Abstract
The technical feasibility of advanced oxidation process, in particular persulfate (PS) oxidation followed by struvite precipitation for landfill leachate treatment and nutrient recovery has been depicted in the current study. Furthermore, the impact of activation of PS with thermal and ultraviolet (UV) irradiation techniques on COD removal efficiency is also investigated. A maximum COD removal efficiency of 96% is accomplished at 65 °C together with supply of UV irradiation. The impact of persulfate dose, pH, and PS/65 °C/UV system on COD and biodegradability is also illustrated in the current study. Additionally, decomposition rate constant values are also ascertained in the present study. Afterwards, nutrient recovery using struvite precipitation is carried out for sustainable utilization of resources. Preliminary treatment of leachate with PS/65 °C/UV system is greatly conducive to recovering high quality struvite crystals. Besides, 94.9%, 83.5%, and 91.3% of PO43− - P, NH4+ - N, and Mg2+ recovery efficiency attained respectively at pH 9.5 and 1.2:1:1 molar ratio of Mg2+: NH4+ - N: PO43− - P. Additionally, all the nutrient recovery studies are validated using chemical equilibrium model Visual MINTEQ. Later, bioavailable fraction of PO43− - P in the recovered struvite is also investigated for utilization as fertilizer. The presence of Cu and Zn in the recovered struvite precipitate enhanced its economic value as a fertilizer. Since Cu and Zn are vital micronutrients for growth of plants. The low soluble values of recovered struvite precipitate confirmed its utilization as slow releasing fertilizer.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Industrialization, rapid urbanization, and economic development indispensably contributed to amplified quality of life standards all over the world, which ascertained to generation of huge quantity of solid waste (Tyagi et al. 2021). Worldwide solid waste generation is expected to be increased to 2.2 billion tons per year by 2025. India is foreseen to generate more than 55 million tons of solid waste per year with an annual growth of 5% (CPCB 2016). Lack of treatment facilities and infrastructure owing to the end of massive quantity of solid waste as untreated in landfills. Approximately 75% of the solid waste generated is being discharged into the landfills (Pujara et al. 2019). Further physico-chemical and biological interactions occurred within the landfill, moisture content of waste and percolation of rainwater led to generation of liquid waste within the landfill and is referred to as leachate (Giang et al. 2018). The characteristics of landfill leachate vary significantly pertaining to weather conditions, moisture content, age, and composition of solid waste. Furthermore, landfill leachate is rich in ammonium nitrogen (NH4+- N), which is toxic to the aquatic species and poses severe risk to the surrounding ecosystems (Sun et al. 2015; Teng et al. 2021). Hence, it has become imperative to bring the NH4+- N content below the permissible limits to evade possible future contamination of water resources (Rani et al. 2020).
Several physicochemical and biological techniques were employed to treat the wastewater containing greater quantity of NH4+- N. Biological techniques such as anammox and nitrification–denitrification methods were harnessed earlier, but greater retention time and regular pH maintenance made scanty application of these techniques (Molinuevo et al. 2009). Physicochemical techniques include stripping of NH4+- N using air, however, ammonium stripped out into the atmosphere contributes to air pollution. Furthermore, huge capital and operating costs also limited the usage of physicochemical techniques to eliminate NH4+- N (Maharaja et al. 2016).
In recent years, magnesium ammonium phosphate (MgNH4PO4. 6H2O) also known as struvite gained ample attention due to its effectiveness to recover the NH4+- N present in the wastewater (Lavanya and Ramesh 2021; Huang et al. 2020). Equi molar concentration of ammonia (NH4+), magnesium (Mg2+), and phosphate (PO43−) is inevitable to precipitate struvite. Nevertheless, isomorphous precipitation of compounds (K- struvite and calcium phosphates) consume NH4+, Mg2+, and PO43− ions present in the system (Huang et al. 2019). Therefore, struvite formation necessitates availability of constituent ions (NH4+, Mg2+, and PO43−) in greater molar ratio than the required stoichiometric ratio as per Eq. (1). Several authors reported the recovery of NH4+- N exist in numerous wastewaters as struvite. Lavanya et al. (2019) depicted the precipitation of struvite from swine wastewater that consists of greater quantities of NH4+- N. Similalrly, Huang et al. (2015) illustrated the recovery of struvite from anaerobically digested supernatant possess excessive amounts of NH4+- N. Consequently, struvite formation fosters the reutilization of removed NH4+- N from wastewaters as fertilizer. However, organic matter present in the wastewaters interferes with struvite precipitation and minimizes its purity and limits the application as a fertilizer. Hence, the elimination of organic matter from wastewater is conducive to nurture the purity of struvite. Furthermore, production of high-quality struvite is of paramount importance to minimize the ecological risk and health hazards associated with it and make it more efficient for fertilizer application (Munir et al. 2017; Oliveira et al. 2018).
Advanced oxidation techniques have drawn attention due to effectiveness and high performance to abate organic pollutants from wastewater (Guan et al. 2018). In the recent past, among numerous advanced oxidation methods, sulfate radical (SO4.−) based approaches draw recognition owing to their efficacy (Wu et al. 2021; Wang et al. 2024). Exclusively, persulfate (PS) is an excellent oxidation agent and can be activated to engender SO4.− radicals. PS activation could be accomplished through numerous ways including heat, ultrasound, ultraviolet (UV) irradiation, alkaline and metal ions to generate SO4.− radicals (Wu et al. 2023; Xie et al. 2023). Despite several methods, heat and UV irradiation techniques are deployed for SO4.− radical production in the current study. Heating (temperature > 30 oC) and UV irradiation both fosters the fission of O-O bond present in PS, which is conducive for SO4.− radical formation in the system (Li et al. 2022; Xia et al. 2020). Thus, SO4.− radical–based advanced oxidation processes gained attention confer to broad pH and high redox potential (2.5 V – 3.6 V) compared to hydroxyl (OH.) radical (1.9 V - 2.8 V) based advanced oxidation techniques (Huang et al. 2020; Tang et al. 2019; Yuan et al. 2020). Furthermore, SO4.− radicals possess greater stability and long life, which accord to foster the oxidation of organic pollutants (Tang et al. 2020).
