Abstract
Associations of perchlorate, thiocyanate, and nitrate exposures with bone mineral density (BMD) in adults have not previously been studied. This study aimed to estimate the associations of individual and concurrent exposure of the three chemicals with adult BMD. Based on National Health and Nutrition Examination Survey (NHANES, 2011–2018), 1618 non-pregnant adults (age ≥ 20 years and 47.0% female) were included in this study. Survey-weighted linear regression models were used to estimate individual urinary perchlorate, thiocyanate, and nitrate concentrations with lumbar spine BMD and total BMD in adults. Then, weighted quantile sum (WQS) regression and Bayesian kernel machine regression (BKMR) models were conducted to evaluate associations of co-occurrence of the three chemicals with adult BMD. In all participants, nitrate exposure was inversely associated with lumbar spine BMD (β = − 0.054, 95%CI: − 0.097, − 0.010). In stratification analyses, significant inverse associations were observed in female and participants older than 40 years old. In WQS regressions, significant negative associations of the weighted sum of the three chemicals with total and lumbar spine BMD (β = − 0.014, 95%CI: − 0.021, − 0.007; β = − 0.011, 95%CI: − 0.019, − 0.004, respectively) were found, and the dominant contributor was nitrate. In the BKMR models, non-linear dose–response associations of nitrate exposure with lumbar spine and total BMD were observed. These findings suggested that environmental perchlorate, thiocyanate, and nitrate exposure may reduce adult BMD and nitrate is the main contributor.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In light of worldwide aging population, osteoporosis and fracture have led to increasing economic burden on health care (Clynes et al. 2020). Bone mineral density (BMD) is a widely used measurement for bone health status (Medina-Gomez et al. 2018). Total body and lumbar spine are two preferred sites suitable for BMD measurement from adolescents to old age (Medina-Gomez et al. 2018). Reduction in BMD is a significant risk factor for osteoporosis (Lane 2006), which increases bone fragility and susceptibility to fracture (Clynes et al. 2020). Although genetic and lifestyle factors are well-known determinants of BMD (Trajanoska and Rivadeneira 2019; Weaver et al. 2016), recent studies indicated that BMD is extremely sensitive to background levels of environmental pollutants (Buha et al. 2019; Yang et al. 2023). The clinical consequences and economic burden of bone density loss require identification and management of potential risk factors.
Perchlorate (Cl \({{\text{O}}}_{4}^{-}\)), thiocyanate (SC \({{\text{N}}}^{-}\)), and nitrate (N \({{\text{O}}}_{3}^{-}\)) are ubiquitous in environment (Kumarathilaka et al. 2016; Qin et al. 2014; Singh et al. 2022). Perchlorate is a strong oxidizing agent and can be formed by natural processes and is extensively used in rocket fuel, fireworks, and other industrial products (Baldieri et al. 2023; Kumar et al. 2022; Sijimol and Mohan 2014). Industrial use of perchlorate is persistent in environmental media (Kumarathilaka et al. 2016). Thiocyanate, a toxic substance containing sulfur, carbon, and nitrogen, is a common pollutant in industrial wastewaters from mine, printing, or coke industries (Wang et al. 2022). Additionally, cyanogenic glucosides in plant foods and cyanide in cigarette smoke and vehicle exhaust can also be metabolized into thiocyanate (Bhandari et al. 2014; Lee and Kwon 2009; Narkowicz et al. 2018; Oluwole and Oludiran 2013). As water-soluble inorganic salt, nitrate concentrations are frequently exceeded the surface and groundwater limits all over the world including China (Bishayee et al. 2022; Zhang et al. 2021). Intensive use of fertilizers in agriculture and industrial waste are the major sources of nitrate pollution in the aquatic environment (Abascal et al. 2022). Besides, nitrate is widely used as preservatives in meat products (Chazelas et al. 2022) and inorganic nitrate is abundant in vegetables (Hord et al. 2009). As results of naturally occurring and anthropogenic contamination, the three chemicals are ubiquitous in environmental media and can cause adverse impacts on human health (Serrano-Nascimento and Nunes 2022). The general public is typically exposed to perchlorate, thiocyanate, and nitrate via dietary ingestion, besides, thiocyanate can be a major product formed from cyanide that enters into the body (Cengiz et al. 2022; Huber et al. 2011; IARC 2018). Urinary concentrations of the perchlorate, thiocyanate, and nitrate are commonly used as biomarkers to evaluate their exposure status (Lau et al. 2013).
As well-known NIS uptake inhibitors, previous studies have demonstrated that perchlorate, thiocyanate, and nitrate can competitively inhibit iodine intake and affect thyroid hormones synthesis (King et al. 2022; Suh et al. 2014). The essential role of thyroid hormones in adult bone maintenance is well established (Williams and Bassett 2018). Functional integrity and structure of adult skeleton is maintained by the bone remodeling cycle (Raggatt and Partridge 2010). The skeleton is an exquisitely sensitive target tissue for thyroid hormones (Bassett and Williams 2016). Circulating thyroid hormones enter the nucleus and regulate bone maintenance via binding and activating the thyroid hormone receptors α and β expressed in the skeleton (Bassett and Williams 2016; Gogakos et al. 2010). Skeletal response to thyroid hormones are incompletely characterized but involve the Ihh-PTHrP feedback loop (Stevens et al. 2000), growth hormone, insulin-like growth factor 1 (O'Shea et al. 2005), fibroblast growth factor receptor signaling pathways (Barnard et al. 2005; Stevens et al. 2003), and the Wnt/beta-catenin pathway (Wang et al. 2007). A series of studies have demonstrated the consequences of thyroid hormone variation on BMD and bone health in adults (Delitala et al. 2020; Williams and Bassett 2018). Thyroid dysfunction including hypothyroidism and hyperthyroidism can influent bone turnover reducing bone density and enhance fracture susceptibility (Delitala et al. 2020; Vestergaard and Mosekilde 2003). Besides, subclinical thyroid disease can also cause BMD reduction and increased risk of fracture (Apostu et al. 2020; Xu et al. 2020). Moreover, variation of thyroid hormones even across normal reference ranges were also related to low BMD and increased risk of fracture (Aubert et al. 2017).
Accordingly, it is reasonable to speculate that perchlorate, thiocyanate, and nitrate exposure may affect BMD. A previous study conducted in threespine stickleback (Gasterosteus aculeatus) found that perchlorate exposure beginning at 21 days post fertilization until sexual maturity at 1 year of age can cause abnormal skeletal morphology of adult fish (Furin et al. 2015). People gradually lose bone mass; previous studies showed that low BMD or deceases of BMD in adults give rise to enhanced risk of osteoporosis in old age (Xie et al. 2022; Xue et al. 2020). Thus, it is important to pay attention to the effects of the three chemical exposures on BMD in adults. However, to date, population-based studies focused on perchlorate, thiocyanate, and nitrate exposure with BMD in adults are scarce. Moreover, previous studies suggested that perchlorate, thiocyanate, and nitrate can work together in inhibiting iodine uptake (Horton et al. 2015; King et al. 2022; Tonacchera et al. 2004). Thus, exposures of the three chemicals should been considered together and multipollutant analysis models are needed.
In this study, urinary perchlorate, thiocyanate, and nitrate concentrations of 1618 non-pregnant adults (≥ 20 years) from the National Health and Nutrition Examination Survey (NHANES) were measured. Lumbar spine BMD and total BMD of them were measured in the mobile examination centers. Based on data of NHANES from 2011 to 2018, relationships of perchlorate, thiocyanate, and nitrate exposures with BMD were estimated.
