Abstract
The presence of sulfur-containing compounds in fuel oil has become a major global issue due to their release of toxic sulfur dioxide. Hydrodesulfurization is a commonly used method for removing sulfur from fuel. However, new desulfurization techniques have been developed recently as hydrodesulfurization (HDS) is ineffective in removing refractory sulfur, e.g., BT, DBT, 4-MDBT. In this study, a series of deep eutectic solvent (DES) using ChCl, salicylic acid, oxalic acid, citric acid, and adipic acid as hydrogen bond acceptors and MeOH, EtOH, BuOH, EG, DEG, and TEG as hydrogen bond donors on different mole ratios were synthesized and then investigated the efficiency of these DESs in extracting sulfur from model and diesel fuel. Densities, viscosity, refractive index, and FTIR spectra of synthesized DESs were recorded. It also included oxidative desulfurization, which is a promising approach offering high selectivity, mild reaction conditions, low cost, and high efficiency. Hydrogen peroxide was selected as the oxidant in this study due to its excellent performance, commercial availability, and high proportion of active oxygen. [Citric acid: TEG] [1:7] and [adipic acid: TEG] [1:8] were found to be the most effective, removing up to 44.07% and 42.53% sulfur from model oil during single-stage extraction at 30 °C using a solvent-to-feed ratio of 1.0 and was increased to 86.87% and 85.06% using successive extraction up to the fourth stage. On oxidation, extraction efficiencies were reported to be 98.98%, 87.79%, and 56.25% and 96.96%, 81.22%, and 44.51% for model oil containing DBT and diesel 1 and diesel 2 with DES [citric acid: TEG] [1:7] and [adipic acid: TEG] [1:8] respectively at 30 °C using a solvent-to-feed ratio of 1.0. The study found that [citric acid: TEG] [1:7] exhibits better extraction performance in the deep desulfurization of fuels at an extraction temperature of 30 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Desulfurization is a process used for removing sulfur compounds from hydrocarbon streams using various methods such as hydrodesulfurization, extractive desulfurization, oxidative desulfurization, adsorptive desulfurization, and biodesulfurization. Desulfurization is necessary for several reasons: harmful gases like sulfur dioxide and nitrogen oxides contribute to air pollution, which causes respiratory difficulties and other health concerns (Nautiya et al. 2019). Sulfur compounds in fuels can corrode engine components and degrade engine efficiency, resulting in higher fuel consumption and emissions (Fig. 1). That is why most countries have employed strict regulations to reduce environmental pollution from combustion engines by controlling sulfur emissions (Saleh et al. 2020). In the European Union, the “Euro IV” standard, in effect since 2005, limits sulfur content in diesel to a maximum of 50 ppm. Ultra-low-sulfur diesel with a limit of 10 ppm sulfur was introduced under the Euro VI standard in September 2014 for diesel engines, and subsequently, in September 2015, they were implemented for gasoline engines (Astle et al. 2019). Desulfurization can help engines run more efficiently and lower emissions by eliminating sulfur from fuels. Also, sulfur is a dangerous contaminant that can damage refinery equipment and affect the quality of refined goods. Desulfurization safeguards refinery equipment and ensures that the resulting product meets quality standards (Silitonga et al. 2012). Another method for desulfurization is biodesulfurization (BDS), which is based upon the potential of bacteria to remove the organosulfur compounds from fuels. The advantage of BDS is that it does not degrade the carbon skeleton of the compounds. Also, it operates at ambient temperature and pressure and low emission and has no generation of unwanted side-products, thereby decreasing its energy cost (Mohebali and Ball 2016). However, a report of which is available in the literature states the requirement and the identification of several bacterial species for diesel oil. Nevertheless, this study needed extensive research to understand its mechanism (Mamuad and Choi 2023). Many researchers used adsorptive desulfurization (ADS) as an alternative to hydrodesulfurization (HDS) process. ADS require a solid adsorbent which is capable to adsorb the sulfur compounds from the fuel. The ADS can be carried out in two ways: (i) physisorption where the organosulfur compounds’ nature is not chemically changed by separation and (ii) reactive adsorption where a chemical reaction occurs between sulfur compounds and solid adsorbent (Javadli and de Klerk 2012). Furthermore, in the ADS process, bentonite was used by many researchers for the adsorptive desulfurization of organosulfur compounds present in diesel fuel. However, a report in the past also stated that adsorptive desulfurization using bentonite also needs improvement, hence demanding extensive research for further modification (Alcaraz et al. 2023). Currently, refineries use the HDS process for removing the sulfur from fuels (Fig. 2).
It is a catalytic process utilizing hydrogen gas to remove the sulfur from crude oils (Silitonga et al. 2012). During the process, the organosulfur compounds present in the crude oil are converted into sulfur-free hydrocarbons and H2S gas. This method includes the application of catalysts such as bimetal Ni–Mo and Co–Mo supported on Al2O3 at a high-temperature range and high pressure (Singh et al. 2016). Nickel-based catalysts are more suitable for feed with high olefin and carbon dioxide as it minimizes side reactions (Simanzhenkov and Idem 2003). The H2S generated is then recovered from the diesel fuel either in the form of elemental sulfur or sulfuric acid (Kulkarni and Afonso 2010).
But the HDS process has some drawbacks. The HDS process requires extreme temperature (300–350 °C), high pressure (20–100 atm of H2), and a large amount of H2. Additionally, owing to the impact of steric hindrance from the alkyl moiety and benzene ring, HDS is not particularly successful in eliminating organic sulfur compounds like BT, DBT, and their derivatives (Majid et al. 2020). While it is possible to attain deep desulfurization by altering parameter conditions, extremely high pressure can cause olefin saturation thus reducing the fuel oil’s octane rating. In contrast, increasing the temperature might cause coke to develop, which deactivates the catalyst (Li et al. 2012). So, there is a need to develop another alternative process for desulfurization. Desulfurization using solvent extraction is an alternative to the HDS process (Farzin Nejad and Miran Beigi 2015).
A novel desulfurization method involves solvent extraction, using a solvent to miscible the organosulfur compounds from the oil and separating the sulfur-containing layer through gravitational separation (Javadli and de Klerk 2012). It is popular due to its simplicity, mild conditions, and minimal fuel loss. However, there are challenges associated with the use of toxic and volatile solvents, which pose significant environmental risks (Atlas et al. 2001). In the past, straight-run gas oil, light cycle oil, coker gas oil, and their mixture called mixed gas oil were studied in a single-stage batch extraction and continuous mode extraction run for the removal of sulfur compounds using the commercial solvent N-methyl-2-pyrrolidone. After optimization of extraction parameters such as extraction temperature, solvent-to-feed ratio, and performance factor for the feedstocks, continuous counter extraction was carried out at the optimized conditions (Kumar et al. 2015). Conventional solvents like acetonitrile, N, N-dimethylformamide, and dimethyl sulfoxide contain a high degree of volatility and, when used extensively, can be hazardous. Therefore, some alternative solvents have been searched in the past few decades by various research groups. Alternative solvents involve supercritical fluid room temperature ionic liquids (RTILs), which contribute to their significance in green chemistry (Kerton and Marriott 2013). And it was found that room-temperature ionic liquids are suitable for desulfurizing fuels. RTILs are composed of organic heterocyclic structures paired with either organic or inorganic anions. The distinct characteristics of ILs have contributed to their extensive utilization as catalysts, solvents, and electrolytes in the gas and oil industry, and examples include the conversion of biomass into chemical platforms, storage of solar energy, and carbon dioxide transformation into fuels (Khan et al. 2018). RTILs themselves act as extractants and/or catalysts in the desulfurization of fuels. It has been studied extensively due to its distinct structure, low vapor pressure, recyclable nature, and low solvent loss in the process (Bhutto et al. 2016). Further information on ionic liquids in EDS can be found in the literature. Under the optimal conditions, the extraction of model gasoline with ILs could enhance the efficiency by up to 95%. The authors also stated that the process becomes complimentary when combined with adsorptive desulfurization using Raney nickel and acetonitrile as solvents (Fazlali et al. 2017). Numerous Keggin-type polyoxometalates entrapped in an RTIL phase were used for catalytic oxidative desulfurization of fuels and 80% removal of sulfur compounds in a period of 3 h (Julião et al. 2017). Pyridinium-based ILs found promising solvents for the desulfurization of fuels. The study also suggests that the size of cation in ILs has a significant impact on its extraction performance and observed the order of its performance with respect to the size of cation as [BPy][BF4] < [HPy][BF4] < [OPy][BF4] (Gao et al. 2018). Numerous studies have focused on the use of imidazolium-based ILs as extractants for desulfurization via solvent extraction. Examples include hexafluorophosphate of 1-butyl-3-methylimidazolium, tetra chloroaluminate, and thiocyanate of 1-butyl-3-methylimidazolium. These are far more environmentally friendly compared to conventional solvents, but their complex synthesis path makes them expensive (Mafi et al. 2018).
