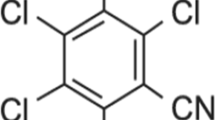
Abstract
Insecticides are widely used for pest control and frequently reach aquatic systems, imposing a risk to the biota. In this work, the effect of environmental concentrations of bifenthrin on the grazing capacity of Simocephalus vetulus (Cladocera) and Argyrodiaptomus falcifer (Copepoda) on phytoplankton was evaluated. Fifteen microcrustacean individuals and a natural phytoplankton assemblage dominated by Cyanobacteria were exposed during 46 h to three concentrations of bifenthrin (C0 0 μg L−1, C1 0.02 μg L−1, and C2 0.05 μg L−1). A significant decrease in both microcrustaceans grazing rates on total phytoplankton was observed in C2 compared to C0 and C1. The filtration rate (ml ind−1 h−1) of S. vetulus decreased significantly for the cyanobacteria Anabaenopsis arnoldii, Dolichospermum circinale, and Glaucospira sp. in C2 compared to C0 and C1. The ingestion rate (org ind−1 h−1) of A. falcifer decreased significantly in C1 and C2 compared to C0 only for A. arnoldii. Regarding phytoplankton morphological groups, the filtration rate of S. vetulus decreased in C1 and C2 compared to C0 for Colonies and Coenobiums in C2 concerning C0 and C1 for Filaments and in C2 compared to C0 for Silicified. For A. falcifer, the ingestion rate was reduced in C2 compared to C0 for Silicified, Flagellated, and Sessile. The results showed that bifenthrin affected both microcrustaceans grazing capacity on phytoplankton, especially at the highest insecticide concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Globally, agricultural land covers about five billion hectares, approximately 38% of the world’s land surface. Around one-third of this surface is used for crop cultivation, while the remaining two-thirds are meadows and pastures used for livestock (FAO 2022). The indiscriminate use of agrochemicals to maximize crop yield has resulted in adverse effects on the environment, including air, water, and soil pollution, as well as harm to non-target organisms and human health (Elahi et al. 2019). This phenomenon is in increase, considering that the pesticides applied have increased from 4.0 to 8.7 million tons between 2019 and 2020 (FAO 2022). Based on the data regarding ecological effects, we know that agricultural insecticide use, in combination with nutrient and habitat degradation, is likely a significant driver of biodiversity loss in aquatic ecosystems affected by agriculture (Stehle and Schulz 2015).
Pesticides have various routes of entry into surface waters, including runoff by precipitations, one of the primary pathways, leaching through soil to groundwater, air transport and deposit by wind or precipitation, or direct application. Ultimately, the extensive use of pesticides in agriculture leads to widespread contamination of surface waters, which can damage biota (Hladik et al. 2015; Sanchez-Bayo and Goka 2014; Sy et al. 2022).
Pesticides are known to harm non-target species at various levels of organization, including individuals, populations, and ecosystems. For instance, at the individual level, pesticides can lead to direct toxicity, which may cause death, stunted growth, or impaired reproduction. Similarly, at the population level, the use of pesticides can lead to a decrease in the number of individuals, causing a decline in population size or even local extinction. Additionally, at the ecosystem level, pesticides can impact community structure, disturb food webs, and reduce biodiversity. Hence, the adverse effects of pesticides can be extensive, leading to significant consequences at multiple levels of organization (Stehle and Schulz 2015). However, the toxicity of pesticides on zooplankton differs depending on various factors, including species, genotype, life stage, and size. Populations in the growth phase are particularly susceptible to the harmful effects of pesticides, but they also can recover promptly from the damage. Pesticides can affect population dynamics by impeding individual survival and reproduction and disrupting the sex ratio (Hanazato 2001).
Zooplankton is a valuable community in aquatic trophic networks as it is a primary food source for numerous aquatic organisms, including fish and larger invertebrates. They consume phytoplankton and bacteria, facilitating the transfer of energy and nutrients between various trophic levels. Additionally, they act as regulators of phytoplankton populations by limiting their growth and preventing the occurrence of algae blooms. Zooplankton also participates in nutrient cycling within aquatic ecosystems by excreting and releasing nutrient-rich fecal pellets that other organisms can utilize as a source of nutrients (e.g., Hutchins et al. 1995; Pace and Cole 2002; Sommer and Lengfellner 2008). Previous studies suggest that the impact of pesticides on zooplankton may result in changes to their swimming behavior and body morphology, which could have a ripple effect on the ecosystem dynamics, such as the elongation of the food chain and the reduction of energy transfer efficiency from primary producers to top predators (Hanazato 2001; Mendoca et al. 2018).
