Abstract
In the past few decades, the excessive and inadequate use of technological advances has led to groundwater contamination, mainly caused by organic and inorganic pollutants, which are highly harmful to human health, agriculture, water bodies, and aquaculture. Among all toxic pollutants, As and F− play a significant role in groundwater contamination due to their excellent reactivity with other elements. To mitigate the prevalence of arsenic and fluoride within the water system, the use of biochar gives an attractive strategy for removing them mainly because of the substantial surface area, pore size, pH, aromatic structure, and functional groups inherent in biochar, which are primarily dependent upon its raw material and pyrolysis temperature. Researcher develops different methods like physiochemical and electrochemical for treating arsenic and fluoride contamination. Among all removal methods, bioadsorption using agricultural waste residues shows effective/feasible removal of As and F− due to its low cost, ecofriendly nature, readily available, and efficient reuse compared with several other harmful synthetic materials that demand costly design specifications. This study discusses current developments in bioadsorption methods for As and F− that use agricultural-based biomaterials and describes the prevailing state of arsenic and fluoride removal strategies that use biomaterials precisely.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
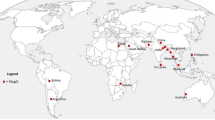
Groundwater is a main source of water to fulfil demand for fresh water for agricultural, domestic, and industrial purposes. Approximately 33.33% of the population relies on fresh groundwater as a primary source of drinking water (International Association of Hydrogeologists 2020). Acquiring a nontoxic and sustainable resource of potable groundwater is necessary to achieving viable development for a country. Moreover, increasing water scarcity problems are closely related to the climate crisis, land use change, population growth, and infrastructure development impacting water management, distribution networks, and water quality problems. Groundwater contamination is the accumulation of foreign entities via anthropogenic activities (Government of Canada 2017). The numerous contaminants, such as organic–inorganic, heavy metals, toxic pesticides, microplastics, and new emerging contaminants, are very hazardous to agriculture, human health, and the sustainable development of the ecosystem (Li and Wu 2019). The damaging effects of contaminated groundwater on human health and agriculture are prolonged and hard to identify. Among several contaminants that infiltrate aquatic ecosystems, organic–inorganic contaminants such as As and F− are identified as crucial, which results in severe health and environmental issues (Rupasinghe et al. 2022). The primary sources of As and F− contaminants are agricultural waste, industrial waste, domestic waste, landfill, mining, sewage waste, medical waste, etc., as depicted in Fig. 1. Subsequently, remediation is very stimulating and expensive once groundwater is contaminated since groundwater is situated in underground geographical profile (Amen et al. 2020).
F− is vigorous in minerals such as fluorspar, fluorite, mica, apatite, rock phosphate, cryolite, and other F− which are richer in igneous rocks, sedimentary formations, and mineralized tones. Groundwater contamination with F− has increased globally in past decades. F− is a flexible element essential for dental care at its lower concentration (up to 0.6 mg/L) and, at the same time, hazardous at its higher concentration (> 1.2 mg/L) (Lizneva et al. 2018). Conversely, prolonged F− intake can result in fluorosis, harmfulness to kidneys, and cancer (Sikdar 2018). As a result, the WHO has set acceptable limits of 1.5 mg/L for F− levels in potable water (Cotruvo 2017; Nath 2018). High F− concentration in groundwater above the acceptable threshold value of 1.5 mg/L is a notable public health alarm in India. Approximately 90% of the rural population in our nation relies on groundwater as a prime source of potable and household water. The alarming rate of fluorosis among a significant rural population is an urgent concern due to the excessive amount of F− in groundwater, as depicted in Fig. 2. Overall, 19 states and districts are affected by high F− concentrations. The severely affected areas are Haryana, Punjab, Delhi, Rajasthan, Gujrat, Uttar Pradesh, West Bengal, Bihar, Jharkhand, Chhattisgarh, Odisha, Telangana, Andhra Pradesh, Tamil Nadu, and Karnataka.
On the other hand, As is a massively poisonous cancer-causing and geogenic metalloid usually detected in diverse parts of the globe (Balali-Mood et al. 2021). In the past decades, there has been a gradual increase in As contamination worldwide due to the unrestricted growth in various industrial accomplishments, coal mining, semiconductor industries, and excessive use of pesticides and chemicals for crop growth (Ahmad et al. 2022). Following the WHO maximum allowable bound of As concentration in fresh consumable water is 0.01 mg/L (Cuong et al. 2022). In the case of an aquatic environment, As primarily occurs in dual oxidation states, specifically, arsenate (As(V)) and arsenite (As(III)). Mainly, As(III) is a principal species having complex mobility and solubility and abundant toxicity compared to As(V). As a result, groundwater contaminants could contaminate the waterbodies and indirectly enter the food chains by cultivating crops and other farming activities (Sakhiya et al. 2023). The spatial analysis indicates that the parts of India showing the highest level of impact are mainly situated on the eastern bank of the Bhagirathi River, covering the districts of Malda, Murshidabad, and Nadia. Furthermore, the western bank of the river, specifically the districts of Howrah, Hoogly, and Burdwan, also exhibits significant vulnerability to the phenomenon mentioned. The presence of As in underground water primarily occurs in aquifers which are limited to a maximum depth of 100 m. It has been observed that the underground aquifers situated at greater depths indicate a noticeable absence of As contamination. In addition to West Bengal, the problem of As contamination in groundwater is prevalent in the states of Bihar, Assam, Haryana, Chhattisgarh, Jharkhand, Karnataka, Punjab, Uttar Pradesh, and West Bengal. The accumulation of As in the regions of Bihar, West Bengal, and Uttar Pradesh is predominantly observed in alluvial deposits depicted in Fig. 3.
An enormous range of practical techniques has been introduced and implemented to remove As and F− from contaminated groundwater, wastewater, and aqueous solution. It includes chemical precipitation/coagulation, ion exchange, membrane separation, electrochemical treatments, adsorption, etc. (Hegde et al. 2020). The merits and demerits of the removal method for As and F− are presented in Table 1. Among the implemented removal techniques, adsorption is a highly effective method for removing contaminants owing to its less expensiveness, eco-friendliness, simplicity in operation, and ready accessibility. A broad range of natural adsorbents and their chemical modification are used to address As and F− from water solutions. Modified adsorbents comprise carbon materials, geomaterials, oxides and hydroxides, industrial waste products and by-products, and biomaterials (Wang and Wang 2019). The techniques mentioned in Table 1 have a moderate to higher removal efficiency for As and F−. Still, they also have operational issues, such as the need for expensive equipment, setting, maintaining ideal conditions, labor costs, and secondary waste production. Moreover, it has been noted that among all the methods the adsorption method is better adapted for removing toxic contaminants because there is a large selection of reasonably priced adsorbents. Additionally, utilizing biomass just like bioadsorbents could significantly lower the cost of removal. The innovation of this technique is receiving an abundance of attention from scientists worldwide to simplify and reduce the cost of the removal processes (Mohan et al. 2014) (Venkatachalam et al. 2023).
There are many studies available that discuss bioadsorption process for the removal of As and F− from contaminated groundwater. These studies cover a wide range of bioadsorbents, including plant biomass, forest biomass, algae-based biomass, microbial biomass, and others. However, there is a lack of extensive research that thoroughly explores the probable and practical utilization of agricultural biomass adsorbents for removal of As and F− in real time applications. This contribution aims to provide a detailed analysis of different bioadsorbents available in the environment specially focused on agriculturally based adsorbents that have been developed for remediation. It covers adsorbent capacity and the fundamental mechanisms that make them effective in removing As and F−. This contribution also explores the major sources of groundwater contamination and affected regions in Indian states with harmful effects on health, agriculture, environment. The synthesis of adsorbents and their modification to increase the biomaterial surface area and pore size is reviewed. It elaborates on innovations, particularly on applying and discovering more biomaterials, which can be prime applicants in water treatment technologies. This information particularizes the industrial application of biomaterial for removing toxic contaminants for large-scale operations and helps to support the prospect of ecological development in developing countries like India. The main aim of the article is to encourage further innovations into agricultural residues that have significant advantages in removing As and F−. It also aims to explore ways to improve their effectiveness and the possibility of increasing commercialization and scalability at a local level. Developing an agricultural-based adsorbent that effectively removes As and F− could be a significant step in tackling the prevalent problem of potable drinking water. The present article emphasizes the need to carefully consider the long-term consequences of such agricultural-based adsorbents to avoid the revival of As and F− into the ecosystem. The present review inspires researchers to come up with ideas for sustainable modifying adsorbents so that they can show improved removal efficacies. A brief discussion on a synthesis of adsorbents, modification of adsorbents, novel biomaterial for removing contaminants, and parameters effects on removal capacity has been discussed.
Biomass-derived bioadsorbents
Bioadsorbents are a group of materials comprising natural and chemically modified substances that have catalytic properties in adsorption. Extensive investigation is being conducted on bioadsorbents in the removal of pollutants like organic and inorganic pollutants (Rupasinghe et al. 2022; De et al. 2018). Bioadsorbent-derived from biochar, known as fine-grained carbon material, is cost-effective and environmentally friendly for better sustainability to resolve environmental problems. Biomaterials obtained from agricultural residues, industrial wastes, and domestic wastes, which are low-cost and readily accessible, have been widely used as adsorbents for removal due to their inclusive properties in the remediation of pollutants (Senewirathna et al. 2022; Nehra et al. 2020). Significant elemental configuration, physicochemical features, and lattice arrangement of biochar mark its better applicability in agriculture, renewable energy sources, carbon sequestration, bio-oils, biofuels, and removal of toxic pollutants from the environment (Dhawane et al. 2018).
Synthesis of bioadsorbents
Biochar preparation involves the conversion of biomass into effective products primarily through thermochemical and biochemical conversion. Recent literature comprehensively lists thermochemical conversion procedures comprising pyrolysis and gasification; however, fermentation and anaerobic incorporation are biochemical translation methods prominently used for biochar fabrication (Valdés-Rodríguez et al. 2022). Pyrolysis is a thermochemical reaction carried out in the absence of an oxygen atmosphere (300 –900 °C) that converts raw biomass into valuable products in solid, liquid, and gaseous fractions (Hegde et al. 2020; Dhawane et al. 2018). Furthermore, it is essential to mention that pyrolysis can be classified into three distinct categories: fast, slow, and flash, based on the heating rate, residence time, and temperature. Temperatures ranging from 450 to 900 °C are used in the fast pyrolysis process with 0.5 to 10 s of relatively short residence time. In contrast to fast and flash pyrolysis techniques, slow pyrolysis involves the utilization of lower temperatures ranging from 250 to 550 °C, along with a prolonged residence time of 450 to 550 s.
Slow pyrolysis yields more valuable biochar (constituting 35–50% of the biomass weight) through three phases involving hemicellulose degradation, moisture evaporation, and lignin alteration compared to fast pyrolysis (Panwar et al. 2019; Tomczyk et al. 2020). The first stage permits light volatiles and moistness to evaporate at 200 °C temperature, which helps to form various functional groups like carbonyl and carboxylic acids (Yaashikaa et al. 2020) The second phase of slow pyrolysis at temperatures extending from 200 and 500 °C involves the deterioration of hemicelluloses and cellulose. The third phase helps to terminate the residual lignin and other organic components with temperatures exceeding 500 °C. A schematic representation of the process of biochar preparation and its application is shown in Fig. 4.
Methods for enhancement of surface properties
In order to boost the efficacy of biochar, it is essential to apply chemical, physical and physiochemical treatments to enhance the basic properties of the carbonaceous materials. It will help to expand porosity, surface area and functional groups like hydroxyl, thiol, amine, and carboxyl (Wang and Wang 2019). There are different ways of modifying biochar discussed in the following sections.
Physical activation
The carbonized biochar shows a significantly limited surface area and pore volume in its pristine state. Thus, its immediate applicability in chemical processes results in poor outcomes. The appearance of pores on the outer layer during pyrolysis contributes to the elimination of volatiles, followed by a blockage of pores by the development of tar (Dhawane et al. 2018). The inadequate surface features of the biochar contribute to a lower adsorption capacity. Thus, to improve the surface features of the biochar, it is exposed to an activation process. Physical activation in biochar modifications applies physical methods like high temperatures and inert gases to enhance the properties of biochar. Applying steam or CO2 at 700 to 900 °C as an oxidizing agent for physical activation is a highly prevalent method for surface modification in the industrial domain. The present technique confers a notable advantage over alternative methodologies, as the activated carbon generated thereby reveals a highly expanded pore structure and remarkable mechanical reliability, exhibiting a superior hardness number (Zoroufchi Benis et al. 2020). The employed practice is recognized as an eco-friendly technology because it can escape the production of secondary wastewater resulting from a lack of chemical treatment during the process.
Chemical activation
Chemical activation method involves treating biochar with acids and bases to formulate pores and boost the surface area of biochar. The most regularly used acids are phosphoric acid, sulfuric acid, nitric acid, hydrochloric acid, citric acid, and oxalic acid (Wang and Wang 2019). This method improved the surface area of activated carbon (1000–3000 m2) with limited energy expenditures, thus often used in several areas like semiconductor technology, water treatment, soil remediation, and chemical process engineering labs. The effectiveness of chemical activation is influenced by the type of chemical reagents, type of biomass used, heating temperature, and heating time. Recently, Nie et al. (2023) reported the chemical activation of Aspergillus oryzae fungal biochar to enhance adsorption capacity using hydrochloric acid as an activating reagent showing a surface area of 114.5 m2/g. Similarly, corn straw biomass was used to produce biochar with thermal treatment at 700 °C using HCL attaining 0.13 cm3/g and 40.24 m2/g, respectively, for pore volume and surface area (Wang et al. 2022). Recently, synthesized activated carbon using sunflower seed husk biomass activated using H3PO4 helps to enhance a surface area of 378.8 m2/g and creates a mesoporous structure (Nguyen et al. 2023). Chemical activation is liable for creating a substantial amplification of pores on the surface, along with numerous fractures that play an integral part in enhancing the overall surface area. The activation can be carried out by two distinct methodologies, particularly dry impregnation and wet impregnation. The surface characteristics are acquired through the dry impregnation technique, in which the initial activating agent is blended with the raw material before thermal treatment and it displays superior features to those obtained via the wet impregnation approach. The improvement of surface properties can be achieved through direct diffusion of the activator without forming damaging chemicals, facilitated through mixing of precursor and activating agent in an inert condition (Dhawane et al. 2018). Commonly used alkaline activating agents are sodium hydroxide and potassium hydroxide. Recently, corn straw (Zea mays L.) modified with NaOH, pyrolyzed at 700 °C for 2 h under N2 atmosphere conditions and showed an effective surface area of 82.64 m2/g which helps to improve the adsorption capacity of adsorbents (Wang et al. 2022). Similarly, banana waste-like stems and leaves were used to synthesize KOH modified biochar with a feedstock ratio of 1:92 g:mL to a banana. The optimum parameter conditions were 1.33 mol/L, 721 ℃, 76 min for modification of biochar which resulted in 36.41% biochar yield ratio and 480.94 mg/g Cd adsorption capacity (Ding et al. 2023). Further, oxidizing agent modifications help enhance oxygen-containing functional groups on the surface of the adsorbent. Biochar modification with hydrogen peroxide (H2O2) improves the carboxyl and hydroxyl functional group for removing aqueous mercury using seaweed-modified biochar (Yang et al. 2023).
