Abstract
In environmental toxicology, combined toxicity has emerged as an important concern. Atrazine (ATZ), dichlorvos (DIC), and imidacloprid (IMD) are the major pesticides, extensively used to control insect, flies, mosquitoes, and weed. Here, we investigate whether the exposure to three different types of pesticides individually and in combination for 24 h alters antioxidant enzyme responses in zebrafish (Danio rerio). Oxidative stress parameters (biochemical and mRNA expression), acetylcholinesterase (AChE) activity, and Metallothionein-II (MT-II) mRNA expression levels were measured. Present work includes toxicological assessment of individual and combined (CMD) exposure of ATZ (185.4 µM), DIC (181 µM), IMD (97.8 µ), and CMD (ATZ 92.7 µM + DIC 90.5 µM + IMD 48.9 µM), in the liver, kidney, and brain of adult zebrafish. Lipid peroxidation (LPO), glutathione (GSH) content, AChE, superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) activity along with mRNA expression of SOD, CAT, GPx, and MT-II were evaluated. Briefly, LPO, GSH content, the activity of AChE, and all antioxidant enzymes enhanced significantly in individual exposure, which was further altered in the CMD group. The mRNA expression of SOD, CAT, GPx, and MT-II in the liver and kidney showed significant down-regulation in all exposed groups. In the brain, significant upregulation in mRNA expression of SOD, CAT, GPx, and MT-II was observed in DIC and IMD groups, while ATZ and CMD showed significant downregulation except for GPx. Findings postulate that the CMD group exhibits synergistic toxic manifestation. The present study provides the baseline data on the combined toxic effects of pesticides and suggests regulating the use of pesticides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The indiscriminate use of pesticides in agriculture has become a major risk to ecology as they enter the environment through spraying or surface run-off after rainfall. The small quantity of pesticide(s) can badly affect the ecosystem due to their presence, multiplicity, toxicity, and persistency (Wang et al. 2017; Nele et al. 2020; Covert et al. 2020; Satyanarayana et al. 2023). Total pesticides retain after use in agricultural fields, and transported to different water sources depends on several factors like topography, weather (especially rainfall), agricultural practices, and chemical properties of pesticides (Olwenn et al. 2021; Kruć-Fijałkowska et al. 2022). In the past few decades, the diversity of agrochemicals increases, resulting in water pollution shifts from being dominated by a single active compound to complex multi-agent polluting factors. The presence of numerous chemicals and agents shows combined effects that can be greater (synergy) or lower (antagonism) than the sum of the effects predicted by single-stressor models (Shukla et al. 2017a; Kruć-Fijałkowska et al. 2022; Falfushynska et al. 2022; Li et al. 2023). Despite this most standardized water-quality criteria and risk assessment protocols focus on the toxicity of single chemical (Van Leeuwen et al. 1996; Barata et al. 2006). Pesticides greatly influence reactive oxygen species (ROS) and cause oxidative stress, which leads to the inactivation of antioxidant enzymes which in-turns, serves as a biomarker for pesticide-induced toxicity. Free radicals/ROS scavengers such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) are enzymetic and protect against their deleterious effects (Blahova et al. 2013; Narasimhamurthy et al. 2022; Ahmed et al. 2022; Ahmad et al. 2022). Glutathione (GSH) is a tripeptide with antioxidative properties because of the thiol group which acts as a reducing agent and minimizes the toxicity of free radical or ROS (Santos and Martinez 2014). Acetylcholinesterase (AChE) is the primary enzyme that catalyzes the hydrolysis of acetylcholine into choline and acetic acid (Rodríguez-Fuentes et al. 2015). AChE activity has been widely utilized in assessing aquatic pollution (Assis et al. 2007; Rodríguez-Fuentes et al. 2015; Benli and Çelik 2021; Guerra et al. 2021; Viana et al. 2022; Mendonça-Soares et al. 2023). Metallothioneins (MTs) are low molecular weight protein and helpful in scavenging ROS (Valavanidis et al. 2006; Moncaleano-Niño et al. 2022). MTs have been extensively studied in heavy metal and pesticide toxicity (Agarwal et al. 2010; Erdogan et al. 2011; Ceyhun et al. 2012; Mai et al. 2020; Moncaleano-Niño et al. 2022).
Atrazine (ATZ) (6-chloro-N-ethyl-N′-(1-methyl ethyl)- 1,3,5-triazine-2,4-diamine) is a chlorinated herbicide of triazine class which is the most extensively used herbicide mainly applied in corn, maize, wheat, sorghum grass, and sugarcane field (Jin et al. 2010; Ahmed et al. 2022; Ahmad et al. 2022). The mode of action of triazine class herbicides includes inhibition of the photosynthetic pathway, specifically the photosystem II by binding the QB-binding site of the D1 protein complex present in the chloroplast thylakoid membrane (Shaner 2014). It is highly persistent and resistant to degradation with a half-life in surface water is about ˃200 days (Ghosh and Philip 2006).
Dichlorvos (DIC) (2, 2-dichloroethyl dimethyl phosphate) is an organophosphate class insecticide widely used in household, agriculture, and livestock parasite purpose in controlling flies, aphids, mites, and caterpillars (Bui-Nguyen et al. 2015; Ogunro 2023). Unrestrained application has resulted in the detection of DIC in freshwater sources which has amplified the risk of exposure thus affecting various non-target organisms including human beings (Falfushynska et al. 2022; Narasimhamurthy et al. 2022). DIC mainly inhibits the activity of the AChE enzyme and leads to an accumulation of acetylcholine and elevated cholinergic activity in the central and peripheral nervous system, which may even lead to death (Bui-Nguyen et al. 2015; Falfushynska et al. 2022; Nachimuthu et al. 2023; Mali et al. 2023). AChE is an enzyme that hydrolyzes the neurotransmitter acetylcholine at neuromuscular junctions and brain cholinergic synapses.
Imidacloprid (IMD) 1-(6-chloro-3-pyridylmethyl)-N-nitroimidazolidin-2-ylideneamine) is a chloronicotinyl neonicotinoid class insecticide used to control pests in cereals, vegetables, tea, and cotton worldwide (Tomizawa and Casida 2005; Mendonça-Soares et al. 2023). It is effective against insects at low application rate and is considered as a replacement for organophosphorus pesticides. It acts as a nicotinic acetylcholine receptor (nAChR) agonist and can lead to central nervous system disorders (Tomizawa and Casida 2005). IMD has been listed by US environmental protection agency, as Class E- evidence of non-carcinogenicity for humans. Imidacloprid usage became a burning issue in environmental research due to its severe toxicity to bees (Ge et al. 2015).