The grazing effects of zooplankton on Cyanobacteria, a bacteria group characterized by having oxygen-evolving photosynthesis as algae and Plantae (Reynolds 2006), are highly variable, and current literature suggests that it may be species-dependent (Frau 2022). For example, some studies like Lazzaro et al. (2003), Von Rückert and Giani (2008), and Lacerot et al. (2013) concluded that zooplankton grazing was null or inefficient in assemblages dominated by Cyanobacteria. In contrast, some studies, such as Nadin-Hurley and Duncan (1976), found that larger Daphnia species could consume filamentous cyanobacteria in bundles resembling spaghetti. Similarly, Panosso et al. (2003) reported positive outcomes when studying the effects of Notodiaptomus iheringi (Wright S.) (Copepoda) on filamentous, single-cell, and small colonies of cyanobacteria, although they preferred other food items. Additionally, Bouvy et al. (2001) and Kâ et al. (2012) demonstrated that certain tropical rotifers such as Brachionus spp., Keratella spp., and some copepods such as Notodiaptomus cearensis (Wright S.) and Thermocyclops decipiens (Kiefer), were able to cut and consume large filamentous cyanobacteria. Dos Santos Severiano et al. (2018) and Diniz et al. (2019) reported that calanoid copepods and cladocerans were effective grazers on colonial and filamentous cyanobacteria.
Cyanobacteria blooms should not be overlooked due to their potential to cause environmental, health, and economic issues, as evidenced by Huisman et al. (2005), Merel et al. (2013), Drobac et al. (2013), and Carmichael et al. (2016), among others. However, little is known about insecticides’ effect on zooplankton capacity of feeding and controlling Cyanobacteria blooms. In this study, we tested the effect of several environmental concentrations of the insecticide bifenthrin on the grazing rates of two microcrustaceans over a natural phytoplankton assemblage dominated by Cyanobacteria. We hypothesized that both microcrustaceans feed on Cyanobacteria, but their grazing capacity is diminished in the presence of bifenthrin, in a concentration-dependent manner.
Material and methods
To achieve the proposed aim, we designed a microcosm experiment with two microcrustacean species: Simocephalus vetulus (O.F. Müller) (Cladocera) and Argyrodiaptomus falcifer (Daday) (Copepoda) feeding on a natural phytoplankton assemblage dominated by Cyanobacteria. Microcrustaceans were collected from shallow isolated lakes in the Paraná River floodplain. The organisms were gradually adapted to develop laboratory stock cultures with 16:8h light darkness photoperiod, 22 ± 1 °C in dechlorinated tap water, and were fed with Tetradesmus obliquus (Turpin) M.J. Wynne prior to the experiment began. The phytoplankton assemblage was obtained from a shallow urban lake that recurrently presents Cyanobacteria blooms (Frau et al. 2019). Microcrustaceans were gradually acclimated to the experimental medium for a week. The experimental design consisted of a control (C0: 0 μg L−1), a low (C1: 0.02 μg L−1), and a high concentration of bifenthrin (C2: 0.05 μg L−1). A commercial formulation (Bifentrin10®, Formulagro S.R.L., Argentina, bifenthrin 10%) was employed. Exposure concentrations were selected based on preliminary mortality tests and environmental reports of bifenthrin in surface water (e.g., Ensminger et al. 2013; Weston et al. 2015; Budd et al. 2020). Bifenthrin concentrations were determined with C18 solid phase extraction (SPE C18) and liquid chromatography coupled to triple quadruple mass spectrometry (LC–MS/MS). The insecticide concentrations were set up in three conditions: phytoplankton alone, S. vetulus addition (15 adults), and A. falcifer addition (15 adults) per replicate. All treatments were replicated three times (n = 27) in 250 ml transparent glass vessels, and the experiment lasted 46 h. The experiment was performed at 22 ± 1 °C and photoperiod of 16:8h light darkness. Samples for phytoplankton quantification were taken at the experiment’s beginning and the end (Fig. 1).
Every 24 h, neonates were removed, and microcrustacean mortality was recorded at the end of the experiment. At the beginning and the end of the experiment, relevant limnological variables like temperature (°C), pH, conductivity (μS cm−1), and dissolved oxygen (DO, mg L−1) were measured with a HACH multiparameter probe. In addition, samples for inorganic nutrient analyses (nitrite-nitrate, ammonium, and soluble reactive phosphorus) were taken and processed following APHA (2005) methods. Samples for bifenthrin quantification were also taken at the beginning and end of the experiment and sent to a specialized lab for its quantification. Phytoplankton counting was performed following the method proposed by Utermöhl (1958) using an inverted microscope NIKON to 400X magnification. Counting was done until 100 individuals of the dominant species were registered in the sample to secure a counting error of < 20% (Venrick 1978). The density obtained was expressed as ind mL−1.