Physiochemical activation
The physiochemical method is a recently developed technique effectively used to prepare bioadsorbents. This technique combines both physical and chemical activation methods to enhance the properties of biomass. Initially, biomass and chemical reagents are mixed and then it is carbonized in an inert atmospheric condition with a supply of steam, carbon dioxide, nitrogen, etc. The adsorbent synthesis from this technique has diversified textural and chemical properties. This method is beneficial when the washing process fails to eliminate the chemicals present on the surface, leading to blockage of the pores (Venkatachalam et al. 2023). Therefore, carbonized substances are subjected to activation more in an environment that promotes oxidation to facilitate the proper formation of pores. The pattern of chemical treatment plays a vital part in defining the characteristics of activated carbon in the physicochemical method. Moreover, the various routes employed in this method significantly impact the resulting properties.
Comparison between biochar modification methods
The adoption of chemical, physical, and physiochemical activation methods for the enhancement of surface properties as discussed above were effective in increasing the surface area and porosity of biochar. Carbon materials modification was found to boost the surface area through a complementary effect between biochar and carbon materials. The elemental composition, precisely the proportion of carbon, nitrogen, and oxygen, significantly impacts the characteristics of biochar. The fundamental characteristics of biochar are determined by the nitrogen-to-carbon ratio, which governs its fundamental nature, and the oxygen-to-carbon ratio, which governs its hydrophilic properties. According to Huang et al. (2021), alkaline modification exhibits a more significant proportion of surface aromaticity and nitrogen-to-carbon ratio but a lesser proportion of oxygen-to-carbon than acid modification. It has been observed that the biochar preparation procedure becomes more complex due to the requirement for additional treatment with alkali or acid solutions. To address the handling of acid or alkali solutions, future research may explore the sequential modification of both acid and alkali. The neutralization process between acidic and alkaline solutions presents a viable treatment option for both solutions. Applying oxidizing agents can enhance oxygen-containing groups. However, oxidizing agents have been prohibited due to cost and treatment factors. A strategy that facilitates the continuous use of oxidizing agent solutions could reduce manufacturing costs. The primary purpose of treatment with an oxidizing agent is to enhance the number of active sites available for adsorption and catalytic reactions. Nevertheless, there have been reports of metallic ion loss from carbon-based materials. Therefore, additional research efforts should be directed toward enhancing the stability of metal ions in biochar. Compared to the discussed techniques for biochar modification, steam and gas refining do not require the consideration of solution usage after modification.
In comparison with all three activation methods, physical activation is a more environmentally friendly choice as it does not need multiple chemicals and is widely used in industries. But the process usually involves significant energy capital and expert apparatus which leads to variations in the resulting biochar based on the individual activation conditions. On the other hand, chemical activation gives better biochar surface properties and adsorption capacity. However, this method leads to the existence of hazardous leftovers and it requires posttreatment for the disposal of leftover chemicals. Similarly, the physiochemical activation method provides a dual approach which creates biochar with improved surface area, porous nature, and active chemical reaction, thus enhancing its efficacy in several applications. Overall, all activation methods have advantages and disadvantages, offering investigators a variety of choices for modifying biochar to meet particular treatment requirements. Furthermore, there is a need to enhance the preparation circumstances of the pyrolysis process and the subsequent steam or gas purging. The choice of modified biochar methods is typically contingent upon the specific environmental application of biochar. Previous research has indicated the need for additional studies to enhance the durability of metal ions in biochar.
The discussion provided in this work highlights the significant potential of modified biochar for remediating the environment. However, it is worth noting that some biochar modification approaches may not prioritize ecological sustainability. Through the modification of biochar with metal salts, functional groups that include oxygen can be significantly increased, resulting in a notable enhancement of its adsorption capacity. Inappropriate application of such modified biochar can lead to the leaching of infused metal ions, causing secondary pollution.
Functional groups like hydroxyl, amine, carboxyl, and thiol can enhance the adsorbent adsorption capacity. The enhanced adsorption capacity can be associated with the existence of a hydroxyl group, which results in an amplified active site for the chemisorption process. The hydroxyl group facilitates electrostatic interaction, coulombic attraction, hydrogen bonding, and ligand exchange (Khan et al. 2022; Naga Babu et al. 2018). Consequently, the most suitable bioadsorbents for the remediation investigations are those having −OH group functionalization. Additionally, the adsorption capacity of biomaterials reliably improved with an enlargement in the surface area of a material. The porosity of the adsorbent correlated with the surface area of the material. The material porous nature helps to aid more active adsorption sites through pore dispersion (Biswal et al. 2022).
Remediation techniques like bioadsorption use naturally available biomaterial obtained from different sectors as bioadsorbents (Biswal et al. 2022). There are numerous biomaterials available naturally, like agricultural biomass waste residues, including corn stalks, rice husks, rice straws, wheat straws, cotton stems, coffee plant waste, tea plant residue, coconut husks, peanut shells, orange peel, banana peel, tree bark, weeds, etc. and microbial biomass like fungi, microalgae yeasts, etc. for the removal process. These all natural materials have considerably low removal efficiency at commercial-scale operations. In addition, chemically modified biomaterials show higher removal efficiency than naturally available biomaterials. Accordingly, bioadsorption remediation method is eco-friendly and cheaper to remove As and F− as all these materials are naturally easily accessible, and biodegradable. Similarly, the inclusive features like high surface area, high porosity, and lower density of different biomaterials enhance its benefits in As and F− removal.
Bioadsorbents used for the removal of F− ions
The utility of artificial materials and chemicals in removing F− ions has restricted the investigation of several bioadsorbent to a definite range for commercial practices. In this part, the brief summarization of different bioadsorbent based on agricultural waste residues has been critically discussed for applying material for industrial-level removal techniques.
The watermelon rinds (Citrullus lanatus) biochar-based adsorbent is utilized for removing F− from groundwater and industrial waste. The watermelon rind increases removal efficiency by 66%, with 9.5 mg/g adsorption at pH 1 and a reaction contact time of 3 h for collected groundwater samples. Similarly, the adsorption capacity of watermelon rind for industrial effluents rises to 70% when the adsorbent dose increases. Thermodynamics studies showed that removing the F− adsorption reared a spontaneous exothermic process. Subsequently, this material was concluded to be used for industrial effluent with low pH (Sadhu et al. 2022). Likewise, zirconium dioxide-biochar (ZrO2/BC) obtained from Camellia oleifera seed shell shows significant removal of F− ions from an aqueous solution (Mei et al. 2020). The bioadsorbent shows an adequate F− removal capacity of 11.04 mg/g at the initial concentration of 10 mg/L, with pH ranging from 3 to 9 with 180 min of contact time and 1.6 gm/L adsorbent dose. Alternatively, adsorption was reduced due to a decline in adsorption sites on the surface of the adsorbent with increased contact time. At the same time, an increase in adsorbent dose of more than 1.6 gm/L drops the adsorption capacity because of the low concentration of F− left over in the solution after adsorption. The pseudo-second-order kinetic model and the Langmuir adsorption isotherm model showed consistency in the adsorption process. The thermodynamic investigations have demonstrated that the adsorption phenomenon is an exothermic reaction that occurs spontaneously. It was concluded that ZrO2/BC effectively removes F− and reduces waste biomass.
The Cucumis pubescens peel of fruit was investigated for the removal of F− from contaminated groundwater (Kazi et al. 2018). The adsorbent indicated efficient removal of F− ions, nearly more than 90%, at a pH range from 3 to 8 with an initial concentration of 8–16 mg/L and 120 min of reaction time with optimum adsorption. A new adsorbent derived from Citrus limetta peels was activated with FeCl3 at two distinct pyrolysis temperatures, 250 °C (AC-CLP250) and 500 °C (AC-CLP500), studied for deflouridation (Siddique et al. 2020). The pseudo-second-order kinetic model and Langmuir adsorption isotherm model indicated consistency in the bioadsorption process. The thermodynamics studies revealed that the adsorption process was a spontaneous endothermic reaction. Langmuir isotherm model well fitted for monolayer adsorption of F− with 4.926 and 9.709 mg/g adsorption for both temperature conditions, respectively. The results indicated that the efficient F− removal efficiency was significantly improved with the rise in temperature ranges from 25 to 45 °C. All results concluded that AC-CLP could be utilized as a bioadsorbent to remove F− ions from the groundwater. Senewirathna et al. (2022) removed F− ions from groundwater samples using activated carbon derived from Palmyrah (Borassus flabellifer) nut shells (PAC). However, the maximum F− removal efficiency was 61% for PAC within 45-min contact time, 0.2 g adsorbent dose, and 1 mg/L initial F− concentration at 30 °C temperature. Further, the adsorption capacity of F− ions rises with an increase in adsorbent dose as the increased dose of adsorbent renovates the active adsorption sites. For the present study, the pseudo-second-order is well-fitted for the kinetics studies, which specify the chemisorption process. PAC could be another source of activated carbon, which is low-cost, eco-friendly, and feasible for efficiently removing F− ions from groundwater. Further, chemically modified domestic tea waste biochar also reduced F− contamination (Roy et al. 2018). The deflouridation was analyzed with varying adsorbent doses from 2 to 10 g/L, pH 2, and temperature (60 °C) for optimization of a central composite design (CCD) parameter using response surface methodology (RSM) and artificial neural network (ANN). Over raw tea waste biochar, the maximum F− removal observed in chemically activated biochar is 98.31%. The nature of the adsorption method was indicated as endothermic and spontaneous. Outcomes concluded that tea waste biochar is low-cost, eco-friendly, and feasible in tea growing areas and could be used as an efficient adsorbent for treating F− contaminated water. In a study, Abeysinghe and Baek (2022) reported on adsorbent made up of milk sludge biochar for the remediation of F− contaminated solution. Physiochemical properties of milk sludge biochar were determined by pyrolysis at a temperature of 500—800 °C to attain the maximum adsorption of F− 485.9 mg/g, and adsorbent removed 93.6% of F− ions when the concentration of F− 100.5 mg/L. The study findings indicate that milk sludge biochar exhibits potential for the remediation of wastewater that contains higher amounts of F−, such as those originating from semiconductor industries. Then, the treated wastewater could be fit for irrigation water applications based on the calcium and F− concentrations. Similarly, acid-activated coffee grounds adsorbent was used for a batch experiment to treat lead and F− from contaminated water collected from industrial waste (Naga Babu et al. 2018). The influence of many working parameters, covering the pH of a solution, biochar dose, temperature, recyclability, and time, was studied regarding removal efficiency. The optimal F− adsorption capacity was about 9.05 mg/g with 20 mg/L of initial concentration, pH 4, dose 0.2 g/100 mL, contact time 105 min, and temperature 30 °C. The experimental study concluded that the F− adsorption process trails the Langmuir isotherm model, signifying monolayer adsorption on the uniform surface. The established method can be effectively applied to domestic and industrial waste sources with inclusive adsorption of around 89% of the initial concentration of F−.
The activated carbon powder from waste palm shell (PSAC) vs. modified carbon with magnesium silicate (MgSiO3) was used to remove F− from in situ and ex situ groundwater (Choong et al. 2020). The highest possible F− adsorption capabilities were observed as 116 mg/g and 150 mg/g for PSAC and MPSAC, correspondingly. The Langmuir isotherm and Freundlich model fit for both activated carbons. A comparison study concluded that MPSAC is highly possible for ex situ wastewater treatment. Pillai et al. (2020) used silica nanoadsorbent (SRH) derived from rice husk for F− removal. Physical and structural properties of silica nanoadsorbent characterized through FTIR, XRD, SEM, and BET. Operational variable parameters affecting on removal efficiency of F−, also isotherm and kinetic study, were examined for the study. Results showed the maximum adsorption capacity of SRH with 72% at pH 8, 60-min residence time, a 4 g adsorbent dose, and 10 g/L initial F− concentration, respectively. The present study concluded that the adsorption capacity of F− and SRH bioadsorbent was intensely reliant on optimized factors. The banana peel powder purified F− from water (Mondal and Roy 2018). Further, desiccated peels of bananas were used as a base for water remediation in the column experiment. The observed results revealed an effective removal of F−, i.e., 86.5% with pH 5 and 10 mg/L initial concentration. At the same time, adsorbents also show an active surface area of 23.98 m2/g and 178.26% porous percentage. Similarly, Wang et al. (2018) studied for removing F− using pomelo peel biochar (PPBC-La) loaded with lanthanum. Approximately 82% of adsorption was achieved after 9 h. The findings of the experimental investigation indicate adsorption statistics were suitably well-defined via pseudo-second-order kinetic and Freundlich isotherm models. In comparison with raw PPBC, PPBC-La displays excellent anion exchangeability, enhancing its adsorption efficacy significantly. The PPBC-La has shown a remarkable F− adsorption of 19.86 mg/g. Manna et al. (2015) have successfully synthesized grafted jute as an efficient adsorbent for removing F−. The increase in F− content of the grafted jute powder was more significant when compared to that of the raw jute powder. The conclusions of the infrared and X-ray photoelectron spectroscopic analyses revealed that most F− accumulation on grafted jute was attributed to the hydrogen bonds, protons, and carbon-F− bonds. In another study, emerging adsorbents were developed for defluoridation using avocado kernel seeds (Salomón-Negrete et al. 2018). The experiment demonstrates that the pyrolysis process adsorbent showed the most superior adsorption characteristics. In contrast, its counterpart obtained with CO2 activation showed dropped efficacy in defluoridation, with the carbonization temperature as the principal synthesis parameter. Brahman et al. (2016) synthesized an adsorbent utilizing a stem of Tecomella undulata for biochar. The researchers studied the impact of pH, biochar dose, initial concentration, contact time, and temperature on the removal efficacy of biochar. The optimal reaction conditions were verified with an optimal adsorption capacity of 6.16 mg/g. Lv et al. (2006) used activated carbon from the Pithacelobium ducle plant. Due to the effective porous nature of activated carbon, which causes intraparticle diffusion, the F− adsorption was limited to physisorption. The adsorbent removal efficiency increases up to 81% at 5 mg/L initial concentration. Still, the process of removal displays an increased rate throughout the initial 40 min of the experimental period. The decline in adsorption rate can be due to the absence of accessible active spots on the surface of the biosorbent. This adsorbent validates the value of plant-based activated carbon as an active component in removing F−. Conversely, the concentration range examined was lesser, demanding further research on removal at greater doses. Mupa et al. (2016) conducted an experimental investigation using silica obtained from rice husk ash to eliminate F− content from water, focusing on its impact on algal biomass Pedistrum boryanum. The experimental findings indicate that the bioadsorbent adsorption capacity was determined to be 25.64 mg/g at pH 7. Carbonyl and hydroxyl functional groups have been observed to enhance adsorption, exhibiting a monitored pseudo-second-order kinetics optimally fitted to the Langmuir isotherm model. Getachew et al. (2015) involved synthesizing activated carbon through a combination of powder obtained from banana peel and coffee husk, which was subsequently improved with an injection of sulphuric acid. The bioadsorbent exhibited remarkable efficacy in reducing F− from a concentration of 10 mg/L, achieving removal efficiencies of 85% for banana peel and 86% for coffee husk. The bioadsorbent showed significant effectiveness in removing F− from actual water samples, with an observed efficiency of 80–84%. Therefore, this bioadsorbent may be considered one of the most promising materials for advancing a reliable water purification technology. In contrast, it is notable that using banana peel powder demanded a prolonged duration of approximately 13 h to achieve the state of equilibrium and attain the permissible threshold of F− concentration. The adsorption of F− experiences a substantial reduction under alkaline pH conditions, owing to the repulsive forces amongst the negatively charged surface and F− ions.
