Abstract
Peroxidase-like based on double transition metals have higher catalytic activity and are considered to have great potential for application in the field of pollutant degradation. First, in this paper, a novel Fe0-doped three-dimensional porous Fe0@FeMn-NC-like peroxidase was synthesized by a simple one-step thermal reduction method. The doping of manganese was able to reduce part of the iron in Fe-Mn binary oxides to Fe0 at high temperatures. In addition, Fe0@FeMn-NC has excellent peroxidase-like mimetic activity, and thus, it was used for the rapid degradation of p-chlorophenol (4-CP). During the degradation process, Fe0 was able to rapidly replenish the constantly depleted Fe2+ in the reaction system and brought in a large number of additional electrons. The ineffective decomposition of H2O2 due to the use of H2O2 as an electron donor in the reduction reactions from Fe3+ to Fe2+ and from Mn3+ to Mn2+ was avoided. Finally, based on the experimental results of LC-MS and combined with theoretical calculations, the degradation process of 4-CP was rationally analyzed, in which the intermediates were mainly p-chloro-catechol, p-chloro resorcinol, and p-benzoquinone. Fe0@FeMn-NC nano-enzymes have excellent catalytic activity as well as structural stability and perform well in the treatment of simulated wastewater containing a variety of phenolic pollutants as well as real chemical wastewater. It provides some insights and methods for the application of peroxidase-like enzymes in the degradation of organic pollutants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phenolic pollutants are widely present in industrial processes such as pharmaceuticals, paper, printing, and dyeing (Zhang et al. 2007). Incompletely treated industrial wastewater often contains phenolics, which have serious effects on human and animal physiology when discharged into the environment and can accumulate in organisms, with the risk of carcinogenicity and teratogenicity (Hu et al. 2021; Villaluz et al. 2019). Among the monochlorophenols, p-chlorophenol (4-CP) is the most toxic (Czaplicka 2004), and chlorophenol-containing wastewater must be treated before it is discharged into the environment. Therefore, it is necessary to find an effective method to remove phenolics from wastewater.
Green chemistry technologies using nano-enzymes as catalysts are receiving more and more attention compared to traditional physical and chemical treatment methods (Liu et al. 2023b; Mishra et al. 2022; Wang and Chen 2020). Among them, peroxidase-like enzymes, which can catalyze the activated decomposition of H2O2 to produce hydroxyl radicals (•OH) with stronger oxidizing ability, have been intensively studied in the field of advanced oxygenation of organic pollutants (Chen et al. 2020; Tang et al. 2023; Yang et al. 2020). As an effective peroxidase-like mimic, metal-based nanomaterials have been successfully applied to remove chlorophenols from laboratory synthetic wastewaters and real industrial wastewaters due to their excellent electron transfer ability (Tang et al. 2023). For example, Xin et al. prepared AA-NiMn-CLDHs@HNTs-Ag nanomotors by assembling AgNPs and NiMn-CLDH nanosheets inside and outside the lumen of natural halloysite nanotubes (HNTs) using a layer-by-layer self-assembly technique, which was able to degrade 95.63% of phenol within 90 min (Xing et al. 2022). Magnetic material Au@Ni/rGO with a size less than 8 nm and core-shell nanostructure was successfully synthesized and exhibited peroxidase-like activity, exhibiting excellent performance in the photodegradation behavior of phenol, 2-chlorophenol, and 2-nitrophenol (Darabdhara and Das 2019). However, the cost of precious metals and the scarcity of the material are also difficulties that have to be faced at the material preparation stage. Iron-based materials, as a peroxidase-like, have received increasing attention from researchers due to their environmental-friendly properties and low cost (Huang et al. 2023; Jiang et al. 2021; Jiao et al. 2020; Wan et al. 2022). Among them, zero-valent iron and iron oxides have been shown to possess peroxidase-like activity, while Fe3O4 is an ideal feedstock for catalysts due to its magnetic properties facilitating material recycling (Baye et al. 2023; Liu et al. 2022). However, the use of iron-based nano-enzymes alone comes with unavoidable drawbacks such as low Fe2+/Fe3+ recovery and high ion leaching. Therefore, it is crucial to improve the stability of iron-based materials and their catalytic properties.
Manganese, as a widespread element in the environment, has a wide variety of valence states and excellent redox properties (Cai et al. 2021; Du et al. 2020; He et al. 2022; Hu et al. 2017). Some studies have shown that Mn2+, Mn3+, and Mn4+ can exhibit peroxidase mimetic activities (Ding et al. 2021; Lv et al. 2023; Meng et al. 2020). However, manganese-based nano-enzymes are often difficult to recover, which limits their wide application in water pollution treatment. Therefore, iron-manganese bimetallic nano-catalytic materials can effectively solve the problems of material recycling as well as material stability, achieving the best of both worlds. Due to the synergistic effect between Fe-Mn, Fe-Mn bimetallic nano-enzymes tend to exhibit stronger catalytic performance than the corresponding monometallic nano-enzymes (Huang et al. 2022; Qu et al. 2021; Wang et al. 2019). This has been widely applied in the field of pollutant degradation and detection and sensing (Liu et al. 2023a; Mao et al. 2023; Martins et al. 2023; Sun et al. 2021; Zhang et al. 2022). For example, Liang et al. showed that redox cycling between Fe3+/Fe2+ and Mn3+/Mn2+ can promote the generation of free radicals, thus showing excellent performance in the degradation of tetracycline (Liang et al. 2023); Liu et al. found that Mn-amplified Fe-N nano-enzymes have more excellent peroxidase-like activity, which is attributed to the strong electron transfer properties of Fe and Mn, which can accelerate the decomposition of H2O2 to produce more •OH (Liu et al. 2023a). For carbon-based materials, high-temperature pyrolysis favors the formation of a large number of carbon vacancies, which can significantly accelerate the redox step (Wei et al. 2023). In addition, bimetallic nano-enzymes were found to have d-orbital coupling features that contribute to the lowering of electron transfer energy barriers in redox (Feng et al. 2023). However, polymetallic nano-enzymes are still poorly studied, and efforts are still needed to fill this research gap.
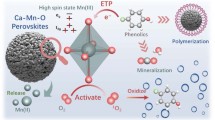
In this work, to further improve the catalytic activity as well as stability of the nano-enzyme, we employed a simple high-temperature pyrolysis reduction method to synthesize a novel Fe0-doped three-dimensional porous FeMn-NC nano-enzyme for the catalytic degradation of 4-CP (Scheme 1a). Among them, Fe0@FeMn-NC exhibited excellent peroxidase-like (POD-like) activity with high H2O2 affinity and catalytic efficiency. Moreover, the presence of Fe0 could effectively replenish the Fe2+ consumed in the reaction, avoiding the ineffective decomposition of H2O2 and enabling more hydroxyl radicals (•OH), superoxide radicals (•O2−), and singlet oxygen (1O2) to be used for the degradation of pollutants (Scheme 1b). Fe0@FeMn-NC nano-enzyme have excellent catalytic activity and structural stability and are effective in treating both simulated wastewater containing various phenolic pollutants as well as real chemical wastewater.
