Abstract
Cadmium (Cd) contamination in soil poses a severe threat to plant growth and development. In contrast, silicon (Si) has shown promise in enhancing plant resilience under Cd-induced stress. In this study, we conducted an integrated investigation employing morphological studies, gene expression analysis, and metabolomics to unravel the molecular mechanisms underlying Cd tolerance in maize plants. Our results demonstrate that Si biofortification significantly mitigated Cd stress by reducing Cd accumulation in plant tissues, increasing Si content, and enhancing maize biomass in Cd-stressed plants resulted in a substantial enhancement in shoot dry weight (+ 75%) and root dry weight (+ 30%). Notably, Si treatment upregulated key lignin-related genes (TaPAL, TaCAD, Ta4CL, and TaCOMT) and promoted the accumulation of metabolites (sinapyl alcohol, phenylalanine, p-coumaryl alcohol, cafeyl alcohol, and coniferaldehyde) essential for cell wall strength, particularly under Cd stress conditions. Si application enriched the signal transduction by hormones and increased resistance by induction of biosynthesis genes (TaBZR1, TaLOX3, and TaNCDE1) and metabolites (brassinolide, abscisic acid, and jasmonate) in the roots and leaves under Cd stress. Furthermore, our study provides a comprehensive view of the intricate molecular crosstalk between Si, Cd stress, and plant hormonal responses. We unveil a network of genetic and metabolic interactions that culminate in a multifaceted defense system, enabling maize plants to thrive even in the presence of Cd-contaminated soil. This knowledge not only advances our understanding of the protective role of Si but also highlights the broader implications for sustainable agricultural practices. By harnessing the insights gained from this research, we may pave the way for innovative strategies to fortify crops against environmental stressors, ultimately contributing to the goal of food security in an ever-changing world. In summary, our research offers valuable insights into the protective mechanisms facilitated by Si, which enhance plants’ ability to withstand environmental stress, and holds promise for future applications in sustainable agriculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal accumulation in soil profiles, which is regarded as non-essential plant components, causes a substantial threat to agricultural sustainability and world food production. These pollutants harm plant growth by interfering with numerous physiological, biochemical, and molecular pathways that are essential for crop development and yield (Shanying et al. 2017) (Elsheery et al. 2023) (Helaly et al. 2018). However, the level of growth loss is dependent on a number of parameters, including conditions for experiments, the individual heavy metals implicated, and the plant species under study (Hussain et al. 2020, 2016). Our study looked into the distinct issues of Si-induced plant development, as well as the associated uptake and transformation under Cd stress. The pollution in agricultural soil with heavy metals has significantly reduced the effectiveness of important plant processes, endangering both yield and growth (Desoky et al. 2020; Imran et al. 2020; Rady et al. 2019). The elevated intensity of heavy metals in the land triggers oxidative stress, which is as regard by a raise in the generation of ROS, including oxygen radicals. These reactive oxygen species (ROS) pose a serious danger to plants because they disrupt several biological processes at the physiological, biochemical, and molecular levels (Imran et al. 2020; Sarwar et al. 2015; Shanying et al. 2017). Cadmium (Cd) pollution has been observed to have the most devastating effect on crops such as wheat (Alzahrani et al. 2019; Sarwar et al. 2015), peas (Kevresan et al. 2001), and rice (Imran et al. 2020). The deleterious effects of Cd pollution on these plant species are amplified because of their sensitivity.
Cd is most poisonous metallic element to both vascular and vertebrates plants and is found in soil, water, and the environment. The extensive distribution of Cd can be linked to a variety of sources, including emission of industries, sewage sludge, use of phosphate (PO4) composts, and release of Cd-containing public garbage (Gratão et al. 2005). Because of the widespread toxicity of Cd, agricultural land and the plants grown on it undergo severe risk all over the world. This widespread problem threatens both agricultural productivity and the overall health of the ecosystem (Grant et al. 2008). Cd uptake by plant roots, followed by translocation to aerial plant parts, has diverse and multifaceted impacts on plants in paddy soil settings, spanning morphological, physiological, and biochemical aspects. This complex interplay of Cd in paddy soils affects an extensive range of essential plant activities, from sprouting to subsequent growth and developmental phases. The most common physical indications of Cd infection in plants include stunted or scrubby root and branch development, a considerable drop in typical biomass amassing yellowing of the leaf, and finally plant death (Uraguchi et al. 2009). Cd stress can also severely disrupt plant development, morphogenesis, photosynthetic, and respiratory functions. According to metabolome analysis, 17 metabolites in maize roots significantly decreased under Cd stress conditions, including GSH, 2-methyl fumarate, thymidine, and glucuronic acid. These metabolites are related to amino acids and include ASP, GLU, GLY, TYR, and ALA. ASP, GLU, GLY, and serine, as well as other metabolites like malic acid, citric acid, and inositol, all experienced significant increases in 10 different metabolites in maize leaves under Cd stress circumstances. These findings imply that Cd stress affects metabolites differently in maize leaves and roots (Wang et al. 2021).
Silicon (Si), the second most common metal in the Earth’s crust, has been overlooked for a long time despite being a crucial element in sophisticated plants (Shi et al. 2014). Nonetheless, recent scientific investigations have revealed solid evidence proving benefits of Si on crop plant growth. Si supplementation proven to enhance crop, plant lenience to numerous stresses faced in their growth conditions, including the harmful effects of heavy metal stress (Wang et al. 2016; Zhu and Gong 2014). Si is the only element that plants can translocate, deposit, and absorb in large amounts without damage (Ma et al. 2001). Wheat, maize, rice, and pak choi are just a few of the more well-known crops that have benefited from a renewed focus in recent years on the function of Si in reducing Cd toxicity in plants (Brassica chinensis L.) (Rizwan et al. 2012; Song et al. 2009). Si improves pak choi (Brassica chinensis L.) resistance to Cd toxicity by efficiently reducing uptake and transport of Cd from roots to shoots (Song et al. 2009). The increased Cd tolerance seen in cotton plants after Si supplementation connected to a number of positive mechanisms. Cotton treated with Si has higher photosynthetic efficiency and increased antioxidant enzyme activity, which aids in the reduction of oxidative stress caused by Cd exposure. Furthermore, Si supplementation inhibits Cd uptake, resulting in lower levels of electrolytic leakage, malondialdehyde, and hydrogen peroxide (H2O2) (Farooq et al. 2013).
