Abstract
Hydrogeological, hydrochemical and isotopic traits of the groundwater in the Quaternary aquifer system in an urban-periurban locality within and encircling the Kolkata-Howrah twin city in the south Bengal Basin have been synthesised to explain the present- and paleo-hydrological processes, surface and groundwater interaction and mixing dynamics of contamination of groundwater. Rock-weathering, evaporation, ion-exchange and active mineral dissolution are the key processes commanding the groundwater chemistry. Freshwater flushing from the recharge zones had thinned the entrapped sea water which has generated the present-day brackish water by a non-uniform fusion. The best-fit line of the plots of δD and δ18O of groundwater samples displays a slope lower than that of local meteoric water line (LMWL) and global meteoric water line (GMWL) which hints that isotopic constitution of the groundwater of the present area is primarily formed by evaporation before or in the recharging process. A wide range of δ18O values in groundwater suggests that these waters are not blended enough to remove dissimilarities in isotope configuration of recharge water. This also suggests that many groundwaters are a result of mixing of present-day recharge and an older integrant recharged under previously cooler climatic conditions. The groundwater samples are more depleted of oxygen at the shallower level. The depleted samples cluster around the Tolly’s nala (canal) where upper aquitard is missing or < 10-m thick. The tritium values range between 0.70 and 15.02 which indicate the occurrence of ‘sub-modern’, ‘a mix of modern and sub-modern water’ and ‘modern water’. It indicates mingling of isotope-depleted water from the Hugli River by means of Tolly’s canal with relatively less-depleted groundwater of Kolkata’s late Pleistocene aquifer. The tritium values and Cl/Br ratio of groundwater samples adjoining Tolly’s canal and elsewhere refer the direct infiltration of ‘modern wastewater and freshwater’ which mixes with the ‘sub-modern water’ in the aquifer system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The demand for groundwater in India and elsewhere is increasing by leaps and bounds owing to the rising population, industrialisation, unpredictable rainfall and change of climate (Banerjee and Sikdar 2022a, 2020; Battista and de LietoVollaro 2017; Sahu and Sikdar 2017; Yan et al. 2016; Howard and Gelo 2002). The increased exploitation of groundwater is leading to declining groundwater levels, land subsidence and water quality degradation from anthropogenic and geogenic sources (Sikdar et al. 2022; Banerjee and Sikdar 2022a, b; Krishana et al. 2021; Galloway and Burbey 2011; Kagabu et al. 2011; Hosono et al. 2011; McDonald et al. 2011; Hoque et al. 2007; Morris et al. 2003). Isotopic signatures are widely used for analysis of hydrological processes like transport, recharge and discharge areas, and mixing mechanism since they give a conventional fingerprint which is inherent to the water molecules (Li et al. 2014; Criss 1999; Clark and Fritz 1997; Gat 1996; Gat and Gonfiantini 1981). Furthermore, the isotope (δD and δ18O) composition of water at various locations is influenced by climatic activities that reveal its origin (Li et al. 2014; Clark and Fritz 1997). Similar methods were employed in Dhaka, Bangladesh (Hoque et al. 2014), sections of south Kolkata, India (McArthur et al. 2018) and Delhi, India (Kumar et al. 2011) to locate recharge zones (Sikdar and Sahu 2009).
The South Bengal Basin’s Quaternary aquifer, which is in the lower deltaic plain of Ganga River, is severely stressed because of unregulated abstraction of subsurface water required for growing population and urbanisation (Banerjee and Sikdar 2022a, b, 2021; Sikdar et al. 2001). The area with megacities, urban-peri-urban areas, agrarian economy and groundwater-dependant industries cannot be sustained if the problems of groundwater in the area are overlooked and undermined.
The aquifer system lying under the two metropolis of south Bengal Basin—Kolkata-Howrah and their surrounding peri-urban areas is experiencing several problems, like (i) declining water level, (ii) land subsidence, (iii) change of groundwater flow direction, (iv) water quality deterioration, (v) salinisation of freshwater, (vi) at places, aquifer changing from confined nature to unconfined and (vii) expansion of groundwater depression (Sikdar et al. 2022; Banerjee and Sikdar 2022a, b, 2021; McArthur et al. 2018). The piezometric surface lying within the extent of the study area has dropped by 34 cm in 2016 due to the groundwater overdraft of 408 × 103 m3/day. The water level has been found to drop by 13–37 cm/year on a long-term basis (Sikdar et al. 2022; Banerjee and Sikdar 2021). As a result, Kolkata and Howrah are on the verge of a catastrophic water crisis (Sikdar et al. 2022; Banerjee and Sikdar 2022a, b, 2021; McArthur et al. 2018; Mukherjee et al. 2018; Sahu and Sikdar 2017, 2011; Sikdar et al. 2001; Sikdar 1996a).
Stable isotopes, δD (D/H) and δ18O (18O/16O) and radioactive isotope, tritium (3H) are useful to study the present and paleo-hydrological processes and surface water-groundwater interaction (Bahir and Misdaq 2007; D’Alessandro et al. 2004; Bourg et al. 2001). Hydrogeological processes and identification of groundwater recharge source have been studied by different researchers by analysing environmental tracers (Meredith et al. 2013; Gonfiantini et al. 1998) including Indo-Gangetic basin (O’Keeffe et al. 2020; Lapworth et al. 2020, 2017; Bonsor et al. 2017; MacDonald et al. 2016; Kumar et al. 2011). However, published works on δ18O, δ2H and 3H distributions in the Bengal Basin are scanty. Several researchers have applied stable isotope techniques to understand hydrogeochemical processes of the aquifer in Bengal Basin (Sikdar and Sahu 2009; Sikdar and Chakraborty 2008; Klump et al. 2006; Harvey et al. 2005; Stüben et al. 2003; Basu et al. 2002; Aggarwal et al. 2000; Shivanna et al. 1999) and in Kolkata city (McArthur et al. 2018) by using isotope signature but in a piecemeal manner.
Studies of stable isotope signatures along with the identification of recharge areas for Kolkata, one of the largest megacities of South Bengal Basin (McArthur et al. 2018), are limited. Comprehensive research related to recharge mechanism, groundwater mixing dynamics, wastewater contamination in view of the analysis of radioactive isotope—tritium, for the densely populated Kolkata city, are scanty. Similar studies of stable and radioactive isotopes are available (Sikdar and Sahu 2009) in and around East Calcutta Wetland (ECW), lying adjacent to the eastern periphery of the city limit. Such studies in and around the rural and peri-urban areas of Howrah city and blocks of Howrah district, Mahestala-Budge Budge areas, have never been carried out. The increased groundwater requirement due to rapid urbanisation and industrialisation of these rural and peri-urban areas demand such kind of scientific studies for understanding the groundwater dynamics. The basin scale studies in Bengal Basin were carried out by several researchers and elaborated the variation on stable and radioactive isotopes in groundwater with respect to depth of the aquifer, recharge areas, season. But the variation in the isotope values, for smaller heterogenous complex hydrogeological system beneath the twin cities and its’ peri-urban areas in South Bengal Basin has never been examined. This research paper attempts to find the answers these novel questions.
Hence, objectives of this study are (a) to use stable and radioactive isotopes combined with hydrogeological and hydrochemical information for better understanding of groundwater system dynamics in the complex groundwater system beneath the world’s largest delta, (b) to use tritium (3H) dating of groundwater for identifying the recharge zones for groundwater and potentially toxic elements, (c) to explain the present hydrological processes, surface and groundwater interaction and mixing dynamics of contamination of groundwater, (d) to improve the overall knowledge and understanding in the relevant hydrogeological field for the megacities of the south Bengal Basin as well as the adjacent rural and peri-urban areas.
Study area
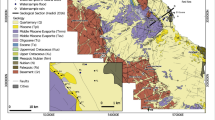
The research area is 436 km2 located on both banks of the meandering Hugli river in the south Bengal basin. The area includes the twin cities Kolkata and Howrah, and their peri-urban areas in South 24-Parganas and Howrah districts (Fig. 1). The increase of built-up area between 1985 and 2016 is about 19% (109 to 192 km2) which has been nearly compensated by the reduction of vegetation cover by 11%, agricultural land by 7% and waterbodies by 1.47% in the study area (Banerjee and Sikdar 2021). Groundwater is abstracted from wells where a screen is mainly placed at 80 to140 m bgl and between 180 and 240 m bgl at places (Sikdar et al. 2022; Banerjee and Sikdar 2022a, 2021).