Furthermore, another alternative to RTILs is found in terms of eutectic-based solvents. Deep eutectic solvents (DESs) have similar characteristics as RTILs, but a major advantage to RTILs is that its easier and simpler synthesis process makes them attractive for desulfurization. Therefore, it is extensively used in the separation process. Cu–Fe/TiO2, a photocatalyst, was utilized in the photooxidative–desulfurization process of model oil and real diesel using H2O2 as an oxidant. The extraction was performed with choline chloride-glycerol–based ionic liquid. A 100% desulfurization was observed in two stages, showing better extraction performance of the photo-catalyst during the process (Fatimah et al. 2015). In another study, choline chloride-glycerol was also studied in the oxidative desulfurization of dibenzothiophene and 4,6-dimethyldibenzothiophene as a model oil. The solvent choline-chloride exhibits excellent performance in the removal of sulfur compounds from the model fuels (Mohd Zaid et al. 2017). DESs have many applications in various fields, such as electrochemistry (Gupta et al. 2016), nanomaterial synthesis (Gu et al. 2017), and carbon dioxide capture (Nisha Saini and Kamal Kumar 2023). In the past few years, a lot of research exploring the application of DESs in various fields, such as organic synthesis (Smith et al. 2014), catalysis (Joarder et al. 2023), food sector (Suthar et al. 2023), waste water treatment (Florindo et al. 2020), nanomaterial exploration (Tomé et al. 2018), and purifying and manufacturing of biodiesel (Zhao and Baker 2013), has been done. Thus, DES has gathered attention in the scientific community as a new class of ionic liquid analog due to its similarities to conventional ionic liquids. Its biodegradability and low-cost elements make it an ideal extraction solvent in the separation industry (Warrag et al. 2018). Deep eutectic solvent is a blend comprising multiple constituents with the ability to create intermolecular forces. The solution thus formed is known as a eutectic solvent. The classification of DES is primarily based on two major constituents: the salt and the complexing agents. When a proton donor and a proton acceptor are combined, a eutectic mixture is formed which exhibits a melting temperature lower than that of its individual compounds. DESs have extremely low vapor pressure, making them suitable for industrial applications. Abbot et al. discovered DES by mixing zinc chloride and quaternary ammonium salts (Abbott et al. 2001). The recorded melting points of the resulting liquids indicate that the minimum melting point, at 23 °C, was due to the presence of choline chloride salt. The general formula of a deep eutectic solvent (DES) can be represented as Cat+X−nY, where Cat+ refers to a quaternary salt, X− represents a Lewis base, Y denotes a molecule capable of forming complexes, and n indicates the number of complexing molecules that interact with the Lewis base. Typically, there are four different varieties of DES. Generally, type 1, 2, and 3 DESs are made up of quaternary salts along with either metal halides (type 1), which were obtained by substituting Group 13 elements and transition metals into the previously studied imidazolium ionic liquids. Hydrated metal halides (type 2), or hydrogen bond donors (type 3), have received significant research attention due to their versatility in forming various hydrogen bond donors and hydrogen bond acceptors. HBDs such as alcohols, amides, and carboxylic acids can interact through hydrogen bonding with HBAs like choline salt and tetraalkylammonium salt, and type IV DES is created by mixing halogenated metal compounds and HBD (Lim et al. 2020). Figure 3 shows some of the commonly used HBDs and HBAs for extractive desulfurization. In a standard solvent extraction process for oxidative desulfurization, an oxidizing agent is added to the feed mixture to convert sulfur compounds into sulfones without any disruption to the C─S bond. The resulting products have increased molecular weight and polarity, enabling the extraction of sulfur through solvent extraction (Betiha et al. 2018). Various oxidants are used, which facilitate the chemical transmission of active oxygen species to sulfur compounds, resulting in the formation of sulfones. Then, these sulfones are separated from fuel by polar solvents like ionic liquids or DES.
Glycol-based DES exhibits better extraction efficiency in the removal of organic sulfur compounds from the model oil. In this case, methyltriphenyl phosphonium bromide and tetraethylene glycol-based DES enhance its extraction capability from 45 to 84% up to the fourth stage (Sudhir et al. 2020). Saini et al. used phenylacetic acid and salicylic acid as hydrogen bond acceptor with triethylene glycol as hydrogen bond donor for synthesizing the DESs and applied their application in the oxidative desulfurization of fuels. Among both the synthesized DESs PAA:TEG and SAA:TEG, PAA:TEG exhibited better performance in desulfurization of fuels (Saini et al. 2024). Recently, mixing-assisted oxidative desulfurization (MAOD) has been reported in the literature which enhances fluid/fluid interfacial area between an oxidant and oil by employing high-shear mixing. In this study, H2O2 and Fe(VI) were used as oxidants, while heteropoly acids (HPA) and acetic acid were used as catalysts. The maximum desulfurization was accomplished as 100% at 40 °C operating temperature, 10,000 rpm agitation speed, and 1:1 PTA to catalyst ratio for this system (Haboc et al. 2023). Hydrogen peroxide and formic acid were also used as oxidant-catalytic systems for the oxidative desulfurization of model fuels. The oxidized fuels were further extracted by using DES choline chloride and tetraethylene glycol (Saini et al. 2022). Hydrogen peroxide combined with Lewis acid-based IL exhibits good extraction performance from real diesel where Lewis acid-based ILs act as both catalyst and solvent (Andevary et al. 2019).
In this work, type 3 DES is used, which includes a series of DES in the different mole ratios using citric acid and adipic acid as HBA and TEG as HBD. Also, in this study, hydrogen peroxide, with formic acid as a catalyst, is used as an oxidant because it forms performic acid, a powerful oxidizing agent, and hydroperoxide generates no harmful by-products, and this was confirmed by various literature studies in the past (Abbott et al. 2003a).
Experimental section
Materials used
Table 1 includes a list of compounds utilized in this investigation, including their Chemical Abstracts Service (CAS) number. The varied compositions of the synthesized DES for this experiment are shown in Table 2, along with a description of how they are physically different. Diesel 1 and diesel 2 were procured from Indian Refinery.
Among these DES synthesized, [citric acid/TEG] [1:7] and [adipic acid/TEG] [1:8] were selected based on their extraction efficiency, as others showed extraction efficiency from 10 to 20%. In comparison, these DESs showed extraction efficiency of up to 40%. So, additional DESs with varying ratios were synthesized, as listed in Table 3. Table 4 contains the calculated molar mass of the designed DESs, and the selected physical properties of DESs are listed in Table 5. All the ratios having colorless liquid appearance were then used for extractive desulfurization, and, based on their extractive efficiencies, [citric acid/TEG] [1:7] and [adipic acid/TEG] [1:8] were selected as final DESs.
The molecular mass of DES was determined as: MWDES (g/mol) = XHBA × MWHBA + XHBD × MWHBD, where XHBA: molar fraction of HBA, MWHBA: molecular mass of HBA (g/mol), and XHBD: molar fraction of HBD, MWHBD: molecular mass of HBD (g/mol). MW of citric acid = 192.194 g/mol, MW of adipic acid = 146.142 g/mol, MW of tri-ethylene glycol = 150.174 g/mol.
DES preparation
The DES was synthesized using a method described in the literature, which involved mixing an HBA and HBD in a specific mole ratio (Abbott et al. 2003b; Saini et al. 2022; Saini et al. 2024). The HBA and HBD were combined in a round-bottom flask and agitated at a temperature of up to 80 °C for 30 min until a homogeneous solution was achieved. The resulting DES was then allowed to cool at room temperature. Figure 4 shows the reaction scheme between HBD and HBA among the synthesized DESs.
