Abstract
We used microbiology and molecular biology techniques to screen out high-temperature and low-temperature-resistant saprobiotics for compost and prepared a compound fermentation bacteria agent to rapidly ferment cattle manure into high-quality organic fertilizer in low-temperature season. Conventional composting and high-throughput techniques were used to analyze the changes of physical and chemical indexes and biodiversity in the process of composting, from which high and low-temperature-resistant strains were obtained, and high-temperature and low-temperature-resistant solid composite bactericides were prepared and added to composting to verify the effects of composite bactericides on composting. The conventional composting cycle took 22 days, and the diversity of microflora increased first and then decreased. Composting temperature and microbial population were the key factors for the success or failure of composting. Two strains of high-temperature-resistant bacteria and six strains of low-temperature-resistant bacteria were screened out, and they were efficient in degrading starch, cellulose, and protein. The high-temperature and low-temperature-resistant solid bacterial agent was successfully prepared with adjuvant. The preparation could make the compost temperature rise quickly at low temperature, the high temperature lasted for a long time, the water content, C/N, and organic matter fell quickly, the contents of total phosphorus and total potassium were increased, and the seed germination index was significantly improved. Improve the composting effect. The solid composite bacterial agent can shorten the composting time at low temperature and improve the composting efficiency and quality.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to the survey data of the Ministry of Agriculture and Rural Affairs of China and the National Bureau of Statistics, the number of cattle raised in China shows a gradual growth trend, and by the end of 2022, the total number of cattle raised in China will reach 102.16 million (Chen et al. 2023) (Xu et al. 2023a). At the same time, livestock and poultry manure has become one of the important causes of pollution in China. The production of cattle manure reached 52.8 million tons in 2020 and exceeded 4 billion tons in 2022 (Zhou et al. 2023a). Cattle manure emits an unpleasant odor, which is easy to breed pathogenic microorganisms. Excessive direct discharge affects the growth of crops, causes serious pollution to the environment, and restricts the survival and development of cattle industry (Xu et al. 2023b).
Composting is the process of decomposing substances that are not easily decomposed in accumulated manure into substances that are beneficial to the environment and soil plants under the action of microorganisms. Composting is the most common and economical way to disposal of manure (Onwosi et al. 2017; Xu, et al. 2023a). However, the traditional composting process has disadvantages such as low efficiency, insufficient mineralization of nitrogenous compounds, large nitrogen emissions, and low compost quality (Cheng et al. 2023; Rao and Parsai 2023). There are many factors that affect composting, mainly including temperature, moisture content, pH, C/N, organic matter, and other nutrients. Optimizing these factors is an effective way to improve the efficiency of composting, which can ensure the smooth progress and improvement of fecal waste fermentation. In order to solve this problem, people often pre-treat compost raw materials; the most common method is to add bacteria to the material. For example, Liang et al. found that adding compound bactericides to the composting process of chicken manure or cow manure accelerates the degradation of antibiotics (Liang et al. 2020); Xie et al. found that adding composite microbial agents to food waste compost can accelerate the biodegradation of organic matter, reduce greenhouse gas emissions in food waste compost, and improve the quality of compost (Xie et al. 2021). Xu et al. found that the addition of thermophilic bactericides in the composting process increased the complexity and diversity of the bacterial and fungal communities, enhanced the mineralization of organic carbon, accelerated the degradation of cellulose, and promoted the humification process of solid organic waste compost (Xu et al. 2019). In summary, the addition of functional bactericides can improve the quality of compost and realize the reuse of resources.
The composting process is widely used in China. But in northern regions of China, such as Hebei and Inner Mongolia, the temperature in autumn and winter is relatively low, and the fermentation of compost is slow. The commercial fermentation agent can generally play a role above 15 ℃, which greatly limits the promotion and application of microbial agents (Mi et al. 2023; Xie et al. 2021). The search for low-temperature-resistant fermentation bacteria is the key factor to solve the composting in the northern region of China. On the other hand, refractory substances such as cellulose and lignin in cattle manure are mainly decomposed in the high-temperature period, but most microorganisms cannot survive when the temperature is too high (Chang et al. 2019; Zhou, et al. 2023a). Therefore, searching for high-temperature-resistant fermentation bacteria is also a key factor to improve the quality of compost (Bao et al. 2021; Sardar et al. 2021). However, there are still few studies on the screening and evaluation of low and high-temperature-resistant bacteria. In this study, we screened high-temperature and low-temperature-resistant bacteria, prepared solid composite microbial agent and added it to cattle manure, and investigated the effect of fermentation bacterial agent on cattle manure composting at low temperature.
Materials and methods
Main reagents
AGAR medium and some chemical reagents were purchased from Fuchen (Tianjin) Chemical Reagents Co., LTD. Tryptone was purchased from Beijing Aoxing Biotechnology Co., LTD. DNA extraction reagent Taq-TM DNA Polymerase MBI EP0702 and SanPrep column DNAJ gel recovery kit SK8131 were purchased from Autobiological (Shanghai) Technology Co. LTD.
Fermentation of cattle manure and sampling
Fresh cattle manure was provided by the experimental cattle farm of Hebei Agricultural University. For indoor composting, the water content of fresh cow manure was close to 60% after dry and wet separation. The cow manure was packed into a plastic box connected with a ventilation tube to allow natural internal ventilation. The top of the plastic box was covered with a breathable woven bag. The compost was fermented for 22 days. Two artificial turnings were conducted on 8 days and 15 days within 22 days of composting. Each sample was evenly mixed and put into a sealed bag. One part was stored at − 20 ℃ for 48 h for microbial screening and physical and chemical index detection, and the other part was stored at − 80 ℃ for microbial diversity analysis.