With this background, the authors in the present study investigated the feasibility of SO4.− radical–based advanced oxidation process combined with struvite precipitation to realize high purity struvite. Consequently, precipitating superior quality struvite precipitates is inevitable to augment its application as a fertilizer. Even though several studies highlighted the possibility of struvite recovery, no study has highlighted the way to enhance its purity. Hence, in this study, authors accentuated the purity aspect of struvite through sequential persulfate oxidation and struvite precipitation. As a result, quality of struvite precipitates obtained with and without SO4.− radical–based advanced oxidation process is assessed in the current study. During the process of precipitating struvite crystals, impact of several processing parameters includes activation of PS and dosage of PS; pH on removal of organic matter along with degradation kinetics followed by study of factors influencing struvite precipitation is ascertained. Likewise, the experimental results obtained from the studies are confirmed using chemical equilibrium model (Visual MINTEQ). Additionally, heavy metal concentration analysis in the obtained struvite is also conducted to evaluate its feasibility as a fertilizer. Besides that, the solubility of struvite precipitate at different pH is verified for identification of best soil conditions for its application.
Materials and methods
Leachate collection
Landfill leachate was collected from Mavallipura site, Bangalore, Karnataka, India. Mavallipura site is used for processing the waste generated from Bangalore city. The leachate is collected in rinsed and clean bottles. Immediately after the collection, the bottles were stored at 4 °C to arrest further degradation. The physico-chemical characteristics (Table 1) of collected leachate are analyzed as per standard methods for examination of water and wastewater (APHA 2012). The leachate sample possesses greater COD and BOD and is rich in NH4+. Furthermore, leachate is poor biodegradable in nature owing to BOD/COD = 0.11, which restrains the deployment of biological techniques for treatment.
Chemicals
Potassium persulfate (K2S2O8) is employed to carry out leachate treatment using advanced oxidation methods. Likewise, other chemicals such as H2SO4 and NaOH are utilized to adjust the pH especially during pH studies. Similarly, potassium dihydrogen phosphate (KH2PO4) is utilized during struvite precipitation experiments. All the chemicals used in the current study are analytical grade. Moreover, all the chemicals are purchased from Merck, India.
Leachate treatment by persulfate/temperature/UV irradiation method
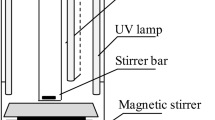
Batch studies are carried out in 1 L capacity reactor with 500 mL effective volume of leachate. UV light of 35 W is fixed to the wooden box. Inside the wooden box, the reactor is placed over the magnetic stirrer for uniform mixing and temperature adjustments (45 °C, 65 °C, and 85 °C). A predetermined quantity of K2S2O8 is added to the reactor consisting of leachate. Later, the aliquot of 5 mL quantity is collected at regular intervals and centrifuged at 3500 rpm for 3 min followed by filtration through 0.45-µm membrane, which is utilized for compositional analysis.
Degradation kinetics
The kinetic analysis is carried out to evaluate rate constant values for organic contaminant removal at diverse experimental circumstances. Organic contaminant removal from landfill leachate by PS usually follow pseudo first order (PFO) kinetics. Therefore, the following equations are utilized to assess the rate constants under various conditions.
Upon integrating Eq. 2 at t = 0, C = Ci and t = t, C = Ct, the equation transforms to
Ci = initial COD concentration (mg/L); Ct = COD concentration at time t (mg/L); k = reaction rate (min−1); t = reaction time (min).
Nutrient (PO4 3− - P and NH4 + - N) recovery through struvite crystallization
The leachate samples with optimum results from PS studies are used for struvite crystallization. For struvite crystallization, definite quantities of reagents were added to the reactor. Subsequently, the whole solution in the reactor is stirred continuously for 45 min and the remaining solution is kept undisturbed for 30 min to promote precipitation. Supernatant quantity of 10 mL is collected for elemental analysis. The precipitates collected are washed thrice with deionized water and allowed for air drying for 24 h.
The combined experimental setup for leachate treatment by persulfate/temperature/UV irradiation method followed by struvite precipitation is provided in Fig. 1.
Chemical equilibrium modelling
Visual MINTEQ is the most widely employed chemical equilibrium model to depict solubility and speciation (Gustafsson 2005; Wu and Zhou 2012). Furthermore, this model is also deployed to identify the equilibrium between various phases (dissolved and undissolved) of compounds in aqueous solution (Çelen et al. 2007). Hence, this model provides a detailed notion of precipitation potential of diverse salts in the solution. The activity coefficients computation in the model is elicited using Davi’s approximation of Debye–Huckle equation.
Saturation index (SI) is the most used parameter to compute the precipitation potential of salts.
where IAP = ion activity product.
Ksp = solubility product constant.
SI = 0 condition signifies that the salt is in equilibrium condition; SI > 0 condition corresponds that the salt is in supersaturated condition, thus precipitation occurs spontaneously; SI < 0 condition denotes that the salt is in undersaturated condition, thereby precipitation does not occur. In the current study, SI of various salts that are likely to form in the aqueous solution is determined for better understanding.
