Abstract
Anthropogenic alterations have paramount impacts on the alpha and beta diversity of aquatic resources, and fishes are predominantly susceptible to such impacts. Mahanadi River, one of the major peninsular rivers of India, has abundant fish resources, which play a significant role in supporting the fishers’ livelihoods. The exploratory study in the river conducted for three consecutive years recorded 148 species under 53 families. Cyprinids dominated the fish diversity with 41 species, followed by Bagrids (9) and Sciaenids (7). One hundred-one species under 29 families were reported from the freshwater stretch. With a total of 111 species reported under 48 families, the estuarine and tidal freshwater stretch was more speciose, due to marine migrant species which advent the estuarine and tidal freshwaters stretch for breeding and feeding purposes. Tikarpara, a conserved site within a sanctuary, was the most species-diverse as well as a species-even site. The study also recorded the extension of the distributional range of 3 fish species and also 4 exotic species from the river. The seasonal variations in diversity indicated that the deviations were not prominent in freshwater sites, whereas in tidal brackish water sites, species richness was relatively higher in post-monsoon, and species evenness was higher during monsoon. Taxonomic distinctness test showed that the average taxonomic distinctness was high for tidal estuarine locations as they harbour taxonomically distant fishes. The hierarchical clustering of sites showed the inordinate effect of river gradient and fragmentation on the fish community structure. Analyzing the key drivers of the assemblage structure of the entire river, salinity was the major deterministic factor, and within the freshwater stretch, the major influences were depth, transparency, and specific conductivity. The study concluded that, despite all of its ecological stresses, Mahanadi still supports rich fish diversity, yet there is a notable shift in the fish community structure. There is a need for integrating molecular and morphological tools for the taxonomic revision of many genera and species for proper in situ and ex situ conservation measures and to formulate future biodiversity management plans addressing to reduce the impacts of the ecological threats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lotic ecosystems and their biodiversity provide conspicuous services to human beings including potable water and food and recreation and also facilitate nutrient cycles and energy flows that connect biological systems from continental interiors to the oceans (Peterson et al. 2001). It is well established that lotic environments especially rivers are highly speciose and contain a disproportionate amount of biodiversity given their relatively small proportion of the worldwide landscape (Sheldon 1988; Allan and Flecker 1993; Ward 1998; Dudgeon et al. 2006; Tedesco et al. 2017). Concomitantly, they are also subjected to frequent, often intense, hydrologic disturbances that are both natural and anthropogenic in nature (Resh et al. 1988; Leigh et al. 2011), and these fast-changing environments with multiple habitat alterations pose significant challenges for sustaining healthy fish communities and maintaining entire ecosystem integrity, as fishes are highly susceptible to anthropogenic impacts (Dudgeon 2019).
Understanding the processes and mechanisms that underpin distributions of biodiversity has been a core objective of ecological research since the advent of ecology (Darwin 1859; Haeckel 1866). Owing to the enormous variability of aquatic biodiversity, understanding their distribution has important implications in studying species’ niches, in the assessment of anthropogenic impacts, and in formulating conservation and management plans for the resources (Gavioli et al. 2022). The most prevalent way to investigate biodiversity patterns is the study of variations in taxonomical species diversity (Colwell & Coddington 1994), and another effective approach is to appraise the three different levels of taxonomical diversity, i.e., alpha (the local diversity), beta (the variation of community composition among sites), and gamma diversity (the regional diversity) using the different measures developed to investigate each level of diversity.
Several perennial and seasonal rivers traverse the Indian subcontinent covering the length and breadth of India. Most Indian rivers harbour extremely rich biodiversity and contribute significantly to the food security and livelihood of India, fisheries being the major contributor. Though considerable effort has been put into studying the fish fauna of Indian rivers, most previous studies tended to assess the diversity by listing the species inhabiting them, and hence, knowledge gaps remain about the different levels of diversity of Indian rivers as well as the diversity drivers at different scales. Despite some recent studies on fish assemblage structure and dispersal dynamics of fish communities, most rivers of India are still unexplored in these detailed aspects of fish diversity and community structure. There are very few studies on the regional drivers of alpha and beta diversity of ichthyofauna in Indian rivers. Hydroelectric and other developmental projects in different rivers of India are leading to increasing alterations to their natural habitats and flow regimes. Other anthropogenic activities including mining, land-use changes, and pollution also contribute to the stress on ecosystems, and all these formed the most important themes with regard to investigations of fish biodiversity in rivers. Many such studies indicated that fish communities are at risk from these impacts as many species have very restricted distribution (Ponniah and Gopalakrishnan 2000) due to river fragmentation and habitat destruction.
Mahanadi River is the third largest river of peninsular India, originating in the Bastar hills in Chhattisgarh in the northernmost boundary of the Eastern Ghats and joining the Bay of Bengal after dividing into branches and forming a delta. Mahanadi River basin is one of the recognized climatic vulnerable regions of India, with noted decadal reduction in stream flow and high modulations in the hydrology posing a threat to this vulnerable coastal ecosystem (Panda et al. 2013). The overall hydrological regimes of the Mahanadi basin have been highly fragmented through the construction of numerous dams, barrages, and weirs and experience pollution as its banks are heavily populated with profound impacts on all forms of biodiversity. River fragmentation has been identified as a significant driver of biodiversity changes in the Neotropics (Su et al. 2021), and a study by Sajina et al. (2022) on the biotic integrity based on fish assemblages of Mahanadi indicated that the ecological health of the river has been impaired in some stretches, attributable to the stressors impacting the river ecosystem.
Diversity measures have potential application in two main areas, conservation and environmental monitoring, as diversity is used as an index of ecosystem well-being (Magurran 1988). Across the globe, rivers are continuously being explored and re-explored by scientists for swotting diversity. There have been many earlier studies on the fish diversity of the Mahanadi, and most of them focussed on a particular stretch or tributary and listed the species present without analyzing the diversity pattern of the river. With the objective to appraise the status of fish diversity and its assemblage structure across the river continuum, the present study was conducted to examine the variation in alpha and beta diversity of fish communities in the Mahanadi River. Effort was also taken to analyze the shifts in diversity, and assemblage pattern in comparison to the historical data on the same is also discussed keeping the important ecological stressors acting on the ecosystem. Hence, the present study is a revisit into the status of fish diversity in the Mahanadi River and how this diversity shifts along the river course.