Determination of physical and chemical indexes
The day of starting composting was recorded as the first day, and samples were collected at regular intervals. The sampling time of physical and chemical indexes was 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 13, 15, 17, 20, and 22 days. The temperature of all sampling sites was measured at 9:00 am and 5:00 pm every day, and the average value was taken as the pile temperature of the day. The pH value was measured by a pH meter. The moisture content was measured by drying at 105 ℃. Total organic carbon and total nitrogen were determined according to Chinese national standard GB13193-91 and agricultural industry standard NYT 297–1995. Organic matter was measured as organic carbon × 1.724. The seed germination index was determined according to the method of Zhang et al. (Zhang et al. 2023a), and each index was repeated three times.
Microbial diversity analysis
The sampling times of high-temperature and low-temperature-resistant bacteria were the early composting period and the high-temperature period, and the sampling times of microbial diversity were the early composting period (93S), the heating period (94S), 1 day before the high-temperature period (95S), and the day of the high-temperature period (96GWS). The strain DNA was extracted according to the instructions of genomic DNA extraction kit (Beijing Solarbio Technology Co., LTD.). The 16S rDNA sequence was amplified by PCR. 16S rRNA and ITS sequencing was performed using the Illumina platform MiSeq from Paisenore Biological Co., LTD. (Shanghai). The effective data of each sample were obtained by computer detection, and the α diversity, species composition, and dominant flora comparison were analyzed. The sequence with the highest abundance (97%) was selected as the representative sequence, the number of OTUs was obtained by Usearch statistics, and dilution curve analysis was performed. The mothur software was used to calculate sample alpha diversity, analyze sample community structure component diagram, sample and species analysis diagram, and analyze the diversity of bacterial flora among samples. The Fisher exact test is used to test the significance of differences in species composition and community outcomes between samples.
Screening and identification of high-temperature and low-temperature-resistant bacteria
The steps for isolation and purification of the high-temperature-resistant bacteria are as follows: the bacteria were cultured at 55 ℃ for 48 h, actinomycetes and fungi were cultured at 55 ℃ for 7 days, and then, the colony morphology was observed. Different single colonies were selected and inoculated into the corresponding liquid medium. After 24 h of incubation in a constant temperature culture at 55 ℃, the cultures were streaking again on solid plates and repeated 3–4 times until purified bacteria, actinomycetes, and fungi were obtained.
The isolation and purification of low-temperature-resistant bacteria were similar to the above steps, but the low-temperature-resistant bacteria were sampled at the beginning of fermentation and at the high-temperature stage and cultured at 25 ℃ and 55 ℃, respectively. After the strains that could grow were screened out, they were cultured at 15 ℃, 20 ℃, 25 ℃, and 30 ℃ to observe whether these bacteria could still grow. In this study, the temperature suitability test determined that 25 ℃ was the culture temperature of low-temperature-resistant bacteria, and 55 ℃ was the culture temperature of high-temperature-resistant strains.
The selected strains were sent to Shanghai Sangon Biotechnology Co., LTD., for 16S rDNA sequencing analysis and BLAST comparison to determine the species of microorganisms. At the same time, the selected strains were cultured, and single colonies were selected for Gram staining and microscopic identification.
Functional verification of high-temperature and low-temperature-resistant bacteria
Degradation tests such as starch hydrolysis test, cellulose degradation test, and protein hydrolysis test were used to screen strains with strong decomposition ability of cattle manure (López-González et al. 2014). When the various bacteria to be screened grew to 80% of the logarithmic phase, they were stored at 4 ℃.
Determination of the growth curve of each bacterium and the number of colonies per unit volume
The OD value of the bacterial solution was measured every 2 h at 600 nm by spectrophotometry. After determining the optimal inoculation time, each strain was grown in shake flasks at 25 ℃ and 55 ℃, respectively. When the bacteria reached the optimal access point, the bacterial solution was obtained, and the number of colonies in the bacterial solution was measured and recorded. The coating plate method was used for determination, and the dilution concentration gradients was 10−4–10−8. Each strain was treated in triplicate and the average value was calculated. The number of the strain contained in each unit volume of the bacterial solution was measured as cfu·mL−1.
Preparation of fixed composite bacterial agent
The inoculation amount of compost is generally 0.5–1%. In this experiment, about 170 kg cow manure was composted, the volume of various microorganisms was 200 mL, and a total of 1600 mL bacterial solution was prepared. The inoculation amount was about 0.94% of which 400 mL was two strains of high-temperature-resistant bacteria and 1200 mL was six strains of low-temperature-resistant bacteria. We mixed fine bran and fine sawdust evenly and divided them into two parts. One part was recorded as X1 is 400 g mixed with high-temperature-resistant bacteria, and 100 g glucose was added; the other part was recorded as X2 which is 1200 g mixed with low-temperature-resistant bacteria, and 300 g glucose was added. Glucose was used as fermentation primer, and an appropriate amount of distilled water was mixed with solid substrate evenly. The water content was 60% and cultured in the incubator for 24 h, respectively. The high-temperature-resistant bacteria were cultured at 55 ℃, and the low-temperature-resistant bacteria were cultured at 25 ℃ and 55 ℃. Take out the mixed solids and dry them in the oven at 25 ℃ and 55 ℃. Thoroughly dried X1 and X2 are mixed evenly at room temperature, and the solid bactericide is prepared.
Effects of combined bacterial agents on compost fermentation
The cattle manure composting with solid compound microbial agent was set as the experimental group (group T), and the natural composting without microbial agent was set as the control group (group C). The composting was carried out in winter (December). At the time of sampling, the initial composting period of groups T and C was T1 and C1, the heating period was T2 and C2, the high-temperature period was T3 and C3, the cooling period was T4 and C4, and the putrefaction period was T5 and C5, and each was repeated three times. The sampling time was 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 13, 15, 17, 20, and 22 days. Physical and chemical indexes, species composition, and diversity were detected according to 2.2, 2.3, and 2.4 to verify the effects of high-temperature and low-temperature tolerance composite microbial agents on cattle manure composting in winter and high-temperature composting stage.
