Abstract
Children aged 3–6 years undergo a critical stage of growth and development and are irreversibly affected by their iodine status. In order to reveal iodine status in preschool children, we detected iodine concentrations in urine samples from 1382 children aged 3–6 years based on a cross-sectional study. The median urinary iodine concentration (UIC) of children was 193.36 μg/L and was 336.96 μg/g·Cr corrected for creatinine. The study developed a link between dietary habits and iodine status, revealing that regular calcium supplement (OR: 1.79, (95% CI: 1.03, 3.12)) increased deficiency risk, while moderate seafood consumption (OR: 0.60, (95% CI: 0.38, 0.95)) decreased it. Additionally, modest intake of shellfish (OR: 0.58, (95% CI: 0.33, 1.00)), vegetables (OR: 0.61, (95% CI: 0.38, 0.97)), and eggs (OR: 0.53, (95% CI: 0.30, 0.95)) was found to protect against excess iodine. The findings underline the importance of balanced diets and various nutrients’ roles in preschoolers’ iodine status.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iodine, an essential trace element, holds paramount significance in human growth and development. Both deficiency and excess iodine pose substantial health risks. In prenatal stages, iodine deficiency hampers fetal neurodevelopment and escalates the probability of fetal miscarriage and stillbirth (Black et al. 2013). During infancy and early childhood, it can result in various Iodine Deficiency Disorders (IDD), including endemic goiter and clinical or subclinical cretinism. Furthermore, inadequate iodine intake during childhood adversely impacts physical and cognitive-behavioral development, leading to developmental delays (Zimmermann et al. 2008). Conversely, excessive iodine may provoke hyperthyroidism, hypothyroidism, goiter, and thyroid autoimmune diseases (Farebrother et al. 2019).
Iodine deficiency (ID) continues to be a major global public health problem, affecting approximately 29% of the global population (Andersson et al. 2012; Delange 2002). Mild iodine deficiency remains prevalent in around 30% of Europe (Pearce et al. 2013), while developed countries like the USA and Australia have experienced a recent decline in iodine intake (Lee et al. 2010). The human body metabolizes iodine in various forms, which are converted to iodide before absorption in the intestine. Iodine uptake primarily relies on dietary iodine intake rather than its chemical form. Considering the inadequacy of dietary sources to meet appropriate iodine requirements in certain regions, including China, the World Health Organization (WHO) recommends iodine fortification of table salt as a cost-effective measure to prevent ID and related thyroid disorders in humans (World Health Organization 2014). Post the implementation of the Universal Salt Iodization (USI) policy in China in 1995 (Sun et al. 2017), the coverage rate of iodized salt in Shanghai reached an impressive 92.3% (Wang et al. 2019). This does not necessarily rule out the possibility of iodine deficiency, while it does indicate a high rate of utilization. Biennial monitoring of iodine status in school-age children has been the norm, primarily measuring Urinary Iodine Concentration (UIC) and Thyroid volume (Tvol) (Wu et al. 2012; Zimmermann and Andersson 2021). However, comprehensive data concerning the iodine intake of preschool children, especially those between 3 and 6 years old, remains conspicuously absent.
Hence, this epidemiological study was designed to fill the research gap by conducting a stratified cluster sampling on randomly selected children aged 3–6 years in Shanghai. The study gathered urine samples and collected sociodemographic and dietary information to illuminate their iodine status and pertinent influencing factors. This research represents the only large-scale epidemiological exploration of iodine status in preschool children in China to date.
Materials and methods
Study population
In this cross-sectional investigation, we employed a multi-stage stratified cluster random sampling strategy (Fig. 1), and six districts in Shanghai were randomly selected as survey sites, encompassing two central urban areas (Jing’an District and Xuhui District), two peripheral urban areas (Yangpu District and Pudong District), and two rural regions (Jiading District and Chongming District). The study spanned five months, from October 2013 to February 2014, and the participants between 3 and 6 years old are recruited from the community. Eligibility criteria required a minimum of two years of uninterrupted local residency, while children diagnosed with thyroid diseases (such as endemic goiter, hyperthyroidism, thyroiditis) or any other condition that might influence UIC were excluded.
Sample size calculation
The sample size was determined employing the formula n =\({\left(\frac{\mu_{\alpha}\times \sigma }{\delta}\right)}^2\), wherein δ represents allowable error, and δ =‾x − μ, with a 95% confidence interval and μα = 1.96 was adopted to ensure precision. As per our preceding work, the median UIC among school-age children in Shanghai in 2012 was 151.0 μg/L. Assigning σ to 150 μg/L and an allowable error of 21 μg/L (relative error of approximately 14%), while considering a missed follow-up rate of 15%, the projected sample size is determined to be 1176. Additionally, considering the financial considerations associated with this program, the final sample size has been established as 1382 children, ranging in age from 3 to 6 years.
Sample information collection
We collected random spot urine samples (mid-stream) from the subjects, which were stored at low temperatures, swiftly transported to the laboratory, dispensed into centrifuge tubes, and preserved at −80°C refrigerator. Height and weight measurements were meticulously taken by trained personnel using a standard uniform protocol recommended by WHO.