Model oil preparation
This study used dibenzothiophene as a representative sample to explore the behavior of sulfur compounds in model oil. Since diesel fuel contains around 75% of aliphatic hydrocarbons and 25% of aromatic hydrocarbons, model oil was prepared by dissolving DBT in aliphatic hydrocarbons such as n-octane. The DBT was added to a bottle, followed by n-octane. The mixture was subjected to ultrasonication for several minutes without heat until the DBT was fully dissolved. For analyzing the concentration of the model feed in an ED-XRF analyzer, a calibration curve was prepared using the certified standard solution of the different concentration ranges up to 600 ppm. Then, the prepared model oil was analyzed for total sulfur content using an ED-XRF analyzer. The prepared model oil exhibited a sulfur content measuring 495 ppm.
Extractive desulfurization process
Solvent and model oil were mixed in a round-bottom flask following a particular mass ratio and vigorously stirred at a particular temperature for a specified time frame. When the feed and solvent interact, the solvent selectively extracts the solute and separates it into two distinct phases based on their density difference. The denser phase settles downward (extract or solvent-rich phase) while the less dense phase rises upward (raffinate or product phases). The selection of the solvent is made to ensure that the solute in the solution has a higher affinity for the added solvent, facilitating easy removal of the solute from the solution. The mixture was then moved to a separating funnel and permitted to undergo sedimentation. The resulting phases were separated and weighed. The raffinate phase was poured into a separating funnel, to which distilled water was added shaken vigorously, and then allowed to settle. The lower layer was discarded, and the upper layer, known as the raffinate hydrocarbon (RHC), was weighed. This step was performed to eliminate any residual solvent from the raffinate phase. The phase with higher density was further analyzed for total sulfur content using an X-ray fluorescence analyzer. Figure 5 shows the process of extractive desulfurization via solvent extraction. The mechanism has also been proposed by several studies in the literature (Naidu and Krishnan 1966; Ciocirlan and Iulian 2009).
The extraction efficiency is evaluated using the equation given below:
Extraction Efficiency (wt.%) = \(\frac{{S}_{i}-{S}_{f}}{{S}_{i}}\times 100\) where Si is the initial sulfur content.
Sf is the final sulfur content.
EDS exhibits significant potential for pre- and post-refining of intermediate cuts obtained from distillation of crude oil. This potential allows for the reduction of higher operating conditions required by the HDS unit to achieve higher desulfurization levels.
Similarly, the chosen DESs [citric acid/TEG] [1:7] and [adipic acid/TEG] [1:8] were utilized to extract sulfur compounds from diesel feeds through oxidative desulfurization.
Oxidative desulfurization process
The effectiveness of DES in oxidative desulfurization was assessed using formic acid as a catalyst and 30% aqueous solution of H2O2 as the oxidant, which changes the sulfur compounds present in fuel into sulfones. The reaction scheme is shown in Fig. 6. The utilization of DES combined with H2O2 as an oxidant in the EODS process was discovered by Ye and Wang (2023). Then, the selected DES was used for extraction. In the standard experiment, a known amount of diesel was introduced into a round-bottom flask, followed by the addition of H2O2 and formic acid. The resulting blend was then stirred vigorously for 30 min at 50 °C, using the ratio of oxidant to fuel being maintained. Subsequently, the layers containing the oxidized compounds and methanoic acid were permitted to undergo settling and then withdrawn separately. Experiments were performed to optimize the mole ratio of oxidant to sulfur and the reaction period for diesel through ODS. The diesel fuel after oxidation was then extracted using selected DES at a specific temperature for a predetermined duration. The resulting mixture was then cooled, allowing the phase to separate. The weight of each phase was then determined. Phases were then analyzed for total sulfur content.
Analytical methods used
Density and viscosity
The density and kinematic viscosity of each DES were assessed over a temperature range of 20–60 °C using Anton Paar Stabinger SVM 3001 Viscometer. Before utilization, the device was calibrated by employing a solution of established density supplied by the maker.
Refractive index
ATAGO RX-7000i is a digital refractometer used to measure the refractive index of the liquid. The refractive index of all the synthesized DES was measured at 20 °C. Performing zero calibration before each use is important for accurate measurement.
Total sulfur analyzer
Energy dispersive X-ray fluorescence (EDXRF) total sulfur analyzer Lab-XSCL 3500 made by Oxford Instrument, China, was used to measure sulfur. It employs an X-ray tube to generate high-energy X-rays that are aimed at the sample. As a result of the interaction, lower-energy X-rays are emitted that have characteristics unique to the elements found in the sample. The detected and processed X-rays are utilized to quantify the sulfur content.
FTIR
Perkin Elmer-make Fourier transform infrared spectroscopy (FTIR) spectrometer detects molecular vibrations of a sample by measuring the amount of infrared radiation using KBr as the pelletizing matrix. It absorbs at different frequencies, resulting in an IR spectrum that shows the sample’s molecular makeup. An interferometer creates an interferogram, which is Fourier-transformed to generate the spectrum. Range, 4000–650 cm−1 (mid-range), accessories—ATR.
Results and discussion
Impact of temperature on the density of the prepared DESs
Figure 7 shows the impact of temperature on density. The relationship between density and temperature demonstrates an apparent decrease. When the liquid’s temperature rises, the intensity of the hydrogen bond diminishes, leading to a reduction in the molecular spacing and available space within the mixture. This linear decrease in density is attributed to the volume expansion of the liquid upon heating, causing the mixture to become less dense. This phenomenon is supported by the various cited literature in the past (Naidu and Krishnan 1966; Saini et al. 2022; Saini et al. 2024).
Impact of temperature on viscosity of the synthesized DESs
Figures 7 and 8 illustrate the impact of temperature on the density and viscosity of all the prepared deep eutectic solvents. Figures 7 and 8 illustrate the physiochemical properties of glycol-based eutectic solvents, and similar trends in densities and viscosities have been observed in related literature (Ciocirlan et al. 2010; Perkins et al. 2014; Li and Li 2015; Mjalli and Naser 2015). The results demonstrate a consistent decrease in dynamic viscosity as temperature increases, demonstrating typical liquid characteristics. The variations in viscosity are influenced by the intermolecular interactions, which depend on the molecule’s size and shape. Positive viscosity deviation is observed when these interactions dominate, whereas mixtures without strong interactions exhibit negative viscosity deviation, while mixtures lacking strong interactions display negative viscosity deviation (Yusof et al. 2014; Li et al. 2017).
With increasing temperature, the viscosity of DESs decreases, suggesting enhanced fluid flow in process streams. The rise in temperature reduces interactions within the individual components and between dissimilar molecules due to the increase in thermal energy. Consequently, the Δη values become less negative as the temperature rises.
FTIR analysis of the synthesized DESs
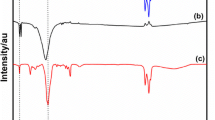
The FTIR spectra of the produced DESs [citric acid/TEG] [1:7] and [adipic acid/TEG] [1:8] were examined in the corresponding mole ratios, and they are presented alongside their particular individual constituents such as citric acid, adipic acid, and TEG in Figs. 9 and 10. The FTIR spectra give the interaction between HBA and HBD. Citric acid, adipic acid, and TEG all show adsorption peaks at 3282 cm−1, 3012 cm−1, and 3384 cm−1 indicating the presence of O–H stretching vibration of the carboxylic and hydroxyl groups, 1749 cm−1 indicating C = O stretching vibrations of carbonyl groups in the carboxylic group, 1698 cm−1 peak is attributed to C = O stretching vibration of the carbonyl group in the ester functional group of citric acid. C–H stretching vibrations at 2876 cm−1 are observed in the aliphatic carbon chain in TEG, and 1691 cm−1 peak in adipic acid is associated with the C = O stretching vibration of the carbonyl group. Stretching vibration of the OH group was noted around 3100–3600 cm−1, indicating strong hydrogen-bonded OH. Both the DESs show broad adsorption peeks at 3391 cm−1 and 3394 cm−1 corresponding to O–H and 2866 cm−1 and 2871 cm−1 indicating C–H stretching vibrations, and 1720 cm−1 and 1723 cm −1 peak attributes to C = O stretching vibration of carbonyl groups in the carboxylic group. Furthermore, the carboxyl group peak becomes narrower, a change that could be associated with the hydrogen bond formation within the DES involving citric acid or adipic acid and TEG (Saini et al. 2022; Saini et al. 2024).
Optimization of conditions
To achieve maximum sulfur removal while minimizing resource consumption, the optimization of conditions was done to find the most suitable mole ratio, DES-model oil ratio, extraction temperature, and extraction duration for desulfurization.
