Abstract
The environmental behavior of microplastics (MPs) has attracted global attention. Research has confirmed that MPs can strongly absorb almost every kind of pollutant and can serve as vectors for pollutant transport. In this research, the sorption isotherms of six organic pollutants with different structure on four virgin plastic particles with different crystallinity were determined. Results indicated that the hydrophobicity (KOW) of organic pollutants and the crystallinity of MPs were the two key factors that affected the sorption process of organic pollutants on MPs. Strong correlations were observed between KOW and the partition coefficient. Hydrophobic partition was one of the major mechanisms regardless of the type of organic chemical (hydrophobic, polar, or dissociable). What is more, the influence of the crystallinity of MPs on the sorption process increased with increasing hydrophobicity of the chemical. Combining this result with analyzing the related literature on the effect of crystallinity, it was concluded that the effect of crystallinity on the sorption of chemicals with strong hydrophobicity was obvious, whereas this effect was negligible for chemicals with weak hydrophobicity. The influence of the crystallinity of MPs on sorption could even exceed the influence of MPs type, so crystallinity should be considered carefully when discussing the sorption capacity of MPs. This study enhances the understanding of the sorption of organic pollutants by MPs.
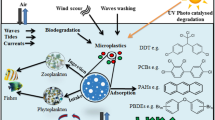
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the past decades, plastic products have been widely used due to their durability, versatility, and low cost, so the global generation of plastic waste has increased rapidly. In 2018, the global total plastic waste reached 380 million tons (Liu et al. 2021). However, on a global scale, only 9% of plastic products are recycled, about 12% are incinerated, and the remaining 79% are discharged into the environment (Pico et al. 2019).
After being discharged into the environment, plastics break down continuously into microsized or nanosized particles known as microplastics (MPs). The large amount of plastic entering the environment poses a threat to human and environmental health (Enfrin et al. 2020; Kogel et al. 2020; Luo et al. 2020, 2023; Rai et al. 2021). Recently, MPs have been found in marine organisms such as fish, mussels, and seabirds (Herzke et al. 2016; Garnier et al. 2019; Hermabessiere et al. 2019; Liu et al. 2020) and in various environmental compartments, including sediments, lakes, rivers, oceans, and urban wastewater (Carr et al. 2016; Wang et al. 2017a, 2017b; Hossain et al. 2023). Therefore, the environmental behavior of MPs has attracted global attention (Zhou et al. 2020; Feng et al. 2023).
Research has confirmed that MPs can strongly adsorb almost every kind of pollutant, including heavy metals, organic pollutants, pharmaceutical products, and personal care products, because of their large surface area and high hydrophobicity. MPs can therefore serve as vectors for pollutant transport (Avio et al. 2015; Guan et al. 2020; Menendez-Pedriza and Jaumot 2020; Wang et al. 2020; Atugoda et al. 2021; Chang et al. 2022; Liu et al. 2022; Luo et al. 2023). The sorption of chemical pollutants on MPs has been investigated intensively. The adsorption process may be influenced by three factors, namely, pollutant, MPs, and aquatic background characteristics, such as pH, ion strength, and dissolved organic matter.
For heavy metals, electrostatic interaction and surface complexation control the adsorption of heavy metals onto MPs. Therefore, heavy metal characteristics such as ionic radius, charge number, and complexing capability and MP characteristics such as the surface potential and functional groups present at the surface exert a considerable effect regardless of the crystallinity of MPs (Tourinho et al. 2019; Zou et al. 2020).
Hydrophobicity (KOW) is a good predictor of sorption of hydrophobic organic pollutants on MPs, and strong correlations between KOW and the partition coefficient (KD) have been observed (Karapanagioti and Klontza 2008; Velez et al. 2018; Jiang et al. 2021). Given that the dominant interaction is related to hydrophobic partition, the water chemistry in terms of, for instance, pH and salinity slightly influences the sorption process of organics to MPs. However, pH and salinity may strongly affect the sorption process for hydrophilic or ionizable organic pollutants because the corresponding sorption mechanisms include electrostatic forces aside from hydrophobic partition. With regard to the effect of the properties of MPs, most studies have focused on surface area, particle size, aging, type of plastics, and crystallinity. Some studies have found that polythene has the strongest sorption capacity among MPs, and others have reported that the order of sorption capacity is polystyrene > PVC > polythene (Huffer and Hofmann 2016; O'Connor et al. 2016; Wu et al. 2019; Tang et al. 2020; Bao et al. 2021). In short, no agreement currently exists about which type of MP has the strongest adsorption capacity despite numerous studies conducted.
