Abstract
To evaluate seaweed as a biomonitoring organism, Fucus was sampled in the Faroe Islands. Nineteen PAHs, including the EPA 16, and four groups of alkylated PAHs were quantified using GC–MS analysis of extracts obtained using a modified QuEchERS method with ultrasonication in acetonitrile, back-extraction into hexane, and Florisil® cleanup. Samples from the harbor of Tórshavn collected at high tide were the most polluted with PAH concentrations between 1.3 × 102 and 1.7 × 102 ng/g wet weight. All samples contained a factor 10 higher concentrations of alkylated PAHs compared to their parent compounds. These results suggest that Fucus might be suitable as a biomonitoring organism for PAH pollution. Differences between samples collected in close proximity and on different days were observed (same range of RSD 14–120% and 60–102%, respectively), suggesting that water exchange, tide levels, and direct exposure to surface diesel pollution have a strong influence on pollutant uptake in Fucus. The findings stress the need for further evaluation of the sampling strategy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mid 1990’s activities related to the ocean have been the major driver of the economy in the Faroe Islands. Currently, fishing and its related industries account for more than 20% of the gross domestic product (The Government of the Faroe Islands 2019). Since 1995, aquaculture and shipping have contributed significantly to the economic growth. This economic expansion has led to increased traffic in the waters surrounding the Faroe Islands, increasing the pollution load, posing a threat to natural habitats and human health (Sorte et al. 2020).
One major group of pollutants related to the marine industries is polycyclic aromatic hydrocarbons (PAHs), which is a diverse group of hydrophobic, organic contaminants comprising at least two fused aromatic rings (Bergamasco et al. 2014; Lawal 2017). In the environment, they are often found with some degree of alkylation and are subjected to various transformations including biodegradation and photooxidation, which influences their physicochemical properties (Andersson and Achten 2015; Boll et al. 2015). PAHs are of environmental concern due to their specific toxicity towards aquatic life including fish, benthic organisms, and marine vertebrates (Honda and Suzuki 2020), not to mention their carcinogenic properties, posing a threat to human health (Ambade et al. 2021). In the Arctic marine environment, they originate from both natural sources including volcanic eruptions, underwater oil seeps and forest fires, and anthropogenic sources such as the use and combustion of coal- and oil-derived products (Balmer et al. 2019; Lawal 2017). The anthropogenic sources of PAHs vary from local sources to long-range transport with wind and ocean currents (AMAP 2004). The growing fishing and shipping industries are therefore also contributing to the increased levels of PAHs in the marine environment surrounding the Faroe Islands, due to the combustion of heavy fuels and diesel oil, and the use of coal tar for impregnation of wooden boats (Van Metre and Mahler 2010). The environmental fate of PAHs in the marine and terrestrial environments in the Arctic is of growing interest, and the understanding of the transport and degradation of PAHs in the Arctic ecosystems remain a scientific challenge (Balmer et al. 2019; Jörundsdóttir et al. 2014; Laender et al. 2011). Thus, it is of relevance to assess the occurrence and distribution of PAHs along the coasts of the Faroe Islands and to evaluate new and relevant biomonitoring organisms for exposure monitoring.
Biomonitoring organisms are used for quantitative determination of specific contaminants in the environment. They provide an integrated assessment of the presence of pollutants by only responding to the fraction of ecotoxicological relevance (Conti et al. 2008). An ideal biomonitoring organism for assessing local pollution needs to be easy to identify, sedentary, abundant, easy to collect, available to sample throughout the year, accumulator of the pollutant, and possible to analyse (Conti and Cecchetti 2003; Rainbow 1995). Blue mussels (Mytilus edulis) are considered ideal for in situ monitoring of lipophilic persistent organic pollutants in the marine environment, as they are sedentary filter feeders (Poulsen et al. 2021) but the accessibility can vary with the sampling location. The widespread occurrence of seaweed, its potential for reflecting passive uptake and its ability to tolerate pollutants makes it a potential alternative biomonitoring organism to blue mussels (Chalkley et al. 2019). Seaweed has been used for biomonitoring of trace metals via passive uptake routes, however the number of studies investigating the use of seaweed as a biomonitoring organism for PAHs are limited to a few recent studies on green, red, and brown macroalgae (Lourenço et al. 2019; Pavoni et al. 2003; Zokm et al. 2022). Furthermore, these studies have been carried out in the Mediterranean and sub-tropical climate, where the abundance of species, distribution, transport, and degradation of PAHs might differ from that of the Arctic.
Fucus is a genus of brown macroalgae which is highly abundant in the intertidal zones along the rocky coasts of the North Atlantic (Jueterbock et al. 2013). Members of the genera Fucus are canopy-forming macroalgae, which due to their large surface area provide liveable habitats for a large variety of marine organisms. Therefore, assessing the accumulation of PAHs in Fucus is not only relevant in relation to environmental monitoring, but also for environmental conservation. Knutzen and Sortland (1982), investigated the uptake of PAHs in F. vesicolosus along the coasts of Norway and found a total PAH concentration for up to 28 PAHs including methylated PAHs of 284–4665 µg/kg dry weight depending on the sampling location. Kirso and Irha (1998) assessed the role of red, green, and brown macroalgae in the distribution of PAHs in the Baltic Sea. Their results showed that 80 – 89% of the initial amount of benzo(a)pyrene added in an ex situ experiment was found in the biomass of F. vesicolosus, which suggests bioconcentration of PAHs. These findings indicate that Fucus as a native species might also serve as a suitable passive biomonitoring organism for PAHs in the Arctic (Conti and Cecchetti 2003; García-Seoane et al. 2018; Rainbow 1995).
The aims of this study were to investigate whether the brown macroalgae Fucus takes up and accumulate PAHs from the surrounding environment, and to assess if it can be used as monitoring organisms for PAH contamination in the Arctic and specifically in the Faroe Islands. This includes comparison of inter-day sampling of the same genera under similar conditions at the same location, and of the same genera sampled at different conditions and locations.
Seaweed belonging to the Fucus genus was sampled in Tórshavn, Runavík, Toftir, and Kirkjubøur, and analyzed within 48 h after sampling. The extraction method for PAHs from seaweed was developed based on a QuEChERS (Quick, Easy, Cheap, Effective, Rugged, Safe) method designed for extraction of PAHs from heavily pigmented fruits (Słowik-borowiec et al. 2022). The chemical analysis was carried out using gas chromatography-mass spectrometry (GC–MS) in selected ion monitoring (SIM) mode. In order to investigate whether the measured PAHs were adsorbed to the surface of the sampled seaweed or absorbed into the tissue, a thorough wash with acetonitrile (ACN) followed by analysis of the liquid and tissue was conducted on two of the samples in parallel with the regular extraction.