Data analysis
Since S. vetulus is a filter-feeding cladoceran, a filtration rate (ml ind−1 h−1) was calculated following Wetzel and Likens (2000). For A. falcifer, an ingestion rate was estimated according to Paffenhöfer (1971), considering that it is a raptorial copepod. Both filtration and ingestion rates, respectively, were estimated considering the total phytoplankton density, the dominant phytoplankton species of the assemblage (≥ 50% of total density), and phytoplankton morphological groups: Sessile, Flagellates, Colonies and Coenobiums, Filaments, and Silicified species (Table 1).
Filtration and ingestion mean rates at the end of the experiment were compared between bifenthrin treatments (C0, C1, and C2) through one-way analyses of variance (ANOVA) with a previous evaluation of parametric application requirements (homoscedasticity and normality). Tukey tests were applied as post-hoc analyses, considering for all the comparisons an α = 0.05.
Results
Experimental conditions
During the experiment, the environmental variables showed low variation between treatments and sampling dates, with a mean temperature of 22 °C, circumneutral pH, conductivity of 2000 μS cm−1, and DO of 8 mg L−1 (P > 0.05 for all of them) (Table 2). Inorganic nutrients concentrations were for ammonium 135 μg L−1 (± 44), for nitrite+nitrate: 23 μg L−1 (± 17), and for soluble reactive phosphorus the concentration was below the detection limit in all the vessels. Bifenthrin concentrations in the medium decreased through time and varied from 0.02 μg L−1 at the beginning to 0.006 μg L−1 at the end in C1 and from 0.05 μg L−1 to 0.008 μg L−1 in C2. No bifenthrin was detected in control vessels.
Regarding phytoplankton, the initial medium accounted for thirty-eight species. Sixteen were Chlorophyceae, eight Cyanobacteria, seven Bacillariophyceae, four Euglenophyceae, two Cryptophyceae, and one Dinophyceae. The total density recorded at the begging of the experiment was 4551 ind mL−1. Cyanobacteria was the dominant group accounting for 50% of total density, followed by Bacillariophyceae (28%), Chlorophyceae (11%), Cryptophyceae (9%), and Dinophyceae (2%). Dominant species were three Cyanobacteria: Anabaenopsis arnoldii Aptekar, Dolichospermum circinale (Rabenhorst ex Bornet & Flahault) Wacklin, Hoffmann & Komárek, Glaucospira sp., and one diatom species Ulnaria acus (Kützing) Aboal, accounting for more than 70% of total density in all treatments. No statistically differences were found in phytoplankton density of the three controls (C0) (control without bifenthrin, control with low bifenthrin concentration and control with high bifenthrin concentration, F = 2.48, p = 0.35). Microcrustacean survival at the experiment’s end was generally higher than 85%. In C0, a mean of 6% of deaths were reported for both microcrustaceans, in C1 (6% for the cladoceran and 13% for the copepod) and C2 (13% for the cladoceran and 27% for the copepod).
Microcrustaceans grazing rates under bifenthrin effects
For S. vetulus, the maximum filtration rate was reported in C1 with 0.78 ml ind−1 h−1 and the lowest in C2 concentration (0.40 ml ind−1 h−1). Regarding A. falcifer, the maximum ingestion rate was registered in the control (C0) with 654 org ind−1 h−1, and the lowest in C2 (169 org ind −1 h−1). The grazing rates on total phytoplankton decreased significantly in C2 compared to C0 and C1 for both, S. vetulus (F = 9.39, P = 0.014) and A. falcifer (F = 12.02, P = 0.008) (Fig. 2a and b, respectively).
Mean values and standard deviations of S. vetulus (a) and A. falcifer (b) filtration and ingestion rates, respectively, at the end of the experiment, by considering the total phytoplankton density registered on each treatment (C0, C1, and C2). Arrows show Tukey test significant differences between treatments
Our specific analysis on the phytoplankton species dominance revealed that for S. vetulus, the highest filtration rates were obtained for Glaucospira sp., followed by A. arnoldii and U. acus, while the lowest was for D. circinale. Simocephalus vetulus filtration rates decreased significantly in C2 compared to C0 and C1 for the three cyanobacteria species: Anabaenopsis arnoldii (F = 26.95, P = 0.001), D. circinale (F = 13.09, P = 0.006), and Glaucospira sp. (F = 15.41, P = 0.04). These differences were found between C0 vs. C2 and C1 vs. C2. No differences in the filtration rate were found for U. acus (F = 0.85, P = 0.47) (Fig. 3a). In contrast, A. falcifer ingestion rate was higher for A. arnoldii, followed by U. acus, and Glaucospira sp. No grazing over D. circinale was registered. A. falcifer ingestion rates decreased significantly in C1 and C2 compared to C0 just for A. arnoldii (F = 8.06, P = 0.02) (Fig. 3b).