Data analysis and processing
Data are presented as mean ± SD. The SPSS19.0 software was used to analyze the data, and the T test was used for significance analysis. GraphPad Prism 5 software was used to draw bar and line graphs. Dilution curves, species composition, and dominant microorganisms were analyzed by QIIME2 software.
Results
Changes in physical and chemical indexes during conventional composting
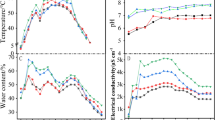
The whole composting period was 22 days, and the temperature first increases and then decreases. On the 4th day, the temperature is higher than 55 ℃ and enters the high-temperature period. On the 7th day, the maximum temperature reaches 60.5 ℃, which lasts for 7 days and begins to decrease, basically meeting the composting temperature requirements of China’s “Hygienic Standard for Harmless Manure (GB7959-87)” (Fig. 1a). The initial pH of compost was 8.06, reached the maximum value of 9.03 on the 7th day, and then gradually decreased. In the later stage of compost, the pH was basically stable between 8.10 and 8.20, which was suitable for the growth and reproduction of bacteria (Fig. 1b). During the whole composting process, the moisture content decreases throughout the composting process. The initial moisture content of the compost was 61.4%, and the moisture content after the compost was 37.3%, which decreased by 24.1% in the whole process (Fig. 1c). The C/N decreased throughout the composting process. The initial C/N was 32, which decreased by 48.4 in the suitable range, and the C/N was 16.4 at the end of the composting (Fig. 1d).
Analysis and evaluation of microbial diversity during composting
Single-sample α diversity analysis can reflect the richness and influence of microbial communities within a sample. The Chao I index, Shannon index, ACE, and Coverage were used to assess the diversity of bacteria and fungi at different compost stages of cattle manure. The results showed that bacterial diversity increased first and then decreased, and 95S was the turning point. The diversity of fungi showed the same trend (Table 1).
In this study, metagenomic sequencing technology was used to analyze the diversity and abundance of bacteria and fungi during cattle manure composting and to explore the changes in microbial community composition at different stages during cattle manure composting. The results showed that the diversity of bacteria and fungi in 96GWS was significantly different from the other three samples. At the phylum level, the difference between 96GWS and 93S bacteria was significant in Bacteroidetes and Firmicutes (P < 0.05), and there was no significant difference in other bacteria. At the genus level, there were significant differences between 96GWS and 93S in unclassified bacteria, Actinomyces, Flavobacterium, Azotomonas, and Fibrovibrio (P < 0.05). There were significant differences between 96GWS and 93S fungi in Ascomycota and Basidiomycota (P < 0.05), and there were no significant differences in other fungi. At the genus level, 96GWS and 93S bacteria showed significant differences between Yeasts and unclassified Microcystomycetes (P < 0.05) (Fig. 2). The results showed that the diversity of bacteria and fungi changed with the change of temperature at different stages of cattle manure compost, and dominant microorganisms that were conducive to rapid fermentation of cattle manure appeared at each stage, mainly including Actinomyces, Azotomonas, Vibrio, Yeasts, and Microcystomycetes.
Metagenomic sequencing was used to analyze the changes of bacterial and fungal diversity in cow manure fermentation. a Cluster tree and bar chart analysis of bacteria and fungi in phylum. b Cluster tree and bar chart analysis of bacteria and fungi in order. c Cluster tree and bar chart analysis of bacteria and fungi in family. d Cluster tree and bar chart analysis of bacteria and fungi in genus
Screening and identification of microorganisms resistant to high temperature and low temperature
The samples of high-temperature and low-temperature-resistant bacteria were obtained from the early composting stage and high-temperature stage, and the plate culture method and temperature adaptability test were used to screen the high-temperature and low-temperature-resistant bacteria. Therefore, 29 strains of microorganisms were obtained, including 20 strains of low-temperature-resistant bacteria and 9 strains of high-temperature-resistant bacteria, which were numbered as follows: initial low-temperature-resistant bacteria DDX1, DDX2, DDX3, DDX4, DDX5, DDX6, DDX7, DDX8, DDX9, DDX10, DDX11; low-temperature-resistant bacteria DGX1, DGX2, DGX3, DGX4, DGX5, DGX6, DGX7, DGX8, DGX9; GX1, GX2, GX3, GX4, GX5, GX6, GX7, GX8, GX9 in high-temperature stage. The species of strains were identified by 16Sr DNA method, among which Escherichia coli, Enterobacter, and Enterobacter cloacae had no significant effect on the fermentation of cattle manure compost, so they were excluded, and then verified by Gram staining, 8 types of strains were identified (Table 2 and Fig. 3).
Finally, the 8 strains of bacteria were selected for the subsequent preparation of complex bacterial agents, The high-temperature-resistant bacteria included GX4 and GX7, and the low-temperature-resistant bacteria included DDX1, DDX2, DDX4, DDX5, DDX6, and DDX7 (Table 3).