Material and methods
Study area
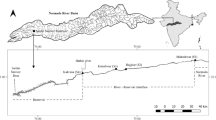
The Mahanadi River, with a total length of 851 km, has one of the largest drainage basins on the east coast of India covering 141,589 km2 (WRIS 2011), extending over major parts of Chhattisgarh and Odisha and smaller parts of Jharkhand, Maharashtra, and Madhya Pradesh. It has a tropical climate with a humid monsoon. Mahanadi estuary is one of the major estuaries in India, which lies in the Cuttack and Puri districts of Odisha and drains into the Bay of Bengal (CWC 2014). The tidal estuarine part of the river covers a length of 40 km and has a basin area of 9 km2 (Sundaray et al. 2009).
Data collection
Eleven sampling stations covering a total distance of about 700 km of the river (including the tidal and brackish water stretch) were selected for the study (Fig. 1). Among the eleven sites, eight sites (S1 to S8) fall in the freshwater stretch, and the three sampling stations viz. Kujang (S9), Bhutumundai (S10), and Paradeep (S11) were selected from the tidal brackish water stretches for the study. As there is no major control structure upstream of the Hirakud Reservoir, site S1 can be considered an unregulated river, whereas the river is more or less regulated downstream of the Hirakud Dam. The selected sites included three sites immediately downstream of a dam, barrage, and weir, and they were S2 (downstream of Hirakud dam), S7 (Kandarpur, downstream of Mundali weir), and S8 (downstream of Jobra barrage). Some of the sites were totally urban (like S3, Sambalpur), whereas site S5 (Tikarpara) was falling within Satkosia Wildlife Sanctuary. The habitat profile of the selected sites is provided in Table 1
Sampling was carried out seasonally for three years (2012–2015) in Mahanadi River. Fish were sampled through experimental fishing. Fishing was done in different mesohabitats, such as pools, riffles, and raceway areas, and it was made sure that uniform effort was used in all sampling stations in terms of gear and duration. Gill netting with three different mesh sizes (small, 20–30 mm; medium, 45–60 mm; and large, 90–100 mm) for a duration of 6 h, and 10 casts of cast nets were done for a single sampling. Fishes were identified in the field, and representative specimens were preserved in 10% formalin and transferred to the laboratory for confirming field identification. The fishes were identified following identification keys given by Talwar and Jhingran (1991) and Jayaram (2010) and related recent taxonomic literature for particular species. In order to assess the fish assemblage structure across our studied stretch of river, we quantified variation in the relative abundance of fishes belonging to different guilds, across the sampling sites. The major criteria considered for categorizing different guilds were trophic level, dwelling habit, species resilience, and tolerance level. FishBase (Froese and Pauly 2022) was followed for categorizing species based on the trophic guild, niche of occurrence, tolerance, and species resilience.
Data analysis
The within-habitat taxonomic diversity or alpha diversity was assessed using simple diversity indices. The fish abundance data were analyzed for assessing the species richness, species dominance or evenness, and species diversity using Primer 7 software. Various indices, viz. Margalef richness index (d), Pielou’s evenness index (J′), Simpson’s index (1 − λ), and Shannon’s index (H′), were estimated for each site. Species-wise relative abundance was estimated from species abundance data pooled for all sites and seasons, as well as for each site, as a percentage of total abundance (Mahajan and Fatima 2017).
Beta diversity is the variation in species composition between areas of alpha diversity, and the alternative approach to the measurement of beta diversity is to investigate the degree of association or similarity of sites or samples using standard ecological techniques of ordination and classification (Greig-Smith 1983; Pielou 1984; Southwood 1978). In order to estimate beta diversity and to measure the differences in fish assemblages along the river continuum, the Jaccard index of similarity was calculated using PAST. As there are a number of sites in the investigation, hierarchical clustering of sites using the Bray–Curtis similarity of fish abundance data was carried out for obtaining a good representation of beta diversity. For depicting the species dominance trends graphically, a k-dominance plot was made using Primer 7.
In order to reflect the community differences among samples, non-metric multi-dimensional scaling (nMDS) plots were constructed for the Bray–Curtis similarities of the square root transformed species abundance data, using Primer 7. To explore how the environmental parameters control or influence the fish faunal distribution, canonical correspondence analysis (CCA) was done using PAST. As there was distinct differentiation of freshwater and brackish water sites, the dbRDA (distance-based redundancy analysis) procedure was carried out for only freshwater sites with fish abundance data as resemblance matrix and the thirteen water quality parameters as predictor variables using PERMANOVA + add-on of Primer 7 software. The water quality data was normalized, whereas fish abundance data were square-rooted before converting to a resemblance matrix.
Results
Present status of fish diversity
Alpha diversity patterns
A total of 40,405 fishes belonging to 147 species under 53 families were obtained as samples from the eleven sampling sites of the Mahanadi River. The eight freshwater sites yielded 33,567 samples belonging to 101 species under 29 families. With a total of 111 species reported under 48 families, the estuarine and tidal freshwater stretch below Jobra was more speciose than the freshwater stretch. The highest values of alpha diversity were found in the estuarine and tidal freshwater stretch from where 46 fish species under 24 families were exclusively reported. This is due to the record of marine migrant species which advent the estuarine and tidal freshwaters stretch for breeding and feeding purposes.
The alpha diversity estimated from the abundance data pooled for all seasons divulged that the species richness (S) was maximum at Bhutumundai (S10) with 74 species, followed by Kandarpur (S7) and Hirakud (S2) with 70 and 68 species, respectively. The Margalef richness index was maximum for the tidal estuarine sites (S10 and S9) and S7 among freshwater sites. Based on Simpson’s index and Shannon’s index, the highest diverse site was S6, which is a conserved site within a sanctuary. The species evenness was also maximum at S6 (Online Resource 1).
Higher taxon diversity
Following the classification of Nelson et al. (2016), there were 22 orders represented among fishes recorded from the river during the study. The species-rich order was Cypriniformes, whereas the family-rich orders were Siluriformes and Perciformes with 11 families each (Online Resource 2). In evolutionary point of view, the three most primitive orders were Elopiformes, Anguilliformes, and Osteoglossiformes, whereas the most advanced order was Tetraodontiformes, following Nelson et al. (2016).
