Abstract
An effective way to reduce herbicide quantity is to use adjuvants in order to optimize the amount of herbicide and improve its control efficiency. In order to screen for efficient herbicide tank-mix adjuvants, improve the control of weeds in maize fields, reduce the amount of effective ingredients, and improve the adsorption and digestion behavior of herbicides in soil, this study evaluated the synergistic effects and soil behavior of four types of tank-mix adjuvants combined with herbicides. Different types of adjuvants can enhance herbicide production. Surface tension was significantly reduced by 13% after the pesticide solution was applied with AgroSpred™ Prime. The contact angle with the foliar surface was significantly reduced and solution wettability improved using Atp Lus 245-LQ-(TH). The permeability of topramezone and atrazine in leaves of Amaranthus retroflexus L. and Digitaria sanguinalis (L.) Scop. was increased by 22–96% after adding either tank-mix adjuvant. The solution drying time and maximum retention on leaves were not affected by the tank-mix adjuvants. Ethyl and methylated vegetable oils can reduce the adsorption of topramezone in the soil, thus reducing its half-life in soil. The tank-mix adjuvants had no significant effect on soil dissipation or adsorption of atrazine. AgroSpred™ Prime and Atp Lus 245-LQ-(TH) have the best synergistic effect on topramezone and atrazine in the control of A. retroflexus L. and D. sanguinalis (L.) Scop. in maize fields.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pesticide application remains the main means to control diseases, pests, and weeds in crop fields. The use of pesticides includes root irrigation, leaf spraying, and foam application on seeds. The wettability and retention period of pesticide solutions on leaf surfaces may be poor. This limits the effectiveness of the pesticides, necessitating their increased use, resulting in the risk of pesticide residues in the environment and adverse effects on human health (Gao et al. 2020; Li et al. 2019). Recent attempts to improve pesticide utilization rates have mainly focused on enhancing pesticide efficacy by adding appropriate adjuvants during pesticide formulation or application. Several studies have confirmed that different types of additives can achieve the purpose of increase drug efficacy in different ways. For example, Zhang et al. studied the effect of methylated seed oil adjuvant topramezone on the bioactivity of Setaria faberi Herrm and Abutilon theophrasti Medic., solution properties, droplet expansion and volatilization, deposition of active components, absorption, and leaf movement (Zhang et al. 2013). Methylated seed oil significantly improved the efficacy of topramezone by 68.9% and promoted absorption efficiency by 45.9% in leaves of A. medic. Zandonadi et al. mixed a foliar fertilizer of manganese sulfate and manganese nitrate with an insecticide (chlorfenapyr) (Zandonadi et al. 2018). The addition of a silicone adjuvant improved the permeation rate by 20% for a blend of chlorfenapyr and manganese sulfate and by 35% for a blend of fipronil and manganese nitrate. Mohassel et al. described that the addition of adjuvants (frigate, citogate, and adigor) to diclofop-methyl and fenoxaprop-p-ethyl reduced the surface tension of the insecticide solution, significantly improving the control efficacy against Avena fatua L. (Mohassel et al. 2011).
After pesticides have entered the environment, they exhibit a series of different environmental behaviors. More attention has been paid to enhancing the mechanism of pesticide activity after mixing with adjuvants, but less research has addressed the environmental behavior of pesticide solutions that have been prepared using adjuvants. Soil and sediment sorption are considered important preludes to other environmental behaviors of atrazine, which control the fate of atrazine in ecosystems (Qu et al. 2017). Dissipation is another key process controlling the environmental transport and fate of atrazine. Swarcewicz et al. reported the decreasing dissipation rate of atrazine in soils in the presence of increasing concentrations of Atpolan 80 emulsifiable concentrate adjuvant (Swarcewicz et al. 2007). These findings were attributed to the increased adsorption of atrazine and the resulting decrease in its availability for dissipation in soils with higher organic matter content. Kucharski showed that the addition of a surfactant as an adjuvant did not affect the residual level of metamitron in soils, while the use of an oil adjuvant resulted in a significant increase in the persistence of the herbicide in soils under field conditions (Kucharski 2009). Therefore, whether different types of additives have different effects on the behavior of the pesticides in the environment requires further investigation.
Atrazine is currently one of the most commonly used herbicides to control a variety of broadleaf and some grass weeds in maize fields (Cui et al. 2021). The long-term and large-scale use of atrazine has accelerated the emergence of atrazine-resistant weed biotypes, and atrazine produces metabolites in the soil that have a lasting impact on the ecological environment. Topramezone is a novel and highly selective phenylmethylpyrazolone herbicide that was developed by BASF. Topramezone has an excellent selectivity and broad-spectrum herbicidal activity (Escobar-Niño et al. 2019). The degradation ability of the topramezone is weak and it is not easy to photolyze; therefore, the stable chemical properties of the topramezone may have toxic effects on the subsequent crops. Long-term excessive use of pesticides leads to higher levels of pesticide residues in the environment, which are harmful to both the environment and human health (Scialli et al. 2014; Martins-Santos et al. 2018). Therefore, there is an urgent need to increase the efficacy of atrazine and topramezone, reduce their usage, and improve environmental and crop safety. Topramezone can be reabsorbed and transported by the crop root system, and atrazine causes groundwater pollution and phytotoxicity in subsequent crops. Therefore, it is also important to detect the adsorption and dissipation behaviors of synergist pesticides in soils (Chang et al. 2022).
