Abstract
In this study, we investigate the toxicity of commercial formulations based on glyphosate, 2,4-D, imidacloprid, and iprodione, in isolation and mixed, on Allium cepa. The mixtures consisted of combinations in the lowest (M1), intermediate (M2), and highest concentrations (M3) of each pesticide. We measured physiological (germination rate, germination speed, and radicular length) and cyto-genotoxic (mitotic index and frequency of aberrant cells) parameters. In addition, we analyzed the cell cycle progression and cell death induction by flow cytometry. When applied in isolation, the pesticides changed the parameters evaluated. M1 and M2 inhibited root length and increased the frequency of aberrant cells. Their genotoxic effect was equivalent to that of pesticides applied in isolation. Furthermore, M1 and M2 caused cell death and M2 changed the cell cycle progression. M3 had the greatest deleterious effect on A. cepa. This mixture inhibited root length and promoted an additive or synergistic effect on the mitotic index. In addition, M3 changed all parameters analyzed by flow cytometry. This research clearly demonstrates that the pesticides tested, and their mixtures, may pose a risk to non-target organisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of pesticides has increased substantially in Brazil since 2008, following an increased demand for food at the national and global scales (Brovini et al. 2021). In addition, the political-economic model adopted by the country’s agrobusiness heavily relies on the use of chemical compounds (Araújo and Oliveira 2017).
The intensive use of pesticides in agriculture has, as a result, the input of it into the water resources, due to soil runoff. Therefore, it is necessary to monitor pesticide levels in the water to protect the environment and people (Barbosa et al. 2015; Albuquerque et al. 2016; Brovini et al. 2021).
In Brazil, Resolution No. 375 of the National Environmental Council (CONAMA 2005) establishes quality standards for water resources to fit their different uses, including protecting of aquatic communities and the water supplied for human use. On the other hand, the Ministry of Health (MS) regulates the pesticide levels allowed in drinking water through Ordinance No. 2,914 of 2011 (MS 2011) and coordinates the National Program for Monitoring the Quality of Drinking Water (Vigiagua). Data from the latter are included in the Sisagua database (Water Quality Monitoring System).
However, CONAMA and MS regulate less than 10% of Brazil’s active ingredients approved for use. In addition, different classes of pesticides interact in the environment because of simultaneous or sequential applications, which may result in additive, synergistic, antagonistic, or potentiation toxic effects, i.e., different from the responses produced by each chemical substance alone (Fioresi et al. 2020; Finkler et al. 2022).
The herbicides glyphosate (replaced glycine chemical group), and 2,4-dichlorophenoxyacetic (aryloxy alkanoic acid chemical group) are the most commercialized pesticides in Brazil and worldwide (IBAMA 2020). Their water concentrations frequently exceed the limits allowed by the legislation (Aranha and Rocha 2019), raising concern about the adverse effects caused in exposed organisms: acute and chronic toxicity (Rodrigues et al. 2019; Silva et al. 2020; Brovini et al. 2021), oxidative stress, DNA damage, and mutations (Bukowska 2006; Rodrigues et al. 2019; Chaufan et al. 2014). In addition, glyphosate is possibly a carcinogen (IARC 2017), and 2,4-D may cause cancer in humans (IARC 2018).
On the other hand, the levels in the water of the insecticide imidacloprid (chemical group neonicotinoid) and of the fungicide iprodione (chemical group dicarboximide) are unregulated by Brazilian legislation. Nevertheless, the eco-cytogenotoxicity of these compounds has been reported by several studies (Karabay and Oguz 2005; Demsia et al. 2007; Bianchi et al. 2015; 2016; Rodríguez et al. 2015; Iturburu et al. 2018; Aragão et al. 2019; Bernardes et al. 2019; Chaufan et al. 2019; Carneiro et al. 2020; Fioresi et al. 2020; Samojeden et al. 2022).