Using a standardized questionnaire, uniformly trained personnel guided the subjects’ guardians to provide essential details, including demographic characteristics and dietary habits of the children. The demographic details incorporated age, gender, outdoor activity duration, passive smoking, education level of parents, and household economic status. Passive smoking was defined as the primary caregiver of the preschool child smoking at least one cigarette daily for over six months.
Dietary information
Guardians were asked to recall the frequency of dietary intake over the past three months using the Food Frequency Questionnaire (FFQ) from the National Nutrition Survey. FFQ data included dietary supplementation (vitamin AD, calcium, iron, zinc), with intake frequency divided into three categories (never, occasionally, frequently), seafood (marine fish and shrimp) consumption, freshwater fish intake, shellfish intake, puffed food consumption, meat and vegetable intake, daily milk consumption, and daily egg intake, with various assessment scales employed for each. Seafood consumption was evaluated by three frequencies: occasional, moderate, and excessive, where occasional consumption was defined as consuming marine fish or shellfish less than once a week, excessive seafood intake was defined as consuming sea fish or shellfish more than six times a week, and moderate was defined as consuming marine fish or shellfish one to six times a week.
Quality control
Urine samples were meticulously collected and dispensed by trained personnel to safeguard against contamination. The polyethylene terephthalate (PET) containers used for collection and dispensing were physicochemical stable, user-friendly, hermetically sealed, iodine-free, and capable of enduring rapid reduction to ultra-low temperatures and prolonged stable storage at low temperatures.
Quality assurance was ensured by testing control iodine samples (two levels, low concentrations GBW09108 and high concentrations GBW09110), reagent blanks, and sample blanks for each batch (60 samples). In this detection, the measured values of control samples of iodine remained within 5% of the specified criteria values. The correlation coefficients (r) of the standard curve exceeded 0.999. Table 1 exhibited limits of detection (LOD), limits of quantification (LOQ), and their detection rates for urinary iodine.
Assessment of urinary iodine and creatinine concentrations
In alignment with the health standard WS/T107.2-2016 in China, UIC was measured via inductively coupled plasma mass spectrometry (Agilent ICP-MS/MS, model: 8900), adhering to the standard addition method, and designating tellurium (Te) as the internal standard. Urine samples were transferred from the −80°C storage to a 4°C refrigerator two days prior to the experiment, allowing the samples to thaw until clear. The sample diluent was a 0.25% solution of tetramethylammonium hydroxide. Ultrapure water with 18.2 MΩ/cm was utilized throughout the procedure.
The WHO’s criteria for evaluating iodine status in children were adhered to. Accordingly, MUI <100 μg/L was defined as iodine deficient (DI), MUI between 100 and 199 μg/L as adequate iodine (AI), 200–299 μg/L as more than adequate iodine (MAI), and MUI ≥300 μg/L as excessive iodine (EI).
The concentration of urinary creatinine was determined using the oxidative enzyme method with a fully automatic biochemical analyzer (Hitachi Diagnostics (Shanghai), model: 7100). To each of the six colorimetric tubes, 2.0 mL of alkaline picric acid solution was added, mixed thoroughly, and allowed to react at room temperature for 20–30 minutes. Upon the addition of 5 mL of ultrapure water, the absorbance at a wavelength of 490 nm was measured. The colorimetric analysis was completed within 0.5 hours, and the absorbance was measured thrice for each concentration, with the mean value being taken. A standard curve was plotted using the mean absorbance values as the vertical coordinate and creatinine concentrations (g/L) as the horizontal coordinate. Under identical conditions as the standard curve, a volume of 0.5 mL of urine sample was dispensed in an Eppendorf (EP) tube, with a blank reagent as the control. The absorbance of the sample was measured after thorough shaking and mixing to determine the concentration of urinary creatinine.
Statistical analysis
Continuous variables with normally distributed data were represented as mean ± standard deviation (SD), whereas categorical variables were presented as frequency (percent). For skewed distribution data, median (interquartile range) denoted concentrated trends. UIC was expressed as median (interquartile range). Variations in demographic characteristics and dietary frequency among preschool children were analyzed using the chi-square test, Mann-Whitney U test, and Wilcoxon rank-sum test. Univariate and multivariate logistic regression models were employed to investigate the associations between dietary factors and iodine intake in preschool children. Notably, covariates in the multivariate logistic regression model were selected from the basic characteristics of preschool children.
Statistical analyses were conducted in SPSS 24.0 (IBM, Chicago, USA) and R 4.2.0 (Lucent Technologies, USA). Two-sided tests were adopted in this study, and p value < 0.05 was regarded as statistically significant.
Results
Baseline characteristics
In this study, we recruited 1382 children aged 3–6 years in Shanghai (Table 2), comprising 725 (52.5%) boys and 657 (47.5%) girls. The cohort included 497 (36.0%) children from the central urban area, 412 (29.8%) children from peripheral urban areas, and 473 (34.2%) children from rural regions. The preschoolers had an average age of (57.99±12.60) months, with an average height-for-age z-score (HAZ) of 0.47±1.39 and a weight-for-age z-score (WAZ) of 0.63±1.46, respectively. Among the participants, 1005 (72.7%) preschool children engaged in outdoor physical activities for 1–3 hours daily, and the rate of exposure to passive smoking was 19.6%. Furthermore, over 70% of the children’s parents had received education at the high school level or above. Additionally, the households of the preschool children exhibited favorable economic status, with 831 (60.2%) families reporting an annual income of 100,000 CNY or higher.
