Abstract
The selection of the appropriate biomonitor species is a crucial criterion for biomonitoring on a broad spatial scale. Mosses Hypnum cupressiforme and Brachythecium spp. and lichen Evernia prunastri were sampled at 22 remote sites over Serbia aiming interspecies comparison of their bioconcentration capacities. The concentration of 16 potentially toxic elements (PTEs), Al, Ba, Cd, Co, Cr, Cu, Fe, Hg, Mn, Ni, P, Pb, S, Sr, V, and Zn, was measured in the samples. Between the co-located mosses, linear regression analysis (type II) showed significant determination coefficients only for a couple of the elements (Cd and S), while for H. cupressiforme vs. lichen, significant regression lines were obtained for a broader set of elements (Ba, Cd, Fe, Hg, Mn, Ni, Sr). The ratio of the PTEs in the mosses discovered higher concentrations in H. cupressiforme than in another moss at some sites and vice versa at other sites. According to the PTE ratios, H. cupressiforme accumulated much more element content than the lichen, but followed a similar spatial pattern. In addition, principal component analysis (PCA) pointed out a different grouping of the PTEs depending on the species tested. The poor correlation of the moss-moss data is perhaps because several species of the genus Brachythecium were sampled, which possibly influenced the average genus accumulation capacity. In addition, morphological features of the mosses (concave vs. flat leaflets, creeping vs. cushiony life form) presumably delegate differences in PTE accumulation. To conclude, it should be careful with using more biomonitor species, even of the same genus, within the same study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last several decades, ambient air pollution has been a leading risk factor for the global burden of diseases, especially in low- and middle-income countries (GBD 2016). Accordingly, the state of air quality (AQ) has increased all over the world. Regulatory networks of AQ monitoring stations are established mainly in population centers near traffic burden sites, in city centers, and at locations of particular concern such as a school, hospital, or particular emissions sources. However, large areas in the interior of countries remain uncovered by any data source on air pollution. Unlike urbanized areas, remote areas are usually characterized by a high variety of plants and animals (biodiversity), which reflect changes in the environment and thus pollution levels. Therefore, biomonitoring is considered a useful tool for representing pollutants and their trends in real-time and retrospective monitoring of environmental pollution (Chaudhary 2022).
Biomonitoring of air pollutants using cryptogams (mosses and lichens) could be a complementary approach to assess long-term atmospheric deposition of the pollutants across urban, industrial, and remote areas (Aničić Urošević et al. 2017 and reference therein). Morphological and structural traits of mosses, such as the presence of rhizoids instead of developed root system, large specific intercepting surface area, and less developed cuticles high cation-exchange capacity (CEC) of the cell walls, these organisms mainly rely on atmospheric deposition for nutrients and water supply (Chakrabortty and Paratkar 2006; Varela et al. 2023). Mosses are tolerant to environmental pollution and may act as accumulative biomonitors, especially those ectohydric mosses—conducting water externally through capillary movement, accomplished with a high amount of metal binding sites (González and Pokrovsky 2014). Mosses accumulate great amounts of contaminants between apoplast and symplast compartments without damaging vital functions of the cells (Vásquez et al. 1999). However, some recent research points out that mosses predominantly have a great capacity to retain particles containing pollutants and their concentrations clearly depict particle deposition patterns in many circumstances (Varela et al. 2023). Lichens also take up nutrients over their complete surface; they lack stomata or waxy cuticles and hence have little control over the uptake or removal of nutrients (Abas 2021; Conti and Cecchetti 2001). They are usually quite sensitive to environmental changes and can play a role as early-warning bioindicators of pollution impacts (Nash 2008; Ochoa-Hueso et al. 2017; Giordani and Brunialti 2015); still, some species can accumulate trace elements far beyond their physiological requirements (Garty 2001; Nimis et al. 2002). Besides numerous case studies, both mosses and lichens also have applications in biomonitoring within international programs. Thus, within the ICP Vegetation program (https://icpvegetation.ceh.ac.uk/), a survey of atmospheric deposition of the air pollutants has been performed every 5 years over remote areas of Europe and Asia by collecting and analyzing four moss species: Hylocomium splendens (Hedw.) Schimp., Pleurozium schreberi (Brid.) Mitt, Hypnum cupressiforme Hedw., and Pseudoscleropodium purum (Hedw.) M. Fleisch. The recommended species are widespread pleurocarpous mosses, the first one with clearly distinct, separate annual growth segments. Still, no one species is universally spread across Europe and Asia to be uniformly collected within the sampling campaigns. Thus, P. schreberi was the most frequently sampled species for heavy metals (ca. 42%), followed by H. splendens (23.5%), H. cupressiforme (19.6%), and P. purum (7.7%), while other moss species constituted 7.1% (Harmens et al. 2015). Fernández et al. (2015) provided an extensive review of the number of genera (11) and species (72), and protocols as well, used within the program. It should be noted that the concentrations of elements may vary considerably between species, thus precluding comparison of the results obtained. Thus, in the studies where more than one species is used, firstly, interspecies calibration should be performed and data transformation by the corresponding intercalibration line (Carballeira et al. 2008). Since lichens are extremely sensitive to environmental stress, especially concerning atmospheric pollution, eutrophication, and climate change that feature was used to study their bioindication potential and scaling of the pollution response. Accordingly, a European guideline proposes a standardized method to assess lichen diversity on tree bark as a measure of air pollution changes (Asta et al. 2002). However, lichens also act as accumulative biomonitors and can be used for assessing trace elements atmospheric levels and their deposition patterns (Bačkor and Loppi 2009), and a guide for collecting lichen bioaccumulation data was proposed (Cecconi et al. 2019). In the guide, the scale for native lichens was built up by analyzing element concentration data for Flavoparmelia caperata (L.) Hale and Xanthoria parietina (L.) Th. Fr. (foliose lichens), while for transplants (active biomonitoring approach), the scale was proposed for Evernia prunastri (L.) Ach. and Pseudevernia furfuracea (L.) Zopf. (fruticose lichens).
Searching for an ideal biomonitor species as well as intercomparison with other recommended biomonitors is always an actual question. It is of great importance for biomonitoring to select and investigate the species that are widely distributed and available for sampling without worrying about the species vanishing due to overharvesting. Species selection is one of the key factors influencing the concentrations of elements in moss tissues (Fernández et al. 2015). Some studies have compared the concentrations of elements derived from several moss species collected within the same sampling site (Wolterbeek et al. 1995; Galsomiès et al. 2003; Carballeira et al. 2008; Schröder and Nickel 2019). Still, the number of moss species involved in interspecies comparisons is very limited relative to the total number of species used (Fernández et al. 2015). They compared mostly those moss species recommended for biomonitoring of air pollution by the Manual for moss monitoring within ICP Vegetation program (https://icpvegetation.ceh.ac.uk/sites/default/files/ICP%20Vegetation%20moss%20monitoring%20manual%202020.pdf). However, the recommended species are not of ubiquitous distribution, and thus some other species were successfully tested for the complementary use, e.g., Abietinella abietina var. abietina (Hedw.) M. Fleisch in mountainous areas (Zechmeister 1998) or Atrichum undulatum (Hedw.) P. Beauv. studied under controlled conditions (Sabovljević et al. 2020). However, the other authors point out that even different genotypes of the same species can react differently to the accuracy and reliability of information obtained (Stanković et al. 2018).