In addition, consensus about the effect of the crystallinity of MPs on the sorption of organic contaminants is lacking. In accordance with the regularity of molecular chain arrangement, polymers can be divided into three categories: crystalline, semicrystalline, and amorphous. Generally, glassy polymers have rigid and condensed structures, whereas the amorphous polymers have relatively expansive flexible structures. Hydrophobic organic contaminants can easily partition in amorphous polymers. Some studies have found that crystallinity considerably affects the sorption of organic pollutants onto MPs, whereas others have indicated that the effect is negligible (Teuten et al. 2009; Liu et al. 2019b).
The key objectives of this study are to explore the origin of these contradictions and gain insights into the effect of crystallinity on the sorption of organic pollutants by MPs. Six organic chemicals that significantly differed in structure, hydrophobicity, ionization, and polarity were selected. Four plastic particles, namely chlorinated polyethylene (CPE) and three polyethylene plastic particles (i.e., LPE, LPE2, and HPE), were adopted as target sorbents. The degree of crystallinity of HPE is higher than the crystallinity of LPE and LPE2 which have a similar degree of crystallinity. CPE is an amorphous polymer. The batch sorption isotherms of the target sorbates onto MPs with different crystallinity were investigated and modeled, and the effects of particle size were studied. Moreover, the studies in this area were collected and analyzed, and their results were combined with those of the current study to determine the specific effect of crystallinity.
Materials and methods
Chemicals and materials
All chemicals purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) were of analytical grade or high purity. The relevant physicochemical properties of the chemicals are shown in Table 1.
Four sorbents (CPE, HPE, LPE, and LPE2) were utilized in this study. HPE was purchased from Cospheric LLC (USA), and the others were bought from Haoying Plastic Material Corporation (Dongguan, China). Before the particles were used in experiments, they were made to pass through a 280-μm sieve (60 mesh).
MP characterization
The morphologies of the MPs were characterized using a scanning electron microscope and an X-ray photoelectron spectrometer. The elemental compositions, surface area, purity, and molecular weight of the MPs were determined. Crystallinity was determined based on the thermogravimetric and differential scanning calorimeter (DSC) curves. The details of the characterization of MPs have been provided in our previous research (Zou et al. 2020).
Adsorption experiments
In the adsorption experiment, 0.01 mol/L of sodium chloride and calcium chloride were dissolved in distilled water as the background solution, and 200 mg/L of sodium azide was added to inhibit the bacteria. The stock solution of the involved chemicals with a concentration range of 0.001–100 mg/L was prepared with this background solution. About 10 mg of MPs were placed in a 42-mL brown glass bottle, and the prepared stock solution of organic pollutants with different concentrations (0.001–100 mg/L) was added. The glass bottles were mixed on a turntable at room temperature at 20 rpm. After equilibration, a 2 mL solution was obtained and filtered to determine the concentration of organic pollutants in the aqueous solution. The detailed analytical methods are given in Supplementary data. Control experiments in which MPs were not added were conducted, and the other processes were similar to those described above. The concentration of organic pollutants measured in the control experiment was adopted as the initial concentration. In accordance with mass conservation, the amount of chemicals on the MPs was determined by the difference in aqueous concentrations between the control and equilibrium experiments. The medium conditions involved in this paper are relatively simple, so duplicate samples were obtained in all the experiments. And the results showed that in most cases the standard deviation of parallel samples was less than 5%. The pH of the medium was measured, and it was between 7.0 and 7.6, staying almost the same over the course of the experiments. In the case of CPE, the pH was a little higher than the other MPs involved here, likely because of its relatively strong nucleophilic ability which has been confirmed in our previous research (Zou et al. 2020).
