Abstract
The wastewater from organic peroxide production has high chemical oxygen demand (COD) concentration and poor biodegradability, so it is necessary to find a cost-effective treatment method. The iron-carbon microelectrolysis (IC-ME) technology was used to pretreat the organic peroxide production wastewater, and the influence of reaction conditions on the removal effect of pollutants and the degradation mechanism were studied. The effects of initial pH, iron filings, iron-carbon ratio, and reaction time on the wastewater treatment were investigated by single-factor and response surface optimization experiments, and the degradation mechanism was analyzed by three-dimensional fluorescence spectroscopy, UV–Vis, and gas chromatography mass spectrometry (GC–MS). The experimental results showed that the COD removal efficiency was 35.67% and the biodegradability of wastewater was increased from 0.113 to 0.173 under the conditions of initial pH of 3.1, the dosage of iron filings of 30.5 g/L, the ratio of iron-carbon of 1.01, and the reaction time of 122.8 min, and the process of IC-ME for degrading COD of wastewater from the production of organic peroxide was consistent with the secondary reaction. The IC-ME process could decompose macromolecular organic compounds such as tyrosine proteins and aromatic proteins, and improve the biodegradability of wastewater. It provides a theoretical reference for the practical application of IC-ME to treat this type of wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organic peroxides are used as initiators in the production of high-pressure polyethylene, and with the wide application of polyethylene, at the same time, the demand for organic peroxides is increasing. The production process of organic peroxides produces a large amount of wastewater, and the wastewater contains specific pollutants such as tert-butanol and tert-butyl hydroperoxide, which makes the wastewater irritating odor, refractory to degradation, high toxicity, and low biodegradability. However, conventional treatment techniques are difficult to deal with, and pre-treatment of organic peroxide production wastewater is required to remove some of the difficult-to-degrade compounds in the wastewater and improve the biodegradability of the wastewater. Common pretreatment methods include advanced oxidation (Sun et al. 2022; Feng et al. 2023; Borba et al. 2022), hydrolysis-acidification (Zhang et al. 2021), coagulation-precipitation (Zhou et al. 2020), etc. However, these pretreatment methods require functional materials or additional processes, which lead to an increase in the cost of wastewater treatment, so proposing economically feasible pretreatment methods for this type of difficult-to-biochemical-degradable wastewater is the focus of research (Ma et al. 2019).
Microelectrolysis is an effective pretreatment technology for the treatment of highly concentrated and difficult to degrade wastewater (Lin et al. 2019). IC-ME technology takes the treated wastewater as the electrolyte and adds iron filings and activated carbon to form a large number of tiny galvanic cells, and the main chemical reactions between iron and carbon are expressed as follows in Eqs. (1)–(4) (Li et al. 2021).
Under acid aeration conditions:
Under alkaline aeration conditions:
The Fe2+ and [H] produced by the primary cell reaction have a reducing effect and can undergo redox reactions with pollutants in the wastewater. Their electrochemical oxidation can be used to remove or transform difficult organic compounds and improve the biodegradability of wastewater (Chen et al. 2019). Meanwhile, primary cells can effectively degrade or remove organic pollutants through adsorption, coagulation, and coprecipitation (Yang et al. 2017). IC-ME is widely used and has good effect on the treatment of pharmaceutical wastewater dye wastewater (Jia et al. 2020), waste leachate (Fu et al. 2021), and electroplating wastewater (Gao et al. 2023).Ma et al. (2019) used a homemade microelectrolysis recirculation system to pretreat industrial park wastewater, and the COD removal of industrial wastewater was 51% under the conditions of 25% filler-wastewater ratio, reflux rate of 16 L/h, HRT of 24 h, and aeration volume of 60 L/h. Zhang et al. (2019) used a peroxodisulfate-coupled IC-ME system to pretreat waste leachate, and the experimental results showed that the COD removal reached 62.91% under the optimal conditions of initial pH 7, Fe–C ratio of 3, and persulfate dosage of 85 mM. Yang et al. (2016) used Fe/Al/C microelectrolysis to pretreat oil refinery wastewater, and the experimental results showed that most of the organic matter in the refinery wastewater was removed, the biodegradability was enhanced, and the biotoxicity was reduced. Zhuang et al. (2019) used IC-ME coupled with Fenton oxidation to pretreat bamboo heat treatment wastewater, COD removal after pretreatment reached 60.29%, BOD5/COD (B/C)increased from 0.037 to 0.32, and wastewater biodegradability was improved. Dinçer et al. (2021) used Fenton oxidation to treat organic peroxide production wastewater, with a COD removal rate of 72.8%, a total organic carbon (TOC) removal of 58.0%, and photo-Fenton oxidation under the same conditions for 5 h resulted in COD removal of 78.8% and TOC removal of 59.2%. However, the study on the treatment of organic peroxide production wastewater using IC-ME has not been reported.