Geomorphologically, the study area lies under the fluvio-deltaic environment of deposition. The meandering River Hulgi, the lifeline of the twin cities, flows from north-east corner to south-west corner of the present study area. Topographically, the area is almost flat, with many depressions. The surface elevation ranges between 1.5 and 9 m amsl with an average of 6 m amsl. The regional slope is from north to south. The major geomorphic units exposed are broadly classified into different units, e.g. deltaic plains, inter-distributary marshes, paleo-channels, younger levees adjacent to the River Hugli and older levees on both sides of the old Tolly’s Canal (Figs. 1 and SI-1) (Banerjee and Sikdar 2021, 2022a, b). Generally, the riverbank of the River Hugli is elevated, having its natural slope away from the riverbed, that is, towards east in Kolkata and towards west in Howrah.
The climate of the study area is predominantly influenced by the northeast and southwest monsoons. Hot lengthy summer with occasional nor’westers, prolonged monsoon from June to October, mild winter and a brief spring are the characteristic features of the climate in the area. The mean annual rainfall of the area is 1668 and 1619 mm during 1966–2018 and 1998–2018 respectively (Banerjee and Sikdar 2022a, 2021; IMD 2020). The area receives maximum rainfall during peak-monsoon period (July–September). In the last few decades, the long-term rainfall in this area shows a decreasing tendency (Banerjee and Sikdar 2022a, 2021).
Geological settings
The sediments of south Bengal Basin were deposited in a fluvio-deltaic environment and have relatively flat landscape (Sikdar et al. 2022, 2013). The sub-surface sedimentology of a few hundred metres consists of Late Pleistocene topography containing paleo-channels and paleo-interfluves covered by sediments of Holocene channels/floodplains (Sikdar et al. 2022; McArthur et al. 2018).
The stratigraphy of this sedimentary deposit (Fig. SI-3) was developed using borehole lithology and geophysical logging by Banerjee and Sikdar (2022a, 2021) and Sikdar et al. 2022. At the upper part of the sediment distribution, there is a confining clay/silty clay layer (aquitard) with an average thickness of 26.30 m (Banerjee and Sikdar 2022a, 2021). Below the upper confining bed, the Quaternary confined aquifer occurs, which is about 300-m thick. It consists of sands of various grades mixed with gravels at places (Banerjee and Sikdar 2022a, 2021; Sikdar 2000). This aquifer is heterogeneous in nature as the lenses of fine-grained sediments in the sandy horizon are spatially uncorrelated (Sikdar et al. 2022; Sahu and Sikdar 2017). The Holocene grey clay and late Pleistocene Last Glacial Maximum Paleosol (LGMP) of the upper part of the sediments prevent infiltration of Hugli river water into underlying aquifer (Sikdar et al. 2022; Sikdar and Dasgupta 1997). The overlying cover of aquitard is thinned down to < 10 m or ceases to exist in some areas; examples include areas along the Tolly’s nala (canal), a palaeo-channel and Mahestala and Budge Budge (M-B) area in the southern and south-western sections respectively of the present study area (Figs. 1 and 5 of Banerjee and Sikdar 2021). Grey sands occur underneath the Tolly’s canal instead of the grey clay/silty clay and LGMP. This grey sand has given a direct path for contaminated water from the canal to enter the aquifer below (McArthur et al. 2018).
In Kolkata and surrounding area, the general characteristics of the aquifer are fresh and saline in nature, high TDS, occasional As and Cl, high Fe content with presence of NO3 in water (CGWB 2006). The discharge rate, transmissivity and storage coefficient of the aquifer beneath Kolkata vary from 20 to 70 m3/h, 1400 to 5610 m2/day and 33 × 10−3 to 20 × 10−5 respectively (Banerjee and Sikdar 2021; Sikdar 1996a, b; CGWB 2006). On the other hand, in Howrah district, discharge, transmissivity and storativity vary from 36 to 211 m3/h, 446 to 2514 m2/day and 45 × 10−4, respectively (CGWB 2004).
Impacts of urbanisation
Between 1980 and 2014, the built-up area in Kolkata increased by 62 km2 while wetland and vegetation cover decreased by about 38.5 km2 and 23.5 km2 respectively (Mukherjee et al. 2018). During the period 1985–2016, the built-up area of Howrah increased by 11 km2, while the adjacent peri-urban and rural areas increased by 40 km2 (Banerjee and Sikdar 2021). Such widespread urbanisation has put a significant stress on total groundwater demand.
It has been estimated that the groundwater abstraction in Kolkata Municipal Corporation (KMC) had jumped from 55 to 219 million litres/day (mld) between 1956 and 1992 (Banerjee and Sinha Roy 1992). The surface water supply had reduced the groundwater withdrawal to 144.3 mld during 2006 (CGWB 2006). The groundwater withdrawal by motor pump fitted wells, both KMC wells and private-owned, was estimated at around 305 mld. Although, the extrapolated groundwater extraction is about 320 mld through 2020 to 2025 period (CGWB 2006). The total requirement of drinking water for the entire population of Kolkata city (consisting of 4.6 million residents and a floating population of 6 million) happens to be about 1262 mld (https://www.downtoearth.org.in/news/city-of-sorrows-12134). Out of the total water supply of around 1209.6 mld, surface water is 1096.2 mld and the rest is groundwater. As per official record, the demand and supply gap is 37.8 mld. Keeping in mind that 35% supply water loss is caused by pipeline leakage, Kolkata can be labelled a ‘water-negative’ city rather than being ‘water-positive’.
Deterioration of Quaternary aquifer system
The indiscriminate abstraction of groundwater has resulted in a significant fall in piezometric levels, resulting in the formation of a groundwater depression in central and south-central Kolkata and changing the directions of the groundwater flow (Sikdar et al. 2022; Banerjee and Sikdar 2021; Sikdar 1996b). Decades of high-capacity pumping has caused the decline of piezometric surface by approximately 14 m and the rate varies from 0.08 to 0.37 m/year (Sikdar et al. 2022; Banerjee and Sikdar 2022a, b, 2021; McArthur et al. 2018). Between 1985 and 2016, the groundwater trough has increased in area by 15 times at an annual rate of 8.6 km2. Thus, the core of the trough has shifted eastward, causing regional groundwater flow inside the city to shift from west to east (Banerjee and Sikdar 2021; Fig. SI-4). In Howrah city, the piezometric surface is declining since 1996 (Patra et al. 2018). Over-withdrawal of groundwater has also brought in water quality deterioration, changes in hydrochemical facies, mixing of wastewater and other potentially toxic elements (PTE), a change in the nature of the aquifer at places from confined to unconfined and an increase in the risk of land subsidence in south Bengal Basin (Sikdar et al. 2022; Banerjee and Sikdar 2022a, b, 2021).
Tolly’s canal receives the majority of south Kolkata’s contaminated water. Chloride, NO3, SO4 and NH4 concentrations in groundwater, as well as Cl/Br mass ratios, have been utilised to demonstrate the extent to which wastewater contaminates the Bengal Basin aquifer (McArthur et al. 2012) and Kolkata city (Banerjee and Sikdar 2022b; McArthur et al. 2018).
Churiyal Khal (canal) (Fig. 1) receives surface runoff (rainwater) and carries municipality discharge of the M-B area. Few localised small north–south trunk channels connect the canal and occasionally these channels along with connecting branches discharge polluted water to the neighbouring low-lying lands or water bodies. During monsoon, water logging occurs in pockets in several areas. The drains’ size and hydraulic capacity are insufficient to convey the sewage load. Due to over-exploitation of groundwater, both rainwater and sewage water from the twin cities may infiltrate into the aquifer at places where the upper aquitard is absent or thin (< 10 m) or a local unconfined aquifer condition has developed (Banerjee and Sikdar 2022b, 2021).