The environmental persistence of pesticides can be estimated by categorizing their half-lives into three groups: low (less than 16 days), moderate (16 to 59 days), and high (over 60 days). Pesticides with very short half-lives can be problematic if they need to be applied frequently within a short timeframe. Pesticides with longer half-lives are more likely to build up after repeated applications. This may increase the risk of environmental contamination (Hanson et al. 2015). Many abiotic and biotic environmental factors affect how long a pesticide remains in the environment, making it challenging to determine a specific half-life (Hanson et al. 2015). Typically, glyphosate remains in surface waters for a period ranging from 2 to 91 days (Battaglin et al. 2014; Castro Berman et al. 2018). The estimated time it takes for 2,4-D to degrade in aerobic aquatic environments is 15 days, while in anaerobic conditions, it can take anywhere from 41 to 333 days. (NPIC 2011). Imidacloprid breaks down rapidly in water in the presence of light, but it remains persistent in water in the absence of light (Flores-Céspedes et al. 2012). In the dark, it breaks down very slowly at a pH between 5 and 7. In contrast, the half-life is about 1 year at pH 9 (Fossen 2006). The half-life of iprodione in aerobic aquatic environments was estimated to be 9 days, and in anaerobic environments, 7–14 days (US EPA 2007).
In view of the scenario described above, it is necessary to evaluate the adverse effects of these pesticides, isolated and combined, on non-target organisms (Finkler et al. 2022). Allium cepa is a plant commonly employed to evaluate water quality. The use of this model organism for the detection of toxic (macroscopic analysis) and cytogenotoxic activity (microscopic analysis) is validated by the International Chemical Safety Program and the United Nations Environmental Program (WHO 1985). Allium cepa is highly sensitive (82%) compared to the Ames test and the rodent carcinogenicity bioassay (Rank and Nielsen 1994). In addition, flow cytometry accurately detects changes in the cell cycle kinetics and measures cell death. This technique has been applied to investigate the effects of xenobiotics on plants (Rayburn and Wetzel 2002; Monteiro et al. 2010; Andrade-Vieira et al. 2012; Ghosh et al. 2015; Ghosh et al. 2016; Fioresi et al. 2020; Souza et al. 2023).
This study documents the effects of commercial formulations based on glyphosate, 2,4-D, imidacloprid and iprodione, and mixtures of them, on A. cepa. Physiological (germination rate, germination speed, and radicular length) and cyto-genotoxic (mitotic index and frequency of aberrant cells) parameters were analyzed, in addition to cell cycle progression and cell death induction (flow cytometry). Realistic concentrations reported on the freshwater and drinking water, and the maximum values allowed by the Brazilian legislation, were tested (Resolution No. 375/2005 of CONAMA and Ordinance No. 2,914/2011 of the Ministry of Health).
Materials and methods
Pesticides
Stock solutions and test solutions were prepared from the commercial formulations of each pesticide. Pesticides were diluted and homogenized in distilled water. The tested concentrations are expressed as microgram of acid equivalent per liter (herbicides) or microgram of active ingredient per liter (imidacloprid and iprodione).
The original DI Roundup commercial formulation, Monsanto, batch no. 0045 20–63560, was used. It contains 445 g/L (44.5% m/v) of N-(phosphonomethyl)glycine di-ammonium salt) and 370 g/L (37% m/v) acid equivalent of N-(phosphonomethyl)glycine), and 751 g/L (75% m/v) of inert ingredients was used. This formulation is classified as hazardous to the environment (class III) but is unlikely to cause acute harm to humans (category 4) (MAPA 2022). The tested concentrations were as follows: (a) 50 µg/L — recorded in tap water (Aranha and Rocha 2019); (b) 65 µg/L — corresponding to the maximum concentration allowed in freshwaters class I and II (CONAMA 2005) and (c) 280 µg/L — maximum concentration allowed in freshwaters class III (CONAMA 2005).
The commercial product Nufarm U46 BR (lot 0800491517499 T/B) containing 806 g/L of dimethylamine salt of 2,4-dichlorophenoxyacetic acid (2,4-D Amine), 670 g/L (67% w/v) of acid equivalent, and 380.9 g/L (38.09% m/v) of other ingredients was used. This formulation is considered hazardous to the environment (class III) and to human health, being classified in category 4 — slightly toxic product (MAPA 2022). The concentrations tested were as follows: (a) 4 μg/L — maximum concentration allowed in freshwaters class I and II (CONAMA 2005), the same value established by Ordinance No. 2,914/2011 (MS 2011); (b) 10 μg/L — found in the tap water of Brazilian municipalities (Aranha and Rocha 2019); (c) 30 μg/L — maximum concentration allowed in class III freshwater (CONAMA 2005).