The individual toxic potential of ATZ, DIC, and IMD has been extensively studied (Celik and Suzek 2009; El-Gendy et al. 2010; Kapoor et al. 2010; Jin et al. 2010; Zhu et al. 2011; Ceyhun et al. 2012; Blahova et al. 2013; Tao et al. 2020; Khaled et al. 2023; Mendonça-Soares et al. 2023). All three pesticides are known to cause oxidative stress, liver ailment, sperm abnormalities, and neurobehavioral abnormalities, alter the antioxidant capacity of the cell, affect the normal functioning of the cell, and even lead to death (Jin et al. 2010; Bui-Nguyen et al. 2015; Ge et al. 2015; Guo-Ping et al. 2020; Azpiazu et al. 2021; Ahmed et al. 2022; Ahmad et al. 2022; Mali et al. 2023). ATZ is known to cause several health effects like ovarian cancer, DNA methylation changes, breast cancer, DNA damage, decreased semen quality, and birth defects. (Zhu et al. 2011; Blahova et al. 2013; Abarikwu et al. 2017; Demirci et al. 2018; Kruć-Fijałkowska et al. 2022). Tao et al. (2020) assessed the individual and combined toxicity of three organophosphate pesticides on freshwater green algae Chlorella pyrenoidosa. They observed a reduction in the malondialdehyde (MDA) content and SOD activity. Similarly, a sub-lethal dose of IMD can alter LPO, GSH, AChE, and ribonucleic acid (RNA) in Japanese quail (Khaled et al. 2023).
The combined toxicity of these pesticides has not been explored yet. However, we have reported oxidative stress caused by the combination of ATZ, DIC, and IMD (commercial grade) in zebrafish (Shukla et al. 2017a). The reason behind this work is to estimate the initiation of toxic manifestations of commonly used pesticides to predict the risk of environmental and health hazards so that proper measures can be taken to prevent toxicity. Therefore, the present work was executed to evaluate the toxic effects caused by ATZ, DIC, and IMD individually and in combination. mRNA expression analyses were carried out for SOD, CAT, GPx, and MT-II along with LPO, GSH, AChE, SOD, CAT, and GPx enzymatic activity in adult zebrafish.
Materials and methods
Chemicals, reagents, kits, and primers
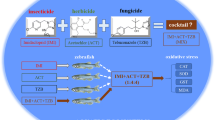
High-purity analytical grade (99% or above) chemicals and reagents were used in this study. Pesticide standards, atrazine (99.6%), dichlorvos (98.8%), and imidacloprid (99.9%) were purchased from Sigma Aldrich USA. RiboZol™, Amresco, USA (Fig. 1 showing the structure of atrazine, dichlorvos, and imidacloprid). qPCR Green Master with UNG-blue dye and Verso cDNA synthesis kit for PCR analysis were acquired from Thermo Fischer Scientific, India. Specific primer sequences for SOD, CAT, GPx, MT-II, and β-Actin were obtained from Eurofins Genomics, India Pvt. Ltd.
Zebrafish
Certified wild species of adult zebrafish (Danio rerio) (4–5 months; length 2.7 cm ± 0.5 cm: weight 0.285–0.385 g) were procured from a pet store and handled following good animal practice as defined by animal welfare bodies. The study was approved by University Research Committee (No. PhD/FS/RA/02). Zebrafish were acclimatized for 15 days to the laboratory environment by keeping them in glass aquaria containing 50 lt of de-chlorinated tap water maintained under controlled temperature (26 ± 2 °C) with constant aeration and maintaining 12 h alternate light and dark cycle. Zebrafish were fed daily on dried blood worms (Taiyomax™) and commercial pellets (Otohime™ A1, size; 75–150 µm). The tank was cleaned every day; feces and other residue were removed from the water on time and no feeding was given 24 h before experimentation.
Experimental protocol
Water physiochemical parameters
Water conditions examined by American Public Health Association (APHA 2005) are as follows: temperature — 26 ± 1.0 °C, pH — 7.0 ± 0.1, dissolved oxygen — 7.2 ± 0.2 mg/L, hardness — 109.0 mg/L, conductivity — 0.2 μohm, alkalinity — 0.27 g/L.
Animal exposure
The acute toxicity test was conducted in a static condition; the experiment was designed as per Organization for Economic Co-operation and Development (OECD 2004) guidelines, and zebrafish were fastened during the experimentation period.
Total 180 adult zebrafish (mixed sex) were divided into five groups (36 fish randomly selected in each group, 18 fish used for biochemical, and 18 for mRNA expression, with random pooling of 06 fish); all experiments were conducted in duplicates. Group I: animals were held in de-chlorinated water, served as control (CTRL) Group II: ATZ, 185.4 µM, Group III: DIC, 181 µM, Group IV: IMD, 97.8 µM, and Group 5: CMD (ATZ 92.7 µM + DIC 90.5 µM + IMD 48.9 µM) (half concentration of each single compound exposure). The study was based on our earlier reported work with these three pesticides (Shukla et al. 2017a).
Tissue collection procedure and homogenization
Zebrafish were sacrificed after 24 h of exposure under a stereomicroscope. For total RNA isolation, the liver, kidney, and brain were removed immediately and stored at − 20 °C in 200 µL of ice-cold ribozol for 48 h. For biochemical studies, the liver, kidney, and brain were minced, and homogenized (2.5% w/v) with ice-cold 0.15% KCl–0.1 M phosphate buffer (pH 7.4) using Potter–Elvehjem homogenizer and stored at − 20 °C.
Biochemical assays
All biochemical parameters were done on Multiskan EX Plate Reader, Thermo Fisher Scientific, Inc., USA, using standard methodologies. The levels of MDA, an end product of lipid peroxidation, were measured with the absorbance coefficient of the malondialdehyde-thiobarbituric acid (MDA-TBA) complex at 532 nm using 1,1,3,3-tetraethoxypropane as the standard (Ohkawa et al. 1979). GSH levels were determined using 5, 5′-dithio-bis (2-nitrobenzoic acid) (DTNB) for color development at 412 nm (Ellman 1959). SOD enzyme activity was measured by the modified method of NADH-phenazine-methosulfate-nitroblue-tetrazoliumformazan inhibition reaction at 550 nm (Kakkar et al. 1984). GPx enzyme activity was measured using GSH as substrate and DTNB as standard by the method of (Flohe and Gunzler 1984) at 420 nm. CAT activity was measured at 570 nm by the method of (Sinha 1972). AChE activity was measured by Ellman et al. (1961), Protein levels were assayed by the method of Lowry et al. (1951) using bovine serum albumin (BSA) as the standard at 690 nm.
Gene expression study
Total RNA extraction and reverse transcription
Isolation and extraction of total RNA were done by Shukla et al. (2017b), and the qualitative and quantitative analyses were performed on NANOGENIUS photometer MAPADA instruments, China. The optical density 260/280 ratio for entire samples ranges from 1.9 to 2.0. Total RNA was reverse transcribed using Verso cDNA synthesis kit as per manufacturer’s instructions using thermal cycler Veriti™, Applied Biosystems, Thermo Fischer Scientific, USA.