Mean values and standard deviations of the filtration and ingestion rates, respectively, of S. vetulus (a), and A. falcifer (b) at the end of the experiment by considering dominant species density registered on each treatment (C0, C1, and C2). Arrows show Tukey test significant differences between treatments
Regarding the morphological groups of phytoplankton, different grazing patterns were found among groups and microcrustacean species. S. vetulus filtrated mainly Colonies and Coenobiums, while A. falcifer ingested mainly Filaments, and Silicified phytoplankton. Simocephalus vetulus filtration rate decreased significantly in C2 and C1 compared to C0 for Colonies and Coenobiums (F = 6.68, P = 0.03), in C2 compared to C0 and C1 for Filamentous (F = 17, P = 0.03) and in C2 vs. C0 for Silicified (F = 6.86, P = 0.02) (Fig. 4a). For A. falcifer, the ingestion rate decreased significantly in C2 compared to C0 for Silicified (F = 10.09, P = 0.012), C2 vs. C0 and C1 for Flagellates (F = 21.52, P = 0.02), and C2 vs. C1 for Sessile (F= 27.18, P= 0.001) (Fig. 4b).
Mean values and standard deviations of the filtration and ingestion rate, respectively of S. vetulus (a), and A. falcifer (b) at the end of the experiment by considering phytoplankton morphological groups density registered on each treatment (C0, C1, and C2). Arrows show Tukey test significant differences between treatments
Discussion
In natural ecosystems, zooplankton populations must cope with natural and anthropogenic structuring pressures acting simultaneously (Hanazato 2001). In this experiment, we proved that the pyrethroid bifenthrin, even at low environmental concentrations, affects the grazing capacity of two zooplankton species on phytoplankton. Numerous studies have reported the impact of insecticides on life history traits and behavior of zooplankton, especially those particularly designed to alter the nervous system such as pyrethroids. Specifically, these chemicals can diminish zooplankton ability to escape fish and other invertebrates. Examples of such studies include Groner and Relyea (2011), Hayasaka et al. (2012), and Gutierrez et al. (2020). However, little is known about the effect of insecticides on the grazing capability of zooplankton, and particularly on Cyanobacteria assemblages.
Microcrustaceans’ grazing preferences in the absence of bifenthrin
Both zooplankton species demonstrated an active grazing effect on the phytoplankton assemblage offered in the absence of bifenthrin (C0). However, some patterns probably more related to their feeding strategies and palatability preferences were found. Indeed, filter-feeders Cladocera use their appendages with sieve-like structures to create water flow, which traps particles larger than the filter mesh size for consumption. In contrast, diaptomid Copepods like A. falcifer are selective raptorial feeders and select food items by considering several morphological and physiological characteristics (Titocci et al. 2022).
In our experiment, S. vetulus efficiently fed on filamentous Cyanobacteria, especially Glaucospira sp. following previous evidence supporting the idea that cladocerans may effectively graze on medium size phytoplankton species ( ̴40 μm of maximum linear dimension, MLD) (Frau, 2022) and particularly on small filamentous cyanobacteria (Kâ et al. 2012). Despite their ability to feed on filamentous organisms has been questioned, with this experiment, we contribute to the idea that they effectively feed on filamentous forms in the absence of the insecticide. Their feeding rate, however, seems to be lower in larger and more complex forms of filamentous species, like A. arnoldii (> 40 μm of MLD), and much larger species, like D. circinale (>> 40 μm MLD). The three species of Cyanobacteria dominant in our experiment have a filament morphological structure, but compared with Glaucospira sp., in D. circinale and A. arnoldii, cells appear linked to each other in bundles (much condensed and larger in D. circinale), possibly affecting grazing. We have a low comprehension of the feeding mechanisms used by S. vetulus to ingest these filaments, and new, more specific experimental designs exploring their feeding mechanism should be performed to contribute to this topic. Both species of Cyanobacteria (A. arnoldii and D. circinale) have been reported as potentially toxic (Bernard et al. 2016), and this may also harm cladoceran feeding (Nandini and Sarma 2023). However, for the shallow lake where we collected the phytoplankton assemblage, although several events of Cyanobacteria blooms have been reported in the past (Frau et al. 2019), no toxins production has been detected recently (Frau et al. 2022, unpublished data). Simocephalus vetulus feeding on U. acus was lower than Cyanobacteria species and was not statistically significant in the absence of bifenthrin. Although several studies performed with marine cladocerans have already proved that they may effectively feed on pennate diatoms like U. acus (e.g., Kim et al. 1989, Wong et al. 2006, Katechakis and Stibor 2004), the evidence for freshwater cladocerans is unconclusive (Frau et al. 2017).