Functional validation results of microorganisms resistant to high temperature and low temperature
The results of starch hydrolysis, fibrinolysis, and proteolysis showed that all the 8 strains had the ability to hydrolyze starch, and the hydrolysis effect was strong. GX4, DDX2, DDX5, DDX6, and DDX7 were significantly hydrolyzed compared with the control (P < 0.01). GX7, DDX1, and DDX4 were significantly hydrolyzed compared with the control (P < 0.05). The value in the table indicates the hydrolysis ability, and the higher the value, the stronger the starch hydrolysis ability. DDX7 has the strongest starch hydrolysis ability, followed by DDX6, and the values of DDX2 and GX4 are 8.69 and 5.6, respectively. The hydrolysis ability of other bacteria is not significantly different about 4.0. All the 8 strains could degrade cellulose, and the degradation effect was strong. Compared with the control group, all the 8 strains hydrolyzed cellulose significantly (P < 0.01). The degradation ability of DDX7 was the strongest, followed by DDX1, GX7, and GX4, and the degradation ability of other bacteria was about 3.0–4.0. Six strains of bacteria had the ability to degrade proteins, and the degradation effect was strong. Compared with the control group, the six strains of bacteria were highly hydrolyzed (P < 0.01). DDX2 had the strongest degradation ability, followed by DDX4, and the degradation ability of other bacteria was about 2.0–4.0. The two strains of heat-resistant bacteria did not hydrolyze proteins (Table 4).
Determination of the growth curve of each bacterium and the number of colonies per unit volume
According to the growth curve of each bacterium, the time of logarithmic growth phase, the time when the logarithmic phase reached 80%, and the OD value were obtained (Table 5). The number of colonies in the unit volume of microbial fluid was detected (Table 6).
Preparation of microbial compound agent
The inoculum amount of compost is generally 0.5–1%. In this experiment, about 170 kg of cattle manure was composted, and the volume of various microorganisms was 200 mL. A total of 1600 mL of bacterial solution was prepared, and the inoculum amount was about 0.94%, including 400 mL of heat-resistant bacteria and 1200 mL of low-temperature-resistant bacteria. After all the bacteria grew to the optimal growth time, according to the preparation method, the solid microbial agent was finally obtained. The agent was dark yellow in color and was associated with a pungent smell of microorganisms.
Effect of solid compound bacterial agent on compost fermentation
Effects of solid compound bacterial agent on physicochemical indexes during compost fermentation
The composting process took 22 days. The temperature of C and group Ts increased first and then decreased. The pile temperatures of groups T and C were 28.3 ℃ and 21.0℃ on the first day, respectively. The temperature of group T increased rapidly and reached the high-temperature stage 2 days earlier than that of group C. The temperature of group T entered the high-temperature stage at 52.8 ℃ on the 4th day and reached the maximum temperature of 59.7 ℃ on the 8th day. The high-temperature stage lasted for 9 days. The temperature of group C was 51.7 ℃ on the 6th day, and the maximum temperature of group C was 56.3 ℃ on the 9th day. The high-temperature stage lasted for 6 days, which met the requirements of Chinese “harmless manure hygiene standard (GB7959-87)” for composting temperature. After the high-temperature period, the temperature of each group showed a downward trend, and the temperature was below 50 ℃ on the 13th day. These results indicated that the addition of microbial agent could rapidly start the initial temperature of compost, increase the maximum temperature of compost, and prolong the high-temperature time (Fig. 4a).
The pH in the compost first increased and then decreased. The initial pH value was around 8.0. With the progress of composting, the pH value of group T was significantly higher than that of group C on the 5th day (P < 0.05), and there was no significant difference between group T and group C at other time points. The pH value reached its maximum on day 10 and then decreased continuously until it reached its lowest value at the end of the study, which was maintained between 8.0 and 8.5. The results showed that adding bacterial agent could accelerate the pH change (Fig. 4b).
The initial moisture content of the compost was about 60%, and the moisture content of the two compost groups showed a decreasing trend. At 0 days and 20 days, there was no significant difference between group T and group C, and at 5 days, 10 days, and 15 days, group T was significantly lower than group C (P < 0.05). At the end of composting, the water content of group C eventually decreased by 18.4%, and that of group T eventually decreased by 24%. The results showed that adding bacteriotics in the composting process of cow manure promoted the decrease of water content (Fig. 4c).
There was no significant difference in C/N between group T and group C at 0 days, but the C/N in group T was significantly lower than that in group C at 5 days (P < 0.01), and the C/N in group T was significantly lower than that in group C at other time points (P < 0.05). The C/N values of group C and group T were 32.0 and 32.4 at the beginning of this experiment and 16.4 and 15.1 at the end of composting, respectively. The test showed that the C/N decreased faster with the addition of bacterial agent (Fig. 4d).
The content of organic matter decreased slowly during the composting process. There was no significant difference between group T and group C at 0 and 20 days, but group T was significantly lower than group C at 5 and 10 days (P < 0.01), and group T was significantly lower than group C at 15 days (P < 0.05). The initial contents of group C and group T were 682.3 and 682.5 g·kg−1, respectively, and the final contents were 567.7 and 559 g·kg−1, respectively, which decreased by 114.6 and 123.5 g·kg−1, respectively. The results showed that the addition of bacterial agent could promote the decomposition of organic matter (Fig. 4e).
GI increased during composting. There was no significant difference between group T and group C at 0 day, but group T was significantly higher than group C at 5, 10, 15, and 20 days (P < 0.01). The initial GI of group C and group T was 26% and 26.3%, respectively, and the final GI was 88.2% and 92.7%, respectively. The two groups showed the largest increase from 0 to 10 days, indicating that the high-temperature period played a key role in GI. At the end of composting, the GI of both groups had exceeded 80%, which could be regarded as the cattle manure had no phytotoxicity or had become mature. The results showed that the seed germination index was accelerated by adding the compound bacterial agent (Fig. 4f).
Effects of microbial compound agents on microbial diversity during compost fermentation
By calculating the alpha diversity index (Observed species, Shannon, Chao I, and Coverage), the diversity data showed that the measured data values increased and tended to be stable with the composting process, indicating that the sequencing data of this experiment was close to saturation. The results showed that the sequencing quantity of this experiment could reflect the diversity composition of bovine fecal microbiota at different composting stages in this experiment (Table 7).