The fungicide Rovral SC, FMC Química do Brasil LTDA, batch no. 021–17-5475, containing 500 g/L of iprodione (3-(3,5-dichlorophenyl)-N-isopropyl-2,4-dioxo imidazolidine-1-carboxamide), is classified as dangerous for the environment (class III) and category 5 — unlikely to cause acute damage to humans (MAPA 2022). The tested concentrations were (a) 15.96 µg/L — chosen because it is lower than the other concentrations; (b) 27.14 µg/L — corresponding to the concentration of iprodione found in freshwaters (Sequinatto et al. 2013) and (c) 47.26 µg/L — defined based on the estimated concentration of the active ingredient in freshwaters according to the total number of applications for coffee (Queiroz et al. 2018).
The tested concentrations of the insecticide Warrant® 700 WG, lot no. 3269–16-1154, containing 700 g/kg imidacloprid (1-(6-chlorine-3-pyridynilmethyl)-N-nitroimidazolidim-2-ylidenoamine), were (a) 0.14 μg/L, (b) 1.13 μg/L, and (c) 4.53 μg/L, found in freshwaters and rural wells (Bortoluzzi et al. 2007; Sequinatto et al. 2013). According to the degree of environmental hazard, the formulation is considered hazardous to the environment (class III) and category 4 —slightly toxic to human health (MAPA 2022).
For pesticide mixtures, the lowest (M1), intermediate (M2), and highest concentrations tested (M3) were combined (Bianchi et al. 2016; Felisbino et al. 2018; Fioresi et al. 2020). The concentrations of pesticides were separated into three groups to determine the effects of each one in the mixture (Table 1).
Trials with A. cepa
Seeds of A. cepa (2n = 16), periform bay variety (ISLA®, lot no. 127536, germination rate of 98%), not treated with pesticides, were used. In all assays, distilled water was used as the negative control.
Phytotoxicity
The trial was conducted according to the US Protection Environmental Agency (US EPA 1996). For the analysis, 15 seeds of A. cepa were arranged in Petri dishes (150 × 15 mm) lined with filter paper with added 4 ml of pesticide solutions and mixtures. The trial was performed in triplicate. Germinated seeds were counted every 24 h until 65% of the seeds in the control group had roots and were at least 20 mm in length. Germination rate (G) was calculated using the formula: G = (N/A) × 100, where N is the total number of germinated seeds and A is the number of seeds placed for germination. The following formula was used to calculate germination speed: IVG = (∑Ni)/(∑Di), where Ni is the number of roots germinated on day i; Di is number of days for germination. The final length of the radicles was measured with the aid of a caliper.
Cytogenotoxicity
The cytogenotoxicity test was conducted according to Mazzeo et al. (2015). Radicles exposed to pesticides and mixtures, and which measured bout 15 mm, were collected, fixed in methanol + acetic acid (3:1, v/v) and stored in a refrigerator. To remove the excess fixator, the roots were washed in distilled water three times, 5 min each time. The radicles were hydrolyzed in HCl 5N at room temperature for 20 min. They were also washed in distilled water three times, for 5 min each time. After this process, the meristematic region of the roots was sectioned on a slide, stained with orcein 2% for 10 min, then covered with coverslip and macerated. Ten slides were prepared for each treatment, and 5000 cells were counted per treatment (500 cells/slides). The slides were analyzed under a light microscope at 400 × magnification. The frequency of dividing cells was determined by calculating the mitotic index (cytotoxic potential). All mitotic and chromosomal abnormalities observed were grouped within the same category: frequency of aberrant cells (Souza et al. 2021).
Flow cytometry
The flow cytometry analyses were conducted in three replicates (three samples) for each treatment, and 12 meristematic regions of A. cepa were evaluated per replicate. Nuclear suspensions were obtained by the method described by Galbraith et al. (1983). The meristems were sectioned with the aid of a scalpel blade in a Petri dish containing 600 μl of LB01 lysis buffer, in a box with crushed ice. The suspensions with the insulated nuclei were filtered with 45 μm nylon mesh to eliminate waste. These were then colored with 50 μl of propidium iodide (1 mg/L) for analysis in a BD FACSCanto II flow cytometer.
The distribution of nuclei in the different phases of the cell cycle was analyzed: G0/G1 — nuclei with 2C DNA content, S — nuclei with 2 — 4C DNA content, and G2/M nuclei with 4C DNA content. The proportion of nuclei/particles in sub-G1 was measured. This represents condensed nuclei with lower fluorescence intensity, due to the difficult access of the propidium iodide to particles with DNA content smaller than 2C (fragmentation of genetic material due to the process of cell death) (Andrade-Vieira et al. 2012; Moreira et al. 2021). The following were determined: the fluorescence intensity of the nuclei in G1 (IFG1), which allows the estimation of the average amount of fluorescence emitted by the G1 nuclei; the dispersion of the frontal light (FSC — forward scatter), to analyze the diameter/nuclear size, and the lateral dispersion of light (SSC — side scatter), to verify the nuclear complexity. In addition, the coefficient of variation of the nuclei in G1 (CV = standard deviation divided by the mean) was also determined.