The tank-mix adjuvant is a pesticide additive that can be directly added to the pesticide bucket before spraying, and can improve the physical and chemical properties of the solution after uniform mixing (Zhang et al. 2021). The types, functions, dosages, and usages of tank mix adjuvants are diverse, with strong flexibility and adaptability, and can be mixed with chemical and microbial pesticides. Tank-mix adjuvants can also improve the spray performance of the solution in many different ways, such as improving surface tension, increasing permeability, improving rain erosion resistance, preventing drift, promoting sedimentation, reducing the risk of phytotoxicity, and resisting photolysis. In recent years, tank-mix adjuvants have been developed rapidly in China and have been successfully applied for plant protection and the spraying of many crops over a large area. The effect of adjuvants on the herbicide efficacy varies depending on the type of adjuvant and herbicide used. Zhao in their study found that, by studying the surface tension and contact angle, PT might be the best surface-active agent for pesticide spraying; however, it did not always show the best effect in regard to practical applications. MO has the greatest effect on EPX absorption in rice shoots and CLR deposition on the plant surface (Zhao et al. 2022). Therefore, the reasonable selection of additives is of great significance in reducing the use of herbicides.
In recent years, due to the increase in herbicide dosage and area, problems such as serious herbicide damage, deterioration of farmland soil environment, and influence of pesticide residues on crop change have gradually emerged in the process of herbicide application. Therefore, agricultural production urgently needs to use the technical advantages of tank-mix adjuvants to reduce the occurrence of the above problems. We hope to screen suitable adjuvants that can improve field efficacy and reduce pesticide residues in the soil through field synergistic studies and soil behavior studies of different adjuvants added to herbicides.
Materials and methods
Adjuvants and materials
The maize cultivar tested was Zhengdan 958 (donor seeds). The soil used for testing was obtained from the West Campus of Hebei Agricultural University, Baoding City (loam, pH 5.94). The weeds used in the study were Digitaria sanguinalis (L.) Scop. and Amaranthus retroflexus L.
Topramezone pesticide (30%) was purchased from BASF China Co., Ltd. (Shanghai, China). Atrazine pesticide (38%) was purchased from Shandong Binnong Technology Co., Ltd. (Binzhou, China). Ethyl and methylated vegetable oils were provided by BASF China Co., Ltd. AgroSpred™ Prime adjuvant was provided by Momentive Trading Co. Ltd. (Shanghai, China). Atp Lus 245-LQ-(TH) adjuvant was provided by CRODA Chemicals Co., Ltd. (Shanghai, China). Maisi adjuvant was provided by Grand AgroChem Co., Ltd. (Beijing, China). Topramezone (98.41% purity) and atrazine (99.16% purity) standards were supplied by Dr. Ehrenstorfer GmbH (Augsburg, Germany). High-performance liquid chromatography (HPLC)–grade acetonitrile and methanol were purchased from Thermo Fisher Chemicals (Shanghai, China). HPLC-grade dichloromethane was purchased from Yongda Chemicals Co., Ltd. (Tianjin, China). Analytically pure formic acid was purchased from Thermo Fisher Scientific Co., Ltd. (China). Analytically pure hydrochloric acid was provided by Kermel Chemicals Co., Ltd. (Tianjin, China). Analytical-grade NaCl was provided by Guangfu Technology Co., Ltd. (Tianjin, China).
Sample pretreatment
By referring to previously described detection methods of Feng et al. and Wang et al., a method was developed to simultaneously analyze topramezone and atrazine pretreatment in the leaves of maize, D. sanguinalis (L.) Scop., and A. retroflexus L. Leaves were collected and put into a knife grinder (Feng et al. 2017; Wang et al. 2018). The mixture was ground into a powder on dry ice. One gram of the sample was accurately weighed (to 0.01 g) and placed in a 50-mL plastic centrifuge tube with a stopper. Five milliliters of 1 mol/L aqueous hydrochloric acid solution was added, followed by vortexing with 10 mL of acetonitrile for 5 min. NaCl (3 g) was added, vortexed for 1 min, and centrifuged at 4000 rpm for 5 min. The supernatant was filtered through a 0.22-μm polytetrafluoroethylene filter for subsequent analysis.
In another experiment, soil (2 g) was accurately weighed (to 0.01 g) and placed in a 50-mL plastic centrifuge tube with a stopper. Two milliliters of ultrapure water was added to the centrifuge tube and vortexed, followed by the addition of 10 mL of 5% formic acid in acetonitrile for extraction. The sample was vortexed for 5 min, NaCl (3 g) was added, and the tube was vortexed again for 5 min and centrifuged at 4000 rpm for 5 min. One milliliter of the supernatant was then collected, placed on a purification column (25 mg primary secondary amine sorbent + 150 mg anhydrous magnesium sulfate), vortexed for 30 s, and centrifuged at 8000 rpm for 5 min. The resulting supernatant (0.5 mL) was mixed with ultrapure water (0.5 mL) and vortexed. The supernatant was filtered through a 0.22-μm polytetrafluoroethylene filter for subsequent analysis.
Ultra-high-performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS)
Quantitative analyses of topramezone and atrazine were performed using UPLC. The apparatus (Shimadzu, Kyoto, Japan) was coupled to a triple-quadrupole mass spectrometer (AB 5500 Triple Quad; Agilent, San Diego, CA, USA) using an electrospray ionization source and multiple reaction modes. A Bonshell C18 (50 mm × 2.1 mm, 2.7 µm particle size) analytical column with a C18 security guard cartridge from Agela Technologies (Torrance, CA, USA) was employed for the chromatographic separation. The flow rate of the two herbicides was 0.3 mL/min and the column temperature was 40 ℃. The injection volume was 2 μL. The differences between the two herbicide methods were as follows: the mobile phases for atrazine were 0.1% aqueous formic acid solution (phase A) and acetonitrile (phase B). Gradient elution to atrazine and optimized as follows: 0.0–0.5 min from 90 to 60% A and held for 2 min, 2.5–3.0 min from 60 to 10% A, held for 2.5 min, and 5.5–6.0 min from 10 to 95% A and held for 1 min. The mobile phases used for topramezone were 0.1% formic acid aqueous solution (phase A) and methanol (phase B). Gradient elution of topramezone was optimized as follows: 0.0–1.0 min from 95 to 30% A and held for 1 min, 2.0–2.5 min from 30 to 10% A and held for 0.5 min, and 3.1–5.0 min of 90% A.