Analytical methods
The pH of the leachate sample is analyzed using pH meter. Phosphorous content in the leachate is analyzed using vanadomolybdo phosphoric acid method using UV/VIS spectrophotometer. Solids concentration in the leachate sample is evaluated using gravimetric analysis. Total organic carbon (TOC) in the leachate sample is determined using Shimadzu TOC analyzer. Heavy metal concentrations present in the leachate sample are measured using ICP-MS. The NH4+- N concentration in the leachate sample is corroborated using Nessler’s reagent spectrophotometric technique.
Solubility of the recovered struvite precipitate
The solubility of the recovered struvite precipitate is analyzed at various pH values (i.e., 5.9, 7 and 8). Solubility experiments are conducted by dissolving 2 g of the recovered struvite precipitate in 100 mL of the deionized water, then the mixture is agitated using arbitrary shaker at 150 rpm for half an hour. Later, the phosphorous content in the solution is determined.
Results and discussion
Impact of PS activation by heat and UV irradiation on COD and TOC removal efficiency
The time dependent impact of heating and UV irradiation on COD and TOC removal from landfill leachate is investigated. Figure 2 depicts the variation of COD and TOC removal efficiency, and COD and TOC reaction kinetics by PS activation using both thermal and UV irradiation techniques. Both heat (Eq. 5) and UV (Eq. 6 and 7) activation distinctly heightened the performance of PS.
As corroborated in Fig. 2a, COD removal efficiency is superior at high heating temperatures. Furthermore, supplementation of UV irradiation in addition to heat greatly contributed to boost COD removal efficiency. For a time of 210, 240, and 270 min with combined temperature (85 °C) and UV irradiation, the COD removal efficiency achieved is 97, 97, and 99% respectively, which signifies the slight variation in COD removal efficiency. Similar patterns of results are observed even for 65 °C and 45 °C with UV irradiation. As a result, 65 °C is considered the optimum temperature for activation of PS for further experiments. Furthermore, at 65 °C with UV irradiation with various time periods of 210, 240, and 270 min, the COD removal efficiency is almost same. Hence, 210 min is considered as optimum period for performing further experiments.
Similarly, Fig. 2b illustrates the variation of TOC removal efficiency over time. As mentioned above, both thermal and UV activation of PS promoted TOC removal efficiency. TOC removal efficiency attained by PS activation with UV at 65 °C and 85 °C for 210 min is 82 and 83% respectively. Therefore, PS activation with UV irradiation at 65 °C is considered as optimum for further studies.
Besides that, the reaction kinetic analysis for COD and TOC by both thermal and UV activation of PS is carried out using PFO reaction and the results are shown in Fig. 2c and 2d, respectively. Additionally, the computed rate constant values for COD and TOC at different PS activation using thermal and UV irradiation conditions are presented in Table 2.
The abovementioned observations are owed to greater occurrence of SO4.− radicals during PS activation at elevated temperature and UV irradiation (Eq. 5, 6 and 7). Elevated temperatures (i.e., > 30 °C) together with UV accord for cleavage of O-O bond present in PS, which is decisive for SO4.− radical formation. Thus, produced SO4.− radicals enables the removal of organic contaminants from landfill leachate. Similarly, augmented reaction rate values (Table 2) during PS activation with rise in thermal and UV irradiation also illustrates the quick bombardment between SO4.− radicals and contaminants, which facilitates the contaminant removal. Additionally, higher temperatures also account for decomposition of organic matter present in the leachate sample (Zhou et al. 2019; Ali and Kim 2016).
Impact of PS dosage on COD removal efficiency
PS concentration is another preliminary factor in the PS/temperature/UV irradiation system. Hence to study the influence of PS dose on COD removal efficiency, PS concentration is varied from 1 to 10 g/L (Fig. 3a). The other parameters include temperature, maintained at 65 °C with the aid of UV irradiation.
As illustrated in Fig. 3a, the COD removal efficiency is raised with PS dosage and reaction time. Increase in PS dose promotes the production of SO4.− radicals, which greatly contributed to degrade organics present in the leachate. Thereby, the COD removal efficiency increased with raise in PS dosage. However, the COD removal efficiency attained at PS dosage of 5 g/L is almost similar to the efficiencies accomplished at 6, 7, 8, 9, and 10 g/L dosages. Thus, 5 g/L PS dosage is considered an optimum considering economical aspects. In addition to that reaction kinetic analysis is performed at numerous PS dosages and reaction time (Fig. 3b). The calculated reaction rate values are furnished in Table 3. Both temperature and UV irradiation contributed to the fission of O-O bond in PS, thereby the readiness of SO4.− is high and aids the degradation of organics in leachate (Eq. 5, and 6). Additionally, enhanced reaction rate values with PS dosages (Table 3) also confirms the rapid production of SO4.− radicals. Furthermore, these SO4.− radicals commence chain reactions that advocate limited or complete degradation of organics. Additionally, alkaline conditions maintained during the studies are also ascribed to produce OH. (Eq. 7), which abets organics removal. On the other facet, augmented PS dosage (i.e., > 5 g/L) surges the SO4.− radicals availability, which raises the interaction among them and form PS ions (Eq. 8). Similarly, at greater PS dosages, evolution of inhibitory compounds like SO42− (Eq. 9) arrests the decomposition of organics. The above highlighted reasons imply that utilization of SO4.− radicals except for organics contributed to sluggish rise in COD efficiency at greater doses of PS (Soubh and Mokhtarani 2016; Zhang et al. 2018; Huang et al. 2020).