In stress holding plants, Si has a vital role in tailoring gene expression rather than serving as a secondary messenger or direct signal. Si actively adjusts the formation and accumulation of RO and nitrogen species (ROS and RNS) in stressed plants. Recent research investigated the upregulatory effects of Si on critical proteins involved in redox balance. In tomato plants under drought stress, Si was observed to increase the expression of the ubiquinol-cytochrome c reductase complex protein in complex III of the mitochondrial electron transport chain (ETC) (Elsheery et al. 2020a, b). This upregulation reduced electron removal from complex III ubisemiquinone, lowering ROS generation within the mitochondria. Furthermore, Si increased the activity of the mitochondrial alternative oxidase, which limits ROS formation by limiting electron leakage from the mitochondrial ETC (Basu and Kumar 2021). Through upregulation of the cellular antioxidant defense mechanism, Si exerts regulatory control over the redox potentials of cells (Das et al. 2018). Si treatment increases antioxidant enzyme and non-enzymatic antioxidant levels and activity in stressed plant shoots and roots. This Si-induced antioxidant defense system enhancement helps the plant stability to withstand and deal with numerous stresses (Kim et al. 2017) (Elsheery et al. 2020a, b). Using Si results in the downregulation of genes necessary for the production of both non-enzymatic and enzymatic antioxidants, according to another research. By aiding efficient scavenging of ROS, these genes play a crucial role in redox potential control. By controlling the expression of many antioxidant-related genes, Si mitigates salt stress in plants (Chung et al. 2020) (Elsheery et al. 2020a, b). There has not been any in-depth study of how Si affects phenylpropanoid metabolism and plant hormones in maize plants under Cd stress, especially in terms of the metabolites in the route of lignin synthesis.
While previous research has focused on Si’s role in mitigating Cd toxicity, little is known about how Si influences phenylpropanoid metabolism and plant hormones in maize plants under Cd stress, particularly in terms of lignin synthesis metabolites. In this study, we sought to elucidate the impact of Cd stress on maize plants with a focus on changes in saccharide molecules and Si content within the plants. Our research closely monitored specific target metabolites and uncovered previously unobserved metabolites. We explored the intricate processes involved in the biosynthesis and transmission of signals by important plant hormones under Cd stress. What distinguishes our study is the development of a unique and interconnected signal regulatory network, shedding light on how Si reduces the impact of Cd stress on plants. These findings represent a significant step forward in understanding plant responses to stress and have the potential to inform new strategies for managing environmental stress in agriculture.
Materials and methods
Soil sampling and preparation
The soil utilized was sourced from the topmost layer, specifically the depth of 0–20 cm of a freely draining grassland field dominated by Medicago sativa L. situated at the Agriculture Research Station in Charsadda, Pakistan, at an elevation of 20 m (34.1682° N, 71.7504° E). The upper part under consideration is characterized as sedimentary and possesses a silty clay loam surface. Average yearly temperature ranges from 12 to 32 °C, while the yearly precipitation is recorded at 513 mm. Notably, there is no documented record of Cd contamination in this soil. This experimental website is under the ownership of the Agriculture Research Station Charsadda, which have a longstanding and esteemed history in the field of farming for over 20 years. No indications of Cd contamination were detected at the location. Subsequently, the soil samples were carefully gathered and placed in air-permeable polyethylene bags, ensuring adequate ventilation, and promptly taken to the laboratory. Prior to utilization, the soil underwent homogenization and sieving with a mesh size of 4 mm, accompanied by the removal of fine roots and other plant residues. The fundamental properties of the soil were determined as pH 8, electrical conductivity of 0.73 m/S, nitrogen (Kjeldahl) content of 475 mg kg−1, phosphorus (Olsen) content of 2.2 mg kg−1, potassium (measured using the Jenway PFP7 Flame Photometer) content of 233 mg kg−1, and carbon content (determined using the Walkley and Black chromic acid wet oxidation technique) of 9600 mg kg−1, respectively. For further details regarding the experimental site, please refer to the work of Shah et al. (2020).
Experimental design and set-up
A completely randomized design pot conducting tests was performed in anon-heated glass house, employing three replicates per treatment. The pots utilized in the experiment had dimensions of 12 cm by 8 cm for the surface area and 18 cm in height, with a total of 12 pots. One kilogram of soil was placed in each pot based on dry weight. The experimental treatments are shown in Table 1.
The pots underwent a preliminary incubation phase in a greenhouse, where they were exposed to Cd stress for a period of 1 month. The Cd and Si were applied in the form of CdCl2 and Si(OH)4 bought from Sigma Chemical Company, St. Louis, MO, USA. The acquisition of maize seeds was facilitated through the National Agricultural Research Center in Islamabad, Pakistan. In each pot, six seeds of the maize variety Silver-2019 were initially sown. Six days of growth resulted in a thinning procedure that left only two seedlings per pot. The plants received watering at intervals of 4 days, ensuring that the soil moisture remained consistently at a gravimetric moisture content of 20% throughout the duration of the experiment. In a carefully monitored growth chamber, the seedlings were raised, which maintained an 8-h dark cycle and 16-h light. The light concentration provided was measured at an average of 124 ± 3 μmol m−2s−1. Additionally, a moisture of 60% and temperature of 22 °C were maintained in the growth chamber to provide optimal conditions for the seedlings’ development.
Measurements
Plant biomass
After a growth period of 40 days, samples were taken from both the plant and the soil. The shoots were carefully severed at the stem’s base, while the soil and root were collected as separate entities. To ensure cleanliness, the roots were washed with tap water and subsequently measured their length with the help of a scale. Following this, the shoot, root, and soil samples were thoroughly dried at an elevated temperature (65 °C) for a duration of 24 h, resulting in over drying. Once dried, the samples were weighed, homogenized, and subjected to ball milling to prepare them for further analysis.
Analyses of saccharide compounds
For sample extraction, a quantity of 20 mg was mixed with ultrapure water with concentration of 1 mL. The resulting mixture was exposed to 30 min of ultrasound treatment, followed by 10 min of centrifugation at 14,000 rpm. Using an injection syringe with a 0.1 m filter, the obtained supernatant was purified. After that, 20 times as much ultrapure water was added to dilute the liquid. A Thermo Scientific Dionex ICS-5000 + ion chromatograph was applied to measure the dilution of saccharide compounds. It was outfitted with a number of different parts, including a single pump, eluent generator, automatic sampler AP, electrochemical detector (DC), and Chromeleon7.2 SR5 chromatographic data analytical software. Injections were made at a rate of 1 mL/min, with a volume of 10 L. Mobile phases A and B comprised of ultrapure water and a 200 mM NaOH solution (where ultrapure water is diluted with 50% NaOH), correspondingly. The gradient elution method was in this manner: 91% A at 0 min; 91% A at 18 min; 0% A at 21 min; 0% A at 31 min; 91% A at 32 min; and 91% A at 40 min.