Regarding feeding on morphological groups, results were consistent with those found with dominant species, adding information from other phytoplankton groups not represented in the previous classification of dominant species. This is, S. vetulus shown to feed predominantly on Colonies and Coenobiums, a morphological group represented by other phytoplankton taxa, like A. delicatissima, T. glabrum, and D. communis. These results are consistent with previous reports (e.g., Frau et al. 2017). In this respect, it has been reported that Cladocera may feed efficiently in a range between 20 and 50 μm of MLD (Moustaka-Gouni and Sommer 2020). The non-statistically significant feeding on single cells (flagellates and sessile forms) is also consistent with previous studies. Because of their small size and high surface-to-volume ratio, these algae can rapidly counteract the zooplankton grazing impact by absorbing nutrients and displaying high growth rates (Litchman et al. 2010; Colina et al. 2016).
For A. falcifer, grazing pattern effects in the absence of bifenthrin were radically different. For this species, only significant ingestion rates were obtained for A. arnoldii. Previous studies reported efficient grazing effects of Copepoda on filamentous Cyanobacteria, like Panosso et al. (2003), who reported that Notodiaptomus iheringi Wright S. fed on filamentous cyanobacteria. Bouvy et al. (2001) and Kâ et al. (2012) also reported that some copepod species like N. cearensis (Wright S.) and Thermocyclops decipiens (Kiefer) can cut large filamentous cyanobacteria into smaller pieces and consume them. Indeed, in our experiment, we found a similar result in those treatments with A. falcifer at the end of the experiment, where A. arnoldii individuals appeared as shorter filaments with some single cells dispersed in the sample.
When phytoplankton morphological groups were considered, the pattern of grazing observed contrasted with S. vetulus. Indeed, this copepod demonstrated to graze efficiently on groups that S. vetulus did not, such as Silicified, Sessile, and Flagellates. A. falcifer has a selective feeding strategy, which means that it can detect and capture single preys. Previous studies have already reported the feeding capability of this copepod on the morphological groups reported here. Rietzler et al. (2002) reported grazing on Silicified species (diatoms) and some other Chlorophyceae, showing a wide range of sizes and phytoplankton species in the gut content. In the same line, Titocci et al. (2022) also reported for Eudiaptomus sp. an effective grazing effect on small-size phytoplankton species like those represented in the Sessile and Flagellate groups. Frau et al. (2017) also reported that Argyrodiaptomus sp. might feed on single cells and diatoms.
The effects of bifenthrin on microcrustacean's grazing behavior
Bifenthrin is an artificial compound from pyrethrins present in extracts from chrysanthemum flowers. It acts as a neurotoxin by binding to voltage-gated sodium channels in neurons. This insecticide is extensively used in both agricultural and urban settings. However, its high toxicity significantly threatens aquatic life, including fish and invertebrates (Riar 2014). Previous studies reported pyrethroids’ effects, particularly bifenthrin, in aquatic biota (Werner and Moran 2008). However, this may be the first study demonstrating the effect of bifenthrin on zooplankton grazing capability on phytoplankton. In this regard, we found a decrease in the grazing capability for both microcrustacean species used in this experiment, especially with the highest concentration of bifenthrin (C2: 0.05 μg L−1).
For S. vetulus, we found that the filtration rate was diminished in the highest concentration of bifenthrin (C2) compared with the control (C0) when dominant species and morphological phytoplankton groups were considered. Similar results were found by Pestana et al. (2010) with Daphnia magna Straus feeding on Chlorella vulgaris Beijerick. In their experiment, Pestana (op. cit.) tested the effect of another neurotoxic insecticide, the imidacloprid, at a higher test concentration (between 2.2 and 8.8 mg L−1). Similarly, Fernandez et al. (1994) proved the negative effect of sublethal concentrations of endosulfan (0.44 mg L−1) and diazinon (0.47 mg L−1) on the filtration rate of D. magna on a monoculture of Nannochloropsis oculata (Droop) D.J. Hibberd. In this experiment, we test a complete phytoplankton assemblage and several times lower insecticide concentrations representing environmental conditions, and a significant effect on the grazing capacity of this Cladocera was also observed.