The MS conditions for atrazine detection were a dry gas temperature of 300 ℃, a curtain air pressure of 20 psi, a collision air pressure of 7 psi, an atomized air pressure of 50 psi, and an auxiliary air pressure of 50 psi. The electrospray voltage was 5500 V in the multiple-ion reaction monitoring mode. The ionization mode was positive. The MS conditions of the topramezone were a dry gas temperature of 550 ℃, a negative ionization mode, with the other conditions the same as those for atrazine. Mass spectral parameters of atrazine and topramezone are listed in Supplementary Table S1.
Effect of different adjuvants on the surface tension of topramezone and atrazine
Pesticide suspensions containing 30% topramezone and 38% atrazine were mixed with distilled water at a dosage of33.3 mg/L and 886.7 mg/L, respectively. The adjuvant solutions were prepared to the recommended dosages (all v/v) of 0.3% for ethyl and methylated vegetable oils 0.03% for AgroSpred™ Prime, 0.1% for Atp Lus 245-LQ-(TH), and 0.2% for Maisi. The control group did not include adjuvants. Twenty milliliters of each preparation was used. Surface tension was determined by the platinum plate method (GB/T 22237–2008) in an automatic tensiometer (JK99B; Powereach, Shanghai, China). Determinations were repeated three times for each treatment. Distilled water was used for calibration (approximately 71 mN/m, 28 ± 1 °C) before measuring each solution.
Effect of different adjuvants on contact angles of topramezone and atrazine
The contact angles of topramezone and atrazine in the absence and presence of each adjuvant were determined using a JC2000DM measuring instrument (Powereach). In a closed environment (temperature 28 ± 1 ℃), 1 µL of each solution was dispensed onto the surface of the paraffin slide with a microinjector. The contact angle was measured within 5 s after the sample was added and was repeated three times for each treatment (Zhang et al. 2015).
Effect of different adjuvants on drying times of topramezone and atrazine
The drying time was tested by the drop-drying method (Lu et al. 2004). In a calm environment (temperature 25 ℃ ± 1 ℃, relative humidity 40%), a micro-sampler was used to collect 1 μL of each treatment solution and drop it onto the surface of a paraffin slide. The time for complete drying of the solution was recorded. Each treatment was repeated three times.
Effect of different adjuvants on maximum retention of topramezone and atrazine
An appropriate amount of intact and uniformly sized maize seeds maize, D. sanguinalis (L.) Scop., and A. retroflexus L. seeds were collected in Petri dishes. The seeds were covered with filter paper and moistened with distilled water to promote germination. Nutrient soil, vermiculite, and plant ash (2:2:1) were thoroughly mixed, moistened, and placed in a bowl. Germinated seeds were planted in pots and sprayed with a nutrient solution. The samples were then allowed to grow in a greenhouse at a temperature of 27 ± 5 ℃ and a humidity of 70 ± 5%.
A previously described immersion method was adopted (Teng et al. 2011). Equal-sized portions of D. sanguinalis (L.) Scop. and A. retroflexus L. leaves (2 cm × 2 cm) without major veins were cut and weighed using a balance with a precision of 1/1000. Leaves were removed using forceps and completely immersed in a beaker containing the solution for 5 s. The leaves were then quickly lifted to the surface and suspended vertically for 15 s. The droplets on the leaf were weighed after they no longer flowed.
Effects of different adjuvants on the permeability of topramezone and atrazine
Plastic flower pot was two-thirds filled with sandy loam. D. sanguinalis (L.) Scop. and A. retroflexus L. seeds were set flat on the soil, covered with 3 cm of soil, and watered for later use. In the seedling tray, the maize seeds were sterilized, germinated at 25 ℃ in the dark, and then transferred to a plastic flowerpot for later use.
In the pesticide spray experiment, pesticides were prepared as described above. Each solution was sprayed using a model 3WP-2000 walking spray tower at the 3rd and 4th leaf stages of maize, and the 2nd and 3rd leaf stages of D. sanguinalis (L.) Scop. and A. retroflexus L. There were five treatments for each plant type, with 10 pots per treatment. The same amount of water was used in the control group. When the pesticide is applied, the fog surface of the fan-shaped sprinkler head is perpendicular to the walking direction. The shelf height was adjusted according to the height of the plant. The stems and leaves in each pot were placed within an effective spray range.
Plant leaf samples were collected randomly and evenly 24 h after pesticide application. Approximately 100 g was sampled at each time point. The sample leaves were rinsed with water, and excess moisture on the leaf surface was absorbed through filter paper and placed in a plastic bag. The samples were stored at – 20 ℃.
Effects of different adjuvants on the adsorption of topramezone and atrazine in soil
The soil sample (2.0 g) was placed in a 50-mL plastic centrifuge tube with a stopper, and 10 mL of the topramezone and atrazine 0.1 mol/L CaCl2 standard solution with a concentration of 5.0 mg/L was added. The water-to-soil ratio was 5:1 (w/w). The centrifuge tube was placed in a shaker at a constant temperature (25 ± 1 ℃) and shaken horizontally for 1, 5, 10, 24, and 48 h. The centrifuge tube was then centrifuged at 4000 rpm for 10 min. The sample supernatant (2.0 mL) was collected and filtered through a 0.22-μm polytetrafluoroethylene filter for UPLC-MS/MS analysis.
The soil sample (2.0 g) was placed in a 50-mL plastic centrifuge tube with a stopper, and then 10 mL of (30% topramezone suspension and 38% atrazine suspension) 0.1 mol/L CaCl2 solution with an initial concentration of C0 (0.5, 1, 2, 3, 4, and 5 mg/L) was added. As recommended, the dosages used (all % v/v) were 0.3% for ethyl and methylated vegetable oils, 0.03% for AgroSpred™ Prime, 0.1% for Atp Lus 245-LQ-(TH), and 0.2% for Maisi. The groups with only ultrapure water or the solution without soil were set as the control groups. Each centrifuge tube was placed in a shaker at a constant temperature (25 ± 1 ℃), shaken for 24 h, removed, and centrifuged at 4000 rpm for 10 min. Aliquots (2.0 mL) of sample supernatant were collected and filtered through a 0.22-μm polytetrafluoroethylene filter for UPLC-MS/MS analysis.