Iodine status among preschool children
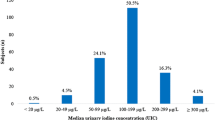
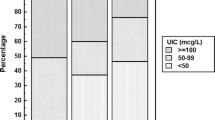
The median urinary iodine concentration (MUIC) in our study cohort was 193.36 (interquartile range:130.84, 269.68) μg/L, demonstrating adequate iodine intake. When corrected for creatinine (Cr), MUIC presented at 336.96 μg/g·Cr (Table 3). In Table 4, a detailed examination of age-dependent differences did not yield statistically significant variations in urinary iodine concentration (UIC) (p = 0.117). Interestingly, gender-wise distribution showed significantly a higher MUIC in boys compared to girls (p < 0.001). Furthermore, discrepancies in UIC among children from different townships were not statistically significant (p = 0.058). Passive smoking appeared to associate with higher MUIC levels (p = 0.009). Socioeconomic factors, such as increasing annual family income, were positively associated with the children’s iodine status, aligning it closer to the WHO recommended optimal range (p = 0.023). Variations in iodine intake were also observed with respect to the educational qualifications of the children’s parents (p = 0.043).
Our findings, benchmarked against WHO’s criteria, identified 13.5% of our cohort as iodine deficient, with a majority (68.6%) demonstrating MUIC in the range of 100–300 μg/L, considered optimal. However, 17.9% of the children exhibited excess iodine consumption (Table 5).
Dietary associations with iodine status in preschool children
Our univariate logistic regression findings (Table S1) suggest intriguing dietary correlations with iodine status among preschool children. We observed that children receiving regular calcium (Ca) supplementation were at an augmented risk of iodine deficiency, as indicated by an odds ratio (OR) of 1.55 (95% confidence interval [CI]: 1.00–2.39), when compared to their counterparts not receiving Ca supplementation. Conversely, moderate intake of seafood (OR: 0.64, 95% CI: 0.44–0.94) reduced the risk of iodine deficiency in preschool children. Furthermore, a dietary intake of more than two eggs per day demonstrated a protective effect against iodine deficiency, evidenced by a reduced OR of 0.51 (95% CI: 0.27–0.98). Intriguingly, a weekly consumption of 1–6 times of puffed foods (OR: 0.70, 95% CI: 0.49–1.00) and more than six servings of sea fish and shrimp (OR: 0.61, 95% CI: 0.37–0.98) emerged as potential protective factors against excess iodine intake. Moreover, daily consumption of 50–100 g vegetables (OR: 0.62, 95% CI: 0.43–0.91) and a single egg (OR: 0.46, 95% CI: 0.29–0.72) also appeared to serve as protective elements against excessive iodine intake. In contrast, a higher meat intake, quantified as more than 100 g per day, corresponded to an increased risk of excessive iodine intake (OR: 1.54, 95% CI: 1.01–2.34).
Refer to Fig. 2 and Table S2, multivariate logistic results reveal that routine calcium (Ca) supplementation indeed elevates the risk of iodine deficiency (DI) in preschool children, reflected by an odds ratio (OR) of 1.79 (95% CI: 1.03–3.12). Conversely, moderate intake of seafood was found to reduce the risk of iodine deficiency, with an OR of 0.60 and a 95% CI ranging from 0.38 to 0.95. While dietary intake of staple food, vegetables, meats, milk, and eggs do not significantly influence the prevalence of iodine deficiency in this population. Of particular interest, a substantially decreased risk of excess iodine (EI) in preschool children was associated with weekly consumption of shellfish 1–6 times (OR: 0.58, 95% CI: 0.33–1.00), daily intake of 50–100 g of vegetables (OR: 0.61, 95% CI: 0.38–0.97), and the consumption of one egg per day (OR: 0.53, 95% CI: 0.30–0.95).
These results underscore the role of diet as a modifiable determinant of iodine status, thereby meriting further research into the potential benefits of diet-centric interventions in managing iodine intake among preschool children.
The aforementioned analyses are adjusted for the following covariates: creatinine (continuous variable, g/L), age (continuous variable, in months), sex (categorical variable: boy, girl), region (categorical variable: central urban, peripheral urban, rural), annual household income (measured in units of 104 CNY/year, categorical variable: <3, 3–10, 10–25, >25), education level of parents (categorical variable: below junior high school, high/vocational school, college/bachelor’s, master’s degree), passive smoking (categorical variable: no, yes), and daily outdoor exercise time (hours/day, categorical variable: <1, 1–3, >3). In the presented results, an asterisk (*) denotes a significant correlation between dietary habits and iodine status, with a p value less than 0.05.
Discussion
Iodine status of preschoolers
Our cross-sectional survey conducted in Shanghai in 2014 aimed to evaluate iodine status in preschool children aged 3–6 years and identify dietary factors that influence their iodine intake. The study found that the iodine intake in Shanghai children is adequate. Furthermore, children’s iodine intake was strongly influenced by various dietary factors, including dietary supplements, seafood, vegetables, meat, milk, and eggs.