In this experiment, the IC-ME method was used to pretreat the wastewater of organic peroxide production. The objectives of this study were as follows: (1) to explore the effects of initial pH, iron filings dosage, iron-carbon mass ratio, and reaction time on the treatment effect of wastewater pollutants during the IC-ME to pretreat organic peroxide production wastewater. (2) The reaction conditions of IC-ME pretreatment wastewater were optimized by the response surface method. (3) The mechanism of IC-ME degradation of pollutants in organic peroxide production wastewater was explored by three-dimensional excitation-emission matrix fluorescence spectroscopy (3D-EEM), ultraviolet and visible spectrophotometry (UV–Vis), and gas chromatography mass spectrometry (GC–MS).
Materials and methods
Materials
The test water was taken from a chemical plant, and its water quality characteristics are shown in Table 1.
The iron filings are taken from a mechanical processing plant, and the columnar activated carbon is made of a product of an environmental protection company in Gongyi (diameter of about 2 ~ 3 mm, length of about 3 ~ 5 mm), and pretreatment is required before use. The iron filings were washed with 10% NaOH alkali to remove the oil on the surface, then activated with 2% dilute sulfuric acid and rinsed with distilled water for use; the activated carbon was soaked with test wastewater for 2 days before use to eliminate the influence of adsorption of the activated carbon itself on the determination results, then rinsed and air-dried for use (Ma et al. 2019).
Equipment and operation
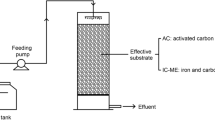
As shown in Fig. 1, the degradation test was carried out using a 250-mL conical flask, the initial pH of the wastewater was adjusted with NaOH (0.1mol/L) and dilute H2SO4 (0.1mol/L), then pretreated activated charcoal and iron filings were added and placed in an oscillator to carry out the reaction, and at the end of the reaction, the COD and UV254 of the wastewater were determined.
Analytical methods
The COD was determined by potassium dichromate method (Jiao et al. 2021), UV254 by UV spectrophotometry using HCAH DR5000 UV spectrophotometer; BOD5 was determined by dilution inoculation method using HACH HQ-10 dissolved oxygen meter and SPX-250B-Z incubator; pH was determined by pHS-25 acidity meter. A fluorescence spectrophotometer (F-7100, Hitachi) was used to determine the three-dimensional fluorescence spectra.
Experimental methods
-
1.
Screening design of influencing factors
The initial pH of the wastewater, the dosage of iron filings (g/L), the mass ratio of iron to carbon, and the reaction time (min) were used as the influencing factors to investigate the effects of the above factors on the removal efficiency of pollutants.
-
2.
Response surface experimental design
Response surface method (RSM) combines mathematical and statistical methods to model and optimize the effects of a few independent variables, and has been widely and effectively used in modeling and optimization of various engineering problems(Al-Sabur 2021).Based on the results of single-factor experiments, response surface analysis was used to investigate the effects of controllable variables on COD removal efficiency, and the initial pH, iron filings dosage, iron-carbon ratio and reaction time were selected as independent variables, with COD removal efficiency as the response value. The Box-Behnken design(BBD) model was used for a 4-factor, 3-level design scheme, as shown in Table 2, to investigate the relationship between the factors and response values, and regression fitting was performed to establish a mathematical model of the process of IC-ME for the treatment of organic peroxide production wastewater (Abdulgader et al. 2020).
Results and discussion
Study on the influencing factors of pretreatment of organic peroxide production wastewater
Effect of initial pH
The raw water COD was 15636.4 mg/L and the UV254 was 2.881. The reaction time was 120 min, the dosage of iron filings was controlled to be 30 g/L, m(Fe)/m(C) = 1:1, to investigate the influence of the initial pH of different wastewaters on the effect of IC-ME. As shown in Fig. 2, the removal efficiency of COD and UV254 in IC-ME showed a trend of increasing first and then decreasing, and the removal efficiency of COD and UV254 reached the maximum value of 34.5% and 79.06%, respectively, at pH = 3. The lower the pH, the larger the potential difference of the iron carbon primary cell, and the reduced [H] and Fe2+ produced, which facilitated the redox reaction with organic matter and improved the removal efficiency of pollutants (Hu et al. 2022). However, too low a pH will accelerate the corrosion of iron and too much H+ will compete for the electrons generated at the anode in the primary cell reaction and polarization will occur, which will reduce the removal efficiency of pollutants (Che et al. 2017). When pH > 3, high pH is favorable to the formation of iron salt flocs, but as a result, the electrode reaction will become weaker and the dissolution of iron will be slower, causing a decrease in the removal efficiency of pollutants (Xu et al. 2016). Meanwhile, COD removal decreased with increasing pH because the redox potential of the IC-ME reaction was higher under acidic conditions than under alkaline conditions (Han et al. 2023).