Impact of PS/temperature/UV irradiation on biodegradability
Biodegradability is a vital parameter that needs to be analyzed during the treatment of leachate with PS/temperature/UV irradiation system. Primarily, leachate possesses refractory substances that are challenging to eliminate by biological systems. However, PS/temperature/UV irradiation system is efficient in degradation of non-biodegradable substances, thus indispensably contributed to enhance the BOD/COD ratio (Fig. 4).
As illustrated in Fig. 4, at the beginning of study, the BOD/COD ratio is low (i.e., 0.11), which raised to 0.48 after treating the leachate for 210 min. Furthermore, higher quantity of radical production owing to PS activation under elevated temperature and UV irradiation imperatively contributed to breakdown and oxidation of the complex organic substances into simpler compounds and ultimately harness to achieve desired leachate treatment (Huang et al. 2020). Mahvi et al. (2012) also illustrated the enhancement in biodegradability owing to greater release of SO4.− radicals during the process.
Impact of initial pH on COD removal efficiency
The influence of pH on COD removal efficiency is evaluated by varying the pH from 3 to 11 using PS/temperature/UV irradiation system (Fig. 5a). During the experiments, PS dose of 5 g/L and temperature of 65 °C with aid of UV irradiation is supplied to the system.
As confirmed from Fig. 5a, the COD removal efficiency is less at pH 3 and pH 5 when compared to pH 7. Nevertheless, for pH 7 and pH 7.56 (i.e., pH of leachate), variation in COD removal efficiency is quite low. The COD removal efficiency attained at reaction time of 210 min, initial pH 7 and 7.56 is 94% and 96% respectively. Therefore, pH 7.56 is considered as optimum and is used for performing further studies. Furthermore, reaction kinetic studies are carried out with varying pH and reaction time and the results are depicted in Fig. 5b. Also, the corresponding reaction rate constant details are illustrated in Table 4.
As mentioned above, the readiness of SO4.− radicals varied with pH of the leachate. During the studies, SO4.− radicals are generated within the system as per Eq. 5, and 6 at pH 3 and 5. Furthermore, S2O82− is deemed as a scavenger of SO4.− radicals in acidic pH (Wei et al. 2018). Therefore, the reaction rate plunged faintly under acidic conditions, which caused lower COD removal efficiency. Likewise, lower reaction rates (Table 4) at pH 3 and 5 are also responsible for achieving lower COD removal efficiency. However, at pH 7 and 7.56, the availability of OH− in the leachate solution is high compared to acidic pH (i.e., 3 and 5), which reacts with SO4.− radicals (Eq. 7) and is responsible for generation of OH. radicals. Therefore, coexistence of SO4.− and OH. radicals at pH 7 and 7.56 together contributed to attain greater COD removal efficiency than that of pH 3 and 5. Moreover, the reaction rate values at pH 7 and 7.56 are higher over other studied pH range. Further OH− ions concentration is higher at pH 7.56 than that of pH 7, which harnessed to attain higher COD removal efficiency. However, at pH 9, alkaline pH led to production of greater quantity of OH. radical over SO4.− radical. Furthermore, the redox potential of SO4.− is 2.5 - 3.6 V, which is greater than the redox potential OH. radical (i.e., 1.9 - 2.8 V) (Oh et al. 2016; Wei et al. 2018). Thus, it is hypothesized that under alkaline pH conditions (i.e., pH 9) degradability of organic substances is slightly lower than that of neutral pH. In addition to that initial pH of leachate is 7.56, which underlines that the alkalinity is majorly due to HCO3− ions. But at pH 9, HCO3− ions are transformed to CO32− ions, which is scavenger of OH. radicals (Soubh and Mokhtarani 2016). Hence, the above emphasized logics caused lower COD removal efficiency values at pH 9 than at neutral pH values.
Nutrient recovery through struvite crystallization
For nutrient recovery, the leachate obtained after PS treatment (PS dose = 5 g/L, temperature = 65 °C, UV light = 35W) is used. Numerous factors influence the precipitation of struvite from leachate. Hence, the influence of various factors on struvite precipitation is investigated and reported in the subsequent sections.
Impact of pH on struvite precipitation
pH is the most governing parameter that impacts the struvite precipitation (Huang et al. 2015). Further pH also regulates the speciation of PO43−- P and NH4+ - N ions, super saturation and solubility of precipitates that are evinced in the solution during the process (Rathod et al. 2014). Therefore, to determine the impact of pH on struvite precipitation, pH is varied from 8 to 11 and the Mg:P molar ratio is maintained at 1:1. The variations of PO43−- P and NH4+ - N recovery efficiencies and purity of struvite with pH is provided in Fig. 6.
Variations of a PO43−- P and NH4+ - N recovery efficiencies with different pH after treatment with PS/temperature/UV irradiation system, b variation of purity of struvite with and without PS/temperature/UV irradiation system, c variation of SI of different salts with pH (experimental condition: Mg2+: NH4+ - N: PO4.3− - P molar ratio = 1:1:1)
As presented in Fig. 6a, the PO43−- P recovery efficiency augmented steadily with a rise in pH. However, the NH4+ - N recovery efficiency increased up to pH 9.5 and attained maximum recovery efficiency of 81.3% at pH 9.5. Nevertheless, increase in pH beyond 9.5 led to diminish the NH4+ - N recovery efficiency compared with pH 9.5. As a result, pH 9.5 is considered optimum for performing further studies.