The effectiveness of co-located cryptogams, lichen and moss species, as the most recommended biomonitors of the air pollution, was often compared as they are both assumed to be cost-effective and are a simple alternative to instrument deposition monitoring (Bargagli et al. 2002; Adamo et al. 2003; Coskun et al. 2008; Balabanova et al. 2013; Ndlovu et al. 2019; Jafarova et al. 2023). However, co-located mosses and lichens as passive biomonitors in situ have not been often studied probably due to a lichen sensitivity to environmental pollution (Bačkor and Loppi 2009).
In this study, two pleurocarpous moss taxa (H. cupressiforme and Brachythecium spp.) were used to assess potentially toxic element (PTE) atmospheric deposition, considering that these taxa are not firmly attached to the substrate and accumulate nutrients mostly from atmospheric deposition, both wet and dry (Thoni et al. 1996; Faus-Kessler et al. 2001; Couto et al. 2003) with the ability to retain particles to a large extent (Varela et al. 2023). Otherwise, H. cupressiforme and B. rutabulum are among the most abundant moss species growing on logs in Central European forests (Müller et al. 2020; Ódor et al. 2005) and rest of the Europe, as well (Fig. 1 SM). In a laboratory experiment, for these species, the total number of binding sites on the surface was similar, ≈ 0.485 mmol g−1 (González and Pokrovsky 2014). Geographic distribution of these two moss species is similar, but they growing in different niches: H. cupressiforme grows mainly on logs within forests preferring shadow, while Brachythecium spp. is more available in open habitats (pastures), as well as in habitats under greater anthropogenic pressure. To our knowledge, these species have never been intercompared in situ relative to element accumulation capability in the samples collected in parallel at the same sites.
In addition, bioconcentration capacity of moss H. cupressiforme is compared with that of an epiphytic lichen E. prunastri (common name: oakmoss), the widely spread fructose species selected due to the thallus in low contact with surface to which attached is, and it is a species frequently used in biomonitoring studies (Dhaouadi et al. 2022; Cansaran-Duman et al. 2011; Jovan and McCune 2005; Pacheco et al. 2008). The comparative use of moss and lichen species, widely applied for biomonitoring purposes, in particular studies has not been explored enough.
The overall goal of this study was to investigate the biomonitoring potential of several co-located cryptogam biomonitors for the assessment of airborne PTEs. The main aim of this study was to make intercomparison of co-located two mosses H. cupressiforme and Brachythecium spp. We hypothesize that the species could be interchangeably used in the same survey due to the similarity of the form (pleurocarpous) and CEC capacity. The other aim was the comparison of moss H. cupressiforme with lichen E. prunastri, which grows on the closest tree, i.e., in the same ambient but without any contact with soil substrate. We hypothesize that PTE concentrations in the lichen should be lower than in the moss but with a similar spatial pattern of distribution across the study area.
Materials and methods
Study area, sampling sites, and species
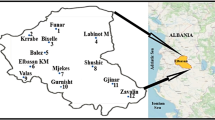
The biomonitoring survey was carried out across the territory of the Republic of Serbia, a country situated at the crossroads of Central and Southeast Europe and the central Balkan. The country covers a total of 88 499 km2 lying between latitudes 41° and 47° N and longitudes 18° and 23° E. The relief is very diverse; from Pannonian Plain (≈100 m a.s.l., represents mainly agricultural area) at the north, which covers one-third of the country; via hilly terrain in the central part; and mountains dominate the southern third of the country (up to 2 656 m a.s.l.). The climate of Serbia can be classified as a warm humid continental or humid subtropical climate (Pecelj et al. 2020; Aničić Urošević et al. 2020).
The sampling took place during autumn 2020 at 22 sites over the territory of Serbia covering different pollution scenarios, from agricultural areas in the north to the hill and the mountain terrains in the south of the country (Fig. 1). The sites overlap with those within the national biomonitoring network established for Moss surveys performed in the frame of the ICP Vegetation program. Regularly, H. cupressiforme samples were collected over the country, as a species recommended by the manual for moss monitoring. However, wherever it was possible, the species of Brachythecium spp. genera (sites 3, 6, 7, 10 ‒ 1, 2 ‒ B. rutabulum (Hedw.) Schimp., and 13, 17 ‒ Sciuro-hypnum populeum (Hedw.) Ignatov & Huttunen, 8, 9 ‒ B. velutinum (Hedw.) Ignatov & Huttunen, 1, 2 ‒ B. albicans (Hedw.) Schimp. as substitute) and lichen E. prunastri (L.) Ach. were sampled, at the closest distance to H. cupressiforme sampling sites. Distribution maps of the biomonitors studied are presented in Fig. 1 SM. H. cupressiforme was mainly collected at felled old trunks and stumps, while Brachythecium spp. was sampled on soil at a distance no more than 200 m from the previous species. The mosses were sampled according to the protocol recommended in the Manual for Moss surveys (Frontasyeva et al. 2015). The samples were collected from open spaces, out of tree canopies, and far from roads, households, and landfills. Five to seven subsamples of the species per sampling site were collected and after, in the laboratory, homogenized in one sample. The moss samples were carefully cleaned from impurities, and only the upper green part of the phylloid was taken for subsequent chemical analysis. The lichen was sampled from oak trees very close (up to 50 m) to H. cupressiforme, following the same rules as the mosses, except the fact that tree crowns could not be avoided and, consequently, the throughfall precipitation effect.
Wherever it was possible within the national biomonitoring network (Moss surveys, ICP Vegetation program) each studied biomonitors’ species were sampled. Unfortunately, there was only one site where all three species were sampled at an acceptable distance from each other (site No. 13).
Chemical analyses
After the cleaning procedure, three subsamples of each moss and lichen species collected at 22 sites were subjected to chemical preparation and analyses. About 0.5 g of the samples were placed in Teflon vessels and digested with 5 mL of trace pure HNO3 (Suprapur, Merck) and 2 mL H2O2 p.a. (Suprapur, Merck). The digestion was performed at 180 °C in the Mars 6 microwave digestion system (CEM, USA). After cooling, the digested samples were quantitatively transferred into flasks and made up to the volume of 50 mL with deionized water. The content of 15 elements, Al, Ba, Co, Cd, Cr, Cu, Fe, Mn, Ni, P, Pb, Sr, S, V, and Zn, was determined using inductively coupled plasma optical emission spectrometry (ICP-OES) PlasmaQuant 9000 Elite (Analytik Jena, Germany). The calibration solutions were prepared from IV-STOCK-13 (Inorganic Ventures, USA) standard solution. All control standards were analyzed after every ten samples. All samples were prepared and analyzed in triplicate.