Impact of different mole ratios on extraction efficiency
The physicochemical properties of DESs vary when they possess different HBA/HBD mole ratios, consequently influencing the extraction efficacy of solvents. The DESs of varying ratios used for the extraction of sulfur from model oil are illustrated in Fig. 11. The impact of the DES ratio showed that increasing the proportion of HBD led to greater desulfurization. Earlier studies with FeCl3-based eutectic solvents have also reported a comparable rise in removal efficiency due to the DES ratio (Yu et al. 2016). Also, on increasing the HBD content, the carbon atom chains also increase, leading to decreased hydrogen bond interactions caused by steric hindrance (Gano et al. 2015a). From Fig. 11, [citric acid/TEG] showed the extraction efficiency of 40.07%, 44.44%, 44.30%, 44.52%, 44.65%, and 44.75%, respectively, in their increasing mole ratio from 1:5 to 1:11 using solvent-to-feed ratio of 1 at a constant extraction temperature of 30 °C and a constant extraction time of 15 min. Similarly, [adipic acid/TEG] showed extraction efficiency of 42.25%, 42.53%, 42.44%, 42.52%, and 42.62%, respectively, in their increasing mole ratio from 1:7 to 1:11 using solvent-to-feed ratio of 1 at a constant extraction temperature of 30 °C and a constant extraction time of 15 min. According to the data, [citric acid/TEG] [1:7] to [1:11] and [adipic acid/TEG] [1:8] to [1:11] demonstrated similar and optimal extraction efficiency, i.e., around 44.07% and 42.53% respectively, reaching the desulfurization saturation point. Moreover, the economy of an industrial extraction process can be influenced by the quantity of components present in synthesized DESs. Hence, in this experiment, the [citric acid/TEG] [1:7] and [adipic acid/TEG] [1:8] combinations prove to be ideal extracting agents for the desulfurization of model oil.
Impact of extraction time on extraction efficiency
The mixing duration during the extraction process is a crucial element that impacts the efficiency of extraction by ensuring equilibrium conditions are achieved. The results presented in Fig. 12 demonstrate that the desulfurization rate increases from 39.07%, 40.52%, to 44.07%, respectively, using solvent-to-feed ratio of 1 at a constant extraction temperature of 30 °C for the DESs [citric acid/TEG] [1:7] as the stirring time is extended from 5 to 15 min, reaching its maximum of 15 min at a constant extraction temperature 30 °C and solvent-to-feed ratio 1. However, beyond this point, the extraction efficiency slightly decreases from 44.07 to 43.91% and further up to 43.96% [Citric acid/TEG] [1:7], i.e., it eventually stabilizes, suggesting saturation of sulfur content in the extraction phase. The observed decline could potentially be attributed to experimental errors leading to minor oil losses (Yusof et al. 2014), and in the case of [adipic acid/TEG] [1:8], extraction efficiency increases from 38.53%, 40.03%, to 42.53% respectively with time from 5 to 15 min, at a constant extraction temperature 30 °C and solvent-to-feed ratio 1, but after 15 min, it remains somewhat constant up to 25 min, i.e., 42.60 and 42.55%, respectively. This observation aligns with similar findings by Tang et al. (Safa et al. 2016) and Li et al. (Tang et al. 2015) utilizing varied DES compositions. Thus, based on these findings, a stirring time of 15 min was deemed optimal for the removal of sulfur compounds from the feed using the synthesized solvents [citric acid/TEG] [1:7] and [adipic acid/TEG] [1:8] at a constant extraction temperature and solvent-to-feed ratio 1.
Impact of extraction temperature on extraction efficiency
Figure 13 illustrates the impact of extraction temperature on the desulfurization performance of synthesized DESs using a solvent-feed ratio of 1 and 15 min of the extraction time. At first, increasing the extraction temperature from 30 °C, 40 °C, 50 °C and 60 °C enhances the efficiency of DES extraction slightly from 44.04%, 44.24%, 44.44%, and 45.25% respectively for [citric acid/TEG] [1:7] DES. However, above 30 °C, the extraction efficiency levels off. Therefore, 30 °C was chosen as the optimal extraction temperature for [citric acid/TEG] [1:7] DES.
Several studies have suggested that raising the extraction temperature from low to room temperature decreases viscosity, facilitating mass transfer and enhancing the efficiency of extraction (Li et al. 2013a; Mokhtar et al. 2014; Liu et al. 2014; Lima et al. 2018). However, elevated temperatures can cause deterioration of the DES and organic sulfur compounds in the fuel. Consequently, desulfurization rates increase from 42.53%, 42.44%, 42.53%, and 42.69%, respectively, and then become constant to some extent for [adipic acid/TEG] [1:8] DES when the temperature exceeds room temperature, i.e., 30 °C, 40 °C, 50 °C, and 60 °C (Khezeli and Daneshfar 2017). Therefore, a lower extraction temperature is more appropriate as it allows the DES to achieve the maximum level of desulfurization primarily due to the exothermic nature of the extraction process. Raising the temperature promotes electrophilic substitution reaction on the DBT aromatic ring. This suggests that the procedure can be conducted at ambient temperature. Similar findings and patterns have been reported in various literature (Li et al. 2013a, b; Mokhtar et al. 2014; Gano et al. 2015a; Shu and Sun 2016; Mohd Zaid et al. 2017; Wang et al. 2020) for other DESs and ILs. Therefore, for the [adipic acid/TEG] [1:8] DES in this study, a temperature of 30 °C was deemed appropriate for extraction.
Impact of solvent-to-feed ratio on extraction efficiency
The extraction efficacy of the DESs increased as the solvent-to-feed ratio improved from 1:1 to 1:4, respectively (Fig. 14). The extraction conditions were set to be at the extraction temperature of 30 °C and the extraction time of 15 min. The extraction efficiencies for [citric acid/TEG] [1:7] were 44.04%, 60.61%, 67.47%, and 78.99% for ratios 1:1, 1:2, 1:3, and 1:4, respectively. Similarly, for [adipic acid/TEG] [1:8], the extraction efficiencies were 42.53%, 54.75%, 66.67%, and 72.85%, respectively, for the same ratios. These results indicate that increasing the number of DESs promotes the extraction process. In this context, the DES ratio was maintained at a constant value of 1, whereas the model oil was varied in order to ensure an economically feasible process for industrial utilization. It is preferable to use a lower solvent-to-feed ratio. However, previous studies have also consistently demonstrated that increasing the ratio of DES and model oil tends to enhance desulfurization performance (Wilfred et al. 2012; Dharaskar et al. 2015; Tang et al. 2015), but it was found that a significant difference in the ratio between DES and model oil had a limited impact on enhancing desulfurization effectiveness. So in the present experiment, a 1:1 DES-model oil ratio was chosen, resulting in a satisfactory desulfurization rate of 44.04% and 42.53% for [citric acid/TEG] [1:7] and [adipic acid/TEG] [1:8] respectively. Thus, optimized solvent-to-feed ratio was selected for the DESs [citric acid/TEG] [1:7] and [adipic acid/TEG] [1:8] for subsequent experiments.
Impact of successive extraction stage
A series of cyclic operations are implemented to enhance the deep desulfurization of fuel oil. These cycles involve retaining the upper oil phase after the initial extraction process and utilizing fresh DES for subsequent extractions under identical conditions. Successive extraction was employed to assess the effectiveness of DES desulfurization under the optimized extraction conditions. As depicted in Fig. 15, extraction efficiency was increased from 44.04, 75.01, 85.00%, 88.00%, and 42.53%, 67.02%, 77.03%, to 85.06%, respectively for the DESs [citric acid/TEG] [1:7] and [adipic acid/TEG] [1:8], as the number of extraction cycles increased from stage 1 to stage 4. Up to four cycles, extraction efficiencies of 86.87% and 85.06% were achieved for [citric acid/TEG] [1:7] and [adipic acid/TEG] [1:8], respectively. These efficiencies can be further improved by escalating the count of extraction cycles (Li et al. 2013a; Zhao et al. 2018).
Impact of nature of HBA in DESs
The structure of HBAs also affects the extraction efficiency of both the DESs [citric acid/TEG] [1:7] and [adipic acid/TEG] [1:8]. The DES [citric acid/TEG] [1:7] found more extraction efficiency than that of DES [adipic acid/TEG] [1:8]. Between the two deep eutectic solvents (DES) [citric acid/TEG] [1:7] is more polar and reactive towards the extraction of sulfur compounds via solvent extraction methods. The polarity and reactivity of a solvent play a crucial role in its ability to extract specific compounds from a mixture. In this case, [citric acid/TEG] [1:7] has citric acid as one of its components. Citric acid contains polar functional groups like carboxylic acid and hydroxyl groups, contributing to its high polarity. These polar functional groups in the citric acid-based DES make it more capable of interacting with and extracting polar sulfur compounds efficiently. The citric acid–based DES may also provide better solubility for sulfur-containing compounds due to the enhanced polar interactions. On the other hand, [adipic acid/TEG] [1:8] contains adipic acid, which also has polar carboxylic acid (–COOH) groups but lacks the hydroxyl (–OH) groups present in citric acid. As a result, it is slightly less polar than the citric acid–based DES (Li et al. 2016; Saini et al. 2024).