Methods
Study population from NHANES
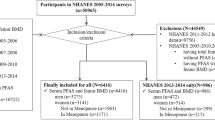
This study was based on the NHANES program, which is a national survey that examines a nationally representative sample of about 5,000 persons each year and became a continuous program since 1999 to meet emerging needs. Data of NHANES was released after data cleaning, documentation and Disclosure Review Board (DRB) reviewing and was publicly available in biennial cycles. In this study, data of four biennial cycles (2011–2012, 2013–2014, 2015–2016, and 2017–2018) was included. Participants (age ≥ 20 years old) with missing information on covariates or BMD examination results were excluded. Besides, those who did not measure urinary perchlorate, thiocyanate, nitrate, iodine, or creatinine concentrations were also excluded. The flow chart of participant selection was presented in Fig. S1. Finally, 1618 participants were included for final analysis. The NHANES protocol was approved by the National Center for Health Statistics (NCHS) Ethic Review Board (previously known as: NCHS Research Ethics Review Board and the NHANES Institutional Review Board).
Measurement of urinary perchlorate, thiocyanate, nitrate, iodine, and creatinine concentrations according to NHANES procedures
Ion chromatography with tandem mass spectrometry (IC-MS/MS) was used to measure urinary perchlorate, thiocyanate, and nitrate concentrations. The urinary iodine concentration was evaluated by inductively coupled plasma dynamic reaction cell mass spectroscopy (ICP-DRC-MS). Besides, an enzymatic method was used to measure the urinary creatinine concentration using Roche/Hitachi Mod P in 2011–2012 and Roche/Hitachi Cobas 6000 chemistry analyzer in 2013–2018. Detailed procedures for urine sample collecting and preparation, chemicals and reagents, equipment and instrumentation, and quality control are available at https://www.cdc.gov/nchs/nhanes/index.htm.
The lower limit of detection (LLOD) was 0.05 ng/mL for perchlorate, 20 ng/mL for thiocyanate, 2.4 ng/mL for iodine, 700 ng/mL for nitrate, and 5.00 mg/dL for creatinine. For target chemical concentrations below LLOD, the imputed fill values (LLOD/sqrt(2)) were used for replacement.
Bone mineral density and covariates from NHANES
Bone measurements (including lumbar spine BMD and total BMD) were obtained using Dual-energy x-ray absorptiometry (DXA). The DXA examinations were conducted by trained and certified radiology technologists. Further details of the DXA examination protocol, quality control, and data editing were located on the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm).
Osteoporosis was defined as a lumbar spine BMD value of more than 2.5 standard deviations (SDs) below the mean of male and female participants aged between 20 and 29 years. Osteopenia was further evaluated as a lumbar spine BMD value that was between 1 and 2.5 SDs below the mean of male and female participants aged between 20 and 29 years (Compston et al. 2019; Kanis 1994; Mäkitie and Zillikens 2022).
Demographics variables of individual family, and household level information were obtained in home by trained interviewers using Computer-Assisted Personal Interviewing (CAPI) system. Information about lifestyles (for example: physical activity, alcohol assumption, and smoking status) were collected using questionnaires, which were administered to participants both at home and in the mobile examination centers (MECs). With regard to physical activity, suggested metabolic equivalent (MET)-minutes scores for the physical activities were given by NHANES survey. We further quantified the physical activity into three categories (insufficient: no regular physical activity or some physical regular activity but less than 500 MET-minutes per week, moderate: 500–1000 MET-minutes regular activity per week, high: more than 1000 MET-minutes regular activity per week) based on the 2008 Physical Activity Guidelines for Americans (CDC 2008). In addition, medical conditions (for example: blood pressure, diabetes, arthritis, and thyroid problem), prescription medication intake, and menopausal status of participants were also collected using questionnaires. In NHANES survey, menopausal status was determined based on the responses to the questionnaire on reproductive health. Women were asked, “Have you had at least one menstrual period in the past 12 months? (Please do not include bleedings caused by medical conditions, hormone therapy, or surgeries)”. Participants answered “No” to this question were subsequently asked, “What is the reason that you have not had a period in the past 12 months?”. Women who answered the reason as menopause/hysterectomy were defined as menopausal women.
Statistical analysis
The NHANES is a complex, multistage, probability sample design survey which requires consideration of weights in statistical analysis (for details, see https://wwwn.cdc.gov/nchs/nhanes/tutorials/Weighting.aspx). Weighted means with standard error (SE) were used to describe the distributions of continuous variables. As for categorical variables, numbers (n) and weighted percentages (%) were used to describe their distributions. Then, distributions of variables were presented by gender. The gender differences of categorical variables were estimated using Rao-Scott χ2 test and gender differences of continuous variables were evaluated by weighted linear regression models.
Distributions of urinary nitrate, perchlorate, and thiocyanate concentrations were presented with geometric means, percentiles, and range. Concentrations of these three chemicals were adjusted for urinary creatinine (CR) concentrations to compensate the variation in urine dilution. Creatinine adjustment of the chemical was based on the following equation (Cone et al. 2009):
CR-adjusted concentrations of the three chemicals were log10-transformed before introduction in regression models to reduce the effect of extreme values.
Covariates were selected using a step-wise backward elimination approach (Atashili and Ta 2007). This method starts with a full model that considers all of the variables to be included in the model. Variables then are deleted one by one if the estimate for exposure-outcome relationship altered less than 10% after dropping the target confounder. All remaining variables that have significant contribution to the outcome were selected as covariates (Table S1). Based on the stepwise backward elimination approach, the final models were adjusted for age at interview, race, gender, body mass index (BMI), education level, family income, alcohol assumption, smoking status, physical activity, prescription medication intake, menopause status, and urinary iodide level of participants.
Firstly, the survey-weighted linear regression model was used to estimate the association of individual perchlorate, thiocyanate, and nitrate exposure with BMD. Logistic regression model was used to estimate the association between perchlorate, thiocyanate, and nitrate exposure with osteopenia and thyroid dysfunction. Stratified analyses by gender and age were then conducted and P value for interaction (interaction term of perchlorate, thiocyanate, and nitrate exposure with gender or age was included in regression models) was estimated. Secondly, the association of co-exposure to the three chemicals with BMD was evaluated using weighted quantile sum (WQS) regression. A weighted index of the three chemicals was constructed using WQS regressions, which represents the whole burden of all three chemicals. Then, the corresponding weight of each chemical to the WQS index was calculated. The WQS regression is a weighted quartile sum approach in conjunction with linear regression, but cannot address the non-linear associations of the three chemicals with BMD (Carrico et al. 2015). Therefore, Bayesian kernel machine regression (BKMR) models were then conducted to estimate the non-linear associations of co-exposure to perchlorate, thiocyanate, and nitrate with BMD and the potential interaction effects among the three chemicals (Bobb et al. 2018).
Sensitive analyses were conducted to estimate the stability of associations of perchlorate, thiocyanate, and nitrate exposure with BMD and osteopenia by the following: (1) Excluding women with hypertension, arthritis, or thyroid problem; (2) excluding postmenopausal women.
Statistical Analysis System (SAS statistical software, 9.4 version, SAS Institute Inc., Cary, USA) and the R software (4.0.3 version, R Development Core Team, Vienna, Austria) were used for statistical analyses.
Results
Basic characteristics
The basic characteristics are presented in Table 1. A total of 1618 participants were included with an average age of 38.9 ± 0.55 years. More than half of these people are non-Hispanic white (66.5%), well-educated (66.1% had an above high school education), and never smoke (59.4%). Of all the participants, 347 (weighted: 23.2%) were diagnosed with hypertension, 121 (weighted: 6.7%) with diabetes, 98 (weighted: 7.9%) with thyroid problem, 7 (0.43%) with osteoporosis, and 327 (20.2%) with osteopenia. Mean lumbar spine and total BMD for all participants was 1.03 (± 0.15) and 1.12 (± 0.11) g/cm, respectively. The median concentration of lumbar spine and total bone mineral content was 57.4 g and 2320.7 g, respectively. Women (n = 760) in this study had significantly higher rate of overweight, higher education level, and less alcohol assumption than male (n = 858) (P < 0.05).