Effect of iron filings dosage
The COD of the raw water was 15,795.0 mg/L and the UV254 was 2.879. The initial pH = 3, m(Fe)/m(C) = 1:1, and the reaction time was 120 min to investigate the effect of different iron filings on the treatment effect of IC-ME. As shown in Fig. 3, as the dosage of iron filings increased from 10 to 60 g/L, the removal efficiency of pollutants showed a trend of slowly increasing and then slowly decreasing. When the dosage of iron filings was 40 g/L, the COD removal efficiency and UV254 removal efficiency reached the maximum value of 37.08% and 80.07%, respectively. It is because with the increase of iron filings, more primary cells are formed and the pollutant removal efficiency increases, but too much iron filings will consume H+ and reduce the production of reduced [H] in the system, resulting in poor pollutant removal effect(Li et al. 2022).
When the dosage of iron filings was 30 g/L, the wastewater COD removal efficiency could reach 34.88%, which is only 2.2% lower than the dosage of 40 g/L. Considering the economy in actual wastewater treatment and reducing the wastewater treatment cost, the dosage of iron filings is selected to be 30 g/L, which not only has good treatment effect, but also reduces the cost of wastewater treatment.
Effect of iron-carbon ratio
The COD of raw water was 15,523.7 mg/L and UV254 was 2.509. The initial pH was controlled to be 3, the dosage of iron filings was 30 g/L, and the reaction time was 120 min, to investigate the effects of different iron-carbon ratios on the treatment effect of IC-ME. As shown in Fig. 4, with the change of iron-carbon ratio from 3:1 to 1:4, the pollutant removal efficiency showed a trend of increasing and then decreasing. m(Fe)/m(C) = 1:1, the COD removal efficiency and UV254 removal efficiency reached the maximum value of 34.07% and 80.47%, respectively. The main reason for this is the variation in the mass ratio of iron to carbon and the lack of iron or carbon in the microelectrolysis process, which forms insufficient primary cells and decreases the pollutant removal efficiency (Han et al. 2019). Therefore, when the same amount of iron and carbon is used, it is possible that at this time the largest number of tiny primary cells are formed and the pollutant removal efficiency is maximum.
Effect of reaction time
The COD of the raw water was 15,523.7 mg/L, and the UV254 was 2.509. The initial pH = 3, m(Fe)/m(C) = 1:1, and the dosage of iron filings was 30 g/L. The effect of reaction time on the effect of IC-ME was investigated. As can be seen from Fig. 5, in the early stage of the reaction, with the extension of the reaction time, the IC-ME redox reaction in the reaction system continued to occur, and the contaminant removal rate gradually increased. The pollutant removal efficiency gradually increased with the prolongation of reaction time. When the reaction proceeded to 120 min, the COD removal efficiency reached the maximum value of 34.03%, at this time the UV254 removal efficiency was 78.29%, after which the pollutant removal efficiency no longer increased. The reason is that the H+ in the system at the beginning of the reaction is high, the Fe2+ and Fe3+ content increases during the reaction process, the electrode degrades the organic matter by primary cell reaction; at the later stage of the reaction, the H+ content in the system decreases, the iron carbon electrode is wrapped by Fe(OH)2, Fe(OH)3, and organic matter, the efficiency of the primary cell reaction decreases, and the COD removal efficiency no longer increases (Wang et al. 2016).
Response surface optimization
The results of the response surface test design are shown in Table 3.
The data were analyzed using Design Expert 12.0 statistical software, and the four factors of initial pH (A), iron filings dosage (B), iron-carbon ratio (C), and reaction time (D) and their interactions on the COD removal efficiency of the target response were obtained (Fard et al. 2021), and the second-order regression equation was used for fitting, and the regression equation for COD removal efficiency was:
Analysis of COD removal rate RSM fitting results
Analysis of variance for the regression equation
As shown in Table 4, the regression equation model ANOVA was analyzed to test the significance of the model. Among them, larger F values and smaller p values can indicate the significant level of the correlation coefficient (Popović et al. 2019). The F value of this COD removal efficiency model was 39.42 with p less than 0.0001, which indicates a good significance of the model. Based on the magnitude of F value (Okolo et al. 2021), it can be concluded that the magnitude of the effect of four factors on COD removal efficiency is A > B > D > C, i.e., initial pH > iron filings dosage > reaction time > iron-carbon ratio. And the misfit term p value = 0.1059 > 0.05, which is not significant, indicates that the residuals are generated by random errors and the model is a good simulation of the experiment with less errors generated by the experiment (Srimoke et al. 2022). The R2 value of the RSM model for COD removal is 0.9753 close to 1.0, indicating a good correlation of the model. The correction coefficient of determination (Adj R2) = 0.9505 indicates that the model explains 95.05% of the variation in response values. The signal-to-noise ratio (Adeq Precision) value is greater than 4, which is within a reasonable range (Sadaf et al. 2022). The difference between Adj R2 and Pred R2 of this model = 0.9505–0.8474 < 0.2, which indicates that the model can significantly represent the variation of each influencing factor during the experiment (Keshmiri-Naqab and Taghavijeloudar 2023); the coefficient of variation (C.V.) value is 1.53% < 4%, which indicates that the test consists of a high reproducibility (Estrada-Vazquez et al. 2019). In conclusion, the quality of this fit is good.