Family Cyprinidae with 41 species dominated the fish fauna of the river system followed by families Bagridae and Sciaenidae (with 7 species each). With 6 species in each, and families Clupeidae and Mugilidae were also prominent in the system. The four exclusive freshwater families which were not recorded from the estuarine and tidal freshwater stretch were Anguillidae, Nemacheilidae, Pangasiidae, and Ritidae.
The dominant carps in the order of abundance were Cirrhinus reba, Labeo calbasu, L. gonius, L. bata, L. catla, C. mrigala, L. rohita, and Bangana dero. Among the barbs, minnows and other minor cyprinids, Amblypharyngodon mola, Osteobrama cotio, Devario devario, Pethia ticto, Puntius sophore, Systomus sarana, and Puntius chola were prominent. The most abundant catfishes were Ailia coila, R. chrysea, Mystus cavasius, Wallago attu, and Sperata aor. The two clupeid species Gonialosa manmina and Gudusia chapra, Nandid Nandus nandus, Ambassid Chanda nama, and Goby Glossogobius giuris were the other abundant species in the river.
Endemic and exotic fishes
Though the fishes of Mahanadi were listed under ichthyofauna of Eastern Ghats (Menon 1951; Devi and Indra 2003), fish fauna of the Mahanadi basin are predominantly Gangetic with very few endemic characteristics in comparison to Western Ghat rivers (Menon 1951). There are some species that are highly endemic to the Mahanadi River. Rita chrysea, or the Mahanadi rita, is a species of nanobagrid catfish endemic to the Mahanadi River system (Menon 1999), and the species is abundantly available in the freshwater stretch of the river. Another endemic species recorded in the study is Tor mosal mahanadicus the Mahanadi mahseer, indigenous to the Mahanadi River system (Mani et al 2010; Khare et al. 2014).
Four exotic/alien species were recorded from Mahanadi viz. North African catfish Clarias gariepinus, Grass carp Ctenopharyngodon idella, Bighead carp Hypophthalmichthys nobilis, and Nile Tilapia Oreochromis niloticus. The invasion coefficient index (Singh et al. 2013) which is the proportional abundance of each species relative to the total abundance, calculated for the four species ranged from 0 to 0.039, indicating that the invasion level of exotics is not to the extent of posing any invasion risk until the study period in the river.
Conservation status of fishes
The conservation status as per the IUCN red list (IUCN 2022) was referred for the species recorded from Mahanadi (Online Resource 3). The only Endangered species, Asian catfish Clarias magur, was recorded throughout the river except for the estuarine sites. Two vulnerable species were Wallago attu and Bagarius yarrelli. Ailia coila, Anguilla bengalensis, Bagarius bagarius, Chitala chitala, Ompok bimaculatus, O. pabda, Parambassis lala, and Protonibea diacanthus were the eight species under the near-threatened category. Out of the total 147 species, 122 were under the least concern category, of which 14 species showed a decreasing population trend.
Diversity variation along the river continuum
k-Dominance
As diversity and dominance are inverse complementary concepts, the k-dominance plot helped to depict the species dominance trends graphically (Fig. 2). The curve for fish abundance of Jobra and Bhutumundai is higher for lower values of k, indicating these sites have less diverse than the other sites. Tikarpara seems to have a lesser curve for all values of k, indicating the site is unambiguously more diverse than the other locations.
Jaccard index of dissimilarity
Beta diversity was estimated in the present study to ascertain how species abundance differs along the river gradient (Table 2). The most downstream site, Paradip showed the lowest value for the Jaccard index of dissimilarity with most other sites (with index value less than 0.1 with all freshwater sites), indicating its unique species composition owing to the marine connection. The index value was above 0.75 between five pairs of freshwater sites, and the Hirakud site showed similarity to three other sites.
Hierarchical clustering of sites
In order to find the similarity among different stretches based on fish species abundance, hierarchical clustering was done using the Bray–Curtis similarity of fish abundance data (Fig. 3). The clustering was so distinctive indicating the diversity pattern along the river continuum explicitly. The most downstream site (S11) located at the proper estuarine region individually formed a separate cluster (C1). All the remaining sites (S1 to S10) formed a big cluster with four sub-clusters; the most upstream site (S1) subclustered (C2) together with three barrage sites S2, S7, and S8 (C3) and remaining freshwater sites S3 to S6 (C4). The two tidal-influenced sites (S9 and S10) also formed a separate sub-cluster (C5). The clustering showed the inordinate effect of river gradient and fragmentation on the fish community structure.
Non-metric multi-dimensional scaling (nMDS)
nMDS was performed to visualize the level of similarities and/or dissimilarities among sites in a distance-based ordination plot to provide insight on the fish community composition of the river. The bubble plot of nMDS formed two bubbles at a 20% similarity level, clearly separating S11, the most downstream site from the rest of the sites. At a 40% similarity level, six bubbles were formed, with all the freshwater sites in all the seasons encompassed in a single bubble, whereas the rest 5 bubbles occupied the three tidal freshwater and estuarine sites. Monsoon samples of the downstream sites showed distinctness from other seasons (Fig. 4).
Taxonomic distinctness
The species richness for individual sampling locations ranged from 50 to 74 and the average taxonomic distinctness (AvTD, △ +) varied from 79.01 to 88. 22 (Fig. 5). The sites S9, S10, and S11 were having higher values for △ + . Funnel plot simulated for different locations with △ + of Mahanadi River showed that the three locations S9, S10, and S11 were lying within the funnel of 95% of simulated values of △ + , whereas the remaining sites were lying below the limit. The values of variation in taxonomic distinctness (VarTD, Ʌ +) ranged from 242.81 to 632.33 with the locations S9, S10, and S11 having the lowest values. The ellipse plot of simulated △ + and Ʌ + pairs indicated that all the sampling locations were within the 95% probability contours (Fig. 5).
Seasonal variations in abundance and diversity
The seasonal variations in diversity indices estimated from site-wise pooled abundance data indicated that the deviations in the richness, diversity, and species evenness measures were not prominent in freshwater sites, and there was no particular pattern in the seasonal deviations. In tidal brackish water sites, species richness was relatively higher in the post-monsoon season, whereas species evenness was higher in the monsoon season. The richness index values were notably low in monsoon in comparison to the other three seasons. The Pielou’s evenness index and Shannon’s diversity index followed similar trend in seasonality (Fig. 6).