Mondal et al. (2015) demonstrated the efficacy of aluminum-impregnated coconut fiber in removing F− from an aqueous solution. The experimental findings show that the biomaterial displays a remarkable removal efficiency of 98% with a 0.05 g/L adsorbent dose, a reaction time of 60 min, and an alkaline pH of 12. The XRD and BET assessments show a 26.3 m2/g surface area, and the alum was evenly infused over the surface, which increases the porous nature of the biomaterial. As a result, an additional F− ions were adsorbed across the active surface. The adsorption process exhibited a pseudo-second-order kinetic behavior and conformed to the Langmuir isotherm model, particularly under heightened intraparticle diffusion. In contrast, the presence of SO42− and PO43− ions elicited a decrease in the removal efficacy to 81.3% and 66.2%, correspondingly. Seid (2017) reported that F− contaminated water can be treated by seeds of cabbage trees. Analyses of defluoridation were carried out at varied pH levels between 2 and 13 and F− concentrations between 1 and 40 mg/L at 20 °C. When F− ions and cabbage seeds encounter, the concentration F− ions, i.e., 10 mg/L, reduced to 3.4 mg/L concentration. Also, spectroscopical analysis depicts F− ions chemically adsorbed and creating a complete complexation on the surface of the seeds. After 60 min, a less active sorbent surface was available, which triggered the adsorption to decrease. The efficacy of Conocarpus erectus plant-derived biochar namely powdered biochar and granular biochar used for removing F− reported by Papari et al. (2017). The investigation displayed superior exclusion efficacy of 98% detected in PBC in comparison to 80% removal efficiency of GBC at different parameters like pH 6 and 20 °C temperature. The observed gaps in adsorption phenomena can be linked with increased PBC surface area. The PBC surface area was determined to be 9.88 m2/g, while the superior adsorption capacity of the material can be identified to a surface area of 5.16 m2/g for PBC. The presence of bicarbonate chloride, and nitrate ions had a visible impact on the adsorption of F− ions, thereby impeding the efficacy of the selective removal procedure. The present investigation confirms that adsorption is associated with the surface area of the biosorbent, which allows powdered biochar to create additional potential for adsorption owing to the reduction in particle size, thereby giving greater accessibility to the adsorption sites. Ravulapalli and Ravindhranath (2017) prepared activated charcoal using the Ficus racemosa plant barks impregnated with HNO3 for defluoridation. The addition of HNO3 helps to increase higher surface area for the adsorption of F− ions. Experimental findings showed 88% removal efficiency with a concentration of F− of 5 mg/L. The existence of additional competitive ions, precisely PO43−, impacts removal efficacy by decreasing from 88 to 75%. Also, the removal efficiency decreased at primary pH conditions due to opposing charges resulting in adsorption surfaces which electrostatic repulsion F− ions.
Citrus limetta (sweet lime) pulp was used for biochar preparation and was pyrolyzed at 250 °C and 500 °C (Ibrahim et al. 2019). Biochar was modified through an iron(III) chloride solution, represented as ACP-250 and ACP-500, for further processing. The prepared adsorbents could remove 82% and 86% of F− with a 12.6 mg/g removal capacity. Therefore, it can be concluded that modified biochar shows significant removal of F− ions from water. Similarly, Zhang and Huang (2019) experimented on a biosorbent made from grape pomace modified with Zr(IV). Bioadsorbent removed 96% of F− contents from water with 19.9 mg/L as an initial concentration. The experimental results indicated that the adsorbent performed well at pH 3 and showed noticeable single-use efficiency. Equally, at a pH of more than 5, the efficacy is reduced owing to the opposition amongst the F− ions and OH− ions which can bind the bioadsorbent practice at a developed scale. Further, Populus alba tree (PAAC) biomass was used for preparing activated carbon and applied to remove F− contents in simulated and real wastewater. Under 10 mg/L F− concentration, 4 g/L PAAC dose shows optimum removal of F− (93.37%). The ultrasonic-assisted PAAC’s monolayer adsorption capability was 77.12 mg/g (Bonyadi et al. 2019). An experiment on wheat straw (Triticum aestivum) to reduce F− content in water samples was conducted by Romar-Gasalla et al. (2017). Wheat straw F− adsorption may be caused by hydrogen bonding without releasing the −OH group. Additionally, residues containing F− and other elements found in wheat straw lead to complexes like CaF2 or TiF. Another study conducted by Deshmukh et al. (2018) used ash from the tea powder to defluoridate water. In addition, distinct sizes of tea ash material were used to analyze F− solutions with concentrations varying from 2 to 6 mg/L. Batch tests discovered material with a tiny particle size had better F− adsorption than the others because of its higher surface area and microporosity. As a result, this substance is promoted as being great for defluoridation. The bioadsorbent has been prepared using Mentha longifolia (mint) leaves to remove F− ions (Sunitha and Reddy 2018). The removal efficiency was observed at different factors like initial F− concentration pH, contact time, and adsorbent dose. Because of the larger surface area of the adsorbent particle, the adsorption capability of the biosorbent with a particle size of 1.4 mm revealed 95% removal of F− with 2–15 mg/L concentration at pH 2 to 10. Tirkey et al. (2018) synthesized a novel adsorbent using Jamun leaf ash (Syzygium cumini) having calcium oxide/hydroxide, calcium carbonate, and verified it for the F− removal process. The adsorbent displayed an admirable F− removal capacity of 4.56 mg/g and efficiency up to 77.8% within 60 min of residence time, having an initial concentration of 6 mg/L, pH 6.5, and 27 °C temperature. Likewise, the XRD shape exposed the feasible creation of Ca(OH)2 and F− complex, and there can be an opportunity for ion exchangeability with F− and OH− ions amongst F− and Ca2+. Later, the experiments were defensible. The adsorption of F− followed pseudo-second-order kinetics and involved intraparticle diffusion. In a batch experiment, Karmakar et al. (2018) used the F− Pistia stratiotes (water lettuce) to remove F− ions. At F− level of 5 mg/L, the best removal efficacy was detected as 38.89%. The F− growth ratio demonstrated that the biosorption process comprised F− bioaccumulation on the biosorbent’s surface, followed by full adsorption. Angelina Thanga Ajisha and Rajagopal (2015) used Delonix regia pods for preparing activated carbon to treat F− contaminated water. The seed pods were pyrolyzed over 1 h at 800 °C to produce activated carbon with a huge porous structure and surface area. In a 300-min contact time, the adsorbent revealed an enhanced removal efficacy observed as 98.4% at acidic pH 2, active dose of 1.5 g/100 mL, and initial F− content of 10 mg/L. With outstanding removal capacity, the experiment results followed the Freundlich isotherm and pseudo-second-order kinetics. Brahman et al. (2016) developed an adsorbent applying the stem of Tecomella undulata. The researchers investigated the impact of pH, biochar dose, starting concentration, contact time, and temperature on biochar’s removal capacity. An optimal reaction condition was discovered, resulting in a maximum adsorption capacity of 6.16 mg/g.
Bombuwala Dewage et al. (2018) investigated the effects of alpha-Fe2O3 and Fe3O4 on high surface area Douglas fir biochar in treating nitrate and F− ions in an aqueous solution. The Freundlich and Langmuir isotherm models were applied for modelling the process of adsorption within the temperature range of 25 to 45 °C. F− Langmuir adsorption capability was determined to be 9 mg/g. Yu et al. (2015) also looked at the potential of a novel lanthanum-modified carbon (LMC) adsorbent derived from Sargassum species. During the initial hour, F− adsorption reached up to about 90%. The findings from the kinetic investigation revealed that both the pseudo-second-order model and the Langmuir isotherm model effectively defined the experimental data. LMC has a substantially greater adsorption capacity comparing several commercial adsorbents, which is 94.34 mg/g at neutral pH. The F− removal efficacy of Musa paradisica carbon modified with Ti(IV) was compared to the original material (Vilakati et al. 2019). The modified material is capable of removing 99% F− ions, whereas the original material observed a removal efficiency of 90% at an initial concentration of 2.05 mg/L at 40 °C. The material adsorption capacity for F− ions was found to decrease under basic pH conditions, primarily due to the presence of repulsive forces in between the detrimental surface spots and F− ions. The adsorption capacity was found to be significant at a pH of 2.04, which can be attributed to the protonation of hydroxyl groups. This protonation leads to electrostatic interactions with fluoride ions (F−). In contrast, when the temperature was set at 50 ºC, the efficiency of F− removal decreased to 52%. Additionally, it was observed that the presence of Cl ions had a detrimental effect on the removal process. The potential for broader application of biosorbents may be constrained by this limitation. Shen et al. (2021) experimented on four magnetic biochar (mBC) derived from rice husk through pyrolysis with pristine magnetic biochar and three other aluminum (Al) and magnesium (Mg) oxides-bonded with magnetic biochar (i.e., Al-mBC, MgmBC, and MgAl-mBC) for dual removal of F− and AsV ion from wastewater. Observed results reveal that F− and AsV adsorption on four mBC depended on pH condition. Both ions competed for adsorption, resulting in lower adsorption capacity than single ion adsorption. According to the findings of an experimental study, MgAl-mBC demonstrated the highest adsorption capacity for AsV and F− ions. Specifically, at a pH of 5 and a temperature of 10 °C, the maximum adsorption capacity was observed to be 34.45 mg/g for AsV ions. Similarly, at a pH of 5 and a temperature of 30 °C, the optimum adsorption capacity for F− ions was observed as 22.11 mg/g. However, the MgAl-based mBC indicates superior AsV adsorption capabilities compared to other mBCs, showing an optimum adsorption capability of 24.30 mg/g. Under the dual condition of adsorption process, it was observed that Al-mBC showed the highest adsorption capacity for F− ions, specifically 19.41 mg/g at a pH of 5 and a temperature of 30 °C. On the other hand, MgAl-mBC revealed even greater enhancement in AsV adsorption compared to other mBCs, displaying a higher adsorption capacity of 24.30 mg/g at a pH of 5 and a temperature of 30 °C. The adsorption isotherm was effectively described by the Langmuir and Freundlich models. The study findings indicate that four modified biochars (mBCs) examined in the research have significantly higher adsorption capacities for AsV and F− compared to other types of biochars commonly used for the remediation of contaminated water. De et al. (2018), attempted a study on the applicability of engineered biochar (EBC) derived from de-oiled Pongamia pinnata waste lignocellulosic biomass seed cake for adsorption of F− from contaminated groundwater. The batch adsorption experiment and EBC characterization studied several parameters like pH of the solution (3–10), initial F− concentrations (5–20 mg/L), and biosorbent dose (0.2–0.7 g) to get optimum equilibrium conditions. The maximum removal efficiency was obtained during 90 min of contact time, pH 7, and bioadsorbent dose of 10 mg/L, respectively. The Langmuir isotherm and pseudo-second-order kinetic models were well-fitted for the study, showing an optimum adsorption capacity of 0.985 ± 0.025 mg/g. The fixed bed column mode caused due to breakthrough curves which were fitted for various mathematical models, and in that mode, the modified dose–response model showed the peak fit with an adsorption of 1.12 ± 0.025 mg/g. Halder et al. (2015) investigated a batch adsorption study on powdered form activated carbon (PAC) derived from the stem of Eichhornia crassipes through steam activation to remove F− ions from synthetically fluoridated water and wastewater. Adsorption process and experimental matrix were optimized by RSM and central composite rotatable design (CCRD). The impact of several factors like adsorbent dosage (2–12 gm/L), pH (2–12), contact time (20–180 min), revolutions per minute (100–300 RPM), and temperature (20–60 °C) were investigated for efficient removal of F− ions. The physical properties showed that surface area as well as total pore volume were approximately equivalent to 97.68 m2/gm and 0.5185 cm3/gm, respectively. The optimum adsorption of F− ions was found at biochar AC dose 7 gm/L, pH 2, temperature 40 °C, RPM 200, and contact time 100 min, respectively. The analysis of variance (ANOVA) presented that the chosen model is well-fitted for the experimental data. Results concluded that removing F− ions using activated carbon derived from E. crassipes stem could be used as a low-cost, eco-friendly, and prominent biomaterial for treating fluoridated wastewater. Similarly, Saikia et al. (2017) used perennial grass (Saccharum ravannae L.) for fabricating activated biochar for removing As and F− by RSM, which is based on CCD. The effect of parameters such as initial concentration, biochar dosage, and contact time on As and F− adsorption removal effectiveness was examined. Among all parameters, biochar adsorbent dose was the maximum effective parameter for removing As and F− from water. Experimental results of CCD showed that activated biochar might have higher removal efficiency for As (72.1%) and moderate (24.80%) for F− from an aqueous solution. Chemisorption types of the adsorption process were predicted for isothermal and kinetic models. Pseudo-second-order model well fitted for both As and F−. Results showed that the activated biochar might be a favorable, cost-effective, and eco-friendly adsorbent for As and F− removal from contaminated water solution. The recent biomaterials used for removing F− ions are presented in Table 2.
Parameters affecting F− removal
The optimum F− removal efficiency of any adsorbents is very much needed for its commercial usage, and its efficiency depends on various parameters such as initial F− concentration, pH, adsorbent dose, contact time, and temperature. Thus, the estimation of the optimal level of individual parameters is important to develop an effective F− removal technology.