Effects of different adjuvants on the dissipation of topramezone and atrazine in soil
The aforementioned concentrations of atrazine and topramezone and the adjuvant were used. The group without any adjuvants and the group with ultrapure water were used as the control groups. The soil samples were collected, dried, and weighed (50 g each), and the solution was added to adjust the soil water content to 60% of the saturated water content. Each treatment was conducted in triplicate. Samples were taken at 2 h, 3 days, 7 days, 14 days, 30 days, 60 days, and 90 days, and the contents of the topramezone and atrazine were detected by QuEChERS (Miguel et al. 2022) pretreatment.
Data analysis
The maximum retention of the solution is calculated as follows:
\({W}_{1}\)—initial blade weight (mg);
\({W}_{0}\)—blade weight when no liquid drops flow out (mg);
\(S\)—leaf surface area (cm2).
The effect of adjuvants on the permeability of pesticides was calculated by detecting the residual amount of pesticides added to different adjuvants in plant leaves after application. The permeability of topramezone and atrazine on the leaves of maize, Macedon, and Amaranthus was calculated as follows:
\({R}_{i}\)—pesticide residue in the leaves after rinsing (mg/kg);
\({R}_{t}\)—pesticide residue in leaves without rinsing (mg/kg).
Excel software was used to analyze and sort out the dissipation data of topramezone in soil, the residual amount of topramezone in Baoding soil was changed and mapped using Origin software, and the correlation between physical and chemical properties and adsorption was analyzed. According to the first-order kinetic equation, the fitting data formulas are
\({C}_{t}\)—residual pesticide concentration during period t (mg/kg);
\({C}_{0}\)— original pesticide deposition (mg/kg);
\({T}_{1/2}\)—dissipation half-life;
\(k\)—dissipation coefficient.
Microsoft Excel was used in order to process the synergic index data, SPSS was used to calculate the 95% confidence intervals, and GraphPad Prism was used to draw the graphs.
Results and discussion
Determination of residue analysis methods for topramezone and atrazine in the five matrices
The standard spectra of the two herbicides are shown in Fig. S8; the peak shape of the chromatogram is sharp and accurate, which proves the feasibility of the method. In the leaves of maize, D. sanguinalis (L.) Scop., and A. retroflexus L., the recovery experiments of topramezone and atrazine at three levels were performed. The concentrations used were 0.01, 0.1, and 1 mg/kg, with five replicates per added concentration. The two compounds in the three matrices were between 74 and 107%, with relative standard deviation (RSD) ranging from 0.8 to 17.8% (Supplementary Table S2). In the soil, recovery experiments of topramezone, atrazine, and their metabolites were performed using concentrations of 0.02, 0.05, and 0.2 mg/kg, with five replicates for each concentration. The compounds in the soil and 0.1 mol/L CaCl2 solution were between 75 and 119%, with RSD ranging from 1.0 to 8.7% (Supplementary Table S2). The experimental results showed good accuracy and precision, meeting the requirements for pesticide residue detection in China. The linearity of topramezone, atrazine, and their metabolites ranging from 0.005–0.2 mg/L was determined. Satisfactory linearity was achieved with a correlation coefficient (R2) > 0.99 (Supplementary Table S3).
Effect of different adjuvants on the surface tension of topramezone and atrazine solutions
As shown in Fig. 1, the surface tension of the solutions was 35.1 mN/M when no adjuvant was added. When an aqueous solution of each adjuvant was added, the surface tension was reduced to 30.1, 22.4, 30.1, and 30.3 mN/M for ethyl and methylated vegetable oils, AgroSpred™ Prime, Atp Lus 245-LQ-(TH), and Maisi, respectively. The smaller the surface tension, the smaller the shrinkage force. Thus, spreading a solution on the surface is easier, as is wetting the solid interface (Loglio et al. 2006). The surface tension of the solution can be reduced by adding approximately 36% AgroSpred™ Prime adjuvant. Followed by ethyl and methylated vegetable oils and Atp Lus 245-LQ-(TH), the lowest surface tension reduction was seen with the Maisi adjuvant agent. The lower the surface tension, the better the wettability, which is conducive to the role of pesticides. Guo used a silicone spray to improve the interface performance of pesticides and demonstrated a significantly improved control effect on scallion rust (Guo 2011).
Effect of different adjuvants on the contact angle of topramezone and atrazine
During the spraying process, a solution forms a certain contact angle (θ) with the leaf surface in the passage from the nozzle of the spray device to the leaf surface. The deposition state of a solution on the target plant leaf surface can be analyzed by the size of the contact angle, which indicates the solution’s wettability and distribution. The contact angle is a quantitative measure of surface wettability and is expressed as the angle between the liquid or vapor interface and the solid surface. Zhang found that spray adjuvants enhance the efficacy of mesotrione by reducing its contact angle on weed leaves and increasing its maximum holding capacity on target plants (Zhang et al. 2015). Meng found that the type and concentration of tank-mix adjuvants would affect the contact Angle (Meng et al. 2023). In our experiment, the contact angle of topramezone and atrazine solutions without any adjuvants was 80.5°. As shown in Fig. 2, after the addition of four kinds of spray adjuvants, the contact angle of the solution decreased significantly. Atp Lus 245-LQ-(TH) most profoundly affected the contact angle (θ = 52.0°), which represented a 28.5% decrease, followed by Maisi adjuvant (θ = 65.8°, 14.7% decrease). Ethyl and methylated vegetable oils (θ = 75.3°) and AgroSpred™ Prime (θ = 75.5°) had no significant effect on the contact angle, with a contact angle decrease of only 5.0–5.2% compared with the adjuvant-free control. According to the grading method of Gaskin et al., the wettability of the solution is defined as follows: θ < 60° indicates good wettability; 60° ≤ θ < 80° indicates medium wettability; 80° ≤ θ < 100° indicates poor wettability; and θ ≥ 100° indicates very poor wettability (Gaskin et al. 2005). The wettability of topramezone and atrazine solutions without adjuvants was poor. After adding ethyl and methylated vegetable oils, AgroSpred™ Prime, and Maisi adjuvants, the wettability was medium. After adding Atp Lus 245-LQ-(TH) adjuvant, the wettability was good.