Materials and methods
Materials and drugs
D( +)-glucosamine hydrochloride, dicyandiamide, 3,3′,5,5′–tetramethylbenzidine (TMB), and ZnCl2 were purchased from Shanghai Maclin Biochemical Co., Ltd. FeCl3•6H2O, MnCl2, H2O2 (30 wt%), glacial acetic acid, anhydrous sodium acetate, anhydrous ethanol, tert-butanol (TBA), p-benzoquinone (PBQ), furfuryl alcohol (FFA), p-chlorophenol (4-CP), p-nitrophenol (4-NP), phenol, paracetamol (PCM), bisphenol A (BPA), hydrochloric acid, NaOH, and 2,2,6,6-tetramethylpiperidine (TEMP) were purchased from Sinophenol Chemical Reagents Co., Ltd. 5,5-Dimethyl-1-pyrroline N-oxide (DMPO) was purchased from Dojindo Laboratories. All chemicals are used as received without further purification. All aqueous solutions were prepared with deionized water (18.25 MΩ·cm, microporous).
Instruments
High-resolution field emission scanning electron microscopy (FESEM, Regulus 8230, Japan) was used to record scanning electron microscopy images. Transmission electron microscopy (TEM) imaging was performed on a JEM-2100F microscope at an acceleration voltage of 200 kV (JEOL, Japan). The specific surface area and pore structure of the materials were measured by the AutosorB-IQ3 N2 adsorption-desorption instrument (Kantha, USA). X-ray photoelectron spectroscopy (XPS) spectra were collected by an ESCALAB250Xi XPS microscope (Thermo, USA). UV-visible absorption spectra were collected by UV-visible spectrophotometer (SHIMADZU, Japan). Raman analysis was performed on a LabRAM HR Evolution (France) spectrometer with an argon ion laser (532 nm) as the excitation source. The XRD patterns of the materials were recorded by an X-ray diffractometer (Rigaku D/MAX2500VL/PC, Japan). The concentration of metal ions leached in solution was determined by atomic absorption spectrometer (Perkin Elmer AA800, USA). Electron paramagnetic resonance (EPR) measurements were carried out on a JES-FA200 spectrometer (JEOL, Japan). The TOC removal rate was measured by Multi N/C 3100 TOC analyzer (Jena, Germany). The intermediates in the reaction process were analyzed by liquid chromatography-four-stage electrostatic field orbital trap mass spectrometry (LC-MS, Thermo, USA).
Synthesis of Fe0@FeMn-NCnanocomposite catalysts
The Fe0@FeMn-NC nanocatalysts required for the experiments were synthesized by a one-step pyrolysis method. Firstly, D( +)-glucosamine hydrochloride and dicyandiamide were used as carbon and nitrogen sources, ZnCl2 as a pore-forming agent, and FeCl3-6H2O and MnCl2 as iron and manganese sources, respectively, which were dissolved into a certain amount of deionized water in a molar ratio of 10:10:2:1:1, respectively, by ultrasonic dissolution. After drying the above solution, the solid obtained was transferred to a tube furnace and heated up to 900 °C at a heating rate of 5 °C/min in an atmosphere of N2. Holding time was 2 h, and then annealed to obtain Fe0@FeMn-NC. Finally, NC-900, Fe-NC, and Mn-NC were prepared by the same scheme described above under the conditions of no metal doping and Fe/Mn ratios of 2:0, 0:2, respectively.
Peroxidase activity measurement
To study the peroxidase-like activity of FeMn-NC nano-enzymes, TMB was selected as the substrate for colorimetric assay. Fe0@FeMn-NC nano-enzymes (40 mg/L) were added to HAc-NaAc buffer solution (pH = 4) containing TMB (0.3 mM) and H2O2 (10 mM). After incubation at room temperature (25 °C) for 5 min to produce the corresponding blue color reaction, 1 mL of the solution was removed from the tube and filtered through a microporous filter (0.45 μm) and transferred to a UV-vis spectrophotometer to measure the absorbance value at 652 nm.
In the steady-state kinetic experiments, we fixed the TMB concentration at 0.3 mM and performed kinetic analysis of different concentrations of H2O2 by varying the H2O2 concentration and recording the absorbance at 652 nm at selected time intervals in a scanning kinetic mode. Apparent kinetic parameters (Km and Vmax) were obtained through typical Miltonian equation and double-reciprocal equation diagrams (Zhang et al. 2021), as shown in Eq. (1):
where V represents the initial reaction rate, Vmax represents the maximum reaction rate, [S] represents the substrate concentration, and Km represents the Michaelis–Menten constant.
4-CP adsorption and degradation experiments
The catalytic performance of the Fe0@FeMn-NC nano-enzyme catalyst was evaluated based on the degradation efficiency of 4-CP in the presence of H2O2. The effects of several important parameters of 4-CP degradation such as H2O2 concentration, initial pH, and catalyst dosing on the 4-CP degradation efficiency were explored. The experimental procedure was as follows: 50 mL of mixture containing catalyst (40 mg/L) and 4-CP (100 mg/L) was added into a 50-mL conical flask, pH was adjusted and stirred continuously for 30 min to reach the adsorption equilibrium, and then, a certain concentration of H2O2 was added with continuous stirring. One milliliter of liquid was removed from the reaction solution at 0, 2, 5, 10, and 30 min, respectively, and filtered through a 0.45-μm membrane to remove the catalyst, and an excess of anhydrous methanol was added to quench the unreacted radicals to inhibit further reaction. Finally, the obtained samples were analyzed by high performance liquid chromatography (HPLC, LC-20A, Shimadzu, Japan) for the determination of 4-CP. The catalysts were collected by filtration at the end of each run and then washed at least five times with ethanol and deionized water, dried, and reserved for the next cycle of experiments. Subsequent degradation experiments with 4-NP, BPA, PCM, Phenol, and actual wastewater were conducted using the same methodology as described above.
DFT calculations
All calculations are based on Materials Studio DMol3 version 2020 (Delley 1990, 2000). Geometry optimization and energy minimization of the compounds were carried out using the Generalized Gradient Approximation (GGA) and the Perdew-Burke-Ernzerhof (PBE) exchange function (Perdew et al. 1996). The cutoff energy of the plane-wave basis set is 380 eV. In the geometric optimization, the energy convergence criterion of the system is set to 1 × 10−5 eV, with a force of less than 0.03 eV/Å per atom. For the 4-CP degradation process, we use Linear Synchronous Transition/Quadratic Synchronous Transition (LST/QST) for the transition state search and verify whether the transition state has only one imaginary frequency by frequency analysis. Then, transition state confirmation is performed based on the Nudged Elastic Band (NEB) method.