Distributions of urinary perchlorate, thiocyanate, and nitrate concentrations
Distributions of urinary nitrate, perchlorate, and thiocyanate concentrations are described with geometric means, quartiles, and range (Table 2). Perchlorate and thiocyanate and were detected in all samples, and nitrate were detectable in 99.9% samples. The median (25th–75th percentiles) creatinine-adjusted concentration for perchlorate, thiocyanate, and nitrate was 2.85 (1.82–4.78) ng/mL, 1,258 (674–2,686) ng/mL, and 46,482 (33,554–66,708) ng/mL, respectively.
Associations of perchlorate, thiocyanate, and nitrate exposure with BMD: linear regression models
Associations of individual perchlorate, thiocyanate, and nitrate exposure with BMD were estimated using survey-weighted linear regression models (Fig. 1). Among all participants, nitrate exposure was associated with reduction of lumbar spine (β = − 0.054, 95%CI: − 0.097, − 0.010) after adjustment of potential confounders. Perchlorate and thiocyanate exposure was inversely related to total BMD though not significant after adjusting for potential confounders. When stratified by gender, significantly negative associations of nitrate exposure with lumbar spine and total BMD in female were observed. However, no significant interaction effect of perchlorate, thiocyanate, or nitrate exposure with gender on BMD was observed (P for interaction > 0.05). When stratified by age, nitrate exposure was inversely associated with lumbar spine and total BMD (β = − 0.063, 95%CI: − 0.124, − 0.001; β = − 0.039, 95%CI: − 0.078, − 0.001, respectively) in participants aged 40–60 years, but no significant interaction effect of perchlorate, thiocyanate, or nitrate exposure with age on BMD was observed (P for interaction > 0.05).
Associations of urinary perchlorate, thiocyanate, and nitrate concentrations with lumbar spine BMD (A) and total BMD (B) (n = 1618). The models were adjusted for age at interview, race, gender, body mass index (BMI), education level, family income, alcohol assumption, smoking status, physical activity, prescription medication intake, menopause status, and urinary iodide level of participants. Psex-int-u: unadjusted interaction P value for chemical exposure and sex, Psex-int-a: adjusted interaction P value for chemical exposure and sex, Page-int-u: unadjusted interaction P value for chemical exposure and age, Page-int-a: adjusted interaction P value for chemical exposure and age. *: P < 0.05
Associations of perchlorate, thiocyanate, and nitrate exposure with osteopenia and thyroid dysfunction: logistic regression models
Associations of perchlorate, thiocyanate, and nitrate exposure with osteopenia were estimated using survey-weighted logistic regression models (Table S2). After adjustment of potential confounders, thiocyanate and nitrate exposures were associated with increased risk of osteopenia though not significant (OR = 1.44, 95%CI: 0.56, 3.66, OR = 1.32, 95%CI: 0.29, 6.00). When stratified by age and gender, no significant association of exposure to the three chemical with osteopenia was found in any of the groups. Associations of exposures to the three chemicals with thyroid dysfunction are presented in Table S3, but no significant associations of the three chemical exposures with thyroid dysfunction were found.
Co-exposure to perchlorate, thiocyanate, and nitrate with BMD: WQS regression analysis
Associations of co-exposure of the three chemicals with BMD are shown in Fig. 2. After adjusting for potential confounders, the regression coefficient for every unit increase of WQS index with lumbar spine BMD was − 0.014 (95%CI: − 0.021, − 0.007). The WQS results suggested that nitrate was the dominant contributor to the reduction of lumbar spine BMD (WQS index weight: 94.0%), followed by perchlorate (WQS index weight: 4.5%) and thiocyanate (WQS index weight: 1.5%) (Fig. 2, Part A). Besides, a significantly inverse association of the weighted sum of the three chemicals with total BMD (β = − 0.011, 95%CI: − 0.019, − 0.004) was found in WQS regression analysis. The main contributor to reduction in total BMD was nitrate (WQS index weight: 77.1%), followed by thiocyanate (WQS index weight: 20.7%) and perchlorate (WQS index weight: 4.6%) (Fig. 2, Part B).
Associations of perchlorate, thiocyanate, and nitrate co-exposure with lumbar spine BMD (A) and total BMD (B) using weighted quantile sum (WQS) regression models. The models were adjusted for age at interview, race, gender, body mass index (BMI), education level, family income, alcohol assumption, smoking status, physical activity, prescription medication intake, menopause status, and urinary iodide level of participants
Co-exposure to perchlorate, thiocyanate, and nitrate with BMD: BKMR model
BKMR model showed a negative overall association of the three chemicals co-exposure with lumbar spine BMD (Fig. 3, Part A). Urinary nitrate concentration (75th percentile vs 25th percentile) was significantly related to decreased lumbar spine BMD when perchlorate and thiocyanate were at their 25th, 50th, or 75th percentile level (Fig. 3, Part B). The exposure–response results also showed a non-linear dose–response association between nitrate exposure and lumbar spine BMD with the other two chemicals at their 50th percentiles, but no significant associations of perchlorate and thiocyanate exposure with lumbar spine BMD were observed (Fig. 3, Part C). The associations between the perchlorate, thiocyanate, or nitrate exposure and lumbar spine BMD showed no differences when the chemical on right longitudinal axis fixed at different levels (25th, 50th, and 75th percentiles) and the remaining chemical at the median (Fig. 3, Part D). Therefore, no significant interaction effect among the three chemicals exposures on lumbar spine BMD was observed. Similarly, co-exposure of the three chemicals was negatively associated with total BMD. A non-linear relationship of nitrate exposure and total BMD was found and no significant interaction effect of the three chemicals exposures on total BMD was found (Fig. 4).
Associations of urinary perchlorate, thiocyanate, and nitrate concentrations with adult lumbar spine BMD using BKMR model. The models were adjusted for age at interview, race, gender, body mass index (BMI), education level, family income, alcohol assumption, smoking status, physical activity, prescription medication intake, menopause status, and urinary iodide level of participants. A The Y-axis represents estimates difference (95% CI) in outcome when all chemicals were fixed at specific percentile (X-axis) comparing to chemicals all at their 50th percentiles. B Estimates and 95% CI of each chemical exposure (25th percentile vs 75th percentile) with outcome when all other chemicals fixed at their 50th percentiles. C The univariate exposure–response association of single chemical exposure with outcome when others fixed at the 50th percentiles. D The bivariate exposure–response associations of single chemical exposure with outcome when the chemical on right longitudinal axis fixed at the 25th (red), 50th (green), and 75th (blue) percentiles and the remaining chemical at the median. Abbreviations: Cl \({{\text{O}}}_{4}^{-}\), perchlorate; SC \({{\text{N}}}^{-}\), thiocyanate; N \({{\text{O}}}_{3}^{-}\), nitrate
Associations of urinary perchlorate, thiocyanate, and nitrate concentrations with adult total BMD using BKMR model. The models were adjusted for age at interview, race, gender, body mass index (BMI), education level, family income, alcohol assumption, smoking status, physical activity, prescription medication intake, menopause status, and urinary iodide level of participants. A The Y-axis represents estimates difference (95% CI) in outcome when all chemicals were fixed at specific percentile (X-axis) comparing to chemicals all at their 50th percentiles. B Estimates and 95% CI of each chemical exposure (25th percentile vs 75th percentile) with outcome when all other chemicals fixed at their 50th percentiles. C The univariate exposure–response association of single chemical exposure with outcome when others fixed at the 50th percentiles. D The bivariate exposure–response associations of single chemical exposure with outcome when the chemical on right longitudinal axis fixed at the 25th (red), 50th (green), and 75th (blue) percentiles and the remaining chemical at the median. Abbreviations: Cl \({{\text{O}}}_{4}^{-}\), perchlorate; SC \({{\text{N}}}^{-}\), thiocyanate; N \({{\text{O}}}_{3}^{-}\), nitrate
Sensitive analysis
Associations of perchlorate, thiocyanate, and nitrate exposure with BMD after excluding participants with hypertension, arthritis, and thyroid problem are presented in Table S4. Similar to results among all participants, nitrate exposure was significantly associated with decreased lumbar spine and total BMD. Moreover, thiocyanate exposure was inversely associated with total BMD though not significant after adjusting for potential confounders. When stratified by gender, nitrate exposure was associated with decreased BMD in male and female though not significant after adjusting for potential confounders. When stratified by age, significant inverse associations of nitrate exposure with lumbar spine and total BMD, thiocyanate exposure with total BMD were observed in participants aged 40–60 after adjusting for potential confounders. Table S5 presents associations of exposure to the three chemicals with osteopenia restricted to women without hypertension, arthritis, and thyroid problem. Significant positive trends of thiocyanate and nitrate exposures were associated with the risk of osteopenia were observed though not significant. When stratified by age and gender, no significant association of exposure to the three chemical with osteopenia was found in any of the groups.