Plant hormone analyses
The research conducted by Li et al. in 2020 was modified by the methodology used in this study. To prepare the samples, leaves and roots were chilled in liquid nitrogen (N) and subsequently crushed into a powder, and 0.1 g of the powdered samples were subjected to an extraction solution of CH3OH, H2O, and formic acid (in a ratio of 80:19:1, v/v/v) in the presence of ultrasound for a duration of 10 min. Following extraction, the samples undergo centrifugation at 12,000 rpm for 5 min. The supernatants obtained were combined with 50 mg of primary secondary amine (obtained from CNW Technologies GmbH, Shanghai, China) in a 2 mL centrifuge tube. After the mixture had been nitrogen-dried, the volume was maintained at 100 L using solution containing 80% methanol (CH3OH). Supernatant was filtered through a 0.22-m organic membrane to ensure purity. For analysis, an Agilent 6465 Triple Quadrupole UPLC–MS/MS system (Ultivo; Agilent Technologies, Santa Clara, CA, USA) was utilized. The program worked at 0.4 mL/min and had an EclipsePlus C18 column (2.1 × 50 mm, 1.8 μm) attached. Acetonitrile and a 0.1% formic acid solution in water made up mobile phases A and B, respectively. As for the gradient elution procedure, it went as follows: 80% A at 0 min, 5% at 4 min, 80% A at 4.1 min, and 5.2 min. Positive and negative ion scanning modes are employed with the UPLC-MS/MS equipment for analysis, as well as multiple reaction monitoring.
Identification of lignin synthesis-related metabolites
A total of 0.1 g of root samples were crushed into a powder using liquid nitrogen. One milliliter of a solution containing 80% CH3OH and 1% CH2O2 was used in the extraction procedure. These mixtures were subjected to shaking for 3 min, followed by treatment with an ultrasonic instrument for 10 min. Afterward, centrifugation was carried out at 10,000 rpm for duration of 5 min. Then, supernatant was poured into a 2 mL centrifugation tube along with 50 mg of C18 and 5 mg of multi-walled carbon nanotubes obtained from Sinopharm Chemical Reagent Co. (Beijing, China). After 2 min of vertexing, at 4 °C, the sample was centrifuged at 10,000 rpm for 5 min. The whole supernatant was filtered through an organic membrane with a 0.22-m pore size to ensure purity. This study used the Agilent 6465 Triple Quadrupole UPLC/MS/MS system, specifically the Agilent Technologies Ultivo model. The system was equipped with a Venusil Hilic column (2.1 × 50, 1.8 μm) for chromatographic separation. The flow rate utilized was set at 0.25 mL/min. Mobile phases A and B were comprised of acetonitrile and a 0.1% formic acid solution in water, respectively. The gradient elution protocol consisted of the following steps: 5% A at 0 min, 95% A at 4 min, 5% A at 4.1 min, and 5% A at 5.2 min. The UPLC-MS/MS system operated in both positive ( +) and negative ( −) ion testing modes, utilizing several reactions observing for analysis purposes.
The HPLC–UV system utilized in this research was provided by Agilent Technologies and featured a Venusil Hilic column (4.6 × 100, 5 μm). For the system, the rate of 1 mL/min was chosen. Acetonitrile and ultrapure water, respectively, made up mobile phases, i.e., A and B. To achieve elution, substance of interest underwent a 5-min isocratic run of 60:40 for both mobile phases. The UV absorption of the eluted compounds was continuously monitored at a wavelength of 217 nm.
Real-time quantitative PCR
To support the findings from the transcriptomics analysis, a qRT-PCR approach was utilized for 10 selected genes. RNA extraction was carried out using the RNAprep pure Plant Kit, following the instructions provided by the manufacturer. The Fast-Quant RT Kit was then used to synthesize cDNA. The expression of the β-actin gene served as a reference for the qRT-PCR analysis, which was operated using SuperReal PreMix Plus (SYBR Green). Primer sequences for the selected genes were obtained from Sangon Biotech (Shanghai, China) and are recorded in Table S1. The specific protocols and procedures for the qRT-PCR analysis were explained earlier by Li et al. in 2020.
Statistical analysis
A differential analysis was carried out via SPSS 26.0 and ANOVA (IBM, Inc., Armonk, NY, USA). GraphPad Prism, version 8.0.0, was used to make the graphs. Using the Tukey’s t-test to assess the separation of means, large differences were uncovered at the p 0.05 level. R 3.5.0 was utilized for the multivariate analysis (R Core Team, 2018).
Results
Si increases the plant biomass and Si content by decreasing Cd uptake
In comparison to the control group (those who were not exposed to Cd Fig. 1a–b), the use of Si increased the root and shoot dry weight by a significant (p 0.05) 39% and 21%, respectively. In contrast, Cd stress led to a notable decrease in shoot’s dry weight by − 19% and in case of root − 29% related to the control group (Fig. 1a–b). Remarkably, the application of Si in Cd-stressed plants exhibited a substantial enhancement in shoot dry weight and root dry weight (Fig. 1a–b). The dry weight of shoot increased by + 75% and root dry weight increased by + 30% compared to the Cd-stressed condition. Under the Cd stress condition, the shoot and root of plants experienced a significant reduction in Si content, exhibiting a decrease of − 25% and − 18%, respectively (Fig. 1e–f). However, exogenous Si application resulted in a decrease shoot and root Cd content in Cd-stressed plants by − 20% and − 26%, respectively (Fig. 1c–d). Additionally, the utilization of Si resulted in a notable augmentation of Si concentration in the root and shoot of Cd-stressed plants, with increase of + 60% and + 26%, respectively.
The effect of silicon (Si) on shoot dry weight (a), root dry weight (b), cadmium concentration in shoot (c), and cadmium concentration in root (d), as well as the presence of Si content in shoot (e) and root (f) of CD-stressed maize plants, was investigated. Results are presented as mean values ± SD (n = 3). One-way ANOVA was used to analyze the statistical differences. Different lowercase letters above the bars indicate significant differences of treatments at the level of p < 0.05
Si act synergistically with saccharide compounds under Cd stress
A substantial increase was observed (p < 0.05) in leaf arabinose (+ 180%) in response to Cd stress, glucose (+ 182%), fructose (+ 40%), and sucrose (+ 107%) content with respect to the control (no Cd; Fig. 2a–h). Similar trends were observed in the roots, with arabinose content increased by + 275%, glucose by + 478%, fructose by + 27%, and sucrose by + 87% (p < 0.05) with respect to the control (no Cd; Fig. 2a–h). Remarkably, the practice of Si to Cd-stressed plants increased the relative content of arabinose even more and glucose in the leaf by + 29% and + 39%, respectively (Fig. 2a–b). No significant elevation was observed in leaf fructose content. Interestingly, the sucrose content in the leaf exhibited a decrease of − 2% in comparison to the control (Cd stress). In the roots, the application of Si in Cd-stressed plants led to significant increases in glucose, fructose, and sucrose content by + 10%, + 7%, and + 2%, respectively (Fig. 2a–h). However, the arabinose content in the roots significantly decreased by − 16% upon Si application (Fig. 2b).