Results and discussion
Physical characterization of the material
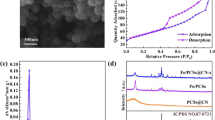
XRD characterization was performed to explore the crystalline phase of the nano-enzymes. As shown in Fig. 1a, all materials exhibited characteristic peaks (broad diffraction peaks at 20–25°) similar to the graphitized carbon nitride of NC-900. The diffraction peaks appearing at 35.4°, 41.2°, and 59.6° are attributed to the (111), (200), and (220) crystal planes of the Fe-Mn oxides, respectively. In Fe-NC and Mn-NC there are only characteristic peaks of Fe-Mn oxides. When Fe-Mn is doped simultaneously, new diffraction peaks appear at 44.7°,65.2°, and 82.5°, corresponding to the (110), (200), and (211) crystal planes of α-Fe, respectively (Gong et al. 2021). This is due to the fact that Fe in Fe oxides can be stripped out and reduced to zero-valent Fe by Mn under high temperature environment (Xu et al. 2023b). Figure 1b shows the Raman spectra of NC-900, Fe-NC, Mn-NC, and Fe0@FeMn-NC, which can reflect their defective information. The D peak (1350 cm−1) corresponds to the sp3 hybridization of carbon atoms, and the G peak (1580 cm−1) is due to the intra-structural scaling of the sp2 hybridization of carbon atoms (Ananthoju et al. 2019). All materials have broad D-band peaks, indicating the presence of abundant defects in the carbon matrix, to play an important role in improving the catalytic activity of the catalysts. In addition, the value of ID/IG decreases significantly with the doping of Fe-Mn; this is due to the emergence of Fe-Mn crystallization, which makes the catalyst gradually tend to be ordered. The porous carbon layer in Fe0@FeMn-NC plays an important role for electron transfer within the system. As shown in Fig. 1c, all the samples exhibit a typical type I adsorption isotherm for microporous materials, associated with the presence of a large number of microporous structures, where intra-microporous adsorption occurs in the low-pressure region, leading to a sharp increase in adsorption, whereas a slow rise in the adsorption plateau suggests that there is a trace amount of hollow and microporous structures in the material (Lai et al. 2016; Liang et al. 2014). The hysteresis loop appears in the region of higher relative pressure, showing a typical type IV adsorption isotherm, which was shown to be related to the presence of meso- and microporous structures in the carbon structure (Zhang et al. 2018). The pore size distribution and specific surface area of the nanocatalysts were calculated by the DFT method, and the results are shown in Fig. 1d and Table S1. The pore sizes are mainly distributed in the range of 0.5–5 nm, belonging to the typical micro-mesoporous materials, and are mainly dominated by micropores distributed below 2 nm. This phenomenon suggests that Fe0@FeMn-NC has formed a layered pore structure that is favorable for increasing the reaction sites and thus enhancing the reaction activity. By comparing the enlarged pore size distribution (inset in Fig. 1d) and the specific surface area (Table S1), it can be seen that the number of micropores and the specific surface area appeared to decrease after loading the metal, which was attributed to the metal particles having a certain blocking effect on the micropores of the material.
Figure S1 shows the SEM images of NC-900, Fe-NC, Mn-NC, and Fe0@FeMn-NC, which exhibit irregular porous features. Similar to NC-900, they all have a large number of graded porous structures, which suggests that Fe/Mn doping does not affect the macrostructure of carbon nitride (Wang and Nan 2020). A granular structure was clearly observed from the TEM images of Fe0@FeMn-NC (Fig. 2a, b), which could be the doped Fe and Mn species (Xu et al. 2023a). The HR-TEM images showed (Fig. 2c, d) that the interfacial spacing of the carbon matrix was 0.34 nm, suggesting that a large amount of graphitized carbon was formed in Fe0@FeMn-NC (He et al. 2024). The 0.22-nm and 0.25-nm interfacial spacing is attributed to the (200) and (111) crystal faces of the Fe–Mn oxides. In addition, the (110) crystal face of α-Fe (with an interfacial spacing of 0.20 nm) can be clearly seen (Xu et al. 2023b). The energy dispersive X-ray spectroscopy (EDS) mapping images in Fig. 2e show a homogeneous distribution of the elements C, N, O, Fe, and Mn on Fe0@FeMn-NC. This is in agreement with our results described in XRD, indicating that Fe0 was successfully synthesized on a carbon matrix where Fe-Mn oxides coexist.
The elemental composition and state of Fe0@FeMn-NC were determined through further characterization by XPS. Significant C, N, and O signals, as well as weak Fe and Mn signals, can be observed in the XPS gross spectrum of Fe0@FeMn-NC (Fig. S2a), which is because the amounts of Fe and Mn are trace compared to carbon and nitrogen during the material synthesis process. Among them, C 1 s was able to be indexed as C-C (284.8 eV), C-N (286.0 eV), and C-O (288.1 eV) structures (Fig. S2b) (He et al. 2023; Zhu et al. 2018). In its high-resolution N 1 s XPS spectra (Fig. S2c), four different types of N, Pyridinic-N (398.5 eV), Graphitic-N (400.9 eV), Oxidized-N (402.7 eV), and M-NX (399.7 eV), were observed, demonstrating that the material forms abundant Fe-NX and Mn-NX, which can provide a large number of active sites for the catalytic reaction to proceed (Deng et al. 2017). In the O 1 s profile (Fig. S2d), it was possible to quickly fit three distinct characteristic peaks indexed as M–O (530.2 eV), M–O-C (531.8 eV), and C-O (533.4 eV), respectively (Duan et al. 2022). The Fe 2p pattern (Fig. 3a) with distinct characteristic peaks of Fe0 (705.4 eV), Fe2+ (710.6 eV and 723.9 eV), and Fe3+ (713.8 eV and 726.9 eV) reconfirms the successful synthesis of zero-valent iron in Fe0@FeMn-NC, which would be more favorable for the activation of peroxide (Lin et al. 2017; Yamashita and Hayes 2008). The spectrum of Mn 2p is shown in Fig. 3b, which can be back-convoluted to the characteristic peaks of Mn2+ (640.2 eV and 652.4 eV), Mn3+ (641.4 eV and 653.7 eV), and Mn4+ (643.0 eV and 655.3 eV) (He et al. 2024). All these results are in agreement with the conclusions obtained in the previous characterization.
Peroxidase activity
TMB was chosen as a chromogenic substrate for Fe0@FeMn-NC nano-enzymes to evaluate the peroxidase-like activity of the nano-enzymes due to its ability to be catalytically oxidized by Fe0@FeMn-NC nano-enzymes to a blue color product (OxTMB) in the presence of H2O2 (Fig. 4a). Compared to the addition of NC-900 only, Fe-NC, and Mn-NC, Fe0@FeMn-NC had the maximum absorbance at 652 nm, indicating the highest peroxidase-like activity (Fig. 4b). It proved that there is a synergistic effect between Fe and Mn. When both are present together, they can effectively increase the peroxidase activity.
a Oxidation of TMB by Fe0@FeMn-NC peroxidase-like produces the blue product Ox-TMB; b UV-vis absorption spectra of NC-900, Fe-NC, Mn-NC, and Fe0@FeMn-NC in the system with TMB + H2O2; steady-state kinetic assay of Fe0@FeMn-NC nano-enzyme: c time-dependent absorption changes at 652 nm in the presence of different concentrations of H2O2; d the velocities were measured with different H2O2 concentrations; e double-reciprocal plots between velocity and H2O2 concentration; f Km and Vmax comparison of different nano-enzymes
To quantitatively assess the peroxidase activity of NC-900, Fe-NC, Mn-NC, and Fe0@FeMn-NC nano-enzymes, the Michaelis-Menten constants (Km) and the maximum reaction rates (Vmax) were determined using double-inverse plots of the initial reaction rates (Fig. 4c–e, Fig. S3, Fig. S4, and Fig. S5). As shown in Fig. 4f, the Km values (3.38, 3.23, and 0.25 mM) of the three nano-enzymes were lower than those of HRP (3.7 mM (Gao et al. 2007)), except for NC-900 (Km = 4.53 mM), when H2O2 was used as substrate. It indicates that Fe/Mn-doped nano-enzymes have more excellent affinity for H2O2 than HRP, and among them, Fe0@FeMn-NC is the best. Most encouragingly, the newly synthesized Fe-Mn bimetallic peroxidase mimic in this study exhibited a higher affinity for H2O2 compared to other classes of peroxidized nano-enzymes previously reported (Fig. S6 and Table S2).
