging affects the intracellular calcium movements due to agonist-induced contractions in permeabilized female rat bladder. Urinary bladder isolated from young and old female Sprague-Dawley rats were used. Small detrusor strips were permeabilized with β-escin. The contractile responses induced with agonists were compared between young and old groups. Carbachol-induced contractions were decreased in permeabilized detrusor from old rats compared to young group. Heparin and ryanodine decreased carbachol-induced contractions in young rats where only heparin inhibited these contractions in olds. Caffeine-induced contractions but not inositol triphosphate (IP3)-induced contractions were decreased in old group compared to youngs. The cumulative calcium response curves (pCa 8–4) were also decreased in old rats. Carbachol-induced calcium sensitization responses did not alter by age where GTP-β-S and GF-109203X but not Y-27632 inhibited these responses. Carbachol-induced contractions decrease with aging in rat bladder detrusor. It can be postulated as IP3-induced calcium release (IICR) is primarily responsible for the contractions in older rats where the decrease in carbachol contractions in aging may be as a result of a decrease in calcium-induced calcium release (CICR), rather than carbachol-induced calcium sensitization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In a healthy and young adult, the function of the urinary bladder is sustained with the relaxation of detrusor smooth muscle and the contraction of urethral sphincter during urine storage, and completely the opposite of this action during micturition. These functions were elicited with the synchronized work of spinal cord, pons and excitator, inhibitor and sensory nerves in the forebrain (Anderson 1993). The innervation of the bladder is sustained with the role of cholinergic, nitrergic, purinergic, monoaminergic and peptidergic mechanisms (de Groat and Yoshimura 2001). Detrusor sensitivity to agonists, its spontaneous activity and the levels of the contractile proteins may vary under conditions like urethral obstruction, incontinence and aging (Hashim and Abrams 2007). Aging causes changes in many organs as well as a significant decrease in filling, storage and emptying functions of the bladder. The symptoms like reduced voiding efficiency and bladder overactivity may bring forth a social problem such as urinary incontinence (Smith 2010; Zhao et al. 2010). Especially in women, urinary incontinence incidence increases with age and the side effects of the present drugs unfortunately fail to carry out a radical therapy.
Investigating the roles of intracellular calcium signalling components in the treatment of urinary incontinence is a new therapeutic approach since the pronounced role of Rho kinase (ROCK) in the regulation of urinary bladder smooth muscle tone and contraction had been demonstrated in many studies (Bing et al. 2003; Wibberley et al. 2003; Kirschstein et al. 2014). Variations on agonist-induced contractions due to aging are also suggested to depend on impairments in intracellular signal transduction pathways (Ordway et al. 1986; Lluel et al. 2000; Gomez-Pinilla et al. 2011; Lowalekar et al. 2012). Furthermore, it was observed that the decreased response of bladder to β-adrenergic stimulation by aging is related to both an inhibition in adenylyl cyclase activity and the changes in guanine nucleotide regulatory protein (G-protein) content or function (Derweesh et al. 2000). However, the underlying intracellular mechanisms of the functional changes that may occur with aging in the bladder have not been enlightened yet.
Smooth muscle contraction is activated by an increase in cytosolic calcium produced by calcium entry through voltage-sensitive calcium channels or by triggering calcium release from intracellular stores. The main intracellular calcium store is the “sarcoplasmic reticulum.” Calcium release from the sarcoplasmic reticulum can be mediated by both ryanodine and inositol triphosphate (IP3) receptors. Ryanodine receptors are activated by an increase in free calcium concentration (calcium-induced calcium release; CICR), while IP3 receptors are activated by IP3 produced as a result of agonist stimulation (IP3-induced calcium release; IICR) (Anderson 1993; Wibo and Godfraind 1994). Agonists activating G protein coupled receptors (GPCRs) increase force in smooth muscle via calcium-dependent myosin light chain phosphorylation. The ratio of activities of calcium/calmodulin-dependent myosin light chain kinase (MLCK) and myosin light chain phosphatase (MLCP) determines the level of myosin light chain phosphorylation and the activation of the muscle. There are mechanisms which can lead to muscle contraction without any necessary change in intracellular calcium. ROCK and protein kinase C (PKC) activation can induce smooth muscle contraction by inhibition of myosin phosphatase activity at constant calcium, and this is called calcium sensitization (Somlyo and Somlyo 2003).