The [citric acid/TEG] [1:7] DES is more suitable for extracting sulfur compounds through solvent extraction methods due to its higher polarity and more robust interactions with polar sulfur-containing compounds.
Diesel desulfurization
Experiments were conducted on diesel 1 and diesel 2 to study the removal of sulfur compounds. Results are shown in Table 6. Diesel 1 and diesel 2 have a total sulfur content of 213 ppm and 422 ppm. The extraction efficiencies are presented in Table 6, with values of 74.64% and 39.81% for [citric acid/TEG] [1:7] and 69.48% and 25.35% for [adipic acid/TEG] [1:8], observed for diesel 1 and diesel 2, respectively. The extraction was carried out at optimized conditions for both the diesel. Diesel 1 was found to have a higher extraction efficiency of 74.64% and 69.48% than diesel 2 for DESs [citric acid/TEG] [1:7] and [adipic acid/TEG] [1:8]. This lower extraction efficacy in diesel 2 was due to the higher concentration of the refractory sulfur compounds than that present in diesel 1 (Saini et al. 2022; Saini et al. 2024).
Oxidative desulfurization
For the effective and prominent oxidation of sulfur compounds, the ratio of oxidant and catalyst was optimized with model oil. The 30% aqueous hydrogen peroxide was used as an oxidant, and 98% formic acid was used as a catalyst. In this study, hydrogen peroxide in the presence of formic acid is used as an oxidant because together, they form performic acid, which is a powerful oxidizing agent because hydroperoxide generates no harmful by-products (Gao et al. 2008; Dharaskar et al. 2015; Gano et al. 2015b; Ahmed Rahma et al. 2017; Mafi et al. 2018; Makoś and Boczkaj 2019). For the optimization study, the amount of the oxidant and catalyst varied during desulfurization experiments. The desulfurization conditions remain constant in the optimization experiments. The oxidation and extraction conditions are stated in Table 6. The study investigated the impact of oxidant quantity on the extraction, altering the molar ratio of H2O2 to the sulfur-containing compound. Desulfurization occurred at 30 °C for 15 min using a DES/model oil ratio of 1:1. The reaction equation in stoichiometric proportions revealed that two moles of H2O2 react with a single mole of DBT, resulting in the formation of a sulfone:

Hence, the molar ratio of the oxidant to the sulfur-containing compound was expected to significantly impact desulfurization. Table 7 shows the impact of oxidant and catalyst on the extraction efficiency.
Impact of concentration of oxidant and catalyst on reaction
Higher H2O2 dosage higher than 0.2 ml has shown improved efficiency, but excessive amounts can decrease efficiency due to non-productive thermal decomposition of the oxidant (Gano et al. 2015a; Saini et al. 2022). Based on the data presented in Table 7, it can be implied that the extraction efficiency improves when the feed is oxidized. This improvement can be attributed to the formation of sulfones, which possess higher polarity. In the absence of a catalyst, the extraction efficiency did not show any significant increase. The introduction of formic acid as a catalyst enhances the oxidation mechanism by reducing the associated energy barrier and promoting the rate of ODS. Increasing the amount of catalyst from 0.4 to 0.8 ml increases the extraction efficiency from 78.18 to 82.42% while the amount of oxidant remains constant, i.e., 0.2 ml. From Table 7, the amount of catalyst reaches the saturation point using 0.6 ml and 0.8 ml of catalyst amount, i.e., 82.22 and 82.42%, respectively. Therefore, the optimum amount of the catalyst for oxidation was selected as 0.6 ml. Increasing the oxidant from 0.2 to 0.6 ml using the constant amount of catalyst 0.6 ml increases the extraction efficiency from 82.22 to 98.98%. This indicates sulfur compounds are converted into sulfones, showing almost complete oxidation at this stage. Therefore, the optimum amount of oxidant and catalyst was observed as 0.6 ml and 0.6 ml, i.e., a 1:1 volume ratio of oxidant and catalyst with respect to a feed of 495 ppm total sulfur.
Impact of oxidation of various feeds on extraction efficiency
Fig. 16 shows the impact of oxidation on the extraction of the feeds used with DESs [citric acid/TEG] [1:7] and [adipic acid/TEG] [1:8] at an extraction temperature of 30° for an extraction time of 15 min. From the given data, it can be concluded that extraction efficiencies of feeds DBT-octane, diesel 1, and diesel 2 on oxidation increase from 44.04, 74.64, 39.81 to 98.98%, 87.79%, 56.25% for the DES [citric acid/TEG] [1:7]. Similarly, with DES [adipic acid/TEG] [1:8], it increases from 42.53, 69.48, and 25.35 to 96.96%, 81.22%, and 44.51%, respectively. The extraction efficiency affects the types and complexity of organosulfur compounds. Because diesel is an intricate blend of refractory sulfur compounds, the extraction efficacy of diesel 1 and diesel 2 was found to be lower than that of model compounds. The extraction efficiency of diesel 2 was found to be much lower than that of diesel 1. This is due to the fact that diesel 2 contains a higher concentration of bulkier organosulfur compounds than diesel 1 (Saini et al. 2022; Saini et al. 2024).
Study of various DESs reported and their extraction efficiency
From Table 8, the order of sulfur removal was found to be TBAC:EG > CHCl/Ph > MIM:PA > TBAB:PEG > TBAB:FA > CA:TEG > AA:TEG.
The order of extraction efficiency was 99.50% > 99.2% > 97.6% > 82.4% > 80.47 > 44.07% > 42.53%.
In the case of DESs used in other studies mentioned earlier in Table 8, TBAC/EG and CHCl/Ph both have maximum extraction efficiency, i.e., 99.5% and 99.2%, respectively, for the removal of benzothiophenes from fuel. However, both extraction experiments have different experimental conditions, such as the extraction temperature, extraction time, extraction cycle, and DES-to-feed ratio. Nevertheless, TBAC/EG has a lower extraction temperature of 30 °C and a lower extraction time of 10 min than that of CHCl/Ph, which requires 40 °C extraction temperature and 40-min extraction time to produce 99% desulfurized feed. Also, TBAC/EG gains 99.5% removal of benzothiophene in five extraction stages compared to CHCl/Ph. Furthermore, the extraction efficiency of MIM/PA was higher, i.e., 97.6%, achieved in the four-extraction stage using 10 min of extraction time in one single extraction stage. However, the extraction temperature above was almost the same, i.e., 30 °C. TBAC/EG (1:2) and MIM/PA (1.75:5) exhibited higher extraction efficiency, but this was achieved through multiple extraction stages, with up to four and five stages involved. Furthermore, when it comes to the reactivity of sulfur compounds, the order is as follows: thiophene > benzothiophene > di-benzothiophene. Thus, removing di-benzothiophene from oil proves to be more challenging compared to thiophene and benzothiophene. This difficulty, combined with the longer extraction time, could explain the higher extraction efficiency observed in cases involving TBAB/FA (1:0.5), TBAC/EG (1:2), TBAB/TEG (1:2), and ChCl/PA (2.5:1) DES.
TBAB/PEG achieved its maximum extraction efficiency (82.4%) in the extraction time of 30 min using DES-to-feed ratio of 1.5. However, in the case of TBAB/FA, the maximum extraction efficiency of 80.47% was achieved in the extraction time of 40 min using a DES-to-feed ratio of 2 at the temperature of 30 °C. Furthermore, in our work, the extraction efficiency for both the DESs CA/TEG and AA/TEG (44.07% and 42.53%) was achieved in the shorter extraction time, i.e., 15 min using DES-to-feed ratio of 1 at a temperature of 30 °C. All the above literature reported that extraction experiments have different operating conditions, such as a higher DES-to-feed ratio and higher extraction time than our work.
The order of the oxidative desulfurization for DESs was CHCl/Gly > CA/TEG > AA/TEG > CHCl/2Gly > L-Pro/OA.