Fish assemblage structure
Fish assemblage structure was examined among the different clusters formed in the hierarchical grouping of sites along the river gradient. The relative abundance of the major ten species in the clusters exposed discrete assemblage structure in each cluster (Online Resource 4). In cluster 1 (the single site cluster of most downstream site), the assemblage was a mix of marine migrants such as anchovies, sardines, and perches, resident species of mullets, and freshwater migrants like bagrid catfish, Mystus gulio. In cluster 2 (the single site cluster of most upstream site), the endemic catfish R. chrysea dominated the fish assemblage along with barb Systomus sarana, freshwater clupeid Gudusia chapra, carp Labeo dero, etc. In cluster 3 (comprising sites immediately downstream of dam, barrage, and weir), the fish assemblage structure consisted of relatively smaller fishes with low population doubling time such as catfishes, freshwater clupeids, barbs, and minor carps. Cluster 5 (formed of two tidal-influenced sites), river sprat Corica soborna dominated the assemblage with other small fishes such as Chanda nama, Aplocheilus panchax, and Pethia ticto.
In order to assess the fish assemblage structure across our studied stretch of river, we quantified variation in the relative abundance of fishes belonging to different guilds, across the sampling sites. The major criteria considered for categorizing different guilds were trophic level, dwelling habit, species resilience, and tolerance level (Online Resource 5). Among the three trophic guilds, omnivores dominated the ecosystem, followed by carnivores, and the least abundant were the herbivores (Fig. 7). The niche occupancy of fishes indicated a prevalence of column-dwelling fishes over benthic and pelagic dwellers. Taking account of the tolerance level of fishes, medium-tolerant ones were the majority of the population. The population doubling time (PDT) of the fishes showed that nearly half of the population were highly resilient with low PDT scores (Fig. 7).
Key drivers of fish community structure
Major environmental factors that influence fish abundance and their distribution were explored for the river using the canonical correspondence analysis (CCA). As the number of species was too high for species-level analysis, guild-wise analysis was done. The first two axes of CCA explained 75.11% of the total variations. CCA biplot (Fig. 8) indicated that salinity, conductivity, hardness, dissolved oxygen, and nitrate-nitrogen are the major deterministic factors for fish distribution in the river. The estuarine sites dominated by omnivorous and tolerant fish guilds were found to be greatly influenced by salinity and conductivity as well as nutrients such as phosphate (P) and nitrate (N).
In order to elucidate the significant environmental drivers influencing fish community structure, dbRDA was carried out only for the freshwater stretch of the river as the CCA plots pointed towards the profound effect of salinity on fish faunal distribution. In the dbRDA plot, the two axes described the percentage of variation in terms of the total fish community structure, viz. axis 1 of the dbRDA accounted for 35.2% of the fitted variation (20% of the total variation) and strongly correlated with depth and transparency. Axis 2 explained 16.8% of the fitted variation (9.5% of total variation) and appears largely associated with specific conductivity and total hardness. Vector overlays showed that conductivity was inversely related to depth and transparency, indicating shallower stretches in the studied river having high conductivity (Fig. 9).
Discussion
The first study on the fish diversity of the Mahanadi River was carried out by Day who reported 146 species mostly collected from the Cuttack region (Day 1889). The other notable works included Hora (1940) which reported 43 species from the headwaters of the river; Chauhan (1947) listed 54 fish species from the Tel River, a tributary of river Mahanadi; Job et al. (1955) reported more than 103 species from Mahanadi and 86 species from different localities both above and below the Hirakud Dam; and Jayaram and Majumder (1976) reported 42 species. More recent studies by Patel et al. (2016) and Singh et al. (2020) compiled and compared the fishes reported by earlier workers, and a maximum of 107 species have been reported so far from the freshwater stretches of the river. Tyagi et al. (2021) provided a checklist of fishes from the entire stretch of Mahanadi with 121 fish species, which included freshwater, estuarine, and marine migrant fishes from the river. The present study recorded 101 species which is the maximum freshwater fish diversity ever recorded in a single study from Mahanadi River.
While comparing the previous species records from the Mahanadi River with the present study, a large number of species were consistently reported by different researchers since Hora (1940), such as Clarias magur, Heteropneustes fossilis, Mystus cavasius, Channa gachua, C. punctata, C. marulius, C. striata, Parambassis ranga, Nandus nandus, Glossogobius giuris, Xenentodon cancila, Lepidocephalichthys guntea, Acanthocobitis botia, and Pachypterus atherinoides. The present record of cyprinid diversity is as rich as the earlier studies with the occurrence of Amblypharyngodon mola, Barilius barna, B. bendelisis, Cabdio morar, Cirrhinus mrigala, Ctenopharyngodon idella, Cirrhinus reba, Pethia ticto, Systomus sarana, Puntius sophore, Rasbora daniconius, Salmostoma bacaila, Garra mullya, Labeo boggut, and Laubuca laubuca. The cyprinid diversity reported by Hora (1940) and Jayaram and Majumdar (1976) was relatively fewer, whereas the fish diversity works in the last two decades (Prakash 2004; Dahire 2008; Patel et al. 2016) explored many Cyprinid species such as Labeo boga, Labeo calbasu, Labeo gonius, Tor tor, and Tor mosal. The occurrence of exotics such as Clarias gariepinus and Oreochromis niloticus was reported since 2004. With the advancements in modern technology, the ichthyofaunal diversity has been largely explored using molecular tools throughout the world. Apart from the few molecular studies based on mitochondrial DNA sequencing for determining the genetic variation among the river populations of certain species (Channa marulius by Habib et al. 2011; 2012, Sperata seenghala by Kumari et al. 2017), there is not much focused molecular level studies on the ichthyofauna of Mahanadi.
The present study recorded the extension of the distributional range of a few fishes. Awaous grammepomus, the scribbled goby, was a new record in the present study from river Mahanadi. There are a few clupeid species, which were only recorded in the recent diversity studies from the river. The Ganges River sprat, Corica soborna, was recorded in the present study as well as in the latest checklist by Tyagi et al. (2021) from Mahanadi, though Whitehead (1972) and Khan (2002) inadvertently stated Mahanadi River as the type locality of the species instead of “Mahananda” River from where the species was originally described by Hamilton (1822). The clupeoid species Pellona ditchela (Family, Pristigasteridae) was reported from the freshwater stretch of the river in our study. This anadromous species has a very broad distribution along the Indo-West Pacific region, and the species have been reported from many Indian waters viz. Andaman and Nicobar Islands (Rajan et al. 2013) and Indian Sundarbans (Khan 2002; Mukherjee et al. 2012). Hooghly-Matlah estuary (Chatterjee et al. 2000; Manna et al. 2014), Narmada estuary (Bhakta et al. 2020), and from Mahanadi by Tyagi et al. (2021) for the first time.