Methods
Groundwater chemistry
Total 231 and 56 number of pumping wells tapping groundwater between the depth of 33 and 140 m bgl and below the depth of 180 m bgl, respectively, was sampled for chemical analysis. Groundwater is not being tapped in between 140 and 180 m bgl due to the increase of finer particles (silty sand to silty clay) (Banerjee and Sikdar 2021) within the study area. Out of 287 wells, only nine wells are tapping water above 50 m bgl which is statistically insignificant for analytical purpose. The strainers of the pumping wells are placed based on the grainsize of the sediments (medium to coarse sand). Therefore, the samples are mainly collected from the depth range of 80–140 m bgl and 180–230 m bgl. Results of chemical analysis of 287 groundwater samples (Fig. 1 of Banerjee and Sikdar 2022a, b; Fig. SI-1) collected from depths between 32 and 229 m bgl have been used in this study. Out of these, 205, 58 and 24 samples are from Kolkata, Howrah and M-B area respectively. The locations of the samples were recorded using a portable GPS device and are shown in Fig. SI-1. Results of two hundred and one samples have been taken from McArthur et al. (2018), while the remaining 86 samples have been collected by the present authors in 2019 from localities not covered by McArthur et al. (2018). These areas are Howrah in the west and M-B area to the south-west of Kolkata (Fig. 1).
After purging, the samples are collected in two 100-mL polythene bottles from wells fitted with hand pumps or motor pumps. Prior to collection, sampling bottles were soaked in 1:1 diluted HCl solution for 24 h, washed three times with deionised waters and were washed again before filling the sampling bottles with the sample water. During sampling, the samples were filtered with Whatman 40 filter paper. In the laboratory, the samples were filtered using 0.45-μm Millipore filter paper and acidified with ultra-pure nitric acid for cation analyses. For cation analysis, one sample is acidified with 0.15 mL of 50% Analar® nitric acid, while the other is not acidified for anion analysis. Decision for the time for purging depends on two factors: depth of piezometric surface and depth of well with the aim that the water sample is collected directly from the aquifer not from the well casing. Analysis of the 86 samples has been carried out at the laboratory of TÜV SÜD, India, and National Institute of Hydrology, Roorkee, India, for cations, anions and metals/metalloid (Fe, Mn and As). Ion chromatography is used to analyse cations and anions, while ICP-OES is used to analyse metals. The total dissolved solid (TDS, mg/L) of these samples is calculated using the following APHA (2005) methodology:
The analytical precision for the measurements of ions is checked by calculating the charge balance error (CBE) using the following formula:
where ΣC and ΣA are the sum of concentrations of cations and anions, respectively, expressed in mill-equivalents/litre. If the CBE is within ± 10%, the analysis is assumed to be good.
Isotope analysis
For groundwater samples taken from 33 to 229 m bgl (Fig. 1), two types of isotope analysis were carried out: stable isotope (δ2H and δ18O) and radioactive isotope [Tritium (3H)]. For stable isotopes, 235 groundwater samples (Kolkata—201, Howrah—23 and M-B area—11) and three Hugli river water were taken into account. Stable isotope sample analysis data of 200 groundwater samples from Kolkata city and three samples from River Hugli were taken from McArthur et al. (2018). Remaining 35 samples of groundwater were collected by the present authors in acid washed polyethylene Tarson bottles. Out of these, 12 samples were used for tritium (3H) analysis. For stable isotope analysis, 100 mL of sample and for tritium analysis, 500 mL of sample were collected. These bottles were washed twice with the respective well water at the site. Sample bottles were tightly sealed and brought to the laboratory at the shortest possible time in ice-box for analysis to avoid any losses due to diffusion and evaporation. Other parameters of these samples, such as pH and electrical conductivity (EC), were also measured.
In the year 2019, δ2H, δ18O and 3H were analysed at the Nuclear Hydrology Laboratory, National Institute of Hydrology Roorkee, India (NIH). Continuous flow isotope ratio mass spectrometry and dual inlet isotope ratio mass spectrometry were used to analyse δ18O and δ2H using standard operating procedures (Brenninkmeijer and Morrison 1987; Epstein and Mayeda 1953). The data are displayed on the following δ scale in concentrations per mil (‰) relative to Vienna Standard Mean Ocean Water (VSMOW):
where Rsample is the 18O/16O or 2H/1H ratio in water sample and Rstandard is the corresponding ratio in VSMOW standard.
The all-inclusive accuracy, founded on ten repeated calculations for each of the samples, is < ± 1.0‰ for δ2H and < ± 0.1‰ for δ18O. To condense 3H in 500 mL of water sample, electrolytic reduction was applied using a 20-cell standard tritium enrichment unit at temperatures ranging from 0 to 5 °C to achieve maximal tritium fractionation and enrichment. As the electrolytic process progresses, the sample volume decreases to 25 mL. The activity of tritium in the reinforced samples, including the standards, was determined in tritium unit (TU) with a 2-sigma error using an ultra-low-level liquid scintillation counter (Quantulus Wallac model 1220). The minimum detection limit is estimated to be 0.4 TU (1σ criteria).
GMWL and LMWL
McArthur et al. (2018); Sikdar and Sahu (2009); Sikdar and Chakraborty (2008); Klump et al. (2006); Sengupta and Sarkar (2006); Mukherjee (2006); Harvey et al. (2005); Stüben et al. (2003); Basu et al. (2002); Aggarwal et al. (2000); and Shivanna et al. (1999) have published work on δ18O, δ2H and 3H distributions in Bengal Basin. Mukherjee (2006) collected 64 groundwater samples, seven river-water and 14 rainwater samples from Gangetic portion of West Bengal during May–August of 2003–2005 and analysed δ18O and δ2H compositions to obtain a local meteoric water line (LMWL). The corresponding equation for this LMWL is δD = 7.24 δ18O + 7.73 (r2 = 0.93). LMWLs are site-specific and act as the covariation of hydrogen and oxygen stable isotope ratios over a long time. Sengupta and Sarkar (2006) also obtained a LMWL whose equation is δD = 7.88 δ18O + 8.93 (r2 = 0.99). Majumder et al. (2011) studied 328 numbers of groundwater samples to appraise the pattern of groundwater flow in Bengal Delta aquifers of Bangladesh applying environmental isotope (2H, 18O, 13C, 3H and 14C) techniques. A distinct LMWL (δ2H = 7.7 δ18O + 10.7‰) was established implementing linear correlation analysis methods to the monthly weighted rainfall isotopic compositions (δ18O and δ2H) (Majumder et. al. 2011). Sikdar et al. (2013) displayed the plots of δD drawn against δ18O for groundwater samples fetched from both shallow together with deep levels in May 2009 from Moyna, N-24 Parganas that defined a line that falls under the LMWL of Sengupta and Sarkar (2006). These LMWLs nearly match the global meteoric water line (GMWL) of Craig (1961) (δD = 8 δ18O + 10). Craig’s GWML displays the relations between δD and δ18O in fresh surface waters all over the world.
In the current study, the GMWL of Craig (1961) (δD = 8 δ18O + 10) and the LMWL of Sengupta and Sarkar (2006) (δD = 7.88 δ18O + 8.93) were used to investigate the isotopic data of water samples in and around Kolkata-Howrah cities.
Results
Ion chemistry
Ion chemistry of groundwater of the study area has been elaborated in Banerjee and Sikdar (2022a, b). However, the results of TDS, chloride, sodium, calcium, fluoride and bromide are presented here to discuss the hydrochemical processes and relation with the isotopic variations. TDS concentrations range from 176 to 3377 mg/L. TDS levels of 500 mg/L, the acceptable limit (AL) and > 2000 mg/L, the permissible limit (PL), of BIS (Bureau of Indian Standard) (2012) are found in approximately 18% and 6.6% of the total number of samples, respectively. TDS < AL concentration were found in 11, 3.5 and 3.5% samples in Kolkata, Howrah and M-B areas respectively. The concentration of TDS > PL is observed up to a depth of 137 m. In the depth span of 195 to 229 m bgl, only 12% of samples with TDS > 500 mg/L exist. Samples having Cl < 25 mg/L account for about 56.5%, whereas Cl > 1000 mg/L is found to occur in samples up to a depth of 137 m. Excessive Cl and TDS concentrations were found in groundwater samples obtained from the M-B region at lower depths, i.e. < 40 m.
Sodium concentration ranges between 15 and 1116 mg/L, the mean value being 192 mg/L. The mean concentration of Na up to 137-m depth is 202 mg/L and between 183 and 229 m depth, it is around 154 mg/L. Calcium concentration varies from 4 to 324 mg/L with a mean of 106 mg/L. The mean concentration of Ca up to 137 m bgl is 112 mg/L whereas the same is 82 mg/L from depth 183 to 229 m. Fluoride concentrations are ≤ 1 mg/L with mean of 0.31 mg/L. The F and Ca concentrations in groundwater show an inverse correlation in the study area (Banerjee and Sikdar 2022b).