Thus, the order of their extraction efficiency was 100 > 98.98 > 96.96 > 13.9 > 10.
From the above table, all the experiments have different experimental conditions such as extraction time, extraction temperature, DES-to-feed ratio, and extraction cycle. However, the oxidant remains the same, i.e., hydrogen peroxide. The catalysts used are different, such as Cu-Fe/TiO2 for CHCL/Gly, HPW for CHCl/2Gly, and formic acid for CA/TEG and AA/TEG. The maximum extraction efficiency of 100% was observed for the DES CHCL/Gly in a longer extraction time of 120 min in two extraction stages. However, it is worth noting that these studies had significantly longer extraction times compared to the timeframes used in this work. In our work, the maximum extraction efficiency of 98.98% and 96.96%, respectively, for both the DESs CA/TEG and AA/TEG was achieved in the lower extraction time of 15 min in one single-stage extraction of DBT in n-octane. However, the reported oxidant and catalyst volume ratio was higher (i.e., 4:1) than that used in our study (i.e., 1:1). The DES used in this study shows more efficiency in less extraction time and temperature.
RT room temperature, Gly glycol, EG ethylene glycol, TEG triethylene glycol, PEG polyethylene glycol, Ph phenol, TBPB tert-butyl-peroxy-benzoate, TBAB tetra butylammonium bromide, TBAC tetrabutylammonium chloride, PA propanoic acid, HPW tungstophosphoric acid, OA oxalic acid, FA formic acid, CA citric acid, AA adipic acid, MIM-1 methylimidazole, Cu-Fe/TiO2 copper-iron/titanium dioxide, L-Pro L-proline.
Recycling of DES
The recycling of deep eutectic solvents (DES) holds great significance and is crucial for industrial purposes. One important aspect involves the elimination of sulfur-based compounds from solvent. This can be achieved through several methods. Firstly, the DES can be heated to eliminate the sulfur compounds. Secondly, the sulfur compounds can be precipitated by diluting the DES with water. Additionally, compounds containing sulfur can be subjected to re-extraction using low-volatility hydrocarbons, like n-pentane or hexane (Gano et al. 2015a; Saini et al. 2022; Saini et al. 2024). Here, DES regeneration was achieved through a process of dilution with water, succeeded by distillation, as described by Shu and Sun (Jiang et al. 2016; Shu and Sun 2016). An equal amount of distilled water and the extracted phase were combined and left for several hours to allow for precipitation. The obtained precipitate was then separated and sent for FTIR analysis, revealing it to be n-octane. The remaining filtrate, consisting of distilled water and DES, was then separated using a rotatory evaporator, or rotavapor, through evaporation at a temperature of 80 °C under reduced pressure. The resulting DES was subsequently subjected to FTIR analysis, and Fig. 17 demonstrates that this method led to the nearly complete recovery of the utilized DES. It also demonstrated that the regenerated DES preserved its initial structures. Extraction using regenerated DES showed a decrease in sulfur removal can be attributed to the dissolution of DBT in DESs, which decreases the extraction efficacy of the DESs, and this can be supported by numerous studies reported in the literature such as Mohd Zaid et al. (2015); Liu et al. (2016); and Almashjary et al. (2018).
Conclusion
The presence of organosulfur compounds in transportation fuels creates a harsh environmental scenario worldwide. HDS is a process commercially used by refineries. In this study, we used extractive oxidative desulfurization via solvent extraction method using deep eutectic solvents, which has many advantages over the HDS process, such as the requirement for lower operating temperature and pressure. Also, deep eutectic solvents have extremely low vapor pressure and easier synthesis methods, and it becomes a promising approach towards desulfurization via solvent extraction. In this study, two types of DESs were synthesized using citric acid and adipic acid as hydrogen bond acceptor and triethylene glycol as hydrogen bond donor. The extraction time, extraction temperature, and DES to oil ratio were studied to optimize desulfurization performance. The best suitable extraction conditions were investigated to be DES-to-oil ratio 1, reaction temperature 30 °C, and reaction time 15 min. Further in the study, oxidation of feed using 30% aqueous hydrogen peroxide and 98% formic acid was observed to enhance the desulfurization rate due to the conversion of sulfur compounds to sulfones. The volume of the oxidant and catalyst was also optimized for better performance in the oxidative desulfurization, and the optimized volume of the oxidant aqueous 30% hydrogen peroxide and catalyst formic acid was 0.6 ml, i.e., a 1:1 volume ratio was required in order to reach deep desulfurization of model feed DBT enriched in n-octane containing 495 ppm total sulfur. The extraction efficiencies for the DESs [Citric acid/TEG] [1:7] and [adipic acid/TEG] [1:8] were observed as 44.04%, 74.64%, 39.81%, 42.53%, 69.48%, and 25.35% for model oil, diesel 1, and diesel 2, respectively, in one single extraction stage at the optimized extraction conditions. This extraction efficiency increases further on oxidation up to 98.98%, 87.79%, 56.25%, and 96.96%, 81.22%, and 44.51% for the DESs [citric acid/TEG] [1:7] and [adipic acid/TEG] [1:8] respectively in one single extraction stage at the optimized extraction conditions. Therefore, between the two DESs, [citric acid/TEG] [1:7] DES was identified as the most effective novel solvent for deep desulfurization of fuels via oxidative desulfurization.
References
Abbott AP, Capper G, Davies DL et al (2001) Preparation of novel, moisture-stable, Lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chains. Chem Commun 1:2010–2011. https://doi.org/10.1039/b106357j
Abbott AP, Capper G, Davies DL et al (2003) Novel solvent properties of choline chloride/urea mixtures. Chem Commun Abbott 1:70–71. https://doi.org/10.1039/b210714g
Abbott AP, Capper G, Davies DL et al (2003) Novel solvent properties of choline chloride / urea mixtures †. Chem Commun 1:70–71
Ahmed Rahma WS, Mjalli FS, Al-Wahaibi T, Al-Hashmi AA (2017) Polymeric-based deep eutectic solvents for effective extractive desulfurization of liquid fuel at ambient conditions. Chem Eng Res Des 120:271–283. https://doi.org/10.1016/j.cherd.2017.02.025
Alcaraz MGT, Choi AES, Dugos NP, Wan MW (2023) A review on the adsorptive performance of bentonite on sulfur compounds. Chem Eng Trans 103:553–558. https://doi.org/10.3303/CET23103093
Almashjary KH, Khalid M, Dharaskar S et al (2018) Optimisation of extractive desulfurization using choline chloride-based deep eutectic solvents. Fuel 234:1388–1400. https://doi.org/10.1016/j.fuel.2018.08.005
Andevary HH, Akbari A, Omidkhah M (2019) High efficient and selective oxidative desulfurization of diesel fuel using dual-function [Omim]FeCl4 as catalyst/extractant. Fuel Process Technol 185:8–17
Astle MA, Rance GA, Loughlin HJ et al (2019) Molybdenum dioxide in carbon nanoreactors as a catalytic nanosponge for the efficient desulfurization of liquid fuels. Adv Funct Mater 29:1808092. https://doi.org/10.1002/adfm.201808092
Atlas RM, Boron DJ, Deever WR, Johnson AR, McFarland BL, Meyer JA (2001) Method for removing organic sulfur from heterocyclic sulfur-containing organic compounds, US H1986 H
Betiha MA, Rabie AM, Ahmed HS et al (2018) Oxidative desulfurization using graphene and its composites for fuel containing thiophene and its derivatives: an update review. Egypt J Pet 27:715–730. https://doi.org/10.1016/j.ejpe.2017.10.006
