Abstract
The use of additives, especially colorants, in food and pharmaceutical industry is increasing dramatically. Currently, additives are classified as contaminants of emerging concern (CECs). Concerns have been raised about the potential hazards of food additives to reproductive organs and fertility. The present study investigates the reproductive toxicity of tartrazine (TRZ), a synthetic colorant, in male rats and aims to explore the curative effect of Ginkgo biloba extract (EGb) against TRZ-induced testicular toxicity. Twenty-four rats were divided into four groups: the control (0.5 ml distilled water), the EGb group (100 mg/kg EGb alone), the TRZ group (7.5 mg/kg TRZ alone), and the TRZ-EGb group (7.5 mg/kg TRZ plus 100 mg/kg EGb). The doses were administered orally in distilled water once daily for 28 days. Toxicity studies of TRZ investigated testicular redox state, serum gonadotropins, and testosterone levels, testicular 17 ß-hydroxysteroid dehydrogenase activity, sperm count and quality, levels of inflammatory cytokines, and caspase-3 expression as an apoptotic marker. Also, histopathological alterations of the testes were examined. TRZ significantly affected the testicular redox status as indicated by the increase in malondialdehyde and the decrease in reduced glutathione, superoxide dismutase, and catalase. It also disrupted serum gonadotropins (follicle stimulating hormone and luteinizing hormone) and testosterone levels and the activity of testicular 17ß-hydroxysteroid dehydrogenase. Additionally, TRZ adversely affected sperm count, motility, viability, and abnormality. Levels of tumor necrosis factor-α, interleukin-1β, interleukin-6, and expression of caspase-3 were increased in the testes. Histopathological examination of the testes supported the alterations mentioned above. Administration of EGb significantly ameliorated TRZ-induced testicular toxicity in rats. In conclusion, EGb protected against TRZ-induced testicular toxicity through antioxidant, anti-inflammatory, and anti-apoptotic mechanisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Infertility and subfertility are serious health problems which affect millions of people worldwide, of which 40–50% of cases are due to male factors (Krausz and Riera-Escamilla 2018). Concerns have been raised about the potential hazards of food additives to reproductive organs and fertility. More than 2500 items of food additives are used to improve the flavor and the artistic attributes of the food making it more attractive to the consumers. The worldwide increase in food consumption intensifies the occurrence of food additives and their metabolic products in the environment (Lempart-Rapacewicz et al. 2023). Recent literatures classified food additives as contaminants of emerging concern (CECs) (Salimi et al. 2017; Han et al. 2022). Colorants are a major class of food additives used widely in food industry and may also be used in non-food consumables such as cosmetics and pharmaceuticals (Delwiche 2004). There is no nutritional value for food colorants; however, interest in their adverse effects is growing (Tanaka 2006). A major group of food colorants is the synthetic azo dyes, including tartrazine (TRZ). FAO/WHO established an allowed daily intake of TRZ of 0–7.5 mg/kg (EFSA panel on food additives and nutrient sources added to food, 2009). Currently, data on TRZ toxicity are contradictory. Some studies reported no adverse effects for TRZ in both human and experimental models (Mpountoukas et al. 2010; Amin et al. 2010). However, several reports documented TRZ toxicity, including nephrotoxicity (Erdemli et al. 2017), neurotoxicity (Essawy et al. 2023), immunotoxicity (Abd-Elhakim et al. 2018), and mutagenic effects (Khayyat et al. 2017) when consumed moderately or in excess, or even at the allowed dose. Furthermore, adverse effects of TRZ on the male reproductive system have been reported. Wopara et al. (2021) mentioned that TRZ can alter testicular function and negatively affect sperm quantity and quality. It also disrupted the pituitary gonadotropins and decreased testosterone level (Mehedi et al. 2009). The toxicity of TRZ may be due to its direct covalent binding to proteins, causing denaturation of the active protein sites and misconfiguration (Saeed et al. 2011). Metabolic biotransformation of the azo bond can generate reactive amines that further generate free radicals and exaggerate oxidative damage, a driving force in TRZ toxicity (Demirkol et al. 2012; Albasher et al. 2020). Inflammation and apoptosis are also prime mechanisms in the pathophysiology of TRZ-induced testicular toxicity (Wopara et al. 2021; Abd-Elhakim et al. 2018).
Owing to their diverse beneficial effects, plant-derived phytochemicals have been used as key sources for the discovery of new drugs. Ginkgo biloba is an ancient tree species that originated from China and is now widespread all over the world. It has traditionally been used as a medicinal plant due to its wide spectrum of health benefits (Noor-E-Tabassum et al. 2022). Various bioactive ingredients with several therapeutic actions have been isolated from Ginkgo biloba, including flavonoids, terpenoids, alkylphenols, carboxylic acids, lignans, and polyprenols (Shu et al. 2020; Liu et al. 2021). The therapeutic efficacy of Ginkgo biloba extract (EGb) in the treatment of health disorders such as bronchitis, stomach discomfort, nervousness, ischemic heart disease, and diabetes mellitus has been reported (Chan et al. 2007). Furthermore, studies reported the beneficial effects of EGb against toxicities in different animal models (Verma et al. 2019; Mohammed et al. 2020; Sherif et al. 2020; Essawy et al. 2022).
It becomes crucial to evaluate the safety limits and the potential toxicity of substances used as food additives. In line with this context, this study further investigates the reproductive toxicity of TRZ in male rats. To the best of our knowledge, the effect of EGb on testicular toxicity of TRZ in rats was not studied up to now. Therefore, the main objective here is to evaluate the potential ameliorative effect of EGb on TRZ-induced toxicity in the testes of rats referring to the mechanisms involved. To achieve our objective, testicular redox status, gonadotropin and testosterone levels, 17 ß-hydroxysteroid dehydrogenase activity, sperm count and quality, inflammatory cytokine levels, and caspase-3 expression were evaluated. As well, histopathological examinations of the testicular tissue were performed.
Materials and methods
Chemical and reagents
Tartrazine (purity ≥ 95%) was procured from Sigma-Aldrich (USA). Ginkgo biloba extract was procured from Puritan Pride (USA). Kits and reagents used in the analyses were purchased from Bio-diagnostic Co. (Egypt). Enzyme-linked immunosorbent assay (ELISA) kits for measuring inflammatory cytokines were procured from Sigma-Aldrich Chemie (Germany). All reagents and chemicals used were of analytical grade.