Effect of different adjuvants on the drying time of topramezone and atrazine solutions
The drying time was reduced and the solution loss caused by natural factors that include wind speed and leaf jitter was reduced. The drying time of topramezone and atrazine solutions without adjuvants was 13.3 min (Fig. 3). After the addition of ethyl and methylated vegetable oils, the drying time was not significantly reduced (12.5 min). The drying times following the addition of the AgroSpred™ Prime, Atp Lus 245-LQ-(TH), and Maisi adjuvant were 11.2, 10.6, and 11.3 min, respectively. The addition of Atp Lus 245-LQ-(TH) had the clearest effect on drying time, which was 15% shorter than that seen without adjuvants, followed by AgroSpred™ Prime and Maisi adjuvants, which shortened the drying time by 10%. The reduction in the drying time of the pesticide solution was due to the presence of adjuvants, which reduce the surface tension of the pesticide solution, increase the surface area of the pesticide drops, and shorten the drying time (Lu et al. 2004).
Effect of different adjuvants on maximum retention of topramezone and atrazine
Plant leaves have a limited retention capacity for externally applied fluid. Excess fluids, such as topramezone and atrazine solutions, would not be retained. In the present study, when no adjuvants were added, the retention of the solution was 14.08 mg/cm2 in A. retroflexus L. leaves and 6.33 mg/cm2 in D. sanguinalis (L.) Scop. leaves (Fig. 4). For A. retroflexus L., the maximum retention of pesticides when ethyl and methylated vegetable oils, AgroSpred™ Prime, Atp Lus 245-LQ-(TH), and Maisi adjuvants were present decreased to 9.35, 12.55, 10.09, and 11.13 mg/cm2, respectively. The reduction was greatest for ethyl and methylated vegetable oils. The addition of the adjuvant reduces the retention of solution on the leaves, perhaps due to the addition of excess adjuvant, leading to a low surface tension that affects the maximum retention of solution on the leaves. Therefore, the amounts of different adjuvants should be controlled during use (Cui et al. 2021). For D. sanguinalis (L.) Scop., the maximum retention of pesticides when ethyl and methylated vegetable oils, AgroSpred™ Prime, Atp Lus 245-LQ-(TH), and Maisi adjuvants were added decreased to 5.51, 8.04, 7.38, and 6.91 mg/cm2, respectively. Through the significant difference analysis of the 95% level, we found that all four of the adjuvants could not have a synergistic effect on the two herbicides at the maximum retention, and even reduced the maximum retention of herbicides in A. retroflexus L. leaves. Regarding the differences in the influence of different additives, Li et al. demonstrated differences in the optimal amounts of different types of additives (Li et al. 2019). Within a certain range of each added compound, the maximum retention of liquid on the leaves increased as more compounds were added. Beyond this range, the maximum retention decreased with an increased addition of each compound. This affected the efficacy of the herbicides.
Effect of different adjuvants on the permeability of topramezone and atrazine
The cutin membrane on plant surfaces, especially the leaf surface, affects the reduction of water in the plant body and the absorption of foliar fertilizer and pesticides by the plant (Li et al. 2017). The cutin membrane is primarily composed of waxy, cutin, and keratinized layers (Xiang et al. 2005). The waxy layer at the outermost layer of the cuticle forms a hydrophobic and low-energy surface, which is not conducive to the wetting and permeation of liquid on the plant surface (Schreiber 1995). This affects the absorption of foreign substances by plants and prevents the invasion of harmful substances from the environment. The main factors that affect the permeability of keratin membranes include temperature, humidity, individual differences, and adjuvant use. Herbicides can enter plants through the leaf epidermis or stomata and, in most cases, diffuse through the cuticle.
When A. retroflexus L. was treated, there was almost no penetration of topramezone in the leaves of the control group without pesticide application (Fig. 5a). When AgroSpred™ Prime and Atp Lus 245-LQ-(TH) adjuvants were used, the permeabilities were increased to 22% and 95%, respectively. The permeability of atrazine in the leaves of the control group was 59%. The addition of tank-mix adjuvants improved the permeability to different degrees. AgroSpred™ Prime had the greatest influence on permeability (96%), followed by Atp Lus 245-LQ-(TH) adjuvant with a permeability of 90%. The findings suggest that when A. retroflexus L. is treated in a maize field, AgroSpred™ Prime or Atp Lus 245-LQ-(TH) adjuvant should be added to the 30% topramezone and 38% atrazine pesticide solutions.
When D. sanguinalis (L.) Scop. was treated, the permeability of topramezone in the leaves of the control group was 41%. Treatment with AgroSpred™ Prime and Maisi adjuvant significantly improved the permeability to 78% and 87%, respectively (Fig. 5b). The atrazine permeability of the control group was 64%. The Atp Lus 245-LQ-(TH) and Maisi adjuvants significantly improved permeability (74% and 86%, respectively). The findings indicate that when D. sanguinalis (L.) Scop. is treated in a maize field, Maisi adjuvant should be added to 30% topramezone and 38% atrazine pesticide solutions.