The ingestion rate of A. falcifer on A. arnoldii decreased in the presence of the highest concentration of bifenthrin (C2). No changes were observed regarding the other dominant phytoplankton species, which is probably related to the high food selectivity that these diaptomids show. On the contrary, when dominant morphological groups were contemplated, the effect of bifenthrin was more conspicuous. Argyrodiaptomus falcifer decreased its grazing capacity in the presence of the higher bifenthrin concentration with Silicified, Flagellates, and Sessile phytoplankton groups. As was indicated in the previous section of the discussion, our results suggests that A. falcifer has a low preference for Cyanobacteria as a food resource, and its preference for grazing over other phytoplankton groups is negatively affected by the presence of bifenthrin. We could not find much evidence of other pesticides acting on copepod grazing rates. However, the tendency observed seems similar, as denoted by Donghui and Guangxing (2014), who found that Sinocalanus tenellus (Kikuchi K.) (marine copepod) showed a decrease in its filtration rates with increased DDT concentrations.
Previous evidence indicates that copepods are less sensitive to pesticides than cladocerans. For instance, Relyea (2005) showed that two copepods (Eurytemora and Mesocyclops) exposed to carbaryl and malathion, as well as common herbicides such as 2,4-D and glyphosate, were less responsive than other zooplankton groups that exhibited high mortality rates. Groner and Relyea (2011) found that malathion and carbaryl had a lower effect on copepods than cladocerans. Similarly, in a review study, Sanchez-Bayo (2006) revised the effect of 468 organic pollutants on planktonic crustaceans and found that cladocerans are much more sensitive than copepods. In our experiment, copepod mortality was slightly higher, especially at the highest bifenthrin concentration (C2). This suggests that the highest mortality observed for A. falcifer in the treatments with bifenthrin could be more related to the effects that this insecticide has on copepods feeding behavior. This is the presence of the insecticide affected its feeding behavior, suggesting a possible secondary effect of starvation.
Conclusions
With this experiment, we contribute to the knowledge that insecticides may affect microcrustaceans grazing capability over phytoplankton, even considering environmental concentrations that may be found and persist for long periods in water. Bifenthrin effects showed to be more relevant for Cladocera than for Copepoda grazing rates over Cyanobacteria, and this may be attributed to the fact that cladocerans are generalist filter feeders. Particularly for A. falcifer, results showed a decreased selectivity over Cyanobacteria (only on A. arnoldii), but bifenthrin effects appear as equal as relevant by negatively affecting feeding on other phytoplankton groups that may be more palatable for this copepod.
Data availability
Data available under reasonable request.
References
APHA (2005) Standard Methods for the Examination of Water and Wastewater, 21st ed.
Bernard C, Ballot A, Thomazeau S, Maloufi S, Furey A, Mankiewicz-Boczek J, Pawlik-Skowrońska B, Capelli C, Salmaso N (2016) Cyanobacteria associated with the production of cyanotoxins. In: Meriluoto J, Spoof L, Geoffrey A (eds) Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis, 1st edn. John Wiley & Sons, U.S
Bouvy M, Pagano M, Troussellier M (2001) Effects of a cyanobacterial bloom (Cylindrospermopsis raciborskii) on bacteria and zooplankton communities in Ingazeira reservoir (northeast Brazil). Aquat Microb Ecol 25:215–227
Budd R, Wang D, Ensminger M, Phillips B (2020) An evaluation of temporal and spatial trends of pyrethroid concentrations in California surface waters. Sci Total Environ 718:137402
Carmichael WW, Boyer GL (2016) Health impacts from cyanobacteria harmful algae blooms: implications for the North American Great Lakes. Harmful Algae 54:194–212
Colina M, Calliari D, Carballo C, Kruk C (2016) A trait-based approach to summarize zooplankton–phytoplankton interactions in freshwaters. Hydrobiologia 767:221–233
Diniz AS, Dos Santos Severiano JS, Melo Júnior M, Dantas EW, Moura AN (2019) Phytoplankton–zooplankton relationships based on phytoplankton functional groups in two tropical reservoirs. Mar Freshw Res 70:721–733