Animals and experimental design
Twenty-four adult male Wistar albino rats (3 months, 180–200 g) were obtained from the facility house of the Faculty of Drug and Pharmacy, Pharos University, Alexandria, Egypt. The animals were housed in stainless steel cages and kept at 25 ± 1 °C and a 12-h dark/light cycle with access to the standard diet and tap water. The handling and experimental procedures of the study comply with and were approved by the Animal Ethical Committee of Alexandria University (approval number AU04190226101). After 2 weeks of acclimatization, the rats were assigned to four groups. The control group was given 0.5 ml of distilled water for 28 consecutive days; the EGb group received 100 mg/kg EGb alone; the TRZ group administered 7.5 mg/kg TRZ alone; and the TRZ-EGb group received 7.5 mg/kg TRZ; and 100 mg/kg EGb separately. The dose of TRZ was selected as the upper limit of the allowed daily intake (0–7.5 mg/kg/day), while the dose of EGb was selected according to Abd El-Maksoud et al. (2019). TRZ and EGb doses were administered orally in 0.5 ml of distilled water once daily for 28 consecutive days. The dose the, route, and the duration of TRZ administration were selected according to Essawy et al. (2023).
Blood collection and preparation of testicular homogenate
After completion of the treatment protocol, the rats were fasted overnight prior to anesthesia (light diethyl ether) for the euthanization. Blood was collected by cardiac puncture in non-heparinized tubes. The blood was centrifuged (3000 rpm for 10 min.) for isolation of the sera which were stored at − 20 °C for measurement of follicle stimulating hormone (FSH), luteinizing hormone (LH), and testosterone levels. For the preparation of testicular tissue lysate, the testes were immediately isolated from the rats and washed with cold saline. A 0.5 g from each testis was homogenized in 5-ml-cold phosphate buffer using a polytron. The homogenates were then centrifuged at 12,000 rpm for 15 min using a cooling centrifuge (Hettich model EBA12R, Germany). The supernatants were stored at − 20 °C for further analysis. A portion of the rat’s testes was immediately fixed in 10% formalin for histological and immunohistochemical examinations.
Determination of the testicular redox state
For evaluation of the testicular redox state, the level of malondialdehyde (MDA), the reduced glutathione (GSH) content, and the activities of superoxide dismutase (SOD) and catalase (CAT) were determined in testicular tissue. MDA was determined spectrophotometrically according to Ohkawa et al. (1979) and is expressed as nmol/g tissue. The GSH content, expressed as µmol/g tissue, was measured as described by Beutler et al. (1963). The activities of SOD and CAT, expressed as U/mg protein, were measured as described by Nishikimi et al. (1972) and Aebi (1984), respectively.
Measurement of reproductive hormones and 17ß-hydroxysteroid dehydrogenase
To evaluate the effect of TRZ with and without EGb on reproductive hormones, serum levels of FSH and LH were determined as described by Simoni et al. (1997) and Kosasa (1981), respectively. They are expressed as mIU/ml. Furthermore, the serum testosterone level (ng/ml) was quantitatively measured using ELISA technique based on competitive binding testosterone in the sample and a defined amount of horseradish peroxidase–conjugated testosterone. The specific activity of 17ß-hydroxysteroid dehydrogenase (17 β-HSD) was estimated in the testicular tissue as described by Penning et al. (1984). Enzyme activity is expressed as U/mg protein.
Determination of testicular inflammatory cytokines
Tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) levels were quantitatively determined in testicular tissue using ELISA kits, as described in the manufacturer’s instructions. They are expressed as pg/g tissue.
Sperm collection and evaluation of sperm quality
For sperm collection, the left testes and the cauda epididymis were immediately isolated from the rats. The epididymis was chopped in 5 ml Ham’s F12 medium, and the sperm suspension was incubated for 5 min at 35 °C. After repeated washings in Ham’s F12 medium, the collected sperm were evaluated for count, motility, viability, and abnormalities. For sperm count (millions/ml), one drop of sperm suspension was transferred to hemocytometer and allowed for 10 min to settle the sperm. The sperm mobility (%) was evaluated in at least 10 hemocytometer slide fields (Slott et al. 1991). Sperm viability (ratio of live and dead sperm) expressed as percentage was assessed according to the method of Talbot and Chacon (1981). Briefly, 0.2 ml of the sperm suspension was added to 1% trypan blue stain and incubated for 15 min at 37 °C, and then 0.1 ml of the mixture was loaded in hemocytometer chamber, allowed to settle for 60 s, and then the viable sperms were counted. Abnormal sperm shapes were observed in eosin/nigrosin-stained smears. One hundred sperms were randomly examined in different fields to measure the percentage of abnormal sperm (Filler 1993).
Histological examination
One testis from each rat was immediately fixed in 10% formalin, dehydrated in ascending grades of ethyl alcohol, fixed, embedded in paraffin, and sectioned at 5-µm thickness. Sections were stained with routine hematoxylin and eosin (H&E) and examined to record the histological changes (Sakr et al. 2011).
Immunohistochemical investigation
Successive 5-μm thick testis paraffin sections were mounted on positively charged slides and incubated at 37 \(^\circ{\rm C}\) to assess the immunoreactivity of caspase-3. Anticaspase-3 antibody (ab4051, Abcam, Inc.) was used, as described in the avidin–biotin complex (ABC) technique (Hsu et al. 1981).
Statistical analysis
All the results are presented as mean ± standard error of the mean (SEM). Statistical Package for the Social Sciences (SPSS, version 28) software was used for the analysis. One-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test was applied to compare between the experimental groups. Differences are significant when p < 0.05.
Results
EGb ameliorated TRZ-induced changes in the testicular redox status
To assess testicular redox state under the influence of TRZ and EGb, level of lipid peroxidation (LPO), as MDA, in addition to the antioxidants GSH, SOD, and CAT were measured (Fig. 1). Oral administration of 7.5 mg/kg TRZ daily for 28 triggered oxidative stress indicated by a significant increase of 301.6% in MDA level compared to the control group. Furthermore, TRZ-treated rats had significantly lower levels of antioxidants. They decreased by 66.9%, 83.9%, and 52.8% for GSH, SOD, and CAT, respectively relative to the control group. Supplementation with EGb plus TRZ significantly improved the redox state of the testes of rats. It decreased the level of MDA by 58.4% while GSH, SOD, and CAT increased by 101.8%, 342.6%, and 31.6%, respectively compared to the TRZ alone group. Notably, no significant changes in testicular redox indices were found in rats treated with EGb alone relative to the control group.