Real-time polymerase chain reaction (RT-PCR)
PCR amplification and quantitation were carried out for SOD, CAT, GPx, MT-II, and β-Actin as housekeeping gene (reference gene) were executed with 100–150 basepairs (bp). β-Actin remains unchanged in exposed groups during the whole procedure. The primer sequence for SOD, CAT, GPx, MT-II, and β-Actin was taken from Jhamtani et al. (2018) (Table 1). RT-PCR was performed by qPCR GreenMaster, UNG-blue dye detection kit using Applied Biosystem ABI® 7500 QPCR System, USA. All the reactions were carried out in a 10-μL reaction mixture in triplicates as per the maker’s instructions.
Data analysis
The data obtained by real-time RT-PCR are presented as threshold cycle (Ct), which is the cycle at which the intensity of fluorescence was determined to be statistically significant Pfaffl (2001). For fold change calculation in target gene expression levels, relative quantification by the “delta-delta Ct Method” was used for comparing relative expression levels between treatments in RT-PCR. The method is based on the relative expression of a target gene vs a reference gene.
Statistical evaluation
The data of individual exposed groups were presented as the mean ± standard error (n = 6), and the statistical significance of mean value in the examined tissue for different groups, viz., control, ATZ, DIC, IMD, and CMD was examined by two-way analyses of variance (ANOVA). A probability of ≤ 0.05 was considered to be significant. The signs *, #, $, and ǂ indicate significant difference from the CTRL, ATZ, DIC, and IMD exposed groups, respectively, which represents as *,#,$,ǂp < 0.05; **,##,$$,ǂǂp < 0.001; ***,###,$$$,ǂǂǂp < 0.005 levels.
Results
Lipid peroxidation levels (LPO), glutathione content (GSH), and acetylcholinesterase (AChE) activity
Considerable enhancement in LPO was observed in all examined tissues of entire exposed groups in comparison with the control group Fig. 2(i). Significant (p < 0.005) enhancement of LPO was observed in the CMD group in all three examined tissue, the group followed by DIC, IMD, and ATZ. Brain suffered with maximum LPO followed by liver and kidney.
(i–iii) Lipid peroxidation, glutathione content, and acetylcholinestrase activity in the liver, kidney, and brain of zebrafish exposed to ATZ, DIC, IMD, and CMD group for 24 h, presented as mean ± SE (n = 6). Difference between groups were considered as significant when p < 0.05. Signs, viz., *, #, $, and ǂ, indicate significant difference from the CTRL, ATZ, DIC, and IMD exposed groups, respectively, which represents *,#,$,ǂp < 0.05; **,##,$$,ǂǂp < 0.001; ***,###,$$$,ǂǂǂp < 0.005 levels
In the liver, reduced (p < 0.005) GSH content was found in individual exposed groups, and no change in the CMD group when compared with control Fig. 2(ii). The ATZ group showed maximum reduction followed by DIC and IMD. In the kidney, the DIC exposed group shows significant (p < 0.005) elevated GSH content, while considerable reduction in the CMD group. However, no alteration was observed in ATZ and IMD groups. In the brain, reduced GSH content was observed in ATZ (p < 0.005) and CMD (p < 0.001), while DIC showed a significant (p < 0.005) increase and no change in the IMD group in comparison with the control group.
In the liver, kidney, and brain, considerable increase in AChE activity was found in the entire exposed groups when compared with control Fig. 2(iii). The CMD group showed significant (p < 0.005) enhancement in AChE activity in comparison with individual exposed groups. The brain shows maximum AChE activity than the liver and kidney.
Antioxidant enzymes activities and gene expression
SOD enzyme activity and mRNA expression
In the liver, a progressive increase in SOD enzyme activity was found in the entire exposed groups to ATZ < DIC < IMD < CMD in comparison with the control group Fig. 3(i). In the kidney, a considerable decline in SOD activity was observed in individual exposed groups, while significant changes (p < 0.05) in the CMD group when compared with the control group. In the brain, significantly diminished SOD activity was noticed in all exposed groups in comparison with the control group. In the CMD group, significant (p < 0.001) elevated SOD activity was observed in comparison with individual exposed groups.
(i) Superoxide dismutase activity in liver, kidney, and brain of zebrafish exposed to ATZ, DIC, IMD, and CMD group for 24 h, presented as mean ± SE (n = 6). Difference between groups were considered as significant when p < 0.05. Signs, viz., *, #, $, and ǂ, indicate significant difference from the CTRL, ATZ, DIC, and IMD exposed groups, respectively, which represents *,#,$,ǂp < 0.05; **,##,$$,ǂǂp < 0.001; ***,###,$$$,ǂǂǂp < 0.005 levels. (ii–iii) Fold change in SOD mRNA expression in individual exposed groups vs control and in the CMD group vs control, respectively
In the liver, mRNA expression of SOD is significantly downregulated in all exposed groups (Fig. 3ii (fold change, ATZ, DIC, and IMD), iii (fold change, CMD)). Maximum downregulation was observed in the CMD group (87.29 fold) followed by DIC (58.97 fold), ATZ (5.32 fold), and IMD (1.7 fold). In the kidney, significant downregulation was observed in all exposed groups. The highest downregulation was observed in the ATZ group (2.7 fold) followed by DIC (2.60 fold), CMD (1.56 fold), and IMD (0.61 fold). In the brain, upregulated in DIC (1.83 fold) and IMD (2.33 fold) while downregulated in ATZ (1.04 fold) and CMD group (0.43 fold) were observed.
CAT enzyme activity and mRNA expression
In the liver and kidney, diminished CAT activity was observed in all exposed groups when compared with the control Fig. 4(i). In the CMD group, noteworthy enhanced activity was observed in the liver when compared with individual exposed groups. In the same way, the CMD group shows elevated activity in the kidney when compared with ATZ and DIC. However, no alteration was observed in comparison to IMD. In the brain, a noteworthy increase in CAT activity was noticed in the DIC, IMD, and CMD groups and a decrease in the ATZ exposed group when compared with the control. In the CMD group, a significant increase in CAT activity was observed when compared with ATZ, while decrease with DIC and no significant change when compared to the IMD group.
(i) Catalase activity in liver, kidney, and brain of zebrafish exposed to the ATZ, DIC, IMD, and CMD groups for 24 h, presented as mean ± SE (n = 6). Difference between groups were considered as significant when p < 0.05. Signs, viz., *, #, $, and ǂ, represent significant difference from the CTRL, ATZ, DIC, and IMD exposed groups, respectively, which represents *,#,$,ǂp < 0.05; **,##,$$,ǂǂp < 0.001; ***,###,$$$,ǂǂǂp < 0.005 levels. (ii–iii) Fold change in CAT mRNA expression in individual exposed groups vs control and in CMD group vs control, respectively
In the liver, mRNA expression of CAT is significantly downregulated in all exposed groups (Fig. 4ii (fold change, ATZ, DIC, and IMD), iii (fold change, CMD)). Maximum downregulation was observed in CMD (29.50 fold) followed by ATZ (16.01 fold), DIC (2.87 fold), and IMD (2.5 fold). In the kidney, significant downregulation was observed in the ATZ group (5.18 fold) followed by IMD (1.69 fold), DIC (0.87 fold), and CMD (0.67 fold). In the brain, upregulation in DIC (0.24 fold) and IMD (0.43 fold) while downregulation in ATZ (3.44 fold) and CMD (0.37 fold) were observed.