Associations of perchlorate, thiocyanate, and nitrate exposure with BMD after excluding postmenopausal women are presented in Table S6. The significantly negatively association between nitrate exposure and lumbar spine BMD still existed (β = − 0.045, 95%CI: − 0.086, − 0.004) after adjustment of potential confounders. Similarly, no significant associations of exposure to the three chemicals with osteopenia were found after excluding postmenopausal women (Table S7).
Discussion
To the best of our knowledge, this is the first study that evaluated associations of exposure to perchlorate, thiocyanate, and nitrate with lumbar spine and total BMD in adults. The results showed that urinary nitrate concentration was significantly inversely associated with lumbar spine and total BMD. Moreover, co-exposure to the three chemicals was inversely associated with BMD and nitrate was the dominant contributor to the reduction of BMD.
Previous studies conducted in animals found that perchlorate exposure was associated with some bony characters, but relevant epidemiological studies are limited (Bernhardt and von Hippel 2008; Bernhardt et al. 2006, 2011; Furin et al. 2015). A previous study conducted in fish found that perchlorate exposure at greater than 12 mg/L caused bony structure abnormalities and a concentration-dependent manner was observed (Bernhardt et al. 2011). Another study gave fish contaminated water with perchlorate (30 or 100 mg/mL) at different time points (beginning at 0, 3, 7, 14, 21, 42, 154, or 305 days post fertilization until sexual maturity at 1 year of age) also found that perchlorate exposure was related to some skeletal traits and concentration of perchlorate was more important than exposure time for disruption of skeletal traits (Furin et al. 2015). These findings suggest that there may be a threshold concentration that triggers a physiological change. In our study, no significant associations of perchlorate exposure (median concentration: 2.1 ng/mL) with lumbar spine BMD and total BMD in adults were observed. Perchlorate has been widely used in firework, fertilizers, and other products (Calderón et al. 2022; Wu et al. 2011). Perchlorate is highly stable in the environment and can be found in water, soil, and foods (Kumarathilaka et al. 2016). General population mainly exposed perchlorate through dietary ingestion (ATSDR 2008). For NHANES participants, food was the major source of perchlorate in most people (Lau et al. 2013). The median urinary perchlorate concentrations in this study are lower than that of adults from other countries (King et al. 2023). These findings indicated that perchlorate pollution in the US is better than other countries. The null associations of perchlorate exposure with BMD in this study may be due to the low exposure level of perchlorate. Besides, the duration and consistency of perchlorate exposure was unknown in this study, chronic exposure of perchlorate is most likely to cause any measurable effects on bone tissue due to the short half-life of perchlorate (NRC 2005).
In this study, no significant associations of thiocyanate exposure with lumbar spine and total BMD were found. Previous studies focused on thiocyanate exposure with bone health are scarce though some studies reported the effect of thiocyanate on iodine uptake inhibition (Allain and McGregor 1993; Bassett and Williams 2016). However, the clinically meaningful reduction of iodine uptake requires a dose of thiocyanate at 200–400 mg (Reiter and Härnulv 1984). Thiocyanate exposure at low level is not likely to have adverse effects on thyroid function in healthy individuals. Moreover, it is worth noting that thiocyanate is a metabolite of multiple sources. On the one hand, thiocyanate can be a metabolite of cyanide in tobacco (Bhandari et al. 2014; Narkowicz et al. 2018), which can impair bone homeostasis (Lu et al. 2021). On the other hand, green vegetables can also be metabolized into thiocyanate (Lee and Kwon 2009; Oluwole and Oludiran 2013). Green vegetable intake is beneficial for bone health (Rondanelli et al. 2021), which may modify the negative effect of thiocyanate on bone health. Considering the complexity of thiocyanate exposure routes, the effect of thiocyanate exposure on BMD may be related to a variety of factors such as dietary pattern and cyanide exposure.
Nitrate exposure was significantly associated with reduction of lumbar spine and total BMD in adult and WQS regression results showed that nitrate was the main contributor in co-exposure of the three chemicals with reduction of BMD. Previous studies on NIS inhibitors and possible health effects mainly focused on perchlorate due to its powerful ability of inhibiting iodide uptake (15 and 240 times to thiocyanate and nitrate, respectively) (Tonacchera et al. 2004). However, a growing number of studies showed that concentrations of thiocyanate and nitrate are much high than perchlorate and may account for a much larger proportion of iodine uptake inhibition (King et al. 2023; Serrano-Nascimento and Nunes 2022; Yu et al. 2022). Nitrate concentration in this study is over 400 and 15,000 times higher than thiocyanate and perchlorate, respectively. High exposure level of nitrate may partly explain the dominant reduction effect of nitrate on BMD in this study.
In BKMR models, nitrate at low concentration was positively associated with BMD though not statistically significant, and significantly negative association was found with increasing of nitrate concentration. Previous studies focused on nitrate exposure with bone health showed conflicting results (Conley et al. 2017; Golchin et al. 2016; Jamal et al. 1998, 2009; Liu et al. 2022). People generally expose to nitrate via ingestion of food and water that are contaminated by it (IARC 2018). Vegetables, especially leafy vegetables, have been identified as a major source of nitrate in diet (ATSDR 2017). Nitrate-rich foods were used to reduce bone loss since dietary nitrate can enhance nitric oxide (NO) bioavailability (Yousefzadeh et al. 2022). The NO-cyclic guanosine monophosphate (cGMP)-protein kinase G (PKG) pathway plays an important role in bone health (Kim et al. 2021). Some studies reported high BMD and lower rates of bone turnover in people using nitrate (Jamal et al. 1998, 2009), and some studies reported no effect of nitrate supplement on bone health (Conley et al. 2017; Golchin et al. 2016). However, the use of related fertilizers can result in high concentrations of nitrate in soil, water, and agricultural products (Luo et al. 2022; Sarkar et al. 2021; Zhang et al. 2015). Human intake of large quantities of nitrate-contaminated water and food may cause disruption of thyroid hormone levels (King et al. 2022; Xie et al. 2019), which is critical for bone health (Zhu et al. 2022). In addition to thyroid hormones, previous studies found that nitrate can also affect steroid hormone levels (Poulsen et al. 2018). The skeleton is a complex tissue, and hormonal control of bone remodeling is elaborate (Bandeira et al. 2010; Noble 2016). Steroid hormones can exert action on osteoblasts by interacting with specific receptor proteins (Huang and Zheng 1999). Disruption of steroid hormones may also attribute to the impact of nitrate exposure on BMD. The discrepancy of nitrate exposure with BMD is likely to depend on the complicated mechanisms of nitrate role in bone health at different levels. Additional studies are needed to confirm the findings in this study and verify the potential mechanisms.