Effect of TRZ with and without EGb treatment on testicular redox state. The experimental groups were tested for levels of MDA (a), GSH (b), SOD (c), and CAT (d). The results are presented as mean ± SEM (n = 5). a and b represent statistical significance versus the control and TRZ groups, respectively using one-way ANOVA followed by the least significant difference (LSD) test. Abbreviations: CAT, catalase; EGb, Ginkgo biloba extract; GSH, reduced glutathione; SOD, superoxide dismutase; and TRZ, tartrazine
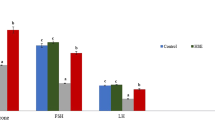
EGb improved TRZ-induced alteration in serum reproductive hormones and testicular 17 β-HSD activity
According to Fig. 2, TRZ administration significantly decreased serum LH and testosterone levels by 63.1 and 52.8%, while serum FSH level increased significantly by 70.9% compared to the control group. Furthermore, testicular 17 β-HSD activity decreased significantly by 37.6% compared to the control group. Interestingly, combined administration of EGb plus TRZ significantly improved these indices. Compared to the TRZ group, LH and testosterone levels and17 β-HSD activity increased by 137.4%, 25.9%, and 23.1%, respectively, while FSH level decreased by 28.9%. No significant changes were observed in these parameters after EGb administration alone.
Effect of TRZ with and without EGb on serum levels of LH (a), testosterone (b), and FSH (c), and testicular 17 β-HSD activity (d). Values are presented as the mean ± SEM (n = 4). a and b represent the statistical significance versus the control and TRZ groups, respectively, using one-way ANOVA followed by the least significant difference (LSD) test. Abbreviations: EGb, Ginkgo biloba extract; FSH, follicle stimulating hormone; LH, luteinizing hormone; and 17 β-HSD, 17-beta-hydroxysteroid dehydrogenase
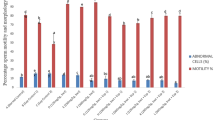
Effect of TRZ with and without EGb on the quantity and quality of sperm
Indices to diagnose the quantity and quality of the sperm were determined to assess the testicular function (Table 1). The results showed significant deterioration in sperm quality in rats administered TRZ. The sperm count, motility, and viability significantly decreased by 54.8%, 49.0%, and 54.9%, respectively while sperm abnormality increased significantly by 140.5% compared to the control group. Concurrent administration of TRZ plus EGb significantly increased the count, motility, viability of sperm and decreased the percentage of sperm abnormalities compared to the TRZ group. It is notable that administration of EGb alone significantly increased the count and decreased the abnormalities of sperm, while the motility and viability of sperms were insignificantly increased compared to normal control.
EGb ameliorated TRZ-induced inflammation in the testes of rats
TRZ was evaluated for its inflammatory effect in the testes of rats by monitoring cytokine levels. According to Fig. 3, TRZ administration significantly increased TNF-α, IL-1β, and IL-6 levels by 81.7%, 110.6%, and 134.1%, respectively compared to the control group. Treatment with EGb plus TRZ significantly decreased the levels of TNF-α, IL-1β, and IL-6 levels by 21.6%, 26.0%, and 26.2% respectively relative to TRZ alone group. Administration of EGb alone does not have an effect on cytokine levels in the testes of rats.
Effect of EGb on TRZ-induced inflammatory mediators in the testes of rats. The experimental groups were assessed for levels of TNF-α (a), IL-1β (b), and IL-6 (c). Values are presented as mean ± SEM (n = 4). a and b represent the statistical significance versus the control and TRZ groups, respectively, using one-way ANOVA followed by the least significant difference (LSD) test. Abbreviations: EGb, Ginkgo biloba extract; IL, interleukin; TNF-α, tumor necrosis factor-alpha; and TRZ, tartrazine
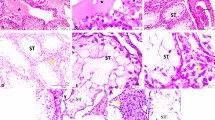
Effect of EGb on TRZ-induced histopathological alterations
Histological alterations of the testes were observed in H&E stained sections. Sections from the control and EGb-treated groups (Fig. 4) showed the normal structure of the seminiferous tubules. The tubules have regular outlines and are surrounded by a thin regular basal lamina. The interstitial tissue showed blood vessels and clusters of normal Leydig cells. Within the seminiferous tubules, the spermatogenic cells are found in an organized manner with spermatogonia, primary spermatocytes, spermatids, and tufts of spermatozoa appeared in the lumen. Sertoli cells appeared elongated with oval basal nuclei. On the other hand, testicular sections from TRZ-treated rats (Fig. 5) revealed severe histopathological alterations, as reflected by deformed and atrophied seminiferous tubules with irregular outline and wide spaces between the tubules. Deformed germ cells with pyknotic nuclei, irregularly arranged and degenerated spermatogenic cells with vacuolations among them, wide separation between germ cells, many Sertoli cells with densely stained nuclei, portions of clustered and abnormal scanty elongated spermatids, and absence of sperm were observed within the seminiferous tubules. In addition, degeneration of Leydig cells and congested blood vessels were observed. Testicular sections of rats treated with TRZ and EGb (Fig. 6) revealed preservation of the normal architecture of many seminiferous tubules with well-organized germ cells, normal appearance of spermatogenic cells and Leydig cells, and improved interstitial tissue.
Photomicrographs of testicular tissue from the control group (a and c) and EGb-treated rats (b and d). Figs. a and b show the normal architecture of seminiferous tubules, interstitial tissue containing normal Leydig cells (Ly), normal blood vessels (bv), normal spermatogonia (black arrow), and spermatids (S), and lumen filled with spermatozoa (Z). Figs. c and d show higher magnification of the seminiferous tubules with normal and complete spermatogenic cells arranged in a regular manner with spermatogonia and primary spermatocytes (yellow arrow), spermatids (S), spermatozoa (Z), and normal Sertoli cells (double red arrow). H&E, scale bar: 200 µm (a and b) and 50 µm (c and d)
Photomicrographs of testicular tissue from rats treated with TRZ alone (a, b, c, and d). Figs. a and b show deformed and atrophied seminiferous tubules with irregular outline, wide spaces between the tubules (filled star), markedly disarranged spermatogenic cells (double black arrow), vacuoles between spermatogenic cells (V), lack of spermatozoa (Z), congested blood vessels (bv), and degenerated Leydig cells (red arrow). Figs. c and d illustrate higher magnification of the seminiferous tubules showing spermatogonia with pyknotic nuclei (green arrow), separation between germ cells (double headed arrow), clustered spermatids (S), and elongated scanty spermatids (curved orange arrow). H&E, scale bar: 200 µm (a and b) and 50 µm (c and d)
Photomicrographs of sections from testes of rats treated with TRZ and EGb. Fig. a shows improved architecture of seminiferous tubules with normal arrangement of spermatogenic cells, spermatogonia (black arrow), normal Leydig cells (Ly), spermatids (S), and sperm (Z). Fig. b shows higher magnification of seminiferous tubules with normal primary spermatocyte (yellow arrow) and Sertoli cells (double red arrow). H&E, scale bar: 200 µm (a) and 50 µm (b)
Effect of TRZ with and without EGb on caspase-3 reactivity in rat’s testis
To examine apoptotic activity in the testes, immunoreactivity of caspase-3 was observed. As shown in Fig. 7, sections in testes from the control (a) and the EGb alone (b) groups revealed absence or weak caspase-3 immunoreactivity. On the contrary, the TRZ-treated group (c) shows strong immunoreactivity for caspase-3. Combined administration of TRZ and EGb (d) significantly decreased caspase-3 expression in the testes compared to the TRZ-only group.