Method
Sampling
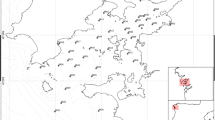
Samples of seaweed were collected at six locations around Tórshavn (TH), Runavík (R), Toftir (TO), and Kirkjubøur (K) in the Faroe Islands (Fig. 1, table S1.1), between the 30 May 2022 and the 4 June 2022. Four of the locations (harbors in Runavík and Tórshavn) were suspected to be polluted with diesel oil and creosote originating from fishing and shipping activities. Kirkjubøur was suspected to be less polluted, due to its location on the west coast, where the water exchange is more rapid, and the density of marine traffic is lower. A sample area located northeast from the harbor of Tórshavn was also suspected to have a low concentration of PAHs and was therefore also sampled as a potential reference site. Toftir, a smaller harbor located south of Runavík, was suspected to be less polluted than Tórshavn and Runavík but have higher pollution than the reference sites. See supplementary information (figure S1.1) for detailed maps showing each sampling location. Each sample consisted of a composite sample from multiple individuals in the same area (maximum radius of 100 m) comprised of four to five increments each consisting of thalli from at least three individuals excluding stem and receptacles exceeding a length of 1 cm. The samples were consistently collected just below surface at the current water level.
Sampling locations. Tórshavn A and B, and Runavík A and C were suspected hotspots for PAH pollution. Toftir and Runavík B were expected to be less polluted. Kirkjubøur and Tórshavn B were chosen as the reference sites. The samples THA-1–THA4 were sampled at Tórshavn A, THB-1 at Tórshavn B, and THC-1 at Tórshavn C. RA-1 + 2 were sampled at Runavík A, RB-1 at Runavík B, and RC-1 at Runavík C. TOA-1 was sampled at Toftir and KA1 + KB1 at the site in Kirkjubøur
Sample names
The two cities and two towns are represented with a 1–2 letter abbreviation Tórshavn (13,083 inhabitants (2017)) (TH), Runavík (3979 (2018)) (R), Toftir (793 (2017)) (TO), and Kirkjubøur (75 (2009)) (K). Different locations within the same city are sorted alphabetically (A, B, and C), and numbering refers to different samples collected at the same location. Further information about the samples can be found in Table 1.
Standards, materials, and chemicals
Standard solutions for calibration containing 18 parent PAHs (EPA16 + benzo[e]pyrene and perylene) and 20 alkylated PAHs (table S5.4) were prepared in HPLC-grade iso-octane from Rathburn (Højbjerg, Denmark). The standard also includes dibenzothiophene and alkylated dibenzothiophene which are heterocyclic sulfur containing compounds. In the following sections, dibenzothiophene is included in the sum of the PAHs (∑PAH19). All samples were analyzed for the 19 parent PAHs. The ones present at concentrations above the detection limit (DL) are listed with concentrations in Table 2 and 3, and ∑PAH19 is the sum of the listed concentrations excluding those with concentrations below DL. An internal standard solution and a recovery solution containing nine and six deuterated PAHs, respectively (S5.4), each at a concentration of approximately 8 µg/mL, were also prepared in HPLC-grade iso-octane from Rathburn (Højbjerg, Denmark). The calibration, internal, and recovery standards used are listed in table S5.1, S5.2 and S5.3, respectively. Exact concentrations of the standard solutions are listed in supplementary information (table S5.5). Hexane (mixtures of isomers) and ACN (HiPerSolv) were both HPLC grade and purchased from VWR Chemicals (Søborg, Denmark). Primary secondary amine (Spera™ PSA) was acquired from Phenomenex (Værløse, Denmark). Florisil® (60–100 mesh) was acquired from Supelco (Søborg, Denmark). Sodium hydrogencitrate sesquihydrate (98.5 purity) (Na2C6H6O7) was purchased from Sigma-Aldrich (Søborg, Denmark), tri-sodium citrate dihydrate (99.0 purity) (Na3C6H5O7) was acquired from VWR (Søborg, Denmark), and magnesium sulphate (MgSO4) (98.0% purity) was purchased from ChemSolute (Roskilde, Denmark).
Method development
The method for sample preparation was based on a modified QuEChERS method for the extraction of PAHs in highly pigmented fruits and vegetables with a water content between 20 and 80% (Goodman 2011; Słowik-borowiec et al. 2022). During the method development, the extraction solvents ACN and n-hexane:acetone 4:1 (v/v) were tested, and ACN showed to be the more efficient solvent for the extraction of PAHs. Extraction by ultrasonication showed to be more efficient than dispersion with Ultra Turrax®, which was also tested. For the up-concentration and additional sample cleanup, a liquid–liquid back-extraction with hexane in ACN was preferred over up-concentration of n-hexane:acetone (4:1) v/v by evaporation, as it was less time consuming, and ACN was the better extraction solvent. Excess pigments were cleaned up by Florisil®, as it showed to be more efficient at removing pigments without removing PAHs compared to graphitized carbon black (GCB) and C18 fused silica. See supplementary information (S6) for a detailed description of the results from the method optimization.
Sample preparation
To avoid contamination of PAHs after sampling, all samples were collected in Teflon bags and stored in a refrigerator at 5 °C within 2 h after sampling. Approximately 100 g of sample was homogenized using a small kitchen blender (Køkkenchef, 500 mL, 300 W, 10069036) after thorough cleaning with tap water to remove epiphytes, particles, and other organisms attached to the sample. Approximately 12.5 g of the homogenized sample was transferred to a 50-mL glass centrifuge tube, and 12.5 mL of ACN and 80 µL of internal standard solution were added. After mixing the solvent with the sample, the samples were placed in an ultrasonication bath for 30 min. After sonication, 1.8 g NaCl, 1.8 g Na3C6H5O7, and 0.9 g Na2C6H6O7 were added to the centrifuge tube, vortexed for 1 min, and afterwards centrifuged at 2000 rpm for 3 min. Approximately 10 mL of the upper organic phase was transferred to a new 50-mL centrifuge tube where 2 mL of hexane and 12.5 mL of tap water were added, vortexed for 1 min, and centrifuged at 2000 rpm for 3 min. The hexane phase of 1–1.5 mL was transferred to a 15-mL glass centrifuge tube containing 80 mg MgSO4 to dry any excess water, and 334 mg Florisil® was added.Footnote 1 The centrifuge tube was vortexed and centrifuged at 2000 rpm for 2 min. Approximately 1 mL of the clean extract was transferred to a 2-mL amber GC vial with screw cap, and 80 µL recovery standard was added. One hundred microliters was transferred to a GC amber vial with a 300 µL insert for analysis, and the remaining sample was stored at − 20 °C.