Data analysis
It was reported that the Freundlich model fit the sorption isotherms of pollutants on microplastics well. And here it was used to fit the data. The Freundlich model is described as \(\text{q}={K}_{F}\times {C}_{W}^{\text{n}}\), where q (mmol/kg) and CW (mmol/L) are the concentrations of organic pollutants sorbed on MPs and in the aqueous solutions at equilibrium, respectively; KF (mmol1−n/Ln/kg) is the Freundlich affinity coefficient; and n is the exponential index. The MPs-water distribution coefficient (KD) is also important in research on sorption, and it is expressed as \({K}_{\text{D}}=\frac{q}{{C}_{\text{W}}}\).
Results and discussion
Summary of the physicochemical properties of MPs
In this study, we selected four sorbents (CPE, HPE, LPE, and LPE2), and three of them (i.e., CPE, HPE, and LPE) were used in our previous research (Zou et al. 2020), where the properties of CPE, HPE, and LPE were characterized and shown in detail. The corresponding characterization results of the sorbent LPE2 are given in Fig. S1, and the major chemical and physical properties of the MPs closely related with this research are listed in Table 2. In brief, the purity of all sorbents was high. HPE had the highest crystallinity (77.9%), and LPE and LPE2 had similar but relatively low crystallinity (38.1% and 45.8%, respectively). CPE is an amorphous substance. The surface morphologies of CPE, LPE, and LPE2 were similar and in all cases rough, possibly because they were commercial materials. Their diameters (between 150 and 250 μm) were also similar. HPE had uniform spherical pellets with relatively smooth surfaces, and the diameter was 27–45 μm. All the sorbents displayed similar surface areas.
Sorption of organic contaminants on MPs
The sorption isotherms of six organic chemicals on HPE and CPE are shown in Fig. 1a and b. For LPE and LPE2, DNT, NAPH, and PHEN were selected as representative sorbates (shown in Fig. 1c and d). The sorption data were modeled with the Freundlich model, and the corresponding coefficients obtained are listed in Table 3. As shown in Table 3, the n values for NAPH, PHEN, and TeCB were close to 1, and the n values for the other chemicals were much smaller than 1. This indicates that hydrophobic partition was the major sorption mechanism of NAPH, PHEN, and TeCB. For the other chemicals, other interactions aside from partition occurred.
The KD in Table 3 can directly reflect the sorption strength of sorbates on MPs, and the sequence was consistent with the order of hydrophobicity (usually quantified by means of KOW, listed in Table 1). This observation agrees with the findings of previous studies (Wang et al. 2020; Jiang et al. 2021). The adsorption of organic pollutants on MPs is known to be mainly related to hydrophobicity. Therefore, KOW and MPs–water distribution coefficient KD were fitted (shown in Fig. 2) to further study the influence of hydrophobicity on the sorption process, and the corresponding equations are listed in Table S1. Because of the deviation of DCP (seen from Fig. 2), it was not included in the KOW-KD fitting.
The correlation between KOW and KD was extremely strong for all sorbents tested. This indicates the outstanding effect of KOW. The chemicals examined in this study included a polycyclic aromatic hydrocarbon, a nitroaromatic hydrocarbon, a phenol, a chlorinated benzene, and a pesticide. Hydrophobicity played a crucial role in the sorption of most organic pollutants by MPs. But it is worth noting that the KD of DCP on CPE and HPE deviated obviously from the fitting curve compared with other sorbate-sorbent combinations. This is most likely because of its dissociation. The pKa of DCP was 7.85, so it is mainly present in its non-dissociated state and partly in its anionic state at the pH of the test medium (about 7.6) (Wen et al. 2022). According to the calculation, about 58.5% of DCP is present in its non-dissociated state at pH 7.6. Then, the KD of DCP was recalculated on the basis of the non-dissociated concentration of DCP. It was found that the log KD increased approximately from 1.8 to 2.0 and still deviated obviously from the fitting curve. In general, the surface of MPs is electronegative especially for CPE (Zou et al. 2020). Therefore, it was speculated that the electrostatic repulsion between the anionic state (about 41.5% at pH 7.6) and the electronegative surfaces of CPE and HPE further weakened the adsorption of DCP on MPs. This observation was in agreement with previous research about the sorption of dissociating organic pollutants on MPs (Wang et al. 2015; Li et al. 2018). It was reported that the electrostatic repulsion of anionic perfluorooctanesulfonate molecules at the surface of PE reduced its partition on PE and thereby decreased its KD (Wang et al. 2015).