Donghui X, Guangxing L (2014) The effects of DDT on the feeding, respiration, survival, and reproduction of Sinocalanus tenellus (Copepoda: Calanoida). Acta Oceanol Sin 33:133–138
Dos Santos SJ, Almeida-Melo VLS, Bittencourt-Oliveira MC, Chia MA, do Nascimento Moura A (2018) Effects of increased zooplankton biomass on phytoplankton and cyanotoxins: a tropical mesocosm study. Harmful Algae 71:10–18
Drobac D, Tokodi N, Simeunovic J, Baltic V, Stanic D, Svircev Z (2013) A review: human exposure to cyanotoxins and their effects 507 on health. Arh Hig Rada Toksikol 64:305–316
Elahi E, Weijun C, Zhang H, Nazeer M (2019) Agricultural intensification and damages to human health in relation to agrochemicals: Application of artificial intelligence. Land Use Policy 83:461–474
Ensminger MP, Budd R, Kelley KC, Goh KS (2013) Pesticide occurrence and aquatic benchmark exceedances in urban surface waters and sediments in three urban areas of California, USA, 2008 – 2011. Environ Monit Assess 185:3697–3710
FAO (2022) FAOSTAT: Pesticides Use. FAO, Rome. http://www.fao.org/faostat/en/#data/RP. Accessed Feb 2023
Fernandez Casalderrey A, Ferrando MA, Andreumoliner E (1994) Effect of sublethal concentrations of pesticides on the feeding behavior of Daphnia magna. Ecotoxicol Environ Saf 27:82–89
Frau D (2022) Grazing impacts on phytoplankton from South American water ecosystems: a synthesis. Hydrobiologia 849:833–860
Frau D, Battauz Y, Alvarenga P, Scarabotti P, Mayora G, Sinistro R (2019) Assessing the relevance of top-down and bottom-up effects as phytoplankton structure drivers in a subtropical hypereutrophic shallow lake. Aquatic Ecol 53:265–280
Frau D, Battauz Y, Sinistro R (2017) Why predation is not a controlling factor of phytoplankton in a Neotropical shallow lake: a morpho-functional perspective. Hydrobiologia 788:115–130
Groner ML, Relyea RA (2011) A tale of two pesticides: how common insecticides affect aquatic communities. Freshwater Biol 56:2391–2404
Gutierrez MF, Rojas Molina F, Frau D, Mayora G, Battauz B (2020) Interactive effects of fish predation and sublethal insecticide concentrations on freshwater zooplankton communities. Ecotoxicol Environ Saf 1967:110497
Hanazato T (2001) Pesticide effects on freshwater zooplankton: an ecological perspective. Environ Pollut 112:1–10
Hayasaka D, Korenaga T, Suzuki K et al (2012) Differences in susceptibility of five cladoceran species to two systemic insecticides, imidacloprid and fipronil. Ecotoxicology 21:421–427
Hladik ML, Kolpin DW (2015) First national-scale reconnaissance of neonicotinoid insecticides in streams across the USA. Environ Chem 12:600–611
Huisman JM, Matthijs HCP, Visser PM (2005) Harmful cyanobacteria, Aquatic ecology series. Springer, The Netheralands
Hutchins DA, Wang WX, Fisher NS (1995) Copepod grazing and the biogeochemical fate of diatom iron. Limnol Oceanogr 40:989–994
Kâ S, Mendoza-Vera JM, Bouvy M et al (2012) Can tropical freshwater zooplankton graze efficiently on cyanobacteria? Hydrobiologia 679:119–138
Katechakis A, Stibor H (2004) Feeding selectivities of the marine cladocerans Penilia avirostris, Podon intermedius and Evadne nordmanni. Mar Biol 145:529–539
Kim SW, Onbé T, Yoon YH (1989) Feeding habits of marine cladocerans in the Inland Sea of Japan. Mar Biol 100:313–318
Lacerot G, Kruk C, Luerling M, Scheffer M (2013) The role of subtropical zooplankton as grazers of phytoplankton under different predation levels. Freshwater Biol 58:494–503
Lazzaro X, Bouvy M, Ribeiro-Filho RA, Oliviera VS, Sales LT, Vasconcelos ARM, Mata MR (2003) Do fish regulate phytoplankton in shallow eutrophic Northeast Brazilian reservoirs? Freshwater Biol 48:649–668
Litchman E, de Tezanos PP, Klausmeier CA, Thomas MK, Yoshiyama K (2010) Linking traits to species diversity and community structure in phytoplankton. Hydrobiologia 653:15–28
Merel S, Walker D, Chicana R, Snyder S, Baurès E, Thomas O (2013) State of knowledge and concerns on Cyanobacterial blooms and cyanotoxins. Environ Int 59:303–327
Moustaka-Gouni M, Sommer U (2020) Effects of harmful blooms of large-sized and colonial cyanobacteria on aquatic food webs. Water 12:1587
Nadin-Hurley CM, Duncan A (1976) A comparison of daphnid gut particles with the sestonic particles in two Thames Valley reservoirs throughout 1970 and 1971. Freshwater Biol 6:109–123
Nandini S, Sarma SSS (2023) Experimental studies on zooplankton-toxic cyanobacteria interactions: a review. Toxics 11:176