GPx enzyme activity and mRNA expression
In the liver, considerable elevated GPx activity was seen in ATZ and CMD group and no alterations in the DIC and IMD when compared with the control Fig. 5(i). Correspondingly, in the kidney, significant elevated activity was observed in individual exposed to the ATZ (p < 0.05) and CMD groups in comparison to the control. However, the suppressed activity was observed in individual exposed to DIC and IMD in comparison with the control. In the brain, significant elevated GPx activity was observed in the DIC, IMD, and CMD groups in comparison to the control. Decreased activity was observed in the ATZ exposed group in comparison with the control. In the CMD group, considerably increased GPx activity was noticed in all three tissues in comparison with the individual exposed group.
(i) Glutathione peroxidase activity in liver, kidney, and brain of zebrafish exposed to the ATZ, DIC, IMD, and CMD groups for 24 h, presented as mean ± SE (n = 6). Difference between groups were considered as significant when p < 0.05. Signs, viz., *, #, $, and ǂ, represent significant difference from the CTRL, ATZ, DIC, and IMD exposed groups, respectively, which represents *,#,$,ǂp < 0.05; **,##,$$,ǂǂp < 0.001; ***,###,$$$,ǂǂǂp < 0.005 levels. (ii–iii) Fold change in SOD mRNA expression in individual exposed groups vs control and in the CMD group vs control, respectively
In the liver, mRNA expression of GPx is considerably downregulated in all exposed groups (Fig. 5ii (fold change, ATZ, DIC, and IMD), iii (fold change, CMD)). The highest downregulation was observed in CMD (31.84 fold) followed by ATZ (22.34 fold), DIC (9.07 fold), and IMD (0.67fold). In the kidney, significant downregulation in DIC (7.99 fold) followed by ATZ (7.36 fold), CMD (4.2 fold), and IMD (1.4 fold). In the brain, GPx mRNA expression significantly upregulated in DIC (14.45 fold), IMD (36.62 fold), and CMD (0.53 fold) while downregulated in ATZ (0.30 fold) were observed.
MT-II mRNA expression
In the liver, mRNA expression of MT-II is significantly downregulated in all exposed groups (Fig. 6i (fold change, ATZ, DIC, and IMD), ii (fold change, CMD)). The highest downregulation was observed in CMD (55.91 fold) followed by ATZ (6.32 fold), DIC (4.5 fold), and IMD (1.01 fold). In the kidney, significant downregulation was observed in DIC (4.25 fold), ATZ (1.73 fold), CMD (1.72 fold), and IMD (0.79 fold). In the brain, significant downregulation in ATZ (0.84 fold) and CMD (0.55 fold) while upregulation in DIC (2.11 fold) and IMD (5.58 fold) were observed.
Discussion
MDA has been widely used for many years as a convenient biomarker for lipid peroxidation of omega-3 and omega-6 fatty acids because of its facile reaction with TBA (Jin et al. 2010; Blahova et al. 2013; Shukla et al. 2017a; Yanhua et al. 2021). The results showed significant MDA generation showing lipid peroxidation in all three examined tissue caused by pesticide exposure. Fascinatingly, in the combined exposure group, maximum lipid peroxidation was observed when compared with the individual exposed groups. An increase in lipid peroxidation due to pesticide exposure may ascribe to triggering ROS. ROS are known to enhance the oxidation of polyunsaturated fatty acids (PUFA) leading to lipid peroxidation and altering endogenous enzyme activity (Jin et al. 2010; Blahova et al. 2013; Shukla et al. 2018; Falfushynska et al. 2022). The liver is a vital organ to metabolize xenobiotics; it encompasses supplementary unsaturated lipids, which experience swift oxidative degradation thus resulting in high MDA generation (El-Gendy et al. 2010; Jhamtani et al. 2018; Olwenn et al. 2021). The kidney is responsible for the filtration and elimination of xenobiotics from the body, therefore may show elevated MDA levels (Abarikwu et al. 2017). We have observed significant LPO in the combined exposed groups as compared with the individual exposed groups. Increased LPO represents the oxidative injury induced by the combination of pesticides. Similar results were reported by other researchers Santos and Martinez (2014) and Ojha et al. (2011). In our earlier reported work with commercial-grade pesticides, we observed similar results of significant LPO in the combined exposed groups (Shukla et al. 2017a). Our results also exhibit significant LPO in the individual exposed groups in comparison with the control groups. Similar results were observed by Celik and Suzek (2009), Jin et al. (2010), Kapoor et al. (2010), and Guerra et al. (2021). The brain is susceptible to oxidative stress due to elevated oxygen utilization with high levels of lipids, which enhances PUFA (Jhamtani et al. 2018). In the brain tissue of combined exposed groups, we have observed a significant increase in LPO, which might be due to the synergistic effects shown by the combination of three pesticides on the occurrence of LPO in the brain. Our findings are in line with the results documented by Bacchetta et al. (2014). We have observed significant LPO in the individual exposed groups when compared with the control group, which demonstrates the neurotoxic nature of the undertaken pesticides. Similar results of increased LPO were reported by other authors caused by individual and combined exposure to pesticides (Celik and Suzek 2009; Kapoor et al. 2010; Blahova et al. 2013; Falfushynska et al. 2022; Ahmed et al. 2022; Ahmad et al. 2022).
GSH is a vital tripeptide with antioxidative properties having a thiol group; it acts as a reductant and can be reversed back in the cell. The depleted GSH content in the liver shows it is utilized by glutathione peroxidase to scavenge ROS on individual exposure to pesticides. Similar results were also reported by Jin et al. (2010) and Kapoor et al. (2010). In combined exposure, the GSH content is near to control which may be due to the protection provided by antioxidant enzymes. An increased GSH content under combined exposure shows its presence in the cell in adequate amounts as required by antioxidant enzymes. Similar results were documented by other authors (Abarikwu et al. 2017; Jhamtani et al. 2018). In the kidney and brain, significantly depleted GSH levels were noticed in the CMD group which is an early effect of ROS generation due to pesticide exposure. In the present work, GSH might be utilized by antioxidant enzymes to eliminate ROS due to combined exposure to pesticides. Analogous to our findings, Demirci et al. (2018), Abarikwu et al. (2017), and Ahmed et al. (2022) have reported a decrease in GSH content.