Zoological Survey of India has exhaustively studied the Mahanadi estuary and reported around 180 species of fishes from the estuary which included more than 150 marine migrant species, indicating the Mahanadi estuarine system as one of the richest and most productive estuaries in India (ZSI 1998). The number of marine migrants recorded in our study was relatively less since our sampling explorations were up to 7 km upstream of the sea mouth point. The estuarine fisheries of the river system play a key role in the livelihood of the local fishermen.
The populations of most diadromous fishes are dwindling in the river system due to increased river fragmentation. There are some diadromous fishes reported in the study such as catadromous eel Anguilla bengalensis, anadromous shad Tenualosa ilisha, and pipe fish Microphis brachyurus. There existed a rich fishery for T. ilisha in the Mahanadi estuary during the 1950s and 1960s (Jhingran and Natarajan 1969), and the construction of many anicuts and dams in the river has impacted the migration of the species greatly (Bhaumik 2013).
The River Continuum Concept (Vannote et al. 1980) describes the entire river system as a continuously integrating series of physical gradients and associated biotic adjustments as the river flows from headwater to mouth, which is a generalized conceptual framework for the characterization of pristine running water ecosystems (Stazner and Higler 1985). The upstream–downstream gradient of changing physical conditions and linked biotic variations postulated by the concept has been documented as the occurrence of a distinct pattern of longitudinal zonation of species in many tropical freshwater systems too (Toham and Teugels 1998; Bhat 2004; Bhat and Magurran 2006). These studies also established that in general, the downstream reaches of rivers are more diverse than upstream ones (Bhat and Magurran 2006). Fish communities in Mahanadi also revealed a strong upstream-to-downstream gradient, along the river. Taxonomic distinctness test indicated that the average taxonomic distinctness (Δ +) for downstream tidal estuarine locations fell within the 95% confidence funnel, whereas the upstream freshwater locations were below it. Δ + is the mean path length through the taxonomic tree connecting every pair of species, and it is high in the downstream stretches because fish communities in downstream tidal estuarine stretches consisted of several species common to upstream reaches in addition to taxonomically distant marine migrant species and estuarine resident fishes. At the same time, the fish communities in the upstream freshwater stretches were mostly comprised of closely related species (dominantly cyprinids and catfishes) resulting in low taxonomic distinctness. As Ʌ + is the variance of pairwise path lengths and reflects the unevenness of the taxonomic tree, the downstream sites of the river yielded lower values of Ʌ + due to the increased evenness in the tree with more regularities in the taxonomic hierarchy. The hierarchical clustering of sites also showed a remarkable salinity gradient indicating the diversity pattern along the river continuum. The separate clustering of unregulated sites and barrage sites indicates that flow regime and connectivity are the factors, influencing riverine fish diversity, as described by Shukla and Bhat (2018).
The seasonality in richness and evenness of fish diversity in tidal freshwater stretches was more prominent than in freshwater stretches, which can be attributed to the connectivity pattern of river and coastal waters. Patterns of connectivity across seasons influence species composition and assemblage structure by influencing the abundance of local immigrations and emigrations (Taylor 1997). Besides connectivity patterns, seasonality of breeding and feeding migration of fishes between freshwater and coastal waters also contributes to the variation. The cause of lower richness and higher evenness values in monsoon in tidal freshwater stretches might be due to increased quantity of freshwater flux and subsequent distribution and dilution of fish abundance to the increased floodplain area (Bower et al. 2019). Studies indicate that the quantity of freshwater flux to the Bay of Bengal has declined at the outlet of the Mahanadi River basin, with high interannual variability (Panda et al. 2013), which would have a reflective impression on the fish fauna and fish assemblage structure. Our study revealed a lack of protuberant seasonal changes in the fish community structure in upstream stretches of the river.
Fish assemblage and food web structure can change across habitat and trophic resource gradients that shift longitudinally in rivers. Knowledge on how lotic food webs vary with environmental changes along longitudinal gradients is important for the management and conservation of river ecosystems (East et al. 2017). Since flow regime and water quality are highly related to each other, any changes in either component may impose influences on the fish community to a certain extent (Cheng et al. 2016; Destouni et al. 2017; Marzin et al. 2012; Schinegger et al. 2012). Analyzing the trophic structure, omnivores dominated over herbivores, and the trend of increasing abundance of omnivores indicated increasing ecological degradation (Sajina et al. 2022).
Anthropogenic activities impacting the aquatic ecosystems affect the inhabiting fish communities greatly. Habitat alterations and destruction are important threats to freshwater biodiversity and distribution patterns (Dudgeon et al. 2006). The onset of the Anthropocene was the period of intense dam building in rivers across the globe (Somanna et al. 2016), and in Mahanadi River, it started with the construction of the multipurpose Hirakud Dam in 1957 and resulted in a total of 254 mainly small- and medium-scale dams within the drainage basin (WRIS 2014). The proliferation of dams and barriers leads to a significant decline in sediment supply up to 67% in the river (Hazra et al. 2020). Since the 1950s, the coastal districts of the delta have also witnessed a rapid increase in population (especially Bhubaneswar city), growth of a port, industrial development along with increased groundwater extraction, small- and medium-scale irrigation projects, and deforestation of mangroves. Besides being a highly fragmented river, Mahanadi is facing various pollution threats as its banks are heavily populated, and it traverses through industrial cities and townships. Mahanadi River is contaminated with sewages, industrial effluents, and agricultural runoff, yet the metal contamination levels detected in the sediment of the river were at the lower level, with mild to no effect on the biota (Samanta et al. 2020). Climatic extremes have also the potential to distress the Mahanadi delta adversely as Odisha is the fifth most flood-prone state in India with exposure to recurrent floods and waterlogging (Hazra et al. 2020).