Statistical analysis
The Shapiro-Willk normality test was applied, and statistical differences between the control and pesticide concentrations within each experimental group were analyzed using ANOVA with subsequent Tukey test (p < 0.05). When the data did not meet the assumptions to perform parametric analysis, the Kruskal–Wallis test was applied, followed by the Dunn’s test (p < 0.05). GraphPad Prism version 9.4 was used for the statistical analysis and elaboration of the figures.
Results and discussion
Phytotoxicity
The goal of applying phytotoxicity assays is to evaluate whether the chemicals present in the growth medium will cause damage to the development, macroscopic structure, and survival of an organism. In this work, the toxic effects of environmentally relevant concentrations of pesticides, and their mixtures, were analyzed by evaluating the germination rate, germination speed and radicular length of A. cepa. The results obtained are presented in Fig. 1.
Phytotoxicity of pesticide glyphosate (GLY), 2,4-D, iprodione (IPR), imidacloprid (IMI), and mixtures (M1, M2, and M3). Group 1: lowest concentrations of each pesticide (50 µg/L GLY, 4 µg/L 2,4-D, 15.96 µg/L IPR, and 0.14 µg/L IMI) and mixture of them (M1). Group 2: intermediate concentrations of each pesticide (65 µg/L GLY, 10 µg/L 2,4-D, 27.14 µg/L IPR, and 1.13 µg/L IMI) and mixture of them (M2). Group 3: highest concentrations of each pesticide (280 µg/L GLY, 30 µg/L 2,4-D, 47.26 µg/L IPR, and 4.53 µg/L IMI) and mixture of them (M3). Asterisks denote statistical significance with respect to the negative control (NC): *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 00001. Treatments followed by number sign differ from each other: #p < 0.05, ##p < 0.01, ###p < 0.001, and ####p < 0.0001
Inhibition of the germination rate is an indicator of acute toxicity. Among the phytotoxic parameters analyzed, it was the least affected, consistent with the results of another study (Aragão et al. 2017). The herbicides did not affect the germination rate of A. cepa (Fig. 1a–c), a monocotyledon plant. Glyphosate and 2,4-D are systemic herbicides of post-emergent action recommended for the control of mono and dicotyledons (glyphosate), or only dicotyledons (2,4-D) (MAPA 2022). Previously, Alves et al. (2021a) had shown that the 2,4-D significantly inhibited the germination rate of the model plant Lactuca sativa (lettuce; dicotyledon), but not of Sorghum bicolor (sorghum; monocotyledon). In another study, seeking positive controls for phytocytogenotoxicity assays, Alves et al. (2021b) again showed that 2,4-D was effective at inhibiting the germination rate of L. sativa, but not of A. cepa.
Glyphosate-based herbicides also seem to have different effects on seed germination deleterious (Shuma et al. 1995; Mondal et al. 2017) or little to no effect (Piotrowicz-Cieślak et al. 2010) depending on the concentrations of the chemical, and the plant species tested. Our results contrast with Alves et al. (2021a, b), who showed that 0.01% glyphosate significantly reduced the percentage of germinated seeds of onion and lettuce, but corroborate the findings of Piotrowicz-Cieślak et al. (2010), who attested that 1–2000 µM of glyphosate did not affect the germination percentage of six species — Lepidium sativum, Sinapis alba, Sorghum saccharatum, Brassica napus, Lupinus luteus, and Avena sativa. According to Piotrowicz-Cieślak et al. (2010), therefore, percent seed germination may not be a good phytotoxicity parameter when glyphosate is employed.
Unlike the herbicides, the fungicide iprodione and the insecticide imidacloprid negatively affected the germination of A. cepa seeds when in certain concentrations. In group 1, iprodione significantly reduced the germination rate with respect to the control. The effect caused by the fungicide differed statistically from the effect of the glyphosate and 2,4-D and M1. In turn, M1 did not change the germination rate when compared with the control, indicating that iprodione, when combined with the other pesticides, did not exert an effect (Fig. 1a). Imidacloprid was the only pesticide isolate that inhibited the germination rate compared to the control in groups 2 and 3. In group 2, the insecticide still exerted a greater effect than all other pesticides and mixture M2. In the same manner, M1, M2, and M3 treatments did not differ from the control (Fig. 1b–c).