The intracellular aspects of contractile mechanisms can be studied in vitro by using smooth muscle preparations having their plasma membrane permeabilized by a chemical agent. Chemically permeabilized smooth muscle is an experimental model for studying the intracellular organelles and signal cascades responsible from muscle contraction (Nasu 1989). β-Escin is a saponin ester which opens holes in the plasma membrane and makes the cell membrane permeable to higher molecular weight compounds (up to 150 kDa), including heparin and IP3, and retains receptor-effector coupling (Kobayashi et al. 1989; Iizuka et al. 1994). Besides this, small endogenous substances such as guanosine-5′-triphosphate (GTP) and calmodulin may escape out of the cell and must be added to the organ bath medium during the experiments (Kitazawa et al. 1989). Permeabilization is a preferable tool for the study of the intracellular signal transduction pathways mediating agonist-induced contraction in smooth muscles. The roles of intracellular calcium stores and signal cascades in smooth muscle contraction and calcium sensitization may be investigated by permeabilization (Somlyo and Somlyo 2003). For instance, the presence of CICR and the importance of ROCK/PKC pathways in carbachol induced calcium sensitization in human bladder (Chambers et al. 1999; Takahashi et al. 2004) and the role of PKC pathway in phorbol ester-induced calcium sensitization in guinea pig bladder have been observed by permeabilization method (Kaneda et al. 1995).
The aim of this study is to examine how aging affects the intracellular calcium movements due to agonist-induced contractions in permeabilized female rat bladder smooth muscle. It is intended that the results obtained in the present study may lead to a positive contribution to the development of new drug molecules in the treatment of urinary incontinence.
Materials and methods
Animal welfare and ethical statement
The study protocol was approved by the Hacettepe University Animal Ethics Committee (No: 2011/63-8). The study was conducted in accordance with “Regulation of the Welfare and Protection of Animals Used for Experimental and Other Scientific Purposes” published by Republic of Turkey Ministry of Food, Agriculture and Livestock (13.12.2011-28141). In total, 60 female Sprague-Dawley rats that were kept under 12-h light/dark period with food and water ad libitum were used.
Tissue preparation
Young (2 to 4 months old) and old (16 to 20 months old) female Sprague-Dawley rats were used in the study. Rats were killed by a sharp blow to the head and bleeding. The urinary bladder was isolated and Hepes buffered modified Krebs’ solution (see below). The mucosa and connective tissues were removed from the bladder under a dissecting microscope. Small strips (150–250 μm in diameter, 3–4 mm in length) of smooth muscle were dissected from the urinary bladder. A small hook was tied to one end of a strip to attach it to the transducer, and a snare of 5/0 surgical silk captured the other end and was used to mount the strip in a fixed position in a 1000-μl chamber in one of a series of small chambers in a Perspex block. The chamber was filled with Hepes buffered modified Krebs’ solution at room temperature and the strips were equilibrated for 30 min under a resting tension of 100 mg. Solution changes were made by moving the Perspex block. After stable responses had been achieved to 80 mM K+ (see below) and 50 μM carbachol in intact tissues, they were moved into relaxing solutions (see below) and incubated for a few minutes. Then, the tissues were permeabilized with 40 μM β-escin in relaxing solution for 30 min at pH 6.8. This was followed by a 4-min wash in relaxing solution before beginning an experiment. Muscle fibers were accepted as permeabilized if the maximum tension obtainable by 100 μM Ca2+ after permeabilization was found to be greater than the tension produced by 80 mM K+ applied in the same strip before permeabilization (Endo et al. 1977). The contractile force was measured by a sensitive force transducer (Swema, Stocholm, Sweden) connected to a computer using Biopac Student Lab Pro 3.7.3 (Commat LTD, Turkey) software.