Photomicrographs of sections in rats’ testes showing immunoreactivity to caspase 3. Sections of the control group (a) and EGb group (b) show a negative or faint positive reaction. Testicular sections from TRZ-treated group (c) show strong immunoreactivity for caspase 3 (red arrows) while TRZ-EGb treated group (d) demonstrated weak expression to caspase 3. Scale bar is 50 µm
Discussion
Food additives are classified as CECs because they are only partially metabolized inside the human body and excreted into the sewage (Birch et al. 2015). Adverse effects associated with the use of food additives on the reproductive system and fertility have been reported (Mehedi et al. 2009; Wopara et al. 2021). Therefore, it is recommended to precisely assess the toxicity of food additives in an attempt to avoid their possible health hazards. Currently, we investigate the toxic effect of TRZ, a widely used synthetic colorant, on the rat testes. Additionally, the study examined the protective effect of EGb, a natural phenolic compound, against TRZ-induced testicular injury. The results confirmed the toxic effect of TRZ, as shown by deterioration of testicular redox status, disruption of reproductive hormones, decline in sperm count and quality, increased production of pro-inflammatory mediators, and increased expression of caspase-3 in addition to histopathological alteration in testicular tissue. Concurrent EGb administration significantly improved the testicular structure and function and protected against TRZ toxic effects.
About 30–80% of men infertility cases have been linked to increased ROS and the oxidative damage they induce (Tremellen 2008). The results of the current study points to oxidative stress as a prime mechanism in mediating the testicular toxicity of TRZ. Oral administration of TRZ triggered oxidative stress in the testes, as shown by the higher level of MDA, a product of LPO that indicates oxidative stress. Our results support previous studies reporting TRZ-induced oxidative stress (Amin et al. 2010; Albasher et al. 2020; Mansour et al. 2021). The testicular tissue is eminently liable to oxidative injury due to its high content of polyunsaturated fatty acids (Sikka 1996). The metabolism of azo dyes catalyzed by reductases and peroxidases, resulting in the formation of semiquinone radicals and aromatic amines, may be the reason behind TRZ-induced oxidative stress (Demirkol et al. 2012). Furthermore, semiquinone radicals promote the formation of other radicals such as superoxide and hydroxyl radicals, and hydrogen peroxide which exaggerate oxidative stress.. Our results illustrated a marked improvement in MDA level in testes of rats co-administered TRZ and EGb. Similarly, previous studies reported that EGb can confer protection against methotrexate-induced testicular damage (Sherif et al. 2020; Mansour et al. 2021). The observed antioxidant effect of EGb is closely related to its constituents of terpenoids and flavonoids, which are highly efficient ingredients in the scavenging of free radicals, including superoxide, hydroxyl, and peroxyl radicals (Yeh et al. 2009).
Depletion of cellular antioxidants is strongly associated with oxidative stress and progression of several disorders. The results reported here revealed decrease in GSH content and activities of SOD and CAT. Overconsumption of antioxidants to remove increased levels of oxidants may explain the decline in the cellular antioxidants. Supplementation of EGb significantly enhanced the measured antioxidants in TRZ-treated rats. EGb possibly ameliorated TRZ-induced oxidative stress through recovery of the testicular antioxidants. In line with our results, EGb was reported to attenuate the testicular toxicity associated with chemotherapies by restoring normal testicular antioxidants along with decreasing the level of MDA (Sherif et al. 2020). EGb has been reported to upregulate testicular antioxidant enzyme by increasing the gene expression of nuclear factor erythroid 2-related factor 2 (Nrf2), a transcription factor that regulates cell oxidative stress and inflammation through the expression of genes encoding detoxifying and antioxidant enzymes (Mansour et al. 2021).
Regulation of the male reproductive function necessitates a multiplex array of endocrine interactions. The most significant regulatory pathway is the hypothalamic-pituitary–gonadal axis, which regulates vital functions through the release of gonadotropins (FSH and LH), in addition to maintaining high levels of testosterone from Leydig cells to control normal spermatogenesis (Anderson and Baird 2002). The results reported here revealed that TRZ administration significantly decreased LH and testosterone levels. Low levels of LH can negatively affect the release of testosterone from Leydig cells. Disruption in reproductive hormones after TRZ administration can markedly affect steroidogenesis and spermatogenesis processes, which can be reflected in the significant decrease in sperm count and quality. Surprisingly, TRZ administration increased the level of FSH in our study. The results reporting the effect of TRZ on the levels of reproductive hormones are contradictory. Elekima and Nwachuku (2019) reported that they had no detrimental effect on reproductive hormones after TRZ administration. Wopara et al. (2021) reported increased levels of FSH, LH, and testosterone when TRZ was administered in combination with erythrosine, a synthetic food colorant. 17β-HSD is an enzyme that catalyzes the synthesis of testosterone in the Leydig cells (Hu et al. 2010). The present data showed a decline in testicular 17β-HSD activity in rats treated with TRZ which may explain the observed decrease in testosterone level in our study. Ayad et al. (2022) reported that the decrease in LH level can negatively affect 17β-HSD activity. Therefore, it can be suggested that the decrease in LH level and subsequent inhibition of 17β-HSD activity can result in a decreased level of testosterone. Administration of EGb plus TRZ for 28 days improved serum FSH, LH, and testosterone levels as well as testicular activity of 17β-HSD. These findings are consistent with those of Mansour et al. (2021), who reported that EGb ameliorated methotrexate-induced disruption of reproductive hormones. EGb conserves the volume and morphology of Leydig cells and increases the level of LH (de Souza Predes et al. 2011). Furthermore, all these parameters were significantly improved by administering quercetin, a bioactive component of EGb, which has been reported to improve testicular steroidogenesis (Martin and Touaibia 2020).