Our research indicated that the median urinary iodine concentration (MUIC) for preschool children in Shanghai in 2014 was 193.36 μg/L. Aligning with the guidelines suggested by the WHO, iodine deficiency (DI) was observed in 13.5% of the children, and iodine excess (EI) in 17.9%. For comparison, the 2014 iodine nutrition survey in Cambodia demonstrated that the MUIC for children aged 0–5 years was 72.0 μg/L, and 63.7% of preschool children exhibiting DI (Laillou et al. 2016). In contrast, a Colombian survey from 2015 to 2016 revealed a MUIC of 365.0 μg/L for children aged 2–5 years, with DI found in only 8.1% of children, but EI was present in a staggering 65.8% (Beer et al. 2021). In South Korea, similar data was observed as the MUIC for children aged 2–7 years was 438.8 μg/L, with only 3.9% DI, but an EI rate of 66.4% (Lee et al. 2014). In Norway, the MUIC for children aged 4–6 years was 132.0 μg/L, demonstrating adequate iodine status with a DI rate of 29.4% (Nerhus et al. 2019).
In comparison with the iodine status of preschool children in other Asian countries (Table 6), iodine status in Shanghai is appropriate, with both iodine deficiency and excess rates falling within a moderate range. The MUIC of preschool children in Shanghai was similar to those in other regions of China (Shan et al. 2021; Wang et al. 2019), with deficiency rates approximately matching those of Hebei and Zhejiang provinces, whereas for excess rates it was slightly higher in Shanghai than in these two regions.
International comparisons reveal varying situations (Censi et al. 2020; Costa Leite et al. 2017; Fuse et al. 2022; Ghattas et al. 2017; Huang et al. 2016; Cesar et al. 2020; Jayatissa et al. 2021; Khatiwada et al. 2016; Ovadia et al. 2017; Vanderpump et al. 2011a). In Lebanon and Israel, the MUIC for school-age children was significantly lower at 66.0 μg/L and 83.0 μg/L, respectively, with iodine deficiency rates soaring up to 74.8% and 62%. In stark contrast, developed countries such as Japan, Spain, and Italy exhibit MUICs for school-age children within the WHO recommended range and maintain lower iodine deficiency rates.
In general, the iodine status of preschool children in Shanghai aligns relatively well with WHO recommended ranges, and both iodine deficiency and excess are rare, which underscores the success of the Universal Salt Iodization (USI) initiative in China.
The World Health Organization (WHO) defines a median urine iodine concentration (MUIC) of less than 100 μg/L as indicative of iodine deficiency (DI), but it is highly likely to be an overestimation of DI rates. In our study, we observed that urine iodine concentration (UIC), assessed through spot retained urine samples, was highly susceptible to dilution in children’s urine, causing its value to be often inaccurate. Generally, urinary creatinine is a measure of kidney function and muscle metabolism. The concentration of urinary creatinine is often used to correct for changes due to dilution or concentration of urine and is therefore commonly used as a correction factor in urine samples. The ratio of urinary iodine to urinary creatinine (UI/Cr), widely accepted as a measure of iodine intake in a single random urine sample, can minimize variations caused by differences in urine volume and dilution of subjects, and offer a superior reference value for portraying iodine intake in populations with sound iodine status (Soldin 2002). In children, especially growing children, this adjustment is important because their muscle mass and kidney function change as they grow. Therefore, we opted for UI/Cr as a robust indicator to assess children’s iodine status in children. Previous studies have also demonstrated the efficacy of UI/Cr as a superior measure. They jointly point out that UI/Cr, when adjusted for age and sex, was significantly outperformed by other estimates in both individual 24-hour iodine excretion estimates and population surveys (Liu et al. 2022; Montenegro-Bethancourt et al. 2015b; Perrine et al. 2014). Overall, correcting UIC with urinary creatinine provides a more stable and reliable indicator of iodine status in children (Montenegro-Bethancourt et al. 2015a). Unfortunately, uniform corrected criteria for evaluating iodine status are yet to be established.
Dietary influences on iodine status
Iodine, being a vital component for thyroid hormone synthesis, is crucial for children’s rapid growth and development. China has consistently implemented Universal Salt Iodization (USI) since 1995, ensuring adequate iodine intake for its population and subsequently being classified as iodine-sufficient by the Iodine Global https://ign.org/. since 2015.
Primary dietary sources of iodine include fish and other seafood (Hess and Pearce 2023). Due to the concentration of iodine from seawater by plants and animals in the ocean, seafood contains high amounts of iodine (Carlsen et al. 2018), and seaweed, for example, is one of the most abundant sources of dietary iodine. Inadequate intake of seafood, which is positively correlated with iodine status, predisposes to DI (Gostas et al. 2020; Krzepiłko et al. 2015; Torres et al. 2017). Given its geographical proximity to the ocean, Shanghai’s abundant seafood contributes significantly to iodine intake for preschool children.