Initial F− concentration
Initial F− concentration is one of the important parameter that affects F− removal efficiency in adsorption studies. Adsorption study helps to define the adsorption capacity of the material, as higher initial concentrations may saturate the available sites present on adsorbent surface within a short period. Moreover, it permits investigation of the connection between initial F− concentration and the rate of adsorption which results in identifying optimal conditions for effective removal. Furthermore, studying initial concentrations leads to evaluating the efficiency and effectiveness of the adsorbent material in real-world scenarios where F− concentrations may vary. Therefore, understanding the initial concentration effect is essential for designing water treatment systems and implementing appropriate strategies for safe drinking water supply in F− affected regions. Eventually, an increase in F− ion concentration increases removal efficiency by providing a higher mass transfer driving force in an adsorption phenomenon. Recent studies by Senewirathna et al. (2022) reported using Palmyrah nut shells derived activated carbon activated with H3PO4 and NaOH for F− removal with a varying F− initial concentration 0.5 to 2.0 mg/L with 61% of removal efficiency value observed at 1 mg/L concentration. Similarly (Sadhu et al. 2022), watermelon rinds biochar used as adsorbent for F− removal reveals maximum adsorption at 50 mg/L with an adsorption capacity of 9.5 mg/g with a variable initial concentration within a range 5–100 mg/L. Adsorption of F− decreases with increase in the initial concentration of F− ions due to less availability of active sites for adsorption for constant adsorbent amount. In another study, Citrus limetta peel used as carbon, observed significant F− adsorption on adsorbent surface when initial F− concentration was low (5 mg/L) compared to the higher concentration (50 mg/L) due to available active sites on the adsorbent surface not sufficient to accumulate the ions under higher concentration of F− (Siddique et al. 2020). Yitbarek et al. (2019) investigated the effect of variable initial F− concentration between 5 and 50 mg/L, by keeping other parameters constant. It was observed that higher adsorption of F− ions took place at lower concentrations and vice versa, which validates that the adsorption capacity of biomass becomes shattered gradually with a rise in initial F− concentration. The adsorption capacity has been progressively improved concerning cumulative initial F− ion concentration until equilibrium adsorption capacity was attained. Recently, Khan et al. (2022) developed a modified pinecone biochar with AlCl3 showing an optimal adsorption capacity of 14.0 mg/g at 40 mg/L with an increase in the concentration of F− ions due to more cooperative adsorption of biochar in which initially the F− ions form coverage on the active sites of the biochar surface, followed by interaction between F− molecules with cumulative initial concentration. The influence of adsorbent was deliberated with a variable initial concentration of F− ion (8–24 mg/L), keeping other parameters constant. Similarly, the peel of fruit biochar used for defluoridation depicts an increasing trend of adsorption capacity from 1.53 to 2.95 mg/g with increasing initial F− ion concentration (8–16 mg/L). Also, observed results show that at higher F− concentrations, a negligible effect was seen on adsorption capacity values because of restricted available active sites on the adsorbent surface (Kazi et al. 2018). Overall, studying the effect of initial F− concentration on the removal efficiency in adsorption processes provides valuable insights for process optimization, performance evaluation, compliance with regulatory standards, understanding kinetics and equilibrium, and assessing cost-effectiveness and scalability.
pH solution
Fundamentally, the pH of an aqueous solution is influenced by the atmosphere and environmental features of the soil (Hegde et al. 2020). The study on effect of pH in F− adsorption is essential since it directly affects the removal efficacy and adsorption capacity. The surface charge of an adsorbent material is pretended by the pH of a solution, which influences its ability to attract and bind F− ions. Understanding the pH dependency helps to optimize the conditions for maximum F− removal. Moreover, pH affects F− ion speciation in solution, with various forms showing different adsorption behaviors. Numerous literature reports are available where the removal process of F− ions was noticed within an extensive pH range. At the same time, more or fewer adsorbents might effort purely with a definite range. Usually, the defluoridation rate lowers with a rise in alkalinity of the pH range of the solution (Naga Babu et al. 2018). Thus, it is essential to study the significance of pH throughout the defluoridation process. The modified biochar powder observed 93.85% removal efficiency with 4.69 mg/g adsorption capacity nearly up to neutral pH 6 with an increasing trend of pH variations from acidic to alkaline state (4–10) due to special effects on the surface charge of the biochar. Also, H+ ions can create obstruction with adsorbate ions regarding the binding of active sites of the adsorbent surface (Bhaumik et al. 2017). Halder et al. (2016) synthesized a modified steam-activated bioadsorbent from Cocos nucifera shell and observed 79.30% maximum removal efficiency at neutral pH 6.27, and it started declining the F− removal. The reduced F− removal was observed due to competition between hydroxyl ions and active adsorption sites.
Adsorbent dosage
Studying the effect of adsorbent dose in the F− removal is essential for numerous reasons. It helps to determine the optimal amount of adsorbent dose required to attain efficient F− removal. By varying the adsorbent dose, researchers can evaluate its influence on the adsorption capacity and efficiency of the process. However, understanding the relationship between adsorbent dose and F− removal can aid in cost optimization by identifying the minimum required dose for effective treatment. Moreover, it also provides insights into the kinetics and mechanisms of the adsorption process, enabling the development of improved F− removal technologies. Finally, it allows for the assessment of any potential adverse effects or limitations associated with high adsorbent doses, such as increased pressure drop or interference with other water treatment processes.
The effects of adsorbent dose varying from 2 to 10 g/L showed maximum removal efficiency at 10 g/L dosage with 98.31% removal within 120 min of reaction time. The removal of F− ions started reducing beyond 10 g/L of dose due to the saturation of active sites on the adsorbent surface (Roy et al. 2018). In another study, the Eichhornia crassipes stem used for the development of activated carbon shows an increasing trend of F− removal with increased adsorbent dose up to equilibrium condition; after that, it started reducing due to conglomeration of particles. The maximum removal efficiency, i.e., 65.06% at the adsorbent dose of 7 g/L, was obtained while keeping all other factors constant. The reduced removal efficiency of adsorbent may be due to overlying active sites at advanced doses (Halder et al. 2015). Similarly, Palmyrah nutshells used as adsorbent for removal of F− displays maximum removal at 0.20 g of adsorbent dose with 61% removal efficiency (Senewirathna et al. 2022). The F− adsorption increases as the adsorbent dose increases and once increased in adsorbent dose renovates active available sites for F− adsorption. Pillai et al. (2020) conducted a batch experiment study for defluoridation with an adsorbent dose varying from 1 to 4 g/L. The removal efficiency was observed, i.e., 72% up to 4 g/L, and then declining towards constant value due to the absence of active spots on the adsorbent surface.
Contact time
Contact time is an important parameter in F− removal process. It refers to the reaction time for which the adsorbate is in contact with the adsorbent material. Adequate contact time permits the adsorbent to interact effectively with the F− ions, leading to their effective removal from the water. Inadequate contact time can result in partial adsorption and ineffective removal efficiency. Equally, extremely long duration may not significantly boost the removal efficiency and can lead to unnecessary delays in water treatment processes. Also, inspecting the effect of contact time is necessary, which is vital to attain equilibrium conditions for designing batch-type experiments and continuous column experiments. Therefore, optimizing the contact time is essential to ensure efficient and economically feasible F− removal. Bhaumik et al. (2017) reported 98.90% removal efficiency within a contact time of 60 min and adsorption of F− ions started decreasing beyond 60 min due to the unavailability of active sites on the adsorbent surface after the equilibrium condition. Similarly, activated biochar produced from perennial grass to remove F− with a contact time of 60 min, keeping the initial concentration and adsorbent dosage constant. The findings indicate a positive correlation between the adsorption rate and contact time, with the highest removal efficiency of 24.80% being attained after 40 min of reaction time. This can be attributed to the saturation of active sites on the surface of the adsorbent (Saikia et al. 2017).
Temperature
Usually, the temperature is not considered an effective parameter in defluoridation process due to its exothermic nature; thus, it operates in a range between 20 and 35 °C. Moreover, when the temperature range exceeds 50 °C, it starts affecting adsorption in different environmental conditions (Hegde et al. 2020). Mostly, F− adsorption studies focus on equilibrium adsorption capacities rather than temperature-dependent adsorption behavior. Temperature is often expected to have a nominal effect on the adsorption process, predominantly for solid adsorbents. However, adjusting and measuring temperature precisely throughout the experiments can be challenging. Overall, considering temperature would need extra resources and experimental circumstances, making it less practical for many researchers in this field.
There are multiple parameters which affect the removal efficiency and adsorption capacity of bioadsorbents as discussed in the above section. Most of the existing research and studies only focused on batch experiments rather than its scalability for large-scale adsorption systems. However, the adsorption capacity of any bioadsorbents directly depends on the pH level of water. This mainly happens due to impacts on the surface charge of the adsorbent and the ions present in the adsorbate. Furthermore, the pH of water significantly affects the adaptability of the bioadsorption process and its operating cost. Hence, adjustment of pH level of water from neutral to acidic or neutral to basic for a large adsorption processing unit to get an optimum pH condition is quite time consuming and increases operational costs for multiple chemicals and monitoring apparatus. Thus, recognizing the impact of adsorption processes on the pH level is important for effective removal of contaminants and for managing the scalability/commercialization of the adsorption operating system.
A study conducted by Meshesha Tulu et al. (2018) used a peanut husk biochar to remove F− and obtained 82.3% at an acidic pH level of 3 for the batch experiments. However, it is not possible in practical conditions and large-scale operations to maintain acidic conditions of water, because it requires more time, multiple chemicals, skilled technicians, etc. and further it requires double purification to maintain its neutral pH range for domestic or other uses. Similarly, the impact of temperature on removal studies depends on the type of adsorbent and adsorbate. It also affects the large-scale operation system of adsorption processes due to high energy consumption. Most of the studies confirmed fluoride’s maximum removal at temperatures ranging from 25 to 40 °C. In a recent study, moringa pods biochar achieved the maximum removal efficacy of 97.5% at 27 °C (Vani et al. 2024). Later, Bharali and Bhattacharyya (2015) developed a biochar which removed almost 80% of F− ions from water at 30 °C, and it was observed that removal efficiency decreases as the temperature rises due to the weakening of adsorptive forces between adsorbent and adsorbate. Likewise, contact time is also a dominant parameter which affects the removal efficacy and adsorption capacity of bioadsorbents. In general, extending a contact time increases the adsorption and removal of adsorbate up to a certain limit, but it then gradually decreases due to unavailability of active sites on the adsorbents. Thus, identifying an equilibrium contact time for the adsorption process is important for the efficient utilization of resources and minimize the operational costs of the system in practical application and scalability.
Bioadsorbents used for As removal
The cotton stalk is used as biochar for removing As(III) from contaminated groundwater (Ahmad et al. 2022). Factors like reaction time, cotton stalk biochar (CSB) dose, and initial As(III) concentrations were considered for adsorption investigations. According to the experimental findings, the highest possible As(III) ability to adsorb was 0.0899 mg/g from 0.2 mg/g concentration with a contact time of 120 min, pH 6. As(III) removal effectiveness lowers as As(III) concentrations’ rise in contaminated groundwater. With an excessive amount of As 200 g/L, the capacity to absorb CSB declined concurrently with a rise in adsorbent dose, i.e., 89.70 g/g and 41.30 g/g at 1 and 3 g/L, respectively. The results of the FTIR analysis showed that the CSB surface contained a variety of functional groups, including amide, carboxyl, hydroxyl, and ketone groups, and AsO3−3 was removed from the water via ion exchange or related processes. The current study shows that adequate adoption capability increases As(III) removal efficiency by about 88%. Mukherjee et al. (2021) produced biochar from rice straw (RSBC) using a gradual pyrolysis technique at 600 °C. The batch adsorption investigation was carried out for variables that could impact, such as adsorption dose, initial concentration, pH, and reaction contact time. The findings of batch adsorption show that 120 min was the time at which maximum adsorption occurred. At a pH of 7, As(V) removal efficiency peaked at more than 3.43%. The initial As(V) concentrations were increased from 10 to 100 g/L, and as a result, the adsorption capacity increased as well from 4.53 to 13.1 g/g. Further, the removal efficiency was reduced from 64.5 to 9.23%, which was made possible by lengthening the particle dispersion path and ensuring more collisions between the individual char particles. The findings of this study demonstrate the viability of utilizing this pyrolysis waste as a powerful new electron-exchanging adsorbent for removing metalloid pollutants from water. Another study conducted by Nguyen et al. (2022) synthesized iron-coated biochar from pomelo peel (PPCI) via slow pyrolysis for the application of removal of As(III) and As(V) from contaminated solution. The characterization of PPCI adsorbent for textural, morphological, surface charge, and surface function with the PPCI adsorbent before and after adsorption was accompanied. According to the experimental findings, As(III) and As(V) reached their maximum adsorption capacities in pH 7, estimated with the help of the Langmuir model depicted As 11.77 mg/g and 15.28 mg/g for both anions. Due to the inner-sphere complexation of As species by Fe in PPCI, PPCI’s adsorption capacity for As(III) and As(V) was found to be substantially more significant in comparison to raw pomelo peel (PP) at 0.033 mg/g and 0.034 mg/g, respectively.
Wen et al. (2021) synthesized porous biochar activated with MnFe2O4 magnetic nanocomposite (BC-MF) to instantly remove both organic and inorganic As species from contaminated water. The experimental outcomes showed outstanding effectiveness for removing p-ASA and As(V), and the p-ASA and As(V) capacity for adsorption were estimated to be approximately 105 and 90 mg/g at an ideal concentration of 10 g/L. With pH ranges from 3 to 7, p-ASA and As(V) were successfully adsorbed, and their adsorption mechanism was accelerated by di- and mono-anionic species of these compounds. According to the characterization findings of the FTIR and XPS examination, removing p-ASA and As(V) was demonstrated to be accomplished via electrostatic attraction and surface complexity. Still, the reduction of p-ASA might also be achieved by hydrogen bonding and π-π interactions. Niazi et al. (2018a) used oak tree wood biochar (OW-BC) in a batch experiment investigation for removing both arsenites As(III)) and arsenate As(V). An adsorbent made from oak wood char was used to extract As(III) and As(V) from the simulated solution and contaminated groundwater. The experimental results show that OW-BC can effectively eliminate As in both situations. The Langmuir model was successfully fitted for both forms of As adsorption at 3.06 and 3.89 mg/g, correspondingly. Similarly, Ahmed et al. (2021) investigated innovative phosphorus (P) biochar modification (PLBC) synthesized via pyrolysis of the dietic herb Taraxacum mongolicum Hand-Mazz (TMHM) and treated with monopotassium phosphate (KH2PO4). As(III) was remediated from contaminated water with modified biochar As an adsorbent. SEM–EDX, TEM, FTIR, and XRD were used to examine biochar before and after P loading. Compared to raw biochar and P-loaded biochar (PLBC), PLBC demonstrated superior As(III) adsorption outcomes. The experimental data best fitted for Langmuir and pseudo-second-order models. Lin et al. (2019) used an impregnating approach to develop a new Fe–Mn-La-impregnated biochar composite (FMLBC) used for treating arsenite contaminated water. According to the findings, the capacity for adsorption of FMLBC (14.9 mg/g) was significantly higher than that of raw biochar (3.73 mg/g) and impregnated biochar (9.48 mg/g). NO3 and SO42 ions did not affect As adsorption, but PO43 favored As elimination. The kinetic adsorption of As on FMLBC was well defined by a second-order model. Luo et al. (2019) produced ultrasonic biochar from waste corncob and then loaded with TD nanoparticles. The experimental batch study examined the influence of initial concentration, pH, adsorbent dosage, and co-existing ions. Results show that the maximum adsorption capacities of observed As (118.06 mg/g) were considerably higher compared to other carbon materials. Experiment results revealed that the As(V) adsorption capacity was above 70% within pH 5.