As illustrated in earlier sections, pH plays a critical role in struvite precipitation. pH governs the speciation of PO43−- P and NH4+ - N ions, which are vital for struvite formation (Zhang et al. 2009; Lavanya and Sri Krishnaperumal Thanga 2021). Generally, PO43−- P ions persist in three different forms (H2PO4−, HPO42−, and PO43−) depending on the pH. H2PO4− species prevail in the solution pH ranges from 5 to 6.5 (Georgantas and Grigoropoulou 2007). Furthermore, HPO42− appears in the solution from pH 7 to 9. Likewise, PO43− persuades in the solution at pH > 10 (Lavanya et al. 2019). In the current study, pH is varied from 8 to 11; hence from pH 8 to 9, HPO42− is present and lower H+ concentration facilitates the struvite formation (Eq. 10) and manifests high PO43−- P recovery efficiency. Similarly, pH > 9.5, promotes the conversion of NH4+ to NH3 (Li et al. 2012) (Eq. 11, reaction favored to right), as a result the NH4+ - N recovery efficiency is reduced beyond pH 9.5.
The purity of the struvite precipitates obtained after the treatment with PS/temperature/UV irradiation system ranges from 89.5 to 69.5% (Fig. 6b). However, the purity of struvite precipitates collected without treatment of leachate by PS/temperature/UV irradiation system varied from 78.4 to 58.2% (Fig. 6b). The abovementioned purity results highlight the necessity of leachate treatment prior to struvite precipitation. Commonly, leachate is rich in organic matter, which hinders the precipitation of high-grade struvite. Therefore, elimination of organic matter from leachate samples is essential to produce good quality struvite. Hence, leachate treatment with PS eliminates the organic matter present in it and contributes to accomplish high quality struvite precipitates.
Further to validate the results obtained from the experiments, chemical equilibrium model Visual MINTEQ is employed. The most prevailed salts in solution consists of Mg2+, NH4+, and PO43− include Mg3(PO4)3, MgHPO4.3H2O, Mg(OH)2, and struvite (Çelen et al. 2007). As a result, the SI of various salts likely to be precipitated in the aqueous solution is determined using Visual MINTEQ (Fig. 6c). Figure 6c depicts the variation of SI with pH. At pH 8, Mg3(PO4)3, MgHPO4.3H2O, and struvite are in oversaturated condition. Furthermore, SI of MgHPO4.3H2O is marginally in oversaturated condition at pH 8, thus contributing to the reduction of purity of struvite. Similarly, Mg3(PO4)3 and struvite are also precipitated but the SI of struvite is greater, as a result the purity of the struvite is high at pH 8. However, Mg(OH)2 is in undersaturation (i.e., soluble) condition at pH 8; hence, precipitate formation is negligible; thus, Mg(OH)2 formation in aqueous solution is not impacting the purity of struvite. Likewise, at pH 8.5, struvite formation is predominant over Mg3(PO4)3. Nonetheless, Mg3(PO4)3 formation is higher in aqueous solution at pH 8.5 over pH 8, thus led to reduction in purity of struvite at pH 8.5 when compared to pH 8. Analogously, from pH 9 to 11, the formation of Mg3(PO4)3 is dominant over struvite. Furthermore, Mg(OH)2 is in oversaturated condition from pH 10.5 onwards. Thus, formation of salts in aqueous solution diminished the purity of the struvite with pH.
Impact of Mg2+: NH4 + - N: PO4 3− - P molar ratio on struvite precipitation
The concomitant recovery potential of PO43− - P, NH4+ - N, and Mg2+ ions is evaluated with varying molar rations (Mg2+: NH4+ - N: PO43− - P) and at pH 9.5. Figure 7 corroborates the impact of Mg2+: NH4+ - N: PO43− - P ratio on nutrient recovery and amount of precipitate formed during the studies.
It is evident from Fig. 7 that the surge in Mg2+: NH4+ - N: PO43− - P molar ratio nurtures the PO43− - P recovery efficiency from 84.4 to 97.6%. However, the PO43− - P recovery efficiency is almost similar to Mg2+: NH4+ - N: PO43− - P molar ratios of 1.5:2:1, 2:3:1, and 2:5:1. At the examined Mg2+: NH4+ - N: PO43− - P molar ratios, the NH4+ - N recovery efficiency is slightly increased initially with Mg2+: NH4+ - N: PO43− - P molar ratio and remained stable at higher molar ratios. Likewise, Mg2+ recovery efficiency also enhanced from 82.3 to 94.1% with raise in Mg2+: NH4+ - N: PO43− - P molar ratio. As the Mg2+: NH4+ - N: PO43− - P molar ratio is increased, the availability of Mg2+ to interact with PO43− - P and NH4+ - N also increases, thus results in attaining higher recovery efficiency and led to higher precipitate formation (Chen et al. 2018). Similarly, Fig. 7 also confirms the higher precipitate formation with enriched Mg2+: NH4+ - N: PO43− - P molar ratio. Albeit, the recovery efficiency of struvite constituent ions is greater at Mg2+: NH4+ - N: PO43− - P molar ratio of 2:5:1, considering the economic aspects 1.2:1:1 is deemed as optimum molar ratio.
Likewise, the experimental results obtained from studies are validated using chemical equilibrium model Visual MINTEQ. The results obtained from Visual MINTEQ are presented in Fig. 8.