Associations of thiocyanate and nitrate exposure with BMD are more pronounced in females and participants older than 40 years in this study. Males are likely to have stronger bones and the lifetime risk of fracture was lower than females (Nguyen et al. 2007). Epidemiological studies have reported a progressively rise of osteoporosis occurrence in males but a sharply increased risk of osteoporosis in females after menopause was found (Tewari et al. 2022). Differences between females and males in bone architecture (Gabel et al. 2017), bone density (Gabel et al. 2017), and sex hormones (Venken et al. 2008) may responsible for the vulnerability of females to osteoporosis. Besides, a gradual increase in the occurrence of osteoporosis was found with advancing age (Clynes et al. 2020). The major reason of increasing risk of osteoporosis in the older is the decline in metabolism combined with reduced calcium absorption and low physical activity (Moayyeri 2008; Pattanaungkul et al. 2000). Females and the elderly are at high risk of BMD reduction and are likely to be more sensitive to environmental perchlorate, thiocyanate, and nitrate exposures. No significant associations between perchlorate, thiocyanate, and nitrate exposure with osteopenia were found in this study. The potential reason for this result is that age range of the population in this study (mean ± SD: 38.9 ± 0.55) usually are not at the risk of osteoporosis or osteopenia. Though no significant associations of perchlorate, thiocyanate, and nitrate exposures with osteopenia were found, it is worth noting that low BMD in youth may be the single most important factors leading to the development of osteoporosis in the elderly (Lane et al. 2000; Xue et al. 2020). Therefore, the effects of environmental perchlorate, thiocyanate, and nitrate exposures on bone health on the young and middle-aged people are also important.
Moreover, no significantly associations of the three analyte exposures with thyroid dysfunction were found in this study. The rate of thyroid dysfunction in US adults reported in this paper are similar to the levels reported in other studies (Zhang et al. 2024). As well-known NIS inhibitors, previous studies reported that the three analyte exposures may affect thyroid hormone levels (King et al. 2023; Suh et al. 2014), but relationships of the three analyte exposures with thyroid function are not consistent (Braverman et al. 2005; Bruce et al. 2013; Chen et al. 2009; Inada et al. 1983; Tarone et al. 2010; Ward et al. 2010). Some studies have shown that perchlorate, thiocyanate, or nitrate exposure increases the risk of thyroid dysfunction (Chen et al. 2009; Inada et al. 1983; Ward et al. 2010), but others have found that exposure of the three chemicals but does not cause thyroid dysfunction (Braverman et al. 2005; Bruce et al. 2013). Variation of thyroid hormones even across normal reference ranges were also related to low BMD and increased risk of osteoporosis (Aubert et al. 2017). However, thyroid hormones were only measured during the biennial cycle of 2011–2012; thus, associations of perchlorate, thiocyanate, and nitrate exposures with thyroid hormones are not included in this study. Further studies are needed to estimate the mediation roles of thyroid hormones in perchlorate, thiocyanate, and nitrate exposure with BMD and osteoporosis.
The main strength of this study is that we evaluated individual and combined effects of perchlorate, thiocyanate, and nitrate exposure on adult BMD. Besides, contributions of the three chemicals for reduction of BMD were identified. Moreover, potential non-linear and interactions of the three chemicals in relation to BMD were also estimated in this study. However, this study has limitations too. Firstly, sensitive windows of vulnerability to perchlorate, thiocyanate, and nitrate exposure with BMD in this study were not evaluated due to its cross-sectional design. Further studies with prospective design are needed to estimate the sensitive window of perchlorate exposure with BMD. Moreover, potential mechanisms of the three chemicals exposures with BMD were not measured in this study; future studies are needed to clarify the potential pathways. Besides, there are still some unmeasured confounding factors though we adjusted for as many confounders as possible. Moreover, this study was conducted among adults; future studies conducted in sensitive populations such as the elderly and postmenopausal women are needed.
Conclusion
This study demonstrated that exposure to a mixture of perchlorate, thiocyanate, and nitrate may reduce BMD of adults and nitrate seems to be the dominant contributor.
Data availability
The data that support the findings of this study are available in National Health and Nutrition Examination Survey (NHANES) at https://www.cdc.gov/nchs/nhanes/index.htm.
References
Abascal E, Gómez-Coma L, Ortiz I, Ortiz A (2022) Global diagnosis of nitrate pollution in groundwater and review of removal technologies. Sci Total Environ 810:152233. https://doi.org/10.1016/j.scitotenv.2021.152233
Allain TJ, McGregor AM (1993) Thyroid hormones and bone. J Endocrinol 139:9–18. https://doi.org/10.1677/joe.0.1390009
Apostu D, Lucaciu O, Oltean-Dan D, Mureșan AD, Moisescu-Pop C, Maxim A et al (2020) The influence of thyroid pathology on osteoporosis and fracture risk: a review. Diagnostics (Basel) 10. https://doi.org/10.3390/diagnostics10030149
Atashili J, Ta MA (2007) SAS® macro for automating the ‘change-in-estimate’ strategy for assessing confounding. Paper 032–2007. SAS Global Forum 2007. https://support.sas.com/resources/papers/proceedings/proceedings/forum2007/032-2007.pdf
ATSDR (2008) Agency for toxic substances and disease registry. Toxicological Profile for Perchlorates 162:1–211. https://www.atsdr.cdc.gov/ToxProfiles/tp162.pdf
ATSDR (2017) Agency for toxic substances and disease registry. Toxicological Profile for Nitrate and Nitrite. https://www.atsdr.cdc.gov/ToxProfiles/tp204.pdf
Aubert CE, Floriani C, Bauer DC, da Costa BR, Segna D, Blum MR et al (2017) Thyroid function tests in the reference range and fracture: individual participant analysis of prospective cohorts. J Clin Endocrinol Metab 102:2719–2728. https://doi.org/10.1210/jc.2017-00294
Baldieri F, Martelli E, Riccio A (2023) A numerical study on carbon-fiber-reinforced composite cylindrical skirts for solid propeller rockets. Polymers (Basel) 15. https://doi.org/10.3390/polym15040908
Bandeira F, Lazaretti-Castro M, Bilezikian JP (2010) Hormones and bone. Arq Bras Endocrinol Metabol 54:85–6. https://doi.org/10.1590/s0004-27302010000200001
Barnard JC, Williams AJ, Rabier B, Chassande O, Samarut J, Cheng SY et al (2005) Thyroid hormones regulate fibroblast growth factor receptor signaling during chondrogenesis. Endocrinology 146:5568–80. https://doi.org/10.1210/en.2005-0762
Bassett JH, Williams GR (2016) Role of thyroid hormones in skeletal development and bone maintenance. Endocr Rev 37:135–87. https://doi.org/10.1210/er.2015-1106
Bernhardt RR, von Hippel FA (2008) Chronic perchlorate exposure impairs stickleback reproductive behaviour and swimming performance. Behaviour 145:537–559. https://doi.org/10.1163/156853908792451511