Experimental procedures
Agonist-induced contractions
After intracellular calcium stores were loaded by activating solution at pCa 6 for 10 min, a contractile response was elicited by carbachol (50 μM) in the presence of GTP (100 μM) and also by IP3 (50 μM) or caffeine (10 mM). Moreover, carbachol responses were obtained in the presence of IP3 receptor blocker heparin (1 mg/ml) and sarcoplasmic reticulum ryanodine channels blocker ryanodine (10 μM).
In another group of experiments, in order to obtain pCa tension curves, calcium was applied cumulatively (pCa 8–4) in the activating solution.
Carbachol-induced calcium sensitization contractions
After intracellular calcium stores were loaded by activating solution at pCa 6 in the presence of both sarcoplasmic reticulum calcium-ATPase pump inhibitor cyclopiazonic acid (CPA;1 μM) and mitochondrial proton pump inhibitor carbonyl cyanide p-trifluromethoxyphenylhydrazone (FCCP; 1 μM) for 10 min to induce calcium sensitization (Durlu-Kandilci and Brading 2006), a contractile response was elicited by carbachol (50 μM) + GTP (100 μM). CPA prevents filling of the sarcoplasmic reticulum where FCCP prevents the mitochondrial Ca2+ uptake, stores that lost their Ca2+ contents during permeabilization. Under these circumstances, calcium sensitization contractile responses are elicited by carbachol. Moreover, calcium sensitization responses were then obtained in the presence of ROCK inhibitor (R)-(s)-trans-N-(4-pyridyl)-4-(1-aminoethyl)-cyclohexanecarboxyamide 2HCl (Y-27632; 1 μM), PKC inhibitor bisindolylmaleimide I (GF 109203 X; 5 μM), and GTP inhibitor guanosine 5′-(β-thio)diphosphate trilithium salt (GTP-β-S; 1 μM).
In another experimental group, GTP activator guanosine 5′-(γ-thio)triphosphate tetralithium salt (GTP-γ-S) and PKC activator phorbol 12,13-dibutyrate-induced calcium sensitization responses were also obtained. After intracellular calcium stores were loaded by activating solution at pCa 6 for 10 min, a contractile response was elicited by GTP-γ-S (50 μM) and phorbol 12,13-dibutyrate (50 μM) in the presence of GTP (100 μM), CPA (1 μM), and FCCP (1 μM).
Drugs and solutions
Drugs used were β-escin (aescin), carbamylcholine chloride (carbachol), creatine phosphokinase, leupeptin, ethylene glycol-bis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), adenosine 5′triphosphate (Na2ATP), ryanodine, heparin, GTP, CPA, calmodulin, FCCP, D-myo-inositol 1,4,5-trisphosphate (IP3), caffeine, GTP-γ-S, GTP-β-S, phorbol 12,13-dibutyrate, dimethylsulphoxide (DMSO) from Sigma (St. Louis, Missouri) and creatine phosphate disodium salt, Y-27632 ve GF-109203X from Calbiochem (Nottingham, UK). All drugs and solutions were prepared by using 18 MΩ-cm deionized water except FCCP, CPA, GF-109203X, ryanodine and phorbol 12,13-dibutyrate. FCCP was dissolved in ethanol, ryanodine was dissolved in methanol, and CPA, GF-109203X, and phorbol 12,13-dibutyrate were dissolved in DMSO but neither vehicle affected the contractions when tested alone. For intact tissues, Hepes buffered modified Krebs’ solution contained (mM) NaCl 126, KCl 6, CaCl2 2, MgCl2 1.2, glucose 14 and HEPES 10.5. The pH was adjusted to 7.2 with NaOH. K+ Krebs’ solution (80 mM) was prepared by replacing NaCl with an equivalent amount of KCl. For permeabilized tissues, relaxing solution contained (mM) K propionate 130, MgCl2 4, Na2ATP 4, tris-maleate 20, creatine phosphate 10, and EGTA 4, and creatine phosphokinase 3.3 units/ml and protease inhibitor leupeptin (1 μM). The pH of this solution was adjusted to 6.8 with KOH. Activating solutions were the same as relaxing solution except that EGTA was lowered to 0.05 mM, free calcium concentration was adjusted to the desired value and 1 μM calmodulin added as specified. GTP (100 μM) was also added when carbachol was used to activate the muscarinic receptors. In experiments with cumulative calcium response curves, EGTA in the activating solution was kept at 10 mM. The free calcium concentration was calculated using a computer programme (“Bound and Determined”, Brooks and Storey 1992) and expressed as the negative logarithm (pCa). When drugs were added to an organ chamber, they were made up in relaxing solution containing 0.05 mM EGTA, and the concentration given is the estimated final concentration. In the experiments with inhibitors, the strips were incubated with this substance for 15 min in relaxing solution and then contraction was elicited.