High levels of inflammatory cytokines can aggravate the progression of testicular diseases (Loveland et al. 2017). The results reported in our study, which agree with Wopara et al. (2021), showed that TRZ-treated rats had higher levels of TNF-α, IL-1β, and IL-6 in the testes, indicating testicular inflammation. Inflammatory cytokines can contribute to testicular cell degeneration, which can adversely affect spermatogenesis and reproductive hormone production (Suescun et al. 2023). Wopara et al. (2021) reported significant upregulation in the expression of testicular TNF-α, IL-1α, and IL-1β genes in rats administered TRZ and erythrosine. TNF-α is an essential cytokine that regulates different processes in the mammalian testes, including spermatogenesis. Additionally, it can also promote the release of IL-1β and IL-6 which can induce oxidative stress and adversely affect spermatogenesis (Hong et al. 2004). Oxidative stress and inflammation are interrelated mechanisms. Excess ROS production has been documented to activate nuclear factor-kappa B (NF-κB), a transcription factor that can mediate upregulation of genes encoding the synthesis of pro-inflammatory mediators and is considered the primary regulator of inflammation (Singh et al. 2022). Therefore, TRZ-induced testicular inflammation can be mediated through ROS-induced upregulation of NF-κB and subsequent increased synthesis of gene products such as inflammatory cytokines. Raposa et al. (2016) reported that TRZ could significantly increase the expression of NF-κB in the liver of male and female mice. Our finding reported the anti-inflammatory effect of EGb by decreasing testicular TNF-α, IL-1β, and IL-6 levels which can be mediated through inhibition of NF-κB expression as previously reported by Fu et al. (2019). The anti-inflammatory effect of EGb was previously reported against methotrexate-induced testicular damage (Mansour et al. 2021).
Infertility is a health problem that encounters about 15% of couples globally of which, the male factor is involved in almost half of the cases (Krausz and Riera-Escamilla 2018). Decreased sperm count, motility, viability, and abnormal morphology are frequent attributes that diagnose male infertility. Our results showed marked decreases in sperm count, motility, and viability, while the percentage of sperm abnormalities significantly increased in rats treated with TRZ. Oxidative damage may be one of the main contributors to decreased sperm count and quality. Sperms are vulnerable to oxidative damage due to their high content of unsaturated lipids in the plasma membrane (Aitken et al. 2012). Peroxidation of lipids and subsequent loss of membrane integrity can cause substantial damage to sperm (O’Flaherty 2020). Furthermore, ROS can adversely affect the blood-testis barrier which contributes to disruption of spermatogenesis and decrease sperm quality (Liu et al. 2022). In light of this evidence, TRZ-induced ROS and oxidative stress may explain the observed deterioration in sperm quality. Similarly, Mehedi et al. (2009) reported a significant decrease in sperm quality in the mice after ingestion of TRZ. The decline in the sperm antioxidant system may be another reason for the observed decrease in the count and quality of the sperm. High level of testosterone is critically essential for spermatogenesis and maintenance of normal sperm quality. Therefore, the decline in sperm quality and quantity may be associated with impaired spermatogenesis due to disruption in testosterone secretion, severe alterations in the seminiferous tubules, and/or excessive sperm apoptosis. Concomitant administration of TRZ and EGb significantly improved sperm quality parameters. In line with our results, Mansour et al. (2021) found that EGb significantly improved the deterioration in sperm quality induced by methotrexate administration. The ameliorative effect of EGb may be attributed to its antioxidant effect.
Testicular dysfunction caused by exposure to TRZ was supported by histopathological examination of H&E stained sections, which revealed severe changes and loss of regular architecture of the testes. Atrophied seminiferous tubules, deformed germ cells, degenerated spermatogenic cells, absence of sperm, and Leydig cell degeneration were observed, which confirm testicular injury. Mehedi et al. (2009) demonstrated similar histological changes in seminiferous tubules and Leydig cells from TRZ-treated mice. These histological alterations can be caused by the direct effect of TRZ and its metabolites on testicular tissue or indirectly by the disruption of pituitary gonadotropins. Furthermore, peroxidation of lipids can decrease membrane fluidity and affect membrane integrity and functionality, causing significant pathological changes (Sadžak et al. 2020). Abd-Elkareem et al. (2021) reported similar deterioration in the testicular tissue of rats administered the food additive monosodium glutamate. Supplementation with EGb significantly improved the histological architecture of the testes of TRZ-treated rats. Seminiferous tubules with well-organized germ cells, normal appearance of spermatogenic cells and Leydig cells, and improved interstitial tissue were observed. EGb has been reported to improve histopathological changes in the testes of rats induced by ischemia/reperfusion injury (Akgül et al. 2008), doxorubicin (Yeh et al. 2009), and methotrexate (Mansour et al. 2021). The radical scavenging, antioxidant, and anti-inflammatory properties of EGb may explain the improvement in testicular tissue.
Apoptosis is a process of precisely controlled cell death that is essential for preserving tissue homeostasis. It is triggered by sequential activation of caspases, a group of endoproteases that become activated when the cell decides to undergo apoptosis. Caspase-3 is a frequently activated protease that catalyzes DNA fragmentation and cleavage of many cellular proteins. To examine the ability of TRZ to induce apoptosis in our study, the immunoreactivity of caspase-3 was examined in the testes of rats. The results showed a strong positive immunoreactivity to caspase-3 which may be attributed to increased oxidative stress. Abd-Elhakim et al. (2018) reported that the increase in caspase-3 expression in rats exposed to TRZ may be due to increased ROS that can initiate the mitochondrial caspase-3 apoptosis cascade. Administration of EGb in the current study exhibited anti-apoptotic action as manifested by downregulation of caspase-3 expression in the testicular tissue. These findings confirm the anti-apoptotic effect of EGb and its potency to inhibit apoptosis by affecting the caspase-3 pathway. The anti-apoptotic effect of EGb was previously documented in the testis and other organs (Yeh et al. 2009; Wu et al. 2015; Gomaa et al. 2020).
Conclusion
In conclusion, this study indicated that exposure to tartrazine, even within the acceptable daily intake range, adversely affected testicular structure and function. It induced oxidative stress, testicular dysfunction, inflammation, and apoptosis, as well as histological deterioration in the testicular architecture. The study also provided evidence for the protective effect of Ginkgo biloba extract against tartrazine-induced testicular toxicity. The mechanism(s) by which Ginkgo biloba extract protected involve antioxidant effect by modulation of the testicular redox sate, anti-inflammatory effect through inhibition of inflammatory mediators, and apoptotic action by downregulation of caspase-3 expression. These data strongly recommend limiting the intake of tartrazine as a synthetic azo dye and highlight the beneficial effects for supplementation of the herbal Ginkgo biloba against tartrazine-induced testicular toxicity. A limitation of our study is that Nrf2, NF-κB, and the gene expression of antioxidant enzymes were not determined currently. A further study is needed including these molecular studies to have a deeper insight and confirms the exact mechanism(s) by which EGb affords its protective effect.