Topramezone did not permeate the maize leaves treated with different adjuvants, whereas atrazine permeated to varying degrees (Fig. 5c). This may reflect the different physical and chemical properties of topramezone and atrazine. The octanol–water partition coefficient of topramezone of log P = − 1.52 indicates that it would be difficult for this herbicide to pass through the stratum cuticle. It would be easily washed away by the water. This phenomenon can also be seen in Fig. 5a and b. When the same adjuvant was added to the solution, the permeability of topramezone in the leaves of A. retroflexus L. and D. sanguinalis (L.) Scop. was lower than that of atrazine. For atrazine, permeability in the control group was 51%. Both the Atp Lus 245-LQ-(TH) and Maisi adjuvants improved the permeability of the solution, with the permeability reaching 72% and 71%, respectively. The findings indicate that when a 38% solution of atrazine is used in maize fields, Atp Lus 245- LQ-(TH) and Maisi adjuvants can be included.
Effect of different adjuvants on the adsorption behavior of topramezone and atrazine in soil
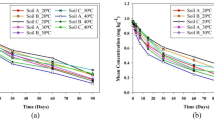
In Fig. S9, it can be seen that the adsorption of pesticides in the soil is a dynamic equilibrium process. In this process, both herbicides showed a trend of fast adsorption first, followed by a slow adsorption, and finally tended to be balanced. They were quickly adsorbed within 0–1 h, and there was no significant difference in the adsorption capacity between 24 and 48 h; therefore, the two herbicides reached adsorption equilibrium within 24 h.
A statistical histogram of the data for the adsorption residues of topramezone with tank-mix adjuvants and topramezone without adjuvants in the soil is shown in Fig. 6. When topramezone was mixed with the ethyl and methyl vegetable oils, it was not adsorbed by the soil and decreased significantly compared to topramezone alone. When topramezone was individually mixed with AgroSpred™ Prime, Atp Lus 245-LQ-(TH), and Maisi adjuvants, there was no obvious trend in the adsorption improvement of topramezone in soil, with significant differences in the adsorption capacity of the same adjuvant at different concentrations of topramezone. Generally, the adjuvants used in this study reduced the soil adsorption capacity of pesticides, thereby improving the utilization of pesticides by microorganisms and accelerating the dissipation of pesticides. A statistical histogram of the data for the adsorption residues of atrazine with and without tank-mix adjuvants in the soil is shown in Fig. 7. When atrazine was used with additives, the direct difference between the treatment and control groups was not significant. The tank-mix adjuvants had no significant adsorption effects on atrazine.
In summary, only ethyl and methylated vegetable oils significantly reduced the adsorption of topramezone in soil. There was a significant difference in the adsorption effects of the two herbicides on soil using the tank-mix adjuvants. These differences may have been affected by the nature of the herbicides themselves. Huggenberger et al. demonstrated that two nonionic surfactants can reduce the adsorption of lindane and diuron, but do not affect the adsorption of atrazine (Huggenberger et al. 1973). The concentration of adjuvants may also affect the fluidity of pesticides (Sanchez-Camazano et al. 1995). The present study used the recommended dosages of adjuvants.
Effects of different adjuvants on the dissipation of topramezone and atrazine in soil
The dissipation kinetic parameters of topramezone and atrazine are listed in Supplementary Table S4. When topramezone was used alone, its half-life in soil was 51.3 days. The half-life in the presence of ethyl and methylated vegetable oils, AgroSpred™* Prime, Atp Lus 245-LQ-(TH), and Maisi adjuvant was 40.8, 45.0, 47.2, and 48.5 days, respectively, representing a respective improvement in the dissipation rate of 20.5, 12.3, 8.0, and 5.5%. Many studies have confirmed that additives promote the dissipation of pesticides in soil. For example, Jing et al. reported that barrel-mixed methylated vegetable oil adjuvant can improve the dissipation of chlorantraniliprole and difenoconazole in soil (Jing et al. 2023).
When atrazine was used alone, its half-life in soil was 8.5 days. The half-life in the presence of ethyl and methylated vegetable oils, AgroSpred™ Prime, Atp Lus 245-LQ-(TH), and Maisi adjuvant was 8.5, 8.1, 8.1, and 8.5 days, respectively. Thus, the addition of adjuvants had little effect on the dissipation of atrazine in the soil. The dissipation of pesticides in soil is affected by many factors. The properties of pesticides themselves may have a more significant impact. For example, Swarcewicz et al. demonstrated no difference between 0.25 and 0.75% in the dissipation effect of atrazine in sandy loam and muddy soil by Atpolan 80 EC treatment (Swarcewicz et al. 2007). In this study, atrazine had a short half-life. Other recent studies have also found that atrazine has a short half-life. For example, Souza et al. described the half-life of atrazine as 4–11 days in mineralized soil (Souza et al. 2022). Zhang et al. reported an atrazine half-life of 9.9 days in phaeozem (Zhang et al. 2020). Finally, other authors reported an atrazine half-life of 6.5–12.9 days in soil from Shandong, China (Fang L. et al. 2012). Currently, there are six metabolites of atrazine (Wang et al. 2022). These include desethylatrazine (DEA), desisopropylatrazine (DIA), diaminochlorotriazine (DEDIA), hydroxyatrazine (HA), deethylhydroxyatrazine (DEHA), and deisopropylhydroxylatrazine (DIHA). These metabolites were detected during the experiments. Three metabolites, DEA, DIA, and HA, were detected. However, their concentrations were low, indicating that they contained this species, and their practical significance was not significant.
Conclusions
In this study, a method was established in order to detect topramezone and atrazine residues in the maize leaves of D. sanguinalis (L.) Scop. and A. retroflexus L and soil. The recovery rate and linear relationship of this method met the requirements for residue detection. The reduction effects of four additives (ethyl and methylated vegetable oil, AgroSpred* Prime, Atp Lus 245-LQ-(TH), and Mais) added to 30% topramezone and 38% atrazine solutions were also studied. The effects of different adjuvants on the surface tension, contact angle, drying time, maximum retention, and permeability of the liquid are discussed. Furthermore, the adsorption and dissipation behaviors of these herbicides in the soil were studied. AgroSpred™ Prime adjuvant significantly reduced the surface tension of the solutions and improved the permeability of the solutions on the surfaces of A. retroflexus L. leaves. Atp Lus 245-LQ-(TH) adjuvant reduced the contact angle between the herbicide solutions and the interface and improved both the wettability and permeability of the solutions on the surfaces of A. retroflexus L. leaves. The application of Maisi adjuvant increased the permeability of the two herbicide solutions on the surfaces of D. sanguinalis (L.) Scop. leaves. However, when using Atp Lus 245-LQ-(TH) and Maisi adjuvants, it is necessary to avoid applying the solution to the maize plant; otherwise, it will increase the permeability of the liquid on the maize leaf surface. In terms of environmental behavior, the addition of ethyl and methylated vegetable oils reduced the adsorption of topramezone by the soil and accelerated its dissipation.