Surface wash with ACN
A study was performed to investigate whether PAHs in Fucus were absorbed into the tissue or adsorbed to the surface. A total of 25 g of Fucus (previously cleaned with tap water) was transferred to a 50-mL centrifuge vial and shaken vigorously for 30 s with 25 mL ACN. The liquid was transferred to another 50-mL glass centrifuge tube, and 160 µL internal standard solution was added. This procedure was then repeated to a total of two extractions that were analyzed separately. Afterwards, 25 mL of tap water and 4 mL of hexane were added to the two ACN samples, and a liquid–liquid extraction was performed. Finally, 160 µL of recovery standard solution was added, vial-mixed, and an aliquot was transferred to an amber GC vial with a 300 µL insert for analysis. The ACN washed samples were left for drying in the fume hood and thereafter treated as the other samples for analysis. The rest of the sampled Fucus were homogenized and analyzed as well.
Chemical analysis
All samples were analyzed using an Agilent Technologies 7890A GC system coupled to an Agilent Technologies 5975 inert XL EI/CI Mass Selective Detector. The samples were injected in splitless mode with an injection volume of 1 µL. The separation of the analytes was performed on a 30-m Zebron ZB5 capillary column (Phenomenex, 0.25-mm inner diameter × 0.25-µm film thickness). Helium was used as the carrier gas at a constant flow of 1.1 mL/min resulting in an average velocity of 38.2 cm/s. The initial temperature was 50 °C, which was held for 1 min and hereafter increased by 25 °C/min until 100 °C, followed by an increase to 315 °C with 12 °C/min. The temperature was kept at 315 °C for 7 min, which resulted in a total analysis time of 28 min. Electron ionization (EI) was performed using an electron energy of 70 eV. The temperature of the EI source was 230 °C and the quadrupole 150 °C. The mass spectrometer was operated in SIM mode for 21 selected mass-to-charge (m/z) ratios, ranging from 83 to 288 m/z. The data acquisition was divided into 12 SIM groups with a dwell time of 20 ms of each m/z (table S7.1).
Quality control/assurance and detection limits
In every batch, at least one method blank (containing 12.5 mL tap water) was treated the same way as the sample, from homogenization to addition of recovery standard, and GC–MS analysis.
To evaluate inter-day precision, a quality control (QC) sample was included in each batch and handled the same way as the regular samples (QC1). The QC1 sample consisted of 12.5 g homogenized Saccharina latissima from multiple individuals and was spiked with 240 µL 1:10 diluted stock solution, containing the 19 PAHs. In this case S. latissima tissue was used as it was deemed to have a comparable matrix to Fucus and due to its large thalli which made it more feasible to get a large enough sample to have several QC1 samples for analysis. DL and limit of quantification (LOQ) were assessed by preparing and analysing a batch of five QC2 samples consisting of a homogenized blend of Fucus sampled on the 4 June 2022 which contained a 10 times dilution of a PAH19 standard solution (table S5.5). The QC2 batch was analyzed 6 June 2022. The five samples of Fucus were also used for intra-day precision. The results are listed in supplementary information S3.
Data treatment and quantification
MassHunter Workstation (Agilent Technologies, ver. B.07.00, 2014, USA) was used for quantification of PAHs. Four-level internal standard curves were used for quantification of each of the PAHs. Linear calibration curves with zero intercept were used as most samples contained target PAHs below the lowest standards. Concentrations were corrected for recovery determined from the surrogate standards added to the sample before extraction according to Poulsen et al. (2021). Alkylated PAHs were quantified based on the patterns exhibited by isomers of one alkylation level as described in the draft of the European Committee for Standardization guideline prEN 15522–2 (Dahlmann and Kienhuis 2016). If the patterns matched, all peaks were integrated and the signal was quantified with the help of the corresponding standard. When a major change in pattern, for example, a drastic increase in peak area for a single peak, was observed, this peak was excluded and the integration window terminated before or started after that peak. All graphs and plots were made using OriginPro 2020 (OriginLab Corporation, 2022). Maps illustrating the sampling locations were created using the ArcGIS Pro 2.5.1©2020 ESRI Inc.
Diagnostic ratios
As a strategy for identifying the source of PAH pollution in the samples, two different diagnostic ratios were calculated. An Ant / (Ant + Phe) ratio < 0.1 classifies the source as petrogenic whereas a ratio > 0.1 classifies the source as pyrogenic. For a Flr / (Flr + Pyr) (Flr = fluoranthene) ratio < 0.4, the source is classified as petrogenic. For a ratio 0.4–0.5, the source is classified as fossil fuel combustion and > 0.5 ratio classifies the source as grass, wood, and coal combustion. The diagnostic ratios were calculated based on suggested ratios from Tobiszewski and Namieśnik (2012).
Results
PAH concentrations and diagnostic ratios
Table 2 shows ∑PAH19 concentrations in Fucus collected at the 13 sampling sites and two diagnostic ratios. The highest ∑PAH19 concentrations and of the most abundant individual PAHs were as follows: Tórshavn > Runavík > Toftir > reference sites. The highest ∑PAH19 concentrations were quantified in samples from Tórshavn (1.3 × 102 ng/g ww), which is consistent with the suspected pollution level. Concentrations of PAHs at the reference sites were < DL. This was also observed for RC-1 and RB-1. For THB-1, none of the measured PAH concentrations exceeded LOQ.
In Tórshavn, the highest concentrations were observed for phenanthrene and pyrene, but relatively high concentrations (> 10% of ∑PAH19) of fluorene were observed in samples from THA-1, THA-2, and THA-4 (Table 2). The high concentrations of phenanthrene compared to anthracene and pyrene compared to fluoranthene demonstrate that the source of PAHs in the Tórshavn samples is of petrogenic origin (De La Torre-Roche et al. 2009; Pies et al. 2008), which is consistent with the observations of diesel spills on the water surface at the site.