Data availability
The data used in the analysis are available from the corresponding author upon reasonable request.
References
Abd El-Maksoud EM, Lebda MA, Hashem AE, Taha NM, Kamel MA (2019) Ginkgo biloba mitigates silver nanoparticles-induced hepatotoxicity in Wistar rats via improvement of mitochondrial biogenesis and antioxidant status. Environ Sci Pollut Res 26(25):25844–25854. https://doi.org/10.1007/s11356-019-06392-4
Abd-Elhakim YM, Hashem MM, El-Metwally AE, Anwar A, Moustafa GG, Ali HA (2018) Comparative haemato-immunotoxic impacts of long-term exposure to tartrazine and chlorophyll in rats. Int Immunopharmacol 63:145–154. https://doi.org/10.1016/j.intimp.2018.08.002
Abd-Elkareem M, Abd El-Rahman MAM, Khalil NSA, Amer AS (2021) Antioxidant and cytoprotective effects of Nigella sativa L seeds on the testis of monosodium glutamate challenged rats. Sci Rep 11(1):13519. https://doi.org/10.1038/s41598-021-92977-4
Aebi H (1984) Catalase in vitro. Meth Enzymol 105:121-126. https://doi.org/10.1016/S0076-6879(84)05016-3
Aitken RJ, Jones KT, Robertson SA (2012) Reactive oxygen species and sperm function-in sickness and in health. J Androl 33(6):1096–1106. https://doi.org/10.2164/jandrol.112.016535
Akgül T, Ayyildiz A, Nuhoğlu B, Karagüzel E, Oğüş E, Yağmurdur H, Ustün H, Germiyanoğlu C (2008) Ginkgo biloba (EGb 761) usage attenuates testicular injury induced by testicular ischemia/reperfusion in rats. Int Urol Nephrol 40(3):685–690. https://doi.org/10.1007/s11255-007-9296-5
Albasher G, Maashi N, Alfarraj S, Almeer R, Albrahim T, Alotibi F, Bin-Jumah M, Mahmoud AM (2020) Perinatal exposure to tartrazine triggers oxidative stress and neurobehavioral alterations in mice offspring. Antioxidants 9(1):53. https://doi.org/10.3390/antiox9010053
Amin AK, Hameid AH II, Abd Elsstar HA (2010) Effect of food azo dyes tartrazine and carmoisine on biochemical parameter related to renal, hepatic function and oxidative stress biomarkers in young male rats. Food Chem Toxicol 48(10):2994–2999. https://doi.org/10.1016/j.fct.2010.07.039
Anderson RA, Baird DT (2002) Male contraception. Endocr Rev 23(6):735–762. https://doi.org/10.1210/er.2002-0002
Ayad B, Omolaoye TS, Louw N, Ramsunder Y, Skosana BT, Oyeipo PI, Du Plessis SS (2022) Oxidative stress and male infertility: evidence from a research perspective. Front Reprod Health 4:822257. https://doi.org/10.3389/frph.2022.822257
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Birch GF, Drage DS, Thompson K, Eaglesham G, Mueller JF (2015) Emerging contaminants (pharmaceuticals, personal care products, a food additive and pesticides) in waters of Sydney estuary, Australia. Mar Pollut Bull 97:56–66. https://doi.org/10.1016/j.marpolbul.2015.06.038
Chan PC, Xia Q, Fu PP (2007) Ginkgo biloba leave extract: biological, medicinal, and toxicological effects. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 25(3):211–244. https://doi.org/10.1080/10590500701569414
de Souza PF, Monteiro JC, Matta SLP, Garcia MC, Dolder H (2011) Testicular histomorphometry and ultrastructure of rats treated with cadmium and Ginkgo biloba. Biol Trace Elem Res 140(3):330–341. https://doi.org/10.1007/s12011-010-8702-5
Delwiche J (2004) The impact of perceptual interactions on perceived flavor. Food Qual Prefer 15(2):137–146. https://doi.org/10.1016/S0950-3293(03)00041-7
Demirkol O, Zhang X, Ercal N (2012) Oxidative effect of tartrazine (Cas No. 1934-21-0) and new coccin (Cas No. 2611-82-7) azo dyes on CHO cells. J Verbrauch Lebensm 7(3):229–236. https://doi.org/10.1007/s00003-012-0782-z
EFSA panel on food additives and nutrient sources added to food (2009) Scientific opinion on the re-evaluation tartrazine (E 102) on request from the European Commission. EFSA J 7(11):1331. https://doi.org/10.2903/j.efsa.2009.1331
Elekima I, Nwachuku OE (2019) Evaluation of acute and chronic toxicity of tartrazine (E102) on steriod reproductive hormones of albino rats. Asian J Res Rep Endocrin 2(1):1–15
Erdemli ME, Gul M, Altinoz E, Zayman E, Aksungur Z, Bag HG (2017) The protective role of crocin in tartrazine induced nephrotoxicity in Wistar rats. Biomed Pharmacother 96:930–935. https://doi.org/10.1016/j.biopha.2017.11.150
Essawy AE, El-Sayed SA, Tousson E, Abd El-gawad HS, Alhasani RH, Abd Elkader HA (2022) Anti-kindling effect of Ginkgo biloba leaf extract and L-carnitine in the pentylenetetrazol model of epilepsy. Environ Sci Pollut Res 29:48573–48587. https://doi.org/10.1007/s11356-022-19251-6
Essawy AE, Mohamed AI, Ali RG, Ali AM, Abdou HM (2023) Analysis of melatonin-modulating effects against tartrazine-induced neurotoxicity in male rats: biochemical, pathological and immunohistochemical markers. Neurochem Res 48(1):131–141. https://doi.org/10.1007/s11064-022-03723-9
Filler R (1993) Methods for evaluation of rat epididymal sperm morphology. Male Reprod Toxicol 3:334–343. https://doi.org/10.1016/B978-0-12-461207-5.50025-0
Fu Z, Lin L, Liu S, Qin M, He S, Zhu L, Huang J (2019) Ginkgo biloba extract inhibits metastasis and ERK/nuclear factor kappa B (NF-κB) signaling pathway in gastric cancer. Med Sci Monit: Int Med J Exp Clin Res 25:6836–6845. https://doi.org/10.12659/MSM.915146
Gomaa DH, Hozayen WG, Al-shafeey MH, Elkelawy AMH, Hashem KS (2020) Ginkgo biloba alleviates cisplatin-mediated neurotoxicity in rats via modulating APP/Aβ/P2X7R/P2Y12R and XIAP/BDNF-dependent caspase-3 apoptotic pathway. Appl Sci 10(14):4786. https://doi.org/10.3390/app10144786