In addition, Hg content in the samples was determined using a direct mercury analyzer, Milestone DMA-80evo (Milestone Srl, Italy). Quality control of all the measurements was ensured by analysis of three certified reference materials, M2 and M3 (moss Pleurozium schreberi with different element content, Finnish Forest Research Institute, Steinnes et al. 1997) and NIST 1547 (peach leaves, National Institute of Standards and Technology, USA), instead of the missing adequate lichen referent material (Table 1 SM). The recovery of the measured element concentrations was satisfactory for the majority of the measured elements and was in the ranges 86‒111% (M2), 81‒110% (M3), and 80‒109% (1547). Chromium was the only element determined with poor recovery (< 75%), and it will be discussed with caution.
Data analysis
The data were processed by STATISTICA 8.0 (StatSoft, Inc., Tulsa, OK, USA), SPSS 21 (IBM Corp., Armonk, NY, USA), and the free software R (R Development Core Team 2014). The Kolmogorov–Smirnov test was used to check the normality of the measured element concentrations in biomonitors. Wilcoxon matched pairs test was used for to assess significant differences in the element content of the biomonitors collected at the same sites. All testing was performed at p < 0.05 significance level. Spearman rank correlation analysis was applied to the dataset, searching for meaningful correlations among the element concentrations in the biomonitors.
Differences in the bioconcentration of elements by two mosses, H. cupressiforme and Brachythecium spp., and by moss, H. cupressiforme and lichen E. prunastri, were estimated by regression analysis, specifically type II recommended for the biomonitor intercalibration (Carballeira et al. 2008). For Model II regressions, neither X nor Y (corresponding to the concentration of elements in the compared biomonitors) is an independent variable, but both are assumed to be dependent on some other parameter which is often unknown. Neither is “controlled,” both are measured, and both include some error. Variables X and Y correspond to the concentration of elements in the compared pairs of biomonitors. As the concentrations of variables were previously unknown to the researcher, the obtained values in the lab were not indeed “independent.” For the previous reason, a type II regression model was applied by calculating the regression as a standardized major axis regression—SMA. Significant correlation coefficient (r) between the variables is a necessary precondition to determine if the hypothesis of a relationship is supported (Legendre 2014).
Principal component analysis (PCA) was applied to the results to reduce multidimensional data to lower dimensions using orthogonal transformation of possibly correlated variables into values of linearly uncorrelated variables. Specifically, in this study, the PCA was used to identify how variables (the PTEs) are grouped in the co-located biomonitors and thus confirm or reject a similar pattern of PTE distribution.
Results and discussion
Element concentrations in moss and lichen samples
Results of this study showed that the standard relative deviation of PTE concentrations for triplicates of the biomonitors’ samples from the same site was low, usually < 15%. Thus, further data processing was performed with the average concentrations of the elements per biomonitor per sampling site (Tables 1 and 2). Descriptive statistics for the element content in the biomonitors over the studied territory are presented in Table 2 SM (Supplementary material). The element concentrations measured in the moss and lichen samples over the study area showed a wide concentration range that should provide the proper interspecies comparison.
Since the element concentrations showed non-normal distribution, we utilized Wilcoxon test which indicated the significant differences (p < 0.05) in the concentrations of a couple of elements (P and S) between the studied mosses H. cupressiforme and Brachythecium spp., which possibly implicate that the majority of the measured elements were accumulated at a comparable level in both mosses. However, Spearman rank correlation analysis showed that the element concentrations between the mosses did not correlate significantly except for Mn and S (R = 0.76 and 0.66, respectively; p < 0.05) (Table 3a SM).
Contrary to the above-mentioned results, the comparison of moss H. cupressiforme and lichen E. prunastri discovered significant differences for the majority of determined elements (Al, Cu, Co, Pb, V, Ba, Cr, Fe, Mn, Sr, P, Ni, Hg; p < 0.05). Spearman correlation analysis founded more significant correlations between the moss and lichen (R = 0.70, 0.60, 0.74, 0.70, and 0.63 for Co, Mn, Sr, Ni, Hg, respectively; p < 0.05) in comparison to the relation moss-moss (Table 3b SM).
Regression analysis and interspecies bioconcentration comparison
H. cupressiforme vs. Brachythecium spp. type II regression analysis was applied for the element concentrations in the co-located mosses, representing measured variables dependent on unknown parameters in situ (Fig. 2a). The SMA slope-fitting provided regression lines, equation, determination coefficient, and significance for each element measured. The obtained regression lines were statistically significant (*p < 0.05) for only Cd and S with coefficients r = 0.7*; 0.7*, respectively. Specifically for Cu, the regression line is also significant with a high value of determination coefficient, but the regression is strongly influenced by one value very distant from the others that can be treated as an outlier. Thus, a good Cu bioconcentration relation between the biomonitor species will be considered with caution. In the international study carried out in three countries where five moss species were intercompared (Wolterbeek et al. 1995), the concentration data suggest the presence of essentially unnoticed outliers, possibly in all of the individual species’ series. This imply that the intercalibration of elemental concentrations in various moss species may not simply lead to a controlled use of more than a single species within the frame work of any survey.
Type II regression lines for the concentrations (mg kg−1) of elements measured in a H. cupressiforme and Brachythecium spp. and b H. cupressiforme and E. prunastri. Red lines represent lines obtained by standardized major axis (SMA) regression method; dashed black lines correspond to lines of slopes equal to 1; data are represented by circles. Significance of the determination coefficient at p < 0.05 are marked with * and at p < 0.001 with **
The slope values of regression lines for the measured elements in this study varied between − 2.48 to 4.53. For Cd*, Cu, Mn, Ni, P, Pb, S*, and Sr, the slope values were in the range 0‒2. According to Carballeira et al. (2008), for the elements with high values of the slopes of the regression lines (> 2), the studied moss species should not be used simultaneously without first being transformed by the corresponding intercalibration line.
In the available literature, there are variety of recommendation regarding interspecies calibration and calibration parameters. Thus, Berg and Steinnes (1997) suggested that regression equations with coefficients r2 ≥ 0.5 could be used to correct data for the interchangeable use of terrestrial mosses Hylocomium splendens and Pleurozium schreberi in a large-scale survey. Contrary, Carballeira et al. (2008) studied differences in the bioconcentration of elements by H. cupressiforme and Pseudoscleropodium purum using regression analysis and recommended determination coefficients close to 1.0 as only suitable for intercalibration of terrestrial mosses within a study. If we follow the latter, stricter criteria, the mosses intercompared in this study could not be interchangeably used for any of 16 measured elements. Although we started research with the hypothesis that the studied moss species can be interchangeably used for biomonitoring purposes since they have a similar physiology (CEC capacity), morphology (pleurocarpous, grow as tufts/mats, similar leaflet form), and habitat (open forest, logs, grassland), it seems that differences in the element accumulation by the studied species are substantial. In an intercomparison study where three moss species were studied in parallel (Galsomiès et al. 2003), the authors discovered the saturation problems for certain PTEs in H. cupressiforme, which do not favor the use of this species in parallel with mostly recommended H. splendens and P. schreberi, i.e., disables the interspecies calibration.