Species composition in compost samples of cattle manure from group C and T at phylum level. A total of 31 phyla were detected, and only the top 10 with the highest abundance are shown in this figure. It can be seen that the changes of various bacterial flora in group C and group T were consistent, but the abundance of bacterial flora was different. Firstly, Proteobacteria was the dominant microorganism with the highest abundance in each stage of each group, especially in the early stage of compost and the warming stage. Secondly, the abundance of Bacteroidetes gradually increased with the progress of compost in each group, from about 11% at the beginning to 32.5% in C3 and 35.5% in T3 in the high-temperature stage, and the proportion decreased with the fall of temperature. Thirdly, Firmicutes accounted for 18% and 24% of the initial abundance of group C and group T, respectively, but decreased to 1% and 3% as the final abundance decreased. Fourthly, actinomycetes did not change much during the whole composting process, accounting for about 8%. Chloroflexi was almost not present in the early composting stage (C1, T1) in groups C and T, but appeared in the high-temperature stage (C3, T3), cooling stage (C4, T4), and ripening stage (C5, T5) with the composting process. The abundance of Chloroflexi increased from 1 to 13% in group C, and Chloroflexi appeared in the cooling stage and ripening stage in group T. When the temperature increased from 1 to 20%, the suitable survival temperature of the strain was 37 ℃. Adding the compound bacterial agent increased the temperature during the high-temperature period and maintained it for a long time, which was not conducive to the growth of the strain. Thermi began to appear after the temperature dropped. The proportion of Tenericutes in group C was about 2%, and that in group T was about 3%, which did not change significantly, but there was still a difference. The proportion of Tenericutes in C1, C2, C3, T1, T2, and T3 was not large, and there was almost no late composting.
Similarly, species composition in compost samples of cattle manure in groups C and T at the genus level. A total of 41 genera were detected, and only the top 10 with the highest abundance are shown in this figure. It can be seen that the changes of various bacterial flora in group C and group T were consistent, but the abundance of bacterial flora was different. From C1 and T1 to C2 and T2, the proportion of Pseudomonas in abundance gradually increased from 17 and 8% to 23% and 24% with the increase of composting temperature, and the increase of the proportion in group T was higher than that in group C. From C2 and T2, the abundance gradually decreased to 1%, and the decrease of the proportion in group T was higher than that in group C, which indicated that Pseudomonas was the dominant microorganism in the warming period. Second, Acinetobacter was present in C1, C2, T1, and T2, and its abundance decreased with the increase of composting temperature, from 10% and 23.4% in C1 and T1, respectively, to 5% and 10% in C2 and T2, and finally to 0%, which was the dominant microorganism in the initial stage of composting. Third, with the increase of composting temperature, the abundance of Psychrobacter decreased continuously. In group C, the proportion of C1 decreased from 17 to 6% in C2, and no mesophilic bacteria were present in C3–C5. In group T, the proportion of Psychrobacter in T1 decreased from 15 to 8% in T2, 1% in T3, and no mesophilic bacteria were present in T4 and T5. With the increase of temperature, the proportion of Ruminofilibacter in C3 increased from 1 to 3.7% in C5, and the proportion of abundance in T3 increased from 7 to 10% in T5. The proportion of Ruminofilibacter in group T was higher, and the increase range was higher, which was the dominant microorganism in the high-temperature stage and the late stage of compost. The proportion of Cellvibrio in C1 and C2 was very small. The proportion of C3 increased to 6% in the high-temperature period and decreased slightly after the high-temperature period. The proportion of T1 and T2 was very small, and the proportion of T3 increased to 5% in the high-temperature period, but remained unchanged after the high-temperature period. The proportion of Luteimonas and B-42 was small in each stage of each group, but it could still be seen as the dominant microorganisms in the high-temperature period and cooling period (Fig. 5).
The histogram of LDA value distribution of significantly different species is used to show the significantly enriched species and their importance degree in each group. There are 94 microorganisms with different classification levels with LDA value greater than 2. In the early stage of compost, there were significant differences in the composition of Pseudomonas in C1, BDZ-13 in C2, Alteromonas in C3, α-Proteobacteria in C4, Tenoxanthomonas in C5, Moraxellaceae in T1, Pseudomonas in T2, Minotrichiura in T3, Agrobacterium in T4, and Chloroflexida in T5 (P < 0.05) (Fig. 6).
Discussion
Cattle manure is considered a valuable source of fertilizer because it is rich in nutrients (nitrogen, phosphorus, and potassium) (Moreira et al. 2023). Reducing the negative impact of cattle manure and improving resource utilization rate have become important problems to be solved urgently in the development of animal husbandry (Alwaneen 2016). However, if not handled properly, it can also cause health hazards, unpleasant odor, and groundwater contamination due to contaminant leaching (Zhou et al. 2023b; Zhu et al. 2023). So far, aerobic composting has been widely adopted as an efficient and environmentally friendly method to convert organic waste into agrologic fertilizer under the influence of a series of microbial activities (Huhe et al. 2017). The technology involves complex biodegradation of solid substrate mixtures by microbial communities composed of various populations under aerobic conditions, and the quality of composting efficiency depends on the control of aerobic composting process parameters. There are many factors affecting aerobic compost, mainly including temperature, moisture content, pH value, C/N ratio, seed germination index, organic matter, and other nutrients (Bernal et al. 2009; Huang et al. 2003; Said-Pullicino et al. 2007). Optimizing these factors can ensure the smooth progress and improvement of fecal waste fermentation and is an effective way to improve composting efficiency.