The collective findings support the use of AgroSpred™ Prime and Atp Lus 245-LQ-(TH) adjuvants for the control of A. retroflexus L. in maize fields. Maisi adjuvant is recommended for the control of D. sanguinalis (L.) Scop. in maize fields. Ethyl and methylated vegetable oils can improve the environmental behavior of topramezone in soil. This research can help in the field of herbicides and tank-mix adjuvant use.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Chang JN, Fang W, Chen L, Zhang PY, Zhang GM, Zhang HB, Liang JS, Wang QY, Ma WF (2022) Toxicological effects, environmental behaviors and remediation technologies of herbicide atrazine in soil and sediment: a comprehensive review. Chemosphere 307:136006. https://doi.org/10.1016/j.chemosphere.2022.136006
Cui K, He L, Zhao Y, Mu W, Lin J, Liu F (2021) Comparative analysis of botrytis cinerea in response to the microbial secondary metabolite benzothiazole using itraq-based quantitative proteomics. Phytopathology 111:1313–1326. https://doi.org/10.1094/PHYTO-11-20-0503-R
Escobar-Niño A, Liñeiro E, Amil F, Carrasco R, Chiva C, Fuentes C, Blanco-Ulate B, Fernández JMC, Eduard S, Fernández-Acero FJ (2019) Proteomic study of the membrane components of signalling cascades of botrytis cinerea controlled by phosphorylation. Sci Rep 9:9860. https://doi.org/10.1038/s41598-019-46270-0
Fang L, Li H, Ding R, F. W, (2012) Evaluation of residues and safe use of atrazine in corn and soil. Adv Pestic 11:33–36. https://doi.org/10.3969/j.issn.1671-5284.2012.05.010
Feng Y, Jin J, Pan J, Qi X (2017) Residues and decline dynamics of topramezone in corn and soil. Agrochemicals 56:664–667. https://doi.org/10.16820/j.cnki.1006-0413.2017.09.013
Gao Y, Lu J, Zhang P, Shi G, Li Y, Zhao J, Liu Z, Yang J, Du F,Fan R (2020) Wetting and adhesion behavior on apple tree leaf surface by adding different surfactants. Colloids Surf B 187. https://doi.org/10.1016/j.colsurfb.2019.110602
Gaskin RE, Steele KD, Forster WA (2005) Characterising plant surfaces for spray adhesion and retention. N.Z Plant Prot 58:179–183
Guo SM (2011) Study on the synergistic effect of agricultural organosilicon sprays auxiliaries on prevention and control of scallion rust disease. Mod Agric Sci Technol 15:177–181. https://doi.org/10.3969/j.issn.1007-5739.2011.15.109
Huggenberger F, Letey J, Farmer WJ (1973) Effect of two nonionic surfactants on adsorption and mobility of selected pesticides in a soil-system. Soil Sci Soc Am J 37:215–219. https://doi.org/10.2136/sssaj1973.03615995003700020018x
Jing J, Zhou Y, Zhang ZY, Wu LX, Zhang HY (2023) Effect of tank-mixed adjuvant on the behavior of chlorantraniliprole and difenoconazole in soil. Heliyon 9:e12658. https://doi.org/10.1016/j.heliyon.2022.e12658
Kucharski M (2009) Changes in application system - influence on herbicides residue in soil and sugar beet roots. J Plant Prot Res 49:421–425. https://doi.org/10.2478/v10045-009-0067-4
Li J, Yang Q, Pan Q, Lu F (2017) Effect of pesticide adjuvants and pesticide reduction on prevention and control of rice and wheat diseases and pests. C.A Agricultural Technology Extension 33:54–56. https://doi.org/CNKI:SUN:ZGNT.0.2017-07-024
Li JJ, Zhang HY, Wang BR, Wang LC, Tao B (2019) Synergistic effect of different adjuvants on atrazine in maize field. J Maize Sci 27:167–174. https://doi.org/10.13597/j.cnki.maize.science.20190626
Loglio G, Noskov B, Pandolfini P, Miller R (2006) Static and dynamic surface tension of marine water: onshore or platform-based measurements by the oscillating bubble tensiometer. Mar Surf Films 93–103. https://doi.org/10.1007/3-540-33271-5_10
Lu M, Wang J, Wang Y, Liu W (2004) The effect of herbicide adjuvants on the physical characteristics and the bioactivity of sulcotrione. Chin J Pestic Sci 6:78–82. https://doi.org/10.3321/j.issn:1008-7303.2004.04.015
Martins-Santos E, Pimenta CG, Campos PRN, Oliveira AG, Mahecha GAB, Oliveira CA (2018) Atrazine affects the morphophysiology, tissue homeostasis and aromatase expression in the efferent ductules of adult rats with mild alterations in the ventral prostate. Chemosphere 193:958–967. https://doi.org/10.1016/j.chemosphere.2017.11.124
Meng YH, Wu QF, Zhou HX, Hu HY (2023) How tank-mix adjuvant type and concentration influence the contact angle on wheat leaf surface. Peer J 11:e16464. https://doi.org/10.7717/peerj.16464
Miguel ÁGC, Diana AVM, Diego ARH (2022) Pesticide-residue analysis in soils by the quechers method: a review. Molecules 27:4323. https://doi.org/10.3390/molecules27134323
Mohassel MHR, Aliverdi A, Rahimi S (2011) Optimizing dosage of sethoxydim and fenoxaprop-p-ethyl with adjuvants to control wild oat. Ind Crops Prod 34:1583–1587. https://doi.org/10.1016/j.indcrop.2011.05.023