The interspecies comparison of PTE content indicated that the biological characteristics of each species, such as living form, and morphology, had a notable influence on the accumulative capacity of mosses, even if species are collected from the same biotope (Chen et al. 2010). Thus, in a recent comprehensive study (Schröder and Nickel 2019) where large and long-term datasets from periodic moss surveys (P. schreberi, Scleropodium purum, and H. cupressiforme) were analyzed, conversion/correction factors between the species are not recommended until further notice. Even in the study with moss bags (Vuković et al. 2015), where the biomonitors’ exposure conditions are much more under the control of researchers than in a passive approach where species are sampled in situ, an interchangeable use of the mosses Sphagnum girgensohnii and H. cupressiforme was not suggested, except conditionally in the case of Cu (r2 = 0.50).
In this study, ratios of the element concentrations in the co-located samples of H. cupressiforme and Brachythecium spp. were also calculated (Table 3). The median ratio suggests that H. cupressiforme accumulates the elements to a greater extent (up to 50%) than Brachythecium spp., except for P, S, and Hg, while for some elements, both species showed a similar accumulation rate (Ba, Cd, Cu, Mn, Sr, Zn). Some authors point out that the results of paired comparison can be directly combined if the species ratio does not differ significantly from 1 (Halleraker et al. 1998). However, the ratios of concentrations of the same element between species can vary from greater than to lower than 1 depending on the sampling site (Reimann et al. 2001). Thus, interspecies calibration cannot be extrapolated to wide regia since it is only valid for the range of concentrations considered (Wolterbeek and Bode 1995).
In this study, at some sites (nos. 6 and 7), multifold higher PTE content was measured in Brachythecium spp. than in H. cupressiforme samples (Table 1). Specifically, at these sites were sampled B. rutabulum species. This finding indicates that there are likely differences in PTE accumulation between the species of the same genus, which was observed by other authors, too. However, because of the limited number of samples, this statement needs further confirmation.
Possible factors influencing different bioconcentration capacities of the studied mosses are morphological peculiarities of the species. In H. cupressiforme, the stems are branched and covered with overlapping leaves; individual leaves are medium-sized, concave sickle-shaped, and taper to the tip that is firmly curled toward the substrate; smooth leaflet surface with elongate cells (Vanicela et al. 2021). Consequently, this species could bioconcentrate higher levels of elements because gaseous exchange can occur on both sides of the phyllidia (Fig. 3) (Glime 2007; Proctor 1982). It should be point out that H. cupressiforme is a complex that includes different varieties and species (Smith 2004) which generates an important morphological and physiological variability that could also be preventing a better intercalibration. B. rutabulum (the most often sampled species of Brachythecium genus) is a creeping moss with medium-sized to large leaves, flat, oval to lanceolate, and gradually or abruptly narrowed to a sharp tip, smooth to slightly plicate leaflet surface with elongate cells (Vanicela et al. 2021). The shape of moss phyllids can affect the efficiency of capturing particles that are the primary source of PTEs measured in moss biomonitors (Vanicela et al. 2021; Di Palma et al. 2017; Tretiach et al. 2011a).
One of the key preconditions for the selection of species for biomonitoring is their element absorption capacity, which in bryophytes depends mainly on the spatial structure of their cushions (Pavlíková et al. 2021). Thanks to the dense spatial structure, the absorption capacity of dry B. rutabulum is quite high, 16.1:1, meaning that the dried plants can absorb an average of 20 g of water with dissolved substances per 1 g of herb (Drobnik and Stebel 2018). However, in a laboratory experiment with four moss species submerged in solutions of different metal concentrations (González and Pokrovsky 2014), from one side, it was shown that B. rutabulum was the most efficient species because it reached the highest percentage of adsorption and had the highest number of available sites for almost all the metals studied. On the other side, the species showed instability in terms of biomass degradation and organic carbon leaching as well, leading to instability at contact with water and metal release. Contrary, H. cupressiforme was the most inert species in terms of biomass degradation and organic carbon leaching and, after Sphagnum species, showed the best performance in the adsorption of Cu, Cd, Ni, Pb, and Zn.
Possible edaphic influence in the uptake of PTEs by the mosses cannot be excluded entirely since H. cupressiforme was sampled mainly on logs. At the same time, Brachythecium spp. was collected on soil. In addition, B. rutabulum shows an open growth form that traps less dust than the more cushiony forms (Glime 2007), while H. cupressiforme forms creeping stems with densely-set branches, most commonly in a pinnate arrangement. Soil particles can be tightly anchored to the thallus’s lower surface, possibly contributing to a higher content of typical soil elements. H. cupressiforme is a species that can derive phosphorus from buried wood (Glime 2007).
H. cupressiforme vs. E. prunastri. From the curiosity, linear regression type II was also applied to the element concentrations in another set of co-located biomonitors from this study—moss H. cupressiforme and lichen E. prunastri (Fig. 2b) without intention of interchangeable use of these biomonitors within the same survey. The significant regression lines (*p < 0.05; **p < 0.01) are achieved for Ba, Cd, Fe, Hg, Mn, Ni, and Sr with coefficients r = 0.66*; 0.67*; 0.61*; 0.61*; 0.65*; 0.9**; 0.74*, respectively. Again, in the case of Cu, one dot (sampling site) strongly influenced the slope and significance of regression line, and it will be assumed as an outlier. Regarding the slope of regression lines, the values between 0 and 2 were obtained for Ba*, Cd*, Cr, Hg*, Mn*, Ni*, P, Pb, S, and Sr* suggesting possible an interchangeable use of the studied biomonitors without data transforming by the corresponding intercalibration line, especially for those elements with significant determination coefficient (Carballeira et al. 2008). However, the absolute PTE content in the biomonitors cannot be neglected. Namely, almost all measured elements, especially Ba, H. cupressiforme accumulated to a higher extent than the lichen. The ratio moss/lichen was discovered twice or thrice higher capacity for the element accumulation by H. cupressiforme, except for Cd, S, and Zn for which the accumulation was about the same level in both biomonitors (Table 3).
Lichen E. prunastri is known as oakmoss due to the thallus remind moss tufts. This biomonitor is not in so close contact with the growing surface as in moss H. cupressiforme. The moss and lichen samples in this study were closely co-located together on the studied sites, the moss growing on a log while the lichen hung from the close-alive tree, which means that both biomonitors were exposed to the same ambient air pollution. In many studies where inter-comparable used moss and lichen biomonitors (Adamo et al. 2007; Giordano et al. 2013; Jafarova et al. 2023), mosses showed higher accumulation capacity for PTEs and microplastic probably due to higher surface/mass ratio and their open habitat position. The mechanisms of PTE entrapment by moss and lichen tissue are similar but not the same. E. prunastri is a fruticose lichen with thallus structured in four layers: the upper cortex, the algal zone, the medulla, and the lower cortex. E. prunastri is an effective particle accumulator for particles less than 20 μm in diameter. Trace elements were mainly concentrated on the cortex of the thallus by the extracellular entrapment of particulate matter occurring within the loose hyphal weft of the medulla (Tretiach et al. 2011b), except Zn, Ca, and K which were also present in the internal layers (Ayrault et al. 2007).