Data analysis
Contractions are expressed as percent of the response to 80 mM KCl (202.1 ± 11.7 mg, N = 52 in young group and 215.4 ± 11.9 mg, N = 50 in old group; N refers to strip numbers) elicited in intact tissues before permeabilization. Data were given as mean ± S.E.M. of n experiments. Statistical analyses were carried out by using one-way analysis of variance (ANOVA) followed by Bonferroni test for comparing multiple groups. Student’s t test was used for comparing two groups. P < 0.05 were accepted as statistically significant.
Results
Agonist-induced contractions
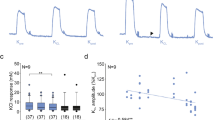
Carbachol-induced contractions were significantly decreased in permeabilized detrusor smooth muscle strips isolated from old group of rats compared to young group as shown Fig. 1. Carbachol-induced contractions were significantly decreased in the presence of sarcoplasmic reticulum IP3 receptor blocker heparin and sarcoplasmic reticulum ryanodine channel blocker ryanodine in young group of rats. In old group of rats, carbachol-induced contractions were significantly decreased in the presence of heparin but did not change in the presence of ryanodine as shown in Fig. 2. IP3-induced contractions did not change in old group of rats but caffeine-induced contractions were decreased in old group of rats compared to young group as shown in Fig. 3.
The contractile response elicited with carbachol (50 μM) + GTP (100 μM) in the absence and presence of heparin (1 mg/ml) or ryanodine (10 μM) in permeabilized detrusor smooth muscle isolated from young and old group of rats (*P < 0.05 compared to control response in young group, **P < 0.05 compared to control response in old group, #P < 0.05 compared to carbachol response in the presence of heparin in young group; n = 6–18)
The cumulative calcium response curves (pCa 8–4) were decreased in permeabilized detrusor smooth muscle isolated from old group of rats compared to young group as shown in Fig. 4.
Carbachol-induced calcium sensitization contractions
Carbachol-induced calcium sensitization responses elicited in the presence of sarcoplasmic reticulum calcium-ATPase pump inhibitor CPA and mitochondrial blocker FCCP did not change in permeabilized detrusor smooth muscle isolated from old group of rats compared to young group as shown in Fig. 5. The calcium sensitization responses in both group did not change with ROCK inhibitor Y-27632 but were significantly inhibited by PKC inhibitor GF-109203X as shown in Fig. 6. Moreover, these responses were decreased in both groups in the presence of G-protein inhibitor GTP-β-S as shown in Fig. 7.
Carbachol-induced calcium sensitization response in the absence and presence of Y-27632 (1 μM) and GF-109203X (5 μM) in permeabilized detrusor smooth muscle isolated from young and old group of rats (*P < 0.05 compared to control response in young group, **P < 0.05 compared to control response in old group; n = 6–15)
Contractions induced by G-protein activator GTP-γ-S (a) and PKC activator phorbol 12,13-dibutyrate (b) were not different in young and old groups as shown in Fig. 8.