In Runavík and Toftir, the same trend was seen where phenanthrene and pyrene were the highest ∑PAH19 concentrations. A high naphthalene concentration of 1.8 ng/g ww was found at TOA-1 compared to 0.25–0.48 ng/g ww at the 12 other sites. Likewise, high relative anthracene concentrations were observed at RA-2, which indicates a mixed source or another source than diesel oil. The fluoranthene-to-pyrene ratio is however lower than the limit of 0.4 which indicates that the PAHs are of mainly pyrolytic origin (De La Torre-Roche et al. 2009). The other samples from Runavík and Toftir are suspected to originate from petrogenic sources.
High variability was observed within sites of close proximity and samples from the same area. THA-1 and THA-2 showed the highest concentration of all ∑PAH19 (except for anthracene) but THA-3 and THA4 had 2–69 times lower concentrations although they were collected in the same area but two days later where the tide level differed 0.3 m (Table 1 and 2). The same was the case for RA-1 and RA-2 that were sampled 3 days apart also at high and low tide.
The mean RSD of ∑PAH19 between samples collected in close proximity at the same day (14% and 120% for THA1 vs THA2 and THA3 vs THA4) was in the same range as the RSD of samples from the same locations collected at different time points (between 60 and 102% RSD for THA1 vs. THA3, THA1 vs. THA4, THA2 vs. THA3, THA2 vs. THA4, and RA1 vs. RA2). For more details, see Table S8.1 in supporting information S8.
Table 3 shows concentrations of alkylated PAHs at all sampling sites. For the alkylated PAHs, the same trend follows as for the parent compounds where the highest concentrations of ∑PAHalk were as follows: Tórshavn > Runavík > Toftir > reference sites. The ∑PAHalk is more than a factor 10 greater than ∑PAH19 for all samples except for TOA-1 where the ∑PAHalk to ∑PAH19 ratio is 6.7. The sample with the lowest ∑PAHalk concentrations was the same sample with the lowest ∑PAH19, RC-1, and RB-1 (2.4 and 4.6 ng/g ww, respectively). In contrast, THB-1 with a low ∑PAH19 of 0.31 ng/g ww showed 51 times higher ∑PAHalk (17 ng/g ww) compared to the reference sites (2.4, 2.6, and 2.2 ng/g ww). For the Tórshavn samples, the highest concentration of alkylated PAHs was observed for alkylated naphthalenes even though the parent PAH is observed in low concentrations. The concentration of alkylated PAHs follows the same trend in concentration as observed for the parent PAHs: THA-2 > THA-1 > THA-4 > THA-3 > THB-1 (Table 3). The same is the case across sites as illustrated in Fig. 2.
For Runavík and Toftir, the most abundant alkylated PAHs were C2 phenanthrenes (data not shown). The distribution between alkylated PAHs at the sites indicates the degree of mainly evaporative weathering where less low-molecular-weight alkylated PAHs are observed (Wang and Fingas 1995). This effect is especially relevant for naphthalene with a steep increase from C1 to C4 naphthalenes.
Figure 3 compares the relative composition of C1–C4 alkylated PAHs between two samples with high ∑PAHalk from Tórshavn and two samples from Runavík with low ∑PAHalk. The alkylated PAH profiles were similar within each of the sites although they were sampled at different days, which indicate same source and similar degree of evaporative weathering. C3-alkylated naphthalenes were the most abundant alkylated PAH group in the samples from Tórshavn. The Runavík samples have higher relative concentration of alkylated phenanthrenes compared to naphthalenes indicating a heavier petroleum product or a more weathered diesel oil sample. The low concentration of alkylated PAHs at Runavík should be considered when evaluating the PAH profiles as a small absolute error will produce a large relative difference.
ACN-washed Fucus
The PAH concentrations (∑PAH19 and ∑PAHalk) in the washed tissue and in the washing liquid are listed in Table 4. ∑PAH19 and ∑PAHalk in the washing liquid were lower compared to the washed tissue. The diagnostic ratios of the washed tissue are comparable with the tissue that had not been washed (data not shown). The concentration of all PAH19 in the washing liquid for THA-3 was < DL, and only phenanthrene and pyrene were > DL (0.47 ng/g ww and 0.78 ng/g ww, respectively) in THA-4 where a decrease in concentrations was observed from the first to the second wash (analyzed ACN). ∑PAHalk > ∑PAH19 in both the washing liquid and in the tissue but same trend was observed where ∑PAHalk in the tissue > ∑PAHalk wash 2 > ∑PAHalk wash 1.
The washing liquid had a green color after 30 s of shaking, which indicates that pigments from inside the Fucus were also extracted. This suggests that the experiment was thorough in washing the outside of the tissue. An important conclusion from this is that the majority of the observed PAHs seem to be absorbed deeper inside the tissue of Fucus and not simply adsorbed to the surface. This emphasizes the relevance of the PAH results from this study in terms of evaluation of Fucus as a biomonitoring organism.
For the washed tissue, the ∑PAH19 concentration was lower in both samples compared to the tissue that has not been washed with can, and no clear trend is seen where specific PAHs are decreasing in concentration after the wash, which indicates that the wash with ACN was not selectively washing away certain PAHs. All the PAHs found in Table 2 and 3 for THA-3 and THA-4 could not be traced in the combined washed tissue and washing liquid. A higher concentration of alkylated fluorene (C1 and C2) was observed in the washed tissue compared to the tissue not washed with ACN in THA-3 (19 vs. 10 ng/g ww). For THA-4, all PAHalk except for naphthalenes were higher in the washed tissue compared to the tissue washed only with tap water.