Leaf arabinose (a), root arabinose (b), leaf glucose (c), root glucose (d), leaf fructose (e), root fructose (f), leaf sucrose (g), and root sucrose (h) of Cd-stressed maize plants were analyzed to assess the counteractive effect of silicon (Si) against cadmium. Results are presented as mean values ± SD (n = 3). Statistical differences were analyzed using one-way ANOVA, and significant differences between treatments were denoted by different lowercase letters above the bars at the level of p < 0.05
Exogenous Si regulates plant hormones and their associated genes in Cd-stressed plants
Cd-stressed plants had a considerable rise in leaf abscisic acid content (+ 75%), brassinolide content (+ 182%), and jasmonic acid content (+ 33%) compared to the control group (without Cd; Fig. 3a–n). Conversely, salicylic acid (SA) content showed a notable decrease (− 3%) in the leaves of Cd-stressed plants compared to the control group (no Cd). Similarly, the roots of Cd-stressed plants displayed similar trends, with significant upregulation of ABA content (+ 100%), BL content (+ 380%), JA content (+ 158%), and SA content (+ 11%) compared to the control group (no Cd). Interestingly, the application of Si to Cd-stressed plants led to sudden increase in ABA, BL, and JA content in both the leaf (+ 5%, + 17%, and + 81%, respectively) and the root (+ 17%, + 352%, and + 6%, respectively; Fig. 3a–n). However, Si application resulted in a gradual decrease in SA content in both the leaf (− 26%) and the root (− 9%) of the Cd-stressed plants (Fig. 3a–n).
In the presence of silicon (Si), Cd-stressed plants exhibited altered levels of antioxidants, including leaf ABA (a), root ABA (b), leaf SA (e), root SA (f), leaf BL (i), root BL (j), leaf JA (m), and root JA (n). Moreover, the expression of genes involved in antioxidant regulation, such as leaf BZR1 (c), root BZR1 (d), leaf LOX3 (g), root LOX3 (h), leaf ICS2 (k), root ICS2 (l), leaf NCDE1 (o), and root NCDE1 (p), showed significant changes in response to Si application under Cd stress. The presented data are represented as mean values ± SD (n = 3), and statistical significance was determined using one-way ANOVA. Notably, lowercase letters above the bars indicate significant differences among treatments at the level of p < 0.05
The comparative expression levels of TaBZR1, TaLOX3, TaICS2, and TaNCDE1 were notably increased in both the leaf (+ 210%, + 419%, + 20%, and + 112%, respectively) and root (+ 86%, + 224%, + 11%, and + 200%, respectively) of Cd-stressed plants when associated to the control group (without Cd; Fig. 3a–n). Upon the exogenous application of Si to Cd-stressed plants, a different pattern of gene expression was observed. The Si application considerably enhanced the expression of TaBZR1 in leaf and root of Cd-stressed plants as compared to other treatments (Fig. 3a–n). However, the treatment of Si also enriched the expression of TaLOX3 in leaf and root of Cd-stressed plants (Fig. 3a–n). Conversely, no significant effect of Si was observed for the relative expression of TaICS2 in the leaf and root of Cd-stressed plants. The application of Si to Cd-stressed plants significantly enhanced the expression of TaNCDE1in the leaf and root as compared to other treatments (Fig. 3a–n).
Si elevates the accumulation of lignin-associated metabolites and the genes in Cd-stressed plants
Cd stress significantly influenced the levels of various lignin-related compounds compared to the control group (no Cd; Fig. 4a–i). Cinnamic acid, phenylalanine, sinapyl alcohol, caffeyl alcohol, coniferaldehyde, p-coumaryl alcohol, and p-coumaraldehyde exhibited significant increases of + 78%, + 74%, + 52%, + 107%, + 64%, + 29%, and + 33%, respectively (Fig. 4a–i). Additionally, there was a notable rise in the total lignin content of the plants exposed to Cd stress of + 33% compared to the control group (no Cd). Conversely, the content of p-coumaric acid, a lignin derivative, displayed a small but significant decrease of − 1% (Fig. 4a–i). Additionally, introduction of Si in Cd-stressed soil had a significant impact on the relative content of lignin-related substances, resulting in distinct changes (upregulation; Fig. 4a–i), except for caffeyl alcohol, p-coumaraldehyde, and coniferaldehyde exhibited significant decrease of − 37%, − 6%, and − 4%, respectively (Fig. 4a–i).
Lignin-related metabolites, including p-coumaric acid (a), sinapyl alcohol (b), p-coumaryl alcohol (c), caffeyl alcohol (d), cinnamic acid (e), coniferaldehyde (f), phenylalanine (g), p-coumaraldehyde (h), and lignin (i), were investigated in maize plants subjected to Cd stress with or without the application of silicon (Si). Additionally, the expression levels of genes involved in the regulation of lignin-related metabolites, such as C3H (j), CAD (k), 4CL (l), PAL (m), HCT (n), and COMT (o), were analyzed. The data are expressed as the mean values ± SD (n = 3) and subjected to statistical analysis using one-way ANOVA. The significance of differences between treatments was determined by identifying distinct lowercase letters above the bars at the p < 0.05 level
Key lignin biosynthesis genes had substantially different relative expression levels in the Cd-stressed soil compared to control group (no Cd; Fig. 4j–o). Specifically, there was a significant increase relative expression of TaCAD, Ta4CL, TaPAL, TaHCT, and TaCOMT by 126%, 47%, 161%, 30%, and 144%, respectively (Fig. 4j–o). Conversely, the relative expression of C3H showed a significant decrease of − 11% compared to the control (no Cd; Fig. 4j–o). The application of Si in Cd-stressed plants resulted in a downregulation of the relative expression levels of TaCAD, Ta4CL, TaPAL, TaHCT, and TaCOMT by − 20%, − 30%, − 13%, and − 29%, respectively (Fig. 4j–o). However, in Cd-stressed plants, exogenous injection of Si resulted in a + 1% increase in the relative expression of TaC3H.