Discussion
Aging is frequently associated with changes in bladder function and, in humans, with the appearance of symptoms such as reduced voiding efficiency and bladder overactivity. This may result in social problems such as incontinence, especially in women, with a restricted therapy due to the side effects of the present drugs used. There are several studies particularly in the rat examining the effects of aging on the bladder detrusor contractility. According to some studies, it seems that the contractile proteins themselves are not affected by aging but several other studies have shown that intracellular mechanisms involving secondary messengers may alter with aging and affect response of the bladder to agonists (Ordway et al. 1986; Sjuve et al. 1997; Derweesh et al. 2000; Lluel et al. 2000; Gomez-Pinilla et al. 2011; Lowalekar et al. 2012). In the present study, by using β-escin permeabilization, we have inquired how aging affects the intracellular calcium movements due to agonist-induced contractions in female rat bladder. Thus, the present data show the difference between young and old female rat bladder detrusor smooth muscle contractions in terms of different steps of intracellular mechanisms.
First of all, after loading the intracellular stores with calcium, we elicited carbachol-induced contractions in the presence of GTP in permeabilized detrusor smooth muscle and observed that there is a significant decrease in old group of rats compared to youngs. This is a similar finding with another study carried out in mouse detrusor where aging impaired bethanechol-induced contractions (Gomez-Pinilla et al. 2011). However, there are studies in disagreement with our data revealing no difference in carbachol-induced contractions in aged Wistar female rats (Lluel et al. 2000) or showing increased contractions to muscarinic agonists in rat bladder (Ordway et al. 1986). Our finding of decreased carbachol-induced contractions may be related with decreased muscarinic receptor density or down regulation by age as suggested earlier in some studies such as Schneider et al. (2005) and Mansfield et al. (2005) or may be as a cause of decreased calcium signaling pathways. We focused on the latter throughout this paper. Therefore, we investigated the effects of sarcoplasmic reticulum IP3 receptor blocker heparin and ryanodine channel blocker ryanodine on carbachol-induced contractions in order to observe whether two different calcium release mechanisms from the main intracellular calcium store sarcoplasmic reticulum changes with aging. According to our results, since heparin inhibited significantly carbachol-induced contractions in both young and old rats but with different ratios, i.e. 27 % inhibition in young rats and 60 % inhibition in old rats, we may suggest that sarcoplasmic reticulum IP3 receptors are more pronounced in the latter group. However, the effect of ryanodine was different in both groups as carbachol-induced contractions were significantly blocked in young rats with 57 % but not in old group. All together, these findings show that IICR is primarily responsible from the contractions in older rats where the decrease in carbachol-induced contractions in aging may be as a result of a decrease in CICR. If we compare the carbachol response in the presence of heparin in young and old rats, then one can see the significant inhibition between these two contractions in regard to old group. This means, since heparin blocks the IP3-induced component of contractions, the remaining calcium-induced contractions are decreased in old group, supporting the idea of carbachol-induced contractions in aging are due to changes in CICR. On the contrary, remaining carbachol responses in the presence of ryanodine are not different from each other in both young and old rats emphasizing once again that IICR is not affected by age differences. Moreover, IP3-induced contractions did not change in old rats where caffeine-induced contractions were decreased in old rats compared to youngs. Caffeine-induced contractile responses are hard to observe in some tissues due to the chemical structure of caffeine as a methylxanthine that is known to inhibit cyclic nucleotide phosphodiesterase and induce cyclic adenosine monophosphate (Belibi et al. 2002; Lindaman et al. 2002). In one of our previous papers, male Wistar albino rat bladders did not contract with 30 mM caffeine but this was with a different rat species and different experimental procedures such as store release experiments (Tugba Durlu-Kandilci and Brading 2007). Since we used female Sprague-Dawley rats under a different experimental protocol, we could observe the contractile response induced by caffeine in the present paper. We may conclude here that these experiments carried out to release calcium directly from sarcoplasmic reticulum also support our conclusion that the decrease in carbachol-induced contractions may be related with the impairment in CICR with age.