Temperature is the most direct and sensitive indicator for successful composting (Huo et al. 2023). If the temperature is too low, the compost will take longer to decompose, resulting in a decrease in production efficiency (Sun et al. 2022). During the composting process, for 22 days, the temperature of the control group (group C) and the experimental group (group T) increased first and then decreased. However, the temperature of group T increased faster and reached the high-temperature stage 2 days earlier than that of group C, indicating that the experimental group may have more effective functional microorganisms, resulting in more organic waste being metabolized and more energy released. Appropriate high temperature and prolonged high temperature time can not only kill pathogens and weed seeds but also improve the safety of compost products (Wu et al. 2020). The pH value is one of the important factors affecting microorganisms (Wang et al. 2023). In this experiment, pH showed a trend of first rising and then decreasing, and the initial rise was caused by the release of ammonia gas. The reason for the decrease in pH during composting may be due to the rapid decomposition of large amounts of labile organic matter by microorganisms, resulting in the production of organic acids and the concomitant consumption of some nitrate nitrogen. In this study, it was found that the pH value increased significantly with the composting process. On the 5th day, the pH value of group T increased slightly faster, reached the maximum on the 10th day, and then the pH value continued to decrease, indicating that the addition of bacterial agent could accelerate the pH change. Changes in carbon and nitrogen are one of the basic characteristics of compost, and C/N is an important indicator of compost maturity, which plays an important role in the growth and metabolism of microorganisms (Xie et al. 2022). In this study, it was found that C/N decreased during the composting process, and group T decreased faster and more than group C, indicating that the addition of bacterial agent was beneficial to the reduction of C/N. The organic matter content in the composting process showed a slow decreasing trend. The results of this study showed that the addition of microbial inoculum had a good effect on improving the degradation rate of organic matter and accelerating the progress of compost maturation (Zhang et al. 2023b). The seed germination index has been used to quickly and effectively assess compost maturity and has been widely accepted by researchers (Kong et al. 2022). The GI was low in the early stages of composting and increased as the composting process progressed, when GI > 80% compost is basically non-toxic to plants. The 85% means the compost is finished and the manure is fully decomposed. In this study, we found that GI increased during the composting process, and high-temperature period played a key role in GI. Moreover, adding compound microbial agent could accelerate seed germination index. In conclusion, the combined microbial agent in this study could promote the decline rate of water content, C/N, and organic matter, significantly improve seed germination index, shorten composting time, and improve composting efficiency.
Compost fermentation mainly consists of four stages: warming period, high-temperature period, cooling period, and putrefied period. Microorganisms play an important role in each stage. It is because of these different microorganisms that a variety of complex changes occur in each period. Inoculating microorganisms is a promising strategy for effective composting, but it often encounters barriers such as long processing times and competition between microbial species (He et al. 2022). To address these challenges, complex microorganisms can be introduced at different stages of the composting process (Mi et al. 2023). Our results showed that bacterial diversity increased first and then decreased during the composting process, with a turning point at 95S. The diversity of fungi showed the same trend. The bacterial and fungal diversity of the other 96GWS was clearly different from the other three samples. This study showed that the diversity of bacteria and fungi changed with the change of temperature at different stages of cattle manure compost, and dominant microorganisms that were conducive to the rapid fermentation of cattle manure appeared at each stage. The Bacillus is the main dominant bacterium in the high-temperature stage of the original compost, which may be related to the growth characteristics of Bacillus with high-temperature and low-temperature resistance, and it can also grow well in a warm environment. Meanwhile, the dominant microorganism in the high-temperature stage was Bacteroidetes by high-throughput sequencing, which was basically consistent with the bacteria screened in this study. It can prolong the high-temperature composting time, which is of great significance to guide the application of microbial agents in composting and further improve the efficiency of composting. Streptococcus thermophilus is a fermentation strain with high yield of exopolysaccharides, which is often used as one of the fermentation agents of yogurt and is widely used in the processing of fermented dairy products, while there are few studies in industrial fermentation. This experiment screened this bacterium during the fermentation period of cow manure at high temperature and found that it has a certain degradation effect on cellulose in manure, so it can continue to study the role of this bacterium in compost.
The addition of microbial inoculants did not significantly affect the abundance of actinomycetes, but Bacteroides were affected. Firmicutes and Bacteroides were more abundant in group T than in group C. Previous findings suggest that these are important bacteria for anaerobic fermentation, breaking down organic matter to hydrogen or acetic acid, which is also similar to findings on cellulose and organic matter degradation during composting (Meng et al. 2019; Song et al. 2021). Pseudomonas was present in all stages of C and group Ts, and the proportion of abundance gradually increased with the increase of composting temperature. The increase amplitude of group T was higher than that of group C, and the decrease amplitude of group T was higher than that of group C in the late composting stage, which was the dominant microorganism in the warming stage. In addition, the abundance of Ruminofilibacter increased with the increase of temperature, with a higher proportion in group T and a higher increase range, which was the dominant microorganism in the high-temperature stage and the late stage of compost. In summary, the addition of microbial agents changed the growth and reproduction of bacteria, had no significant effect on species composition, significantly changed species abundance, and improved the richness of the composting ecosystem and the key composting microbial functional populations.
Conclusion
In this study, through the establishment of common cattle manure compost, we screened out thermoresistant bacteria Streptococcus thermophilus and Bacillus licheniformis, which can efficiently degrade starch, cellulose, and protein, and low-temperature-resistant bacteria Bacillus licheniformis, Bacillus megaterium, Bacillus cereus, and Bacillus thuringiensis. The prepared high-temperature and low-temperature-resistant compost fermentation solid bacterial agent can make the compost temperature increase quickly. When high temperature lasted for a long time, water content, C/N, and organic matter decreased rapidly, which significantly increased seed germination index and shortened composting time. High-temperature and low-temperature-resistant solid microbial agent could accelerate the composting process in winter, significantly change the diversity and structure of microbial community, and improve the efficiency and quality of composting. This study provided a new method to solve the problem of slow fermentation of cattle manure in winter in large-scale cattle farms, which was of great significance for the resource reuse of cattle manure.