Response surface analysis
The initial pH, iron filings dosage, iron-carbon mass ratio, and reaction time and the effects of interaction between various factors on COD removal rate were investigated. The response surface and contour can visually show the influence of the interaction of influencing factors on the removal of organic pollutants by microelectrolysis reaction. From Fig. 6a, b, and d, it can be seen that the slope of the surface is large, indicating that the initial pH, iron-carbon dosage, and iron-carbon mass ratio of the solution have a great influence on the test results, which is consistent with the results of the analysis of variance of the regression equation. Figure 6e and f show that the slope of the response surface is small, indicating that the reaction time has little influence on the experimental results. It can be seen from Fig. 6 that the contour plots of each plot are oval, indicating that the interaction between the factors is significant and has a greater impact on the removal effect of pollutants(Fseha et al. 2023).
Model verification and optimization
Under the conditions of initial pH of 3.1, iron filing dosage of 30.5 g/L, iron-carbon mass ratio of 1.01, and reaction time of 122.8 min, the predicted COD removal of organic peroxide production wastewater by IC-ME was 35.67%, and three sets of parallel tests were conducted under optimal operation conditions, and the obtained COD removal efficiencies were 35.17%, 35.57%, and 35.35%, the experimental values are close to the predicted values, indicating a good fit of the prediction model (Fseha et al. 2023). Moreover, the B/C of the raw water was 0.113, and the B/C of the wastewater pretreated with IC-ME technology was 0.173 under the optimal process treatment conditions, which improved the biodegradability of the wastewater. The model can more accurately reflect the effects of various factors on the removal of organic peroxide production wastewater by IC-ME and has some practical value.
Kinetics of IC-ME for COD degradation
Under the conditions of initial pH of 3.1, iron filings dosage of 30.5 g/L, and iron-to-carbon mass ratio of 1.01, the kinetic tests of COD degradation by IC-ME were carried out under different reaction times (15 min, 30 min, 45 min, 60 min, 75 min, 90 min, 105 min, and 120 min), and the composite function and time relationship of COD concentration were analyzed. The common kinetic models are as follows (Maher et al. 2019):
where Ct is the COD concentration (mg/L) at the moment of t (min), C0 is the initial COD concentration (mg/L). K0, K1, and K2 are the rate constants for the zero-, one-, and two-stage reactions, respectively.
The kinetics of COD removal by IC-ME at kinetic reaction levels of 0, 1, and 2 are shown in Fig. 7A, B, and C, respectively. The results of rate constant (K) and regression coefficient (R2) are shown in Table 5, which indicates that the COD removal is best suited to the two-stage kinetic model, and the kinetics of COD removal is two-stage kinetics with a K1 value of 0.0208 min−1, and an R2 value of 0.9818 (Shokri and Nasernejad 2023). The kinetic correlation coefficient of the second-stage reaction for IC-ME was higher than that of the other reaction stages, indicating that the second-stage reaction was a better fit for the kinetic equation of COD degradation by IC-ME (Niu et al. 2023).
Mechanistic study on the treatment of organic peroxide production wastewater by IC-ME
3D-EEM analysis
The 3D-EEM of organic peroxide production wastewater pretreated by IC-ME are shown in Fig. 8. The color of the fluorescence peaks in the 3D-EEM reflects the degree of contamination of the water, and the darker the color indicates the more serious contamination of the water by organic contamination. According to the FRI analysis method, region I (Ex/Em = 200 ~ 250 nm/280 ~ 330 nm) is tyrosine-based proteins; region II (Ex/Em = 200 ~ 250 nm/330 ~ 380 nm) is tryptophan-based proteins (Zhou et al. 2013). As shown in the figure, two obvious fluorescence peaks appeared in the 3D-EEM of the influent water: the Ex/Em = 270/310, which belonged to tyrosine-like, and the Ex/Em = 215/300 for aromatic protein-like substances.1 Compared with the fluorescence graph of the raw water, weak fluorescence peaks appeared in the 3D fluorescence spectrogram of the effluent water, and the obvious peaks were weakened, indicating that IC-ME pretreatment had a good treatment effect on macromolecular organic compounds such as tyrosine proteins and aromatic protein-like substances in wastewater.