The results of the germination speed experiments are as follows: in group 1, only imidacloprid at 0.14 μg/L caused an adverse effect compared to the control and glyphosate (Fig. 1d). In group 2, iprodione at 27.14 μg/L and imidacloprid at 1.13 μg/L significantly reduced germination speed in relation to the control. Imidacloprid also reduced germination speed compared to all other pesticides, and therefore caused the greatest adverse effect. The M2 mixture also differed from the control and had an effect similar to isolated iprodione and imidacloprid (Fig. 1e). In group 3, the 2,4-D at 30 μg/L and imidacloprid at 4.53 μg/L reduced the germination speed compared to the control, while M3 did not change this parameter (Fig. 1f).
Unlike germination rate and germination speed, radicular length is considered the most sensitive phytotoxic parameter. Considering the lowest concentrations of pesticides and M1 (group 1), 4 µg/L of 2,4-D and 0.14 µg/L of imidacloprid and M1 reduced the root length of A. cepa. M1 had a comparable effect to that of the isolated pesticides (Fig. 1g). In group 2, the 2,4-D at 10 μg/L and M2 significantly decreased radicular length with respect to the control. The effect of M2 did not differ statistically from the effect of isolated pesticides, except for iprodione (Fig. 1h). In group 3, the 2,4-D at 30 μg/L and M3 resulted in shorter roots of A. cepa when compared to the control. The effect of M3 also differed statistically from iprodione (Fig. 1i).
As described above and observed in Fig. 1 h–i, 2.4-D was the only pesticide which, in all concentrations assessed, significantly reduced the length of the roots in relation to the control. These data are consistent with Alves et al. (2021b) and Grabinska-Sota et al. (2003), who experimented with other plant species (Sinapis alba, Lepidium sativum, Avena sativa, Triticum aestivum). By mimicking the action of natural auxin, 2,4-D inhibits the production of the hormone ethylene. Thus, abscisic acid is produced, which inhibits cell proliferation and elongation, hindering growth, damaging tissue, and causing cell death (Marcato et al. 2017).
All mixtures also reduced the radicular length of A. cepa. Plant root growth depends on cell proliferation and elongation during development and differentiation processes. Therefore, the effect observed in mixtures is an indication that the chemicals in pesticides interfere with the genetic material, mitotic machinery, and cell death induction mechanisms (Fioresi et al. 2020).
Cyto-genotoxicity
The results of the mitotic index analysis are presented in Fig. 2a–c. When in isolation, only the herbicides glyphosate and 2,4-D decreased the proportion of cells in division with respect to the control. According to Türkoğlu (2012), a reduction in the mitotic index indicates interference in the functioning of the enzymes that mediate cell cycle, including DNA polymerase, DNA gyrase, RNA polymerase, and kinases, resulting in inhibition of DNA synthesis or blockade of the G1 and G2 phases of the cell cycle and inhibition of microtubule formation.
Mitotic index (a–c) and frequency of aberrant cells (d–e) induced by glyphosate (GLY), 2,4-D, iprodione (IPR), imidacloprid (IMI), and their mixtures (M1, M2, and M3). Group 1: lowest concentrations of each pesticide (50 µg/L GLY, 4 µg/L 2,4-D, 15.96 µg/L IPR, and 0.14 µg/L IMI) and mixture of them (M1). Group 2: intermediate concentrations of each pesticide (65 µg/L GLY, 10 µg/L 2,4-D, 27.14 µg/L IPR, and 1.13 µg/L IMI) and mixture of them (M2). Group 3: highest concentrations of each pesticide (280 µg/L GLY, 30 µg/L 2,4-D, 47.26 µg/L IPR, and 4.53 µg/L IMI) and mixture of them (M3). Asterisks denote statistical significance with respect to the negative control (NC): *p < 0.05, **p < 0.01, and ****p < 0.0001. Treatments followed by number sign differ from each other: #p < 0.05, ##p < 0.001, and ####p < 0.0001
Considering the lowest concentrations of pesticides (group 1), the effects of the herbicides tested did not differ statistically from each other, but differed from iprodione and imidacloprid (p < 0.05) (Fig. 2a). The M1 mixture significantly reduced the mitotic index in relation to the control, and its effect was comparable to that of the isolated pesticides (Fig. 2a). The mitotic index after the M2 treatment did not differ from the control, indicating that the effect of 2,4-D at 10 μg/L was canceled out when associated with other pesticides (Fig. 2b). On the other hand, M3, which combines the highest concentrations of pesticides, drastically reduced the rate of dividing cells concerning the control and also compared to all pesticides when isolated (Fig. 2c). This result indicates that the mixture has an additive or synergistic effect that is more powerful than the pesticides alone. In the additive effect, the toxicity of the mixture is the sum of the individual effects. A synergistic effect occurs when the mixture produces more adverse effects than the isolated chemical substances and the additive effect (Finkler et al. 2022).