Catalytic degradation of 4-CP byFe0@FeMn-NCnano-enzymes
To demonstrate the actual catalytic performance of Fe0@FeMn-NC nano-enzyme, 4-CP was selected as a target pollutant to test the degradation effect. We explored the factors affecting the degradation of 4-CP, such as nano-enzyme species, nano-enzyme dosing, pH, and H2O2 concentration. Figure 6a shows the removal efficiency of 4-CP in different reaction systems. Since all four materials have rich microporous structures, they showed excellent adsorption effects before the addition of H2O2 and basically reached the adsorption equilibrium after 30 min. The concentration of 4-CP in the Fe0@FeMn-NC system decreased rapidly after the addition of H2O2. This system was able to remove 94.62% of 4-CP within 2 min, and the removal rate was as high as 98.91% after 30 min of oxidation. It indicates that Fe0@FeMn-NC has a strong H2O2 activation ability and can accelerate the oxidative degradation of 4-CP by H2O2.
The effect of different Fe0@FeMn-NC dosages on the removal efficiency of 4-CP is shown in Fig. 5b. Firstly, the removal efficiency of 4-CP increased with the increase of Fe0@FeMn-NC concentration, from 62.65% at 5 mg/L to 98.91% at 40 mg/L. Subsequently, continuing to increase the dosage of Fe0@FeMn-NC did not improve the removal of 4-CP. The removal rate of Fe0@FeMn-NC at 80 mg/L decreased to 98.76% at 60 min, which showed a certain inhibitory effect on the degradation of 4-CP, suggesting that excessive Fe0 would lead to the ineffective decomposition of H2O2.
Factors affecting the degradation of 4-CP: a type of nano-enzymes; b dosage of nano-enzymes; c pH; d H2O2 concentration. In addition to the condition to be controlled, other reaction conditions: pH = 4, [4-CP] = 100 mg/L, [nano-enzyme] = 40 mg/L, [H2O2] = 10 mM, adsorption time = 30 min, reaction time = 30 min
It is well known that pH has a significant effect on the formation of hydroxyl radicals and the degradation process of pollutants in Fenton/Fenton-like systems (Masomboon et al. 2009; Wang et al. 2022). With similarity to the Fenton system, the Fe0@FeMn-NC in this study had a higher degradation effect at low pH (3–6) (Fig. 5c), which can be attributed to the following factors: (1) low pH is favorable for the generation of ·OH (Kim et al. 2010); (2) it is beneficial to strengthen the catalytic oxidation ability of ·OH; and (3) facilitates the activation of Fe species (Xia et al. 2022).
In addition, the H2O2 concentration has a crucial effect on the degradation of 4-CP. In this study, the effect of different H2O2 concentrations on the degradation of 4-CP was investigated by varying the amount of H2O2 dosed in the system, and the results are shown in Fig. 5d. When the H2O2 concentration was 1 mM, the degradation of 4-CP by Fe0@FeMn-NC was 90.11% at 60 min. With the increase of H2O2 concentration, the degradation efficiency of 4-CP gradually increased and reached a maximum of 98.91% at 10 mM. Subsequently, the final degradation efficiency no longer increased after the concentration continued to increase. This indicates that the dosage of H2O2 has reached the optimum at 10 mM, and due to the limitation of the number of active sites, it is difficult to decompose the excess H2O2 effectively, and then, the degradation efficiency cannot be improved.
Furthermore, the performances of different catalysts in the degradation of 4-CP reported in selected literature are summarized in Table 1. It is noteworthy that, in comparison with previous studies, Fe0@FeMn-NC prepared by one-step pyrolytic reduction method in this study has excellent 4-CP degradation ability, and its performance is no less than that of conventional non-homogeneous catalysts. This fully demonstrates that our synthesized Fe0@FeMn-NC is an excellent catalyst capable of efficiently degrading organic pollutants in wastewater.
Mechanistic studies
Toward revealing the internal mechanism of Fe0@FeMn-NC nano-enzymatic catalysis, different radical burst experiments were designed according to the strong oxidizing radicals that may be generated during the classical H2O2-based advanced oxidation process, and the results are shown in Fig. S7a. Tert-Butanol (TBA), p-benzoquinone (PBQ), and furfuryl alcohol (FFA) were used as scavengers for •OH, •O2− and 1O2, respectively (Ma et al. 2021; Yun et al. 2018; Zhu et al. 2020). Firstly, the degradation rate of 4-CP decreased from 98.91 to 63.55% within 60 min with the addition of 50 mM TBA. The degradation rate further decreased to 60.25% when the concentration of TBA was increased to 100 mM. The degradation rate of 4-CP was likewise significantly inhibited by 50 mM FFA (degradation rate decreased to 69.72%). In contrast, the effect of PBQ on the removal of 4-CP was less pronounced. Fifty millimolar PBQ merely reduced the degradation of 4-CP to 78.69%. Finally, based on the inhibition effect of different quenchers on 4-CP degradation, we obtained the contribution of •OH, •O2−, and 1O2 to the degradation of 4-CP (Fig. S7b). It can be seen that •OH and 1O2 are the main ROS within the Fe0@FeMn-NC/H2O2 system, and •O2− is the minor one.
The role of •OH, •O2−, and 1O2 in the system was further demonstrated by the determination of free radicals in the reaction by electron paramagnetic resonance spectroscopy. To the reaction system, 5,5-dimethyl-l-pyrrolidine N-oxide (DMPO) was added for the detection of •OH and •O2− and 2,6,6-tetramethylpiperidine (TEMP) was used for the detection of 1O2. The results are shown in Fig. 6a–c, where the signals of •OH, •O2−, and 1O2 were detected. This further confirms that there are not only radical pathways of •OH and •O2− but also non-radical pathways of 1O2 during the degradation of 4-CP.
EPR spectra of •OH (a) and •O2− (b) with DMPO as trapping agent and 1O2 (c) with TEMP as trapping agent in Fe0@FeMn-NC/H2O2 system; d comparison of single-molecule orbital energy levels of 4-CP and its degradation intermediates (HOMO, LUMO, and LUMO-HOMO energy gaps); e the reaction barriers ΔG (kcal/mol) of 4-CP, its degradation intermediates, and transition states obtained by transition state search
In this study, Fe0 was introduced on the basis of the classical Fenton system (e.g., Eqs. (2)–(4)). Since Fe0@FeMn-NC was added in solid form, a two-stage reaction was involved, starting with a non-homogeneous process of H2O2 on the surface of Fe0@FeMn-NC, where Fe0 transfers two electrons to the H2O2 to be oxidized to Fe2+ (Eq. (5)). Notably, competitive adsorption of 4-CP on the catalyst surface resulted in a decrease in the number of active sites exposed to the catalyst surface, thus prolonging the first stage of the reaction process. The second stage involves a rapid degradation process of the pollutant, mainly by Fe2+ catalyzing a homogeneous oxidation reaction on or near the catalyst surface. Strongly oxidizing •OH is rapidly produced in this phase.
Meanwhile, Fe2+ is regenerated from Fe3+ by reaction Eqs. (3) and (6), which promote the formation of •OH. However, Fe0 not only promotes the reduction of Fe3+ on the catalyst surface but also generates H2O2 in situ in the presence of dissolved oxygen to further oxidize the pollutant (Eq. (7)). In addition, 1O2 generated by disproportionation and auto-decomposition between free radicals contributes significantly to the degradation of 4-CP (Eqs. (8)–(9)). Manganese doping resulted in the appearance of a significant increase in the degradation efficiency of 4-CP. On the one hand, it is because similar to iron ions, Mn2+ can also participate in the chain reaction of free radicals in the catalytic system and can catalyze the decomposition of H2O2 to produce •OH (Eq. (10)). On the other hand, manganese atoms can make the iron atoms in the Fe-Mn binary oxides separate and be reduced to zero-valent iron under high temperature environment, thus effectively improving the activation efficiency of H2O2. Eventually, 4-CP is co-oxidized by •OH, •O2−, and 1O2 to produce various intermediates and eventually mineralized to CO2 and H2O (Eq. (11)).