Isotope analysis
The summary of the isotope analysis of 235 samples (Fig. 1) is given in Table 1. δ18O and δD have average values of − 5.0‰ and − 33.7‰ respectively. In the shallow part of the entire area (depth, 33–137 m bgl), the mean value of δ18O and δD is − 5.2‰ and − 35.2‰ respectively. Between 183 and 229 m bgl, the mean values of δ18O and δD are − 4.0‰ and − 26.0‰ respectively. Table 1 shows the range and mean values of δ18O and δD for groundwater and river water (river Hugli samples) collected during pre-monsoon, monsoon and post-monsoon periods. Water samples taken from boreholes with upper clay thickness < 10 m (unconfined system) and > 10 m (confined system) were separated to determine the range and the mean value of δ18O and δD (Table 1). Summary of isotopic composition of groundwater samples for Kolkata, Howrah and M-B area from shallow (33 to 137 m bgl) and deeper (183 to 229 m) aquifer is given in Table 2.
The stable isotope plots’ best fit lines (Fig. 2) have a slope that is lower than both the GMWL of Craig (1961) and the LMWL of Sengupta and Sarkar (2006). The following are the obtained LMWL equations for the groundwater samples taken in the areas of Kolkata, Howrah and M-B:
-
δD = 7.4899δ18O + 4.1990; R2 = 0.97; (Kolkata)
-
δD = 8.2842δ18O + 5.2304; R2 = 0.94; (Howrah)
-
δD = 6.4935δ18O -1.6542; R2 = 0.82; (M-B)
Cross-plot of δ18O vs δD based on stable isotope analysis of samples from the present region of investigation. GMWL is the global meteoric water line of Craig (1961) while LMWL is the local meteoric water line of Sengupta and Sarkar (2006) for Barasat, some 25 km northeast of the present region of investigation
Depth plots drawn against the concentration of δ18O and δD (Fig. 3a, b) show that the groundwater samples < 140 m bgl are more depleted (more negative values of δ18O) of oxygen than the samples from deeper level (> 183 m bgl). Two samples, KOL52 and KOL68 with screen placed at deeper level (> 140 m bgl), have δ18O value of − 6.5 and − 6.7‰, respectively (Fig. 3b).
Tritium (TU) content of the 12 groundwater samples varies between 0.70 and 15.02 TU with error of 0.10 to 0.20 (Fig. 3c). The sample having TU > 15 was collected during peak monsoon period and from the unconfined aquifer system adjacent to Tolly’s canal. The depth profile shows higher TU value at depths between 75 and 140 m bgl while samples having low TU values occur at shallow (75–90 m bgl) and deep (200–225 mbgl) depths. There are total 30 samples out of 235 samples which have depleted δ18O having value of < − 6.1‰. All these 30 samples are from Kolkata and mainly congregate on each side of Tolly’s canal (Fig. 4).
Discussions
Hydrochemical systematics
Gibbs diagram (Gibbs 1970) which is commonly used to demonstrate the link between water composition and aquifer lithological properties (Marandi and Shand 2018) has three fields (evaporation dominance, rock dominance or rock–water interaction and precipitation dominance). The present area’s groundwater samples lie in between evaporation dominance and rock dominance (Fig. 5). The ratio of Na+ to (Na+ + Ca2+) is in the range of 0.25 and 0.9, meaning that sodium concentrations in groundwater are frequently greater than calcium concentrations. The Cl−/(Cl− + HCO3−) ratio ranges between 0.05 and 0.90, suggesting that the amount of Cl− and HCO3− in groundwater varies. Therefore, the main process controlling the groundwater chemistry in the studied region is rock weathering in conjunction with evaporation.
The samples do not plot on the field of precipitation dominance suggesting that precipitation has little impact on groundwater composition. Some water samples were plotted outside of the three fields (Fig. 5a), indicating that some other factor, such as ion exchange, may influence the chemical composition of groundwater.
The Facies 8 (Na-Cl) groundwater samples plot around the line of equality in the Na versus Cl plot (Fig. 14 of Banerjee and Sikdar 2022a), indicating that mineral dissolution is occurring in the aquifer (Sikdar et al. 2001; cf. Llyod and Heathcote 1985).
Silicate weathering, evaporite dissolution and ion exchange processes in groundwater are further investigated using the Ca/Na vs. Mg/Na plot (Fig. 6) and the Na/Cl vs. Cl plot (Fig. 7). Figure 6 displays that silicate weathering is the most important mechanism influencing water chemistry. Figure 7 also indicates that silicate weathering is the dominant process for the samples having Na/Cl ratio greater than that of rainfall (0.86) (Hem 1989) but those with Na/Cl > 2 indicates the twin effect of silicate weathering and ion exchange processes (Herczeg and Edmunds 2000).
The south Bengal Basin, including the current region, was submerged in brackish to seawater during the Late Quaternary period (Sikdar et al. 2001; Hait et al. 1996a, b, 1994a, b; Barui and Chanda 1992; Sen and Banerjee 1990). As a result, seawater with high Cl concentration was probably trapped in the sediments as they were deposited and later modified in the aquifer in a confined condition. Later the trapped seawater was diluted by freshwater infiltrating into the aquifer through the recharge zones present in the north of the current area (Sikdar et al. 2001). Also, direct recharge of meteoric water in localised pockets where the upper aquitard is either absent or exceedingly thin because of scouring action of past channels caused dilution of the trapped seawater (Fig. SI-2). Thus, the mixing of seawater and freshwater gave rise to the brackish water of present-day. This mixing was non-uniform because of the wide variation in the concentration of Cl (127 to 1598 mg/L) of the brackish water (Banerjee and Sikdar 2022a). The majority of the samples of groundwater occur in the salinisation field, implying limited freshening of modified connate water (Fig. 8). Plots of groundwater samples near the marine water mixing line (red line in Fig. 8) indicate a delayed phase of aquifer flushing by freshwater. The presence of Facies 2 (Na-HCO3) water surrounding tiny areas of Facies 1 (Ca-Mg-HCO3) water (Fig. 4 of Banerjee and Sikdar 2022a; Fig. SI-5) suggests dynamic flushing of freshwater in the aquifer system where ion exchange occurs (Sikdar et al. 2001).
Scatter diagram of Cl vs. a Na, b Ca. The red line represents mixing line between dilute groundwater end-member (Na 67, Cl 1.8, Ca 36 mg/L) and seawater end-member (Na 10155, Cl 18290, Ca 388 mg/L) (from Ravenscroft et al. 2013; McArthur et al. 2012; Sikdar and Sahu 2009; Ravenscroft and McArthur 2004)
Isotope signature
Stable isotopic signatures of recharge sources
A study on stable isotopes in Hugli river water (McArthur et al. 2018) found that the least depleted δ18O is − 6.1‰, whereas the volume-weighted annual mean value for δ18O of local rainfall is − 6.2‰. Hugli river water has δ18O and δD values ranging from − 6.1 to − 7.9‰ and − 41.6 to − 51.3‰, respectively. The values of δ18O and δD fluctuate seasonally in response to varying proportions of base-flow and rainfall-runoff that reaches the river (McArthur et al. 2018). The stable isotope plots of Hugli river water (Fig. 2) are below the GMWL and LMWL suggesting evaporation has an enrichment effect.
Previous isotopic studies in South 24-Parganas district of West Bengal (Misra 2001; Shivanna et al. 1999) indicate that δ2H and δ18O values of shallow groundwater (aquifer depth < 70 m) along with deep groundwater (aquifer depth > 70 m) exist in two separate groups but occur on the LMWL or above it, revealing that evaporation has not affected these waters. Moving north-west from the previously studied South-24 Parganas, in the study area, stable isotope data plots indicate a different hydrogeological condition. The best-fit line of δ2H—δ18O plots of the present area’s groundwater has a slope lower than LMWL and GMWL (Fig. 2) indicating that evaporation of rainwater occurred before or at the time of infiltration and/or intermixing of the infiltrated water with the pre-existent soil moisture which had faced various cycles of evaporation and wetting (Allison 1982).