Bernhardt RR, von Hippel FA, Cresko WA (2006) Perchlorate induces hermaphroditism in threespine sticklebacks. Environ Toxicol Chem 25:2087–96. https://doi.org/10.1897/05-454r.1
Bernhardt RR, von Hippel FA, O’Hara TM (2011) Chronic perchlorate exposure causes morphological abnormalities in developing stickleback. Environ Toxicol Chem 30:1468–78. https://doi.org/10.1002/etc.521
Bhandari RK, Oda RP, Petrikovics I, Thompson DE, Brenner M, Mahon SB et al (2014) Cyanide toxicokinetics: the behavior of cyanide, thiocyanate and 2-amino-2-thiazoline-4-carboxylic acid in multiple animal models. J Anal Toxicol 38:218–25. https://doi.org/10.1093/jat/bku020
Bishayee B, Chatterjee RP, Ruj B, Chakrabortty S, Nayak J (2022) Strategic management of nitrate pollution from contaminated water using viable adsorbents: an economic assessment-based review with possible policy suggestions. J Environ Manag 303:114081. https://doi.org/10.1016/j.jenvman.2021.114081
Bobb JF, Claus Henn B, Valeri L, Coull BA (2018) Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Health 17:67. https://doi.org/10.1186/s12940-018-0413-y
Braverman LE, He X, Pino S, Cross M, Magnani B, Lamm SH et al (2005) The effect of perchlorate, thiocyanate, and nitrate on thyroid function in workers exposed to perchlorate long-term. J Clin Endocrinol Metab 90:700–6. https://doi.org/10.1210/jc.2004-1821
Bruce GM, Corey LM, Mandel JH, Pleus RC (2013) Urinary nitrate, thiocyanate, and perchlorate and serum thyroid endpoints based on NHANES 2001 to 2002. J Occup Environ Med 55:52–8. https://doi.org/10.1097/JOM.0b013e31826bb774
Buha A, Jugdaohsingh R, Matovic V, Bulat Z, Antonijevic B, Kerns JG et al (2019) Bone mineral health is sensitively related to environmental cadmium exposure- experimental and human data. Environ Res 176:108539. https://doi.org/10.1016/j.envres.2019.108539
Calderón R, Palma P, Arancibia-Miranda N, Kim UJ, Silva-Moreno E, Kannan K (2022) Occurrence, distribution and dynamics of perchlorate in soil, water, fertilizers, vegetables and fruits and associated human exposure in Chile. Environ Geochem Health 44:527–535. https://doi.org/10.1007/s10653-020-00680-6
Carrico C, Gennings C, Wheeler DC, Factor-Litvak P (2015) Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J Agric Biol Environ Stat 20:100–120. https://doi.org/10.1007/s13253-014-0180-3
CDC (2008) Centers for disease control and prevention. Physical activity guidelines for Americans. U.S. Department of Health and Human Services. Available from: https://health.gov/paguidelines/guidelines/
Cengiz MF, Sen F, Bilgin AK, Boyaci-Gunduz CP (2022) Determination of exposure to major iodide ion uptake inhibitors through drinking waters. Environ Res 204:112345. https://doi.org/10.1016/j.envres.2021.112345
Chazelas E, Pierre F, Druesne-Pecollo N, Esseddik Y, Szabo de Edelenyi F, Agaesse C et al (2022) Nitrites and nitrates from food additives and natural sources and cancer risk: results from the NutriNet-Santé cohort. Int J Epidemiol 51:1106–1119. https://doi.org/10.1093/ije/dyac046
Chen Y, McNabb FM, Sible JC (2009) Perchlorate exposure induces hypothyroidism and affects thyroid-responsive genes in liver but not brain of quail chicks. Arch Environ Contam Toxicol 57:598–607. https://doi.org/10.1007/s00244-009-9304-0
Clynes MA, Harvey NC, Curtis EM, Fuggle NR, Dennison EM, Cooper C (2020) The epidemiology of osteoporosis. Br Med Bull 133:105–117. https://doi.org/10.1093/bmb/ldaa005
Compston JE, McClung MR, Leslie WD (2019) Osteoporosis. Lancet 393:364–376. https://doi.org/10.1016/s0140-6736(18)32112-3
Cone EJ, Caplan YH, Moser F, Robert T, Shelby MK, Black DL (2009) Normalization of urinary drug concentrations with specific gravity and creatinine. J Anal Toxicol 33:1–7. https://doi.org/10.1093/jat/33.1.1
Conley MN, Roberts C, Sharpton TJ, Iwaniec UT, Hord NG (2017) Increasing dietary nitrate has no effect on cancellous bone loss or fecal microbiome in ovariectomized rats. Mol Nutr Food Res 61. https://doi.org/10.1002/mnfr.201600372
Delitala AP, Scuteri A, Doria C (2020) Thyroid hormone diseases and osteoporosis. J Clin Med 9. https://doi.org/10.3390/jcm9041034
Furin CG, von Hippel FA, Postlethwait J, Buck CL, Cresko WA, O’Hara TM (2015) Developmental timing of perchlorate exposure alters threespine stickleback dermal bone. Gen Comp Endocrinol 219:36–44. https://doi.org/10.1016/j.ygcen.2015.02.016
Gabel L, Macdonald HM, McKay HA (2017) Sex differences and growth-related adaptations in bone microarchitecture, geometry, density, and strength from childhood to early adulthood: a mixed longitudinal HR-pQCT study. J Bone Miner Res 32:250–263. https://doi.org/10.1002/jbmr.2982
Gogakos AI, Duncan Bassett JH, Williams GR (2010) Thyroid and bone. Arch Biochem Biophys 503:129–36. https://doi.org/10.1016/j.abb.2010.06.021
Golchin N, Hohensee C, LaCroix A, Gray SL (2016) Nitrate medications, fractures, and change in bone mineral density in postmenopausal women: results from the women’s health initiative. J Bone Miner Res 31:1760–6. https://doi.org/10.1002/jbmr.2838
Hord NG, Tang Y, Bryan NS (2009) Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr 90:1–10. https://doi.org/10.3945/ajcn.2008.27131
Horton MK, Blount BC, Valentin-Blasini L, Wapner R, Whyatt R, Gennings C et al (2015) CO-occurring exposure to perchlorate, nitrate and thiocyanate alters thyroid function in healthy pregnant women. Environ Res 143:1–9. https://doi.org/10.1016/j.envres.2015.09.013
Huang WH, Zheng MH (1999) Steroid hormones and bone. Histol Histopathol 14:1257–68. https://doi.org/10.14670/hh-14.1257
Huber DR, Blount BC, Mage DT, Letkiewicz FJ, Kumar A, Allen RH (2011) Estimating perchlorate exposure from food and tap water based on US biomonitoring and occurrence data. J Expo Sci Environ Epidemiol 21:395–407. https://doi.org/10.1038/jes.2010.31
IARC (2018) International agency for research on cancer. Ingested nitrate and nitrite. https://publications.iarc.fr/_publications/media/download/2875/f7a3a4932fab3d0e9b930dba0b6eb2c4e385a18e.pdf
Inada M, Nishikawa M, Kawai I (1983) Hypothyroidism associated with positive results of the perchlorate discharge test in elderly patients. Am J Med 74:1010–5. https://doi.org/10.1016/0002-9343(83)90803-3
Jamal SA, Browner WS, Bauer DC, Cummings SR (1998) Intermittent use of nitrates increases bone mineral density: the study of osteoporotic fractures. J Bone Miner Res 13:1755–9. https://doi.org/10.1359/jbmr.1998.13.11.1755
Jamal SA, Goltzman D, Hanley DA, Papaioannou A, Prior JC, Josse RG (2009) Nitrate use and changes in bone mineral density: the Canadian Multicentre Osteoporosis Study. Osteoporos Int 20:737–44. https://doi.org/10.1007/s00198-008-0727-7
Kanis JA (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report WHO Study Group. Osteoporos Int 4:368–81. https://doi.org/10.1007/bf01622200
Kim SM, Yuen T, Iqbal J, Rubin MR, Zaidi M (2021) The NO-cGMP-PKG pathway in skeletal remodeling. Ann N Y Acad Sci 1487:21–30. https://doi.org/10.1111/nyas.14486