Also, the fact of how long a biomonitor was exposed to air pollution cannot be neglected. The difference in age of apical segments of H. cupressiforme and branches of E. prunastri, which were sampled and further processed in the laboratory, possibly contributed to the versatility of the PTE concentrations in the biomonitors. E. prunastri is a candidate for an annual cycle with isotomic dichotomous branching. Still, a small amount of error in age determinations is introduced by irregularities in the branching and previous physical damage to the thallus (Stone and McCune 1990). Moss H. cupressiforme has no clear annual growth segments, but some authors point out that the green apical segment of the moss phyllids, usually taken for chemical processing, could be 3 years old (Gramatica et al. 2006). However, the measurements of annual growth rate should be performed if the study is carried out on a wide geographical scale due to great differences in moss biomass production at various sites (Zechmeister 1998).
Correlation and principal component analyses of the element concentrations in the co-located biomonitors
Correlation analysis was applied to the data set of the PTEs within the studied mosses, H. cupressiforme and Brachythecium spp., and the correlation between some element concentrations (Al, Co, Cr, Fe, Ni, V) was extremely significant (p < 0.001) (Fig. 4), unlike the fact that the same element concentrations were not significantly correlated between the species (Table 3 SM). The result indicates that these elements in both mosses originate mainly from the same source—probably the geogenic one. Correlation coefficients are slightly higher between the above-mentioned elements in H. cupressiforme than in Brachythecium spp., except in the case of Ni. This imply that a local soil signature is stronger for H. cupressiforme moss.
The results of PCA applied to the PGE dataset obtained in the co-located mosses H. cupressiforme and Brachythecium spp. are presented as a biplot (Fig. 5a). The first two PCs describe 71.1% of the total variance. Naturally derived elements mainly contribute to PC1, while anthropogenically derived elements are reflected in PC2. The PCA distinguished the PTEs into two groups: Cu, Co, V, Fe, Cr, Mn, Al, Ni, and Pb oriented to H. cupressiforme samples and P, S, Sr, Hg, Zn, Cd, and Ba directed to Brachythecium spp. samples. Long vectors of the variables suggest that the variable significantly contributes to the variance explained by the PCs. The PTE group in the fourth quadrant represents the elements highly intercorrelated, and which are likely of geogenic origin. Another group of elements present in the first quadrant is also closely intercorrelated, but not so close as the first group. These elements are rather associated with anthropogenic pollution sources. It seems that Brachythecium spp. rather reflect anthropogenic pollution than H. cupressiforme which possibly has anchored soil particles at its phyllids. It should be noted that H. cupressiforme at sampling site 9 significantly contribute to PC1 and influenced the data in the fourth quadrant while the influence of the other studied species is discernible in PC2. In addition, Brachythecium spp. at sampling site 7 strongly contribute to PC1 and influenced the data in the first quadrant, while the other species has not any contribution at the same site. These results confirm that the PTEs were accumulated differently by the studied species.
Figure 5b shows the results of PCA applied to the co-located moss and lichen species. The first two PCs describe 56.8% of the total variance. The biplot shows that many of the elements are pointing toward H. cupressiforme, except Hg and S which are oriented to E. prunastri. These two elements are usually emitted as gaseous pollutants and thus possibly easier captured by the lichen (Monaci et al. 2022; Root et al. 2021) whose position in habitat (hanging on the tree) possibly influences higher exposure to airborne pollutants (Jafarova et al. 2023). H. cupressiforme at sampling site 12 significantly contribute to PC1 and strongly influenced the elements in the first quadrant. In the same time, E. prunastri at the same site significantly contribute to PC2. Generally, the scores of the studied moss and lichen from the same sites are strongly associated with the variables that significantly contribute to different PCs, which suggests the species’ different accumulation mechanisms of the elements.
Unfortunately, all three biomonitors studied could be collected only at one sampling site (site no. 13) (Fig. 1). The element concentrations in the biomonitors followed the order H. cupressiforme > Brachythecium spp. > > > E. prunastri (Fig. 6). The lichen accumulated substantially lower PTE content than the mosses.
In this study, the biomonitors’s PTE content showed similar spatial distribution patterns (in the mosses and lichen) and origin of the elements within the samples (in the mosses). Still, this study points out significant differences between the PTE concentrations in the co-located biomonitor samples which imply that the tested species should not be replaceable use within a study. However, because of the sampling limitation, these results need further confirmation.
Conclusion
The comparative use of two mosses, H. cupressiforme and Brachythecium spp., and lichen, E. prunastri, sampled at 22 remote sites over Serbia discovered substantial differences in PTE accumulation by the biomonitors used. Between the co-located mosses, linear regression analysis type II showed significant determination coefficients for only Cd and S (r = 0.79 and 0.7, respectively). Thus, the studied mosses cannot be interchangeably used within the same study. The ratio of the PTEs in the mosses discovered higher concentrations in H. cupressiforme than in the other moss at some sites and vice versa at several other sites. The reason possibly lies in the fact that several species of the genus Brachythecium (B. rutabulum, B. albicans, B. velutinum) were sampled which perhaps influence the average genus accumulation capacity. In addition, morphological features of the mosses delegate differences in PTE accumulation.
Otherwise, between the co-located moss H. cupressiforme and lichen E. prunastri, significant determination coefficients were found for a wider set of elements Ba, Cd, Cu, Fe, Hg, Mn, Ni, and Sr then in the pair moss-moss biomonitor. The PTE ratios showed that the moss accumulated much higher elements concentrations than the lichen, but they followed a similar spatial pattern. In addition, PCA distinguishes different groups of the PTEs depending on the species tested.