Han Y, Hu LX, Liu T, Liu J, Wang YQ, Zhao JH, Liu YS, Zhao JL, Ying GG (2022) Non-target, suspect and target screening of chemicals of emerging concern in landfill leachates and groundwater in Guangzhou. South China Sci Total Environ 837(6):155705. https://doi.org/10.1016/j.scitotenv.2022.155705
Hong CY, Park JH, Ahn RS, Im SY, Choi HS, Soh J, Mellon SH, Lee K (2004) Molecular mechanism of suppression of testicular steroidogenesis by pro-inflammatory cytokine tumor necrosis factor alpha. Mol Cell Biol 24(7):2593–2604. https://doi.org/10.1128/MCB.24.7.2593-2604.2004
Hsu SM, Raine L, Fanger HX (1981) Use of avidin-biotin peroxidase complex (ABC) in immune peroxidase techniques: a comparison between ABC and unlabelled antibody (PAP) procedures. J Histochem Cytochemy 29(4):577–580. https://doi.org/10.1177/29.4.6166661
Hu GX, Liang G, Chu Y, Li X, Lian QQ, Lin H, He Y, Huang Y, Hardy DO, Ge RS (2010) Curcumin derivatives inhibit testicular 17beta-hydroxysteroid dehydrogenase 3. Bioorg Med Chem Lett 20(8):2549–2551. https://doi.org/10.1016/j.bmcl.2010.02.089
Khayyat L, Essawy A, Sorour J, Soffar A (2017) Tartrazine induces structural and functional aberrations and genotoxic effects in vivo. PeerJ 5:e3041. https://doi.org/10.7717/peerj.3041
Kosasa TS (1981) Measurement of human luteinizing hormone. J Reprod Med 26:201–206. https://doi.org/10.1172/JCI105762
Krausz C, Riera-Escamilla A (2018) Genetics of male infertility. Nat Rev Urol 15(60):369–384. https://doi.org/10.1038/s41585-018-0003-3
Lempart-Rapacewicz A, Kudlek E, Brukało K, Rapacewicz R, Lempart L, Dudziak M (2023) The threat of food additive occurrence in the environment- a case study on the example of swimming pools. Foods 12(6):1188. https://doi.org/10.3390/foods12061188
Liu L, Wang Y, Zhang J, Wang S (2021) Advances in the chemical constituents and chemical analysis of Ginkgo biloba leaf, extract, and phytopharmaceuticals. J Pharm Biomed Anal 193(300):113704. https://doi.org/10.1016/j.jpba.2020.113704
Liu JB, Li ZF, Lu L, Wang ZY, Wang L (2022) Glyphosate damages blood-testis barrier via NOX1-triggered oxidative stress in rats: long-term exposure as a potential risk for male reproductive health. Environ Int 159:107038. https://doi.org/10.1016/j.envint.2021.107038
Loveland KL, Klein B, Pueschl D, Indumathy S, Bergmann M, Loveland BE, Hedger MP, Schuppe HC (2017) Cytokines in male fertility and reproductive pathologies: Immunoregulation and beyond. Front Endocrinol 8:307. https://doi.org/10.3389/fendo.2017.00307
Mansour DF, Saleh DO, Ahmed-Farid OA, Rady M, Bakeer RM, Hashad IM (2021) Ginkgo biloba extract (EGb 761) mitigates methotrexate-induced testicular insult in rats: targeting oxidative stress, energy deficit, and spermatogenesis. Biomed Pharmacother 143(1):112201. https://doi.org/10.1016/j.biopha.2021.112201
Martin LJ, Touaibia M (2020) Improvement of testicular steroidogenesis using flavonoids and isoflavonoids for prevention of late-onset male hypogonadism. Antioxidants 9(3):237. https://doi.org/10.3390/antiox9030237
Mehedi N, Ainad-Tabet S, Mokrane N, Addou S, Zaoui C, Kheroua O, Saidi D (2009) Reproductive toxicology of tartrazine (FD and C Yellow No. 5) in Swiss albino mice. Am J Pharmacol Toxicol 4(4):130–135. https://doi.org/10.3844/ajptsp.2009.130.135
Mohammed NA, Abdou HM, Tass MA, Alfwuaires M, Abdel-Moneim AM, Essawy AE (2020) Oral supplements of Ginkgo biloba extract alleviate neuroinflammation, oxidative impairments and neurotoxicity in rotenone-induced parkinsonian rats. Curr Pharm Biotechnol 21(12):1259–1268. https://doi.org/10.2174/1389201021666200320135849
Mpountoukas P, Pantazaki A, Kostareli E, Christodoulou P, Kareli D, Poliliou S, Mourelatos C, Lambropoulou V, Lialiaris T (2010) Cytogenetic evaluation and DNA interaction studies of the food colorants amaranth, erythrosine and tartrazine. Food Chem Toxicol 48(10):2934–2944. https://doi.org/10.1016/j.fct.2010.07.030
Nishikimi M, Rao NA, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46(2):849–854. https://doi.org/10.1016/s0006-291x(72)80218-3
Noor-E-Tabassum DR, Lami MS, Chakraborty AJ, Mitra S, Tallei TE, Idroes R, Mohamed AA, Hossain MJ, Dhama K, Mostafa-Hedeab G, Emran TB (2022) Ginkgo biloba: a treasure of functional phytochemicals with multi-medicinal applications. Evid Based Complement Alternat Med 2022:8288818. https://doi.org/10.1155/2022/8288818
O’Flaherty C (2020) Reactive oxygen species and male fertility. Antioxidants 9(4):287. https://doi.org/10.3390/antiox9040287
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Penning TM, Mukharji I, Barrows S, Talalay P (1984) Purification and properties of a 3α-hydroxysteroid dehydrogenase of rat liver cytosol and its inhibition by anti-inflammatory drugs. Biochem J 222(3):601–611. https://doi.org/10.1042/bj2220601
Raposa B, Pónusz R, Gerencsér G, Budán F, Gyöngyi Z, Tibold A, Hegyi D, Kiss I, Koller Á, Varjas T (2016) Food additives: sodium benzoate, potassium sorbate, azorubine, and tartrazine modify the expression of NFκB, GADD45α, and MAPK8 genes. Physiol Int 103(3):334–343. https://doi.org/10.1556/2060.103.2016.3.6
Sadžak A, Mravljak J, Maltar-Strmečki N, Arsov Z, Baranović G, Erceg I, Kriechbaum M, Strasser V, Přibyl J, Šegota S (2020) The structural integrity of the model lipid membrane during induced lipid peroxidation: the role of flavonols in the inhibition of lipid peroxidation. Antioxidants 9(5):430. https://doi.org/10.3390/antiox9050430