King L, Huang Y, Li T, Wang Q, Li W, Shan Z et al (2022) Associations of urinary perchlorate, nitrate and thiocyanate with central sensitivity to thyroid hormones: a US population-based cross-sectional study. Environ Int 164:107249. https://doi.org/10.1016/j.envint.2022.107249
King L, Wang Q, Xia L, Wang P, Jiang G, Li W et al (2023) Environmental exposure to perchlorate, nitrate and thiocyanate, and thyroid function in Chinese adults: a community-based cross-sectional study. Environ Int 171:107713. https://doi.org/10.1016/j.envint.2022.107713
Kumar KS, Kavitha S, Parameswari K, Sakunthala A, Sathishkumar P (2022) Environmental occurrence, toxicity and remediation of perchlorate - a review. Chemosphere 311:137017. https://doi.org/10.1016/j.chemosphere.2022.137017
Kumarathilaka P, Oze C, Indraratne SP, Vithanage M (2016) Perchlorate as an emerging contaminant in soil, water and food. Chemosphere 150:667–677. https://doi.org/10.1016/j.chemosphere.2016.01.109
Lane NE (2006) Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol 194:S3-11. https://doi.org/10.1016/j.ajog.2005.08.047
Lane JM, Russell L, Khan SN (2000) Osteoporosis. Clin Orthop Relat Res 139–50. https://doi.org/10.1097/00003086-200003000-00016
Lau FK, deCastro BR, Mills-Herring L, Tao L, Valentin-Blasini L, Alwis KU et al (2013) Urinary perchlorate as a measure of dietary and drinking water exposure in a representative sample of the United States population 2001-2008. J Expo Sci Environ Epidemiol 23:207–14. https://doi.org/10.1038/jes.2012.108
Lee J, Kwon H (2009) Conversion of apricot cyanogenic glycosides to thiocyanate by liver and colon enzymes. Toxicol Res 25:23–28. https://doi.org/10.5487/tr.2009.25.1.023
Liu W, Meng Z, Wang G (2022) The efficacy of nitrates for bone health: a systematic review and meta-analysis of observational and randomized controlled studies. Front Endocrinol (Lausanne) 13:833932. https://doi.org/10.3389/fendo.2022.833932
Lu Y, Di YP, Chang M, Huang X, Chen Q, Hong N et al (2021) Cigarette smoke-associated inflammation impairs bone remodeling through NFκB activation. J Transl Med 19:163. https://doi.org/10.1186/s12967-021-02836-z
Luo F, Yan XJ, Hu XF, Yan LJ, Cao MY, Zhang WJ (2022) Nitrate quantification in fresh vegetables in shanghai: its dietary risks and preventive measures. Int J Environ Res Public Health 19. https://doi.org/10.3390/ijerph192114487
Mäkitie O, Zillikens MC (2022) Early-onset osteoporosis. Calcif Tissue Int 110:546–561. https://doi.org/10.1007/s00223-021-00885-6
Medina-Gomez C, Kemp JP, Trajanoska K, Luan J, Chesi A, Ahluwalia TS et al (2018) Life-course genome-wide association study meta-analysis of total body BMD and assessment of age-specific effects. Am J Hum Genet 102:88–102. https://doi.org/10.1016/j.ajhg.2017.12.005
Moayyeri A (2008) The association between physical activity and osteoporotic fractures: a review of the evidence and implications for future research. Ann Epidemiol 18:827–35. https://doi.org/10.1016/j.annepidem.2008.08.007
Narkowicz S, Jaszczak E, Polkowska Ż, Kiełbratowska B, Kotłowska A, Namieśnik J (2018) Determination of thiocyanate as a biomarker of tobacco smoke constituents in selected biological materials of human origin. Biomed Chromatogr 32. https://doi.org/10.1002/bmc.4111
Nguyen ND, Ahlborg HG, Center JR, Eisman JA, Nguyen TV (2007) Residual lifetime risk of fractures in women and men. J Bone Miner Res 22:781–8. https://doi.org/10.1359/jbmr.070315
Noble D (2016) Regulation of bone metabolism. Prog Biophys Mol Biol 122:83–84. https://doi.org/10.1016/j.pbiomolbio.2016.10.001
NRC (2005) The national academies. In: Health implications of perchlorate ingestion. Committee to assess the health implications of perchlorate ingestion, board on environmental studies and toxicology, division on earth and life studies. The National Academies Press, Washington, DC
O’Shea PJ, Bassett JH, Sriskantharajah S, Ying H, Cheng SY, Williams GR (2005) Contrasting skeletal phenotypes in mice with an identical mutation targeted to thyroid hormone receptor alpha1 or beta. Mol Endocrinol 19:3045–59. https://doi.org/10.1210/me.2005-0224
Oluwole OS, Oludiran AO (2013) Normative concentrations of urine thiocyanate in cassava eating communities in Nigeria. Int J Food Sci Nutr 64:1036–41. https://doi.org/10.3109/09637486.2013.825697
Pattanaungkul S, Riggs BL, Yergey AL, Vieira NE, O’Fallon WM, Khosla S (2000) Relationship of intestinal calcium absorption to 1,25-dihydroxyvitamin D [1,25(OH)2D] levels in young versus elderly women: evidence for age-related intestinal resistance to 1,25(OH)2D action. J Clin Endocrinol Metab 85:4023–7. https://doi.org/10.1210/jcem.85.11.6938
Poulsen R, Cedergreen N, Hayes T, Hansen M (2018) Nitrate: an environmental endocrine disruptor? A review of evidence and research needs. Environ Sci Technol 52:3869–3887. https://doi.org/10.1021/acs.est.7b06419
Qin X, Zhang T, Gan Z, Sun H (2014) Spatial distribution of perchlorate, iodide and thiocyanate in the aquatic environment of Tianjin, China: environmental source analysis. Chemosphere 111:201–8. https://doi.org/10.1016/j.chemosphere.2014.03.082
Raggatt LJ, Partridge NC (2010) Cellular and molecular mechanisms of bone remodeling. J Biol Chem 285:25103–8. https://doi.org/10.1074/jbc.R109.041087
Reiter B, Härnulv G (1984) Lactoperoxidase antibacterial system: natural occurrence, biological functions and practical applications. J Food Prot 47:724–732. https://doi.org/10.4315/0362-028x-47.9.724
Rondanelli M, Faliva MA, Barrile GC, Cavioni A, Mansueto F, Mazzola G et al (2021) Nutrition, physical activity, and dietary supplementation to prevent bone mineral density loss: a food pyramid. Nutrients 14. https://doi.org/10.3390/nu14010074
Sarkar S, Mukherjee A, Duttagupta S, Bhanja SN, Bhattacharya A, Chakraborty S (2021) Vulnerability of groundwater from elevated nitrate pollution across India: insights from spatio-temporal patterns using large-scale monitoring data. J Contam Hydrol 243:103895. https://doi.org/10.1016/j.jconhyd.2021.103895
Serrano-Nascimento C, Nunes MT (2022) Perchlorate, nitrate, and thiocyanate: environmental relevant NIS-inhibitors pollutants and their impact on thyroid function and human health. Front Endocrinol (Lausanne) 13:995503. https://doi.org/10.3389/fendo.2022.995503
Sijimol MR, Mohan M (2014) Environmental impacts of perchlorate with special reference to fireworks–a review. Environ Monit Assess 186:7203–10. https://doi.org/10.1007/s10661-014-3921-4
Singh S, Anil AG, Kumar V, Kapoor D, Subramanian S, Singh J et al (2022) Nitrates in the environment: a critical review of their distribution, sensing techniques, ecological effects and remediation. Chemosphere 287:131996. https://doi.org/10.1016/j.chemosphere.2021.131996
Stevens DA, Hasserjian RP, Robson H, Siebler T, Shalet SM, Williams GR (2000) Thyroid hormones regulate hypertrophic chondrocyte differentiation and expression of parathyroid hormone-related peptide and its receptor during endochondral bone formation. J Bone Miner Res 15:2431–42. https://doi.org/10.1359/jbmr.2000.15.12.2431
Stevens DA, Harvey CB, Scott AJ, O’Shea PJ, Barnard JC, Williams AJ et al (2003) Thyroid hormone activates fibroblast growth factor receptor-1 in bone. Mol Endocrinol 17:1751–66. https://doi.org/10.1210/me.2003-0137