Although all biomonitors tested are used extensively in biomonitoring surveys, their replicable use for PTE estimation should be done with caution and depending on the element studied.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abas A (2021) A systematic review on biomonitoring using lichen as the biological indicator: a decade of practices, progress and challenges. Ecol Indic 121:107197. https://doi.org/10.1016/j.ecolind.2020.107197
Adamo P, Crisafulli P, Giordano S, Minganti V, Modenesi P, Monaci F, Pittao E, Tretiach M, Bargagli R (2007) Lichen and moss bags as monitoring devices in urban areas. Part II: Trace element content in living and dead biomonitors and comparison with synthetic materials. Environ Pollut 146(2):392–399. https://doi.org/10.1016/j.envpol.2006.03.047
Adamo P, Giordano S, Vingiani S, Castaldo Cobianchi R, Violante P (2003) Trace element accumulation by moss and lichen exposed in bags in the city of Naples (Italy). Environ Pollut 122:91–103
Aničić Urošević M, Vuković G, Tomašević M (2017) Biomonitoring of Air Pollution Using Mosses and Lichens, A passive and active approach, state of the art research and perspectives. Nova Science Publishers, New York (ISBN: 978-1-53610-051-8)
Aničić Urošević M, Krmar M, Radnović D, Jovanović G, Jakšić T, Vasić P, Popović A (2020) The use of moss as an indicator of rare earth element deposition over a large area. Ecol Indic 109:105828. https://doi.org/10.1016/j.ecolind.2019.105828
Asta J, Erhardt W, Ferretti M, Fornasier F, Kirschbaum U, Nimis PL, Purvis OW, Pirintsos S, Scheidegger C, Van Haluwyn C, Wirth V (2002) European guideline for mapping lichen diversity as an indicator of environmental stress. Br Lichens, pp 1–20. Available online: https://www.researchgate.net/publication/303255153 (accessed on 10 April 2024)
Ayrault S, Clochiatti R, Carrot F, Daudin L, Bennett J (2007) Factors to consider for trace element deposition biomonitoring surveys with lichen transplants. Sci Tot Environ 372(2–3):717–727. https://doi.org/10.1016/j.scitotenv.2006.10.032
Bačkor M, Loppi S (2009) Interactions of lichens with heavy metals. Biol Plant 53:214–222. https://doi.org/10.1007/s10535-009-0042-y
Balabanova B, Stafilov T, Šajn R, Bačeva K (2013) Comparison of response of moss, lichens and attic dust to geology and atmospheric pollution from copper mine. Int J Environ Sci Te 11(2):517–528. https://doi.org/10.1007/s13762-013-0262-8
Bargagli R, Monaci F, Borghini F, Bravi F, Agnorelli C (2002) Mosses and lichens as biomonitors of trace metals. A comparison study on Hypnum cupressiforme and Parmelia caperata in a former mining district in Italy. Environ Pollut 116:279–287
Berg T, Steinnes E (1997) Use of mosses (Hylocomium splendens and Pleurozium schreberi) as biomonitors of heavy metal deposition: from relative to absolute deposition values. Environ Pollut 98(1):61–71. https://doi.org/10.1016/S0269-7491(97)00103-6
Cansaran-Duman D, Atakol O, Aras S (2011) Assessment of air pollution genotoxicity by RAPD in Evernia prunastri L. ach. from around iron-steel factory in Karabuk. Turkey J Environ Sci 23:1171–1178. https://doi.org/10.1016/S1001-0742(10)60505-0
Carballeira CB, Aboal JR, Fernández JA, Carballeira A (2008) Comparison of the accumulation of elements in two terrestrial moss species. Atmos Environ 42:4904–4917. https://doi.org/10.1016/j.atmosenv.2008.02.028
Cecconi E, Fortuna L, Benesperi R, Bianchi E, Brunialti G, Contardo T et al (2019) New interpretative scales for lichen bioaccumulation data: the Italian proposal. Atmosphere 10(3):1–19. https://doi.org/10.3390/atmos10030136
Chakrabortty S, Paratkar GT (2006) Biomonitoring of trace element air pollution using mosses. Aerosol Air Qual Res 6:247–258. https://doi.org/10.4209/aaqr.2006.09.0002
Chaudhary N (2022) Chapter 7-Pollution biomarkers in environmental biomonitoring: an insight into air pollution, in: New Paradigms in Environmental Biomonitoring Using Plants, Editor(s): Supriya Tiwari, Shashi Agrawal, pp 165–180, Elsevier, ISBN 9780128243510, https://doi.org/10.1016/B978-0-12-824351-0.00009-2
Chen YE, Yuan S, Su YQ, Wang L (2010) Comparison of heavy metal accumulation capacity of some indigenous mosses in Southwest China cities: a case study in Chengdu city. Plant Soil Environ 56(2):60–66. https://doi.org/10.17221/160/2009-PSE
Conti ME, Cecchetti G (2001) Biological monitoring: lichens as bioindicators of air pollution assessment-a review. Environ Pollut 114(3):471–492. https://doi.org/10.1016/S0269-7491(00)00224-4
Coskun M, Steinnes E, Coskun M, Cayir A (2008) Comparison of epigeic moss (Hypnum cupressiforme) and lichen (Cladonia rangiformis) as biomonitor species of atmospheric metal deposition. Bull Environ Contam Toxicol 82(1):1–5. https://doi.org/10.1007/s00128-008-9491-9
Couto JA, Fernández JA, Aboal JR, Carballeira A (2003) Annual variability in heavy-metal bioconcentration in moss: sampling protocol optimization. Atmos Environ 37(25):3517–3527. https://doi.org/10.1016/S1352-2310(03)00447-3
Dhaouadi S, Khalloufi N, Ayati K, Ayeb N, Béjaoui M (2022) Use of lichen species for air pollution biomonitoring: Case of Dar-Chichou forest (Cap-Bon, North-East Tunisia). Environ Sustain Indic 16:100211. https://doi.org/10.1016/j.indic.2022.100211
Di Palma A, Capozzi F, Spagnuolo V, Giordano S, Adamo P (2017) Atmospheric particulate matter intercepted by moss-bags: relations to moss trace element uptake and land use. Chemosphere 176:361–368. https://doi.org/10.1016/j.chemosphere.2017.02.120
Drobnik J, Stebel A (2018) Brachythecium rutabulum, a neglected medicinal moss. Hum Ecol 46:133–141. https://doi.org/10.1007/s10745-017-9961-y
Faus-Kessler T, Dietl C, Tritschler J, Peichl L (2001) Correlation patterns of metals in the epiphytic moss Hypnum cupressiforme in Bavaria. Atmos Environ 35(2):427–439. https://doi.org/10.1016/S1352-2310(00)00119-9
Fernández JA, Boquete MT, Carballeira A, Aboal JR (2015) A critical review of protocols for moss biomonitoring of atmospheric deposition: sampling and sample preparation. Sci Tot Environ 517:132–150. https://doi.org/10.1016/j.scitotenv.2015.02.050
Frontasyeva M, Harmens H in collaboration with the participants (2015) Monitoring of Atmospheric Deposition of Heavy Metals, Nitrogen and Pops in Europe Using Bryophytes. Monitoring Manual, 2015 Survey. https://icpvegetation.ceh.ac.uk/sites/default/files/ICP%20Vegetation%20moss%20monitoring%20manual%202020.pdf. Accessed 17 June 2024