Furthermore, to validate the struvite precipitation at Mg2+: NH4+ - N: PO43− - P molar ratio of 1.2:1:1, FTIR and XRD analyses are carried out and the results are presented in Fig. 9. XRD pattern (Fig. 9a) of the precipitates collected after the studies corresponded well with standard struvite pattern (PDF#71–2089). Furthermore, sharp peaks observed in XRD spectrum also illustrates that the struvite formed is crystalline in nature. Figure 9b shows the FTIR pattern of precipitates obtained at Mg2+: NH4+ - N: PO43− - P molar ratio of 1.2:1:1 and at pH 9.5. From FTIR spectrum, the major peaks are noticed at 3697, 3600, 3291, 2357, 1647, 1536, 1450, 1058, 869, and 440 cm−1. The peaks observed at 3697 and 3600 cm−1 are owing to asymmetric stretching vibration of N-H and similarly 1647 cm−1 is obliged to symmetric bending vibration of N-H existing in NH4+ units. Likewise, the peak at 3291 cm−1 is caused by H-O-H stretching vibration of H2O molecules. Further the peak noticed at 2357 cm−1 is due to H-O-H stretching vibration of cluster of H2O molecules, whereas the peak at 1647 cm−1 is occurred by bending vibration of H-O-H bond. Additionally, the peak detected at 1058 cm−1 is due to asymmetric stretching vibration of PO43−, while the peak discovered at 440 cm−1 is instigated by symmetric bending vibration of PO43− units. The peak spotted at 869 cm−1 is owed to distortion of OH associated with Mg2+, whereas peak at 440 cm−1 occurred due to metal oxygen bond prevailed in the precipitate (Chauhan and Joshi 2013).
Phosphorous bioavailability in the recovered struvite precipitate
Phosphorous bioavailability is the vital factor that needs to be determined to utilize the recovered precipitate as fertilizer. PO43− - P fraction soluble in 2% citric acid is considered readily available fraction for plants to utilize. The bioavailable PO43− - P fraction in the precipitate obtained after leachate treatment with PS/temperature/UV irradiation system followed by struvite precipitation performed at Mg2+: NH4+ - N: PO43− - P molar ratio 1.2:1:1 and pH 9.5 is analyzed and is estimated as 90.5%. Furthermore, commercially available super phosphate consists of 87% bioavailable PO43− - P fraction. Since the struvite precipitate obtained in the present study consists of 90.5% bioavailable fraction, utilization of this precipitate is beneficial.
Estimation of heavy metal content, solubility in the recovered struvite precipitate
Estimation of heavy metals concentration in the recovered struvite precipitate is imperative to utilize them as fertilizer. The precipitates recovered from leachate at Mg2+: NH4+ - N: PO43− - P molar ratio 1.2:1:1 and pH 9.5 after treatment with PS/temperature/UV irradiation system is considered for analysis.
The presence of heavy metal concentration is detrimental and causes adverse impacts on health. Therefore, in the wake of these effects, the heavy metal concentration in the recovered struvite precipitate is determined. Furthermore, it is noticed that (from Table 5) the heavy metal concentrations such as As, Cd, Cr, Pb, and Ni are zero, whereas Cu and Zn concentrations are observed as 10.54 and 24.56 mg/Kg respectively in the recovered precipitate. Therefore, recovered struvite precipitate embodied varying quantities of heavy metals. Furthermore, the recovered struvite precipitate contains detectable quantities of Cu and Zn, which boosts the value of struvite and is conducive for utilization as fertilizer, since Cu and Zn are the micronutrients essential for plant growth.
Additionally, solubility and nutrient content in the recovered struvite precipitate are also analyzed to ascertain fertilizer potential. Table 6 provides the solubility values of recovered struvite precipitate at different pH. The solubility of the struvite varied from 42.29 to 48.79%. The solubility of the chemical fertilizers usually varies from 60 to 95% (Massey et al. 2009; Reza et al. 2019). Based on the solubility test results, solubility values of the recovered struvite precipitate are lower than the conventional chemical fertilizers. This highlights that recovered struvite precipitate owing to low solubility releases nutrients slowly into the environment over high soluble chemical fertilizers.
Conclusions
In the present study, two step landfill leachate treatment has been performed. In the first step leachate treatment is carried out using PS/temperature/UV irradiation system followed by struvite precipitation to recover the nutrients present in the leachate. The major motive for deploying PS/temperature/UV irradiation system is to eliminate the organic matter present in the leachate and to recover high quality struvite precipitates. Thereby, the impact on purity of struvite precipitation could be minimized significantly. During the process, the influence of temperature and UV irradiation on PS activation has been investigated. Both temperature (65 °C) and UV irradiation contributed to achieve 96% COD removal efficiency. Similarly, optimum dosage of PS to attain maximum COD removal efficiency is found to be 5 g/L. Additionally, biodegradability of the leachate has been enhanced from 0.11 to 0.48 owing to treatment with PS/temperature/UV irradiation system. A pH of 7.56 is considered an optimum for PS/temperature/UV irradiation system. Likewise, the Mg2+: NH4+ - N: PO43− - P molar ratio of 1.2:1:1 is deemed as optimum keeping economical aspects in consideration. All the struvite precipitation studies are validated using chemical equilibrium model named Visual MINTEQ. The bioavailable fraction of PO43− - P in the recovered struvite precipitate is examined as 90.5%. Analogously, heavy metals including Cu and Zn are present in the recovered struvite. The existence of micronutrients (i.e., Cu and Zn) also magnified struvite fertilizer potential value. Further low soluble values of recovered struvite also emphasize the application of it as slow releasing fertilizer. Hence, the dual processes together PS/temperature/UV irradiation system followed by struvite precipitation is advantageous for effective recovery of nutrients and as well as for leachate treatment.