We then investigated the cumulative calcium contractions (pCa 8–4) in permeabilized detrusor of both group of rats and observed a significant inhibition in calcium response curves in old group of rats compared to young ones. In permeabilized smooth muscle preparations, the contraction elicited by addition of calcium to the organ bath medium primarily shows the response of contractile proteins directly to added amount of calcium and secondarily shows the calcium release induced by loading intracellular calcium stores, i.e. mainly the sarcoplasmic reticulum (Endo et al. 1982; Volpe et al. 1986). Therefore, the inhibition in cumulative calcium contractions in old group in our study may be as a result of an inhibition of contractile proteins and/or the impairment in CICR by aging. Our results are in parallel with a study in mouse detrusor proposing a reduction in the size of intracellular calcium stores as a result of aging (Gomez-Pinilla et al. 2011). Aging has been shown not to be associated with pronounced changes in the cellular contractile proteins in rat detrusor by an earlier study (Sjuve et al. 1997), but in our case, we may suggest the opposite.
Contractile mechanisms in smooth muscle is generally thought to be activated by an increase in cytosolic calcium produced by calcium entry through voltage-sensitive calcium channels or by calcium release from the sarcoplasmic reticulum. Since in permeabilized smooth muscles the process gets rid of the ion channels in the plasma membrane, one can easily focus on intracellular stores as we did in the first part of the present study. There are, however, physiological mechanisms in which intracellular calcium and MLCK activity are constant and agonists activating G protein coupled receptors may cause a contraction, that is calcium sensitization. Calcium sensitization involves inhibition of MLCP and two pathways have been implicated. Inhibition of MLCP can be induced directly by ROCK or by phosphorylation of the phosphatase inhibitor CPI-17 through PKC (Somlyo and Himpens 1989; Somlyo 2002). These mechanisms may be easily studied by permeabilized smooth muscle preparations which allow one to investigate the different levels of molecular control since the outer and the inner mediums contain the same ionic contents. Under constant calcium, sarcoplasmic reticulum calcium-ATPase pump inhibitor CPA and mitochondrial blocker FCCP in the medium, we elicited carbachol-induced calcium sensitization responses in both old and young group of rats where we observed no change in terms of a contractile response. Moreover, the carbachol-induced calcium sensitization responses in both group did not change with ROCK inhibitor Y-27632 but were significantly inhibited by PKC inhibitor GF-109203X in similar ratios; i.e. 37 % inhibition in young ones and 41 % inhibition in old ones. We may propose that both in young and old rats PKC pathway is major in terms of calcium sensitization mechanisms, rather than ROCK pathway. These results are in controversy with a latest paper by Kirschstein et al. (2014) suggesting the increased role of ROCK in carbachol-induced contractions in human detrusor with aging. Under our experimental conditions, the calcium sensitization responses were decreased in both young and old groups in the presence of G-protein inhibitor GTP-β-S, confirming that the contractile responses were originated from G-protein coupled receptors.
Moreover, in parallel with our carbachol-induced calcium sensitization contractile responses, we also elicited contractions induced by G-protein activator GTP-γ-S which were not different in young and old rats. However, GTP-γ-S-induced contractile responses were in matching ratios with the carbachol-induced calcium sensitization contractions, suggesting that we could elicit pure sensitizing contractile responses with this agonist. In agreement with our findings about the involvement of PKC pathway, we also elicited contractions with PKC activator phorbol 12,13-dibutirate in both young and old rats that were not different from each other.