Discussion
PAH concentrations in Fucus
The results indicate that Fucus takes up PAHs and that it can be used as a biomonitoring organism for parent and alkylated PAHs in the marine environment. Other studies examining PAH concentrations in Fucus or comparable brown algae growing in the intertidal zone found between 88 and 2010 ng/g dw total PAHs in S. furactum on the coast of Brazil (Lourenço et al. 2019), 349–2109 ng/g dw napthalene and methylnaphthalenes as well as 284–4665 ng/g dw of total PAHs in F. vesiculosus samples from the coast of Norway (Knutzen and Sortland 1982) and 2–5 ng/g dw total PAHs where phenanthrene was the most abundant (29%) in F. virsoides from the lagoon of Venice (Pavoni et al. 2003). When comparing PAH content in living tissue, the most common way is to compare measurement per dry weight (dw). Water content was, however, not measured for all samples in this study. The conversion from g ww to dw is therefore estimated based on the water content from selected individuals of Fucus (mean water content 83%, SD ± 1.8) (table S4.1) in order to compare them with other studies. The phenanthrene found in this study converted with the water content estimate to 465 ng/g dw and is much higher than the most comparable study from Pavoni, but in the range given by the other studies.
The take-up of PAHs is underestimated if only parent PAHs are considered. The inclusions of alkylated PAHs increases the total PAH concentration tenfold for most samples. Alkylated PAHs are not nearly as well researched as their corresponding parent PAHs, and our results demonstrate that the inclusion of alkylated PAHs is important to avoid underestimation of PAH exposure to the marine environment especially when the main sources are of petrogenic origin such as diesel oil spills (Barron and Holder 2003; Du et al. 2022). Furthermore, some studies suggest that alkylated PAHs might pose a larger threat to the environment and human health than their parent compounds (Kang et al. 2016; Lam et al. 2018; Sun et al. 2014). Because of the low interference of matrix components and high concentration of mixtures of alkylated PAHs observed in this study, an unexpected benefit from examining Fucus might also be the possibility of comparing the alkylated PAH concentrations with the concentration of the corresponding parent PAHs.
A clear difference of PAH concentration in Fucus is seen between the suspected high concentration areas compared to the suspected low concentration reference sites. For Fucus sampled at location 4 in Tórshavn and at location 2 in Runavík, the concentrations are comparable to the low concentrations found at the reference sites. This indicates that the pollution might be very local. The difference in PAH concentration in Fucus that was sampled at close proximity at different days is suspected to be explained by at least one of the three factors: bioconcentration, water exchange, and exposure to the source of PAHs.
The comparison between samples within this study is constrained by the limited availability of data explained by the low number of samples. Therefore, the conclusions drawn from the results of this study are related to a high degree of uncertainty. The interpretation of the results presented in the following represents trends and descriptive insights in the differences between samples rather than statistically validated conclusions.
Bioconcentration
The low concentrations or absence of PAHs in the extraction solvent after both the first and second surface wash with ACN (wash 1 and wash 2) and the high concentrations of PAHs in the washed tissue indicate that both the parent and alkylated PAHs are absorbed by Fucus. According to Kirso and Irha (1998), Fucus vesiculus can bioconcentrate benzo(a)pyrene with a very high uptake and only low degradation 10 days after the exposure in ex situ experiments. Assuming different species in the Fucus genus behave similarly, the results indicate that Fucus is able to accumulate PAHs with limited degradation.
Water exchange and exposure
The exposure and water exchange seem to explain the variations in the PAH concentrations across days and between samples of close proximity. This is supported by results obtained by Garcia and Martins (2021) who examined the PAH concentration in sediment at sheltered and non-sheltered areas. They concluded that the PAH concentration is heavily affected by marine hydrodynamics, energy, and/or dilution processes and that the observed concentration was higher in areas with low water exchange.
This could explain the differences in PAH concentrations between two geographically close sampling locations (RA vs. RB and THA vs. THB), but does not, however, explain the inter-day concentration difference observed in Fucus at Tórshavn 1 and Runavík 1 (THA-1 and THA-2 > THA-3 and THA-4 and RA-1 > RA-2), which are both in sheltered harbors where water exchange is low and the concentration therefore not suspected to differ much from day to day.
Another factor influencing the exposure of Fucus to PAHs is the tide levels. The results show that samples from the same locations collected at different days with different tide levels (different time of the day) contain notably different levels of PAHs. THA-1 and THA-2, which were collected at high tide, show ∑PAH19 of 1.3 × 102 and 1.7 × 102 ng/g ww whereas THA-4, which was collected at low tide, contains ∑PAH19 of 57 ng/g ww (Table 2). Since the pattern of the alkylated PAHs is similar between THA-2 and THA-4 (Table 3), a similar source can be assumed. This is also the case for RA-1 (∑PAH19 = 12 ng/g ww) and RA-2 (∑PAH19 = 3.8 ng/g ww) that were collected at high and low tides, respectively.
Benes and Bracken (2016) demonstrated that F. vesiculosus growing in the upper intertidal zone has a significantly higher nutrient uptake rate compared to F. vesiculosus growing in the lower intertidal zone. The reason for this difference is that nutrient uptake only happens when the macroalga is submerged under water. Thus, individuals growing in the upper intertidal zone that are submerged for a shorter period have adjusted the uptake rate, so the total nutrient uptake is comparable to the individual growing in the lower intertidal zone. This increase in activity could also lead to increase in uptake of PAHs by Fucus growing in the upper intertidal zone, as these individuals are exposed to air during low tide, and in direct contact with the oil pollution on the surface of the water during high tide.
Thus, the results indicate that an important factor for accumulation of PAHs is the time of direct exposure to the PAHs such as a diesel oil slick on the water surface, which is probably why the Fucus sampled at high tide contains the highest ∑PAH19 and ∑PAHalkyl. The differences in PAH concentrations might thus be due to sampling at different tidal currents. All Fucus samples were collected from the water surface, independently of the time of day, and therefore, different individuals were picked, depending on the current water level in the harbor. This suggests that individuals growing at different tidal heights will not be accumulating the same amount of PAHs, and tidal height or growing height needs to be taken into account in order for inter-day samples to be comparable.
All samples have low naphthalene concentrations, which is more likely to dissolve in water than the larger PAHs, and more likely to evaporate from the surface (Baumard et al. 1999; Maxin and Kögel‐Knabner 1995; Whitehouse 1984). An additional experiment was conducted where the two kelp species, S. latissima and Alaria esculenta, growing further down the water column, from Tórshavn and Runavík, were analyzed for PAHs. For these samples, the PAH concentrations were below the DL except for phenanthrene, which was the most abundant parent PAH in that area and naphthalene, which is the most water soluble. Phenanthrene and naphthalene concentrations were just above DL in two S. latissima and one A. esculenta sample from Tórshavn (data not shown).