In a laboratory experiment, Verma et al. (2019) utilized dry waste leaf litter from Tectona and Lagerstroemia speciosa to make biochar, which was subsequently used to remove As(III) and As(V) from water. The data from experiments linked satisfactorily to the Freundlich model and Temkin model for As(III) by LB 800 and TB 800, respectively. Similarly, the Langmuir model was successfully linked for As(V) exclusion using TB 800 and LB 800. The kinetics study indicated that removing As(III) and As(V) adsorption involved endothermic and exothermic processes. The adsorption capacities for As(III) and As(V) were determined as 666.7 and 454.54 g/g for TB 800 and LB 800, and 1250 and 714.28 g/g for TB 800 and LB 800. Further, the use of raw biochar derived from aspen wood (WB) and rice husk (RB) was pyrolyzed at two different temperatures of 400 °C and 700 °C to remove calcium-As compounds (Zama et al. 2022). The findings demonstrate the biochar formation at pH 6.5, where As(III) removal and Ca/As precipitation dropped to 58.1% of As(III) on wood biochar versus 25.4% in solutions without controlled biochar. Observations showed that rice husk biochar (RB4 and RB7) had removal rates of 3.2% and 2.9% at pH 6.5, whereas wood biochar (WB4 and WB7) were better at holding precipitates with removal rates of 0.1% and 0.3%, respectively. The outcomes show that biochar can effectively enhance Ca/As precipitation by eliminating As(III) from water without modification. Similarly, in order to remove As from an aqueous solution, Wang et al. (2015) developed magnetic biochar pinewood biomass and naturally generated hematite mineral. Hematite-modified biochar performed significantly better at removing As in a study than unmodified biochar, probably because the c-Fe2O3 particles on the carbon surface acted As sites of sorption via electrostatic attraction. Zhang et al. (2017) assessed As(V) adsorption using water hyacinth biochar (MWBC), made by impregnating iron on MWBC. The pyrolysis temperature varying 250 °C and 450 °C were considered for the experiment. The MWBC2501 demonstrated the maximum As(V) adsorption capacity of 7.41 mg/g. As removal declined as pH increased more than 10, the removal amount of MWBC2501 stretched up to 100% with a pH range between 3 and 10. This is due to significant interaction among HASO42− and OH ions in an alkaline pH spectrum. The adsorptive capability of As(III) 2.0 mg/g and As(V) 3.1 mg/g were improved. In an experiment, biomass obtained from cottonwood was used to remove As (Zhang et al. 2013). While preparing magnetic biochar, cottonwood was pre-treated using FeCl3H2O and pyrolyzed at 600 °C heating temperature. Magnetic biochar was found to have an adequate adsorption capacity of 3.15 mg/g. According to the findings, As adsorption occurred primarily through the surface binding of metal oxide to As saturated at the outermost layer of biomaterial, which was potentially identified by the generation of mono and bidentate complexes. The study by He et al. (2018) involved synthesizing and characterizing ferrous-impregnated biochar intending to remove As from water. The present investigation attempts to assess the ability to absorb iron modified maize straw biochar compared to original straw biochar. Investigations indicate that iron modified maize straw exhibits a remarkable capacity for absorption (6.80 mg/g) for As(V) due to its large surface area, thermal resistance, and reactivity of functional groups, above that of pristine biochar (0.017 mg/g). Similarly, Lin et al. (2017) investigated on uptake of As through virgin maize stem, iron magnesium modified maize stem (FMBC), and Fe/Mn dual oxide biochar (FMO). The utilization of iron-magnesium oxides resulted in a significant enlargement of the surface area of biochar, thereby improving its capacity to absorb for As(III) (8.25 mg/g) in FMBC, in comparison to the unmodified biochar together with other sorbents. The iron oxides present on the biochar surface exhibited a strong affinity towards As(V). Alkurdi et al. (2019) conducted a comparative study to check the efficacy of biochar as well as bone char for eliminating As. In the current investigation, the pinewood biomass was subjected to pre- and post-pyrolysis therapies through dual metal ions of iron and magnesium to facilitate the elimination of As(V) from the water-based medium. The experimental results indicate the As(V) sorption capacity of the post-pyrolysis pinewood biochar was significantly higher (3.44 mg/g) than the original pinewood biochar (0.50 mg/g). Co-precipitation process appears as an important part of the genesis of bimetallic oxides As opposed to the formation of binary metal oxides on the biochar substrate. This phenomenon may have resulted in an augmentation of the As(V) sorption capacity. The principal mode of As(V) retention is governed by electromagnetic attraction and surface complexity. Similarly, Wang et al. (2016) studied on pinewood biochar mixtures by implementing pre- and post-pyrolysis remedies with MnCl2·4H2O and Ni(NO3)2·6H2O. The experimental findings indicate that the pre-treated Ni/Mn biochar’s surface area was 125 m2/g. In contrast, the post-treated Ni/Mn biochar exhibited a surface area of 282 m2/g. The pristine biochar exhibited a substantially reduced adsorption capacity for As. Therefore, the impregnation of biochar with Ni and Mn oxides significantly enhanced As(V) retention. The observed phenomenon of As(V) adsorption can be attributed to assembling inner-sphere complexes, wherein mono- and bidentate complexes with –OH groups play a crucial role. The dominant accumulation mechanism observed in this study is due to electrostatic attraction for Ni/Mn pristine biochar. At the same time, surface complexation and anion exchange were found to be the primary mechanisms for Ni/Mn pristine biochar. The study’s findings indicate that the maximum adsorption capacity of As was observed as 0.513 mg/g and 6.52 mg/g for As(V) by Ni/Mn pristine and Ni/Mn modified biochar correspondingly. Further, Frišták et al. (2018) pertained to the chemical activation of grapefruit seed biochar through nitric acid to remove As from a replicated sample. The results indicate that the nitric acid modified biochar exhibits a significantly improved adsorption capacity of 34.9 mg/g, which is 6.8 times greater compared to that of the pristine grape seed biochar (GSBC) with an adsorption capacity of 5.1 mg/g. However, the adsorption capacity of modified biochar is 0.9 times less than air-activated biochar, which has an adsorption capacity of 39.4 mg/g. Further, corn stalk biochar modified by phosphoric acid and potassium hydroxide is used for treating As-contaminated water (Hussain et al. 2020). The functional groups identified in acid-modified cornstalk were alkynes, alcoholic, alkyl halide, and amide. The Fourier transform infrared (FTIR) spectra obtained from the cornstalk biochar (BCSB) treated with potassium hydroxide (KOH) revealed the presence of various functional groups such as phenolic, alcoholic carboxylic, amide, ester, and others. The results indicate that the BCSB exhibited a moderate increase in As removal efficiency (ranging from 90 to 99%) compared to the ACSB (ranging from 87 to 98%) under the experimental conditions of pH 5. In another study, hematite-modified pinewood biochar (HMB) used for removing As from water (Wang et al. 2015). The biochar was synthesized using pine wood and hematite (Fe2O3) suspension by pyrolysis at a temperature of 600 °C. The surface areas of HPB and PB were observed to be 209 and 1193 m2/g, respectively. The adsorption capacity of HPB for As was approximately twice As high As that of PB, with a value of 429 mg/g compared to 265 mg/g, respectively. The thermal processing of natural hematite resulted in the conversion of the crystal structure to ɣ-Fe2O3, which showed exceptional binding affinity towards As and, consequently, enhanced the As removal efficiency of HPB. Mohan et al. (2007) studied biomass modified with AlCl3 solution and subsequent pyrolysis at 600 °C. The resulting product was a biochar/AlOOH nanocomposite employed to remove As(V) from water-based media. The Langmuir model has successfully predicted the optimum adsorption capacity of As(V) on biochar/AlOOH nanocomposite, which has been determined to be 17.4 mg/g. This value is comparatively higher than the sorption capacity of Al2O3, which ranges from 0.17 to 8.9 mg/g and is similar to that of activated Al2O3, which ranges from 9.2 to 15.9 mg/g. It is imperative to acknowledge that AlOOH exhibits sustainable properties, and its nanoparticles tend to aggregate in aqueous solutions, thereby decreasing its adsorption efficiency and surface area. After impregnation onto the biochar substrate, the AlOOH flakes are evenly distributed across the surface, thereby producing heightened As adsorption. Van Vinh et al. (2015) developed raw biochar from pine cones at a temperature of 500 °C. The biochar was eventually modified via Zn(NO3)2 loading to assess its adsorption potential for As(III) in aqueous solutions. The experimental results indicate that the pine cone biochar, loaded with Zn, exhibited a significant increase in both pore size (0.028 cm3/g) and surface area (11.5 m2/g) in comparison to the unmodified biochar having pore size 0.016 cm3/g and surface area of 6.6 m2/g, respectively. The present investigation evaluated the adsorption options of modified pine cone biochar with Zn and raw pine cone biochar, which were observed as 0.007 mg/g and 0.0057 mg/g, respectively. Bismuth (Bi)-impregnated biochar was synthesized by impregnating rice husk material with Bi oxide solution and subsequently pyrolyzing it at varying temperatures (400 °C, 500 °C, and 600 °C) (Zhu et al. 2016). The Bi-BC500 composite material, comprising bismuth nanoparticles covered on biochar, showed a substantially high superficial area of 190 m2/g and porous size of 0.019 cm3/g at 500 °C. The present study clarified that the maximum adsorption of As(III) onto Bi-BC500 was observed at pH 9.3, with an adsorption capacity of 16 mg/g. This value was significantly higher than the unmodified BC500 and several other adsorbents extensively documented in the scientific literature. The ligand exchange mechanism played a crucial part in the detoxification of As from contaminated water by Bi-BC500.The pyrolysis of municipal solid waste (MSW) biomass at 500 °C followed by activation of the resultant biochar using a 2 M KOH solution was conducted by Jin et al. (2014) to eliminate As(V) from contaminated aqueous solution. The application of potassium hydroxide treatment resulted in a visible enhancement of phenolic-hydroxyl groups, as evidenced by the developed spectral peak at a point of 3352/cm, along with carboxylic groups at 1015/cm. The experimental findings show 49 m2/g surface area for KOH activated biochar which is three times more compared to the raw biochar of 14 m2/g area. The KOH-activated MSW-500 biochar exhibited a superior adsorption capacity for As(V) with a maximum value of 30.9 mg/g 1.3 times greater than the pristine MSW-500 biochar capacity of 24.5 mg/g. Wu et al. (2018) conducted a study for applying Fe(II) and CaCO3 in different proportions, along with rice husk biomass that undergone pyrolysis at 400 °C. The objective was a modification in rice husk biochar with Fe(II) and CaCO3 to remove As(III) from the solution. The proportion of 0.1 M of Fe(II) and 1% of CaCO3 exhibited a maximum adsorption capacity of 6.34 mg/g for As(III). The calcium-modified red hectorite clay (Ca-MRHBC) exhibited As(III) sorption through electrostatic attraction, complexation, and physical adsorption mechanisms. In a separate investigation, the adsorption of As by unmodified maize stem biochar, as well as modified maize stem biochar that has been modified with iron/manganese (Fe/Mn) (FMBC), and iron/manganese binary oxides (FMO), was assessed. The improvement in surface area of biochar by Fe/Mn oxides resulted in a 8.25 mg/g adsorption for As(III) related to unmodified biochar and other sorbents. The surface-bound iron oxides exhibited a pronounced affinity towards As(V). In contrast, the presence of Mn oxides facilitated the removal of As(V) by encouraging the oxidation of both As species. Thus, the Fe–Mn oxides played a pivotal part in improving the As adsorption capacity of biochar. The utilization of bamboo biomass as a raw material for the fabrication of chitosan and zero-valent Fe-supported biochar (CZVI-biochar) was investigated by Zhou et al. (2014). Similarly, eight distinct ZVI-biochar composites (BBCF) were synthesized by evaluating diverse permutations of the chitosan: Fe mass ratio of biochar. When a ratio of 1:1:2 combined to prepared biochar, the BBCF exhibited a remarkable 96% depletion of the As(V) which is significantly greater than that of the pristine and chitosan-modified biochars. The notable As(V) removal efficiency achieved by the biochar-based composite material at a 1:1:2 ratio can be associated with the electrostatic attraction of As(V) and ZVI nanoparticles on the surface of the composite biochar. Zhou et al. (2017) report the development of a novel, sustainable, and economically viable biochar-based sorbent. The adsorbent was synthesized by combining chestnut biochar and pyrolyzed at 450 °C, with gelatin and iron oxide solutions. The synthesized biomaterial was subjected to experimental investigation to evaluate its efficacy in eliminating As(V) from water. The scanning electron microscopy (SEM) images of MG-CSB revealed the presence of tiny balloon-shaped structures on its surface, prominently missing on the outer surface of the raw biochar. They demonstrate the existence of gelatin moieties and Fe(III) on the surface of biomaterial. The maximum sorption capacity was observed for As(V), i.e., 42.7 mg/g by MG-CSB which was significantly higher than raw CSB and other sorbents. A synthesis of nano-scale zero-valent iron and biochar was conducted by infusing nZVI particles onto pinewood at a 600 °C pyrolysis temperature (Wang et al. 2017). The total area of nZVI/BC composite material, which has a specific surface area of 211.7 m2/g, was comparatively poorer than unmodified pinewood biochar, which has a specific surface area of 334.8 m2/g. This phenomenon can be attributed to the presence of impregnated nZVI, which may have partially blocked the pore spaces on the biochar surface, leading to a reduction in the surface area by 13.6 m2/g. The nZVI/BC composite demonstrated remarkable adsorption effectiveness towards As(V) (124 mg/g) due to the nZVI infused on biochar. The removal of As using novel encapsulated biochar via zirconium hydroxide (ZBC) was studied (Peng et al. 2022). The present research reports observing ZBC’s adsorption capacity towards As(III) and As(V) at pH 6.5 and 8.5, respectively, found to be 107.6 and 40.8 mg/g. The adsorption occurrence of As(III) and As(V) onto ZBC was found to be principally governed by electrostatic attraction. Advanced imaging techniques such As SEM and elemental plotting enabled the characterization of biochar fixed with nZVI/BC post-adsorption of As. The results of Feng et al. (2021) indicate that Fe-modified biochar (FeBC) exhibits enhanced removing efficiencies for As(III) and As(V), with an apparent rise from 86.4% and 99.2% to > 99.9% in an environment of oxygen. The present investigation looked into the pivotal function of O2 in effectively eliminating As from aqueous solutions via FeBC. The exclusive drop of As(V) was observed, whereas the rise of As(III) oxidation was witnessed upon exposure to O2. The analysis involved the complexation of both forms of arsenic species with iron oxides/hydroxides on iron modified biochar. In another study, MnO2 modified composite biochar (MBC) was invented to As contaminants from groundwater (Zama et al. 2017). The findings of the study suggest that the adsorption process of As(III) onto biochar is primarily governed by electrostatic attraction and precipitation. The biochar activated by KOH has demonstrated notable adsorption of 30.98 mg/g for both As(V) and As(V). Furthermore, the As elimination methods facilitated by KOH-activated biochar were attributed to complex reactions involving complexation and π-π interactions. The disordered small flake graphite structure of KOH-activated biochar exhibited a π-acceptor behavior, while As(V) functioned As an electron donor. The chitosan-magnetic-graphene oxide (CMGO) nanocomposite was synthesized by Sherlala et al. (2019) for its use in adsorbing As. The outcomes obtained regarding the characterizations indicate that the synthesis of CMGO nanocomposites has yielded a remarkable outcome, featuring a large specific surface area of 152.38 m2/g The empirical findings have demonstrated that the effectiveness of As elimination was enhanced with the increase in the cumulative dose of adsorbent and contact duration. The optimal pH of 7.3 yielded the maximum adsorption capacity of 45 mg/g and removal efficiency of 61%. Examining the adsorption isotherm reveals that the adsorption data conforms precisely to the Langmuir isotherm model, signifying a homogeneous process. Priyadarshni et al. (2020) developed innovative biochar@Fe and biochar@Cu nanoparticles equilibrated with rice husk biochar to eliminate total As (As III + V) and microbial pollutants from water. The mixtures comprising biochar@Fe and biochar@Cu exhibited a remarkable As removal effectiveness of over 95%, subject to the contact time, pH, and co-existing anions. The adsorption method was observed as fitting to the pseudo-second-order kinetic model and the Freundlich adsorption isotherm, with an optimal adsorption capacity of 20.32 mg/g. The recent biomaterials used for removing As are presented in Table 3.
Critical analysis and future scope
The safety of potable drinking water and its resources has gained significant importance in developing countries like India to fulfil the demand for fresh water. Thus, the need for sustainable water purification technology continues to improve dynamically. The adverse effect of toxic contaminants like As and F− on water systems demands technological advancements to manage water resources effectively. Despite the limitations of conventional technologies in various applications, there is an increasing demand for significant advancement in innovative remediation methods, including bioadsorption to address the issue of contaminated groundwater with As and F−. The researcher provides novel approaches for remediating contaminated groundwater and encourages advancements in the specific areas of expertise identified in the literature. To determine the ideal method for achieving the desired outcome, it is essential to consider different parameters such as simple operative techniques and applicability, the ions concentration, adsorbent dose, pH of a solution, the groundwater features, affordability, and the future effects on environmental impacts.