Since Fe0@FeMn-NC nano-enzymes have excellent catalytic degradation activity for 4-CP, it indicates that a large number of intermediate products are bound to be generated within this reaction system. And how to identify and characterize the intermediate products during the reaction process is crucial for exploring the degradation pathway of 4-CP. In this experiment, the intermediates during the degradation of 4-CP in the Fe0@FeMn-NC/H2O2 system were analyzed by LC-MS and combined with the analysis of 4-CP degradation pathways by Liu et al. and Lei et al. (Hong et al. 2022; Lei et al. 2021). It was initially deduced that the intermediates for the degradation of 4-CP may include phenol (m/z = 96), benzoquinone (m/z = 108), hydroquinone (m/z = 110), maleic acid (m/z = 116), malic acid (m/z = 116), 4-chlorocatechol (m/z = 144), and 2,4,6-trichlorophenol (m/z = 195), as shown in Fig. S8.
To successfully predict the interaction modes during the degradation of 4-CP, we calculated the highest occupied molecular orbital energy (HOMO), the lowest unoccupied molecular orbital energy (LUMO), and the LUMO-HOMO energy gap of the ground state (S) and the degradation intermediate state (IM) of 4-CP. Besides, the transition state (TS) energy barriers of the corresponding reactions were calculated, and the results are shown in Fig. 6d, e. Molecules with high HOMO energies favor reactions with electrophilic molecules, while molecules with lower LUMO energies show affinity for nucleophilic reagents (Liu et al. 2014). Thus, •OH (− 5.219 eV) with the lowest LUMO orbital energy is able to easily replace the hydrogen in the neighboring and interstitial positions of the hydroxyl group on the benzene ring by electrophilic addition reactions. This results in the conversion of 4-CP to 4-chlorocatechol (IM1-1) and 4-chlororesorcinol (IM1-2). The lower LUMO orbital energy of 1O2 (− 4.573 eV) can effectively dechlorinate 4-CP and generate hydroquinone (IM2) through the electrophilic addition reaction of •OH. In turn, p-chlorophenol in solution is subjected to the action of the dechlorinated chlorine radicals in the system to produce trace amounts of 2,4,6-trichlorophenol (IM1-3) with a reaction energy barrier of 712.9 kcal/mol. unstable hydroquinone (− 5.043 eV for HOMO) is readily oxidized to p-benzoquinone (IM3). Immediately thereafter, due to the lower LUMO-HOMO energy gap (2.822 eV), p-benzoquinone is highly susceptible to oxidative ring-opening by electrophilic •OH and 1O2 to form, for example, maleic acid and malic acid, and these small-molecule acids are able to be further mineralized ultimately to CO2 and H2O. In summary, the degradation process of 4-CP in the Fe0@FeMn-NC/H2O2 system was summarized in Fig. S9.
Repeatability and stability studies
As to evaluate the stability and TOC removal rate of Fe0@FeMn-NC nano enzymes, the degradation rates of 4-CP and TOC were determined under five cycling experiments, and the results are shown in Fig. S10. The 4-CP degradation rates in the five cycling experiments were maintained above 90%, while the TOC removal rates were maintained above 40%. This indicates that the Fe0@FeMn-NC nano-enzyme still has good catalytic activity under repeated use. In addition, the ion leaching of Fe-NC, Mn-NC, and Fe0@FeMn-NC in five-cycle experiments was compared (Fig. 7a, b), and the results showed that the doping of Fe with Mn could reduce the leaching of metal ions, and it was gradually decreased in several cycles. The above results further proved that the nano-enzymes have good stability and environmental safety.
Fe and Mn leaching concentrations of Fe-NC, Mn-NC (a) and Fe0@FeMn-NC (b) during degradation of 4-CP (100 mg/L); c effects of Fe0@FeMn-NC/H2O2 system on the degradation of 4-NP, BPA, PCM, and Phenol (10 mg/L); d changes in raw water in the workshop of a chemical plant before and after the reaction. Reaction conditions: pH = 4, [nano-enzyme] = 40 mg/L, [H2O2] = 10 mM, adsorption time = 30 min, reaction time = 30 min
Degradation of multiple pollutants and treatment of actual wastewater
The degradation efficiency of the Fe0@FeMn-NC/H2O2 system for a variety of phenolic pollutants and its application in real wastewater were investigated. As shown in Fig. 7c, Fe0@FeMn-NC exhibited high adsorption rates for 4-CP, 4-NP, BPA, PCM, and Phenol. With the addition of H2O2 after 30 min of adsorption equilibrium, the final degradation rates of 4-NP, BPA, PCM, and Phenol were 95.04%, 98.21%, 99.73%, and 100%, respectively. It shows that the Fe0@FeMn-NC/H2O2 system has the potential to treat a wide range of phenolic pollutants. Subsequently, raw workshop water from a chemical plant was used as the target effluent to study the degradation effect of the Fe0@FeMn-NC/H2O2 system in a real wastewater environment (Table S3 and Fig. 7d). Firstly, its initial indicators were determined, and 4-CP, 4-NP, BPA, PCM, and Phenol were detected in this wastewater. Secondly, the pH was adjusted to 4.0, and Fe0@FeMn-NC and H2O2 were successively added to it. Eventually, after 1 h of reaction, the removal of 4-CP, 4-NP, BPA, PCM, and Phenol reached 90.94%, 87.70%, 85.50%, 77.47%, and 93.98%, respectively. In addition, the indexes of turbidity and COD also decreased by 69.92% and 56.70%. This study demonstrates that Fe0@FeMn-NC is a promising material for the treatment of high concentration organic wastewater.