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ali TU, Kim D-J (2016) Phosphorus extraction and sludge dissolution by acid and alkali treatments of polyaluminum chloride (PAC) treated wastewater sludge. Bioresour Technol 217:233–238. https://doi.org/10.1016/j.biortech.2016.02.017
APHA (2012) Standard methods for the examination of water and wastewater. Washington, DC
Çelen I, Buchanan JR, Burns RT et al (2007) Using a chemical equilibrium model to predict amendments required to precipitate phosphorus as struvite in liquid swine manure. Water Res 41:1689–1696. https://doi.org/10.1016/j.watres.2007.01.018
CPCB (2016) The national action plan for municipal solid waste management
Chauhan CK, Joshi MJ (2013) In vitro crystallization, characterization and growth-inhibition study of urinary type struvite crystals. J Cryst Growth 362:330–337. https://doi.org/10.1016/j.jcrysgro.2011.11.008
Chen Y, Liu C, Guo L et al (2018) Removal and recovery of phosphate anion as struvite from wastewater. Clean Technol Environ Policy 20:2375–2380. https://doi.org/10.1007/s10098-018-1607-2
Georgantas DA, Grigoropoulou HP (2007) Orthophosphate and metaphosphate ion removal from aqueous solution using alum and aluminum hydroxide. J Colloid Interface Sci 315:70–79. https://doi.org/10.1016/j.jcis.2007.06.058
Giang NV, Kochanek K, Vu NT, Duan NB (2018) Landfill leachate assessment by hydrological and geophysical data: case study NamSon, Hanoi. Vietnam J Mater Cycles Waste Manag 20:1648–1662. https://doi.org/10.1007/s10163-018-0732-7
Guan R, Yuan X, Wu Z et al (2018) Principle and application of hydrogen peroxide based advanced oxidation processes in activated sludge treatment: a review. Chem Eng J 339:519–530. https://doi.org/10.1016/j.cej.2018.01.153
Gustafsson JP (2005) Visual MINTEQ, ver 2.32 user guide. KTH, Department of Land and Water Resources, Stockholm, p 550. https://vminteq.com/
Huang H, Liu J, Ding L (2015) Recovery of phosphate and ammonia nitrogen from the anaerobic digestion supernatant of activated sludge by chemical precipitation. J Clean Prod 102:437–446. https://doi.org/10.1016/j.jclepro.2015.04.117
Huang H, Li J, Li B et al (2019) Comparison of different K-struvite crystallization processes for simultaneous potassium and phosphate recovery from source-separated urine. Sci Total Environ 651:787–795. https://doi.org/10.1016/j.scitotenv.2018.09.232
Huang H, Guo G, Tang S et al (2020) Persulfate oxidation for alternative sludge treatment and nutrient recovery: an assessment of technical and economic feasibility. J Environ Manage 272:111007. https://doi.org/10.1016/j.jenvman.2020.111007
Lavanya A, Ramesh SKT (2021) Crystal seed-enhanced ammonia nitrogen and phosphate recovery from landfill leachate using struvite precipitation technique. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-14950-y
Lavanya A, Sri KrishnaperumalThanga R (2021) Effective removal of phosphorous from dairy wastewater by struvite precipitation: process optimization using response surface methodology and chemical equilibrium modeling. Sep Sci Technol 56:395–410. https://doi.org/10.1080/01496395.2019.1709080
Lavanya A, Ramesh ST, Nandhini S (2019) Phosphate recovery from swine wastewater by struvite precipitation and process optimization using response surface methodology. Desalin WATER Treat 164:134–143. https://doi.org/10.5004/dwt.2019.24447
Li W, Ding X, Liu M et al (2012) Optimization of process parameters for mature landfill leachate pretreatment using MAP precipitation. Front Environ Sci Eng 6:892–900. https://doi.org/10.1007/s11783-012-0440-9
Li N, Wu S, Dai H et al (2022) Thermal activation of persulfates for organic wastewater purification: heating modes, mechanism and influencing factors. Chem Eng J 450:137976. https://doi.org/10.1016/j.cej.2022.137976
Maharaja P, Gokul E, Prabhakaran N et al (2016) Simultaneous removal of NH4+-N and refractory organics through sequential heterogeneous Fenton oxidation process and struvite precipitation: Kinetic study. RSC Adv 6:4250–4261. https://doi.org/10.1039/c5ra20492e
Mahvi AH, Roodbari AA, NabizadehNodehi R et al (2012) Improvement of landfill leachate biodegradability with ultrasonic process. PLoS ONE 7:3–9. https://doi.org/10.1371/journal.pone.0027571
Massey MS, Davis JG, Ippolito JA, Sheffield RE (2009) Effectiveness of recovered magnesium phosphates as fertilizers in neutral and slightly alkaline soils. Agron J 101:323–329. https://doi.org/10.2134/agronj2008.0144er
Molinuevo B, García MC, Karakashev D, Angelidaki I (2009) Anammox for ammonia removal from pig manure effluents: effect of organic matter content on process performance. Bioresour Technol 100:2171–2175. https://doi.org/10.1016/j.biortech.2008.10.038
Munir MT, Li B, Boiarkina I et al (2017) Phosphate recovery from hydrothermally treated sewage sludge using struvite precipitation. Bioresour Technol 239:171–179. https://doi.org/10.1016/j.biortech.2017.04.129