A recent study conducted by Hussain et al. (2024) used a low-cost waste coconut fiber for removing F− from groundwater in real-time conditions in the Greater Noida region of India. The outcomes of the results show a nominal removal efficiency of about 70% at a pH level of 2 for 50 mg/L F− initial concentration. Remarkably, this study demonstrates that coconut fiber is not just highly effective but also a cost-effective and bearable prime to synthesize these bioadsorbents for sustainable water purification. But at the same time, it could not help to remove F− completely. Thus, we need to precisely focus on developing low-cost adsorbents for complete removal of contaminants with sustainable treatment methods. Moreover, Vani et al. (2024) studied the removal of F− and As removal from groundwater by combining two sustainable methods bioadsorption and ultrafiltration using waste Moringa oleifera seed powder (MP) and sorghum husk (SH). The findings indicated that a combination of 10 g/L MP with SH returned the most significant F− (97.20%) and As (78.63%) removal rates. Based on initial cost evaluation, it has been determined that the combined method is cost-effective and well-suited for removing potentially harmful elements from water. The investigation highlights the promising applications of affordable absorption methods for ensuring access to safe and clean water consumption, especially in commercial, agricultural, and urban regions.
Exploring novel strategies to address the issue of contaminated groundwater, researchers are investigating feasible bioadsorption/bioremediation/membrane and integrating multiple techniques to remove contaminants like As and F− completely. These approaches utilize the inherent adsorption properties of biological materials to effectively remove contaminants from drinking water and in situ groundwater. These methods frequently employ biomaterials like agro-waste, microbial biomass, and plant-based materials, which are readily available, sustainable, and affordable. Through the use of fixed-bed reactors or permeable barriers, the biomaterials can be immobilized to efficiently adsorb contaminants from water, leading to a significant decrease in pollutant concentrations. In addition, bioadsorption processes can be improved by the formation of biofilms, where microorganisms hold to the surface of adsorbents. This enhances the adsorption capacity and aids in the biodegradation of contaminants. In addition, the study also looks into methods for modifying the surface and genetic engineering of microbial strains to improve the efficiency and specificity of adsorption for specific contaminants. In conclusion, the sustainable bioadsorption methods present a hopeful prospect for the purification of groundwater with lesser cost and effective removal of contaminants by integrating multiple methods. These approaches not only provide environmental advantages but also effectively eliminate pollutants.
Furthermore, the chosen method must be relevant to the specific environmental surroundings, effective in addressing the toxic specific contaminants, and appropriate for the prevailing operational circumstances. Biomass-based carbon materials play a significant role in the remediation of contaminated groundwater, but their full potential has not yet been exploited. In recent years, biochar has attracted a lot of attention. The numerous carbonaceous materials, classified according to pore size, have demonstrated their potential in various chemical processes. A strong alternative for practical applications is biochar manufactured from various biomaterials. Among all the activation techniques for biochar, chemical activation before pyrolysis produces biochar with the highest surface area and pore volume. Still, the process is highly energy-intensive and produces secondary hazardous wastewater that needs additional treatment. Recently, many researchers developed emerging new and novel adsorbents to remove toxic groundwater contaminants effectively. Chemically modified biomass based adsorbents have significant removal efficiency.
The efficacy of the adsorption process is reliant on the judicious use of the optimal adsorbent, thus demonstrating the significance of this essential stage. The primary criteria for selecting an adsorbent are its affordability, ability to remove a significant amount of pollutants, efficacy against a wide range of contaminants, and minimal environmental impact. Extensive research has been conducted on conventional and unconventional adsorption techniques and capacities. The adsorbent exhibits decreased effectiveness in removing contaminants Associated with inadequate raw materials and modification techniques utilized during biochar preparation. This has decreased the adsorbent’s porosity, pore volume, and surface area, limiting its adsorption capacity. The existing adsorption methods exhibit several limitations, such as the substantial initial cost of the adsorbent, challenges associated with segregating pollutants from the adsorbent, reuse of adsorbent, and secondary waste generation. Most adsorbents used to remove contaminants are not readily accessible and, therefore, cannot be effectively employed on an industrial scale.
According to recent advance searches, there appears to be a lack of clarity regarding properly utilizing biomass-based adsorbents, which may require further research guidance. It is necessary to use additional advanced adsorbents, such as carbon nanotubes, graphene, and nanosheets, to address the issue of As and F− contamination in groundwater and wastewater. It is necessary to develop novel innovations in the adsorption process, like applying integrated approach adsorption and bioremediation using agricultural waste biomass to complete the removal of As and F− from groundwater, minimize secondary waste generation, and maintain environmental sustainability.
The combined use of experimental data and high numbers computation is viable for developing machine learning (ML) and artificial intelligence (AI) models that can predict the characteristics of porous materials. These models can facilitate designing, discovering, and optimizing adsorbents. The use of data-driven ML techniques, such as artificial neural networks (ANNs), random forest, and support vector machines (SVM) can facilitate the identification of complicated correlations between the structural, physicochemical, and process properties of porous materials and their consequential properties, such as the adsorption and selectivity. The mentioned models can generate numerical forecasts regarding those characteristics for porous materials that have not yet been synthesized.
Traditional process optimization modeling techniques have multiple constraints like more time and high computational load. On the other hand, the ML approach offers a more accurate, precise, practical and cost-effective alternative to traditional modeling techniques like statistical computation and RSM, which require multiple experimentations (Agrawal et al. 2024). Machine learning has the potential to make accurate predictions without extensive experimentation; thus, it helps to save valuable time and resources. It also demonstrates proficiency in handling intricate, comprehensive, and multidimensional information. Machine learning techniques are becoming increasingly prevalent in materials discovery due to the exponential expansion of research data.
Applying ML algorithms enables precise predictions by utilizing biochar properties, parametric conditions, and types of contaminants. This offers helpful information for enhancing biochar attributes and optimizing its adsorption capabilities. This provides valuable insights into adjusting the features of biochar and enhancing its absorbent effectiveness. The ability of biochar to adsorb contaminants from contaminated water has also been studied using ML techniques such as ANN, DT, SVM, KNN, XGB, and RF (Nie et al. 2023). In a previous study, Zhu et al. (2019) conducted a comparison of the efficacy of ANNs and random forests (RFs) in predicting the adsorption of multiple elements like arsenic, cadmium, copper, zinc, nickel, and lead on biochar. The study used a dataset of 353 adsorption tests and found that RF (R2 ≈ 0.973) demonstrated better predictions in comparison with ANN (R2 ≈ 0.948). However, Da et al. (2022) found that the multilayer perceptron ANN achieved impressive results (R2 ≈ 0.99, RMSE = 3.75) in predicting the capacity of biochar to absorb uranium over time. These results exceeded compared to the other methods such as RF, LR, and SVM.
A preliminary analysis has been conducted regarding the remediation use of biomass-based adsorbents using several adsorbents at small-scale operations for several contaminants. Still, there is a need to study novel agricultural-based adsorbents that can be used for large-scale operations, and enhancement of the reuse cycles of adsorbents is also required. Future studies may consider the economic discourse and expenses of treating groundwater containing As and F− through agricultural adsorbents, which help to increase the economy of farmers and society.
In India, farmers often burn biomass from agricultural waste residues in their fields without knowing their negative impact on the environment. It has a significant adverse effect on soil quality, fertility, and air quality, and it also contributes to global warming, which is very harmful to the environment and aquatic ecosystems. However, these agricultural crop residues can be converted into valuable commodities like biochar, fertilizer, manure, etc. Farm agro-waste in large-scale operations holds great potential for increasing the rural economy. Adsorption materials that are made from crop waste can be affordable alternatives to traditional adsorbents for application in water treatment technology and soil remediation. Generally, in a country like India, every state has its problems with potable drinking water and contaminated groundwater and they have different agricultural residues. The development of adsorbents for local problems with local solutions ultimately helps local farmers. So addressing the problem of local region by developing adsorbents from local agricultural residues. Thus, it also helps farmers with the cost value of their agricultural waste and again if we develop an adsorption operating system there itself, it reduces the cost of adsorption. If we find this kind of local solution for local farmers will help to produce waste to energy, mitigating climate crises, etc. and significantly increase the cost economy of local farmers. Furthermore, they can experience the positive effects of environmentally friendly agriculture waste management, which includes cost reduction and social responsibility. Likewise, agro-based adsorbents can support the growth of dispersed, community-driven economies. This approach also allows for the simultaneous tackling of environmental issues and the enhancement of the existence of farmers in rural India. It is therefore suggested that further research be conducted in this area.
Conclusion
In the present review, we have discussed the various techniques used to remove As and F−. Similarly, we also examined the significance of bioadsorption, bioadsorbents, and the positive and negative aspects of several methods used in published research to remove As and F− from contaminated water. Factors like ion concentration, adsorbent dose, and pH influence the removal process were reviewed. Using biomass-based materials to remove both toxic pollutants has received considerable attention. We also addressed these materials physical and chemical nature and efficiency over traditional materials. The biomaterials were helpful since they offered a sustainable method of removal. The biomaterials were modified in various ways to boost the effectiveness of the pristine state. Thus, the above brief investigation revealed many novel techniques for eliminating toxic pollutants. Future studies will focus on developing innovative biomaterials with certain functional groups, like hydroxyl, thiol, amine, and carboxylic acid. Biomaterials can be found easily, and using them to remove pollutants is safe.
Conventional bioadsorbents which were developed till now are not able to effectively remove A and F− from water. Efficiency is one of the key factors and the parameters at which it gives a maximum removal are other factors that significantly affect the removal efficiency of any bioadsorbents. Availability of raw materials is also another prominent factor for developing bioadsorbents. Agricultural biomass is available abundantly according to FAO. However, other biowaste like tamarind seeds and leaves of any plants are lesser available and there is not a regulated amount of biowaste generation. Moreover, the availability of crop residues can be done based on cropping patterns, crop yield, and harvesting waste in a given area, and thus, we can analyze how much amount of crop waste is generated for a particular crop. Therefore, we need to come up with more practical adsorbents in operating conditions that will give maximum adsorption capacity and removal. So that we can commercialize and scale up those processes to solve actual drinking water problems. Hence, extra attention needs to be paid to studying such biosorbents. The investigation gap was found in enhancing the execution of adsorbents due to their enormous scale for increased removal efficiencies. Multiple properties needed for the fabrication of effective adsorbents, such as altering the adsorbent surface by chemical treatment or creating composites with metal oxides, have been described to resolve issues, and it provides an opportunity for the future development of novel materials.
Data availability
All the appropriate data are presented in the paper.
References
Abeysinghe S, Baek K (2022) Fluoride-contaminated water remediation using biochar derived from dairy processing sludge. Chem Eng J 446:136955. https://doi.org/10.1016/j.cej.2022.136955
Abid M, Niazi NK, Bibi I et al (2016) Arsenic(V) biosorption by charred orange peel in aqueous environments. Int J Phytoremediation 18:442–449. https://doi.org/10.1080/15226514.2015.1109604
Agrafioti E, Kalderis D, Diamadopoulos E (2014) Arsenic and chromium removal from water using biochars derived from rice husk, organic solid wastes and sewage sludge. J Environ Manage 133:309–314. https://doi.org/10.1016/j.jenvman.2013.12.007
Agrawal P, Gnanaprakash R, Dhawane SH (2024) Prediction of biodiesel yield employing machine learning: interpretability analysis via Shapley additive explanations. Fuel 359. https://doi.org/10.1016/j.fuel.2023.130516
Ahmad I, Farwa U, Khan ZUH et al (2022) Biosorption and health risk assessment of arsenic contaminated water through cotton stalk biochar. Surfaces and Interfaces 29:101806. https://doi.org/10.1016/j.surfin.2022.101806
Ahmed W, Mehmood S, Núñez-Delgado A, et al (2021) Adsorption of arsenic (III) from aqueous solution by a novel phosphorus-modified biochar obtained from Taraxacum mongolicum Hand-Mazz: Adsorption behavior and mechanistic analysis. J Environ Manage 292. https://doi.org/10.1016/j.jenvman.2021.112764