Multivariate analysis
Principal component analysis depicted that the clustering positions of plant biomass, saccharides compounds, plant hormones, genes encoding plant hormones, lignin-associated metabolites, and genes shifted significantly from the control position in the response to Cd stress with and without Si, suggesting that the Cd stress with and without Si changed all the plant traits (Fig. 5a). The first two components exhibit 82.8% of the total variance under Cd stress with and without Si. The first component explains 70.4% of the total variance and the second component explains 12.4% of the total variance. Thirty-eight plant traits were considerably increased under Cd stress and were clustered in distinct group I (Fig. 5b). All these traits were strongly correlated and increased under Cd stress while under Si application, no changes were observed in these traits except for root SA, ICS2 gene, caffeoyl alcohol, 4CL, and HCT gene as compared to control. The other groups of traits (shoot dry weight, root Si, shoot Si, root dry weight, and p-coumaric acid) were clustered in group II and were significantly reduced under Cd stress; nevertheless, these traits were increased in Si treatment under Cd stress.
Discussion
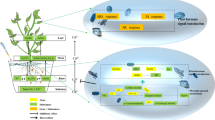
Plant growth can be hampered by Cd stress, which can also disrupt plant metabolism and reduce crop output (Zulfiqar et al. 2022). To minimize Cd toxicity exposure and guarantee food safety, maize plants must minimize Cd absorption. The use of Si modifies several processes, including the secondary metabolism and antioxidant system, to lessen the negative effects of biotic and abiotic stress (Ahanger et al. 2020). The impact of Si treatment on maize plant resistance methods to heavy metal-induced stress is little understood, although further study in this area is needed. Our study looked into the distinct issues of Si-induced plant development, as well as the related uptake and alteration under Cd stress. Si additionally aided in maintaining the structural integrity of the cell wall by controlling the pathway of lignin biosynthesis in the root, likewise, increase in resistance by modifying plant hormone signal transduction. These results demonstrated that Si helps to improve maize plants’ resistance to Cd stress, which is closely connected to synergistically control of Si/Cd absorption, homeostasis, and plant-hormone-associate signal pathway in pepper plants (Fig. 6).
Principal component analysis (PCA) (a) and heat map (b) were used to assess various parameters, including plant biomass and Si content, saccharide compounds, plant hormones, and their associated genes, as well as lignin-associated metabolites and their corresponding genes, in maize plants exposed to Cd stress with or without Si application
When exposed to Cd stress, weight of root and shoot drastically decreased (Fig. 1a–b). The biomass accumulation declined as the plants were exposed to increased Cd accumulation. This can be because of preventing the absorption of carbohydrate molecules. The presence of Cd was found to increase the levels of arabinose in the current study, fructose (C12H22O11), glucose (C6H12O6), and fructose (C6H12O6) in the maize roots and leaves compared to Si and control treatments. The combination of Cd and Se (Cd + Si) substantially enhanced the levels of glucose and sucrose in the roots when compared to Cd treatment alone. According to Shahid et al. (2019), sodium selenate increased the N and C metabolism caused by arsenic and/or Cd stress by lowering the levels of Cd and arsenic, which aided in sugar accumulation and reduced oxidative stress. Transcriptomics research revealed that the metabolism of starch and sucrose in leaves subjected to various treatments changed significantly (control, Cd + selenium) but not in the roots (Sevcikova et al., 2017). Accumulation of soluble sugar in leaves that are under abiotic stress can improve plants maintain water status and remove ROS (Sevcikova et al. 2017; Shah et al. 2020).
Furthermore, to enhance the Si level in the eatable sections of yields, Si has been employed as a foliar spray or as a base fertilizer. Plant species, patterns of utilization and concentration, physiological circumstances, translocation, membrane transporter activity, and mechanisms all affect how Si is absorbed, translocated, and distributed in plants (Gupta et al. 2016; Shah et al. 2023a; Jiang et al. 2023). In this study, the concentration of Si increased significantly with increasing Si content. Numerous investigations have observed that using Si can lower plant absorption and transformation while also mitigating metabolic disturbances caused by heavy metals. Amount of Cd was decreased in the higher parts of the pepper because of nano-ability Se’s to prevent the collection of Cd in the roots. The root system also contained more Cd than the rest of the plant combined (Fig. 2a–c). As the Si concentration increases in this research, Si content rose considerably. According to numerous studies, the use of Si can lessen plant uptake and transformation as well as ameliorate metabolic problems brought on by heavy metals. The amount of Cd was greatly reduced in the upper regions of the maize as a result of Si ability to prevent the buildup of Cd in the roots. Additionally, the roots had a higher concentration of Cd than the shoots (Fig. 1c–d). A potential mechanism for reducing Cd buildup in plant tissues could be to bind Cd and convert it to other non-absorptive form. This investigation showed that Si taken in through roots can change into an organic state very quickly. Si compounds may combine with Cd to form a complex that effectively holds Cd in place and prevents its upward translocation. Other crops, such as rice (Oryza sativa L.) and bok choy (Brassica chinensis) (Guo et al. 2021; Hussain et al. 2020), also exhibit the same phenomenon.
Moreover, there has been no research into negative impact of Si on the regulation of genes and metabolite strangled in the lignin production pathway in maize plants. Previous studies revealed that the genes for the CAD, 4CL, COMT, PAL, C3H, and HCT encode substances in phenylpropanoid pathway. Maize plant lignin levels are positively related to gene levels. The biosynthesis of lignin promotes plant development and nutrient and water transport. According to Fig. 4, after treatment with Cd and Si, the contents of TaPAL, TaCAD, Ta4CL, and TaCOMT dramatically increased. The most effective was Cd and Si. According to research, tobacco lignin production increased the level of PAL (Silva et al. 2018). The MYB transcription factors activate the 4CL lignin biosynthetic gene, which triggers the production of lignin. Increased amounts of CAD, C3H, COMT, 4CL, and HCT were found to be involved in lignin production. This caused lignin to build up and the cell walls to thicken, which improved osmotic protection agents and reduced the salt effect on apples (Chen et al. 2019; Basit et al. 2023). In the biosynthesis of monolignol, cinnamyl alcohol dehydrogenase catalyzes the creation of cafeyl alcohol, p-coumaryl alcohol, and coniferyl alcohol (Barros et al. 2019). To ensure healthy growth and survival, fixation modifies the distribution of metabolites because plants are constantly evolving in response to a changing environment (Dong et al. 2021; Shah et al. 2023b). Si biofortification reduced Cd damage and increased maize plant growth by improving the ultrastructure of the mitochondria and plasma membrane in the tip of root. According to Zhao et al. Si enhanced the shape of roots and controlled how well they absorbed nutrients. This could have a significant impact on lowering the rate at which Cr is absorbed by roots, lowering Cr stress, and preserving plant growth (Zhao et al. 2019; Ali et al. 2023). In our research, different Cd and Si were more effective than Cd/selenium alone at increasing the levels of p-coumaryl alcohol, phenylalanine, sinapyl alcohol, and cafeoyl alcohol in the maize root, which induce lignin biosynthesis (Fig. 4a–i). Such metabolites were found to be more abundant in the lignin biosynthetic pathway and are thought to determine the properties of lignin, making them suitable for lignin engineering to enhance plant growth and utilization (Wang et al. 2019). Under Cd stress, Cui et al. found that Si enhanced lignin content and wall thickness, reduced ROS levels, and induced the expression of Cd-related and lignin production genes, all of which contributed to an increase in the walls’ mechanical strength (Cui met al. 2018). Therefore, we draw the conclusion that the use of Si caused the expression of genes and metabolites related to lignin, improving automated power to cell wall and blocking Cd incorporation in the cells.