Groundwater samples collected between 33 and 137 m bgl are slightly more depleted (mean δ18O − 5.2‰) compared to samples collected between 183 and 229 m bgl (mean δ18O − 4.0‰). Groundwater samples collected from the unconfined system are slightly more depleted (mean δ18O − 5.2‰) than those from the confined system (mean δ18O − 4.9‰). This suggests that evaporation occurs prior to infiltration of rainwater into the aquifer system. However, the range and average mean of δ2H and δ18O values of shallow and deep groundwater, samples from unconfined and confined system (Table 1) are almost similar. No significant isotopic variation is observed as the recharge area for both are quite close, the local climatic condition is quite similar and similarity in hydrostratigraphy. The wide range of δ18O (− 3 to − 8‰) and δ2H (− 19.7 to − 54.7%) values indicates groundwater recharged from multiple recharge sources with varied residence time and different isotopic signatures. The study area has several recharge mechanisms. They are (a) recharge to the locally unconfined system through direct precipitation; (b) recharge to the locally unconfined system through river water—thirty groundwater samples of Kolkata having depleted δ18O (< − 6.1‰) cluster around Tolly’s canal resulted from the mixing of isotopically depleted Hugli river water and less-depleted groundwater from late Pleistocene aquifer; (c) several distant recharge areas for the confined system; (d) artificial groundwater recharge systems in the city areas; (e) return flow from the irrigation practice in the rural areas.
The deuterium excess (d-excess) gives information about source of the initial moisture, effects of monsoon evaporation and moisture recirculation from large inland water bodies (Krishana et al. 2021; Négrel et al. 2007; Clark and Fritz 1997; Dansgaard 1964). The plots of d-excess and ẟ18O of groundwater samples are presented in Fig. 9. The d-excess values range between − 2.2 and 9.3‰ and the mean is 6.5‰. The poor correlation (R2 varies between 0.02 and 0.19) between ẟ18O and D-excess (Fig. 9) reflects the influence of evaporation of surface water before infiltration (Liet al. 2014). The d-excess of Hugli River water samples ranges from 6.8 to 7.4‰ with a mean value of 7.03‰. This suggests that the river water is subjected to evaporation resulting in the stable isotope composition occurring below the GMWL and the LMWL (Fig. 2). The large range of δ18O values (Table 1) in groundwater indicates that there has not been enough mixing of Hugli River water to homogenise variances in recharge water’s isotopic composition.
Tritium isotope: age indicator
Tritium (3H) dating of groundwater has been used in the current study to detect the recharge zones and the source of contaminants. 3H is produced in the environment by natural reactions between cosmic neutrons and the atmospheric 14N. Once created, it joins together with oxygen to create a water molecule, which then falls as rain. The tritium content in the groundwater in the recharge region is about equal to the tritium value of the recharging water.
After being recharged, the concentration of 3H begins to decline in accordance with its 12.32-year half-life. The 3H level thus falls exponentially over time away from the recharge zone as groundwater moves towards the discharge zone. Groundwater devoid of 3H is anticipated to be older than 50 years.
Tritium content of groundwater from shallow and deep aquifer in and around East Calcutta Wetland (ECW), adjacent to the present study area, varies from < 0.76 TU to 6.6 TU and < 0.53 TU to 4.5 TU, respectively (Sikdar and Sahu 2009). The tritium value of the present study ranges between 0.7 and 8.74 TU, except two samples having tritium > 10 TU located at the bank of Tolly’s Canal where locally unconfined aquifer system exists. Six samples out of twelve are having tritium ranges between 0.7 and 2.6 TU. This low TU value indicates that this groundwater has long residence time and that recharging may have occurred further away. The 3H content of today’s precipitation in India is expected to be approximately 1.33 to 6.32 TU at Kozhikode, Kerala; 1.59 to 9.21 TU at Hyderabad (www.isohis.iaea.org); and 5.3 TU in 1995 in Dhaka, Bangladesh (Stute 1999). In the Tuscon basin, southwest USA tritium in rain increased because of industrial tritium release in 1970s, which was ultimately washed out by 1991. Since then, the 3H content in rain is 4.6 to 6.7 TU, which represents natural cosmogenic production (Eastoe et al. 2004). The groundwater in the present study area has a 3H value range that is similar to that of rainwater from Kozhikode, Tuscon basin and ECW which suggests that the aquifer is directly recharged by monsoonal precipitation.
According to Abdullah et al. (2018), groundwater age estimation using tritium provides semi-quantitative ‘ball park’ values as follows:
-
< 0.8 TU denotes sub-modern water (pre-1950s).
-
0.8 to 5 TU denotes mixture of sub-modern and modern water.
-
> 5 to 15 TU denotes modern water (< 5 to 10 years).
-
15 to 30 TU denotes bomb tritium.
-
30 TU denotes recharge generation from the 1960s to 1970s.
Tritium content of the 12 groundwater samples varies between 0.70 and 15.02 TU with error of 0.10 to 0.20 TU (Figs. 3c and 10). The sample having TU > 15 was collected during peak monsoon period and from the unconfined aquifer system adjacent to Tolly’s canal. The depth profile (Fig. 3c) shows that samples with higher TU value occur at depth < 140 m bgl while those with lower TU value occur at shallow and deep depths.
Figure 10 reveals that samples KOL33, KOL86 and KOL113, all of which are found adjacent to the Tolly’s canal, exhibit δ18O < − 6.1‰ and 3H > 5 TU. The age of these groundwater samples is < 5 to 10 years. The absence of the upper aquitard has allowed the modern water to infiltrate into the late Pleistocene aquifer samples from wells SB49 (Dhulagori Toll) and SB57 (Chengail) (Fig. 1) exhibit 3H value of 6.05 and 8.74 TU respectively. These values indicate the existence of modern water in the aquifer system in both of the places. At Dhulagori, the near-absence of the upper aquitard has allowed direct infiltration of modern water (Fig. SI-2). At Chengail, modern water may not have directly infiltrated from the surface into the aquifer because of the presence of the upper aquitard but may have migrated from neighbouring Pujali area where the upper aquitard is practically absent (Figs. 1 and SI-2) Numerical groundwater modelling (Fig. 15 of Sikdar et al. 2022) has also shown that modern water with potentially toxic elements (PTEs) has migrated from Pujali to Chengail. The 3H value of sample KOL79 (Figs. 1 and 10) is 0.70 TU and the δ18O is − 4.5‰ indicating the presence of sub-modern water. The 3H content of the remaining seven samples ranges between 1.5 and 3.4 TU indicating a mix of modern and sub-modern water (Fig. 3c).
Figure 11 shows tritium levels in groundwater together with salinity (as measured by electrical conductivity). The salinity induced by salt dissolution rises with the age of groundwater. However, higher salinity and tritium levels suggest evaporative enrichment within the aquifer system (Krishana et al. 2021).
Cl/Br and tritium as an indicator of recharge sources
In the present area, Cl/Br mass ratios and Cl concentrations in groundwater are used as a proxy for wastewater infiltration (Banerjee and Sikdar 2022b; McArthur et al. 2018, 2016, 2012; Katz et al. 2011). The average Cl/Br ratio is higher in samples at depths < 140 m bgl than the groundwater samples at > 180 m bgl for Kolkata, Howrah and M-B areas (Fig. 8a of Banerjee and Sikdar 2022b). Out of the 279 samples examined, around 29% have Cl/Br < 268 (lower limit of 288 ± 20, to account for analytical uncertainty; Banerjee and Sikdar 2022b; McArthur et al. 2018), with organic degradation being the cause of the surplus Br (Fig. 12). Around 30% of the samples had Cl/Br > 308 (upper limit of 288 ± 20 to account for analytical uncertainty; Banerjee and Sikdar 2022b; McArthur et al. 2018) with the majority of them congregating adjacent to the Tolly’s canal, signifying wastewater contamination (Fig. 12). Furthermore, several samples had NO3/Cl > 0.0002 and SO4/Cl > 0.14, indicating that wastewater from sewage and septic tank leaks had contaminated groundwater in the area (Banerjee and Sikdar 2022b).