Conclusion
In this study, an Fe0-loaded Fe-Mn bimetallic peroxidase mimetic (Fe0@FeMn-NC) was successfully prepared by a simple one-step thermal reduction method with Fe and Mn co-doping. Fe and Mn exist in the valence states of Fe3+/Fe2+/Fe0 and Mn4+/Mn3+/Mn2+, which act as the key active sites catalyzing the decomposition of H2O2 to produce •OH and •O2−, as well as 1O2, for the degradation and even mineralization of 4-CP. Among them, the role of Fe0 should not be neglected, which promptly replenishes the constantly consumed Fe2+ in the reaction and brings in a large number of additional electrons. This avoids the ineffective decomposition of H2O2 due to the use of H2O2 as an electron donor during the reduction reaction of Fe3+ to Fe2+ and Mn3+ to Mn2+. The degradation process of 4-CP, whose intermediates are mainly p-chloro-catechol, p-chloro resorcinol, and p-benzoquinone, was reasonably hypothesized based on the results of LC-MS detection and combined with theoretical calculations. The Fe0@FeMn-NC/H2O2 system also showed high degradation efficiency for simulated wastewater containing multiple phenolic pollutants as well as real chemical plant wastewater. The successful application of Fe0-loaded Fe0@FeMn-NC nano-enzyme in the degradation of 4-CP presented in this work provides some insights and methods for the application of peroxidase-like enzymes in the degradation of organic pollutants.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Ananthoju B, Biroju RK, Theis W, Dryfe RAW (2019) Controlled electrodeposition of gold on graphene: maximization of the defect-enhanced raman scattering response. Small 15(48):1901555. https://doi.org/10.1002/smll.201901555
Baye AF, Nguyen HT, Kim H (2023) Fe0/Fe3C-assisted Fe3O4 redox sites as robust peroxidase mimics for colorimetric detection of H2O2. Sens Actuators B-Chem 377:133097. https://doi.org/10.1016/j.snb.2022.133097
Cai M, Zhang Y, Dong C, Wu W, Wang Q, Song Z, Shi Y, Wu L, Jin M, Dionysiou DD, Wei Z (2021) Manganese doped iron-carbon composite for synergistic persulfate activation: reactivity, stability, and mechanism. J Hazard Mater 405:124228. https://doi.org/10.1016/j.jhazmat.2020.124228
Chen Q, Zhang X, Li S, Tan J, Xu C, Huang Y (2020) MOF-derived Co3O4@Co-Fe oxide double-shelled nanocages as multi-functional specific peroxidase-like nanozyme catalysts for chemo/biosensing and dye degradation. Chem Eng J 395:125130. https://doi.org/10.1016/j.cej.2020.125130
Czaplicka M (2004) Sources and transformations of chlorophenols in the natural environment. Sci Total Environ 322(1–3):21–39. https://doi.org/10.1016/j.scitotenv.2003.09.015
Darabdhara G, Das MR (2019) Dual responsive magnetic Au@Ni nanostructures loaded reduced graphene oxide sheets for colorimetric detection and photocatalytic degradation of toxic phenolic compounds. J Hazard Mater 368:365–377. https://doi.org/10.1016/j.jhazmat.2019.01.010
Delley B (1990) An all-electron numerical method for solving the local density functional for polyatomic molecules. J Chem Phys 92(1):508–517. https://doi.org/10.1063/1.458452
Delley B (2000) From molecules to solids with the DMol3a pproach. J Chem Phys 113(18):7756–7764. https://doi.org/10.1063/1.1316015
Deng Y, Dong Y, Wang G, Sun K, Shi X, Zheng L, Li X, Liao S (2017) Well-defined ZIF-derived Fe-N codoped carbon nanoframes as efficient oxygen reduction catalysts. ACS Appl Mater Interfaces 9(11):9699–9709. https://doi.org/10.1021/acsami.6b16851
Ding Y, Cui K, Guo Z, Cui M, Chen Y (2021) Manganese peroxidase mediated oxidation of sulfamethoxazole: integrating the computational analysis to reveal the reaction kinetics, mechanistic insights, and oxidation pathway. J Hazard Mater 415:125719. https://doi.org/10.1016/j.jhazmat.2021.125719
Du J, Xiao G, Xi Y, Zhu X, Su F, Kim SH (2020) Periodate activation with manganese oxides for sulfanilamide degradation. Water Res 169:115278. https://doi.org/10.1016/j.watres.2019.115278
Duan Z, Zhang W, Lu M, Shao Z, Huang W, Li J, Li Y, Mo J, Li Y, Chen C (2020) Magnetic Fe3O4/activated carbon for combined adsorption and Fenton oxidation of 4-chlorophenol. Carbon 167:351–363. https://doi.org/10.1016/j.carbon.2020.05.106
Duan L, Liu X, Zhang H, Liu F, Liu X, Zhang X, Dong L (2022) A novel way for hydroxyl radicals generation: biochar-supported zero-valent iron composite activates oxygen to generate hydroxyl radicals. J Environ Chem Eng 10(4):108132. https://doi.org/10.1016/j.jece.2022.108132
Feng J, Yang X, Du T, Zhang L, Zhang P, Zhuo J, Luo L, Sun H, Han Y, Liu L, Shen Y, Wang J, Zhang W (2023) Transition metal high-entropy nanozyme: multi-site orbital coupling modulated high-efficiency peroxidase mimics. Adv Sci 10(33):2303078. https://doi.org/10.1002/advs.202303078
Gan Q, Hou H, Liang S, Qiu J, Tao S, Yang L, Yu W, Xiao K, Liu B, Hu J, Wang Y, Yang J (2020) Sludge-derived biochar with multivalent iron as an efficient Fenton catalyst for degradation of 4-Chlorophenol. Sci Total Environ 725:138299. https://doi.org/10.1016/j.scitotenv.2020.138299
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S, Yan X (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2(9):577–583. https://doi.org/10.1038/nnano.2007.260
Gong L, Qiu X, Tratnyek PG, Liu C, He F (2021) FeNX(C)-coated microscale zero-valent iron for fast and stable trichloroethylene dechlorination in both acidic and basic pH conditions. Environ Sci Technol 55(8):5393–5402. https://doi.org/10.1021/acs.est.0c08176
Haghmohammadi M, Sajjadi N, Beni AA, Hakimzadeh SM, Nezarat A, Asl SD (2023) Synthesis of activated carbon/magnetite nanocatalyst for sono-Fenton-like degradation process of 4-chlorophenol in an ultrasonic reactor and optimization using response surface method. J Water Process Eng 55:104216. https://doi.org/10.1016/j.jwpe.2023.104216
He L, Li H, Wang J, Gao Q, Li X (2022) Peroxymonosulfate activation by Co-doped magnetic Mn3O4 for degradation of oxytetracycline in water. Environ Sci Pollut Res 29(26):39249–39265. https://doi.org/10.1007/s11356-022-18929-1
He T, Pan X, Zhou W, Ding H, Liu M, Xiang M, Lou Q, Han L, Zhang Y, Wu Y, Chen Y (2023) Construction of high-content α-iron on zero-valent iron @ biochar composite for the ultra-efficient removal of oxytetracycline hydrochloride: a key step of ammonium bicarbonate pretreatment. Sep Purif Technol 323:124378. https://doi.org/10.1016/j.seppur.2023.124378
He Y, Qin H, Wang Z, Wang H, Zhu Y, Zhou C, Zeng Y, Li Y, Xu P, Zeng G (2024) Fe-Mn oxycarbide anchored on N-doped carbon for enhanced Fenton-like catalysis: importance of high-valent metal-oxo species and singlet oxygen. Appli Catal B-Environ 340:123204. https://doi.org/10.1016/j.apcatb.2023.123204