These results indicate that the seaweed must be in direct contact with the source of PAHs, in this case oil slicks on the water surface, in order to take them up. Therefore, individuals growing in the intertidal zone are more likely to reflect surrounding surface pollution. This could affect how well Fucus reflects the surrounding pollution and therefore how well it is suited as a biomonitoring organism. However, our results indicate that the concentrations of PAHs in Fucus reflect the source of PAHs as well as the suspected level of pollution on the sampling site. To support this, further research is needed to understand the interactions between Fucus and marine PAH pollution. An important aspect is investigation of how well the concentrations of PAHs in Fucus reflect the concentrations and composition of PAHs found in water. This investigation would also add to the understanding of Fucus as an accumulative passive bioindicator (Polechońska and Klink 2023). Other important investigations include elucidation of the effects of species, PAH sources, sampling depths, and tidal levels on the uptake of PAHs in macroalgae such as Fucus. It is too early to make a definite conclusion on the suitability of Fucus as biomonitoring organism. However, we hypothesize that the suspected up-concentration of PAHs in Fucus reflects the condition of the surrounding environment. The sampled species is the one factor where we can make the most direct assumptions about the effects of the measured PAH concentrations. Few studies focus on the direct effects of PAHs on kelp or Fucus in particular. One laboratory setup where Fucus vesiculosus was exposed to a mixture of diesel and seawater found only a short-term increase on the level of lipid peroxidation and no change to metabolic activity or catalase concentration (Ryzhik et al. 2019). Due to the short length of the study (6 days), no conclusion could be drawn about the overall health of the algae. Interestingly, the study found a 350% increase in hydrocarbon-oxidizing bacteria on the thalli of the algae when exposed to the diesel-water mixture. Another intertidal kelp species (Lessonia spicata) was found to be sensitive to PAHs especially during the sporophyte formation, with the EC50 for the inhibition of the formation of sporophytes being 0.04 µg/L PAHEPA16 though this species might be particularly susceptible to reactive oxygen species which are created in photosynthetic organisms when exposed to PAHs (Espinoza-González et al. 2021). As a primary producer and a foundation species, Fucus is a food source and an important habitat for many invertebrate species like Idotea balthica or Tonina fluviatilis, especially in the form of shed detritus. PAHs attached to the detritus could further contribute the PAH load on the sediment. These invertebrates were found to either directly graze on Fucus or eat its detritus (Kahma et al. 2023). Alkylated PAHs were found to also have similar toxic effects as parent PAHs to shrimp, microalgae, and a bacterial system although there might be further unidentified toxic mechanisms (Cong et al. 2021; Kang et al. 2016). This class of substances should be paid attention to as they were found in higher concentrations than their non-alkylated counterparts in this study and are the predominant form of PAHs in crude oil. Overall, it can be safely assumed that the Fucus and species directly connected to it in the ecosystem will be negatively affected by the exposure to PAHs. The magnitude of these effects is difficult to gauge due to the complexity of the exposure scenario.
Conclusion
Fucus take up PAHs at measurable concentrations at sites with high PAH pollution levels such as the inner harbors of Tórshavn and Runavík in the Faroe Islands. These results suggest that Fucus is a suitable biomonitoring organism for PAH pollution. Our results however demonstrate that position in the inter-tidal zone and PAH sources are key factors that affect uptake of PAHs in Fucus and that it reflects only very local pollution levels. More research is needed to determine effects of the different factors on the uptake of PAHs. The differences in PAH concentrations in samples taken in close proximity could reflect very local surface water contamination. This hypothesized ability is an advantage; however, the assessment requires knowledge about the underlying hydrodynamics, water exchange, and dilution processes. Overall, the use of Fucus as a biomonitoring organism for PAHs and their alkylated homologues seems promising, but further criteria for the sampling strategy need to be assessed.
Data availability
Further data supporting the findings of this study are available in Supplementary Information. Raw data are available from the corresponding author upon reasonable request.
Notes
PSA was used in TFA-1, TFA-2, TAA, TLA, TLB, TLC, TLD, and TLE to further cleanup but was afterwards left out because of suspected pollution of pyrene and phenanthrene.
References
AMAP (2004) AMAP Assessment 2002: Persistent Organic Pollutants in the Arctic. Arctic Monitoring and Assessment Programme (AMAP). Oslo, Norway. pp xvi +310. www.amap.no/documents/doc/amap-assessment-2002-persistent-organic-pollutants-in-the-arctic/96
Ambade B, Sethi SS, Kumar A, Sankar TK, Kurwadkar S (2021) Health risk assessment, composition, and distribution of polycyclic aromatic hydrocarbons (PAHs) in drinking water of Southern Jharkhand, East India. Arch Environ Contam Toxicol 80:120–133. https://doi.org/10.1007/S00244-020-00779-Y/TABLES/4
Andersson JT, Achten C (2015) Time to say goodbye to the 16 EPA PAHs? Toward an up-to-date use of PACs for environmental purposes. Polycyclic Aromat Compd 35:330–354. https://doi.org/10.1080/10406638.2014.991042
Balmer JE, Hung H, Yu Y, Letcher RJ, Muir DCG (2019) Sources and environmental fate of pyrogenic polycyclic aromatic hydrocarbons (PAHs) in the Arctic. Emerg Contam 5:128–142. https://doi.org/10.1016/j.emcon.2019.04.002
Barron MG, Holder E (2003) Are exposure and ecological risks of PAHs underestimated at petroleum contaminated sites? Hum Ecol Risk Assess 9:1533–1545. https://doi.org/10.1080/10807030390251029