Additionally, under Cd stress, the accumulation of Si can control the signal transduction of plant hormones that have a coordinated impact on the lignin metabolic path. Plant hormones are crucial for crop output and stress tolerance, as well as for coordinating responses to environmental change (Haider et al. 2021). By altering plant hormones and allocating nutritional components, the root shape and metal transporters were modified (Betti et al. 2021). The resistance, quality features, nutrients, and shelf life of many crops are all improved by foliar sprayer plant hormones such as JA, BR, and SA (Bürger et al. 2019; Garcia-Garcia et al. 2020). In contrast to other therapies like BZR1, LOX3, and NCDE1, our research shows that Cd + Si can greatly boost gene expression with regard to hormonal plants in the roots and leaves. Under Cd stress, these genes also increased the levels of JA, ABA, and BR in the leaves and roots of plants. Si considerably boosted the amount of jasmonic acid and ABA-related genes in our earlier investigation of pepper fruit (Li et al. 2020) (Hewedy et al. 2023).
Considerably, raising the amount of jasmonic acid detected at 48 h below 50 mg/L Cd and 50 mg/L Cd + 0.1 mmol/L MeJA treatment in the Capsicum frutescens var. fasciculatum seedlings, low MeJA absorptions can counteract the negative effects of Cd (Yan et al. 2013). By creating more osmotic chemicals, including cuticles, proline, polyphenols, lignin, and waxes, JAs improve the crop’s ability to fend off water loss (Wasternack et al. 2013). The key enzyme in the pathway that results in the production of phenolic compounds, PAL, is activated more frequently when JAs are present, and this increases gene expression (Wasternack et al. 2013). The mechanism of BRs reduces the toxicity of heavy metals by enhancing the antioxidant system, upregulating MAPK expression, and elevating Pro (proline), antioxidants, K, NA, and osmolytes levels (Ahammed et al. 2020; Rajewska et al. 2016). By modifying antioxidant defenses, Cu stress was mitigated by ABA and defended the glandular trichomes, which helped plants develop and become more tolerant of oxidative stress (Zehra et al. 2020). Through the collaboration of SA, ABA, and JA, they work together to improve plant metabolism and stress tolerance (Peres et al. 2019). Additionally, a variety of phytohormone signal pathways, including JA, can control how phenylpropanoid metabolism is regulated (Dong et al. 2021).
In our comprehensive scientific investigation, we have unveiled the intricate and pivotal role of Si in mitigating Cd stress in maize plants. This research sheds profound light on the mechanisms underlying Si-induced resistance, offering a new perspective on the complex interplay between Cd toxicity, plant metabolism, and crop output. Si treatment emerges as a compelling tool to effectively minimize Cd absorption while orchestrating vital changes in secondary metabolism and antioxidant responses. Additionally, Si’s contribution to preserving cell wall integrity and influencing lignin biosynthesis extends our understanding of plant defense strategies against heavy metal stress. The pivotal role of Si in modulating plant hormone signaling pathways further underscores its potential as a powerful tool in enhancing plant resilience. This research underscores Si as a promising and multifaceted solution in the quest for sustainable and resilient agricultural practices, with significant implications for future agricultural advancements and environmental well-being.
Conclusion
Our study delves into the intricate mechanisms by which Si effectively mitigates the deleterious impact of Cd stress on maize plants. By significantly reducing Cd uptake and accumulation, Si bolsters plant resilience and vitality. Furthermore, Si-induced sugar accumulation in both leaves and roots enhances plant biomass, a key factor in Cd-stressed environments. The thickening of cell walls, as indicated by the elevated levels of specific metabolites and lignin-related gene expression, contributes to plant integrity and Cd resistance. The upregulation of essential genes and hormones associated with stress response, such as TaBZR1, TaLOX3, ABA, BR, and JA, underscores Si’s role in fortifying maize plants under challenging conditions. Our findings provide valuable insights into the novel pathways through which Si operates to enhance crop tolerance to environmental stressors, promising innovative strategies for sustainable agriculture and food security. Importantly, our research enhances our understanding of Si’s protective effects in abiotic stress management. By focusing on the novelty of our data, we underscore Si’s potential to revolutionize the mechanisms underpinning its Cd-alleviating properties. These insights could have broader implications for improving agricultural practices, safeguarding crop yields, and bolstering plant resilience to environmental stress.
Data availability
The data supporting the conclusions of this article are included within the article. Any queries regarding these data may be directed to the corresponding author.