Influence of the crystallinity of MPs
To explore the effect of crystallinity, we selected DNT, NAPH, and PHEN as representatives and investigated their sorption on four MPs (i.e., CPE, HPE, LPE, and LPE2) with different crystallinity. As shown in Fig. 3, the sequence of the sorption strength of sorbates on MPs was consistent with the order of crystallinity. CPE, which was an amorphous substance, had the strongest sorption capacity among the four MPs, while HPE, being the MPs with the highest crystallinity, had the weakest sorption capacity. LPE and LPE2 with low crystallinity had a moderate sorption affinity to the sorbates. Furthermore, the sorption strengths of the different sorbents dramatically differed for PHEN and were discernibly different for NAPH. However, this difference seemed negligible for DNT. Considering that their surface areas were relatively close, the difference in their adsorption capacity possibly resulted from crystallinity.
On the basis of the analysis above, we fitted crystallinity and KD (Fig. 4) and found that for PHEN and NAPH, the correlation between crystallinity and KD was good, indicating that crystallinity played a crucial role in the sorption by MPs, especially for strong hydrophobic PHEN. However, no correlation was found between crystallinity and KD for DNT, indicating the insignificance of crystallinity. Research has shown that amorphous polymers are conducive to the diffusion and partition of organic chemicals (Andrady 2017; Zuo et al. 2019). According to the discussion above, the main mechanism of the sorption of organic pollutants on MPs is hydrophobic partition. Therefore, crystallinity showed the greatest influence on the strong hydrophobic pollutants such as PHEN and moderate influence on the moderate hydrophobic pollutants such as NAPH. Nevertheless, for pollutants with weak hydrophobicity such as DNT, in addition to hydrophobic partition, a variety of sorption mechanisms are generally involved such as electrostatic interaction. Therefore, the influence of crystallinity weakened correspondingly.
In addition, particle size may have had an effect because the particle size of the four sorbents was different. Another sorbent with the same physical and chemical properties as HPE, except for particle size (212–240 µm), was compared with HPE (27–45 µm) to exclude the possible effect of particle size. The sorption isotherms of the two sorbents with different diameters for DNT and PHEN are displayed in Fig. S2. Particle size exerted a negligible effect on the sorption process although a large difference in particle size existed between the two sorbents, which was consistent with the results observed in Fig. 3. CPE with a diameter of 150–250 μm showed higher sorption capacity than HPE with a diameter of 27–45 μm and LPE and LPE2 with a diameter of 150–250 μm. After excluding the possible causes of surface area and particle size, the crystallinity of MPs was found to have a considerable effect on the sorption of organic pollutants by MPs, especially for the organic chemicals with a large KOW.
Insights into the effect of crystallinity
According to the discussion above, the more hydrophobic the pollutants are, the larger the effect of crystallinity on sorption. For pollutants with weak hydrophobicity, the influence of crystallinity weakens correspondingly.
To further confirm our inference, we analyzed related literature on the effect of crystallinity, as shown in Table 4. We found that studies where the concerned sorbates had weak hydrophobicity and dissociation ability, such as antibiotics and pesticides, concluded that crystallinity has a negligible influence on sorption. In studies where the target chemicals were strongly hydrophobic, such as polycyclic aromatic hydrocarbons and chlorobenzenes, crystallinity was considered a predominant factor.
This discovery further confirms our preliminary conclusion. The degree to which crystallinity affected the sorption varied with the hydrophobicity of organic pollutants. In addition, it may explain the inconsistencies in the sequence of the sorption capacity of different types of MPs obtained in different studies. Possibly, crystallinity was disregarded in these studies. The sorption capacity of MPs can be very different due to different crystallinity even though some MPs have the same monomer.
Conclusions
In this study, it was indicated that the KOW of organic pollutants and the crystallinity of MPs were two key factors that affected the sorption process of organic pollutants on MPs. Hydrophobic partition was one of the major mechanisms regardless of whether the organic chemicals were hydrophobic, polar, or dissociable. The general finding is the larger the value of KOW, the stronger the sorption. In addition, the degree of the effect of crystallinity varied with the hydrophobicity of the sorbates. The influence of crystallinity on the sorption process increased with increasing hydrophobicity of the chemical. In summary, this study answered the two questions raised at the beginning of this paper: firstly, for the sorption of chemicals with strong hydrophobicity, the effect of crystallinity is obvious, whereas this effect is difficult to observe for chemicals with weak hydrophobicity. Therefore, some studies have concluded that the effect of crystallinity is prominent, whereas others have reported that crystallinity has no effect on sorption. This might also be due to the different chemicals studied. Secondly, the influence of the crystallinity of MPs is sometimes even greater than the influence of MPs type, so crystallinity should be considered carefully when discussing the sorption capacity of MPs. The study enhances our understanding of the sorption of organic pollutants by MPs.