Baumard P, Budzinski H, Garrigues P, Narbonne JF, Burgeot T, Michel X, Bellocq J (1999) Polycyclic aromatic hydrocarbon (PAH) burden of mussels (Mytilus sp.) in different marine environments in relation with sediment PAH contamination, and bioavailability. Mar Environ Res 47:415–439. https://doi.org/10.1016/S0141-1136(98)00128-7
Benes KM, Bracken MES (2016) Nitrate uptake varies with tide height and nutrient availability in the intertidal seaweed Fucus vesiculosus. J Phycol 52:863–876. https://doi.org/10.1111/jpy.12454
Bergamasco A, Culotta L, De Stefano C, Orecchio S, Sammartano S, Barreca S (2014) Composition, distribution, and sources of polycyclic aromatic hydrocarbons in sediments of the Gulf of Milazzo (Mediterranean Sea, Italy). Polycyclic Aromat Compd 34:397–424. https://doi.org/10.1080/10406638.2014.900642
Boll ES, Nejrup J, Jensen JK, Christensen JH (2015) Chemical fingerprinting of hydrocarbon-contamination in soil †. https://doi.org/10.1039/c3em00631j
Chalkley R, Child F, Al-Thaqafi K, Dean AP, White KN, Pittman JK (2019) Macroalgae as spatial and temporal bioindicators of coastal metal pollution following remediation and diversion of acid mine drainage. Ecotoxicology and environmental safety 182. https://doi.org/10.1016/J.ECOENV.2019.109458
Cong Y, Wang Y, Zhang M, Jin F, Mu J, Li Z, Wang J (2021) Lethal, behavioral, growth and developmental toxicities of alkyl-PAHs and non-alkyl PAHs to early-life stage of brine shrimp, Artemia parthenogenetica. Ecotoxicol Environ Saf 220:112302. https://doi.org/10.1016/J.ECOENV.2021.112302
Conti ME, Cecchetti G (2003) A biomonitoring study: trace metals in algae and molluscs from Tyrrhenian coastal areas. Environ Res 93:99–112. https://doi.org/10.1016/S0013-9351(03)00012-4
Conti ME, Iacobucci M, Cecchetti G, Alimonti A (2008) Influence of weight on the content of trace metals in tissues of Mytilus galloprovincialis (Lamarck, 1819): a forecast model. Environ Monit Assess 141:27–34. https://doi.org/10.1007/s10661-007-9875-z
Dahlmann G, Kienhuis P (2016) Oil Spill Sampling and the Bonn-Oil Spill Identification Network: A Common Method for Oil Spill Identification. Handbook of Environmental Chemistry, vol 41. Springer Verlag, pp 237–254. https://doi.org/10.1007/698-2015-366
De La Torre-Roche RJ, Lee WY, Campos-Díaz SI (2009) Soil-borne polycyclic aromatic hydrocarbons in El Paso, Texas: analysis of a potential problem in the United States/Mexico border region. J Hazard Mater 163:946–958. https://doi.org/10.1016/j.jhazmat.2008.07.089
Danish Meteorological Institute (DMI) (2021) Ministry of Climate, Energy and Utilities. Copenhagen. www.dmi.dk/publikationer/
Du L, Zhang L, Liu R, Gu Y (2022) Is polycyclic aromatic hydrocarbon concentration significantly underestimated in scrubber effluent discharge? Ocean Coast Manag 220:106093. https://doi.org/10.1016/j.ocecoaman.2022.106093
Espinoza-González C, Meynard A, Núñez A, Castañeda F, Oyarzo-Miranda C, Latorre-Padilla N, Rivas J, Contreras-Porcia L (2021) Assessment of the independent and combined effects of copper and polycyclic aromatic hydrocarbons on gametogenesis and sporophyte development of the kelp Lessonia spicata (Phaeophyceae, Ochrophyta). J Appl Phycol 33:4023–4034. https://doi.org/10.1007/S10811-021-02552-7/FIGURES/4
Garcia MR, Martins CC (2021) A systematic evaluation of polycyclic aromatic hydrocarbons in South Atlantic subtropical mangrove wetlands under a coastal zone development scenario. J Environ Manag 277:111421. https://doi.org/10.1016/j.jenvman.2020.111421
García-Seoane R, Fernández JA, Villares R, Aboal JR (2018) Use of macroalgae to biomonitor pollutants in coastal waters: optimization of the methodology. Ecol Ind. https://doi.org/10.1016/j.ecolind.2017.09.015
Goodman N (2011) Agilent QuEChERS Selection Guide Accuracy Starts Here. hvcse.vn/upload/tailieu/5990-8590en-agilent-quechers-selection-guide-8974.pdf
Honda M, Suzuki N (2020) Toxicities of polycyclic aromatic hydrocarbons for aquatic animals. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph17041363
Jörundsdóttir HÓ, Jensen S, Hylland K, Holth TF, Gunnlaugsdóttir H, Svavarsson J, Ólafsdóttir Á, El-Taliawy H, Rigét F, Strand J, Nyberg E, Bignert A, Hoydal KS, Halldórsson HP (2014) Pristine Arctic: background mapping of PAHs, PAH metabolites and inorganic trace elements in the North-Atlantic Arctic and sub-Arctic coastal environment. Sci Total Environ 493:719–728. https://doi.org/10.1016/j.scitotenv.2014.06.030
Jueterbock A, Tyberghein L, Verbruggen H, Coyer JA, Olsen JL, Hoarau G, Alexander Jueterbock C (2013) Climate change impact on seaweed meadow distribution in the North Atlantic rocky intertidal. Ecol Evol 3:1356–1373. https://doi.org/10.1002/ece3.541
Kahma TI, Norkko A, Rodil IF (2023) Macrofauna community dynamics and food webs in the canopy-forming macroalgae and the associated detrital subsidies. Estuaries Coasts 46:1345–1362. https://doi.org/10.1007/s12237-023-01196-9
Kang HJ, Lee SY, Kwon JH (2016) Physico-chemical properties and toxicity of alkylated polycyclic aromatic hydrocarbons. J Hazard Mater 312:200–207. https://doi.org/10.1016/j.jhazmat.2016.03.051
Kirso U, Irha N (1998) Role of algae in fate of carcinogenic polycyclic aromatic hydrocarbons in the aquatic environment, In: Ecotoxicology and Environmental Safety. Academic Press, pp 83–89. https://doi.org/10.1006/eesa.1998.1671
Knutzen J, Sortland B (1982) Polycyclic aromatic hydrocarbons (PAH) in some algae and invertebrates from moderately polluted parts of the coast of Norway. Water Res 16(4):421–428
Laender FD, Hammer J, Hendriks AJ, Soetaert K, Janssen CR (2011) Combining monitoring data and modeling identifies PAHs as emerging contaminants in the Arctic. Environ Sci Technol 45:9024–9029. https://doi.org/10.1021/ES202423F/SUPPL_FILE/ES202423F_SI_001.PDF
Lam MM, Bülow R, Engwall M, Giesy JP, Larsson M (2018) Methylated PACs are more potent than their parent compounds: a study of aryl hydrocarbon receptor–mediated activity, degradability, and mixture interactions in the H4IIE-luc assay. Environ Toxicol Chem 37:1409–1419. https://doi.org/10.1002/etc.4087