Other foods as milk and eggs are also enriched in iodine (Leung and Braverman 2014; Vanderpump et al. 2011b; Witard et al. 2022; Zimmermann 2009) and represent vital elements that shape iodine concentrations in human body (Johner et al. 2013; von Oettingen et al. 2017). Eggs are found to serve unique functions that agglutinate required nutrients for early growth and development (Röttger et al. 2012) and are capable of supplying 15% of daily iodine requirement for children in USA. Although a UK study indicated that eggs were significantly linked to increased UIC in school-aged children (Remer et al. 2006), another UK adolescent study indicated an inverse relationship between UIC and egg intake (Vanderpump et al. 2011b). Interestingly, we discovered that eggs serve as a protective factor against both iodine deficiency (DI) and iodine excess (EI) in preschool children. It seems that dietary intake of eggs allows children’s UIC to fluctuate within the optimal range for organism, where the exact mechanism warrants further investigation. In multiple national surveys, dairy products emerge as major influencers of iodine status, with inadequate intake leading to DI in humans (Haldimann et al. 2005; Lee et al. 2016; Pearce et al. 2004; Qin et al. 2023; Sakai et al. 2022). In contrast, despite their significant iodine content, our study found no substantial relationship between dairy product consumption and iodine intake among preschool children, possibly attributed to the diverse dietary structure of the Chinese population and the less dominant role of dairy products, while overseas children derive above 40% of iodine intake from dairy products (Abt et al. 2018; Dineva et al. 2021).
Furthermore, vegetables and meat, albeit containing smaller amounts of iodine, contribute to iodine intake. The iodine content in these foods is influenced by various factors including soil environment, irrigation methods, fertilizers, and animal species (Ershow et al. 2018). Variety of vegetables varies in iodine content. For instance, root vegetables hardly contain iodine (Stråvik et al. 2021), and Brassica vegetables (broccoli, cabbage, cauliflower, kale, radish, canola) carry trace concentrations of thiocyanate, a competitive sodium/iodine transporter inhibitor, which may be prone to facilitate goiter and provoke DI when consumed in massive quantities (Felker et al. 2016; Leung et al. 2011). With its northern subtropical monsoon climate, Shanghai experiences ample rainfall and alkaline soils, which undergo abundant iodine content through intensive reductive leaching and oxidative precipitation by long-term tillage, fertilization, and irrigation.
Contrary to prior studies suggesting that the supplementation of micronutrients such as iron (Fe), zinc (Zn), and selenium (Se) leads to an elevation in urinary iodine concentrations (Eftekhari et al. 2006; Nazeri et al. 2018; Stråvik et al. 2023; Stråvik et al. 2021), our research findings indicate that the supplementation of Fe and Zn had no discernible impact on iodine intake among children aged 3–6 years, in line with the observations made by O’Kane (O’Kane et al. 2018). Nevertheless, our study did reveal that regular calcium (Ca) supplementation appeared to exacerbate iodine loss in preschool children. The calcium pathway assumes a crucial role in maintaining the normal functioning of the thyroid, whereby the regulation of both iodine uptake and elimination occurs via the Ca2+-dependent transient receptor potential canonical 2 (TRPC2) and Anoctamin 1 pathways (Löf et al. 2012; Viitanen et al. 2013). Increased intracellular Ca2+ concentration resulted in reduced iodine uptake and heightened excretion of iodine ions within the thyroid follicular lumen (Adu-Afarwuah et al. 2021). The above are speculative reasons, and further investigation and more detail on the underlying physiological mechanisms behind these associations remain necessary.
Limitations of this study include the lack of detailed information on iodized salt consumption in households and potential recall bias, as a retrospective food frequency method was employed. We also ignore iodine provided by drinking water. Furthermore, as a cross-sectional study, it only provides a snapshot of iodine status in 2014. It is worth mentioning that we are currently conducting a follow-up study to examine the current status of iodine intake in children in Shanghai.
Despite these limitations, this study is the first large epidemiological survey to evaluate iodine status and its determinants in Chinese preschool children aged 3–6. Our findings provide a scientific basis for children’s growth and development and offer valuable insights for the implementation of USI policy. Additionally, it helps elucidate the variations in iodine status due to differences in dietary structure between China and other regions, suggesting that the “Chinese diet” structure may contribute to optimal trace element requirements.
Conclusion
Following a decade of universal salt iodization (USI), this cross-sectional study unveiled that the children aged 3–6 years in Shanghai indicates suitable iodine intake, with minimal prevalence of both iodine deficiency and excess, validating the efficacy of the USI policy in China. In addition, the study determined that iodine status was closely related to various foods in the daily diet. These findings underscore the need for a balanced diet and the potential roles of different nutrients in iodine metabolism among preschool children.
Data availability
The data that support the findings of this study are not openly available due to the inclusion of information that could compromise the subject’s privacy, but are available from the corresponding author on reasonable request.