Saeed GMS, Sayeed AS, Ashraf S, Batool FN, Ali R, Nas A, Siddiqi R (2011) Investigations of in vitro digestibility of proteins bound to food colors. J Pharm Nutr Sci 1(1):34–40. https://doi.org/10.6000/1927-5951.2011.01.01.07
Sakr SA, Lamfon AH, Essawy AE (2011) Ginger (Zingiber Officinale) extract ameliorates metalaxyl fungicide induced nephrotoxicity in albino mice. Afr J Pharm Pharmacol 5(2):104–112. https://doi.org/10.5897/AJPP.9000163
Salimi M, Esrafili A, Gholami M, JonidiJafari A, RezaeiKalantary R, Farzadkia M, Kermani M, Sobhi H (2017) Contaminants of emerging concern: a review of a new approach in AOP technologies. Environ Monit Assess 189:414. https://doi.org/10.1007/s10661-017-6097-x
Sherif IO, Al-Mutabagani LA, Sarhan OM (2020) Ginkgo biloba extract attenuates methotrexate-induced testicular injury in rats: cross-talk between oxidative stress, inflammation, apoptosis, and miRNA-29a expression. Integr Cancer Ther 19:1534735420969814. https://doi.org/10.1177/1534735420969814
Shu P, Sun M, Li J, Zhang L, Xu H, Lou Y, Ju Z, Wei X, Wu W, Sun N (2020) Chemical constituents from Ginkgo biloba leaves and their cytotoxicity activity. J Nat Med 74(1):269–274. https://doi.org/10.1007/s11418-019-01359-8
Sikka SC (1996) Oxidative stress and role of antioxidants in normal and abnormal sperm function. Front Biosci 1(5):e78–e86. https://doi.org/10.2741/a146
Simoni M, Gromol J, Nieschlag E (1997) The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev 18(6):739–773. https://doi.org/10.1210/edrv.18.6.0320
Singh A, Kukreti R, Saso L, Kukreti S (2022) Mechanistic insight into oxidative stress-triggered signaling pathways and type 2 diabetes. Molecules 27(3):950. https://doi.org/10.3390/molecules27030950
Slott VL, Suarez JD, Perreault SD (1991) Rat sperm motility analysis: methodologic considerations. Reprod Toxicol 5(5):449–458. https://doi.org/10.1016/0890-6238(91)90009-5
Suescun MO, Rival C, Theas MS, Calandra RS, Lustig L (2023) Involvement of tumor necrosis factor-alpha in the pathogenesis of autoimmune orchitis in rats. Biol Reprod 68(6):2114–2121. https://doi.org/10.1095/biolreprod.102.011189
Talbot P, Chacon RS (1981) A triple-stain technique for evaluating normal acrosome reactions of human sperm. J Exp Zool 215(2):201–208. https://doi.org/10.1002/jez.1402150210
Tanaka T (2006) Reproductive and neurobehavioural toxicity study of tartrazine administered to mice in the diet. Food Chem Toxicol 44(2):179–187. https://doi.org/10.1016/j.fct.2005.06.011
Tremellen K (2008) Oxidative stress and male infertility- a clinical perspective. Hum Reprod Update 14(3):243–258. https://doi.org/10.1093/humupd/dmn004
Verma S, Ranawat P, Sharma N, Nehru B (2019) Ginkgo biloba attenuates aluminum lactate-induced neurotoxicity in reproductive senescent female rats: behavioral, biochemical, and histopathological study. Environ Sci Pollut Res 26(26):27148–27167. https://doi.org/10.1007/s11356-019-05743-5
Wopara I, Modo EU, Mobisson SK, Olusegun GA, Umoren EB, Orji BO, Mounmbegna PE, Ujunwa SO (2021) Synthetic food dyes cause testicular damage via up-regulation of pro-inflammatory cytokines and down-regulation of FSH-R and TESK-1 gene expression. JBRA Assist Reprod 25(3):341–348. https://doi.org/10.5935/1518-0557.20200097
Wu C, Zhao X, Zhang X, Liu S, Zhao H, Chen Y (2015) Effect of Ginkgo biloba extract on apoptosis of brain tissues in rats with acute cerebral infarction and related gene expression. Genet Mol Res 14(2):6387–6394. https://doi.org/10.4238/2015.June.11.14
Yeh YC, Liu TJ, Wang LC, Lee HW, Ting CT, Lee WL, Hung CJ, Wang KY, Lai HC, Lai HC (2009) A standardized extract of Ginkgo biloba suppresses doxorubicin-induced oxidative stress and p53-mediated mitochondrial apoptosis in rat testes. Br J Pharmacol 156(1):48–61. https://doi.org/10.1111/j.1476-5381.2008.00042.x
Author information
Authors and Affiliations
Contributions
Amina Essawy and Heba Abdou contributed to the conception and design of the study. Materials preparation and data collection were performed by Shreen Mattar and Nema Mohammed. Data analysis was performed by Amina Essawy, Shreen Mattar, Nema Mohammed, Wessam Abdel-Wahab, and Heba Abdou. Wessam Abdel-Wahab, Nema Mohammed, and Shreen Mattar prepared the figures and tables. The first draft of the manuscript was written and critically revised by Wessam Abdel-Wahab and Amina Essawy. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The following statement was provided for the ethical approval: The procedures of the experiment are compatible with the Institutional Animal Care and Use Committee (IACUC) at Alexandria University (approval number AU04190226101).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Informed consent
Not applicable.
Competing interests
The authors declare no competing interests..
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Essawy, A., Matar, S., Mohamed, N. et al. Ginkgo biloba extract protects against tartrazine-induced testicular toxicity in rats: involvement of antioxidant, anti-inflammatory, and anti-apoptotic mechanisms. Environ Sci Pollut Res 31, 15065–15077 (2024). https://doi.org/10.1007/s11356-024-32047-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32047-0