Taking account of the above-mentioned natural and anthropogenic threats confronted by the Mahanadi River, the crucial impending challenge for fish diversity conservation is to assess the extinction risk of threatened and vulnerable species in such a changing environment. There is a great need to formulate future biodiversity management plans addressing to reduce the impacts of ecological threats. River habitat loss has a direct consequence on fish diversity (Aarts et al. 2004; Barletta et al. 2010) and is a serious social and economic issue in all developing countries including India. Habitat management and conservation need to shift to a more pro-active approach supported by better scientific methods. For taking up ex situ and in situ conservation measures to preserve the germplasm of diverse fish fauna of the river, long-term management plans are needed, and extensive surveys and studies are to be carried out to generate information on breeding behaviour, migration, and spawning grounds. Efforts are to be taken for captive breeding and seed production of threatened fishes for initiating river ranching programmes.
Conclusion
The present study showed that Mahanadi still supports rich fish faunal diversity, yet there is a notable shift in the fish community structure. It also underlined the occurrence of a strong upstream-to-downstream gradient, along the river continuum. The key drivers of the alpha and beta diversity of fish fauna of the river were the ecological parameters that enact much influence over the fish community structure. As rivers are germplasm reserves of native fish fauna, immediate measures are to be taken to conserve the dwindling fish diversity. There are only a few focused molecular-level studies on the ichthyofauna of Mahanadi, and there is a need for integrating molecular and morphological tools for taxonomic revision of many genera and species distributed in the river so that they are precisely defined for proper in situ and ex situ conservation measures.
Data availability
The study is a result of institutional project of the ICAR-CIFRI, and the data would be available on legitimate request.
References
Aarts BG, Van Den Brink FW, Nienhuis PH (2004) Habitat loss as the main cause of the slow recovery of fish faunas of regulated large rivers in Europe: the transversal floodplain gradient. River Res Appl 20(1):3–23
Allan JD, Flecker AS (1993) Biodiversity conservation in running waters. Bioscience 43:32–42
Barletta M, Jaureguizar AJ, Baigun C, Fontoura NF, Agostinho AA, Almeida-Val VMF, Val AL, Torres RA, Jimenes-Segura LF, Giarrizzo T, Fabré NN, Batista VS, Lasso C, Taphorn DC, Costa MF, Chaves PT, Vieira JP, Corrêa MFM (2010) Fish and aquatic habitat conservation in South America: a continental overview with emphasis on neotropical systems. J Fish Biol 76:2118–2176. https://doi.org/10.1111/j.1095-8649.2010.02684.x
Bhakta D, Solanki S, Vadhel N, Meetei WA, Kamble SP, Chandra G, Samanta S, Das BK (2020) Finfish diversity of River Narmada and its tributaries. Proc Zool Soc 73:7. https://doi.org/10.1007/s12595-020-00336-4
Bhat A (2004) Patterns in the distribution of freshwater fishes in rivers of Central Western Ghats, India and their associations with environmental gradients. Hydrobiologia 529:83–97
Bhat A, Magurran AE (2006) Taxonomic distinctness in a linear system: a test using a tropical freshwater fish assemblage. Ecography 29(1):104–110
Bhaumik U (2013) Decadal studies on hilsa and its fishery in India- a review. J Interacad 17(2):377–405
Bower LM, Keppeler FW, Cunha ER, Quintana Y, Saenz DE, Lopez-Delgado EO, Bokhutlo T, Arantes CC, Andrade MC, Robertson CR, Mayes KB, Winemiller KO (2019) Effects of hydrology on fish diversity and assemblage structure in a Texan coastal plains river. Trans Am Fish Soc 148:207–218. https://doi.org/10.1002/tafs.10129
Chatterjee TK, Ramakrishna Talukdar S, Mukherjee AK (2000) Fish and fisheries of Digha coast of West Bengal, Rec Zool Surv India. Occasional Paper 188:1–87
Chauhan BS (1947) Fish fauna of the Balangir district (formerly Patna state). Orissa Rec Indian Mus 51:395–416
Cheng S, Herricks EE, Tsai W, Chang F (2016) Assessing the natural and anthropogenic influences on basin-wide fish species richness. Sci Total Environ 572:825–836
Colwell RK, Coddington JA (1994) Estimating terrestrial biodiversity through extrapolation. Philos Trans R Soc London B Biol Sci 345(1311):101–118
CWC (2014) Mahanadi basin. New Delhi: Ministry of Water Resources, Govt. of India. https://www.india-wris.nrsc.gov.in/
Dahire V (2008) Fish diversity in the riverine resources of Janjgir- Champa district of Chhattisgarh, India. M.F.Sc Thesis, Dept. of Fisheries, IGKV, Raipur, p 78
Darwin C (1859) On the origin of species by means of natural selection, or, the preservation of favoured races in the struggle for life. J. Murray, London, p 502p
Day F (1889) Fauna of British India, including Ceylon and Burma, Fishes, 1 and 2. Taylor and Francis, London
Destouni G, Fischer I, Prieto C (2017) Water quality and ecosystem management: data-driven reality check of effects in streams and lakes. Water Resour Res 53(8):6395–6406
Devi KR, Indra TJ (2003) An updated checklist of ichthyofauna of Eastern Ghats. Zoos’ Print J 18(4):1067–1070
Dudgeon D (2019) Multiple threats imperil freshwater biodiversity in the Anthropocene. Curr Biol 29(19):R960–R967. https://doi.org/10.1016/j.cub.2019.08.002
Dudgeon D, Arthington AH, Gessner MO, Kawabata ZI, Knowler DJ, Lévêque C, Naiman RJ, Prieur-Richard AH, Soto D, Stiassny ML (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev 81:163–182
East JL, Wilcut C, Pease AA (2017) Aquatic food-web structure along a salinized dryland river. Freshw Biol 62(4):681–694
Froese R, Pauly D (Eds) (2022) FishBase. World Wide Web electronic publication. www.fishbase.org, version (02/2022)