Lawal AT (2017) Polycyclic aromatic hydrocarbons. A review. Cogent Environ Sci. https://doi.org/10.1080/23311843.2017.1339841
Lourenço RA, Magalhães CA, Taniguchi S, Siqueira SGL, Jacobucci GB, Leite FPP, Bícego MC (2019) Evaluation of macroalgae and amphipods as bioindicators of petroleum hydrocarbons input into the marine environment. Mar Pollut Bull 145:564–568. https://doi.org/10.1016/j.marpolbul.2019.05.052
Maxin CR, Kögel-Knabner I (1995) Partitioning of polycyclic aromatic hydrocarbons (PAH) to water-soluble soil organic matter. Eur J Soil Sci 46:193–204. https://doi.org/10.1111/j.1365-2389.1995.tb01827.x
Pavoni B, Caliceti M, Sperni L, Sfriso A (2003) Organic micropollutants (PAHs, PCBs, pesticides) in seaweeds of the lagoon of Venice. Oceanol Acta 26:585–596. https://doi.org/10.1016/S0399-1784(03)00052-5
Pies C, Hoffmann B, Petrowsky J, Yang Y, Ternes TA, Hofmann T (2008) Characterization and source identification of polycyclic aromatic hydrocarbons (PAHs) in river bank soils. Chemosphere 72:1594–1601. https://doi.org/10.1016/j.chemosphere.2008.04.021
Polechońska L, Klink A (2023) Macrophytes as passive bioindicators of trace element pollution in the aquatic environment. Wiley Interdiscip Rev: Water. https://doi.org/10.1002/wat2.1630
Poulsen R, Gravert TKO, Tartara A, Bensen HK, Gunnarsen KC, Dicová K, Nielsen NJ, Christensen JH (2021) A case study of PAH contamination using blue mussels as a bioindicator in a small Greenlandic fishing harbor. Mar Pollut Bull 171. https://doi.org/10.1016/j.marpolbul.2021.112688
Rainbow PS (1995) Biomonitoring of heavy metal availability in the marine environment. Mar Pollut Bull 31:183–192. https://doi.org/10.1016/0025-326X(95)00116-5
Ryzhik I, Pugovkin D, Makarov M, Roleda MY, Basova L, Voskoboynikov G (2019) Tolerance of Fucus vesiculosus exposed to diesel water-accommodated fraction (WAF) and degradation of hydrocarbons by the associated bacteria. Environ Pollut 254:113072. https://doi.org/10.1016/J.ENVPOL.2019.113072
Słowik-borowiec M, Szpyrka E, Ksi P, Podbielska M (2022) Simultaneous determination of multi-class pesticide residues and PAHs in plant material and soil samples using the optimized QuEChERS method and tandem mass
Sorte S, Rodrigues V, Borrego C, Monteiro A (2020) Impact of harbour activities on local air quality: a review. Environ Pollut 257. https://doi.org/10.1016/J.ENVPOL.2019.113542
Sun Y, Miller CA, Wiese TE, Blake DA (2014) Methylated phenanthrenes are more potent than phenanthrene in a bioassay of human aryl hydrocarbon receptor (AhR) signaling. Environ Toxicol Chem 33:2363–2367. https://doi.org/10.1002/etc.2687
The Government of the Faroe Islands (2019) Economy of the Faroe Islands [WWW Document]. URL https://www.faroeislands.fo/economy-business/economy/. Accessed 11.22.22
Tobiszewski M, Namieśnik J (2012) PAH diagnostic ratios for the identification of pollution emission sources. Environ Pollut. https://doi.org/10.1016/j.envpol.2011.10.025
Van Metre PC, Mahler BJ (2010) Contribution of PAHs from coal–tar pavement sealcoat and other sources to 40 U.S. lakes. Sci Total Environ 409:334–344. https://doi.org/10.1016/J.SCITOTENV.2010.08.014
Wang Z, Fingas M (1995) Differentiation of the source of spilled oil and monitoring of the oil weathering process using gas chromatography-mass spectrometry. J Chromatogr A 712:321–343. https://doi.org/10.1016/0021-9673(95)00546-Y
Whitehouse BG (1984) The effects of temperature and salinity on the aqueous solubility of polynuclear aromatic hydrocarbons. Mar Chem 14:319–332. https://doi.org/10.1016/0304-4203(84)90028-8
Zokm GME, Ismail MM, Okbah MAE (2022) Seaweed as bioindicators of organic micropollutants polycyclic aromatic hydrocarbons (PAHs) and organochlorine pesticides (OCPs). Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-18634-z
Acknowledgements
The authors acknowledge the technical assistance and academic advice from Jette Petersen and Peter Christensen. We thank laboratory technician Erla Eliasson at University of the Faroe Islands for helping out with the logistics. The authors also thank the rest of the students in the Arctic Pollution research course for contributing to the experience.
Funding
Open access funding provided by Copenhagen University Our work was financially supported by grants including Professor Sigurd Torvborgs Grant and Danish Chemical Society. Ida Huusmann Knøfler received funding from Smediefonden to cover travel expenses. Sigurd Christiansen was supported by the Carlsberg Foundation (grant number CF20-0637).
Author information
Authors and Affiliations
Contributions
Ida Huusmann Knøfler: conceptualization, methodology, data curation and analysis, investigation, writing original draft, and funding acquisition. Kirstine Evald Andersson: conceptualization, methodology, data curation and analysis, investigation, writing original draft, and visualization. Richard Leonard Becker: conceptualization, methodology, validation, data curation and analysis, investigation, writing original draft, and visualization. Sigurd Christiansen: supervision and review and editing. Nikoline Juul Nielsen: project administration, conceptualization, supervision, review and editing, and funding acquisition. Jan H. Christensen: project administration, conceptualization, supervision, review and editing, and funding acquisition.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors agreed with the content, and all gave explicit consent to submit this scientific paper. The authors have not submitted the manuscript to a preprint server before submitting it to Environmental Science and Pollution Research.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Roland Peter Kallenborn
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Knøfler, I.H., Andersson, K.E., Becker, R.L. et al. Is Fucus a suitable biomonitoring organism for polycyclic aromatic hydrocarbon contamination? A study from the Faroe Islands. Environ Sci Pollut Res 31, 26699–26712 (2024). https://doi.org/10.1007/s11356-024-32658-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32658-7