Alchouron J, Navarathna C, Chludil HD et al (2020) Assessing South American Guadua chacoensis bamboo biochar and Fe3O4 nanoparticle dispersed analogues for aqueous arsenic(V) remediation. Elsevier B.V
Ali S, Rizwan M, Shakoor MB et al (2020) High sorption efficiency for As(III) and As(V) from aqueous solutions using novel almond shell biochar. Chemosphere 243:125330. https://doi.org/10.1016/j.chemosphere.2019.125330
Alkurdi SSA, Herath I, Bundschuh J et al (2019) Biochar versus bone char for a sustainable inorganic arsenic mitigation in water: What needs to be done in future research? Environ Int 127:52–69. https://doi.org/10.1016/j.envint.2019.03.012
Amen R, Bashir H, Bibi I et al (2020) A critical review on arsenic removal from water using biochar-based sorbents: The significance of modification and redox reactions. Chem Eng J 396. https://doi.org/10.1016/j.cej.2020.125195
Angelina Thanga Ajisha M, Rajagopal K (2015) Fluoride removal study using pyrolyzed Delonix regia pod, an unconventional adsorbent. Int J Environ Sci Technol 12:223–236. https://doi.org/10.1007/s13762-013-0485-8
Annadurai ST, Arivalagan P, Sundaram R et al (2019) Batch and column approach on biosorption of fluoride from aqueous medium using live, dead and various pretreated Aspergillus niger (FS18) biomass. Surfaces and Interfaces 15:60–69. https://doi.org/10.1016/j.surfin.2019.01.013
Baig JA, Kazi TG, Shah AQ et al (2010) Biosorption studies on powder of stem of Acacia nilotica: Removal of arsenic from surface water. J Hazard Mater 178:941–948. https://doi.org/10.1016/j.jhazmat.2010.02.028
Balali-Mood M, Naseri K, Tahergorabi Z et al (2021) Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol 12:1–19. https://doi.org/10.3389/fphar.2021.643972
Bharali RK, Bhattacharyya KG (2015) Biosorption of fluoride on Neem (Azadirachta indica) leaf powder. J Environ Chem Eng 3:662–669. https://doi.org/10.1016/j.jece.2015.02.007
Bhaumik R, Mondal NK, Chattoraj S (2017) An optimization study for defluoridation from synthetic fluoride solution using scale of Indian major carp Catla (Catla catla): An Unconventional Biosorbent. J Fluor Chem 195:57–69. https://doi.org/10.1016/j.jfluchem.2017.01.015
Biswal BK, Vijayaraghavan K, Tsen-Tieng DL, Balasubramanian R (2022) Biochar-based bioretention systems for removal of chemical and microbial pollutants from stormwater: A critical review. J Hazard Mater 422:126886. https://doi.org/10.1016/j.jhazmat.2021.126886
Bombuwala Dewage N, Liyanage AS, Pittman CU et al (2018) Fast nitrate and fluoride adsorption and magnetic separation from water on Α-Fe2O3 and Fe3O4 dispersed on Douglas fir biochar. Bioresour Technol 263:258–265. https://doi.org/10.1016/j.biortech.2018.05.001
Bonyadi Z, Kumar PS, Foroutan R et al (2019) Ultrasonic-assisted synthesis of Populus alba activated carbon for water defluorination: Application for real wastewater. Korean J Chem Eng 36:1595–1603. https://doi.org/10.1007/s11814-019-0373-0
Brahman KD, Kazi TG, Baig JA et al (2016) Biosorptive removal of inorganic arsenic species and fluoride from aqueous medium by the stem of Tecomella undulate. Chemosphere 150:320–328. https://doi.org/10.1016/j.chemosphere.2016.02.017
Cai H, Xu L, Chen G et al (2016) Removal of fluoride from drinking water using modified ultrafine tea powder processed using a ball-mill. Appl Surf Sci 375:74–84. https://doi.org/10.1016/j.apsusc.2016.03.005
Chai WS, Cheun JY, Kumar PS et al (2021) A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J Clean Prod 296:126589. https://doi.org/10.1016/j.jclepro.2021.126589
Chang HYH, Kuo YL, Liu JC (2019) Fluoride at waste oyster shell surfaces – role of magnesium. Sci Total Environ 652:1331–1338. https://doi.org/10.1016/j.scitotenv.2018.10.238
Choong CE, Wong KT, Jang SB et al (2020) Fluoride removal by palm shell waste based powdered activated carbon vs. functionalized carbon with magnesium silicate: Implications for their application in water treatment. Chemosphere 239:124765. https://doi.org/10.1016/j.chemosphere.2019.124765
Cotruvo JA (2017) 2017 Who guidelines for drinking water quality: first addendum to the fourth edition. J Am Water Works Assoc 109:44–51. https://doi.org/10.5942/jawwa.2017.109.0087
Cuong DV, Wu PC, Liou SYH, Hou CH (2022) An integrated active biochar filter and capacitive deionization system for high-performance removal of arsenic from groundwater. J Hazard Mater 423:127084. https://doi.org/10.1016/j.jhazmat.2021.127084
Da TX, Ren HK, He WK et al (2022) Prediction of uranium adsorption capacity on biochar by machine learning methods. J Environ Chem Eng 10:108449. https://doi.org/10.1016/j.jece.2022.108449
De D, Santosha S, Aniya V et al (2018) Assessing the applicability of an agro-industrial waste to engineered bio-char as a dynamic adsorbent for Fluoride Sorption. J Environ Chem Eng 6:2998–3009. https://doi.org/10.1016/j.jece.2018.04.021
Deshmukh MA, Dodamani AS, Karibasappa GN et al (2018) Tea ash - a new medium for water defluoridation. Indian J Public Heal Res Dev 9:152–157. https://doi.org/10.5958/0976-5506.2018.00028.1
Dhawane SH, Kumar T, Halder G (2018) Recent advancement and prospective of heterogeneous carbonaceous catalysts in chemical and enzymatic transformation of biodiesel. Energy Convers Manag 167:176–202. https://doi.org/10.1016/j.enconman.2018.04.073
Ding E, Jiang J, Lan Y et al (2023) Optimizing Cd2+ adsorption performance of KOH modified biochar adopting response surface methodology. J Anal Appl Pyrolysis 169:105788. https://doi.org/10.1016/j.jaap.2022.105788
Feng Y, Xu Y, Xie X et al (2021) The dual role of oxygen in redox-mediated removal of aqueous arsenic(III/V) by Fe-modified biochar. Bioresour Technol 340:125674. https://doi.org/10.1016/j.biortech.2021.125674
Frišták V, Moreno-Jimenéz E, Fresno T, Diaz E (2018) Effect of physical and chemical activation on arsenic sorption separation by grape seeds-derived biochar. Separations 5:1–9. https://doi.org/10.3390/separations5040059
Getachew T, Hussen A, Rao VM (2015) Defluoridation of water by activated carbon prepared from banana (Musa paradisiaca) peel and coffee (Coffea arabica) husk. Int J Environ Sci Technol 12:1857–1866. https://doi.org/10.1007/s13762-014-0545-8
Giri AK, Patel RK, Mahapatra SS, Mishra PC (2013) Biosorption of arsenic (III) from aqueous solution by living cells of Bacillus cereus. Environ Sci Pollut Res 20:1281–1291. https://doi.org/10.1007/s11356-012-1249-6
Goswami R, Kumar M (2018) Removal of fluoride from aqueous solution using nanoscale rice husk biochar. Groundw Sustain Dev 7:446–451. https://doi.org/10.1016/j.gsd.2017.12.010
Halder G, Sinha K, Dhawane S (2015) Defluoridation of wastewater using powdered activated carbon developed from Eichhornia crassipes stem: optimization by response surface methodology. Desalin Water Treat 56:953–966. https://doi.org/10.1080/19443994.2014.942375
Halder G, Khan AA, Dhawane S (2016) Fluoride sorption onto a steam-activated biochar derived from Cocos nucifera shell. Clean - Soil, Air, Water 44:124–133. https://doi.org/10.1002/clen.201400649
He R, Peng Z, Lyu H et al (2018) Synthesis and characterization of an iron-impregnated biochar for aqueous arsenic removal. Sci Total Environ 612:1177–1186. https://doi.org/10.1016/j.scitotenv.2017.09.016
Hegde RM, Rego RM, Potla KM et al (2020) Bio-inspired materials for defluoridation of water: a review. Chemosphere 253:126657. https://doi.org/10.1016/j.chemosphere.2020.126657
Huang WH, Lee DJ, Huang C (2021) Modification on biochars for applications: a research update. Bioresour Technol 319:. https://doi.org/10.1016/j.biortech.2020.124100
Hussain M, Imran M, Abbas G et al (2020) A new biochar from cotton stalks for As (V) removal from aqueous solutions: its improvement with H3PO4 and KOH. Environ Geochem Health 42:2519–2534. https://doi.org/10.1007/s10653-019-00431-2
Hussain A, Maitra J, Saifi A et al (2024) A sustainable approach for fluoride treatment using coconut fiber cellulose as an adsorbent. Environ Res 244:117952. https://doi.org/10.1016/j.envres.2023.117952
Ibrahim M, Siddique A, Verma L et al (2019) Adsorptive removal of fluoride from aqueous solution by biogenic iron permeated activated carbon derived from sweet lime waste. Acta Chim Slov 66:123–136. https://doi.org/10.17344/acsi.2018.4717
Jha PK, Tripathi P (2021) Arsenic and fluoride contamination in groundwater: a review of global scenarios with special reference to India. Groundw Sustain Dev 13:100576. https://doi.org/10.1016/j.gsd.2021.100576
Jin H, Capareda S, Chang Z et al (2014) Biochar pyrolytically produced from municipal solid wastes for aqueous As(V) removal: Adsorption property and its improvement with KOH activation. Bioresour Technol 169:622–629. https://doi.org/10.1016/j.biortech.2014.06.103
KamathiMwangi C, Mwangi WI, Wanjau NR et al (2016) Remediation of fluoride laden water by complexation with triethylamine modified maize tassels. Environ Nat Resour Res 6:44. https://doi.org/10.5539/enrr.v6n1p44
Karmakar S, Mukherjee J, Mukherjee S (2018) Biosorption of fluoride by water lettuce (Pistia stratiotes) from contaminated water. Int J Environ Sci Technol 15:801–810. https://doi.org/10.1007/s13762-017-1439-3
Kayastha V, Patel J, Kathrani N et al (2022) New Insights in factors affecting ground water quality with focus on health risk assessment and remediation techniques. Environ Res 212:113171. https://doi.org/10.1016/j.envres.2022.113171
Kazi TG, Brahman KD, Baig JA, Afridi HI (2018) A new efficient indigenous material for simultaneous removal of fluoride and inorganic arsenic species from groundwater. J Hazard Mater 357:159–167. https://doi.org/10.1016/j.jhazmat.2018.05.069
Khan BA, Ahmad M, Iqbal S et al (2022) Effectiveness of the engineered pinecone-derived biochar for the removal of fluoride from water. Environ Res 212:113540. https://doi.org/10.1016/j.envres.2022.113540
Kumar R, Sharma P, Sharma PK et al (2023) Rice husk biochar - a novel engineered bio-based material for transforming groundwater-mediated fluoride cycling in natural environments. J Environ Manage 343:118222. https://doi.org/10.1016/j.jenvman.2023.118222
Kushwaha R, Singh RS, Mohan D (2023) Comparative study for sorption of arsenic on peanut shell biochar and modified peanut shell biochar. Bioresour Technol 375:128831. https://doi.org/10.1016/j.biortech.2023.128831
Letechipia JO, González-Trinidad J, Júnez-Ferreira HE et al (2023) Removal of arsenic from semiarid area groundwater using a biosorbent from watermelon peel waste. Heliyon 9:1–12. https://doi.org/10.1016/j.heliyon.2023.e13251
Li P, Wu J (2019) Sustainable living with risks: meeting the challenges. Hum Ecol Risk Assess 25:1–10. https://doi.org/10.1080/10807039.2019.1584030
Li C, Chen N, Zhao Y et al (2016) Polypyrrole-grafted peanut shell biological carbon as a potential sorbent for fluoride removal: Sorption capability and mechanism. Chemosphere 163:81–89. https://doi.org/10.1016/j.chemosphere.2016.08.016
Lin L, Qiu W, Wang D et al (2017) Arsenic removal in aqueous solution by a novel Fe-Mn modified biochar composite: Characterization and mechanism. Ecotoxicol Environ Saf 144:514–521. https://doi.org/10.1016/j.ecoenv.2017.06.063
Lin L, Song Z, Khan ZH et al (2019) Enhanced As(III) removal from aqueous solution by Fe-Mn-La-impregnated biochar composites. Sci Total Environ 686:1185–1193. https://doi.org/10.1016/j.scitotenv.2019.05.480
Lizneva D, Yuen T, Sun L et al (2018) Emerging concepts in the epidemiology, pathophysiology, and clinical care of osteoporosis across the menopausal transition. Matrix Biol 71–72:70–81. https://doi.org/10.1016/j.matbio.2018.05.001
Luo M, Lin H, He Y et al (2019) Efficient simultaneous removal of cadmium and arsenic in aqueous solution by titanium-modified ultrasonic biochar. Bioresour Technol 284:333–339. https://doi.org/10.1016/j.biortech.2019.03.108
Lv L, He J, Wei M et al (2006) Factors influencing the removal of fluoride from aqueous solution by calcined Mg-Al-CO3 layered double hydroxides. J Hazard Mater 133:119–128. https://doi.org/10.1016/j.jhazmat.2005.10.012
Manna S, Saha P, Roy D et al (2015) Defluoridation potential of jute fibers grafted with fatty acyl chain. Appl Surf Sci 356:30–38. https://doi.org/10.1016/j.apsusc.2015.08.007
Mei L, Qiao H, Ke F et al (2020) One-step synthesis of zirconium dioxide-biochar derived from Camellia oleifera seed shell with enhanced removal capacity for fluoride from water. Appl Surf Sci 509:144685. https://doi.org/10.1016/j.apsusc.2019.144685
Meilani V, Lee JI, Kang JK et al (2021) Application of aluminum-modified food waste biochar as adsorbent of fluoride in aqueous solutions and optimization of production using response surface methodology. Microporous Mesoporous Mater 312:110764. https://doi.org/10.1016/j.micromeso.2020.110764
Meshesha Tulu M, Yimer AM, Jebessa AG (2018) Preparation and evaluation of adsorption effectiveness of peanut husk for the removal of fluoride ion from aqueous solution. Mod Chem Appl 06: https://doi.org/10.4172/2329-6798.1000261
Mohan D, Pittman CU, Bricka M et al (2007) Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J Colloid Interface Sci 310:57–73. https://doi.org/10.1016/j.jcis.2007.01.020
Mohan D, Kumar S, Srivastava A (2014) Fluoride removal from ground water using magnetic and nonmagnetic corn stover biochars. Ecol Eng 73:798–808. https://doi.org/10.1016/j.ecoleng.2014.08.017
Mondal NK, Roy A (2018) Potentiality of a fruit peel (banana peel) toward abatement of fluoride from synthetic and underground water samples collected from fluoride affected villages of Birbhum district. Appl Water Sci 8:1–10. https://doi.org/10.1007/s13201-018-0729-3
Mondal NK, Bhaumik R, Datta JK (2015) Removal of fluoride by aluminum impregnated coconut fiber from synthetic fluoride solution and natural water. Alexandria Eng J 54:1273–1284. https://doi.org/10.1016/j.aej.2015.08.006
Mukherjee S, Thakur AK, Goswami R et al (2021) Efficacy of agricultural waste derived biochar for arsenic removal: Tackling water quality in the Indo-Gangetic plain. J Environ Manage 281:111814. https://doi.org/10.1016/j.jenvman.2020.111814
Mupa M, Gwaku F, Gwizangwe I (2016) Pediastrum boryanum immobilized on rice husk ash silica as biosorbent for fluoride removal from drinking water. Indian J Sci Technol 9. https://doi.org/10.17485/ijst/2016/v9i48/89127
Naga Babu A, Reddy DS, Kumar GS et al (2018) Removal of lead and fluoride from contaminated water using exhausted coffee grounds based bio-sorbent. J Environ Manage 218:602–612. https://doi.org/10.1016/j.jenvman.2018.04.091
Nath KJ (2018) Arsenic and fluoride contamination in groundwater: mitigation strategies. Gr Water Dev - Issues Sustain Solut 267–278. https://doi.org/10.1007/978-981-13-1771-2_15
Nehra S, Raghav S, Kumar D (2020) Biomaterial functionalized cerium nanocomposite for removal of fluoride using central composite design optimization study. Environ Pollut 258:113773. https://doi.org/10.1016/j.envpol.2019.113773
Nguyen TH, Loganathan P, Nguyen TV et al (2022) Arsenic removal by pomelo peel biochar coated with iron. Chem Eng Res Des 186:252–265. https://doi.org/10.1016/j.cherd.2022.07.022
Nguyen TKT, Nguyen TB, Chen WH et al (2023) Phosphoric acid-activated biochar derived from sunflower seed husk: Selective antibiotic adsorption behavior and mechanism. Bioresour Technol 371:128593. https://doi.org/10.1016/j.biortech.2023.128593