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
References
Ahn S, Werner D, Karapanagioti HK, McGlothlin DR, Zare RN, Luthy RG (2005) Phenanthrene and pyrene sorption and intraparticle diffusion in polyoxymethylene, coke, and activated carbon. Environ Sci Technol 39:6516–6526
Andrady AL (2017) The plastic in microplastics: a review. Mar Pollut Bull 119:12–22
Atugoda T, Vithanage M, Wijesekara H, Bolan N, Sarmah AK, Bank MS, You S, Ok YS (2021) Interactions between microplastics, pharmaceuticals and personal care products: implications for vector transport. Environ Int 149:106367
Avio CG, Gorbi S, Milan M, Benedetti M, Fattorini D, d’Errico G, Pauletto M, Bargelloni L, Regoli F (2015) Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ Pollut 198:211–222
Bao ZZ, Chen ZF, Zhong Y, Wang G, Qi Z, Cai Z (2021) Adsorption of phenanthrene and its monohydroxy derivatives on polyvinyl chloride microplastics in aqueous solution: model fitting and mechanism analysis. Sci Total Environ 764:142889
Carr SA, Liu J, Tesoro AG (2016) Transport and fate of microplastic particles in wastewater treatment plants. Water Res 91:174–182
Chang J, Fang W, Liang J, Zhang P, Zhang G, Zhang H, Zhang Y, Wang Q (2022) A critical review on interaction of microplastics with organic contaminants in soil and their ecological risks on soil organisms. Chemosphere 306:135573
Enfrin M, Lee J, Gibert Y, Basheer F, Kong L, Dumee LF (2020) Release of hazardous nanoplastic contaminants due to microplastics fragmentation under shear stress forces. J Hazard Mater 384:121393
Feng Q, An C, Chen Z, Lee K, Wang Z (2023) Identification of the driving factors of microplastic load and morphology in estuaries for improving monitoring and management strategies: a global meta-analysis. Environ Pollut 333:122014
Garnier Y, Jacob H, Guerra AS, Bertucci F, Lecchini D (2019) Evaluation of microplastic ingestion by tropical fish from Moorea Island, French Polynesia. Mar Pollut Bull 140:165–170
Guan J, Qi K, Wang J, Wang W, Wang Z, Lu N, Qu J (2020) Microplastics as an emerging anthropogenic vector of trace metals in freshwater: significance of biofilms and comparison with natural substrates. Water Res 184:116205
Guo X, Wang X, Zhou X, Kong X, Tao S, Xing B (2012) Sorption of four hydrophobic organic compounds by three chemically distinct polymers: role of chemical and physical composition. Environ Sci Technol 46:7252–7259
Hermabessiere L, Paul-Pont I, Cassone AL, Himber C, Receveur J, Jezequel R, El Rakwe M, Rinnert E, Riviere G, Lambert C, Huvet A, Dehaut A, Duflos G, Soudant P (2019) Microplastic contamination and pollutant levels in mussels and cockles collected along the channel coasts. Environ Pollut 250:807–819
Herzke D, Anker-Nilssen T, Nost TH, Gotsch A, Christensen-Dalsgaard S, Langset M, Fangel K, Koelmans AA (2016) Negligible impact of ingested microplastics on tissue concentrations of persistent organic pollutants in northern fulmars off coastal Norway. Environ Sci Technol 50:1924–1933
Hossain MB, Yu J, Nur A-AU, Banik P, Jolly YN, Mamun MA, Paray BA, Arai T (2023) Distribution, characterization and contamination risk assessment of microplastics in the sediment from the world’s top sediment-laden estuary. J Environ Manag 344:118472
Huffer T, Hofmann T (2016) Sorption of non-polar organic compounds by micro-sized plastic particles in aqueous solution. Environ Pollut 214:194–201
Jiang H, Xiong Q, Chen X, Pan W, Dai Y (2021) Carrier effect of S-metolachlor by microplastics and environmental risk assessment. J Water Process Eng 44:102451
Karapanagioti HK, Klontza I (2008) Testing phenanthrene distribution properties of virgin plastic pellets and plastic eroded pellets found on Lesvos island beaches (Greece). Mar Environ Res 65:283–290