UV–Vis analysis
UV–visible light (wavelength range 190 ~ 450 nm) was used to scan the raw water as well as the wastewater pretreated by IC-ME, and the UV–Vis are shown in Fig. 8.
From Fig. 9, it can be seen that the organic peroxide production wastewater has obvious absorption peaks between the wavelengths of 190–300 nm, which may be caused by unsaturated cyclic aromatic hydrocarbon compounds such as benzene rings and heterocycles in the organic peroxide production wastewater. After the treatment with IC-ME, the absorbance of the wastewater was reduced between the wavelengths of 220 and 290 nm, indicating that the unsaturated cyclic aromatic hydrocarbon compounds such as benzene ring and heterocyclic were destroyed and the structure tended to be simpler (Hou et al. 2022). After wavelength 300 nm, the absorption peaks did not change, indicating that the pollutants in the wastewater were degraded (Lu et al. 2021).
GC–MS analysis
Changes in organic species and content of organic peroxide production wastewater before and after IC-ME treatment were analyzed using GC–MS, as shown in Fig. 10. By comparison, the number of chromatographic peaks in the effluent was significantly reduced and there was a significant decrease in height (Zhou et al. 2020). When the retention time was between 0 and 5 min, most of the pollutants in the wastewater before and after treatment were alkanes and furans, and the relative content was reduced after IC-ME treatment, and the types of pollutants did not change (Ulhaq et al. 2021); when the retention time was between 5 and 19 min, the most organic species were mainly organic acids, ketones, alcohols, and ester compounds, and after treatment, there were new acids and ketones substances, in this interval, the relative content of pollutants is significantly reduced; in the retention time between 19 and 26 min, the wastewater before treatment of high boiling point, large molecules of organic substances are mainly acids, esters, anilines, benzene rings, and other substances, and the relative content of the substances in this interval is greatly reduced after treatment, indicating that IC-ME can open the ring and break the chain of large molecules, degraded into small molecules, thereby improve the biochemistry of wastewater (Zhou et al. 2020). This is the same as the results of 3D-EEM and UV–Vis analysis.
nt water.
Conclusion
-
1.
The optimal conditions for the pretreatment of organic peroxide production wastewater by IC-ME were obtained by using single-factor test and response surface optimization test: the initial pH was 3.1, the dosage of iron filings was 30.5 g/L, the mass ratio of iron and carbon was 1.01, and the reaction time was 122.8 min. Under these conditions, the COD removal efficiency of IC-ME in the degradation of organic peroxide production wastewater was 35.67% and the biodegradability of wastewater increased from 0.113 to 0.173. The kinetic test showed that the process of IC-ME for degrading COD of organic peroxide production wastewater was in accordance with the secondary reaction.
-
2.
The three-dimensional fluorescence spectra, UV–Vis, and GC–MS analyses of the wastewater before and after the treatment of the IC-ME process showed that the IC-ME decomposed the large organic molecules, such as tyrosine-like proteins and aromatic proteins, through the opening of the ring chain breakage, and transformed them into the small organic pollutants that were easy to be biodegradable, so as to increase the biodegradability of the wastewater.
-
3.
The pretreatment of organic peroxide production wastewater by IC-ME s technology is of practical value by optimizing the process conditions and providing reference for actual wastewater treatment.