As a conclusion, although carbachol-induced contractions decrease with aging, the underlying mechanism is not related with calcium sensitization component of these responses. According to our data, IICR is primarily responsible from the contractions in older rats where the decrease in carbachol contractions in aging may be as a result of a decrease in CICR. As in the case with elder women, since detrusor impairment leading to incontinence is a social and an irritating problem, our findings may be of importance for developing new drug targets.
References
Anderson KE (1993) Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol Rev 45(3):253–308
Belibi FA, Wallace DP, Yamaguchi T, Christensen M, Reif G, Grantham JJ (2002) The effect of caffeine on renal epithelial cells from patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol JASN 13(11):2723–2729
Bing W, Chang S, Hypolite JA, DiSanto ME, Zderic SA, Rolf L, Wein AJ, Chacko S (2003) Obstruction-induced changes in urinary bladder smooth muscle contractility: a role for Rho kinase. Am J Physiol Ren Physiol 285(5):F990–F997
Brooks SP, Storey KB (1992) Bound and determined: a computer program for making buffers of defined ion concentrations. Anal Biochem 201:119–126
Chambers P, Neal DE, Gillespie JI (1999) Ryanodine receptors in human bladder smooth muscle. Exp Physiol 84(1):41–46
de Groat WC, Yoshimura N (2001) Pharmacology of the lower urinary tract. Annu Rev Pharmacol Toxicol 41:691–721
Derweesh IH, Wheeler MA, Weiss RM (2000) Alterations in G-proteins and beta-adrenergic responsive adenylyl cyclase in rat urinary bladder during aging. J Pharmacol Exp Ther 294(3):969–974
Durlu-Kandilci NT, Brading AF (2006) Involvement of Rho kinase and protein kinase C in carbachol-induced calcium sensitization in beta-escin skinned rat and guinea-pig bladders. Br J Pharmacol 148(3):376–384
Endo MKT, Yagi S, Iino M, Kakuta Y (1977) Some properties of chemically skinned smooth muscle fibers. In: Casteels R et al (eds) Excitation-contraction coupling in smooth muscle. Elsevier, Amsterdam, pp 199–209
Endo M, Yagi S, Iino M (1982) Tension-pCa relation and sarcoplasmic reticulum responses in chemically skinned smooth muscle fibers. Fed Proc 41(7):2245–2250
Gomez-Pinilla PJ, Pozo MJ, Camello PJ (2011) Aging differentially modifies agonist-evoked mouse detrusor contraction and calcium signals. Age (Dordr) 33(1):81–88
Hashim H, Abrams P (2007) Overactive bladder: an update. Curr Opin Urol 17(4):231–236
Iizuka K, Ikebe M, Somlyo AV, Somlyo AP (1994) Introduction of high molecular weight (IgG) proteins into receptor coupled, permeabilized smooth muscle. Cell Calcium 16(6):431–445
Kaneda T, Shimizu K, Nakajyo S, Urakawa N (1995) Effect of phorbol ester, 12-deoxyphorbol 13-isobutylate (DPB), on muscle tension and cytosolic Ca2+ in rat anococcygeus muscle. Jpn J Pharmacol 69(3):195–204
Kirschstein T, Protzel C, Porath K, Sellmann T, Kohling R, Hakenberg OW (2014) Age-dependent contribution of Rho kinase in carbachol-induced contraction of human detrusor smooth muscle in vitro. Acta Pharmacol Sin 35(1):74–81
Kitazawa T, Kobayashi S, Horiuti K, Somlyo AV, Somlyo AP (1989) Receptor-coupled, permeabilized smooth muscle. Role of the phosphatidylinositol cascade, G-proteins, and modulation of the contractile response to Ca2+. J Biol Chem 264(10):5339–5342
Kobayashi S, Kitazawa T, Somlyo AV, Somlyo AP (1989) Cytosolic heparin inhibits muscarinic and alpha-adrenergic Ca2+ release in smooth muscle. Physiological role of inositol 1,4,5-trisphosphate in pharmacomechanical coupling. J Biol Chem 264(30):17997–18004