Data availability
Data will be made available on request.
References
Alwaneen WS (2016) Cow manure composting by microbial treatment for using as potting material: an overview. Pak J Biol Sci 19(1):1–10. https://doi.org/10.3923/pjbs.2016.1.10
Bao Y, Feng Y, Qiu C, Zhang J, Wang Y, Lin X (2021) Organic matter- and temperature-driven deterministic assembly processes govern bacterial community composition and functionality during manure composting. Waste Manag 131:31–40. https://doi.org/10.1016/j.wasman.2021.05.033
Bernal MP, Alburquerque JA, Moral R (2009) Composting of animal manures and chemical criteria for compost maturity assessment. A Review Bioresour Technol 100(22):5444–5453. https://doi.org/10.1016/j.biortech.2008.11.027
Chang R, Guo Q, Chen Q, Bernal MP, Wang Q, Li Y (2019) Effect of initial material bulk density and easily-degraded organic matter content on temperature changes during composting of cucumber stalk. J Environ Sci 80:306–315. https://doi.org/10.1016/j.jes.2017.10.004
Chen S, Zhang H, Zhai J, Wang H, Chen X, Qi Y (2023) Prevalence of clinical mastitis and its associated risk factors among dairy cattle in mainland China during 1982–2022: a systematic review and meta-analysis. Front Vet Sci 10:1185995. https://doi.org/10.3389/fvets.2023.1185995
Cheng L, Wang L, Wang X, Ou Y, Liu H, Hou X, Yan L, Li X (2023) The various effect of cow manure compost on the degradation of imazethapyr in different soil types. Chemosphere 337:139325. https://doi.org/10.1016/j.chemosphere.2023.139325
He, Jing, Nengmin Zhu, Yansheng Xu, Li Wang, Jiaqiang Zheng, and Xia Li (2022) The microbial mechanisms of enhanced humification by inoculation with Phanerochaete chrysosporium and Trichoderma longibrachiatum during biogas residues composting. Bioresource Technology 351.https://doi.org/10.1016/j.biortech.2022.126973.
Huang G, Zhong L, Zhang Z, Wu Q (2003) Physicochemical changes and maturity evaluation of solid organic waste compost. Ying Yong Sheng Tai Xue Bao 14(5):813–818
Huhe, C. Jiang, Y. Wu, and Y. Cheng (2017) Bacterial and fungal communities and contribution of physicochemical factors during cattle farm waste composting. Microbiologyopen 6(6).
Huo XJ, YanZhou MJ, Chen JL, Zhou, and C. L. Zheng, (2023) Potassium-rich mining waste addition can shorten the composting period by increasing the abundance of thermophilic bacteria during high-temperature periods. Sci Rep 13(1):6027. https://doi.org/10.1038/s41598-023-31689-3
Kong Y, Wang G, Chen W, Yang Y, Ma R, Li D, Shen Y, Li G, Yuan J (2022) Phytotoxicity of farm livestock manures in facultative heap composting using the seed germination index as indicator. Ecotoxicol Environ Saf 247:114251. https://doi.org/10.1016/j.ecoenv.2022.114251
Liang J, Jin Y, Wen X, Mi J, Wu Y (2020) Adding a complex microbial agent twice to the composting of laying-hen manure promoted doxycycline degradation with a low risk on spreading tetracycline resistance genes. Environ Pollut 265(Pt A):114202. https://doi.org/10.1016/j.envpol.2020.114202
López-González JA, Vargas-García Mdel C, López MJ, Suárez-Estrella F, Jurado M, Moreno J (2014) Enzymatic characterization of microbial isolates from lignocellulose waste composting: chronological evolution. J Environ Manage 145:137–146. https://doi.org/10.1016/j.jenvman.2014.06.019
Meng Q, Yang W, Men M, Bello A, Xu X, Xu B, Deng L, Jiang X, Sheng S, Wu X, Han Y (2019) Zhu H (2019) Microbial community succession and response to environmental variables during cow manure and corn straw composting. Front Microbiol. 10:529
Mi H, Shen C, Ding T, Zheng X, Tang J, Lin H, Zhou S (2023) Identifying the role of array electrodes in improving the compost quality of food waste during electric field-assisted aerobic composting. Bioresour Technol 388:129763. https://doi.org/10.1016/j.biortech.2023.129763
Moreira SG, Hoogenboom G, Nunes MR, Martin-Ryals AD, Sanchez PA (2023) Circular agriculture increases food production and can reduce N fertilizer use of commercial farms for tropical environments. Sci Total Environ 879:163031. https://doi.org/10.1016/j.scitotenv.2023.163031
Onwosi CO, Igbokwe VC, Odimba JN, Eke IE, Nwankwoala MO, Iroh IN, Ezeogu LI (2017) Composting technology in waste stabilization: on the methods, challenges and future prospects. J Environ Manage 190:140–157. https://doi.org/10.1016/j.jenvman.2016.12.051
Rao JN, Parsai T (2023) A comprehensive review on the decentralized composting systems for household biodegradable waste management. J Environ Manage 345:118824. https://doi.org/10.1016/j.jenvman.2023.118824
Said-Pullicino D, Erriquens FG, Gigliotti G (2007) Changes in the chemical characteristics of water-extractable organic matter during composting and their influence on compost stability and maturity. Bioresour Technol 98(9):1822–1831. https://doi.org/10.1016/j.biortech.2006.06.018
Sardar MF, Zhu C, Geng B, Ahmad HR, Song T, Li H (2021) The fate of antibiotic resistance genes in cow manure composting: shaped by temperature-controlled composting stages. Bioresour Technol 320(Pt B):124403. https://doi.org/10.1016/j.biortech.2020.124403
Song C, Li W, Cai F, Liu G (2021) Chen C (2021) Anaerobic and microaerobic pretreatment for improving methane production from paper waste in anaerobic digestion. Front Microbiol 6(12):688290. https://doi.org/10.3389/fmicb.2021.688290