References
Ahammed GJ, Li X, Liu A, Chen S (2020) Brassinosteroids in plant tolerance to abiotic stress. J Plant Growth Regul 39(4):1451–1464
Ahanger MA, Bhat JA, Siddiqui MH, Rinklebe J, Ahmad P (2020) Integration of silicon and secondary metabolites in plants: a significant association in stress tolerance. J Exp Bot 71(21):6758–6774
Ali A, Shah T, Haider G, Awan MI, Gohar M, Munsif F, Ahmad I (2023) Strigolactone-mediated oxidative stress alleviation in Brassica rapa through upregulating antioxidant system under water deficit conditions. J Plant Growth Regul:1–13
Alzahrani Y, Rady MMJE, Safety E (2019) Compared to antioxidants and polyamines, the role of maize grain-derived organic biostimulants in improving cadmium tolerance in wheat plants. Ecotoxicol Environ Safety 182:109378
Barros J, Escamilla-Trevino L, Song L, Rao X, Serrani-Yarce JC, Palacios MD, Engle N, Choudhury FK, Tschaplinski TJ, Venables BJ, Mittler R, Dixon RA (2019) 4-Coumarate 3-hydroxylase in the lignin biosynthesis pathway is a cytosolic ascorbate peroxidase. Nat Commun
Basit F, Bhat JA, Alyemeni MN, Shah T, Ahmad P (2023) Nitric oxide mitigates vanadium toxicity in soybean (Glycine max L.) by modulating reactive oxygen species (ROS) and antioxidant system. J Hazard Mater 451:131085
Basu S, Kumar G, Biochemistry (2021) Exploring the significant contribution of silicon in regulation of cellular redox homeostasis for conferring stress tolerance in plants. Plant Physiol Biochem 166:393–404
Betti C, Della Rovere F, Piacentini D, Fattorini L, Falasca G, Altamura MM (2021) Jasmonates, ethylene and brassinosteroids control adventitious and lateral rooting as stress avoidance responses to heavy metals and metalloids. Biomolecules
Chen K, Song M, Guo Y, Liu L, Xue H, Dai H, Zhang Z (2019) MdMYB46 could enhance salt and osmotic stress tolerance in apple by directly activating stress-responsive signals. Plant Biotechnol J 17(12):2341–2355
Chung YS, Kim K-S, Hamayun M, Kim Y (2020) Silicon confers soybean resistance to salinity stress through regulation of reactive oxygen and reactive nitrogen species. Frontiers Plant Sci 10:1725
Cui J, Liu T, Li Y, Li F (2018) Selenium reduces cadmium uptake into rice suspension cells by regulating the expression of lignin synthesis and cadmium-related genes. Sci Total Environ 644:602–610
Das P, Manna I, Biswas AK, Bandyopadhyay M, Research, P (2018) Exogenous silicon alters ascorbate-glutathione cycle in two salt-stressed Indica rice cultivars (MTU 1010 and Nonabokra). Environ Sci Pollution Res 25:26625–26642
Desoky E-SM, Merwad A-RM, Semida WM, Ibrahim SA, El-Saadony MT, Rady MM, Safety, E (2020) Heavy metals-resistant bacteria (HM-RB): potential bioremediators of heavy metals-stressed Spinacia oleracea plant. Ecotoxicol Environ Safety 198:110685
Dong NQ, Lin HX (2021) Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J Integr Plant Biol 63(1):180–209
Elsheery NI, Helaly MN, El-Hoseiny HM, Alam-Eldein SM (2020a) Zinc oxide and silicone nanoparticles to improve the resistance mechanism and annual productivity of salt-stressed mango trees. Agronomy 10(4):558
Elsheery NI, Sunoj V, Wen Y, Zhu J, Muralidharan G, Cao K (2020b) Foliar application of nanoparticles mitigates the chilling effect on photosynthesis and photoprotection in sugarcane. Plant Physiol Biochem 149:50–60
Elsheery NI, Helaly MN, El-Hefnawy SF, Elhamahmy MM, Abdelrazik EM, Sardarov YB, Ahmad P, Zivcak M, Brestic M, Allakhverdiev SI (2023) 5-Aminolevulinic acid (ALA) reduces arsenic toxicity stress in wheat (Triticum aestivum L.). J Plant Growth Regul 42(6):3303–3322
Farooq MA, Ali S, Hameed A, Ishaque W, Mahmood K, Iqbal ZJE, safety, e (2013) Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol Environ Safety 96:242–249
Grant C, Clarke J, Duguid S, Chaney RL (2008) Selection and breeding of plant cultivars to minimize cadmium accumulation. Sci Total Environment 390(3):301–310
Gratão PL, Polle A, Lea PJ, Azevedo RA (2005) Making the life of heavy metal-stressed plants a little easier. Functional Plant Biol 32(6):481–494
Guo Y, Mao K, Cao H, Ali W, Lei D, Teng D, Chang C, Yang X, Yang Q, Niazi NK, Feng X, Zhang H (2021) Exogenous selenium (cadmium) inhibits the absorption and transportation of cadmium (selenium) in rice. Environ Pollut 268(Pt A):115829
Gupta M, Gupta S (2016) An overview of selenium uptake, metabolism, and toxicity in plants. Front Plant Sci 7:2074
Haider FU, Liqun C, Coulter JA, Cheema SA, Wu J, Zhang R, Wenjun M, Farooq M (2021) Cadmium toxicity in plants: impacts and remediation strategies. Ecotoxicol Environ Saf 211:111887
Helaly MN, El-Sheery NI, El-Hoseiny H, Rastogi A, Kalaji HM, Zabochnicka-Świątek M (2018) Impact of treated wastewater and salicylic acid on physiological performance, malformation and yield of two mango cultivars. Sci Hortic 233:159–177
Hewedy OA, Elsheery NI, Karkour AM, Elhamouly N, Arafa RA, Mahmoud GA-E, Dawood MF-A, Hussein WE, Mansour A, Amin DH (2023) Jasmonic acid regulates plant development and orchestrates stress response during tough times. Environ Exp Bot 208:105260
Hussain S, Khan F, Cao W, Wu L, Geng M (2016) Seed priming alters the production and detoxification of reactive oxygen intermediates in rice seedlings grown under sub-optimal temperature and nutrient supply. Front Plant Sci 7:439
Hussain B, Lin Q, Hamid Y, Sanaullah M, Di L, Khan MB, Yang X (2020) Foliage application of selenium and silicon nanoparticles alleviates Cd and Pb toxicity in rice (Oryza sativa L.). Sci Total Environ 712:136497
Imran M, Hussain S, El-Esawi MA, Rana MS, Saleem MH, Riaz M, Ashraf U, Potcho MP, Duan M, Rajput IAJB (2020) Molybdenum Supply Alleviates the Cadmium Toxicity in Fragrant Rice by Modulating Oxidative Stress and Antioxidant Gene Expression 10(11):1582
Jiang Y, Wei C, Jiao Q, Li G, Alyemeni MN, Ahmad P et al (2023) Interactive effect of silicon and zinc on cadmium toxicity alleviation in wheat plants. J Hazard Mater 131933
Kevresan S, Petrovic N, Popovic M, Kandrac J (2001) Nitrogen and protein metabolism in young pea plants as affected by different concentrations of nickel, cadmium, lead, and molybdenum. J Plant Nutrition 24(10):1633–1644