Out of 12 groundwater samples (Figs. 1 and 10 inset), four samples (SB3, SB21, SB45, SB66) have Cl/Br < 268 but tritium concentration between 0.8 and 5 TU indicating mixing of modern and sub-modern water but no infiltration of wastewater (Fig. 12). SB57 has Cl/Br < 268 and 8.74 TU of tritium, indicating infiltration of modern freshwater. Samples KOL33 and KOL113, which are located adjacent to Tolly’s canal (Figs. 1 and 10 inset), have Cl/Br > 308 and tritium content of 15.02 and 1.90TU respectively. Groundwater sample KOL86 that is also located adjacent to Tolly’s canal has Cl/Br ratio of 284.5 and tritium content of 13.45 TU. Therefore, the tritium values and Cl/Br ratio of groundwater samples adjacent to Tolly’s canal indicate infiltration of modern wastewater/freshwater into the aquifer system which mixes with the sub-modern groundwater. This is further corroborated by the presence of high concentrations of arsenic (10–79 μg/L) in groundwater of few wells adjacent to Tolly’s canal as a result of a pollution plume emerging from a plant site where Paris Green, an arsenic bearing pesticide, was produced between 1965 and 1985 (Banerjee and Sikdar 2022a; McArthur et al. 2018).
The current work indicates that in certain pockets, the upper clay/silty clay is absent or its thickness is < 10 m (Figs. 1 and SI-2). These pockets serve as ‘stratigraphic shortcut’ for pollution plumes, including wastewater (Cl/Br > 308), to enter the aquifer and deteriorate groundwater quality (Banerjee and Sikdar 2022b; McArthur et al. 2018). In other areas, such as Park Street-Park Circus, where the upper aquitard exists, the presence of wastewater in the confined aquifer may be due to vertical leakage of wastewater through the aquitard caused by a rise in lithostatic pressure in the aquitard as well as in the aquifer due to heavy groundwater pumping.
SB49, which is on the ‘Sepatage mixing line’ (Fig. 12), contains 6.5TU of tritium and is located adjacent to Dhulagarh and Sankaril Industrial Estate (Figs. 1 and 10 inset), where the upper aquitard is either < 10-m thick or non-existent (Figs. 1 and SI-2). Therefore, the wastewater has infiltrated the aquifer over a decadal time span. Furthermore, if left untreated, industrial waste containing heavy metals may eventually reach the groundwater pumping level in the future. In the contemporary context, the implication is that if the sewage water includes PTEs, some of them may migrate to Kolkata’s well intakes. This is consistent with the findings of Banerjee and Sikdar (2022a, 2021), Sahu and Sikdar (2011), Sikdar and Banerjee (2003), Ghosh et al. (2001) and Sikdar et al. (2001), who identified the presence of PTEs such as chromium, cadmium, lead, copper and nickel in Kolkata’s groundwater.
Conclusions
The groundwater chemistry of the Quaternary aquifer under the Kolkata-Howrah twin-city in the South Bengal basin is primarily controlled by silicate weathering, evaporation, ion exchange and to some extent mineral dissolution. The Cl/Br ratios of groundwater samples show that surface contamination from anthropogenic Cl is present up to 140 m bgl while elevated Cl at deeper level is obtained from marine water, rather than wastewater. Approximately 30% of the samples with Cl/Br > 308 (upper limit of marine mixing water) were polluted by sewage effluent and septic tank leaks. For the deep groundwater samples with elevated Cl, it is likely that flushing of freshwater from the recharge zones diluted the high-Cl marine water trapped during syn-sedimentation through heterogenous mixing. The presence of high Cl concentration in ‘brackish’ groundwater may indicate the presence of a remnant of seawater that underwent chemical alterations during its confinement period. This suggests that groundwater flow is restricted, and flushing is minimal.
The stable isotope (δ18O and δD) data of groundwater samples show a slope lower than the LMWL and the GMWL, indicating that evaporation occurs before or during the recharge process or that there was some intermixing of the invading water with the pre-existing soil moisture, which had experienced a number of rounds of alternate evaporation and wetting. The varying values of groundwater δ18O suggest that these groundwaters were not properly mixed to blend differences in the isotopic composition of the recharge water. This also demonstrates that many groundwaters are produced by mixing the most recent recharge with an earlier unit recharged under colder climatic conditions than the present day.
The depth plots versus the concentration of stable isotopes reveal that the shallower level groundwater samples are more depleted of δ18O than the deeper level samples. The wide range of δ18O and δ2H values indicates groundwater recharged from multiple recharge sources with different residence times, resulting in different isotopic fingerprints. About 12.80% groundwater samples having depleted δ18O mainly cluster on either side of Tolly’s canal where the aquifer is locally unconfined. The tritium levels of groundwater in the study area vary from 0.70 to 15.02 TU, indicating the presence of ‘sub-modern’, ‘mix of modern and sub-modern water’ and ‘modern water’. This shows a connection between infiltrating isotopically depleted water from the Hugli river via Tolly’s canal and less-deficient groundwater from Kolkata’s late Pleistocene aquifer. The tritium values and Cl/Br ratios of groundwater samples adjacent to Tolly’s canal and elsewhere indicate direct infiltration of ‘modern wastewater and freshwater’ into the aquifer system where it mixes with the ‘sub-modern water’. In the context of the current investigation, the assumption is that if the wastewater contains PTEs, some of it may end up in city-well intakes.
Data availability
Data provided in the supplementary material.
References
Abdullah TO, Ali SS, Al-Ansari NA, Knutsson S (2018) Possibility of groundwater pollution in Halabja Saidsadiq hydrogeological basin, Iraq using modified DRASTIC model based on AHP and tritium isotopes. Geosciences 8:36. https://doi.org/10.3390/geosciences8070236
Aggarwal PK, Basu AR, Kulkarni KM, Froehlich K, Tarafdar SA, Ali M, Hussain A (2000) A report on isotope hydrology of groundwater in Bangladesh: implications for characterization and mitigation of arsenic in groundwater. International Atomic Energy Agency-TC Project BGD/8/016, 64.
Allison GB (1982) The relationship between 18O and deuterium in water and sand columns undergoing evaporation. J Hydrol 55:163–176
APHA (2005) (American Public Health Association) Standard methods for the examination of water and wastewater, 21st edn. DC, USA, Washington
Bahir M, Misdaq MA (2007) Water recharge problems in a semi-arid zone (climatic and anthropogenic impacts): the case of the Essasouira aquifers system (Mogador, Morocco). The Icfai Journal of Earth Sciences 1(2):46–60
Banerjee S, Sikdar PK (2020) Land subsidence due to leakage of aquitard-aquifer pore water in an under-construction tunnel of East-West Metro Railway Project, Kolkata. J of Geol Soc of India 96(5):467–474
Banerjee S, Sikdar PK (2021) Hydrogeological characterization of the Quaternary aquifer of south Bengal Basin in India and the impact of urbanization on the groundwater resources of the system. Hydrogeol J 29:1463–1484
Banerjee S, Sikdar PK (2022a) Hydrochemical fingerprinting and effects of urbanisation on the water quality dynamics of the Quaternary aquifer of south Bengal Basin. India Environmental Earth Sciences 81:134. https://doi.org/10.1007/s12665-022-10258-3
Banerjee S, Sikdar PK (2022b) Cl/Br mass ratio and water quality index from the Quaternary aquifer of south Bengal Basin in India. Environ Sci and Pollut Res. https://doi.org/10.1007/s11356-022-19758-y
Barui NC, Chanda S (1992) Late quaternary pollen analysis in relation to paleoecology, biostratigraphy and daring of Calcutta peat. Proc of Indian Natl Sci Acad 58B(4):191–200
Basu AR, Jacobsen SB, Poreda RJ, Dowling CB, Aggarwal PK (2002) Response to Harvey, 2002: groundwater flow in the Ganges delta. Science 296:15–63
Battista G, de LietoVollaro R (2017) Correlation between air pollution and weather data in urban areas: assessment of the city of Rome (Italy) as spatially and temporally independent regarding pollutants. Atmos Environ 165:240–247
BIS (Bureau of Indian Standard) 10500 (2012) Indian standard drinking water specification, 2nd revision, pp 1–16
Bonsor HC, MacDonald AM, Ahmed KM, Burgess WG et al (2017) Hydrogeological typologies of the Indo-Gangetic basin alluvial aquifer. South Asia Hydrogeol J 25(5):1377–1406. https://doi.org/10.1007/s10040-017-1550-z
Bourg C, Stievnard M, Jouzl J (2001) Hydrogen and oxygen isotopic composition of aqueous salt solutions by gas-water equilibrium method. Chem Geol 173:331–337
Brenninkmeijer CAM, Morrison PD (1987) An automated system for isotopic equilibration of CO2 and H2O for 18O analysis of water. Chem Geol 66:21–26
CGWB (2004) Hydrogeology report on Kolkata Municipal Corporation. Central Ground Water Board
CGWB (2006) Hydrogeology report on Kolkata Municipal Corporation. Central Ground Water Board
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. Lewis Publishers, New York
Craig H (1961) Isotopic variations in meteoric waters. Science 133:1702–1703
Criss RE (1999) Principles of stable isotope distribution. Oxford University Press, New York
D’Alessandro W, Federico C, Longo M, Parello F (2004) Oxygen isotope composition of natural waters in the Mt. Etna Area J Hydrol 296(2004):282–299
Dansgaard W (1964) Stable isotopes in precipitation. Tellus 16:436–468
Eastoe CJ, Gu A, Long A (2004) The origins, ages and flow paths of groundwater in Tucson Basin’ results of a study of multiple isotope system. Water Sci. and Appl. Groundwater Recharge in a Desert Environment: The Southwestern United States. 9. https://doi.org/10.1029/009WSA12
Epstein S, Mayeda T (1953) Variations of O18 content of waters from natural sources. Geochim Cosmochim Acta 4:213–224
Galloway D, Burbey T (2011) Regional land subsidence accompanying groundwater extraction. Hydrogeology J 19:1459–1486
Gat JR (1996) Oxygen and hydrogen isotopes in the hydrologic cycle. Annu Rev Earth Planet Sci 24:225–262
Gat JR, Gonfiantini R (1981) Stable isotope hydrology. Deuterium and oxygen-18 in the water cycle. IAEA, Tech. Rep., Ser., 210, Vienna.