The assessment of AChE enzyme activity is used as a biomarker for aquatic pollution. It is an endogenous enzyme found in all tissue and abundantly in the brain, which makes it a target enzyme for different xenobiotics (Rodríguez-Fuentes et al. 2015; Benli and Çelik 2021; Viana et al. 2022). Pesticide causes oxidative stress which in turn alters AChE activity. Effects of dichlorvos and other organophosphates pesticides on AChE activity have been studied (Gultekin et al. 2001; Lionetto et al. 2003; Assis et al. 2007; Rodríguez-Fuentes et al. 2015; Benli and Çelik 2021; Viana et al. 2022), while the combined toxic effects of pesticides on AChE activity have not been explored. We have observed the induction of AChE enzyme activity in the entire examined tissue due to pesticide exposure. The changes were more prominent in the brain than liver and kidney in all exposed groups, which shows the neurotoxic nature of DIC, IMD, and ATZ individually as well as in the combined group. The increased AChE activity due to pesticide exposure indicates the initiation of oxidative injury in the examined tissue. However, the results are not parallel with the findings of other authors wherein they reported a decrease in AChE activity after pesticide exposure in zebrafish (Assis et al. 2007; Rodríguez-Fuentes et al. 2015; Guerra et al. 2021). However, the combination of fipronil (insecticide) and 2,4-D (herbicide) increases the activity of the AChE enzyme in zebrafish (Viana et al. 2022). Similarly, sulfoxaflor (insecticide) exposure causes a significant increase in AChE activity in the brain of zebrafish (Benli and Çelik 2021). In the same way, a significant increase in AChE activity was observed due to IMD exposure in the zebrafish brain (Mendonça-Soares et al. 2023). In our work, individual and combined exposure shows the initiation of toxic manifestations due to ROS generation along with the commencement of endogenous detoxification process as shown by AChE activity in all examined tissue in zebrafish. This is further correlated with elevated lipid peroxidation levels and altered endogenous enzyme activity in individual and combined pesticide exposure. Therefore, AChE can be regularly used as an important biomarker in the determination of pesticide-induced toxicity.
The vital antioxidant enzymes, viz., SOD, CAT, and GPx, provide the first-line of defence against free radicals induced by toxicants such as pesticides (Zhu et al. 2011; Al-Sawafi and Yan 2013; Ge et al. 2015). SOD leads to the conversion of superoxide anion (O2•−) into hydrogen peroxide (H2O2) and molecular oxygen (O2). Furthermore, CAT and GPx convert H2O2 into H2O and bring down lipid hydroperoxides to their consequent alcohols.
In the liver, notable elevation in SOD enzyme activity with down-regulated mRNA levels was observed in all exposed groups. The liver is responsible for the breakdown of xenobiotics in the body. Elevated SOD activity in the liver shows compensatory effects in response to the lipid peroxidation induced by individual and combined pesticide exposure. Analogous results were postulated by Azpiazu et al. (2021); Yanhua et al. (2021), Guo-Ping et al. (2020), and observed significant alteration caused by the combination of pesticides. SOD mRNA expression in the liver on exposure shows downregulation indicating the inability to neutralize superoxide anions. Increased SOD activity and suppressed mRNA expression show inconsistent response between enzyme activity and gene expression as reported by Jhamtani et al. (2018) and Karaca et al. (2014). The decrease in SOD activity and suppressed mRNA expression in all exposed groups in the kidney may be due to high levels of ROS, which is further supported by elevated LPO. The depletion of SOD activity was maximum in the brain might be due to enhanced LPO due to pesticide exposure. Upregulated mRNA expression in the DIC and IMD exposed groups showed attenuated effects due to maximum lipid peroxidation, while the ATZ and CMD groups exhibit SOD combat-induced lipid peroxidation.
Depleted CAT activity and mRNA expression in liver and kidney tissues as well as in brain tissue on individual ATZ exposure and gene mRNA expression of the CMD group show the underlying mechanism of pesticide (oxidant) susceptibility in exposed zebrafish. Enhanced sensitivity arises as a direct consequence due to accumulation of hydrogen peroxide in tissues, i.e., an inability to maintain cellular antioxidant levels which leads to impaired activity as observed by high lipid peroxidation. Similar to our results, suppressed CAT activity was also observed by Ojha et al. (2011) and Wu et al. (2018). The elevated enzyme activity in the brain of DIC, IMD, and CMD shows the repair mechanism and elimination of H2O2 by CAT (Shukla et al. 2017a).
Kim et al. (2018), and Mai et al. (2020) have observed altered CAT mRNA expression and CAT enzyme activity caused by the combination of pesticides. Elevated CAT activity shows protection against the adverse effects of toxicants as it functions to reduce ROS and prevent tissue damage.
In the combined group, we observed a significant increase in the GPx enzyme activity in all the examined tissue. The overproduction of ROS has been shown to result in an attenuation of GPx in the cells. In our earlier reported work with commercial-grade pesticides, we observed similar results of enhanced GPx enzyme activity in the combined exposed groups (Shukla et al. 2017a). The mRNA levels downregulated in the liver and kidney in all exposed groups contribute to increased free radical species which lead to direct tissue damage caused by individual and combined exposure to pesticides. Upregulated mRNA expression observed in the brain of the DIC, IMD, and CMD groups represents the high ROS scavenging ability of cells to eliminate lipid hydroperoxides as a consequence of enhanced LPO. Similar results were also reported by Kim et al. (2018) on combined exposure.
MTs are known to reduce the dangerous effects of free radicals by releasing zinc into the neuronal membrane. MTs are endogenous enzymes that are shown to scavenge hydroxyl radicals because of their cysteinyl thiolate group. MT contains zinc/cadmium ions, which help in scavenging superoxide and hydroxyl produced by the xanthine oxidase reaction. MT-II mRNA expression was performed to determine its relevance in detoxifying pesticide-induced toxicity. We have observed upregulation in the MT-II mRNA levels caused by individual DIC and IMD pesticides. Upregulation in MT-II mRNA levels in response to the individual exposed brain of DIC and IMD may be considered as a defence mechanism similar to that of other examined antioxidant enzymes’ mRNA levels. The results are correlated with Chen and Maret (2001) findings; they explained MT can act as an oxidative stress indicator, in which oxidation of MT release zinc to further decrease toxicity via thionein and GSH. Therefore, increased MT levels could enhance the antioxidative effectiveness of cells under stress responses. However, significant downregulation was observed in all the examined tissue of the CMD group, with a maximum in the liver. Similar downregulated results were demonstrated by the combination of different pesticides with a metal (Moncaleano-Niño et al. 2022). Fascinatingly, within our study, the levels of MT-II mRNA expression are parallel with the levels of SOD, CAT, and GPx. Our MT-II results further back the incorporation of assessing MT gene in pesticide-induced toxicity.