Lindaman BA, Hinkhouse MM, Conklin JL, Cullen JJ (2002) The effect of phosphodiesterase inhibition on gallbladder motility in vitro. J Surg Res 105(2):102–108
Lluel P, Palea S, Barras M, Grandadam F, Heudes D, Bruneval P, Corman B, Martin DJ (2000) Functional and morphological modifications of the urinary bladder in aging female rats. Am J Physiol Regul Integr Comp Physiol 278(4):R964–R972
Lowalekar SK, Cristofaro V, Radisavljevic ZM, Yalla SV, Sullivan MP (2012) Loss of bladder smooth muscle caveolae in the aging bladder. Neurourol Urodyn 31(4):586–592
Mansfield KJ, Liu L, Mitchelson FJ, Moore KH, Millard RJ, Burcher E (2005) Muscarinic receptor subtypes in human bladder detrusor and mucosa, studied by radioligand binding and quantitative competitive RT-PCR: changes in ageing. Br J Pharmacol 144(8):1089–1099
Nasu T (1989) Actions of some drugs on skinned smooth muscle preparations. Gen Pharmacol 20(2):123–131
Ordway GA, Esbenshade TA, Kolta MG, Gerald MC, Wallace LJ (1986) Effect of age on cholinergic muscarinic responsiveness and receptors in the rat urinary bladder. J Urol 136(2):492–496
Schneider T, Hein P, Michel-Reher MB, Michel M (2005) Effects of ageing on muscarinic receptor subtypes and function in rat urinary bladder. N-S Arch Pharmacol 372(1):71–78
Sjuve R, Uvelius B, Arner A (1997) Old age does not affect shortening velocity or content of contractile and cytoskeletal proteins in the rat detrusor smooth muscle. Urol Res 25(1):67–70
Smith PP (2010) Aging and the underactive detrusor: a failure of activity or activation? Neurourol Urodyn 29(3):408–412
Somlyo AV (2002) New roads leading to Ca2+ sensitization. Circ Res 91(2):83–84
Somlyo AP, Himpens B (1989) Cell calcium and its regulation in smooth muscle. FASEB J Off Publ Fed Am Soc Exp Biol 3(11):2266–2276
Somlyo AP, Somlyo AV (2003) Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83(4):1325–1358
Takahashi R, Nishimura J, Hirano K, Seki N, Naito S, Kanaide H (2004) Ca(2+) sensitization in contraction of human bladder smooth muscle. J Urol 172(2):748–752
Tugba Durlu-Kandilci N, Brading AF (2007) Intracellular calcium stores in beta-escin skinned rat and guinea-pig bladders. Eur J Pharmacol 566(1-3):172–180
Volpe P, Salviati G, Chu A (1986) Calcium-gated calcium channels in sarcoplasmic reticulum of rabbit skinned skeletal muscle fibers. J Gen Physiol 87(2):289–303
Wibberley A, Chen Z, Hu E, Hieble JP, Westfall TD (2003) Expression and functional role of Rho-kinase in rat urinary bladder smooth muscle. Br J Pharmacol 138(5):757–766
Wibo M, Godfraind T (1994) Comparative localization of inositol 1,4,5-trisphosphate and ryanodine receptors in intestinal smooth muscle: an analytical subfractionation study. Biochem J 297(Pt 2):415–423
Zhao W, Aboushwareb T, Turner C, Mathis C, Bennett C, Sonntag WE, Andersson KE, Christ G (2010) Impaired bladder function in aging male rats. J Urol 184(1):378–385
Acknowledgments
This study was supported by 2012 L’Oreal Turkey “Women in Science” Grants (NT Durlu-Kandilci).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Durlu-Kandilci, N.T., Denizalti, M. & Sahin-Erdemli, I. Aging changes agonist induced contractile responses in permeabilized rat bladder. AGE 37, 69 (2015). https://doi.org/10.1007/s11357-015-9807-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11357-015-9807-8