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- IC-ME:
-
Iron-carbon microelectrolysis
- COD:
-
Chemical oxygen demand
- TOC:
-
Total organic carbon
- BOD:
-
Biochemical oxygen demand
- B/C:
-
BOD5/COD
- RSM:
-
Response surface methodology
- BBD:
-
Box-Behnken design
- 3D-EEM:
-
Three-dimension excitation-emission matrix fluorescence spectroscopy
- UV-Vis:
-
Ultraviolet and visible spectrophotometry
- GC-MS:
-
Gas chromatography mass spectrometry
References
Abdulgader M, Yu QJ, Zinatizadeh AA, Williams P, Rahimi Z (2020) Application of response surface methodology (RSM) for process analysis and optimization of milk processing wastewater treatment using multistage flexible fiber biofilm reactor. J Environ Chem Eng 8(3):103797. https://doi.org/10.1016/j.jece.2020.103797
Al-Sabur R (2021) Tensile strength prediction of aluminium alloys welded by FSW using response surface methodology – comparative review. Mater Today: Proceedings 45:4504–4510. https://doi.org/10.1016/j.matpr.2020.12.1001
Borba FH, Hahn CL, Mayer I, Seibert D, Guimarães RE, Inticher JJ, Zorzo CF, Kreutz GK (2022) New hybrid strategy of the photo-Fered-Fenton process assisted by O3 for the degradation of wastewater from the pretreatment of biodiesel production. Chemosphere 306:135470. https://doi.org/10.1016/j.chemosphere.2022.135470
Che JG, Wan JB, Huang XP, Wu RW, Liang K (2017) Pretreatment of piggery digestate wastewater by ferric-carbon micro-electrolysis under alkalescence condition. Korean J Chem Eng 34(9):2397–2405. https://doi.org/10.1007/s11814-017-0144-8
Chen YH, Lin T, Chen W (2019) Enhanced removal of organic matter and typical disinfection byproduct precursors in combined iron–carbon micro electrolysis-UBAF process for drinking water pre-treatment. J Environ Sci 78:315–327. https://doi.org/10.1016/j.jes.2018.11.010
Dinçer AR, Çifçi Dİ, Cinkaya DD, Dülger E, Karaca F (2021) Treatment of organic peroxide containing wastewater and water recovery by fenton-adsorption and fenton-nanofiltration processes. J Environ Manag 299:113557. https://doi.org/10.1016/j.jenvman.2021.113557
Estrada-Vazquez C, Salinas-Pacheco A, Peralta-Reyes E, Poggi-Varaldo HM, Regalado-Mendez A (2019) Parametric optimization of domestic wastewater treatment in an activated sludge sequencing batch reactor using response surface methodology. J Environ Sci Health Part A-Toxic/Hazard Subst Environ Eng 54(12):1197–1205. https://doi.org/10.1080/10934529.2019.1631087
Fard MB, Hamidi D, Yetilmezsoy K, Alavi J, Hosseinpour F (2021) Utilization of Alyssum mucilage as a natural coagulant in oily-saline wastewater treatment. J Water Process Eng 40:101763. https://doi.org/10.1016/j.jwpe.2020.101763
Feng H, Zhang Z, Kuang Q, Chen S, Huang D, Zhou X (2023) The transformation of dissolved organic matter and formation of halogenated by-products during electrochemical advanced oxidation pretreatment for shale gas produced water. J Hazard Mater 455:131614. https://doi.org/10.1016/j.jhazmat.2023.131614
Fseha YH, Shaheen J, Sizirici B (2023) Phenol contaminated municipal wastewater treatment using date palm frond biochar: optimization using response surface methodology. Emerg Contam 9(1):100202. https://doi.org/10.1016/j.emcon.2022.100202
Fu S, Jia H, Meng X, Guo ZJ, Wang J (2021) Fe-C micro-electrolysis-electrocoagulation based on BFDA in the pre-treatment of landfill leachate: enhanced mechanism and electrode decay monitoring. Sci Total Environ 781:146797. https://doi.org/10.1016/j.scitotenv.2021.146797
Gao JS, Wang HX, Yang YL, Lv JF, Wen JH, Zhu J, Zhou JF (2023) Identification of microbial communities and functional genes in an anaerobic-anoxic-oxic (A2O) process in responding to the iron-carbon micro-electrolysis (ICME) pre-treatment of electroplating wastewater based on high-throughput sequencing. Colloids and Surfaces c: Environ Aspects 1:100009. https://doi.org/10.1016/j.colsuc.2023.100009
Han YH, Qi MM, Zhang L, Sang YM, Liu ML, Zhao TT, Niu JF, Zhang SQ (2019) Degradation of nitrobenzene by synchronistic oxidation and reduction in an internal circulation microelectrolysis reactor. J Hazard Mater 365:448–456. https://doi.org/10.1016/j.jhazmat.2018.11.036