Ghosh NC, Chatterjee TK, Gupta A, Saha D (2001) Heavy metal concentration in groundwater of Greater Calcutta (Kolkata) West Bengal India. Indian J Geo. 73(1):55–66
Gibbs RJ (1970) Mechanisms controlling world’s water chemistry. Science 170:1088–1090
Gonfiantini R, Frohlich K, Araguas-Araguas L, Rozanski K (1998) Isotopes in groundwater hydrology. Isotope tracers in catchment hydrology. . Elsevier Science B.V, Amsterdam, The Netherlands, pp 203–225
Hait AK, Das HK, Chakrabarty S, Ray AK, Chanda S (1994a) Late Quaternary Palaeontological changes in Coastal Digha – a note based on floral and faunal record. Indian J of Earth Sci 21(3):192–198
Hait AK, Das HK, Ghosh S, Ray AK, Chanda S (1994b) On the occurrence of mangrove biota and some faunal remains from the sediments of Haldia, West Bengal in relation to its past environment. J Palynol 30:73–78
Hait AK, Das HK, Ghosh S, Ray AK, Chanda S (1996a) Environmental variations in Late Quaternary sequence of Kolaghat, West Bengal. India Current Science 70(12):1089–1093
Hait AK, Das HK, Ghosh S, Ray AK, Chanda S (1996b) New dates of Pleisto-Holocene subcrop samples from South Bengal. India Indian J of Earth Sciences 23(2):79–82
Harvey CF, Swartz CH, Badruzzaman ABM, Keon-Blute N, Yu W, Ali MA, Jay J, Beckie R, Niedan V, Brabander D, Oates PM, Ashfaque KN, Islam S, Hemond HF, Ahmed MF (2005) Groundwater arsenic contamination on the Ganges Delta: biogeochemistry, hydrology, human perturbations, and human suffering on a largescale. Compt Rendus Geosci 337:285–296
Hem JD (1989) Study and interpretation of the chemical characteristics of natural water. US Geological Survey, Water Supply Paper, p 2254
Herczeg A, Edmunds WM (2000) Inorganic ions as tracers. In: Cook P, Herczeg A (eds) Environmental tracers in subsurface hydrology. Kluwer Academic Publishers, Boston, pp 31–77
Hoque MA, Hoque MM, Ahmed KM (2007) Declining groundwater level and aquifer dewatering in Dhaka metropolitan area, Bangladesh: causes and quantification. Hydrogeol J 15:1523–1534
Hoque MA, McArthur JM, Sikdar PK, Ball JD, Molla TN (2014) Tracing recharge to aquifers beneath an Asian megacity with Cl/Br and stable isotopes: the example of Dhaka. Bangladesh Hydrogeol J 22(7):1549–1560. https://doi.org/10.1007/s12665-020-8892-x
Hosono T, Nakano T, Shimizu Y, Onodera S, Taniguchi M (2011) Hydrogeological constraints on nitrate and arsenic contamination in Asian metropolitan groundwater. Hydrol Proc 25:2742–2754
Howard KWF, Gelo KK (2002) Intensive groundwater use in urban areas: the case of megacities. In: Llamas R, Custodio E (eds) Intensive use of groundwater: challenges and opportunities. Balkema, Lisse, The Netherlands, pp 35–58
IMD (2020) India Meteorological Department. Free Download Yearly Gridded data Binary File. https://www.imdpune.gov.in/Clim_Pred_LRF_New/Grided_Data_Download.html. Accessed on 25 Dec 2020
Kagabu M, Shimada J, Delinom R, Tsujimura M, Taniguchi M (2011) Groundwater flow system under a rapidly urbanizing coastal city as determined by hydrogeochemistry. J Asian Earth Sci 40:226–239
Katz BG, Eberts SM, Kauffman LJ (2011) Using Cl/Br ratios and other indicators to assess potential impacts on groundwater quality from septic systems: a review and examples from principal aquifers in the United States. J Hydrol 397:151–166
Klump S, Kipfer R, Cirpka OA, Harvey CF, Brennwald MS, Ashfaque KN et al (2006) Groundwater dynamics and arsenic mobilization in Bangladesh assessed using noble gases and tritium. Environ Sci Technol 40:243–250
Krishana G, Kumar B, Sudarsan N, Rao MS, Ghosh NC, Taloor AK et al (2021) Isotopes (δ18O, δD and 3H) variations in groundwater with emphasis on salinization in the state of Punjab. India Sci of the Total Environment 789:148051. https://doi.org/10.1016/j.scitotenv.2021.148051
Kumar M, Rao MS, Kumar B, Ramanathan A (2011) Identification of aquifer-recharge zones and sources in an urban development area (Delhi, India), by correlating isotopic tracers with hydrological features. Hydrogeology J. 19:463–474. https://doi.org/10.1007/s10040-010-0692-z
Lapworth DJ, Krishan G, MacDonald AM, Rao MS (2017) Groundwater quality in the alluvial aquifer system of northwest India: new evidence of the extent of anthropogenic and geogenic contamination. Sci Total Environ 599–600:1433–1444
Lapworth DJ, Dochartaigh BÓ, Nair T, O’Keeffe J, Krishan G, MacDonald AM, Khan M, Kelkar N, Choudhary S, Krishnaswamy J, Jackson CR (2020) Characterising groundwater-surface water connectivity in the lower Gandak catchment, a barrage regulated biodiversity hotspot in the mid-Gangetic basin. J Hydrol 594:125923. https://doi.org/10.1016/j.jhydrol.2020.125923
Li SL, Yue FJ, Liu CQ, Ding H, Zhao ZQ, Li X (2014) The O and H isotope characteristics of water from major rivers in China. Chin J Geochem 34(1):28–37. https://doi.org/10.1007/s11631-014-0015-5
Llyod JW, Heathcote JA (1985) Natural inorganic hydrochemistry in relation to groundwater. Clarendon Press, Oxford
MacDonald A, Bonsor H, Ahmed K, Burgess W, Basharat M, Calow R, Dixit A, Foster S, Krishan G et al (2016) Groundwater depletion and quality in the Indo-Gangeticbasin mapped from in situ observations. Nat Geosci 9:762–766
Majumder RK, Halim MA, Saha BB, Ikawa R, Nakamura T, Kagabu M, Shimada J (2011) Groundwater flow system in Bengal Delta, Bangladesh revealed by environmental isotopes. Environmental Earth Sciences 64(5):1343–1352. https://doi.org/10.1007/s12665-011-0959-2
Marandi A, Shand P (2018) Groundwater chemistry and the Gibbs Diagram. Appl Geochem 97:209–212. https://doi.org/10.1016/j.apgeochem.2018.07.009
McArthur JM, Sikdar PK, Hoque MA, Ghosal U (2012) Waste-water impacts on groundwater: Cl/Br ratios and implications for arsenic pollution of groundwater in the Bengal Basin and Red River Basin. Vietnam Sci Total Environ 437:390–402
McArthur JM, Ghosal U, Sikdar PK, Ball J (2016) Arsenic in groundwater: the deep Late Pleistocene aquifers of the western Bengal Basin. Environmental Sci and Technol 50(7):3469–3476
McArthur JM, Sikdar PK, Leng MJ, Ghosal U, Sen I (2018) Groundwater quality beneath an Asian megacity on a Delta: Kolkata’s (Calcutta’s) disappearing arsenic and present manganese. Environ Sci and Technol 52(9):5161–5172
McDonald R, Douglas I, Revenga C, Hale R, Grimm N, Gronwall J, Fekete B (2011) Global urban growth and the geography of water availability, quality, and delivery. Ambio 40:437–446
Meredith K, Hollins S, Hughes C (2013) The influence of groundwater/surface water exchange on stable water isotopic signatures along the Darling River. Groundwater and Ecosystems. CRC Press, Boca Raton, FL, USA, NSW, Australia, p 57
Misra AK (2001) Hydrogeology and groundwater resource management in South 24 Parganas District and Calcutta Municipal Corporation area, West Bengal. Unpublished Ph.D. Thesis, Jadavpur University, India.