Oh WD, Dong Z, Lim TT (2016) Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: current development, challenges and prospects. Appl Catal B Environ 194:169–201. https://doi.org/10.1016/j.apcatb.2016.04.003
Oliveira V, Labrincha J, Dias-Ferreira C (2018) Extraction of phosphorus and struvite production from the anaerobically digested organic fraction of municipal solid waste. J Environ Chem Eng 6:2837–2845. https://doi.org/10.1016/j.jece.2018.04.034
Pujara Y, Pathak P, Sharma A, Govani J (2019) Review on Indian Municipal Solid Waste Management practices for reduction of environmental impacts to achieve sustainable development goals. J Environ Manage 248:109238. https://doi.org/10.1016/j.jenvman.2019.07.009
Rani A, Negi S, Hussain A, Kumar S (2020) Treatment of urban municipal landfill leachate utilizing garbage enzyme. Bioresour Technol 297:122437. https://doi.org/10.1016/j.biortech.2019.122437
Rathod M, Mody K, Basha S (2014) Efficient removal of phosphate from aqueous solutions by red seaweed, Kappaphycus alverezii. J Clean Prod 84:484–493. https://doi.org/10.1016/j.jclepro.2014.03.064
Reza A, Shim S, Kim S et al (2019) Nutrient leaching loss of pre-treated struvite and its application in Sudan grass cultivation as an eco-friendly and sustainable fertilizer source. Sustain 11:1–14. https://doi.org/10.3390/su11154204
Soubh A, Mokhtarani N (2016) The post treatment of composting leachate with a combination of ozone and persulfate oxidation processes. RSC Adv 6:76113–76122. https://doi.org/10.1039/c6ra09539a
Sun H, Peng Y, Shi X (2015) Advanced treatment of landfill leachate using anaerobic-aerobic process: organic removal by simultaneous denitritation and methanogenesis and nitrogen removal via nitrite. Bioresour Technol 177:337–345. https://doi.org/10.1016/j.biortech.2014.10.152
Tang S, Li X, Zhang C et al (2019) Strengthening decomposition of oxytetracycline in DBD plasma coupling with Fe-Mn oxide-loaded granular activated carbon. Plasma Sci Technol 21:025504. https://doi.org/10.1088/2058-6272/aaeba6
Tang S, Tang J, Yuan D et al (2020) Elimination of humic acid in water: comparison of UV/PDS and UV/PMS. RSC Adv 10:17627–17634. https://doi.org/10.1039/d0ra01787f
Teng C, Zhou K, Peng C, Chen W (2021) Characterization and treatment of landfill leachate: a review. Water Res 203:117525. https://doi.org/10.1016/j.watres.2021.117525
Tyagi VK, Kapoor A, Arora P et al (2021) Mechanical-biological treatment of municipal solid waste: case study of 100 TPD Goa plant. India J Environ Manage 292:112741. https://doi.org/10.1016/j.jenvman.2021.112741
Wang Y, Lin Y, He S, Wu S, Yang C (2024) Singlet oxygen: properties, generation, detection, and environmental applications. J Hazar Mater 461:132538. https://doi.org/10.1016/j.jhazmat.2023.132538
Wei LL, Chen WM, Bin LQ et al (2018) Treatment of dinitrodiazophenol industrial wastewater in heat-activated persulfate system. RSC Adv 8:20603–20611. https://doi.org/10.1039/c8ra01995a
Wu Y, Zhou S (2012) Improving the prediction of ammonium nitrogen removal through struvite precipitation. Environ Sci Pollut Res 19:347–360. https://doi.org/10.1007/s11356-011-0520-6
Wu S, Shen L, Lin Y et al (2021) Sulfite-based advanced oxidation and reduction processes for water treatment. Chem Eng J 414:128872. https://doi.org/10.1016/j.cej.2021.128872
Wu S, Yang Z, Zhou Z et al (2023) Catalytic activity and reaction mechanisms of single-atom metals anchored on nitrogen-doped carbons for peroxymonosulfate activation. J Hazar Mater 459:132133. https://doi.org/10.1016/j.jhazmat.2023.132133
Xia X, Zhu F, Li J et al (2020) A review study on sulfate-radical-based advanced oxidation processes for domestic/industrial wastewater treatment: degradation, efficiency, and mechanism. F Chem 8:592056. https://doi.org/10.3389/fchem.2020.592056
Xie J, Yang C, Li X et al (2023) Generation and engineering applications of sulfate radicals in environmental remediation. Chem 339:139659. https://doi.org/10.1016/j.chemosphere.2023.139659
Yuan D, Zhang C, Tang S et al (2020) Fe3+-sulfite complexation enhanced persulfate Fenton-like process for antibiotic degradation based on response surface optimization. Sci Total Environ 727:138773. https://doi.org/10.1016/j.scitotenv.2020.138773
Zhang T, Ding L, Ren H, Xiong X (2009) Ammonium nitrogen removal from coking wastewater by chemical precipitation recycle technology. Water Res 43:5209–5215. https://doi.org/10.1016/j.watres.2009.08.054
Zhang L, Liu H, Zheng Z et al (2018) Continuous liquid fermentation of pretreated waste activated sludge for high rate volatile fatty acids production and online nutrients recovery. Bioresour Technol 249:962–968. https://doi.org/10.1016/j.biortech.2017.10.103
Zhou Z, Liu X, Sun K et al (2019) Persulfate-based advanced oxidation processes (AOPs) for organic-contaminated soil remediation: a review. Chem Eng J 372:836–851. https://doi.org/10.1016/j.cej.2019.04.213
Author information
Authors and Affiliations
Contributions
Addagada Lavanya: data curation, methodology, conceptualization, writing—original draft, review and editing, visualization.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ricardo A. Torres-Palma
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lavanya, A. Treatment and nutrient recovery from landfill leachate by sequential persulfate oxidation and struvite precipitation: An evaluation of technical feasibility. Environ Sci Pollut Res 31, 55022–55034 (2024). https://doi.org/10.1007/s11356-024-34825-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-34825-2