The genotoxic activity of pesticides and mixtures was investigated by analyzing the frequency of aberrant cells (Fig. 2d–f). In general, the isolated pesticides, except for imidacloprid at 0.14 μg/L (Fig. 2d) and glyphosate at 65 μg/L and 280 μg/L (Fig. 2e–f), induced a greater genotoxic effect than the control (Fig. 2d–f).
The genotoxicity of pesticides or commercial formulations used in this research has been reported in different studies. Kier and Kirkland (2013) reviewed the genotoxicity of glyphosate and the commercial formulations based on this herbicide, concluding that both have no mutagenic or genotoxic activity on mammalian cells both in vivo and in vitro. In contrast, only high doses of glyphosate caused positive results in non-mammalian systems. In these cases, its genotoxicity was due to the effects of surfactants on commercial formulations and not to the active ingredient itself. In the case of the aquatic biota, Rodrigues et al. (2019) showed that glyphosate, its main degradation product aminomethylphosphonic acid (AMPA), the commercial formulation ATN, and the surfactant polyethoxylated tallow amine (POEA) induced DNA damage on zebrafish larvae. Nevertheless, only the surfactant caused adverse effects in the gonadal cells of the rainbow trout (RTG-2). The difference between the results was explained by the physiological differences among the organisms involved and the fact that those were different species.
In model plants, some studies indicate that glyphosate increases chromosomal aberrations. However, the concentrations tested in those studies are higher than those investigated in the present study: 0.36–7.2 mg/ml (Truta et al. 2011), 5–30 mg/L (Mercado and Caleño 2020), 1.34–13.40 mg/L (Vieira et al. 2022), and 1.56 and 11.66 mg/ml (Finkler et al. 2022).
Bukowska (2006), in his review article, clarified the general mechanisms of action involved in the toxic action of 2,4-D, which involves the induction of oxidative stress, lipid peroxidation, depletion of ATP concentration, NADPH and GSH, modulation of antioxidant system activity, induction of homologous recombination, A → G mutation, chromosomal aberrations, exchange between sister chromatids, breaks in the DNA molecule, and activity-dependent apoptosis and caspases.
When it comes to other pesticides, the data on the toxic effects of iprodione on non-target organisms are scarce, and it is difficult to determine their mechanism of cytogenotoxic action (Chaufan et al 2019). The aneugenic and/or clastogenic effect of the iprodione was reported for some species of model plants (Gadeva and Dimitrov 2008; Aragão et al. 2019; Bernardes et al. 2019; Fioresi et al. 2020). Bernardes et al. (2019) documented the mutagenic activity of iprodione on A. cepa. It caused changes in single sequence repeat (SSR) and inter-simple sequence repeat (ISSR) markers, indicating the induction of deletions and insertions in the DNA molecule. In turn, Chaufan et al. (2019) also demonstrated that iprodione has different biological targets, being able to induce mitotic abnormalities and micronuclei of aneugenic and/or clastogenic origin. They explained those results as corresponding to an induction in oxidative stress in Hep-2 cells (human larynx epidermoid carcinoma cells).
According to Bianchi et al. (2015), the genotoxic action of imidacloprid is due to the presence of an electronegative pharmacophore (N-nitroimine substitute = NNO2), located on the nitroguanidine part that binds covalently to the DNA, causing chromosomal damage. Imidacloprid significantly increased the micronuclei index in Tradescantia and the F1 cells of A. cepa (Rodríguez et al. 2015), and chromosomal aberrations in onion (Bianchi et al. 2016). Karabay and Oguz (2005), Demsia et al. (2007), Bianchi et al. (2015), and Iturburu et al. (2018) also identified imidacloprid as a clastogenic agent.