Similarly, other endogenic antioxidant enzymes, viz., SOD, CAT, and GPx, also helps in scavenging free radicals induced by individual and combination of pesticides. The suppression of MT-II in the present work represents impairment in the defence mechanism against the toxic effects of pesticides and the inability of the cell in MT-II protein synthesis. The results indicate a direct correlation with examined SOD, CAT, and GPx mRNA expression. In the brain, DIC and IMD show upregulation indicating the role of metallothionein in xenobiotic detoxification. The alteration in MT mRNA expression levels in different exposed groups is similar to the antioxidant enzymes studied. Similar to our results, Ceyhun et al. (2012) and Selcuk et al. (2018) have reported upregulated MT mRNA expression due to dichlorvos and imidacloprid exposure. The result shows that MT-II plays not only a significant role in detoxifying metal toxicity but also toxicity induced by pesticide(s).
Conclusion
Our results revealed that combined exposure exhibits maximum alterations in all examined tissues. All three organs were drastically affected in individual exposure groups; however, CMD exposure further elevates the toxic manifestations; thus, it is offering support to our previously reported work in which combined pesticide toxicity causes hepatotoxicity, nephrotoxicity, and neurotoxicity rather than individual target organ toxicity. In conclusion, the combination of pesticide exposure is furthermore destructive than single pesticide exposure, even at half concentration. Further from the findings, it can be understood that the assessment of single pesticide toxicity underrates the impact of combined pesticides toxicity on aquatic system and living organisms; therefore, it is required to anticipate the risk of combined toxicity so that the utility of various pesticides can be regulated to minimize their environmental and health hazard.
Data availability
If required, the data and materials will be provided.
References
Abarikwu SO, Queen CD, Rex-Clovis CN et al (2017) Effects of co-exposure to atrazine and ethanol on the oxidative damage of kidney and liver in Wistar rats. Ren Fail 39:588–596. https://doi.org/10.1080/0886022X.2017.1351373
Agarwal R, Raisuddin S, Tewari S et al (2010) Evaluation of comparative effect of pre-and post treatment of selenium on mercury-induced oxidative stress, histological alterations, and metallothionein mRNA expression in rats. J Biochem Mol Toxicol 24:123–135. https://doi.org/10.1002/jbt.20320
Ahmad M, Riaz U, Iqbal S, Ahmad J, Rasheed H, Al-Farraj ASF, Al-Wabel MI (2022) Adsorptive Removal of Atrazine From Contaminated Water Using Low-Cost Carbonaceous Materials: A Review. Frontiers in Materials 9:909534. https://doi.org/10.3389/fmats.2022.909534
Ahmed YH, AbuBakr HO, Ahmad IM et al (2022) Histopathological, immunohistochemical, and molecular alterations in brain tissue and submandibular salivary gland of atrazine-induced toxicity in male rats. Environ Sci Pollut Res 29:30697–30711. https://doi.org/10.1007/s11356-021-18399-x
Al-Sawafi AG, Yan Y (2013) Bioconcentration and antioxidant status responses in zebrafish (Danio Rerio) under atrazine exposure. Intl J Chem Engg Appli 4:204–208. https://doi.org/10.7763/IJCEA.2013.V4.295
APHA (2005) Standard methods for the examination of water and waste water, 21st edn. American Public Health Association, Washington, DC
Assis CR, Amaral IP, Castro PF et al (2007) Effect of dichlorvos on the acetylcholinesterase from tambaqui (Colossoma macropomum) brain. Environ Toxicol Chem 7:1451–1453. https://doi.org/10.1897/06-488R1.1
Azpiazu C, Bosch J, Bortollotti L et al (2021) Toxicity of the insecticide sulfoxaflor alone and in combination with the fungicide fluxapyroxad in three bee species. Sci Rep 11:6521. https://doi.org/10.1038/s41598-021-86036-1
Bacchetta C, Andrea R, Analia A, Mirta C, Maria JP, Jimena C (2014) Combined toxicological effects of pesticides: a fish multi-biomarker approach. Ecol Indic 36:532–538. https://doi.org/10.1016/j.ecolind.2013.09.016
Barata C, Baird DJ, Nogueira AJ et al (2006) Toxicity of binary mixtures of metals and pyrethroid insecticides to Daphnia magna straus. Implications for multi-substance risks assessment. Aquat Toxicol 78:1–14. https://doi.org/10.1016/j.aquatox.2006.01.013
Benli PP, Çelik M (2021) In vivo effects of neonicotinoid-sulfoximine insecticide sulfoxaflor on acetylcholinesterase activity in the tissues of zebrafish (Danio rerio). Toxics 9:73. https://doi.org/10.3390/toxics9040073
Blahova J, Plhalova L, Hostovsky M et al (2013) Oxidative stress responses in zebrafish (Danio rerio) after subchronic exposure to atrazine. Food Chem Toxicol 61:82–85. https://doi.org/10.1016/j.fct.2013.02.041
Bui-Nguyen TM, Baer CE, Lewis JA et al (2015) Dichlorvos exposure results in large scale disruption of energy metabolism in the liver of the zebrafish, (Danio rerio). BMC Genom 16:853. https://doi.org/10.1186/s12864-015-1941-2
Celik I, Suzek H (2009) Effects of sub acute exposure of dichlorvos at sublethal dosages on erythrocytes and tissue antioxidant defense systems and lipid peroxidation in rats. Ecotoxicol Environ Saf 72:905–908. https://doi.org/10.1016/j.ecoenv.2008.04.007
Ceyhun SB, Ercument A, Birsen K, Kubra A, Orhan E (2012) Chronic toxicity of pesticides to the mRNA expression levels of metallothioneins and cytochrome P450 1A genes in rainbow trout. Toxicol Ind Health 162–169. https://doi.org/10.1177/074823371140948
Chen Y, Maret W (2001) Catalytic selenols couple the redox cycles of metallothionein and glutathione. Eur J Biochem 268:3346–3353. https://doi.org/10.1046/j.1432-1327.2001.02250.x
Covert SA, Megan ES, Sarah MS, Wesley WS (2020) Pesticide mixtures show potential toxicity to aquatic life in U.S. streams, water years 2013–2017. Sci Total Environ 745:141285. https://doi.org/10.1016/j.scitotenv.2020.141285
Demirci O, Guven K, Asma D, Oqut S, Ugurlu P (2018) Effects of endosulfan, thiamethoxam, and indoxacarb in combination with atrazine on multi-biomarkers in Gammaru kischineffensis. Ecotoxicol Environ Saf 147:749–758. https://doi.org/10.1016/j.ecoenv.2017.09.038
El-Gendy KS, Aly NM, Mahmoud FH, Kenawy A, El-Sebae AK (2010) The role of vitamin C as antioxidant in protection of oxidative stress induced by imidacloprid. Food Chem Toxicol 48:215–221. https://doi.org/10.1016/j.fct.2009.10.003