Hong P, Zhang K, He J, Li Y, Wu Z, Xie C, Liu J, Kong L (2022) Selenization governs the intrinsic activity of copper-cobalt complexes for enhanced non-radical Fenton-like oxidation toward organic contaminants. J Hazard Mater 435:128958. https://doi.org/10.1016/j.jhazmat.2022.128958
Hu E, Zhang Y, Wu S, Wu J, Liang L, He F (2017) Role of dissolved Mn(III) in transformation of organic contaminants: non-oxidative versus oxidative mechanisms. Water Res 111:234–243. https://doi.org/10.1016/j.watres.2017.01.013
Hu H, Miao K, Luo X, Guo S, Yuan X, Pei F, Qian H, Feng G (2021) Efficient Fenton-like treatment of high-concentration chlorophenol wastewater catalysed by Cu-Doped SBA-15 mesoporous silica. J Clean Prod 318:128632. https://doi.org/10.1016/j.jclepro.2021.128632
Huang S, Qiao Z, Sun P, Qiao K, Pei K, Yang L, Xu H, Wang S, Huang Y, Yan Y, Cao D (2022) The strain induced synergistic catalysis of FeN4 and MnN3 dual-site catalysts for oxygen reduction in proton-/anion- exchange membrane fuel cells. Appl Catal B-Environ 317:121770. https://doi.org/10.1016/j.apcatb.2022.121770
Huang H, Geng W, Wu X, Zhang Y, Xie L, Ma T, Cheng C (2023) Spiky artificial peroxidases with V−O−Fe pair sites for combating antibiotic-resistant pathogens. Angew Chem Int Ed e202310811. https://doi.org/10.1002/anie.202310811
Jiang G, Wang Z, Zong S, Yang K, Zhu K, Cui Y (2021) Peroxidase-like recyclable SERS probe for the detection and elimination of cationic dyes in pond waterY. J Hazard Mater 408:124426. https://doi.org/10.1016/j.jhazmat.2020.124426
Jiao L, Xu W, Zhang Y, Wu Y, Gu W, Ge X, Chen B, Zhu C, Guo S (2020) Boron-doped Fe-N-C single-atom nanozymes specifically boost peroxidase-like activity. Nano Today 35:100971. https://doi.org/10.1016/j.nantod.2020.100971
Kim JY, Lee C, Sedlak DL, Yoon J, Nelson KL (2010) Inactivation of MS2 coliphage by Fenton’s reagent. Water Res 44(8):2647–2653. https://doi.org/10.1016/j.watres.2010.01.025
Lai Q, Zhao Y, Liang Y, He J, Chen J (2016) In situ confinement pyrolysis transformation of ZIF-8 to nitrogen-enriched meso-microporous carbon frameworks for oxygen reduction. Adv Func Mater 26(45):8334–8344. https://doi.org/10.1002/adfm.201603607
Lei M, Gao Q, Zhou K, Gogoi P, Liu J, Wang J, Song H, Wang S, Liu X (2021) Catalytic degradation and mineralization mechanism of 4-chlorophenol oxidized by phosphomolybdic acid/H2O2. Sep Purif Technol 257:117933. https://doi.org/10.1016/j.seppur.2020.117933
Liang F, Liu Z, Jiang X, Li J, Xiao K, Xu W, Chen X, Liang J, Lin Z, Li M, Wu X, Wang H (2023) NaOH-modified biochar supported Fe/Mn bimetallic composites as efficient peroxymonosulfate activator for enhance tetracycline removal. Chem Eng J 454:139949. https://doi.org/10.1016/j.cej.2022.139949
Liang J, Zhou RF, Chen XM, Tang YH, Qiao SZ (2014) Fe-N decorated hybrids of CNTs grown on hierarchically porous carbon for high-performance oxygen reduction. Adv Mater 26(35):6074-+. https://doi.org/10.1002/adma.201401848
Lin J, Sun M, Liu X, Chen Z (2017) Functional kaolin supported nanoscale zero-valent iron as a Fenton-like catalyst for the degradation of Direct Black G. Chemosphere 184:664–672. https://doi.org/10.1016/j.chemosphere.2017.06.038
Liu H, Chen Q, Zhang S, Li X (2014) Relationship of mineralization of amino naphthalene sulfonic acids by Fenton oxidation and frontier molecular orbital energies. Chem Eng J 247:275–282. https://doi.org/10.1016/j.cej.2014.03.019
Liu J, Ghanizadeh H, Li X, An L, Qiu Y, Zhang Y, Chen X, Wang A (2022) Facile synthesis of core\shell Fe3O4@mSiO2(Hb) and its application for organic wastewater treatment. Environ Res 203:111796. https://doi.org/10.1016/j.envres.2021.111796
Liu B, Wang Z, Wei T, Liu Z, Li J (2023a) Bimetallic FeMn-N nanoparticles as nanocatalyst with dual enzyme-mimic activities for simultaneous colorimetric detection and degradation of hydroquinone. J Environ Chem Eng 11(3):110186. https://doi.org/10.1016/j.jece.2023.110186
Liu C, Zheng J, Zhang B, Zhong X, Wang W, Li Z (2023b) BSA-Cu3(PO4)2 hybrid nanoflower-an efficient and low-cost nanoenzyme for decolorization of organic pollutants. Anal Bioanal Chem 415(9):1687–1698. https://doi.org/10.1007/s00216-023-04563-4
Lv X, Foda MF, He J, Zhou J, Cai J (2023) Robust and facile label-free colorimetric aptasensor for ochratoxin A detection using aptamer-enhanced oxidase-like activity of MnO2 nanoflowers. Food Chem 401:134144. https://doi.org/10.1016/j.foodchem.2022.134144
Ma J, Zhang S, Duan X, Wang Y, Wu D, Pang J, Wang X, Wang S (2021) Catalytic oxidation of sulfachloropyridazine by MnO2: effects of crystalline phase and peroxide oxidants. Chemosphere 267:129287. https://doi.org/10.1016/j.chemosphere.2020.129287
Mao YW, Zhang J, Zhang R, Li JQ, Wang AJ, Zhou XC, Feng JJ (2023) N-doped carbon nanotubes supported Fe-Mn dual-single-atoms nanozyme with synergistically enhanced peroxidase activity for sensitive colorimetric detection of acetylcholinesterase and its inhibitor. Anal Chem 95(22):8640–8648. https://doi.org/10.1021/acs.analchem.3c01070
Martins MBMS, Correa GA, Moniz T, Medforth CJ, de Castro B, Rebelo SLH (2023) Nanostructured binuclear Fe(III) and Mn(III) porphyrin materials: tuning the mimics of catalase and peroxidase activity. J Catal 419:125–136. https://doi.org/10.1016/j.jcat.2023.02.001
Masomboon N, Ratanatamskul C, Lu MC (2009) Chemical oxidation of 2,6-dimethylaniline in the Fenton process. Environ Sci Technol 43(22):8629–8634. https://doi.org/10.1021/es802274h
Meng Y, Zhao K, Zhang Z, Gao P, Yuan J, Cai T, Tong Q, Huang G, He D (2020) Effects of crystal structure on the activity of MnO2 nanorods oxidase mimics. Nano Res 13(3):709–718. https://doi.org/10.1007/s12274-020-2680-5
Mishra P, Lee J, Kumar D, Lauro RO, Costa N, Pathania D, Kumar S, Lee J, Singh L (2022) Engineered nanoenzymes with multifunctional properties for next-generation biological and environmental applications. Adv Func Mater 32(8):2108650. https://doi.org/10.1002/adfm.202108650
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77(18):3865–3868. https://doi.org/10.1103/PhysRevLett.77.3865
Qu M, Qin G, Fan J, Du A, Sun Q (2021) Theoretical insights into the performance of single and double transition metal atoms doped on N-graphenes for N2 electroreduction. Appl Surf Sci 537:148012. https://doi.org/10.1016/j.apsuse.2020.148012
Sun M, He M, Jiang S, Wang Y, Wang X, Liu T, Song C, Wang S, Rao H, Lu Z (2021) Multi-enzyme activity of three layers FeOx@ZnMnFeOy@Fe-Mn organogel for colorimetric detection of antioxidants and norfloxacin with smartphone. Chem Eng J 425:131823. https://doi.org/10.1016/j.cej.2021.131823