Gavioli A, Milardi M, Soininen J, Soana E, Lanzoni M, Castaldelli G (2022) How does invasion degree shape alpha and beta diversity of freshwater fish at a regional scale? Ecol Evol 12(11):e9493. https://doi.org/10.1002/ece3.9493
Greig-Smith (1983) Quantitative plant ecology, 3d ed. - Blackwell, London
Habib M, Lakra WS, Mohindra V, Khare P, Barman AS, Singh A, Lal KK, Punia P, Khan AA (2011) Evaluation of cytochrome b mtDNA sequences in genetic diversity studies of Channa marulius (Channidae: Perciformes). Mol Biol Rep 38:841–846. https://doi.org/10.1007/s11033-010-0175-2
Habib M, Lakra WS, Mohindra V, Lal KK, Punia P, Singh RK Khan, AA (2012) Assessment of ATPase 8 and ATPase 6 mtDNA sequences in genetic diversity studies of Channa marulius (Channidae: Perciformes). Proc Natl Acad Sci India Sect B Biol Sci 82:497–501. https://doi.org/10.1007/s40011-012-0061-x
Haeckel E (1866) Generelle morphologie der organismen, (vol 2). Allgemeine Entwicklungsgeschichte. Georg Reimer, p 606
Hamilton F (1822) An account of the fishes found in the river Ganges and its branches. Archibald Constable & Company, Edinburgh, p 405
Hazra S, Das S, Ghosh A, Raju PK Patel A (2020) The Mahanadi Delta: a rapidly developing delta in India. Deltas in the Anthropocene. Springer, pp 53–77
Hora SL, (1940) On a collection of fish from the headwaters of the Mahanadi River, Raipur district, CP
IUCN (2022) The IUCN red list of threatened species. Version 2022–1. https://www.iucnredlist.org
Jayaram KC (2010) The freshwater fishes of the Indian region. Narendra Publishing House, New Delhi, India
Jayaram KC, Majumdar N (1976) On a collection of fish from the Mahanadi. Rec Zool Surv India 69:305–323
Jhingran VG, Natarajan AV (1969) Derivation of average lengths of different age-groups in fishes. J Fish Res Board Can 26(11):3073–3076. https://doi.org/10.1139/f69-290
Job TJ, David A, Das KN (1955) Fish and fisheries of the Mahanadi in relation to the Hirakud Dam. Indian J Fish 2(1):1–3ti
Khan RA (2002) Fish faunal resources of Sunderban Estuaries System with special reference to the biology of some commercially important species. Rec Zool Surv India Occ Paper 209:1–150 ((Published by Director, Zool. Surv. India, Kolkata))
Khare P, Mohindra V, Barman AS, Singh RK, Lal KK (2014) Molecular evidence to reconcile taxonomic instability in mahseer species (Pisces: Cyprinidae) of India. Org Divers Evol 14:307–326
Kumari P, Pavan-Kumar A, Kumar G, Alam A, Parhi JJ, Gireesh-Babu P, Chaudhari A, Krishna G (2017) Genetic diversity and demographic history of the giant river catfish Sperata seenghala inferred from mitochondrial DNA markers. Mitochondrial DNA Part A 28(6):920–926. https://doi.org/10.1080/24701394.2016.1209195
Leigh C, Stewart-Koster B, Sheldon F, Burford MA (2011) Understanding multiple ecological responses to anthropogenic disturbance: rivers and potential flow regime change. Ecol Appl 22:250–263
Magurran AE (1988) Ecological diversity and its measurement. University Press, Cambridge, p 177p
Mahajan M, Fatima S (2017) Frequency, abundance, and density of plant species by list count quadrat method. Int J Multidiscip Res 3(7):1–8
Mani I, Kumar R, Kushwaha B, Singh M, Nagpure NS, Srivastava PK, Lakra WS (2010) Cytogenetic characterization of an endemic mahseer, Tor mosal mahanadicus (David, 1953; Teleostei: Cyprinidae). The Nucleus 53:109–114
Manna RK, Roshith CM, Das SK, Suresh VR, Sharma AP (2014) Salinity regime and fish species distribution in the Hooghly-Matlah Estuary. In: Sinha RK, Ahmed,B (eds) Rivers for life - Proceedings of the International Symposium on River Biodiversity: Ganges-Brahmaputra-Meghna River System, Ecosystems for Life, A Bangladesh-India Initiative, IUCN, International Union for Conservation of Nature, p 340
Marzin A, Archaimbault V, Belliard J, Chauvin C, Delmas F, Pont D (2012) Ecological assessment of running waters: do macrophytes, macroinvertebrates, diatoms and fish show similar responses to human pressures? Ecol Indic 23:56–65
Menon AGK (1951) Further studies regarding Hora’s Satpura hypothesis. The role of Eastern Ghats in the distribution of Malayan fauna and flora to peninsular India. Proc Nat Inst Sci India 17(6):475–497
Menon AGK (1999) Check list–fresh water fishes of India, Rec. Zool. Sur. India, Miscellaneous Publication, Occasion Paper No 175, p 366
Mukherjee S, Chaudhuri A, Sen S, Homechaudhuri S (2012) Effect of Cyclone Aila on estuarine fish assemblages in the Matla River of the Indian Sundarbans. J Trop Ecol 28:405–415. https://doi.org/10.1017/S026646741200020X
Nelson JS, Grande TC, Wilson MV (2016) Fishes of the world. John Wiley & Sons
Panda DK, Kumar A, Ghosh S, Mohanty RK (2013) Streamflow trends in the Mahanadi River basin (India): linkages to tropical climate variability. J Hydrol 495:135–149
Patel G, Chari MS, Kumar S, Bhakta D, Behera S, Verma K, Chawan RR, Kumar V, Ahmed T (2016) Fish fauna diversity of Mahanadi River in Raigarh district. Chhattisgarh J Exp Zool India 19(1):1285–1289
Peterson BJ, Wollheim WM, Mulholland PJ, Webster JR, Meyer JL, Tank JL, Martí E, Bowden WB, Valett HM, Hershey AE (2001) Control of nitrogen export from watersheds by headwater streams. Science 292:86–90
Pielou EC (1984) The interpretation of ecological data: a primer on classification and ordination. John Wiley & Sons
Ponniah AG, Gopalakrishnan A (eds) (2000) Endemic fish diversity of Western Ghats. Proceedings of the workshop on “Germplasm inventory, evaluation and gene banking of freshwater fishes of Western Ghats” organised by National Bureau of Fish Genetic Resources during 12th – 13th October, 1998 at Central Marine Fisheries Research Institute, Cochin, Kerala, p 347
Prakash (2004) Fish diversity in the water resources of northern part of Raipur district of Chhattisgarh state. M.F.Sc Thesis Dept. of Fisheries, IGKV, Raipur