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Ellman GL, Courtney KD, Andres VJR, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharm 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Erdogan O, Saltuk BC, Deniz E, Ercument A (2011) Impact of deltamethrin exposure on mRNA expression levels of metallothionein A, B and cytochrome P450 1A in rainbow trout muscles. Gene 484:13–17. https://doi.org/10.1016/j.gene.2011.05.026
Falfushynska H, Khatib I, Kasianchuk N et al (2022) Toxic effects and mechanisms of common pesticides (Roundup and chlorpyrifos) and their mixtures in a zebrafish model (Danio rerio). Sci Total Environ 833:155236. https://doi.org/10.1016/j.scitotenv.2022.155236
Flohe L, Gunzler WA (1984) Assays of glutathione peroxidase. Meth Enzymol 105:114–121. https://doi.org/10.1016/S0076-6879(84)05015-1
Ge W, Yan S, Wang J et al (2015) Oxidative stress and DNA damage induced by imidacloprid in zebrafish (Danio rerio). J Agric Food Chem 63:1856–1862. https://doi.org/10.1021/jf504895h
Ghosh PK, Philip L (2006) Environmental significance of atrazine in aqueous systems and its removal by biological processes. Global NEST J 8:159–178
Guerra LJ, Amaral AMB, Quadros VA et al (2021) Biochemical and behavioral responses in zebrafish exposed to imidacloprid oxidative damage and antioxidant responses. Arch Environ Contam Toxicol 81:255–264. https://doi.org/10.1007/s00244-021-00865-9
Gultekin F, Deliba SN, Yasar S, Kilinc I (2001) In vivo changes in antioxidant systems and protective role of melatonin and a combination of vitamin C and vitamin E on oxidative damage in erythrocytes induced by chlorpyrifos-ethyl in rats. Arch Toxicol 75:88–96. https://doi.org/10.1007/s002040100219
Guo-Ping Z, Fang-Wei Y, Jin-Wang L, Han-Zhu X, Fa-Zheng R, Guo-Fang P, Yi-Xuan L (2020) Toxicities of neonicotinoid-containing pesticide mixtures on nontarget organisms. Environ Tox Chem 39:1884–1893. https://doi.org/10.1002/etc.4842
Jhamtani RC, Shukla S, Dahiya MS, Agarwal R (2018) Impact of co-exposure of aldrin and titanium dioxide nanoparticles at biochemical and molecular levels in zebrafish. Environ Toxicol Pharmacol 58:141–155. https://doi.org/10.1016/j.etap.2017.12.021
Jin Y, Zhang X, Shu L et al (2010) Oxidative stress response and gene expression with atrazine exposure in adult female zebrafish (Danio rerio). Chemosphere 78:846–852. https://doi.org/10.1016/j.chemosphere.2009.11.044
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Ind J Biochem Biophys 21:130–132
Kapoor U, Srivastava MK, Bhardwaj S, Srivastava LP (2010) Effect of imidacloprid on antioxidant enzymes and lipid peroxidation in female rats to derive its no observed effect level (NOEL). J Toxicol Sci 35:577–581. https://doi.org/10.2131/jts.35.577
Karaca M, Varışlı L, Korkmaz K, Ozaydın O, Percin F, Orhan H (2014) Organochlorine pesticides and antioxidant enzymes are inversely correlated with liver enzyme gene expression in Cyprinus carpio. Toxicol Lett 230:198–207. https://doi.org/10.1016/j.toxlet.2014.02.013
Khaled AO, Mahmoud MIS, Ahmed NS (2023) Biomarkers of imidacloprid toxicity in Japanese quail, Coturnix coturnix Japonica. Environ Sci Pollut Res 30:5662–5676. https://doi.org/10.1007/s11356-022-22580-1
Kim K, Hwang-Ju J, Sung-Deuk C et al (2018) Combined toxicity of endosulfan and phenanthrene mixtures and induced molecular changes in adult Zebrafish (Danio rerio). Chemosphere 194:30–41. https://doi.org/10.1016/j.chemosphere.2017.11.128
Kruć-Fijałkowska R, Dragon K, Drożdżyński D, Górski J (2022) Seasonal variation of pesticides in surface water and drinking water wells in the annual cycle in western Poland, and potential health risk assessment. Sci Rep 12:3317. https://doi.org/10.1038/s41598-022-07385-z
Li W, Wang B, Yuan Y, Wang S (2023) Spatiotemporal distribution patterns and ecological risk of multi-pesticide residues in the surface water of a typical agriculture area in China. Environ Monit Assess 870:161872. https://doi.org/10.1016/j.scitotenv.2023.161872
Lionetto MG, Caricato R, Giordano ME et al (2003) Integrated use of biomarkers (acetylcholinesterase and antioxidant enzymes activities) in Mytilus galloprovincialis and Mullus barbatus in an Italian coastal marine area. Mar Pollut Bull 46:324–330. https://doi.org/10.1016/S0025-326X(02)00403-4
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mai H, Cachot J, Clérandeau C, Martin C, Mazzela N, Gonzalez P, Morin B (2020) An environmentally realistic pesticide and copper mixture impacts embryonic development and DNA integrity of the Pacific oyster, Crassostrea gigas. Environ Sci Pollut Res 27:3600–3611. https://doi.org/10.1007/s11356-018-3586-6
Mali H, Shah C, Raghunandan BH, Anil SP, Darshan P, Ujjval T, Subramanian RB (2023) Organophosphate pesticides an emerging environmental contaminant: pollution, toxicity, bioremediation progress, and remaining challenges. J Environ Sci 127:234–250. https://doi.org/10.1016/j.jes.2022.04.023
Mendonça-Soares S, Fortuna M, Freddo N et al (2023) Behavioral, biochemical, and endocrine responses of zebrafish to 30-min exposure with environmentally relevant concentrations of imidacloprid-based insecticide. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-023-27667-x
Moncaleano-Niño AM, Gómez-Cubillos MC, Luna-Acosta A et al (2022) Monitoring metallothionein-like protein concentrations and cholinesterase activity in tropical cup oysters as biomarkers of exposure to metals and pesticides in the southern Caribbean, Colombia. Environ Sci Pollut Res 29:52157–52183. https://doi.org/10.1007/s11356-021-17644-7
Nachimuthu KS, Ramasamy A, Murugesan T et al (2023) Toxicity analysis and biomarker response of quinalphos organophosphate insecticide (QOI) on eco-friendly exotic Eudrilus eugeniae earthworm. https://doi.org/10.1007/s10661-022-10834-x
Narasimhamurthy RK, Andrade D, Mumbrekar KD (2022) Modulation of CREB and its associated upstream signaling pathways in pesticide-induced neurotoxicity. Mol Cell Biochem 477:5. https://doi.org/10.1007/s11010-022-04472-7
Nele M, Stefan R, Michael T, Barbara G (2020) Mixture toxicity in the Erft River: assessment of ecological risks and toxicity drivers. Environ Sci Eur 32:51. https://doi.org/10.1186/s12302-020-00326-5
OECD (2004) OECD Guidelines for the Testing of Chemicals, Section 2. Effects on Biotic Systems. Organisation for Economic Co-operation and Development, Paris. www.oecd-ilibrary.org date of access 14 Jan 2022