Kogel T, Bjoroy O, Toto B, Bienfait AM, Sanden M (2020) Micro- and nanoplastic toxicity on aquatic life: determining factors. Sci Total Environ 709:136050
Li J, Zhang K, Zhang H (2018) Adsorption of antibiotics on microplastics. Environ Pollut 237:460–467
Liu FF, Liu GZ, Zhu ZL, Wang SC, Zhao FF (2019a) Interactions between microplastics and phthalate esters as affected by microplastics characteristics and solution chemistry. Chemosphere 214:688–694
Liu G, Zhu Z, Yang Y, Sun Y, Yu F, Ma J (2019b) Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater. Environ Pollut 246:26–33
Liu P, Wu X, Liu H, Wang H, Lu K, Gao S (2020) Desorption of pharmaceuticals from pristine and aged polystyrene microplastics under simulated gastrointestinal conditions. J Hazard Mater 392:122346
Liu P, Shi Y, Wu X, Wang H, Huang H, Guo X, Gao S (2021) Review of the artificially-accelerated aging technology and ecological risk of microplastics. Sci Total Environ 768:144969
Liu P, Dai J, Bie C, Li H, Zhang Z, Guo X, Zhu L (2022) Bioaccessibility of microplastic-associated antibiotics in freshwater organisms: highlighting the impacts of biofilm colonization via an in vitro protocol. Environ Sci Technol 56:12267–12277
Luo H, Li Y, Zhao Y, Xiang Y, He D, Pan X (2020) Effects of accelerated aging on characteristics, leaching, and toxicity of commercial lead chromate pigmented microplastics. Environ Pollut 257:113475
Luo H, Tu C, He D, Zhang A, Sun J, Li J, Xu J, Pan X (2023) Interactions between microplastics and contaminants: a review focusing on the effect of aging process. Sci Total Environ 899:165615
Mato Y, Isobe T, Takada H, Kanehiro H, Ohtake C, Kaminuma T (2001) Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ Sci Technol 35:318–324
Menendez-Pedriza A, Jaumot J (2020) Interaction of environmental pollutants with microplastics: a critical review of sorption factors, bioaccumulation and ecotoxicological effects. Toxics 8:40
O’Connor IA, Golsteijn L, Hendriks AJ (2016) Review of the partitioning of chemicals into different plastics: consequences for the risk assessment of marine plastic debris. Mar Pollut Bull 113:17–24
Pico Y, Alfarhan A, Barcelo D (2019) Nano- and microplastic analysis: focus on their occurrence in freshwater ecosystems and remediation technologies. TrAC-Trend Anal Chem 113:409–425
Rai PK, Lee J, Brown RJC, Kim KH (2021) Environmental fate, ecotoxicity biomarkers, and potential health effects of micro- and nano-scale plastic contamination. J Hazard Mater 403:123910
Schwarzenbach RP, Gschwend PM, Imboden DM (2003) Environmental organic chemistry. Wiley-Inter-science, New York
Siri C, Liu Y, Masset T, Dudefoi W, Oldham D, Minghetti M, Grandjean D, Breider F (2021) Adsorption of progesterone onto microplastics and its desorption in simulated gastric and intestinal fluids. Environ Sci Process Impacts 23:1566–1577
Tang Y, Liu Y, Chen Y, Zhang W, Zhao J, He S, Yang C, Zhang T, Tang C, Zhang C, Yang Z (2020) A review: research progress on microplastic pollutants in aquatic environments. Sci Total Environ 766:142572
Teuten EL, Saquing JM, Knappe DR, Barlaz MA, Jonsson S, Bjorn A, Rowland SJ, Thompson RC, Galloway TS, Yamashita R, Ochi D, Watanuki Y, Moore C, Viet PH, Tana TS, Prudente M, Boonyatumanond R, Zakaria MP, Akkhavong K, Ogata Y, Hirai H, Iwasa S, Mizukawa K, Hagino Y, Imamura A, Saha M, Takada H (2009) Transport and release of chemicals from plastics to the environment and to wildlife. Philos Trans R Soc Lond B Biol Sci 364:2027–2045
Tourinho PS, Koci V, Loureiro S, van Gestel CAM (2019) Partitioning of chemical contaminants to microplastics: sorption mechanisms, environmental distribution and effects on toxicity and bioaccumulation. Environ Pollut 252:1246–1256