Morris BL, Seddique AA, Ahmed KM (2003) Response of the DupiTila aquifer to intensive pumping in Dhaka, Bangladesh. Hydrogeol J 11:496–503
Mukherjee A (2006) Deep groundwater flow and chemistry in the arsenic affected western Bengal Basin, West Bengal, India. Ph.D. Thesis, University of Kentucky.
Mukherjee S, Bebermeier W, Schütt B (2018) An overview of the impacts of land use land cover changes (1980–2014) on urban water security of Kolkata. Land 7:91
Négrel PH, Roy S, Petelet-Giraud E, Millot R, Brenot A (2007) Long Term Fluxes of Dissolved and Suspended Matter in the Ebro River Basin (spain) 2007(342):249–260
O’Keeffe J, Moulds S, Scheidegger JM, Jackson CR, Nair T, Mijic A (2020) Isolating the impacts of anthropogenic water use within the hydrological regime of North India. Earth Surf Process Land 45(5):1217–1228. https://doi.org/10.1002/esp.4799
Patra S, Sahoo S, Mishra P, Mahapatra SC (2018) Impacts of urbanization on land use /cover changes and its probable implications on local climate and groundwater level. Journal of Urban Management 7:70–84
Ravenscroft P, McArthur JM (2004) Mechanism of regional pollution of groundwater by boron: the examples of Bangladesh and Michigan, USA. Appl Geochem 19:1413–1430
Ravenscroft P, McArthur JM, Hoque MA et al (2013) Stable groundwater quality in deep aquifers of southern Bangladesh: the case against sustainable abstraction; Sci. Total Environ 454–455:627–638
Sahu P, Sikdar PK (2011) Groundwater potential zoning of a periurban wetland of south Bengal Basin. India Environ Monit Assess 174:119–134
Sahu P, Sikdar PK (2017) Effect of pumping on hydrologic system of a young satellite city in south Bengal Basin through numerical modelling: past, present and future. Sustain Water Resour Manag 3(3):321
Sen PK, Banerjee M (1990) Palyoplankton stratigraphy and environmental changes during Holocene in the Bengal Basin. India Record Palaeobotany and Palynology 62:25–35
Sengupta S, Sarkar A (2006) Stable isotope evidence of dual (Arabian and Bay of Bengal) vapour sources in monsoonal precipitation over north India. Earth Planet Sci Lett 250:511–521
Shivanna K, Sharma S, Sinha UK, Nair AR, Navada SV, Ray A, Talukdar T, Mehta BC, Ghosh AK (1999) Arsenic pollution in ground water of West Bengal. Proc. Workshop on Groundwater Pollution and Its Protection with Special Reference to Arsenic Contamination. CGWB, Calcutta.
Sikdar PK (1996a) Hydrogeology of the area in and around Calcutta and Howrah Municipal Corporation with special emphasis on the management of groundwater resources. Thesis submitted for the degree of Doctor of Philosophy (Science) of the University of Calcutta
Sikdar PK (1996b) Development dynamics and management of groundwater in and around Calcutta, India. Indian J Geol 71(3):173–186
Sikdar PK (2000) Geology of the Quaternary aquifers of the twin city of Calcutta-Howrah. J Geol Soc India 56:169–181
Sikdar PK, Banerjee S (2003) Genesis of arsenic in groundwater of Ganga Delta -an anthropogenic model. Jour. Human Settlements. April, 10–24.
Sikdar PK, Chakraborty S (2008) Genesis of arsenic in groundwater of North Bengal Plain using PC A: a case study of English Bazar Block, Malda District West Bengal India. Hydrol Process. 22(12):1796–1809
Sikdar PK, Dasgupta SP (1997) Pollution-risk analysis of groundwater of Calcutta and Howrah cities. Indian Minerals 50(4):397–402
Sikdar PK, Sahu P (2009) Understanding wetland sub-surface hydrology using geologic and isotopic signatures. Hydrol Earth Syst Sci 13:1313–1323
Sikdar PK, Sarkar SS, Palchoudhury S (2001) Geochemical evolution of groundwater in Quaternary aquifer of Calcutta and Howrah. India Journal of Asian Earth Sciences 19:579–594
Sikdar PK, Sahu P, Sinha Ray SP, Sarkar A, Chakraborty S (2013) Migration of arsenic in multi-aquifer system of Bengal Basin: analysis via numerical modeling. Environ Earth Sci 70(4):1863–1879
Sikdar PK, Banerjee S, Chakraborty S (2022) Understanding the past-present-future hydrogeologic system through numerical groundwater modeling of south Bengal Basin. India Front Water 3:801299. https://doi.org/10.3389/frwa.2021.801299
Stüben D, Berner Z, Chandrashekharam D, Karmakar J (2003) Arsenic enrichment in groundwater of West Bengal, India: geochemical evidence for mobilization of As under reducing conditions. Appl Geochem 18(9):1417–1434
Stute M (1999) Tritium (3H) in precipitation in Bangladesh. Groundwater arsenic contamination in the Bengal delta plain of Bangladesh. In: Jacks G, Bhattacharya P, Khan AA (eds) Proceedings of the KTH-Dhaka University Seminar, University of Dhaka, Bangladesh, pp 109–117
Yan ZW, Wang J, Xia JJ, Feng JM (2016) Review of recent studies of the climatic effects of urbanization in China. Adv Clim Chang Res 7(3):154–168
Acknowledgements
The authors express their thanks to the Director, Indian Institute of Social Welfare and Business Management (IISWBM) for providing continual support and infrastructure for carrying out the present research work. The authors are grateful to the Kolkata Municipal Corporation; Director of State Water Investigation Directorate, West Bengal; Pioneer Tubewells Industries Pvt. Ltd., Kolkata; Aqua Solutions Pvt. Ltd., Kolkata; and Drilltech Consultant Pvt. Ltd., Kolkata, for agreeing to share pertinent geological and hydrogeological information necessary for the research work. Also, the authors appreciate the service of Mr. Rohit Basu Dhar and Mr. Ritaban Mitra during the research work.
Author information
Authors and Affiliations
Contributions
Data generation and processing, interpretation and writing of the paper have been carried out by Sumanta Banerjee. However, Prof. Sikdar (present guide of my ongoing PhD) has reviewed the paper critically at interpretation stage, presentation of the figures and several other aspects. Prof. S Kumar, as specialist of isotope studies, has also carried out the technical review of this research paper.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Xianliang Yi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Banerjee, S., Sikdar, P.K. & Kumar, S. Hydrochemical systematics and isotope (δ18O, δD and 3H) variations of aquifer system of southern Bengal Basin: implications for groundwater pollution. Environ Sci Pollut Res 31, 23858–23875 (2024). https://doi.org/10.1007/s11356-024-32692-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32692-5