Suh M, Abraham L, Hixon JG, Proctor DM (2014) The effects of perchlorate, nitrate, and thiocyanate on free thyroxine for potentially sensitive subpopulations of the 2001–2002 and 2007–2008 National Health and Nutrition Examination Surveys. J Expo Sci Environ Epidemiol 24:579–87. https://doi.org/10.1038/jes.2013.67
Tarone RE, Lipworth L, McLaughlin JK (2010) The epidemiology of environmental perchlorate exposure and thyroid function: a comprehensive review. J Occup Environ Med 52:653–60. https://doi.org/10.1097/JOM.0b013e3181e31955
Tewari P, Sweeney BF Jr, Lemos JL, Shapiro L, Gardner MJ, Morris AM et al (2022) Evaluation of Systemwide improvement programs to optimize time to surgery for patients with hip fractures: a systematic review. JAMA Netw Open 5:e2231911. https://doi.org/10.1001/jamanetworkopen.2022.31911
Tonacchera M, Pinchera A, Dimida A, Ferrarini E, Agretti P, Vitti P et al (2004) Relative potencies and additivity of perchlorate, thiocyanate, nitrate, and iodide on the inhibition of radioactive iodide uptake by the human sodium iodide symporter. Thyroid 14:1012–9. https://doi.org/10.1089/thy.2004.14.1012
Trajanoska K, Rivadeneira F (2019) The genetic architecture of osteoporosis and fracture risk. Bone 126:2–10. https://doi.org/10.1016/j.bone.2019.04.005
Venken K, Callewaert F, Boonen S, Vanderschueren D (2008) Sex hormones, their receptors and bone health. Osteoporos Int 19:1517–25. https://doi.org/10.1007/s00198-008-0609-z
Vestergaard P, Mosekilde L (2003) Hyperthyroidism, bone mineral, and fracture risk–a meta-analysis. Thyroid 13:585–93. https://doi.org/10.1089/105072503322238854
Wang L, Shao YY, Ballock RT (2007) Thyroid hormone interacts with the Wnt/beta-catenin signaling pathway in the terminal differentiation of growth plate chondrocytes. J Bone Miner Res 22:1988–95. https://doi.org/10.1359/jbmr.070806
Wang L, An X, Xiao X, Li N, Xie D, Lai F et al (2022) Treatment of thiocyanate-containing wastewater: a critical review of thiocyanate destruction in industrial effluents. World J Microbiol Biotechnol 39:35. https://doi.org/10.1007/s11274-022-03481-4
Ward MH, Kilfoy BA, Weyer PJ, Anderson KE, Folsom AR, Cerhan JR (2010) Nitrate intake and the risk of thyroid cancer and thyroid disease. Epidemiology 21:389–95. https://doi.org/10.1097/EDE.0b013e3181d6201d
Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R et al (2016) The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int 27:1281–1386. https://doi.org/10.1007/s00198-015-3440-3
Williams GR, Bassett JHD (2018) Thyroid diseases and bone health. J Endocrinol Invest 41:99–109. https://doi.org/10.1007/s40618-017-0753-4
Wu Q, Oldi JF, Kannan K (2011) Fate of perchlorate in a man-made reflecting pond following a fireworks display in Albany, New York, USA. Environ Toxicol Chem 30:2449–55. https://doi.org/10.1002/etc.648
Xie L, Zhang Y, Qu Y, Chai L, Li X, Wang H (2019) Effects of nitrate on development and thyroid hormone signaling pathway during Bufo gargarizans embryogenesis. Chemosphere 235:227–238. https://doi.org/10.1016/j.chemosphere.2019.06.177
Xie R, Huang X, Liu Q, Liu M (2022) Positive association between high-density lipoprotein cholesterol and bone mineral density in U.S. adults: the NHANES 2011-2018. J Orthop Surg Res 17:92. https://doi.org/10.1186/s13018-022-02986-w
Xu N, Wang Y, Xu Y, Li L, Chen J, Mai X et al (2020) Effect of subclinical hyperthyroidism on osteoporosis: a meta-analysis of cohort studies. Endocrine 69:39–48. https://doi.org/10.1007/s12020-020-02259-8
Xue S, Kemal O, Lu M, Lix LM, Leslie WD, Yang S (2020) Age at attainment of peak bone mineral density and its associated factors: The National Health and Nutrition Examination Survey 2005–2014. Bone 131:115163. https://doi.org/10.1016/j.bone.2019.115163
Yang Y, Li R, Cai M, Wang X, Li H, Wu Y et al (2023) Ambient air pollution, bone mineral density and osteoporosis: results from a national population-based cohort study. Chemosphere 310:136871. https://doi.org/10.1016/j.chemosphere.2022.136871
Yousefzadeh N, Jeddi S, Kashfi K, Ghasemi A (2022) Long-term inorganic nitrate administration protects against ovariectomy-induced osteoporosis in rats. Excli J 21:1151–1166. https://doi.org/10.17179/excli2022-5082
Yu J, Guo J, Zhang H, Cheng X (2022) The association between environmental perchlorate, nitrate, and thiocyanate exposure and oral pain in NHANES. Front Public Health 10:829466. https://doi.org/10.3389/fpubh.2022.829466
Zhang Q, Sun J, Liu J, Huang G, Lu C, Zhang Y (2015) Driving mechanism and sources of groundwater nitrate contamination in the rapidly urbanized region of south China. J Contam Hydrol 182:221–30. https://doi.org/10.1016/j.jconhyd.2015.09.009
Zhang X, Zhang Y, Shi P, Bi Z, Shan Z, Ren L (2021) The deep challenge of nitrate pollution in river water of China. Sci Total Environ 770:144674. https://doi.org/10.1016/j.scitotenv.2020.144674
Zhang X, Wang Y, Wang H, Zhang X (2024) Trends in prevalence of thyroid dysfunction and its associations with mortality among US participants, 1988–2012. J Clin Endocrinol Metab 109:e657–e666. https://doi.org/10.1210/clinem/dgad558
Zhu S, Pang Y, Xu J, Chen X, Zhang C, Wu B et al (2022) Endocrine regulation on bone by thyroid. Front Endocrinol (Lausanne) 13:873820. https://doi.org/10.3389/fendo.2022.873820
Acknowledgements
We appreciate the people who contributed to the NHANES data we studied.
Funding
This work was funded by National Natural Science Foundation of China (NSFC) (Grant No. 82103891), Natural Science Foundation of Guangdong Province (Grant No. 2020A1515010521), and Discipline construction project of Guangdong Medical University (4SG23286G). Author Ming Shi has received research support from Guangdong Medical University. Author Li Li has received research support from NSFC and Natural Science Foundation of Guangdong Province.
Author information
Authors and Affiliations
Contributions
Juxiao Li: conceptualization, data curation, methodology, software, formal analysis, writing—original draft. Bohai Du: methodology, data curation, investigation. Yuhan Wang: data curation, investigation. Jiahuang Qiu: supervision. Ming Shi: conceptualization, resources. Muhong Wei: conceptualization, methodology, writing—review and editing. Li Li: conceptualization, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participant
All participants provided written informed consent and study procedures were approved by the National Center for Health Statistics (NCHS) Ethic Review Board (previously known as: NCHS Research Ethics Review Board and the NHANES Institutional Review Board).
Consent for publication
The manuscript is approved by all authors for publication.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Du, B., Wang, Y. et al. Environmental perchlorate, thiocyanate, and nitrate exposures and bone mineral density: a national cross-sectional study in the US adults. Environ Sci Pollut Res 31, 34459–34472 (2024). https://doi.org/10.1007/s11356-024-33563-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-33563-9