M1 and M2 also significantly increased the frequency of aberrant cells in relation to the control (p < 0.01). This effect was comparable to that exerted by the isolated pesticides (Fig. 2d–e). In group 3, even though 2,4-D at 30 μg/L, the iprodione at 47.26 μg/L and imidacloprid at 4.53 μg/L are genotoxic concerning the control, the frequency of aberrant cells in M3 indicates that it did not induce this effect (Fig. 2f). Iprodione was the only pesticide that, in isolation, induced greater genotoxicity than the mixture (< 0.05) (Fig. 2f). Its effect was reduced in association with other pesticides, indicating an antagonistic effect (Finkler et al. 2022). On the other hand, the low frequency of aberrant cells reported for M3 may also reflect its high cytotoxic action expressed by the inhibition of the mitotic index. A smaller number of dividing cells may make it impossible to observe mitotic and chromosomal abnormalities.
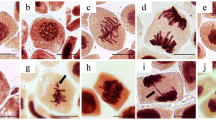
In Fig. 3, the micrographs represent the phases of the normal mitotic cycle of A. cepa and the main abnormalities observed. The frequencies of the specific changes are presented in Table 2. As demonstrated, chromosomal adhesion and micronucleus were the alterations most observed. Chromosomal adhesion is due to abnormal chromatin condensation. The intense connections between chromosomes cause cell death (Fiskesjö 1985). This abnormality is, therefore, an initial step in apoptosis. If it persists until the anaphase, chromosomal adhesion can generate bridges and breaks, manifesting as chromosomal aberrations in the next cell generations (Fioresi et al. 2020). Micronuclei result from unrepaired clastogenic and/or aneugenic damage in the parental cells (Fenech et al. 2020).
Phases of the normal cell cycle of A. cepa (a–e) and the main abnormalities observed (f–j). a Interphase. b Prophase. c Metaphase. d Anaphase. e Telophase. f Micronucleated cell. g–h Metaphase with chromosomal adherence. i Anaphase with chromosomal bridge. f Telophase with fragment and loss chromosomal
Flow cytometry
The flow cytometry technique detects minimal differences in nuclear DNA content and makes it possible to quantify the distribution of cells in the different phases of the cycle. Therefore, the method provides relevant information on the effects of chemical substances on the cell cycle. Flow cytometry also can measure cell death induction (Moreira et al. 2021). In this work, the isolated pesticides and mixtures altered the percentage of cells in sub-G1, G1, S, and G2/M. Higher concentrations of pesticides and M3 caused more significant effects. (Figs. 4 and 5).
Frequency of cells in each phase of the cell cycle (subG1, G0/G1, S, and G2/M) of A. cepa after exposure to pesticides glyphosato (GLY), 2,4-D, iprodione (IPR) and imidacloprid (IMI), and their mixtures (M1, M2, and M3). Group 1: lowest concentrations of each pesticide (50 µg/L GLY, 4 µg/L 2,4-D, 15.96 µg/L IPR, and 0.14 µg/L IMI) and mixture of them (M1). Group 2: intermediate concentrations of each pesticide (65 µg/L GLY, 10 µg/L 2,4-D, 27.14 µg/L IPR, and 1.13 µg/L IMI) and mixture of them (M2). Group 3: highest concentrations of each pesticide (280 µg/L GLY, 30 µg/L 2,4-D, 47.26 µg/L IPR, and 4.53 µg/L IMI) and mixture of them (M3). Asterisks denote statistical significance with respect to the negative control (NC): *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. Treatments followed by number sign differ from each other: #p < 0.05, ##p < 0.01, ###p < 0.001 and ####p < 0.0001
Representative histograms of flow cytometry analysis. a Negative control and b M3. Note in the control the low coefficient of variation of nuclei in G1 and a relatively high percentage of nuclei in S + G2/M, in addition to a low percentage of G1 subparticles. M3 altered all parameters analyzed in flow cytometry. Note the decrease in cells entering division (S + G2/M), the increased coefficients of variation of nuclei in G1, and the percentage of G1 subparticles. The red line indicates the position of the negative control G1 peak. Note the shift of this peak to the left after exposure of A. cepa root cells to M3
The intensity of the fluorescence emitted by isolated chromosomes, chromosomal fragments, cell fragments, nuclear fragments, condensed nuclei, and other smaller particles is lower than the intensity that comes from a normal G1 nucleus. These particles are collectively called G1-subparticles (sub-G1) (Andrade-Vieira et al. 2012). Many of these events are by-products of the cell death process, characterized by nuclear condensation with subsequent DNA fragmentation (Andrade-Vieira et al. 2012; Moreira et al. 2021). Except for glyphosate at 65 μg/L, iprodione at 27.14 μg/L (Fig. 4b), and glyphosate at 280 μg/L (Fig. 4c), the tested pesticides considerably increased the frequency of sub-G1 particles concerning the control (Fig. 4a–c). All mixtures also significantly increased the percentage of G1-subparticles compared to the control (Fig. 4a–c). Compared to the isolated pesticides, M1 did not cause a statistically significant result compared with glyphosate, iprodione, and imidacloprid. However, it caused less effects (p < 0.01) than the 2,4-D at 4 μg/L (Fig. 4a). The effect of none of the isolated pesticides differed statistically from that of M2 (Fig. 4b). The M3 mixture promoted greater damage than the glyphosate (p < 0.001), 2,4-D (p < 0.05) and iprodione (p < 0.0001) isolates, suggesting an additive or synergistic effect. However, the response induced by this mixture was comparable to the isolated use of imidacloprid (Fig. 4c).