Han Y, Xu H, Zhang L, Ma X, Man Y, Su Z, Wang J (2023) An internal circulation iron–carbon micro–electrolysis reactor for aniline wastewater treatment: parameter optimization, degradation pathways and mechanism. Chin J Chem Eng. https://doi.org/10.1016/j.cjche.2023.05.009
Hou ZL, Zhou X, Zhao ZL, Dong WY, Wang HJ, Liu HG, Zeng ZW, Xie J (2022) Advanced aromatic organic compounds removal from refractory coking wastewater in a step-feed three-stage integrated A/O bio-filter: Spectrum characterization and biodegradation mechanism. J Environ Manag 322:116140. https://doi.org/10.1016/j.jenvman.2022.116140
Hu MY, Luo TL, Li QL, Xie YF, Liu G, Wang LJ, Peijnenburg WJGM (2022) Remediation of low C/N wastewater by iron–carbon micro-electrolysis coupled with biological denitrification: Performance, mechanisms, and application. J Water Process Eng 48:102899. https://doi.org/10.1016/j.jwpe.2022.102899
Jia YP, Zhang Z, Tong ZW, Wang W, Zhang LH (2020) Study on efficiency and mechanism of iron-carbon microelectrolysis treatment of dyeing wastewater. CIESC J 71(4):1791–1801. https://doi.org/10.11949/0438-1157.20190997
Jiao N, Liu J, Edwards B, Lv Z, Cai R, Liu Y, Xiao X, Wang J, Jiao F, Wang R, Huang X, Guo B, Sun J, Zhang R, Zhang Y, Tang K, Zheng Q, Azam F, Batt J, Cai W-J, He C, Herndl GJ, Hill P, Hutchins D, LaRoche J, Lewis M, MacIntyre H, Polimene L, Robinson C, Shi Q, Suttle CA, Thomas H, Wallace D, Legendre L (2021) Correcting a major error in assessing organic carbon pollution in natural waters. Sci Adv 7(16):eabc7318. https://doi.org/10.1126/sciadv.abc7318
Keshmiri-Naqab R, Taghavijeloudar M (2023) Could organoclay be used as a promising natural adsorbent for efficient and cost-effective dye wastewater treatment? J Environ Manag 342:118322. https://doi.org/10.1016/j.jenvman.2023.118322
Li X, Jia Y, Qin Y, Zhou MH, Sun JH (2021) Iron-carbon microelectrolysis for wastewater remediation: preparation, performance and interaction mechanisms. Chemosphere 278:130483. https://doi.org/10.1016/j.chemosphere.2021.130483
Li X, Zhang JJ, Qin Y, Zhang XL, Zou W, Ding LJ, Zhou MH (2022) Enhanced removal of organic contaminants by novel iron–carbon and premagnetization: performance and enhancement mechanism. Chemosphere 303:135060. https://doi.org/10.1016/j.chemosphere.2022.135060
Lin WT, Li J, Zhang XL, Dong WY (2019) Pretreatment of evaporated condensate generated during metal cutting process by Fe-C micro-electrolysis. Water Sci Technol 79(3):580–588. https://doi.org/10.2166/wcc.2018.165
Lu X, Xie SQ, Li S, Zhou J, Sun WQ, Xu YH, Sun YJ (2021) Treatment of purified terephthalic acid wastewater by ozone catalytic oxidation method. Water 13(14):14. https://doi.org/10.3390/w13141906
Ma C, Ran ZL, Yang ZQ, Wang L, Wen C, Zhao B, Zhang HW (2019) Efficient pretreatment of industrial estate wastewater for biodegradability enhancement using a micro-electrolysis-circulatory system. J Environ Manag 250:109492. https://doi.org/10.1016/j.jenvman.2019.109492
Maher EK, O’Malley KN, Heffron J, Huo J, Mayer BK, Wang Y, McNamara PJ (2019) Analysis of operational parameters, reactor kinetics, and floc characterization for the removal of estrogens via electrocoagulation. Chemosphere 220:1141–1149. https://doi.org/10.1016/j.chemosphere.2018.12.161
Niu L, Zhao XL, Tang Z, Cai YQI, Lei QT, Hou DYI, Niu HY, Wang LJI, Giesy JP, Wu FC (2023) Novel ternary Cu0-coupled core-shell Fe0/C nanoparticles micro-electrolysis system toward degradation of organic pollutants: synergistic effects and removal mechanism. Sci Total Environ 892:164587. https://doi.org/10.1016/j.scitotenv.2023.164587
Okolo BI, Adeyi O, Oke EO, Agu CM, Nnaji PC, Akatobi KN, Onukwuli DO (2021) Coagulation kinetic study and optimization using response surface methodology for effective removal of turbidity from paint wastewater using natural coagulants. Sci Afr 14:e00959. https://doi.org/10.1016/j.sciaf.2021.e00959
Popović S, Karadžić M, Cakl J (2019) Optimization of ultrafiltration of cutting oil wastewater enhanced by application of twisted tapes: response surface methodology approach. J Clean Prod 231:320–330. https://doi.org/10.1016/j.jclepro.2019.05.184
Sadaf S, Singh AK, Iqbal J, Kumar RN, Sulejmanović J, Habila MA, Pinê Américo-Pinheiro JH, Sher F (2022) Advancements of sequencing batch biofilm reactor for slaughterhouse wastewater assisted with response surface methodology. Chemosphere 307:135952. https://doi.org/10.1016/j.chemosphere.2022.135952