Kim Y-H, Khan AL, Waqas M, Lee I-J (2017) Silicon regulates antioxidant activities of crop plants under abiotic-induced oxidative stress: a review. Frontiers Plant Sci 8:510
Li D, Zhou C, Zhang J, An Q, Wu Y, Li JQ, Pan C (2020) Nanoselenium foliar applications enhance the nutrient quality of pepper by activating the capsaicinoid synthetic pathway. J Agric Food Chem 68(37):9888–9895
Ma JF, Miyake Y, Takahashi E (2001) Silicon as a beneficial element for crop plants. Studies in Plant Science 8:17–39
Peres A, Soares JS, Tavares RG, Righetto G, Zullo MAT, Mandava NB, Menossi M (2019) Brassinosteroids, the sixth class of phytohormones: a molecular view from the discovery to hormonal interactions in plant development and stress adaptation. Int J Mol Sci
Rady MM, Elrys AS, El-Maati MFA, Desoky E-SMJPP (2019) & Biochemistry. Interplaying Roles of Silicon and Proline Effectively Improve Salt and Cadmium Stress Tolerance in Phaseolus Vulgaris Plant 139:558–568
Rajewska I, Talarek M, Bajguz A (2016) Brassinosteroids and response of plants to heavy metals action. Front Plant Sci
Rizwan M, Meunier JD, Miche H, Keller C (2012) Effect of silicon on reducing cadmium toxicity in durum wheat (Triticum turgidum L. cv. Claudio W.) grown in a soil with aged contamination. J Hazard Mater 209:326–334
Sarwar N, Ishaq W, Farid G, Shaheen MR, Imran M, Geng M, Hussain SJE (2015) Zinc–cadmium interactions: impact on wheat physiology and mineral acquisition. Ecotoxicol Environmental Safety 122:528–536
Sevcikova H, Maskova P, Tarkowska D, Masek T, Lipavska H (2017) Carbohydrates and gibberellins relationship in potato tuberization. J Plant Physiol 214:53–63
Shah T, Munsif F, D’amato R, Nie L (2020) Lead toxicity induced phytotoxic impacts on rapeseed and clover can be lowered by biofilm forming lead tolerant bacteria. Chemosphere 246:125766
Shah T, Ali A, Haider G, Asad M, Munsif F (2023a) Microplastics alter soil enzyme activities and microbial community structure without negatively affecting plant growth in an agroecosystem. Chemosphere 322:138188
Shah T, Asad M, Khan Z, Amjad K, Alsahli AA, D'amato R (2023b) Strigolactone decreases cadmium concentrations by regulating cadmium localization and glyoxalase defense system: Effects on nodules organic acids and soybean yield. Chemosphere 139028
Shahid MA, Balal RM, Khan N, Zotarelli L, Liu GD, Sarkhosh A, Fernández Zapata JC, Martínez Nicolás JJ, Garcia-Sanchez F (2019) Selenium impedes cadmium and arsenic toxicity in potato by modulating carbohydrate and nitrogen metabolism. Ecotoxicol Environ Saf 180:588–599
Shanying H, Xiaoe Y, Zhenli H, Baligar VCJP (2017) Morphological and Physiological Responses of Plants to Cadmium Toxicity: a Review 27(3):421–438
Shi Y, Zhang Y, Yao H, Wu J, Sun H, Gong HJPP (2014) & Biochemistry. Silicon Improves Seed Germination and Alleviates Oxidative Stress of Bud Seedlings in Tomato under Water Deficit Stress 78:27–36
Song A, Li Z, Zhang J, Xue G, Fan F, Liang Y (2009) Silicon-enhanced resistance to cadmium toxicity in Brassica chinensis L. is attributed to Si-suppressed cadmium uptake and transport and Si-enhanced antioxidant defense capacity. J Hazard Mater 172(1):74–83
Team RC, Team MRC, Suggests MASS, Matrix S (2018) Package Stats. The R Stats Package
Uraguchi S, Mori S, Kuramata M, Kawasaki A, Arao T, Ishikawa S (2009) Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J Experimental Botany 60(9):2677–2688
Wang Y, Hu Y, Duan Y, Feng R, Gong HJAPP (2016) Silicon Reduces Long-Term Cadmium Toxicities in Potted Garlic Plants 38:1–9
Wang JP, Matthews ML, Naik PP, Williams CM, Ducoste JJ, Sederof RR, Chiang VL (2019) Flux modeling for monolignol biosynthesis. Curr Opin Biotechnol 56:187–192
Wang R, Lin K, Chen H, Qi Z, Liu B, Cao F, Chen H, Wu FJP (2021) Metabolome analysis revealed the mechanism of exogenous glutathione to alleviate cadmium stress in maize (Zea mays L) seedlings. Plants 10(1):105
Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in annals of botany. Ann Bot 111(6):1021–1058
Yan ZZ, Chen J, Li XZ (2013) Methyl jasmonate as modulator of Cd toxicity in Capsicum frutescens var. fasciculatum seedlings. Ecotoxicol Environ Saf 98:203–209
Zehra A, Choudhary S, Wani KI, Naeem M, Khan MMA, Aftab T (2020) Exogenous abscisic acid mediates ROS homeostasis and maintains glandular trichome to enhance artemisinin biosynthesis in Artemisia annua under copper toxicity. Plant Physiol Biochem 156:125–134
Zhao Y, Hu C, Wang X, Qing X, Wang P, Zhang Y, Zhang X, Zhao X (2019) Selenium alleviated chromium stress in Chinese cabbage (Brassica campestris L. ssp. Pekinensis) by regulating root morphology and metal element uptake. Ecotoxicol Environ Saf 173:314–321
Zhu Y, Gong H (2014) Beneficial effects of silicon on salt and drought tolerance in plants. Agron Sustain Dev 34:455–472
Zulfiqar U, Ayub A, Hussain S, Waraich EA, El-Esawi MA, Ishfaq M, Maqsood MF (2022) Cadmium toxicity in plants: Recent progress on morpho-physiological effects and remediation strategies. J Soil Sci Plant Nutr 1–58
Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2023R350), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
TS, ZK, and PA drafted the experimental design, and TS and ZK performed the experiments. SRK, AI, and MA analyzed the data and helped in paper writing. AA and PA revised and edited the language of this manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
All authors consent to participate in the manuscript publication.
Consent for publication
All authors approved the manuscript to be published.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shah, T., Khan, Z., Khan, S.R. et al. Silicon inhibits cadmium uptake by regulating the genes associated with the lignin biosynthetic pathway and plant hormone signal transduction in maize plants. Environ Sci Pollut Res 30, 123996–124009 (2023). https://doi.org/10.1007/s11356-023-31044-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-31044-z