References
Abt E, Spungen J, Pouillot R, Gamalo-Siebers M, Wirtz M (2018) Update on dietary intake of perchlorate and iodine from U.S. food and drug administration's total diet study: 2008–2012. J Expo Sci Environ Epidemiol 28:21–30
Adu-Afarwuah S, Arnold CD, Maleta K, Ashorn P, Ashorn U, Jorgensen JM, Fan Y-M, Nkhoma M, Bendabenda J, Matchado A, Dewey KG (2021) Consumption of multiple micronutrients or small-quantity lipid-based nutrient supplements containing iodine at the recommended dose during pregnancy, compared with iron and folic acid, does not affect women’s urinary iodine concentration in rural Malawi: a secondary outcome analysis of the iLiNS DYAD trial. Public Health Nutr 24:3049–3057
Andersson M, Karumbunathan V, Zimmermann MB (2012) Global iodine status in 2011 and trends over the past decade. J Nutr 142:744–750
Beer RJ, Herrán OF, Villamor E (2021) Median urinary iodine concentration in Colombian children and women is high and related to sociodemographic and geographic characteristics: results from a nationally representative survey. J Nutr 151:940–948
Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R, Uauy R (2013) Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382:427–451
Carlsen MH, Andersen LF, Dahl L, Norberg N, Hjartåker A (2018) New iodine food composition database and updated calculations of iodine intake among Norwegians. Nutrients 10(7):930. https://doi.org/10.3390/nu10070930
Censi S, Manso J, Barollo S, Mondin A, Bertazza L, De Marchi M, Mian C, On Behalf Of The Food And Nutrition Hygiene Services Sian (2020) Changing dietary habits in veneto region over two decades: still a long road to go to reach an iodine-sufficient status. Nutrients 12(8):2399. https://doi.org/10.3390/nu12082399
Cesar JA, S Santos IS, Black RE, Chrestani MAD, Duarte FA, Nilson EAF (2020) Iodine status of Brazilian school-age children: a national cross-sectional survey. Nutrients 12(4):1077. https://doi.org/10.3390/nu12041077
Copyright © World Health Organization (2014) Geneva
Costa Leite J, Keating E, Pestana D, Cruz Fernandes V, Maia ML, Norberto S, Pinto E, Moreira-Rosário A, Sintra D, Moreira B, Costa A, Silva S, Costa V, Martins I, Castro Mendes F, Queirós P, Peixoto B, Carlos Caldas J, Guerra A, Fontoura M, Calhau C (2017) Iodine status and iodised salt consumption in Portuguese school-aged children: The iogeneration study. Nutrients 9(5):458. https://doi.org/10.3390/nu9050458
Delange F (2002) Iodine deficiency in Europe and its consequences: an update. Eur J Nucl Med Mol Imaging 29(Suppl 2):S404–S416
Dineva M, Rayman MP, Bath SC (2021) Iodine status of consumers of milk-alternative drinks v. cows' milk: data from the UK National Diet and Nutrition Survey. Br J Nutr 126:28–36
Eftekhari MH, Simondon KB, Jalali M, Keshavarz SA, Elguero E, Eshraghian MR, Saadat N (2006) Effects of administration of iron, iodine and simultaneous iron-plus-iodine on the thyroid hormone profile in iron-deficient adolescent Iranian girls. Eur J Clin Nutr 60:545–552
Ershow AG, Skeaff SA, Merkel JM, Pehrsson PR (2018) Development of databases on Iodine in foods and dietary supplements. Nutrients 10(1):100. https://doi.org/10.3390/nu10010100
Farebrother J, Zimmermann MB, Andersson M (2019) Excess iodine intake: sources, assessment, and effects on thyroid function. Ann NY Acad Sci 1446:44–65
Felker P, Bunch R, Leung AM (2016) Concentrations of thiocyanate and goitrin in human plasma, their precursor concentrations in brassica vegetables, and associated potential risk for hypothyroidism. Nutr Rev 74:248–258
Fuse Y, Ito Y, Shishiba Y, Irie M (2022) Current iodine status in Japan: a cross-sectional nationwide survey of schoolchildren, 2014–2019. J Clin Endocrinol Metab 107:e2065–e2079
Ghattas H, Francis S, El Mallah C, Shatila D, Merhi K, Hlais S, Zimmermann M, Obeid O (2017) Lebanese children are iodine deficient and urinary sodium and fluoride excretion are weak positive predictors of urinary iodine. Eur J Nutr 56:749–755
Gostas DE, Larson-Meyer DE, Yoder HA, Huffman AE, Johnson EC (2020) Dietary relationship with 24 h urinary iodine concentrations of young adults in the mountain west region of the United States. Nutrients 12(1):121. https://doi.org/10.3390/nu12010121
Haldimann M, Alt A, Blanc A, Blondeau K (2005) Iodine content of food groups. J Food Compos Anal 18:461–471
Hess SY, Pearce EN (2023) Iodine: Physiology, dietary sources, and requirements. In: Caballero B (ed) Encyclopedia of human nutrition (fourth edition). Academic Press, Oxford, pp 273–281
Huang CJ, Tseng CL, Chen HS, Garabwan C, Korovo S, Tang KT, Won JG, Hsieh CH, Wang FF (2016) Iodine nutritional status of school children in Nauru 2015. Nutrients 8(9):520. https://doi.org/10.3390/nu8090520
Jayatissa R, Okosieme OE, Ranasinghe S, Carter JL, Gunatunga IP, Lazarus JH, Premawardhana LD (2021) Thyroid autoimmunity and dysfunction in Sri Lankan children and adolescents after 22 years of sustained universal salt iodization. Thyroid 31:1105–1113