Galsomiès L, Ayrault S, Carrot F, Deschamps C, Letrouit-Galinou MA (2003) Interspecies calibration in mosses at regional scale—heavy metal and trace elements results from Ile-de-France. Atmos Environ 37(2):241–251. https://doi.org/10.1016/S1352-2310(02)00831-2
Garty J (2001) Biomonitoring atmospheric heavy metals with lichens: theory and application. Crit Rev Plant Sci 20:309–371. https://doi.org/10.1080/20013591099254
Giordani P, Brunialti G (2015) Sampling and interpreting lichen diversity data for biomonitoring purposes. In: Upreti DK, Divakar PK, Shukla V, Bajpai R (eds) Recent Advances in Lichenology. Springer, Delhi, pp 19–46 (ISBN: 978-81-322-2180-7)
Giordano S, Adamo P, Spagnuolo V, Tretiach M, Bargagli R (2013) Accumulation of airborne trace elements in mosses, lichens and synthetic materials exposed at urban monitoring stations: towards a harmonisation of the moss-bag technique. Chemosphere 90(2):292–299. https://doi.org/10.1016/j.chemosphere.2012.07.006
Glime J (2007) Bryophyte ecology. Digital version. http://www.bryoecol.mtu.edu. Accessed 17 June 2024
Global Burden of Diseases ‒ GBD (2016) Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1659-724.https://doi.org/10.1016/S0140-6736(15)00128-2
González AG, Pokrovsky OS (2014) Metal adsorption on mosses: toward a universal adsorption model. J Colloid Interface Sci 415:169–178. https://doi.org/10.1016/j.chemosphere.2012.07.006
Gramatica P, Battaini F, Giani E, Papa E, Jones RJ, Preatoni D, Cenci RM (2006) Analysis of mosses and soils for quantifying heavy metal concentrations in Sicily: a multivariate and spatial analytical approach. Environ Sci Pollut Res Int 13(1):28–36. https://doi.org/10.1065/espr2006.01.006
Halleraker JH, Reimann C, De Caritat P, Finne TE, Kashulina G, Niskavaara H, Bogatyrev I (1998) Reliability of moss (Hylocomium splendens and Pleurozium schreberi) as a bioindicator of atmospheric chemistry in the Barents region: interspecies and field duplicate variability. Sci Tot Environ 218:123–139. https://doi.org/10.1016/S0048-9697(98)00205-8
Harmens H, Norris DA, Sharps K, Mills G, Alber R, Aleksiayenak Y, Blum O, Cucu-Man S-M, Dam M, De Temmerman L, Ene A, Fernández JA, Martinez-Abaigar J, Frontasyeva M, Godzik B, Jeran Z, Lazo P, Leblond S, Liiv S, Magnússon SH, Maňkovská B, Pihl Karlsson G, Piispanen J, Poikolainen J, Santamaria JM, Skudnik M, Spiric Z, Stafilov T, Steinnes E, Stihi C, Suchara I, Thöni L, Todoran R, Yurukova L, Zechmeister HG (2015) Heavy metal and nitrogen concentrations in mosses are declining across Europe whilst some “hotspots” remain in 2010. Environ Pollut 200:93–104. https://doi.org/10.1016/j.envpol.2015.01.036
Jafarova M, Grifoni L, Aherne J, Loppi S (2023) Comparison of lichens and mosses as biomonitors of airborne microplastics. Atmosphere 14(6):1007. https://doi.org/10.3390/atmos14061007
Jovan S, McCune B (2005) Air quality bioindication in the greater Central Valley of California, with epiphytic macrolichen communities. Ecol Appl 15:1712–1726. https://doi.org/10.1890/03-5368
Legendre P (2014) Package “lmodel 2”, R package version 1.7–2. http://cran.r-project.org/web/packages/lmodel2/lmodel2.pdf. Accessed 17 June 2024
Monaci F, Ancora S, Paoli L, Loppi S, Wania F (2022) Lichen transplants as indicators of gaseous elemental mercury concentrations. Environ Pollut 313:120189. https://doi.org/10.1016/j.envpol.2022.120189
Müller J, Ulyshen M, Seibold S, Cadotte M, Chao A, Bässler C et al (2020) Primary determinants of communities in deadwood vary among taxa but are regionally consistent. Oikos 129:1579–1588. https://doi.org/10.1111/oik.07335
Nash TH (2008) Lichen Biology. 2nd Edition, Cambridge University Press, Cambridge. https://doi.org/10.1017/CBO9780511790478
Ndlovu N, Frontasyeva M, Newman R, Maleka P (2019) Moss and lichen biomonitoring of atmospheric pollution in the Western Cape Province (South Africa). Am J Anal Chem 10:86–102. https://doi.org/10.4236/ajac.2019.103008
Nimis PL, Scheidegger C, Wolseley PA (2002) Monitoring with lichens-monitoring lichens: an introduction. In: Nimis PL, Scheidegger C, Wolseley PA (eds) Monitoring with Lichens-Monitoring Lichens. NATO Science Series, vol 7. Springer, Dordrecht, pp 1-4. https://doi.org/10.1007/978-94-010-0423-7_1
Ochoa-Hueso R, Munzi S, Alonso R, Arróniz-Crespo M, Avila A, Bermejo V, Bobbink R, Branquinho C, Concostrina-Zubiri L, Cruz C, Cruz de Carvalho R, De Marco A, Dias T, Elustondo D, Elvira S, Estébanez B, Fusaro L, Gerosa G, Izquieta-Rojano S, Lo Cascio M, Marzuoli R, Matos P, Mereu S, Merino J, Morillas L, Nunes A, Paoletti E, Paoli L, Pinho P, Rogers IB, Santos A, Sicard P, Stevens CJ, Theobald MR (2017) Ecological impacts of atmospheric pollution and interactions with climate change in terrestrial ecosystems of the Mediterranean Basin: Current research and future directions. Environ Pollut 227:194–206. https://doi.org/10.1016/j.envpol.2017.04.062
Ódor P, van Dort K, Aude E, Clausen JH, Christensen M (2005) Diversity and composition of dead wood inhabiting bryophyte communities in European beech forests. Bol Soc Esp Briol 26–27:85–102. https://doi.org/10.1016/j.biocon.2006.02.004
Pacheco AMG, Freitas MC, Baptista MS, Vasconcelos MTSD, Cabral JP (2008) Elemental levels in tree bark and epiphytic-lichen transplants at a mixed environmental in mainland Portugal, and comparisons with an in situ lichen. Environ Pollut 151:326–333. https://doi.org/10.1016/j.envpol.2007.06.038
Pavlíková I, Motyka O, Plášek V, Bitta J (2021) Monitoring of heavy metals and nitrogen concentrations in mosses in the vicinity of an integrated iron and steel plant: case study in Czechia. Appl Sci 11:8262. https://doi.org/10.3390/app11178262
Pecelj M, Lukić M, Filipović D, Protić B, Bogdanović U (2020) Analysis of the universal thermal climate index during heat waves in Serbia. Nat Hazards Earth Syst Sci 20:2021–2036. https://doi.org/10.5194/nhess-20-2021-2020
Proctor MCF (1982) Physiological ecology: water relations, light and temperature responses, carbon balance. In: Smith AJE (Ed.), Bryophyte Ecology. Chapman & Hall, London, pp 333–381. https://doi.org/10.1007/978-94-009-5891-3
R Development Core Team (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing. http://www.R-project.org. Accessed 15 June 2024