Sun P, Liu B, Ahmed I, Yang J, Zhang B (2022) Composting effect and antibiotic removal under a new temperature control strategy. Waste Manag 153:89–98. https://doi.org/10.1016/j.wasman.2022.08.025
Wang Y, Wei Y, Zhou K, Gao X, Chang Y, Zhang K, Deng J, Zhan Y, Li J, Li R, Li J, Xu Z (2023) Regulating pH and Phanerochaete chrysosporium inoculation improved the humification and succession of fungal community at the cooling stage of composting. Bioresour Technol 384:129291. https://doi.org/10.1016/j.biortech.2023.129291
Wu N, Xie S, Zeng M, Xu X, Li Y, Liu X, Wang X (2020) Impacts of pile temperature on antibiotic resistance, metal resistance and microbial community during swine manure composting. Sci Total Environ 744:140920. https://doi.org/10.1016/j.scitotenv.2020.140920
Xie X, Wang Y, Wei Z, Zhang Y, Zhang C, Zhang S, Yang H, Zhang X, Zhao Y (2021) Continuous insulation strategy of organic waste composting in cold region: based on cold-adapted consortium. Bioresour Technol 335:125257. https://doi.org/10.1016/j.biortech.2021.125257
Xie Y, Zhou L, Dai J, Chen J, Yang X, Wang X, Wang Z, Feng L (2022) Effects of the C/N ratio on the microbial community and lignocellulose degradation, during branch waste composting. Bioprocess Biosyst Eng 45(7):1163–1174. https://doi.org/10.1007/s00449-022-02732-w
Xu J, Jiang Z, Li M, Li Q (2019) A compost-derived thermophilic microbial consortium enhances the humification process and alters the microbial diversity during composting. J Environ Manage. 243:240–249. https://doi.org/10.1016/j.jenvman.2019.05.008
Xu M, Sun H, Chen E, Yang M, Wu C, Sun X, Wang Q (2023a) From waste to wealth: innovations in organic solid waste composting. Environ Res 229:115977. https://doi.org/10.1016/j.envres.2023.115977
Xu Z, Li R, Zhang X, Liu J, Xu X, Wang S, Lan T, Zhang K, Gao F, He Q, Pan J, Quan F, Zhang Z (2023b) Mechanisms and effects of novel ammonifying microorganisms on nitrogen ammonification in cow manure waste composting. Waste Manag 169:167–178. https://doi.org/10.1016/j.wasman.2023.07.009
Zhang J, Wu Z, Huang Y, Zhan X, Zhang Y, Cai C (2023a) Industrial-scale composting of swine manure with a novel additive-yellow phosphorus slag: variation in maturity indicators, compost quality and phosphorus speciation. Bioresour Technol 384:129356. https://doi.org/10.1016/j.biortech.2023.129356
Zhang Z, Yang H, Wang B, Chen C, Zou X, Cheng T, Li J (2023b) Aerobic co-composting of mature compost with cattle manure: organic matter conversion and microbial community characterization. Bioresour Technol 382:129187. https://doi.org/10.1016/j.biortech.2023.129187
Zhou L, Yang X, Wang X, Feng L, Wang Z, Dai J, Zhang H, Xie Y (2023a) Effects of bacterial inoculation on lignocellulose degradation and microbial properties during cow dung composting. Bioengineered 14(1):213–228. https://doi.org/10.1080/21655979.2023.2185945
Zhou S, Jia P, Xu W, Shane Alam S, Zhang Z (2023b) A novel composting system for mitigating ammonia emissions and producing nitrogen-rich organic fertilizer. Bioresour Technol 386:129455. https://doi.org/10.1016/j.biortech.2023.129455
Zhu L, Huang C, Li W, Wu W, Tang Z, Tian Y, Xi B (2023) Ammonia assimilation is key for the preservation of nitrogen during industrial-scale composting of chicken manure. Waste Manag 170:50–61. https://doi.org/10.1016/j.wasman.2023.07.028
Funding
The study was supported by the Hebei Provincial Science and Technology Key Projects (22327303D).
Author information
Authors and Affiliations
Contributions
Tao Peng: conceptualization, methodology, data curation, formal analysis, investigation, visualization, writing—original draft. Shilin Yue: methodology, investigation, visualization. Wenshuai Mao: review and editing. Qing Yang: review and editing, resources. Guojun Jiang: conceptualization, methodology, review and editing, supervision, project administration, resources.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
The authors declare they have consented to the submission.
Consent for publication
The authors give their consent for the publication.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Diane Purchase
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
(1) The high-temperature-resistant bacteria Streptococcus thermophilus and Bacillus licheniformis P8-B2 and low-temperature-resistant bacteria Bacillus licheniformis, Bacillus giant, Bacillus cereus, and Bacillus thuringiensis were screened to degrade starch, cellulose, and protein efficiently.
(2) The high and low-temperature-resistant compost fermentation solid microbial composite agent was successfully prepared. The preparation can make the compost temperature rise quickly, the high-temperature duration is long, the water content, C/N, and organic matter decrease quickly, improve the total phosphorus and total potassium content and seed germination index, shorten the composting time, and improve the composting efficiency.
(3) High-throughput sequencing found that composting temperature and microbial quantity were the key factors affecting composting, resulting in large differences in microbial composition and abundance at different stages.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, T., Yue, S., Mao, W. et al. Preparation of high-temperature and low-temperature-resistant solid microbial agent for cattle manure fermentation and effect on composting. Environ Sci Pollut Res 31, 29017–29032 (2024). https://doi.org/10.1007/s11356-024-32830-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32830-z