Johner SA, Thamm M, Nöthlings U, Remer T (2013) Iodine status in preschool children and evaluation of major dietary iodine sources: a German experience. Eur J Nutr 52:1711–1719
Khatiwada S, Lamsal M, Gelal B, Gautam S, Nepal AK, Brodie D, Baral N (2016) Anemia, iron deficiency and iodine deficiency among Nepalese school children. Indian J Pediatr 83:617–621
Krzepiłko A, Zych-Wężyk I, Molas J (2015) Alternative ways of enriching the human diet with iodine. J Pre Clin Clin Res. 9:167–171
Laillou A, Sophonneary P, Kuong K, Hong R, Un S, Chamnan C, Poirot E, Berger J, Wieringa F (2016) Low urinary iodine concentration among mothers and children in Cambodia. Nutrients 8:172
Lee J, Kim JH, Lee SY, Lee JH (2014) Iodine status in Korean preschool children as determined by urinary iodine excretion. Eur J Nutr 53:683–688
Lee KW, Shin D, Cho MS, Song WO (2016) Food group intakes as determinants of iodine status among US adult population. Nutrients 8(6):325. https://doi.org/10.3390/nu8060325
Lee SY, Leung AM, He X, Braverman LE, Pearce EN (2010) Iodine content in fast foods: comparison between two fast-food chains in the United States. Endocr Pract 16(6):1071–1072. https://doi.org/10.4158/EP10180.CO
Leung AM, LaMar A, He X, Braverman LE, Pearce EN (2011) Iodine status and thyroid function of Boston-area vegetarians and vegans. J Clin Endocrinol Metab 96:E1303–E1307
Leung AM, Braverman LE (2014) Consequences of excess iodine. Nat Rev Endocrinol 10:136–142
Liu Z, Lin Y, Wu J, Chen D, Wu X, Lan Y, Chen Z (2022) Is the urinary iodine/creatinine ratio applicable to assess short term individual iodine status in Chinese adults? Comparison of iodine estimates from 24-h urine and timed-spot urine samples in different periods of the day. Nutr Metab (Lond) 19:27
Löf C, Sukumaran P, Viitanen T, Vainio M, Kemppainen K, Pulli I, Näsman J, Kukkonen JP, Törnquist K (2012) Communication between the calcium and cAMP pathways regulate the expression of the TSH receptor: TRPC2 in the Center of Action. Mol Endocrinol 26:2046–2057
Montenegro-Bethancourt G, Johner SA, Stehle P, Neubert A, Remer T (2015a) Iodine status assessment in children: spot urine iodine concentration reasonably reflects true twenty-four-hour iodine excretion only when scaled to creatinine. Thyroid 25:688–697
Montenegro-Bethancourt G, Johner SA, Stehle P, Neubert A, Remer T (2015b) Iodine status assessment in children: spot urine iodine concentration reasonably reflects true twenty-four-hour iodine excretion only when scaled to creatinine. Thyroid 25:688–697
Muñoz-Serrano A, González-González A, Tenías-Burillo JM, Falero-Gallego P, Cañete R (2014) Iodine-deficiency levels in school children aged between 6 and 12. Horm Res Paediatr 82:313
Nazeri P, Dalili H, Mehrabi Y, Hedayati M, Mirmiran P, Azizi F (2018) Breast milk iodine concentration rather than maternal urinary iodine is a reliable indicator for monitoring iodine status of breastfed neonates. Biol Trace Elem Res 185:71–77
Nerhus I, Wik Markhus M, Nilsen BM, Øyen J, Maage A, Ødegård ER, Midtbø LK, Frantzen S, Kögel T, Graff IE, Lie Ø, Dahl L, Kjellevold M (2018) Iodine content of six fish species, Norwegian dairy products and hen's egg. Food Nutr Res 62. https://doi.org/10.29219/fnr.v62.1291
Nerhus I, Odland M, Kjellevold M, Midtbø LK, Markhus MW, Graff IE, Lie Ø, Kvestad I, Frøyland L, Dahl L, Øyen J (2019) Iodine status in Norwegian preschool children and associations with dietary iodine sources: the FINS-KIDS study. Eur J Nutr 58:2219–2227
O’Kane SM, Mulhern MS, Pourshahidi LK, Strain JJ, Yeates AJ (2018) Micronutrients, iodine status and concentrations of thyroid hormones: a systematic review. Nutr Rev 76:418–431
Ovadia YS, Arbelle JE, Gefel D, Brik H, Wolf T, Nadler V, Hunziker S, Zimmermann MB, Troen AM (2017) First Israeli national iodine survey demonstrates iodine deficiency among school-aged children and pregnant women. Thyroid 27:1083–1091
Pearce EN, Pino S, He X, Bazrafshan HR, Lee SL, Braverman LE (2004) Sources of dietary iodine: bread, cows’ milk, and infant formula in the Boston area. J Clin Endocrinol Metab 89:3421–3424
Pearce EN, Andersson M, Zimmermann MB (2013) Global iodine nutrition: where do we stand in 2013? Thyroid 23:523–528
Perrine CG, Cogswell ME, Swanson CA, Sullivan KM, Chen TC, Carriquiry AL, Dodd KW, Caldwell KL, Wang CY (2014) Comparison of population iodine estimates from 24-hour urine and timed-spot urine samples. Thyroid 24:748–757
Qin Y, Cifelli CJ, Agarwal S, Fugoni VL (2023) Dairy food consumption is beneficially linked with iodine status in US children and adults: National Health and Nutrition Examination Surveys 2001-2018. Public Health Nutr 26(9):1828–1839. https://doi.org/10.1017/S136898002300071X
Remer T, Fonteyn N, Alexy U, Berkemeyer S (2006) Longitudinal examination of 24-h urinary iodine excretion in schoolchildren as a sensitive, hydration status-independent research tool for studying iodine status. Am J Clin Nutr 83:639–646