Velez JFM, Shashoua Y, Syberg K, Khan FR (2018) Considerations on the use of equilibrium models for the characterisation of HOC-microplastic interactions in vector studies. Chemosphere 210:359–365
Wang F, Shih KM, Li XY (2015) The partition behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanesulfonamide (FOSA) on microplastics. Chemosphere 119:841–847
Wang J, Peng J, Tan Z, Gao Y, Zhan Z, Chen Q, Cai L (2017a) Microplastics in the surface sediments from the Beijiang River littoral zone: composition, abundance, surface textures and interaction with heavy metals. Chemosphere 171:248–258
Wang W, Ndungu AW, Li Z, Wang J (2017b) Microplastics pollution in inland freshwaters of China: a case study in urban surface waters of Wuhan, China. Sci Total Environ 575:1369–1374
Wang F, Zhang M, Sha W, Wang Y, Hao H, Dou Y, Li Y (2020) Sorption behavior and mechanisms of organic contaminants to nano and microplastics. Molecules 25:1827
Wen Q, Wang Y, Zeng Z, Qi F, Gao P, Huang Z (2022) Covalent organic frameworks-derived hierarchically porous n-doped carbon for 2,4-dichlorophenol degradation by activated persulfate: the dual role of graphitic. J Hazard Mater 426:128065
Wu P, Cai Z, Jin H, Tang Y (2019) Adsorption mechanisms of five bisphenol analogues on PVC microplastics. Sci Total Environ 650:671–678
Xu B, Liu F, Brookes PC, Xu J (2018) Microplastics play a minor role in tetracycline sorption in the presence of dissolved organic matter. Environ Pollut 240:87–94
Zhou L, Wang T, Qu G, Jia H, Zhu L (2020) Probing the aging processes and mechanisms of microplastic under simulated multiple actions generated by discharge plasma. J Hazard Mater 398:122956
Zou J, Liu X, Zhang D, Yuan X (2020) Adsorption of three bivalent metals by four chemical distinct microplastics. Chemosphere 248:126064
Zuo LZ, Li HX, Lin L, Sun YX, Diao ZH, Liu S, Zhang ZY, Xu XR (2019) Sorption and desorption of phenanthrene on biodegradable poly(butylene adipate co-terephtalate) microplastics. Chemosphere 215:25–32
Funding
This work is supported by Science and Technology Development Plan Project of Jilin Province, China (20240101053JC), and National Natural Science Foundation of China (42130705).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design.
Dongmei Zhang: Conceptualization; supervision; writing, original draft; reviewing and editing
Zining Zhang: Investigation, validation and data curation
Hui Liu: Investigation and validation
Jiying Zou: Investigation and paper revision
Xiuping Liu: Investigation and validation
Longyu Yin: Investigation and validation
Ya-nan Zhang: Paper revision
Jiao Qu: Project administration, supervision and reviewing
Willie J.G.M. Peijnenburg: Reviewing and editing
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Guilherme Luiz Dotto
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors confirmed that the work described has not been published before, that it is not under consideration for publication elsewhere, and that its publication has been approved by all co-authors.
Highlights
• Sorption of six organic chemicals on four virgin plastic particles was investigated.
• Hydrophobic partition was one of the major mechanisms for most organic chemicals.
• KOW and the crystallinity were the two key factors affecting the sorption process on MPs.
• The degree to which crystallinity affected the sorption varied with hydrophobicity.
• Crystallinity should be considered when discussing the sorption capacity of MPs.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, D., Zhang, Z., Liu, H. et al. Insights into the effect of crystallinity on the sorption of organic pollutants to microplastics. Environ Sci Pollut Res 31, 42202–42211 (2024). https://doi.org/10.1007/s11356-024-33929-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-33929-z