Tang C, Fang T, Chen S, Zhang D, Yin J, Wang H (2023) Citrate-functionalized osmium nanoparticles with peroxidase-like specific activity for highly efficient degradation of phenolic pollutants. Chem Eng J 464:142726. https://doi.org/10.1016/j.cej.2023.142726
Villaluz FDA, de Luna MDG, Colades JI, Garcia-Segura S, Lu MC (2019) Removal of 4-chlorophenol by visible-light photocatalysis using ammonium iron(II) sulfate-doped nano-titania. Process Saf Environ Prot 125:121–128. https://doi.org/10.1016/j.psep.2019.03.001
Wan K, Jiang B, Tan T, Wang H, Liang M (2022) Surface-mediated production of complexed center dot ·OH radicals and Fe=O species as a mechanism for iron oxide peroxidase-like nanozymes. Small 18(50):2204372. https://doi.org/10.1002/smll.202204372
Wang L, Chen Y (2020) Luminescence-sensing Tb-MOF nanozyme for the detection and degradation of estrogen endocrine disruptors. ACS Appl Mater Interfaces 12(7):8351–8358. https://doi.org/10.1021/acsami.9b22537
Wang X, Nan Z (2020) Highly efficient Fenton-like catalyst Fe-g-C3N4 porous nanosheets formation and catalytic mechanism. Sep Purif Technol 233:116023. https://doi.org/10.1016/j.seppur.2019.116023
Wang B, Zou J, Shen X, Yang Y, Hu G, Li W, Peng Z, Banham D, Dong A, Zhao D (2019) Nanocrystal supracrystal-derived atomically dispersed Mn-Fe catalysts with enhanced oxygen reduction activity. Nano Energy 63:103851. https://doi.org/10.1016/j.nanoen.2019.06.047
Wang Y, Wang H, Wang L, Cai B, Chen H (2022) Removal of high-concentration 4-Chlorophenol (4-CP) in wastewater using carbon-based heterogeneous catalytic oxidation: performance and mechanism. J Clean Prod 346:131176. https://doi.org/10.1016/j.jclepro.2022.131176
Wei S, Sun Y, Qiu YZ, Li A, Chiang CY, Xiao H, Qian J, Li Y (2023) Self-carbon-thermal-reduction strategy for boosting the Fenton-like activity of single Fe-N4 sites by carbon-defect engineering. Nat Commun 14(1):7549–7549. https://doi.org/10.1038/s41467-023-43040-5
Xia Q, Zhang D, Yao Z, Jiang Z (2022) Revealing the enhancing mechanisms of Fe-Cu bimetallic catalysts for the Fenton-like degradation of phenol. Chemosphere 289:133195. https://doi.org/10.1016/j.chemosphere.2021.133195
Xing N, Lyu Y, Yang J, Zhang X, Han Y, Zhao W, Ng DHL, Li J (2022) Motion-based phenol detection and degradation using 3D hierarchical AA-NiMn-CLDHs@HNTs-Ag nanomotors. Environ Sci-Nano 9(8):2815–2826. https://doi.org/10.1039/d2en00322h
Xu L, He Z, Wei X, Shang Y, Shi J, Jin X, Bai X, Shi X, Jin P (2023a) Facile-prepared Fe/Mn co-doped biochar is an efficient catalyst for mediating the degradation of aqueous ibuprofen via catalytic ozonation. Chem Eng J 461:142028. https://doi.org/10.1016/j.cej.2023.142028
Xu Z, Sun M, Xu X, Cao X, Ippolito JA, Mohanty SK, Ni B-J, Xu S, Tsang DCW (2023b) Electron donation of Fe-Mn biochar for chromium(VI) immobilization: key roles of embedded zero-valent iron clusters within iron-manganese oxide. J Hazard Mater 456:131632. https://doi.org/10.1016/j.jhazmat.2023.131632
Yamashita T, Hayes P (2008) Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl Surf Sci 254(8):2441–2449. https://doi.org/10.1016/j.apsusc.2007.09.063
Yang H, Xue W, Liu M, Yu K, Yu W (2020) Carbon doped Fe3O4 peroxidase-like nanozyme for mitigating the membrane fouling by NOM at neutral pH. Water Res 174:115637. https://doi.org/10.1016/j.watres.2020.115637
Yun ET, Lee JH, Kim J, Park HD, Lee J (2018) Identifying the nonradical mechanism in the peroxymonosulfate activation process: singlet oxygenation versus mediated electron transfer. Environ Sci Technol 52(12):7032–7042. https://doi.org/10.1021/acs.est.8b00959
Zeng T, Zhang X, Wang S, Ma Y, Niu H, Cai Y (2014) Assembly of a nanoreactor system with confined magnetite core and shell for enhanced Fenton-like catalysis. Chem –Eur J 20(21):6474–6481. https://doi.org/10.1002/chem.201304221
Zhang WH, Quan X, Zhang ZY (2007) Catalytic reductive dechlorination of p-chlorophenol in water using Ni/Fe nanoscale particles. J Environ Sci 19(3):362–366. https://doi.org/10.1016/S1001-0742(07)60060-6
Zhang M, Wu D, Ye Y, Wu L, Yao Z, Ma X, Wang L, Zhang Z, Xiang S (2018) Thermal conversion of MOF@MOF: synthesis of an N-doped carbon material with excellent ORR performance. ChemPlusChem 83(11):1044–1051. https://doi.org/10.1002/cplu.201800392
Zhang L, Liu Z, Deng Q, Sang Y, Dong K, Ren J, Qu X (2021) Nature-inspired construction of MOF@COF nanozyme with active sites in tailored microenvironment and pseudopodia-like surface for enhanced bacterial inhibition. Angew Chem Int Ed 60(7):3469–3474. https://doi.org/10.1002/anie.202012487
Zhang C, Zhang X, Ye Y, Ni P, Chen C, Liu W, Wang B, Jiang Y, Lu Y (2022) Manganese-doped iron coordination polymer nanoparticles with enhanced peroxidase-like activity for colorimetric detection of antioxidants. Analyst 147(2):238–246. https://doi.org/10.1039/d1an01953h
Zhu W, Li Y, Dai L, Li J, Li X, Li W, Duan T, Lei J, Chen T (2018) Bioassembly of fungal hyphae/carbon nanotubes composite as a versatile adsorbent for water pollution control. Chem Eng J 339:214–222. https://doi.org/10.1016/j.cej.2018.01.134
Zhu MP, Yang JCE, Duan X, Zhang DD, Wang S, Yuan B, Fu ML (2020) Interfacial CoAl2O4 from ZIF-67@γ-Al2O3 pellets toward catalytic activation of peroxymonosulfate for metronidazole removal. Chem Eng J 397:125339. https://doi.org/10.1016/j.cej.2020.125339
Funding
The authors acknowledge the financial support from the Key Science and Technology Projects of Anhui Province (202003a07020004) and National Key R&D Program of China (2019YFC0408500).
Author information
Authors and Affiliations
Contributions
XL: conceptualization, methodology, validation, formal analysis, investigation, software, visualization, and writing—original draft. HL: methodology and investigation. ZL: investigation. MC: methodology. KC: writing—review and editing. ZG: writing—review and editing. ZD: writing—review and editing. BW: writing—review and editing. XC: conceptualization, supervision, project administration, funding acquisition, investigation, and writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors listed have approved the manuscript that is enclosed.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: George Z. Kyzas
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lv, X., Liu, H., Li, Z. et al. Critical role of zero-valent iron in the efficient activation of H2O2 for 4-CP degradation by bimetallic peroxidase-like. Environ Sci Pollut Res 31, 10838–10852 (2024). https://doi.org/10.1007/s11356-023-31754-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-31754-4