Shokri A, Nasernejad B (2023) Treatment of spent caustic wastewater by electro-Fenton process: Kinetics and cost analysis. Process Saf Environ Prot 172:836–845. https://doi.org/10.1016/j.psep.2023.02.077
Srimoke W, Kanokkantapong V, Supakata N, Limmun W (2022) Optimising zero-valent iron from industrial waste using a modified air-Fenton system to treat cutting oil wastewater using response surface methodology. Arab J Chem 15(11):104213. https://doi.org/10.1016/j.arabjc.2022.104213
Sun W, Lv H, Ma L, Tan X, Jin C, Wu H, Chen L, Liu M, Wei H, Sun C (2022) Use of catalytic wet air oxidation (CWAO) for pretreatment of high-salinity high-organic wastewater. J Environ Sci 120:105–114. https://doi.org/10.1016/j.jes.2021.06.027
Ulhaq I, Ahmad W, Ahmad I, Yaseen M, Ilyas M (2021) Engineering TiO2 supported CTAB modified bentonite for treatment of refinery wastewater through simultaneous photocatalytic oxidation and adsorption. J Water Process Eng 43:102239. https://doi.org/10.1016/j.jwpe.2021.102239
Wang LQ, Yang Q, Wang DB, Li XM, Zeng GM, Li ZJ, Deng YC, Liu J, Yi KX (2016) Advanced landfill leachate treatment using iron-carbon microelectrolysis- Fenton process: Process optimization and column experiments. J Hazard Mater 318:460–467. https://doi.org/10.1016/j.jhazmat.2016.07.033
Xu XY, Cheng Y, Zhang TT, Ji FY, Xu X (2016) Treatment of pharmaceutical wastewater using interior micro-electrolysis/Fenton oxidation-coagulation and biological degradation. Chemosphere 152:23–30. https://doi.org/10.1016/j.chemosphere.2016.02.100
Yang R, Zhu J, Li Y, Zhang H (2016) A study on the preparation of regular multiple micro-electrolysis filler and the application in pretreatment of oil refinery wastewater. Int. J. Environ. Res. Public Health 13:457. https://doi.org/10.3390/ijerph13050457
Yang ZM, Ma YP, Liu Y, Li QS, Zhou ZY, Ren ZQ (2017) Degradation of organic pollutants in near-neutral pH solution by Fe-C micro-electrolysis system. Chem Eng J 315:403–414. https://doi.org/10.1016/j.cej.2017.01.042
Zhang W, Li X, Yang Q, Wang D, Wu Y, Zhu X, Wei J, Liu Y, Hou L, Chen C (2019) Pretreatment of landfill leachate in near-neutral pH condition by persulfate activated Fe-C micro-electrolysis system. Chemosphere 216:749–756. https://doi.org/10.1016/j.chemosphere.2018.10.168
Zhang Z, Yu Y, Xi H, Zhou Y (2021) Review of micro-aeration hydrolysis acidification for the pretreatment of toxic and refractory organic wastewater. J Clean Prod 317:128343. https://doi.org/10.1016/j.jclepro.2021.128343
Zhou J, Wang JJ, Baudon A, Chow AT (2013) Improved fluorescence excitation-emission matrix regional integration to quantify spectra for fluorescent dissolved organic matter. J Environ Qual 42(3):925–930. https://doi.org/10.2134/jeq2012.0460
Zhou X, Hou Z, Song J, Lv L (2020) Spectrum evolution of dissolved aromatic organic matters (DAOMs) during electro-peroxi-coagulation pretreatment of coking wastewater. Sep Purif Technol 235:116125. https://doi.org/10.1016/j.seppur.2019.116125
Zhuang XW, Zhou W, Hong YY, Li WY, Pan X, Chen SW (2019) Experiment on pretreatment of waste water from bamboo heat treatment by combination of iron-carbon micro-electrolysis and Fenton method. Nord Pulp Pap Res J 34(3):354–361. https://doi.org/10.1515/npprj-2018-0030
Funding
The National Natural Science Foundation of China (51568034),The research was supported by the Open Foundation of Key Laboratory of Yellow River Water Environment in Gansu Province (20JR2RA0002).
Author information
Authors and Affiliations
Contributions
Zichun Yan: conceptualization, writing—review and editing, paper administration; Shilong Xie: formal analysis, visualization, writing—original draft; Mingxia Yang: conceptualization, methodology, investigation.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Weiming Zhang
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, Z., Xie, S. & Yang, M. Effect and mechanism of iron-carbon micro-electrolysis pretreatment of organic peroxide production wastewater. Environ Sci Pollut Res 31, 11886–11897 (2024). https://doi.org/10.1007/s11356-023-31057-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-31057-8