Niazi NK, Bibi I, Shahid M et al (2018a) Arsenic removal by Japanese oak wood biochar in aqueous solutions and well water: Investigating arsenic fate using integrated spectroscopic and microscopic techniques. Sci Total Environ 621:1642–1651. https://doi.org/10.1016/j.scitotenv.2017.10.063
Niazi NK, Bibi I, Shahid M et al (2018b) Arsenic removal by perilla leaf biochar in aqueous solutions and groundwater: An integrated spectroscopic and microscopic examination. Environ Pollut 232:31–41. https://doi.org/10.1016/j.envpol.2017.09.051
Nie Y, Zhao C, Zhou Z et al (2023) Hydrochloric acid-modified fungi-microalgae biochar for adsorption of tetracycline hydrochloride: Performance and mechanism. Bioresour Technol 383:129224. https://doi.org/10.1016/j.biortech.2023.129224
Panwar NL, Pawar A, Salvi BL (2019) Comprehensive review on production and utilization of biochar. SN Appl Sci 1:1–19. https://doi.org/10.1007/s42452-019-0172-6
Papari F, Najafabadi PR, Ramavandi B (2017) Fluoride ion removal from aqueous solution, groundwater, and seawater by granular and powdered Conocarpus erectus biochar. Desalin Water Treat 65:375–386. https://doi.org/10.5004/dwt.2017.20271
Peng Y, Azeem M, Li R et al (2022) Zirconium hydroxide nanoparticle encapsulated magnetic biochar composite derived from rice residue: Application for As(III) and As(V) polluted water purification. J Hazard Mater 423:127081. https://doi.org/10.1016/j.jhazmat.2021.127081
Pillai P, Dharaskar S, Shah M, Sultania R (2020) Determination of fluoride removal using silica nano adsorbent modified by rice husk from water. Groundw Sustain Dev 11:100423. https://doi.org/10.1016/j.gsd.2020.100423
Priyadarshni N, Nath P, Nagahanumaiah CN (2020) Sustainable removal of arsenate, arsenite and bacterial contamination from water using biochar stabilized iron and copper oxide nanoparticles and associated mechanism of the remediation process. J Water Process Eng 37:101495. https://doi.org/10.1016/j.jwpe.2020.101495
Ragul V, Chitra B, Valliammai CT et al (2022) Experimental investigation on defluoridation competency of mesoporous Prosopis juliflora wood based biomaterials. Results Mater 15:100306. https://doi.org/10.1016/j.rinma.2022.100306
Ravulapalli S, Ravindhranath K (2017) Defluoridation studies using active carbon derived from the barks of Ficus racemosa plant. J Fluor Chem 193:58–66. https://doi.org/10.1016/j.jfluchem.2016.11.013
Romar-Gasalla A, Coelho GF, Nóvoa-Muñoz JC et al (2017) Wheat straw as a bio-sorbent for arsenate, chromate, fluoride, and nickel. Water (switzerland) 9:1–11. https://doi.org/10.3390/w9090690
Roy P, Mondal NK, Bhattacharya S et al (2013) Removal of arsenic(III) and arsenic(V) on chemically modified low-cost adsorbent: Batch and column operations. Appl Water Sci 3:293–309. https://doi.org/10.1007/s13201-013-0082-5
Roy S, Sengupta S, Manna S, Das P (2018) Chemically reduced tea waste biochar and its application in treatment of fluoride containing wastewater: Batch and optimization using response surface methodology. Process Saf Environ Prot 116:553–563. https://doi.org/10.1016/j.psep.2018.03.009
Rupasinghe NKLC, Senanayake SMAE, Nanayakkara KGN (2022) Development, characterization and mechanisms study of protonated sawdust biochar-chitosan composite bead biosorbent for defluoridation of contaminated groundwater. Bioresour Technol Reports 17:100946. https://doi.org/10.1016/j.biteb.2022.100946
Sadhu M, Bhattacharya P, Vithanage M, Padmaja Sudhakar P (2022) Adsorptive removal of fluoride using biochar – A potential application in drinking water treatment. Sep Purif Technol 278:119106. https://doi.org/10.1016/j.seppur.2021.119106
Saikia R, Goswami R, Bordoloi N et al (2017) Removal of arsenic and fluoride from aqueous solution by biomass based activated biochar: optimization through response surface methodology. J Environ Chem Eng 5:5528–5539. https://doi.org/10.1016/j.jece.2017.10.027
Sakhiya AK, Vijay VK, Kaushal P (2023) Development of rice straw biochar through pyrolysis to improve drinking water quality in arsenic and manganese contaminated areas. Surfaces and Interfaces 36:102582. https://doi.org/10.1016/j.surfin.2022.102582
Salomón-Negrete MÁ, Reynel-Ávila HE, Mendoza-Castillo DI et al (2018) Water defluoridation with avocado-based adsorbents: synthesis, physicochemical characterization and thermodynamic studies. J Mol Liq 254:188–197. https://doi.org/10.1016/j.molliq.2018.01.084
Sari NA, Ishak CF, Bakar RA (2014) Characterization of oil palm empty fruit bunch and rice husk biochars and their potential to adsorb arsenic and cadmium. Am J Agric Biol Sci 9:450–456. https://doi.org/10.3844/ajabssp.2014.450.456
Seid TM (2017) Biosorption of fluoride ion from water using the seeds of the cabbage tree (Moringa stenopetala). African J Environ Sci Technol 11:1–10. https://doi.org/10.5897/ajest2016.2197
Senewirathna DSGD, Thuraisingam S, Prabagar S, Prabagar J (2022) Fluoride removal in drinking water using activated carbon prepared from palmyrah (Borassus flabellifer) nut shells. Curr Res Green Sustain Chem 5:100304. https://doi.org/10.1016/j.crgsc.2022.100304
Shakoor MB, Niazi NK, Bibi I et al (2019) Exploring the arsenic removal potential of various biosorbents from water. Environ Int 123:567–579. https://doi.org/10.1016/j.envint.2018.12.049
Shen Z, Jin J, Fu J et al (2021) Anchoring Al- and/or Mg-oxides to magnetic biochars for co-uptake of arsenate and fluoride from water. J Environ Manage 293:112898. https://doi.org/10.1016/j.jenvman.2021.112898
Sherlala AIA, Raman AAA, Bello MM, Buthiyappan A (2019) Adsorption of arsenic using chitosan magnetic graphene oxide nanocomposite. J Environ Manage 246:547–556. https://doi.org/10.1016/j.jenvman.2019.05.117
Siddique A, Nayak AK, Singh J (2020) Synthesis of FeCl3-activated carbon derived from waste Citrus limetta peels for removal of fluoride: an eco-friendly approach for the treatment of groundwater and bio-waste collectively. Groundw Sustain Dev 10:100339. https://doi.org/10.1016/j.gsd.2020.100339
Sikdar PK (2018) Groundwater development and management: issues and challenges in South Asia. Groundw Dev Manag Issues Challenges South Asia 1–539. https://doi.org/10.1007/978-3-319-75115-3
Srinivasulu D (2023) Senna auriculata L. flower petal biomass: An alternative green biosorbent for the removal of fluoride from aqueous solutions. Acta Ecol Sin 43:72–81. https://doi.org/10.1016/j.chnaes.2021.09.015
Sun Y, Wang T, Bai L et al (2022) Application of biochar-based materials for remediation of arsenic contaminated soil and water: Preparation, modification, and mechanisms. J Environ Chem Eng 10:108292. https://doi.org/10.1016/j.jece.2022.108292
Sunitha V, Reddy BM (2018) Defluoridation of water using mentha longifolia (mint) as bioadsorbent. J Ind Geophys Union 22:207–211
Tirkey P, Bhattacharya T, Chakraborty S (2018) Optimization of fluoride removal from aqueous solution using Jamun (Syzygium cumini) leaf ash. Process Saf Environ Prot 115:125–138. https://doi.org/10.1016/j.psep.2017.10.022
Tomar V, Prasad S, Kumar D (2014) Adsorptive removal of fluoride from aqueous media using citrus limonum (lemon) leaf. Microchem J 112:97–103. https://doi.org/10.1016/j.microc.2013.09.010
Tomczyk A, Sokołowska Z, Boguta P (2020) Biochar physicochemical properties: pyrolysis temperature and feedstock kind effects. Rev Environ Sci Biotechnol 19:191–215. https://doi.org/10.1007/s11157-020-09523-3
Upendra RS, Khandelwal P, Amiri ZR et al (2015) Optimization of fluoride removal system using Ocimum sp. Leaves and ragi seed husk by applying bio-statistical tools. J Environ Res Dev 9:1109
Valdés-Rodríguez EM, Mendoza-Castillo DI, Reynel-Ávila HE et al (2022) Activated carbon manufacturing via alternative Mexican lignocellulosic biomass and their application in water treatment: Preparation conditions, surface chemistry analysis and heavy metal adsorption properties. Chem Eng Res Des 187:9–26. https://doi.org/10.1016/j.cherd.2022.08.039
Van Vinh N, Zafar M, Behera SK, Park HS (2015) Arsenic(III) removal from aqueous solution by raw and zinc-loaded pine cone biochar: equilibrium, kinetics, and thermodynamics studies. Int J Environ Sci Technol 12:1283–1294. https://doi.org/10.1007/s13762-014-0507-1
Vani B, Hymavathi M, Kalyani S et al (2024) Effective removal of fluoride and arsenic from groundwater via integrated biosorption and membrane ultrafiltration. Water Sci Eng. https://doi.org/10.1016/j.wse.2024.04.001
Venkatachalam CD, Sekar S, Sengottian M et al (2023) A critical review of the production, activation, and morphological characteristic study on functionalized biochar. J Energy Storage 67:107525. https://doi.org/10.1016/j.est.2023.107525
Verma L, Siddique MA, Singh J, Bharagava RN (2019) As(III) and As(V) removal by using iron impregnated biosorbents derived from waste biomass of Citrus limmeta (peel and pulp) from the aqueous solution and ground water. J Environ Manage 250:109452. https://doi.org/10.1016/j.jenvman.2019.109452
Vilakati BR, Sivasankar V, Nxumalo EN et al (2019) Fluoride removal studies using virgin and Ti (IV)-modified Musa paradisiaca (plantain pseudo-stem) carbons. Environ Sci Pollut Res 26:11565–11578. https://doi.org/10.1007/s11356-018-2691-x
Wang J, Wang S (2019) Preparation, modification and environmental application of biochar: a review. J Clean Prod 227:1002–1022. https://doi.org/10.1016/j.jclepro.2019.04.282
Wang S, Gao B, Zimmerman AR et al (2015) Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour Technol 175:391–395. https://doi.org/10.1016/j.biortech.2014.10.104
Wang S, Gao B, Li Y (2016) Enhanced arsenic removal by biochar modified with nickel (Ni) and manganese (Mn) oxyhydroxides. J Ind Eng Chem 37:361–365. https://doi.org/10.1016/j.jiec.2016.03.048
Wang S, Gao B, Li Y et al (2017) Adsorptive removal of arsenate from aqueous solutions by biochar supported zero-valent iron nanocomposite: batch and continuous flow tests. J Hazard Mater 322:172–181. https://doi.org/10.1016/j.jhazmat.2016.01.052
Wang J, Chen N, Feng C, Li M (2018) Performance and mechanism of fluoride adsorption from groundwater by lanthanum-modified pomelo peel biochar. Environ Sci Pollut Res 25:15326–15335. https://doi.org/10.1007/s11356-018-1727-6
Wang Y, Miao J, Saleem M et al (2022) Enhanced adsorptive removal of carbendazim from water by FeCl3-modified corn straw biochar as compared with pristine, HCl and NaOH modification. J Environ Chem Eng 10:107024. https://doi.org/10.1016/j.jece.2021.107024
Wen Z, Xi J, Lu J et al (2021) Porous biochar-supported MnFe2O4 magnetic nanocomposite as an excellent adsorbent for simultaneous and effective removal of organic/inorganic arsenic from water. J Hazard Mater 411:124909. https://doi.org/10.1016/j.jhazmat.2020.124909
Wendimu G, Zewge F, Mulugeta E (2017) Aluminium-iron-amended activated bamboo charcoal (AIAABC) for fluoride removal from aqueous solutions. J Water Process Eng 16:123–131. https://doi.org/10.1016/j.jwpe.2016.12.012
Wu J, Huang D, Liu X et al (2018) Remediation of As(III) and Cd(II) co-contamination and its mechanism in aqueous systems by a novel calcium-based magnetic biochar. J Hazard Mater 348:10–19. https://doi.org/10.1016/j.jhazmat.2018.01.011
Yaashikaa PR, Kumar PS, Varjani S, Saravanan A (2020) A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol Reports 28:e00570. https://doi.org/10.1016/j.btre.2020.e00570
Yadav B, Garg A, Santra PK et al (2014) Use of aralu ( Ailanthus exelsa ) stem charcoal for defluoridation of drinking water. Int J Bioassays 1884–1888. https://doi.org/10.21746/j.ijbio.2023.136121
Yang W, Dou Z, Liu Y et al (2023) Gaseous mercury capture using seaweed biochars modified by clean ultraviolet/hydrogen peroxide advanced oxidation process. J Clean Prod 389:136121. https://doi.org/10.1016/j.jclepro.2023.136121
Yitbarek M, Abdeta K, Beyene A et al (2019) Experimental evaluation of sorptive removal of fluoride from drinking water using natural and brewery waste diatomite. Process Saf Environ Prot 128:95–106. https://doi.org/10.1016/j.psep.2019.05.052
Yu Y, Wang C, Guo X, Paul Chen J (2015) Modification of carbon derived from Sargassum sp. by lanthanum for enhanced adsorption of fluoride. J Colloid Interface Sci 441:113–120. https://doi.org/10.1016/j.jcis.2014.10.039
Zama EF, Zhu YG, Reid BJ, Sun GX (2017) The role of biochar properties in influencing the sorption and desorption of Pb(II), Cd(II) and As(III) in aqueous solution. J Clean Prod 148:127–136. https://doi.org/10.1016/j.jclepro.2017.01.125
Zama EF, Li G, Tang YT et al (2022) The removal of arsenic from solution through biochar-enhanced precipitation of calcium-arsenic derivatives. Environ Pollut 292:118241. https://doi.org/10.1016/j.envpol.2021.118241
Zhang Y, Huang K (2019) Grape pomace as a biosorbent for fluoride removal from groundwater. RSC Adv 9:7767–7776. https://doi.org/10.1039/C9RA00109C
Zhang M, Gao B, Varnoosfaderani S et al (2013) Preparation and characterization of a novel magnetic biochar for arsenic removal. Bioresour Technol 130:457–462. https://doi.org/10.1016/j.biortech.2012.11.132
Zhang L, Qin X, Tang J et al (2017) Review of arsenic geochemical characteristics and its significance on arsenic pollution studies in karst groundwater, Southwest China. Appl Geochemistry 77:80–88. https://doi.org/10.1016/j.apgeochem.2016.05.014
Zhou Y, Gao B, Zimmerman AR et al (2014) Biochar-supported zerovalent iron for removal of various contaminants from aqueous solutions. Bioresour Technol 152:538–542. https://doi.org/10.1016/j.biortech.2013.11.021
Zhou Z, Liu YG, Liu SB et al (2017) Sorption performance and mechanisms of arsenic(V) removal by magnetic gelatin-modified biochar. Chem Eng J 314:223–231. https://doi.org/10.1016/j.cej.2016.12.113
Zhu N, Yan T, Qiao J, Cao H (2016) Adsorption of arsenic, phosphorus and chromium by bismuth impregnated biochar: Adsorption mechanism and depleted adsorbent utilization. Chemosphere 164:32–40. https://doi.org/10.1016/j.chemosphere.2016.08.036
Zhu X, Li Y, Wang X (2019) Machine learning prediction of biochar yield and carbon contents in biochar based on biomass characteristics and pyrolysis conditions. Bioresour Technol 288:121527. https://doi.org/10.1016/j.biortech.2019.121527
Zoroufchi Benis K, Motalebi Damuchali A, Soltan J, McPhedran KN (2020) Treatment of aqueous arsenic – a review of biochar modification methods. Sci Total Environ 739:139750. https://doi.org/10.1016/j.scitotenv.2020.139750
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. Shubhangi Umare: literature survey, judicial literature study, and written original draft. Ajay K. Thawait: review the article and editing. Sumit H. Dhawane: conceptualization, formal analysis, editing, supervision.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participant
Not applicable.
Consent for publication
All respective authors read and permitted the final manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Xianliang Yi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Umare, S., Thawait, A.K. & Dhawane, S.H. Remediation of arsenic and fluoride from groundwater: a critical review on bioadsorption, mechanism, future application, and challenges for water purification. Environ Sci Pollut Res 31, 37877–37906 (2024). https://doi.org/10.1007/s11356-024-33679-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-33679-y