Bhutto AW, Abro R, Gao S et al (2016) Oxidative desulfurization of fuel oils using ionic liquids: a review. J Taiwan Inst Chem Eng 62:84–97. https://doi.org/10.1016/j.jtice.2016.01.014
Ciocirlan O, Iulian O (2009) Density, viscosity and refractive index of the dimethyl sulfoxide + o-xylene system. J Serbian Chem Soc 74:317–329. https://doi.org/10.2298/JSC0903317C
Ciocirlan O, Iulian O, Croitoru O (2010) Effect of temperature on the physico-chemical properties of three ionic liquids containing choline chloride. Rev Chim 8:1–3
Dharaskar SA, Wasewar KL, Varma MN, Shende DZ (2015) Imidazolium ionic liquid as energy efficient solvent for desulfurization of liquid fuel. Sep Purif Technol 155:101–109. https://doi.org/10.1016/j.seppur.2015.05.032
Farzin Nejad N, Miran Beigi AA (2015) Efficient desulfurization of gasoline fuel using ionic liquid extraction as a complementary process to adsorptive desulfurization. Pet Sci 12:330–339
Fatimah H, Zaid M, Kait F et al (2015) Photooxidative – extractive deep desulfurization of diesel using Cu – Fe / TiO 2 and eutectic ionic liquid. Fuel 156:54–62. https://doi.org/10.1016/j.fuel.2015.04.023
Fazlali A, Shahebrahimi Y, Aliyari N, Mohammadi AH (2017) Oil products desulfurization by 1-butyl-3-methylimidazolium tetrachloroaluminate ionic liquid: experimental study and thermodynamic modelling. J Mol Liq 237:437–446. https://doi.org/10.1016/j.molliq.2017.04.101
Florindo C, Monteiro NV, Ribeiro BD et al (2020) Hydrophobic deep eutectic solvents for purification of water contaminated with bisphenol-A. J Mol Liq 297:111841. https://doi.org/10.1016/j.molliq.2019.111841
Gano ZS, Mjalli FS, Al-Wahaibi T et al (2015a) Extractive desulfurization of liquid fuel with FeCl3-based deep eutectic solvents: experimental design and optimization by central-composite design. Chem Eng Process - Process Intensif 93:10–20. https://doi.org/10.1016/j.cep.2015.04.001
Gano ZS, Mjalli FS, Al-Wahaibi T, et al (2015b) Extractive desulfurization of liquid fuel with FeCl3-based deep eutectic solvents: experimental design and optimization by central-composite design. Chem Eng Process Process Intensif 93. https://doi.org/10.1016/j.cep.2015.04.001
Gao H, Luo M, Xing J, Wu Y, Li Y, Li W, Liu Q, Liu H (2008) Desulfurization of fuel by extraction with pyridinium-based ionic liquids. Ind EngChemRes 47:8384–8388
Gao S, Zheng J, Ge G, Luo J (2018) Cu–catalyzed tandem oxidation of n-substituted indolines to isatins. ChemistrySelect 3:13178–13181. https://doi.org/10.1002/slct.201803312
Gu Q, Wen G, Ding Y et al (2017) Reduced graphene oxide: a metal-free catalyst for aerobic oxidative desulfurization. Green Chem 19:1175–1181. https://doi.org/10.1039/c6gc02894b
Gupta AK, Ibrahim S, Al Shoaibi A (2016) Advances in sulfur chemistry for treatment of acid gases. Prog Energy Combust Sci 54:65–92. https://doi.org/10.1016/j.pecs.2015.11.001
Haboc MM, Dugos NP, Choi AES, Wan MW (2023) A review on the current and potential oxidant-catalyst systems in mixing-assisted oxidative desulfurization. Chem Eng Trans 103:559–564. https://doi.org/10.3303/CET23103094
Hao L, Wang M, Shan W et al (2017) L-proline-based deep eutectic solvents (DESs) for deep catalytic oxidative desulfurization (ODS) of diesel. J Hazard Mater 339:216–222. https://doi.org/10.1016/j.jhazmat.2017.06.050
Javadli R, de Klerk A (2012) Desulfurization of heavy oil. Appl Petrochemical Res 1:3–19. https://doi.org/10.1007/s13203-012-0006-6
Jiang W, Li H, Wang C, Liu W, Guo T, Liu H, Zhu W, Li H (2016) Synthesis of ionic-liquid-based deep eutectic solvents for extractive desulfurization of fuel. Energy Fuels 30:8164–8170. https://doi.org/10.1021/acs.energyfuels.6b01976
Joarder S, Bansal D, Meena H, et al (2023) Bioinspired green deep eutectic solvents: preparation, catalytic activity, and biocompatibility. J Mol Liq 376. https://doi.org/10.1016/j.molliq.2023.121355
Julião D, Valença R, Ribeiro JC et al (2017) Efficient eco-sustainable ionic liquid-polyoxometalate desulfurization processes for model and real diesel. Appl Catal A Gen 537:93–99. https://doi.org/10.1016/j.apcata.2017.02.021
Kerton F, Marriott R (2013) Alternative solvents for green chemistry. The Royal Society of Chemistry
Khan AS, Man Z, Bustam MA et al (2018) Efficient conversion of lignocellulosic biomass to levulinic acid using acidic ionic liquids. Carbohydr Polym 181:208–214. https://doi.org/10.1016/j.carbpol.2017.10.064
Khezeli T, Daneshfar A (2017) Synthesis and application of magnetic deep eutectic solvents: novel solvents for ultrasound assisted liquid-liquid microextraction of thiophene. Ultrason Sonochem 38:590–597. https://doi.org/10.1016/j.ultsonch.2016.08.023
Kulkarni PS, Afonso CAM (2010) Deep desulfurization of diesel fuel using ionic liquids : current status and future challenges. Green Chem 12(7):1139–1149. https://doi.org/10.1039/c002113j
Kumar S, Srivastava VC, Raghuvanshi R et al (2015) Removal of refractive sulfur and aromatic compounds from straight-run, fluidized catalytic cracking, and coker gas oil using n -methyl-2-pyrrolidone in batch and packed-bed extractors. Energy Fuels 29:4634–4643. https://doi.org/10.1021/acs.energyfuels.5b00834
Li C, Li D, Zou S, Li Z, Yin J, Wang A, Cui Y, Yao Z, Zhao Q (2013a) Extraction desulfurization process of fuels with ammonium-based deep eutectic solvents. Green Chem 15:2793–2799. https://doi.org/10.1039/c3gc41067f
Li C, Li D, Zou S et al (2013b) Extraction desulfurization process of fuels with ammonium-based deep eutectic solvents. Green Chem 15:2793–2799. https://doi.org/10.1039/c3gc41067f
Li JJ, Xiao H, Tang XD, Zhou M (2016) Green carboxylic acid-based deep eutectic solvents as solvents for extractive desulfurization. Energy Fuels 30. https://doi.org/10.1021/acs.energyfuels.6b00471
Li Z, Fu Y, Zhou A, Yang C (2017) Desulfurization of model FCC gasoline by extraction with ionic liquid and conventional extraction solvents. Pet Sci Technol 35:1699–1705. https://doi.org/10.1080/10916466.2017.1358280
Li Z, Li C (2015) Deep desulfurization of fuels based on an oxidation/extraction process with acidic deep eutectic solvents. Green Chem 17:4552–4559. https://doi.org/10.1039/c5gc00709g
Li Z, Li C, Chi Y, et al (2012) Extraction process of dibenzothiophene with new distillable amine- based protic ionic liquids
Lim CY, Majid MF, Rajasuriyan S, Zaid HFM, Jumbri K, Chong FK (2020) Desulfurization performance of choline chloride-based deep eutectic solvents in the presence of graphene oxide. Environ - MDPI 7:1–17. https://doi.org/10.3390/environments7110097
Lima F, Gouvenaux J, Branco LC et al (2018) Towards a sulfur clean fuel: deep extraction of thiophene and dibenzothiophene using polyethylene glycol-based deep eutectic solvents. Fuel 234:414–421. https://doi.org/10.1016/j.fuel.2018.07.043
Liu C, He Q, Zhang Z et al (2014) Efficient extractive desulfurization of fuel oils using N-pyrrolidone/alkylphosphate-based ionic liquids. Chinese J Chem 32:410–416. https://doi.org/10.1002/cjoc.201400146
Liu W, Jiang W, Zhu W et al (2016) Oxidative desulfurization of fuels promoted by choline chloride-based deep eutectic solvents. J Mol Catal A Chem 424:261–268. https://doi.org/10.1016/j.molcata.2016.08.030
Mafi M, Dehghani MR, Mokhtarani B (2018) Liquid-liquid equilibrium data for extractive desulfurization using 1-butyl-3-methyl imidazolium thiocyanate, n-alkane and thiophene. Fluid Phase Equilib 456:109–115. https://doi.org/10.1016/j.fluid.2017.10.017
Majid MF, Mohd Zaid, Hayyiratul Fatimah, Kait CF, Jumbri K, Chiau Yuan Lim, Sarrthesvaarni Rajasuriyan (2020) Futuristic advance and perspective of deep eutectic solvent for extractive desulfurization of fuel oil: a review. J Mol Liq 306. https://doi.org/10.1016/j.molliq.2020.112870