Röttger AS, Halle I, Wagner H, Breves G, Dänicke S, Flachowsky G (2012) The effects of iodine level and source on iodine carry-over in eggs and body tissues of laying hens. Arch Anim Nutr 66:385–401
Sakai N, Esho OY, Mukai M (2022) Iodine concentrations in conventional and organic milk in the northeastern U.S. Dairy 3(2):211–219. https://doi.org/10.3390/dairy3020017
Shan X, Liu C, Luo X, Zou Y, Huang L, Zhou W, Qin Q, Mao D, Li M, Yang L (2021) Iodine nutritional status and related factors among Chinese school-age children in three different areas: a cross-sectional study. Nutrients 13(5):1404. https://doi.org/10.3390/nu13051404
Soldin OP (2002) Controversies in urinary iodine determinations. Clin Biochem 35:575–579
Stråvik M, Gustin K, Barman M, Skröder H, Sandin A, Wold AE, Sandberg AS, Kippler M, Vahter M (2021) Infant iodine and selenium status in relation to maternal status and diet during pregnancy and lactation. Front Nutr 8:733602. https://doi.org/10.3389/fnut.2021.733602
Stråvik M, Gustin K, Barman M, Levi M, Sandin A, Wold AE, Sandberg A-S, Kippler M, Vahter M (2023) Biomarkers of seafood intake during pregnancy—pollutants versus fatty acids and micronutrients. Environ Res 225:115576
Sun D, Codling K, Chang S, Zhang S, Shen H, Su X, Chen Z, Scherpbier RW, Yan J (2017) Eliminating iodine deficiency in China: achievements, challenges and global implications. Nutrients 9:361
Torres MT, Francés L, Vila L, Manresa JM, Falguera G, Prieto G, Casamitjana R, Toran P (2017) Iodine nutritional status of women in their first trimester of pregnancy in Catalonia. BMC Pregnancy Childbirth 17:249
Vanderpump MP, Lazarus JH, Smyth PP, Laurberg P, Holder RL, Boelaert K, Franklyn JA (2011a) Iodine status of UK schoolgirls: a cross-sectional survey. Lancet 377:2007–2012
Vanderpump MPJ, Lazarus JH, Smyth PP, Laurberg P, Holder RL, Boelaert K, Franklyn JA (2011b) Iodine status of UK schoolgirls: a cross-sectional survey. Lancet 377:2007–2012
Viitanen TM, Sukumaran P, Löf C, Törnquist K (2013) Functional coupling of TRPC2 cation channels and the calcium-activated anion channels in rat thyroid cells: implications for iodide homeostasis. J Cell Physiol 228:814–823
von Oettingen JE, Brathwaite TD, Carpenter C, Bonnell R, He X, Braverman LE, Pearce EN, Larco P, Larco NC, Jean-Baptiste E, Brown RS (2017) Population survey of iodine deficiency and environmental disruptors of thyroid function in young children in Haiti. J Clin Endocrinol Metab 102:644–651
Wang Z, Zang J, Shi Z, Zhu Z, Song J, Zou S, Jin W, Jia X, Guo C, Liu S (2019) Iodine status of 8 to 10 years old children within 20 years following compulsory salt iodization policy in Shanghai, China. Nutr J 18:63
Witard OC, Bath SC, Dineva M, Sellem L, Mulet-Cabero AI, van Dongen LH, Zheng JS, Valenzuela C, Smeuninx B (2022) Dairy as a source of iodine and protein in the UK: implications for human health across the life course, and future policy and research. Front Nutr 9:800559. https://doi.org/10.3389/fnut.2022.800559
World Health Oraganization (2014) WHO guidelines approved by the guidelines review committee, Guideline: fortification of food-grade salt with iodine for the prevention and control of iodine deficiency disorders. World Health Organization
Wu Y, Li X, Chang S, Liu L, Zou S, Hipgrave DB (2012) Variable iodine intake persists in the context of universal salt iodization in China. J Nutr 142:1728–1734
Zimmermann MB, Jooste PL, Pandav CS (2008) Iodine-deficiency disorders. Lancet 372:1251–1262
Zimmermann MB (2009) Iodine deficiency. Endocr Rev 30:376–408
Zimmermann MB, Andersson M (2021) Global endocrinology: global perspectives in endocrinology: coverage of iodized salt programs and iodine status in 2020. Eur J Endocrinol 185:R13–r21
Funding
This study received financial support from the National Key R&D Program of China (Grant No. 2017YFC1600500) and the National Basic Research Program of China (“973” Program; Grant No. 2012CB525001). The sponsor had no role in the study design, data analysis and interpretation, manuscript preparation, review, or approval.
Author information
Authors and Affiliations
Contributions
JL: conceptualization, formal analysis, writing original draft and editing. JL: methodology and software. YW: investigation, formal analysis, and visualization. AL: investigation and visualization. YW: formal analysis and software. YL: methodology and data curation. CY: conceptualization, supervised the study, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
This research was approved by the Medical Ethics Committee of Xinhua Hospital affiliated with Shanghai Jiao Tong University School of Medicine, and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from children’s parents recruited in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, ., Liu, JX., Wang, YQ. et al. Iodine status and associated dietary factors among preschool children in Shanghai. Environ Sci Pollut Res 30, 121823–121833 (2023). https://doi.org/10.1007/s11356-023-30942-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30942-6