Ogunro OB (2023) Redox-regulation and anti-inflammatory system activation by quercetin-3-O-β-Dglucopyranoside-rich fraction from Spondias mombin leaves: biochemical, reproductive and histological study in rat model of dichlorvos toxicity. RPS Pharmacy and Pharmacology Reports 2:2. https://doi.org/10.1093/rpsppr/rqad016
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Annu Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Ojha A, Yaduvanshi N, Srivastava N (2011) Effect of combined exposure of commonly used organophosphate pesticides on lipid peroxidation and antioxidant enzymes in rat tissues. Pestic Biochem Physiol 99:148–156. https://doi.org/10.1016/j.pestbp.2010.11.011
Olwenn M, Martin S, Sibylle E et al (2021) Ten years of research on synergisms and antagonisms in chemical mixture: a systematic review and quantitative reappraisal of mixture studies. Environ Int 146:106206. https://doi.org/10.1016/j.envint.2020.106206
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29:e45. https://doi.org/10.1093/nar/29.9.e45
Rodríguez-Fuentes G, Rubio-Escalante FJ, Noreña-Barroso E, Escalante-Herrera KS, Schlenk D (2015) Impacts of oxidative stress on acetylcholinesterase transcription, and activity in embryos of zebrafish (Danio rerio) following Chlorpyrifos exposure. Comp Biochem Physiol C Toxicol Pharmacol 172–173:19–25. https://doi.org/10.1016/j.cbpc.2015.04.003
Santos KC, Martinez CB (2014) Genotoxic and biochemical effects of atrazine and Roundup, alone and in combination, on the Asian clam Corbicula fluminea. Ecotoxicol Environ Saf 100:7–14. https://doi.org/10.1016/j.ecoenv.2013.11.014
Satyanarayana GNV, Kumar A, Pandey AK, Sharma MT, Natesan M, Reddy MKM (2023) Evaluating chemicals of emerging concern in the Ganga River at the two major cities Prayagraj and Varanasi through validated analytical approaches. Environ Sci Pollut Res 30:1520–1539. https://doi.org/10.1007/s11356-022-22226-2
Selcuk O, Serdar A, Harun A (2018) Imidacloprid exposure cause the histopathological changes, activation of TNF-α, iNOS, 8-OHdG biomarkers, and alteration of caspase 3, iNOS, CYP1A, MT1 gene expression levels in common carp (Cyprinus carpio L). Toxicol Rep 5:125–133. https://doi.org/10.1016/j.toxrep.2017.12.019
Shaner DL (2014) Herbicide handbook-atrazine, 10th edn. Weed Science Society of America, Lawrence, KS, pp 54–55
Shukla S, Jhamtani RC, Dahiya MS, Agarwal R (2017a) Oxidative injury caused by individual and combined exposure of neonicotinoid, organophosphate and herbicide in zebrafish. Toxicol Rep 4:240–244. https://doi.org/10.1016/j.toxrep.2017.05.002
Shukla S, Jhamtani RC, Dahiya MS, Agarwal R (2017) A novel method to achieve high yield of total RNA from zebrafish for expression studies. Int J Bioassay 6:5383–5385. https://doi.org/10.21746/ijbio.2017.05.004
Shukla S, Jhamtani RC, Agarwal R (2018) Emergence of zebrafish as a biomarker for pesticide poisoning in forensic toxicology research. Toxicol Int 25:68–77. https://doi.org/10.18311/ti/2018/22803
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47:389–394. https://doi.org/10.1016/0003-2697(72)90132-7
Tao MT, Bian ZQ, Zhang J, Wang T, Shen HY (2020) Quantitative evaluation and the toxicity mechanism of synergism within three organophosphorus pesticide mixtures to Chlorella pyrenoidosa†. Environ Sci: Processes Impacts 22:2095–2103
Tomizawa M, Casida JE (2005) Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu Rev Pharmacol Toxicol 45:247–268. https://doi.org/10.1146/annurev.pharmtox.45.120403.095930
Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M (2006) Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf 64:178–189. https://doi.org/10.1016/j.ecoenv.2005.03.013
Van Leeuwen CJ, Bro-Rasmussen F, Feijtel TC et al (1996) Risk assessment and management of new and existing chemicals. Environ Toxicol Pharmacol 20:243–299. https://doi.org/10.1016/S1382-6689(96)00072-5
Viana NP, da Silva LCM, Portruneli N et al (2022) Biocentration and toxicological impacts of fipronil and 2,4-D commercial formulations (single and mixture) in the tropical fish, Danio rerio. Environ Sci Pollut Res 29:11685–11698. https://doi.org/10.1007/s11356-021-16352-6
Wang Y, Lv L, Yu Y, Yang G, Xu Z, Wang Q, Cai L (2017) Single and joint toxic effects of five selected pesticides on the early life stages of zebrafish (Denio rerio). Chemosphere 170:61–67. https://doi.org/10.1016/j.chemosphere.2016.12.025
Wu S, Xinfang L, Xinju L, Guiling Y, Xuehua A, Qiang W, Yanhua W (2018) Joint toxic effects of triazophos and imidacloprid on zebrafish (Danio rerio). Environ Pollut 235:470–481. https://doi.org/10.1016/j.envpol.2017.12.120
Yanhua W, Lu L, Chao X, Dou W, Guiling Y, Xinquan W, Hongbiao W, Qiang W (2021) Mixture toxicity of thiophanate-methyl and fenvalerate to embryonic zebrafish (Danio rerio) and its underlying mechanism. Sci Total Environ 756:143754. https://doi.org/10.1016/j.scitotenv.2020.143754
Zhu LS, Shao B, Song Y, Xie H, Wang JH, Liu W, Hou XX (2011) DNA damage and effects on antioxidative enzymes in zebra fish (Danio rerio) induced by atrazine. Toxicol Mech Meth 21:31–36. https://doi.org/10.3109/15376516.2010.529186
Acknowledgements
The authors are grateful to Vice Chancellor, National Forensic Sciences University, Gandhinagar, for overall support. Saurabh Shukla is thankful to the University Grants Commission (UGC), New Delhi, for the award of Junior and Senior Research Fellowships.
Author information
Authors and Affiliations
Contributions
The authors contributed to the study conception and design, material preparation, data collection, and analysis; the draft was performed by Saurabh Shukla; data collection and analysis were performed by Reena C Jhamtani; study design, editing, and manuscript were revised by Rakhi Agarwal. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Declarations
The experiment was designed, and animals were kept as per Organization for Economic Co-operation and Development guidelines.
Ethics approval
The study was approved by the university research committee no. PhD/FS/RA/02.
Consent to participate
The authors give consent to participate.
Consent for publication
The authors give consent to publish the data provided in the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shukla, S., Jhamtani, R.C. & Agarwal, R. Biochemical and gene expression alterations due to individual exposure of atrazine, dichlorvos, and imidacloprid and their combination in zebrafish. Environ Sci Pollut Res 30, 118291–118303 (2023). https://doi.org/10.1007/s11356-023-30160-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30160-0