Qu MJ, Li HD, Li N, Liu GL, Zhao JW, Hua YM, Zhu DW (2017) Distribution of atrazine and its phytoremediation by submerged macrophytes in lake sediments. Chemosphere 168:1515–1522. https://doi.org/10.1016/j.chemosphere.2016.11.164
Sanchez-Camazano M, Arienzo M, Sanchez-Martin MJ, Crisanto T (1995) Effect of different surfactants on the mobility of selected non-ionic pesticides in soil. Chemosphere 31:3793–3801. https://doi.org/10.1016/0045-6535(95)00253-5
Schreiber L (1995) A mechanistic approach towards surfactant/wax interactions - effects of octaethyleneglycolmonododecylether on sorption and diffusion of organic-chemicals in reconstituted cuticular wax of barley leaves. Pestic Sci 45:1–11. https://doi.org/10.1002/ps.2780450102
Scialli AR, DeSesso JM, Breckenridge CB (2014) Developmental toxicity studies with atrazine and its major metabolites in rats and rabbits. Birth Defects Res. Part B 101:199–214. https://doi.org/10.1002/bdrb.21099
Souza A J d, Pereira A P d A, Pedrinho A, Andreote FD, Tornisielo VL, Tizioto PC, Coutinho LL, Regitano JB (2022) Land use and roles of soil bacterial community in the dissipation of atrazine. Sci Total Environ 827. https://doi.org/10.1016/j.scitotenv.2022.154239
Swarcewicz M, Skórska E, Paździoch W (2007) The effect of atpolan 80 ec on atrazine residues in the soil. Pol J Chem Technol 9:5–8. https://doi.org/10.2478/v10026-007-0042-7
Teng CH, Zhang LB, Wang QY, Tao B (2011) Research on synergism of oraanosi|icone adjuvants on atrazine. J Northeast Agric Univ 42:71–75. https://doi.org/10.3969/j.issn.1005-9369.2011.01.013
Wang B, Hou Z, Liu L, Guo H, Liu S, Lu Z (2018) Residual analysis of nicosulfuron, atrazine, mcpa-isooctyl and its metabolite in corn and soil. Mod Agrochem 17:40–44. https://doi.org/10.3969/j.issn.1671-5284.2018.06.012
Wang K, Wei LB, Ren YS (2022) Simultaneous determination of nicosulfuron, atrazine and their metabolites in soybean plants and soil by dispersive solid-phase extraction coupled with ultra-high performance liquid chromatography-tandem mass spectrometry. Plant Prot 49:231–237. https://doi.org/10.16688/j.zwbh.2022566
Xiang J, Chen X, Zhou X (2005) Research progress in plant cuticle wax genes. Lett Biotechnol 16:224–227. https://doi.org/10.3969/j.issn.1009-0002.2005.02.034
Zandonadi CHS, Burkhardt J, Hunsche M, Cunha JPARd (2018) Tank-mix of chlorantraniliprole and manganese foliar fertilizers: impact on rheological characteristics, deposit properties and cuticular penetration. Crop Prot 106:50–57. https://doi.org/10.1016/j.cropro.2017.12.011
Zhang J, Lü HP, Cao LD, Liu YJ, Zhao P, Li FM, Huang QL (2015) Synergism of six spray adjuvants on me sotrione in controlling echinochloa crus-galli and am aranthus retroflexus. Chin J Pestic Sci 17:348–356. https://doi.org/10.3969/j.issn.1008-7303.2015.03.15
Zhang CH, Zhang ZJ, Yao DF, Liu KY (2021) Application of tank-mixing adjuvant for reducing herbicide application and increasing efficiency in maize field. J Maize Sci 29:115–121. https://doi.org/10.13597/j.cnki.maize.science.20210417
Zhang J, Jaeck O, Menegat A, Zhang Z, Gerhards R, Ni H (2013) The mechanism of methylated seed oil on enhancing biological efficacy of topramezone on weeds. PLoS One 8.https://doi.org/10.1371/journal.pone.0074280
Zhang JP, Xu YC, Liang S, Ma XL, Lu ZB, Sun P, Zhang H, Sun FJ (2020) Synergistic effect of klebsiella sp. Fh-1 and arthrobacter sp. Nj-1 on the growth of the microbiota in the black soil of Northeast China. Ecotoxicol Environ Saf 190. https://doi.org/10.1016/j.ecoenv.2019.110079
Zhao PY, Zheng L, Li YY, Wang CJ, Cao LD (2022) Tank-mix adjuvants regulate the deposition, absorption, and permeation behavior of pesticide solutions on rice plant. Agriculture 12:1119. https://doi.org/10.3390/agriculture12081119
Funding
This research was supported by the Natural Science Foundation of Hebei Province (grant number C2021204102) and Starting Scientific Research Foundation for the Introduced Talents of Hebei Agricultural University (grant numberYJ2020039).
Author information
Authors and Affiliations
Contributions
The experiments were conceived and planned by Xiaoxiao Feng, Jingao Dong, and Yingchao Liu; the manuscript was written by Kai An and Xiaoxiao Feng; experiments were performed by Kai An, Jiaxing Ji, Xinyue Wang, Minhao Pang, Tiantian Liu, Sijia Wang, and Huiru Shi. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
The author agrees to publication in the journal indicated below and also to publication of the article in English by Environmental Science and Pollution Research.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ester Heath
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
An, K., Feng, X., Ji, J. et al. Synergistic mechanism and environmental behavior of tank-mix adjuvants to topramezone and atrazine. Environ Sci Pollut Res 31, 20246–20257 (2024). https://doi.org/10.1007/s11356-024-32389-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32389-9