Rajan PT, Sreeraj CR, Immanuel TITUS (2013) Fishes of Andaman Andaman and Nicobar Islands: a checklist. J Andaman Sci Assoc 18(1):47–87
Resh VH, Brown AV, Covich AP, Gurtz ME, Li HW, Minshall GW, Reice SR, Sheldon AL, Wallace JB, Wissmaar RC (1988) The role of disturbance in stream ecology. J N Am Benthol Soc 7:433–455
Sajina AM, Sudheesan D, Samanta S, Paul SK, Bhowmick S, Nag SK, Kumar V, Das BK (2022) Development and validation of fish-based index of biotic integrity for assessing ecological health of Indian rivers Mahanadi and Kathajodi-Devi. Aquat Ecosyst Health Manag 25(2):25–35
Samanta S, Kumar V, Nag SK, Raman RK, Saha K, Bandyopadhyay S, Mohanty BP, Das BK (2020) Metal contaminations in sediment and associated ecological risk assessment of river Mahanadi. India Environ Monit Assess 192:1–17
Schinegger R, Trautwein C, Melcher A, Schmutz S (2012) Multiple human pressures and their spatial patterns in European running waters. Water Environ J 26(2):261–273
Sheldon AL (1988) Conservation of stream fishes: patterns of diversity, rarity, and risk. Conserv Biol 2:149–156
Shukla R, Bhat A (2018) Nestedness patterns and dispersal dynamics in tropical central Indian stream fish metacommunities. Freshw Sci 37(1):147–158. https://doi.org/10.1086/695849
Singh AK, Kumar D, Srivastava SC, Ansari A, Jena JK, Sarkar UK (2013) Invasion and impacts of alien fish species in the Ganga River. India Aquat Ecosyst Health Manag 16(4):408–414
Singh TK, Swain SK, Guru BC (2020) Fish diversity of Mahanadi River (Odisha part), threats and conservation measures. Int J Life Sci 8:2355–371 (ISSN:2320–7817(p) | 2320–964X(o))
Somanna K, Reddy ST, Rao SM (2016) Geomorphology and evolution of the modern Mahanadi Delta using remote sensing data. Int J Sci Res 5(2):1329–1335
Southwood TRE (1978) Ecological methods, 2d ed. - Halstead Press, New York. p 391
Stazner B, Higler B (1985) Questions and comments on the river continuum concept. Can J Fish Aquat Sci 42:1038–1044
Su G, Logez M, Xu J, Tao S, Villéger S, Brosse S (2021) Human impacts on global freshwater fish biodiversity. Science 371(6531):835–838. https://doi.org/10.1126/science.abd3369
Sundaray SK, Nayak BB, Bhatta D (2009) Environmental studies on river water quality with reference to suitability for agricultural purposes: Mahanadi River estuarine system, India–a case study. Environ Monit Assess 155:227–243
Talwar PK, Jhingran AG (1991) Inland fishes of India and adjacent countries. Volumes I & II. Oxford & IBH Publishing, New Delhi
Taylor CM (1997) Fish species richness and incidence patterns in isolated and connected stream pools: effects of pool volume and spatial position. Oecologia 110(4):560–566
Tedesco P, Beauchard O, Bigorne R, Blanchet S, Buisson L, Conti L, Cornu JF, Dias MS, Grenouillet G, Hugueny B, Jézéquel C (2017) A global database on freshwater fish species occurrence in drainage basins. Sci Data 4:170141. https://doi.org/10.1038/sdata.2017.141
Toham AK, Teugels GG (1998) Diversity patterns of fish assemblages in the Lower Ntem River basin (Cameroon), with notes on potential effects of deforestation. Archiv Für Hydrobiologie 141(4):421–446
Tyagi LK, Chandran R, Mandal S, Mayekar TS, Bisht AS, Singh SK, Lal KK (2021) A checklist of fishes of Mahanadi River. India J Indian Fish Assoc 48(1):1–11
Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE (1980) The river continuum concept. Can J Fish Aquat Sci 37(1):130–137
Ward JV (1998) Riverine landscapes: biodiversity patterns, disturbance regimes, and aquatic conservation. Biol Conserv 83:269–278
Whitehead PJP (1972) A synopsis of the Clupeoid fishes of India. J Mar Biol Assoc India 14(1):160–256
WRIS, 2011. River basins. Water Resource Information System of India http://india-wris.nrsc.gov.in/wrpinfo/index.php?title=Basins
WRIS, 2014. Dams in Mahanadi. Water Resource Information System of India. http://india-wris.nrsc.gov.in/wrpinfo/index.php?title=Dams_in_Mahanadi
ZSI (1998) Fauna of Mahanadi estuary (Orissa). Zoological Survey of India, Kolkata. VIII+1–218
Acknowledgements
The authors are grateful to ICAR, New Delhi and the Director, ICAR-CIFRI for the support to conduct the research. The authors thank the institute personnel who assisted during the research. The cooperation rendered by the fishers and local authority during sample collection is gratefully acknowledged. Thanks are due to Dr. Vaisakh G. for helping to prepare the map of the river. The work was carried out under an institutionally funded project “Monitoring and benchmarking ecosystem health of major river systems in India” of ICAR-Central Inland Fisheries Research Institute, Barrackpore, India.
Funding
This study was funded by the Indian Council of Agricultural Research, New Delhi.
Author information
Authors and Affiliations
Contributions
SAMConceptualization, data collection and field work, and article drafting.
SMNArticle drafting.
DSData collection.
SSWork co-ordination and data collection.
SKPData collection and field work (fish diversity studies).
SBData collection and field work.
VKData analysis.
BKDOverall guidance.
Corresponding author
Ethics declarations
Ethical approval
The study was conducted as per the guidelines of the institute ethical committee.
Consent to participate
The authors gave their consent to participate in the study.
Consent for publication
The authors consented to publish the study.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Thomas Hein
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
A. M., S., Nair, S.M., Sudheesan, D. et al. Reconnoitre on ichthyofauna of Mahanadi River of India: shifting diversity down the river continuum and linking ecological traits with patterns in biodiversity. Environ Sci Pollut Res 31, 5684–5698 (2024). https://doi.org/10.1007/s11356-023-31411-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-31411-w