Reimann C, Niskavaara H, Kashulina G, Filzmoser P, Boyd R, Volden T, Tomilina O, Bogatyrev I (2001) Critical remarks on the use of terrestrial moss (Hylocomium splendens and Pleurozium schreberi) for monitoring of airborne pollution. Environ Pollut 113:41–57. https://doi.org/10.1016/S0048-9697(98)00205-8
Root TH, Jovan S, Fenn M, Amacher M, Hall J, Shaw DJ (2021) Lichen bioindicators of nitrogen and sulfur deposition in dry forests of Utah and New Mexico, USA. Ecol Indic 127:107727. https://doi.org/10.1016/j.ecolind.2021.107727
Sabovljević M, Weidinger M, Sabovljević A, Stanković J, Adlassnig W, Lang I (2020) Metal accumulation in the acrocarp moss Atrichum undulatum under controlled conditions. Environ Pollut 256:113397. https://doi.org/10.1016/j.envpol.2019.113397
Schröder W, Nickel S (2019) Moss species-specific accumulation of atmospheric deposition? Environ Sci Eur 31:78. https://doi.org/10.1186/s12302-019-0262-5
Smith AJE (2004) The moss flora of the British Isles. Cambridge University Press, Cambridge
Stanković JD, Sabovljević AD, Sabovljević MS (2018) Bryophytes and heavy metals: a review. Acta Bot Croat 77(2):109–118. https://doi.org/10.2478/botcro-2018-0014
Steinnes E, Rühling Å, Lippo H, Mäkinen A (1997) Reference materials for large scale metal deposition surveys. Accred Qual Assur 2:243–249. https://doi.org/10.1007/s007690050141
Stone DF, McCune B (1990) Annual Branching in the Lichen Evernia prunastri in Oregon. Bryologist 93(1):32. https://doi.org/10.2307/3243544
Thoni L, Schnyder N, Krieg F (1996) Comparison of metal concentration in three species of mosses and metal freights in bulk precipitations. Fresenius J Anal Chem 354:703–708. https://doi.org/10.1007/s0021663540703
Tretiach M, Candotto Carniel F, Loppi S, Carniel A, Bortolussi A, Mazzilis D, Del Bianco C (2011a) Lichen transplants as a suitable tool to identify mercury pollution from waste incinerators: a case study from NE Italy. Environ Monit Assess 175:589–600. https://doi.org/10.1007/s10661-010-1553-x
Tretiach M, Pittao E, Crisafulli P, Adamo P (2011b) Influence of exposure sites on trace element enrichment in moss-bags and characterisation of particles deposited on the biomonitor surface. Sci Total Environ 409:822–830. https://doi.org/10.1016/j.scitotenv.2010.10.026
Vanicela BD, Nebel M, Stephan M, Riethmüller C, Gresseret GT (2021) Quantitative analysis of fine dust particles on moss surfaces under laboratory conditions using the example of Brachythecium rutabulum. Environ Sci Pollut Res 28:51763–51771. https://doi.org/10.1007/s11356-021-14218-5
Varela Z, Boquete MT, Fernández JA, Martínez-Abaigar J, Núñez-Olivera E, Aboal JR (2023) Mythbusters: unravelling the pollutant uptake processes in mosses for air quality biomonitoring. Ecolo Indic 148:110095. https://doi.org/10.1016/j.ecolind.2023.110095
Vásquez MD, López J, Carballeira A (1999) Uptake of heavy metals to the extracellular and intracellular compartments in three species of aquatic bryophyte. Ecotoxicol Environ Saf 44:12–24. https://doi.org/10.1006/eesa.1999.1798
Vuković G, Aničić Urošević M, Goryainova Z, Pergal M, Škrivanj S, Samson R, Popović A (2015) Active moss biomonitoring for extensive screening of urban air pollution: magnetic and chemical analyses. Sci Tot Environ 521–522:200–210. https://doi.org/10.1016/j.scitotenv.2015.03.085
Wolterbeek HT, Kuik P, Verburg T, Herpin U, Markert B, Thoni L (1995) Moss interspecies comparisons in trace element concentrations. Environ Monit Assess 35:263–286
Wolterbeek HTh, Bode P (1995) Strategies in sampling and sample handling in the context of large-scale plant biomonitoring surveys of trace element air pollution. Sci Total Environ 176:33–43. https://doi.org/10.1016/0048-9697(95)04828-6
Zechmeister H (1998) Annual growth of four pleurocarpous moss species and their applicability for biomonitoring heavy metals. Environ Monit Assess 52:441–451. https://doi.org/10.1023/A:1005843032625
Sitography
ICP Vegetation program ‒ international research programme investigating the impacts of air pollutants on crops and (semi-)natural vegetation. https://icpvegetation.ceh.ac.uk/. Accessed 15 June 2024
Manual for moss monitoring, ICP Vegetation program. https://icpvegetation.ceh.ac.uk/sites/default/files/ICP%20Vegetation%20moss%20monitoring%20manual%202020.pdf. Accessed 15 June 2024
Euro+Med PlantBase. https://europlusmed.org/. Accessed 15 June 2024
Acknowledgements
We are grateful to the Ministry of Science, Technological Development and Innovation of the Republic of Serbia for the financial support provided to the Institute of Physics Belgrade (document: 0801–116/1) and University of Novi Sad (grant nos. 451-03-66/2024-03/200125 and 451-03-65/2024-03/200125) and to the bilateral cooperation of the Institute of Physics Belgrade with Joint Institute for Nuclear Research, Dubna, Russia (ref: JINR-Serbia_P05), which enabled the realization of this study.
Funding
This paper was made possible through of bilateral cooperation of the Institute of Physics Belgrade with Joint Institute for Nuclear Research, Dubna, Russia (ref: JINRSerbia_P05) and the financial support provided to the Institute of Physics Belgrade (document: 0801–116/1) and University of Novi Sad (grant nos. 451–03-66/2024–03/200125 & 451–03-65/2024–03/200125).
Author information
Authors and Affiliations
Contributions
Mira Aničić Urošević: Conceptualization; investigation; resources; supervision; methodology, data processing; writing, original draft. Miloš Ilić: Methodology, sampling; sample analyses; writing, review and editing. Dragan Radnović: Methodology, sampling; writing, review and editing. Kostya Vergel: Writing—review and editing. Nikita Yushin: Methodology—analyses. Omari Chaligava: Methodology, data processing; writing, review and editing. Inga Zinicovscaia: Writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gerhard Lammel
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aničić Urošević, M., Ilić, M., Radnović, D. et al. Comparative biomonitoring of airborne potentially toxic elements using mosses (Hypnum cupressiforme, Brachythecium spp.) and lichen (Evernia prunastri) over remote areas. Environ Sci Pollut Res 31, 48296–48312 (2024). https://doi.org/10.1007/s11356-024-34353-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-34353-z