Pace ML, Cole JJ (2002) Comparative and experimental approaches to top-down and bottom-up regulation of bacteria. Microb Ecol 43:107–117
Paffenhöfer GA (1971) Grazing and ingestion rate of nauplii, copepodites and adults of the marine copepod Calanus helgolandicus. J Mar Biolog Assoc UK 11:286–298
Panosso R, Carlsson P, Kozlowsky-Suzuki S, Azevedo MFO, Granéli E (2003) Effect of grazing by a neotropical copepod, Notodiaptomus, on a natural cyanobacterial assemblage and on toxic and non-toxic cyanobacterial strains. J Plankton Res 25:1169–1175
Pestana JLP, Loureiro S, Baird DJ, Soares AMVM (2010) Pesticide exposure and inducible antipredator responses in the zooplankton grazer, Daphnia magna Straus. Chemosphere 78:241–248
Relyea RA (2005) The impact of insecticides and herbicides on the biodiversity and productivity of aquatic communities. Ecol Appl 15:618627
Reynolds C (2006) Ecology of phytoplankton. University Press, Cambridge, UK
Riar NK (2014) Bifenthrin. In: Wexler P (ed) Encyclopedia of toxicology, 3rd edn. Academic Press, U.S
Rietzler AC, Matsumura Tundisi T, Tundisi JG (2002) Life cycle, feeding and adaptive strategy implications on the co-occurrence of Argyrodiaptomus furcatus and Notodiaptomus iheringi in Lobo-Broa reservoir (Brazil). Braz J Biol 62:93–105
Sanchez-Bayo F (2006) Comparative acute toxicity of organic pollutants and reference values for crustaceans. I. Branchiopoda, Copepoda and Ostracoda. Environ Pollut 139:385e420
Sanchez-Bayo F, Goka K (2014) Pesticide residues and bees – a risk assessment. PloS One 9:e94482
Sommer U, Lengfellner K (2008) Climate change and the timing, magnitude, and composition of the phytoplankton spring bloom. Glob Chang Biol 14:1199–1208
Stehle S, Schulz R (2015) Agricultural insecticides threaten surface waters at the global scale. PNAS 112:5750–5755
Sy ND, Wheeler SS, Reed M et al (2022) Pyrethroid insecticides in urban catch basins: a potential secondary contamination source for urban aquatic systems. Environ Pollut 314:120220
Titocci J, Bon M, Fink P (2022) Morpho-functional traits reveal differences in size fractionated phytoplankton communities but do not significantly affect zooplankton grazing. Microorganisms 10:182
Utermöhl H (1958) Zur Vervollkommnung der quantitative Phytoplankton: methodik. Mitt. - Int. Ver Theor Angew Limnol 9:1–38
Venrick EL (1978) How many cells to count? In: Sournia A (ed) Phytoplankton manual. UNESCO, Paris
Von Rückert G, Gianni A (2008) Biological interactions in the plankton community of a tropical eutrophic reservoir: is the phytoplankton controlled by zooplankton? J Plankton Res 10:1157–1168
Werner I, Moran K (2008) Effects of pyrethroid insecticides on aquatic organisms. In: Gan J, Spurlock F, Hendley P, Weston DP (eds) Synthetic Pyrethroids: Occurrence and Behavior in Aquatic Environments. American Chemical Society, Washington D.C
Weston DP, Chen D, Lydy MJ (2015) Stormwater-related transport of the insecticides bifenthrin, fi pronil, imidacloprid, and chlorpyrifos into a tidal wetland, San Francisco Bay, California. Sci Total Environ 527–528:18–25
Wetzel R, Likens (2000). Limnological analyses. 3ed. Springer, New York. 1
Wong CK, Liu XJ, Siu YY, Hwang JS (2006) Study of selective feeding in the marine cladoceran Penilia avirostris by HPLC pigment analysis. J Exp Mar Biol Ecol 331:21–32
Acknowledgements
The authors thank C. Mora for her assistance in nutrients samples processing and M.R. Repetti for her assistance in bifenthrin quantification. The authors also thank anonymous reviewer who improve the manuscript with his/her suggestions.
Funding
This manuscript was supported by project PEICID-2020-194 granted by Agencia Santafesina de Ciencia, Tecnología e Innovación (ASaCTeI).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. D.F. made formal analyses, writing, and original draft preparation. V.A., B.L., and M.F.G. made the experimental set-up. M.F.G. made the founding acquisition. All authors reviewed and agreed with the present version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Frau, ., Andrade, V.S., Lares, B.A. et al. Effects of bifenthrin on microcrustaceans grazing behavior on a phytoplankton assemblage dominated by Cyanobacteria. Environ Sci Pollut Res 31, 3754–3762 (2024). https://doi.org/10.1007/s11356-023-31365-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-31365-z