Makoś P, Boczkaj G (2019) Deep eutectic solvents based highly efficient extractive desulfurization of fuels – eco-friendly approach. J Mol Liq 296. https://doi.org/10.1016/j.molliq.2019.111916
Mamuad RY, Choi AES (2023) Biodesulfurization processes for the removal of sulfur from diesel oil: a perspective report. Energies 16. https://doi.org/10.3390/en16062738
Mjalli FS, Naser J (2015) Viscosity model for choline chloride-based deep eutectic solvents. Asia-Pacific J Chem Eng 10:273–281. https://doi.org/10.1002/apj.1873
Hadj-Kali MK, Mulyono S, Hizaddinb HF, Wazeer I, El-blidi L, Ali E, Hashim MA, AlNashef IM (2016) Removal of thiophene from mixtures with n-heptane by selective extraction using deep eutectic solvents. Ind Eng Chem 55:8415–8423. https://doi.org/10.1021/acs.iecr.6b01654
Mohd Zaid HF, Chong FK, Abdul Mutalib MI (2017) Extractive deep desulfurization of diesel using choline chloride-glycerol eutectic-based ionic liquid as a green solvent. Fuel 192:10–17. https://doi.org/10.1016/j.fuel.2016.11.112
Mohd Zaid HF, Chong FK, Abdul Mutalib MI (2015) Photooxidative-extractive deep desulfurization of diesel using Cu-Fe/TiO2 and eutectic ionic liquid. Fuel 156:54–62. https://doi.org/10.1016/j.fuel.2015.04.023
Mohebali G, Ball AS (2016) Biodesulfurization of diesel fuels - past, present and future perspectives. Int Biodeterior Biodegrad 110:163–180. https://doi.org/10.1016/j.ibiod.2016.03.011
Mokhtar WNAW, Bakar WAWA, Ali R, Kadir AAA (2014) Deep desulfurization of model diesel by extraction with N, N-dimethylformamide: optimization by Box-Behnken design. J Taiwan Inst Chem Eng 45:1542–1548. https://doi.org/10.1016/j.jtice.2014.03.017
Naidu PR, Krishnan VR (1966) Viscosities of binary liquid mixtures. Proc Indian Acad Sci - Sect A 64:229–236. https://doi.org/10.1007/BF03049393
Nautiya NBR, Yadav P, Kumar S, Singh R (2019) Up-gradation of light cycle oil using solvent extraction route. Indian J Chem Technol 26:458–461
Saini N, Kumar K (2023) Deep eutectic solvents in CO2 capture. In: Kumar A, Nadda SS, SK (eds) CO2 Phillic Polymenrs, Nanocomposites and Solvents. Elsevier, pp 193–216
Perkins SL, Painter P, Colina CM (2014) Experimental and computational studies of choline chloride-based deep eutectic solvents. J Chem Eng Data 59:3652–3662. https://doi.org/10.1021/je500520h
Safa M, Mokhtarani B, Mortaheb HR (2016) Deep extractive desulfurization of dibenzothiophene with imidazolium or pyridinium-based ionic liquids. Chem Eng Res Des 111:323–331. https://doi.org/10.1016/j.cherd.2016.04.021
Saini N, Nautiyal BR, Singh R (2022) Extractive oxidative desulfurization of fuels using choline chloride and tetraethylene glycol-based eutectic solvent. Pet Sci Technol 40:1772–1796
Saini N, Yadav P, Singh K, et al (2024) Desulfurizing fuels using alcohol-based DESs using ECODS method. Johnson Matthey Technol Rev 68. https://doi.org/10.1595/205651324x16964075320630
Saleh TA, Sulaiman KO, AL-Hammadi SA (2020) Effect of carbon on the hydrodesulfurization activity of MoCo catalysts supported on zeolite/ active carbon hybrid supports. Appl Catal B Environ 263. https://doi.org/10.1016/j.apcatb.2019.04.062
Shu C, Sun T (2016) Extractive desulfurisation of gasoline with tetrabutyl ammonium chloride-based deep eutectic solvents. Sep Sci Technol 51:1336–1343. https://doi.org/10.1080/01496395.2016.1155602
Silitonga AS, Atabani AE, Mahlia TMI (2012) Review on fuel economy standard and label for vehicle in selected ASEAN countries. Renew Sustain Energy Rev 16:1683–1695. https://doi.org/10.1016/j.rser.2011.12.006
Simanzhenkov V, Idem RO (2003) Crude oil chemistry. https://doi.org/10.1201/9780203014042
Singh R, Kunzru D, Sivakumar S (2016) Monodispersed ultrasmall NiMo metal oxide nanoclusters as hydrodesulfurization catalyst. Appl Catal B Environ 185:163–173. https://doi.org/10.1016/j.apcatb.2015.12.013
Smith EL, Abbott AP, Ryder KS (2014) Deep eutectic solvents (DESs) and their applications. Chem Rev 114:11060–11082. https://doi.org/10.1021/cr300162p
Sudhir N, Yadav P, Nautiyal BR et al (2020) Extractive desulfurization of fuel with methyltriphenyl phosphonium bromide- tetraethylene glycol-based eutectic solvents. Sep Sci Technol 55:554–563. https://doi.org/10.1080/01496395.2019.1569061
Suthar P, Kaushal M, Vaidya D, et al (2023) Deep eutectic solvents (DES): an update on the applications in food sectors. J Agric Food Res 14. https://doi.org/10.1016/j.jafr.2023.100678
Tang X, Zhang Y, Li J, et al (2015) Deep extractive desulfurization with arenium ion deep eutectic solvents. https://doi.org/10.1021/acs.iecr.5b00291
Tomé LIN, Baião V, da Silva W, Brett CMA (2018) Deep eutectic solvents for the production and application of new materials. Appl Mater Today 10:30–50. https://doi.org/10.1016/j.apmt.2017.11.005
Wang Q, Zhang T, Zhang S et al (2020) Extractive desulfurization of fuels using trialkylamine-based protic ionic liquids. Sep Purif Technol 231:115923. https://doi.org/10.1016/j.seppur.2019.115923
Warrag SEE, Adeyemi I, Rodriguez NR et al (2018) Effect of the type of ammonium salt on the extractive desulfurization of fuels using deep eutectic solvents. J Chem Eng Data 63:1088–1095. https://doi.org/10.1021/acs.jced.7b00832
Wilfred CD, Kiat CF, Man Z et al (2012) Extraction of dibenzothiophene from dodecane using ionic liquids. Fuel Process Technol 93:85–89. https://doi.org/10.1016/j.fuproc.2011.09.018
Ye W, Wang T (2023) Extractive desulfurization of liquid fuel with three-body NMP/BEN/H2O deep eutectic solvents. Energy Fuels 37:4973–4985. https://doi.org/10.1021/acs.energyfuels.2c04072
Yu F, Liu C, Yuan B et al (2016) Energy-efficient extractive desulfurization of gasoline by polyether-based ionic liquids. Fuel 177:39–45. https://doi.org/10.1016/j.fuel.2016.02.063
Yusof R, Abdulmalek E, Sirat K, et al (2014) Tetrabutylammonium bromide (TBABr)-based deep eutectic solvents (DESs) and their physical properties. Molecules 8011–8026. https://doi.org/10.3390/molecules19068011
Zhao H, Baker GA (2013) Ionic liquids and deep eutectic solvents for biodiesel synthesis: a review. J Chem Technol Biotechnol 88:3–12. https://doi.org/10.1002/jctb.3935
Zhao X, Zhu G, Jiao L et al (2018) Formation and extractive desulfurization mechanisms of aromatic acid based deep eutectic solvents: an experimental and theoretical study. Chem - A Eur J 24:11021–11032. https://doi.org/10.1002/chem.201801631
Acknowledgements
The authors kindly acknowledge the Director, CSIR-IIP, Dehradun, Uttarakhand, for his permission to publish these results. The authors are also thankful to the analytical sciences division of CSIR-IIP for providing the analysis.
Author information
Authors and Affiliations
Contributions
Nisha Saini: conceptualization, investigation, methodology, supervision, visualization, review, and editing of the original draft. Mansi Negi: experimental write-up and analysis. Pooja Yadav: analysis. Rajkumar Singh: data review.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
All authors have consent to participate as per their contribution.
Consent for publication
All authors have approved the final version of the manuscript for publication.
Competing Interests
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: George Z. Kyzas
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saini, N., Negi, M., Yadav, P. et al. Oxidative desulfurization of fuels using alcohol-based DESs. Environ Sci Pollut Res (2024). https://doi.org/10.1007/s11356-024-33093-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11356-024-33093-4