The effect of pesticides and mixtures on the frequency of cells in phase G0/G1 is shown in Fig. 4d–e. The lowest concentrations of pesticides and the M1 mixture failed to change the proportion of cells in this phase (Fig. 4d). The 2,4-D at 10 and 30 μg/L and imidacloprid at 1.13 and 4.53 μg/L increased the frequency of cells in G0/G1 with respect to the control, as did M2 and M3 (Fig. 4e–f). This result indicates that in response to the genotoxic action of 2,4-D, imidacloprid, and mixtures, a cascade of signal transduction was triggered, culminating in a transient interruption of the cell cycle in G0/G1, to allow for repair before progressing to the subsequent phase of the cycle (Fioresi et al. 2020; Moreira et al. 2021). These results also show that no isolated pesticide differed statistically from M2 (Fig. 4e), while the M3 mixture promoted a greater adverse effect than iprodione at 47.26 μg/L (p < 0.05) (Fig. 4f).
The lowest concentrations of pesticides and M1 did not interfere with the percentage of cells replicating their DNA (Fig. 4g). Considering the intermediate concentrations, only imidacloprid at 1.13 μg/L reduced the frequency of cells in phase S (Fig. 4h). Glyphosate at 280 μg/L increased the proportion of cells in phase S concerning the control and, in contrast, 2,4-D at 30 μg/L, imidacloprid at 4.53 μg/L and M3 were associated with a decrease in the frequency of cells in this phase (Fig. 4i). The M3 treatment differed statistically from the treatments involving isolated glyphosate, iprodione, and imidacloprid but had a similar effect to 2,4-D (Fig. 4i).
According to Moreira et al. (2021), the accumulation of cells in phase S indicates the blockade of DNA replication by activation of the intra-S checkpoint. Our results are consistent with other studies that reported the same effect for the active ingredient (George and Shukla 2013) and a commercial formulation based on glyphosate (Marc et al. 2004), in vitro and in vivo, respectively. George and Shukla (2013) showed that the accumulation of cells in S was due to increased expression of G1/S cyclins. Marc et al. (2004) presented evidence that the herbicide inhibited the dephosphorylation of the residue of Tyr 15 of CDK1/cyclin B, preventing its activation and G2/M transition because of the interference of the herbicide on DNA replication. In the present study, a direct implication of increasing the percentage of cells in this phase, induced by 280 μg/L of glyphosate, was a significant decrease in the number of cells in G2/M. This result supports the inhibition of the mitotic index. These effects could also partly explain the negative response for the genotoxicity index since mitotic and chromosomal abnormalities are measured on dividing cells.
The effect of pesticides and mixtures on the frequency of cells in the G2/M phase is shown in Fig. 4j–l. Figure 4 j shows that although all pesticides decreased the percentage of cells in G2/M with respect to the control, the M1 mixture did not have the same action. Considering the intermediate concentrations of pesticides (group 2), only 2,4-D at 30 μg/L and mixture M2 reduced the frequency of cells in G2/M (Fig. 4k). And, except for iprodione at 47.26 μg/L, all pesticides decreased the frequency of cells with 4C DNA content. Mixture M3 produced the same effect, comparable to the effect of glyphosate, 2,4-D, and imidacloprid isolated (Fig. 4l).
Checkpoint activation is a mechanism that ensures cell survival. Nevertheless, severe lesions activate cell death mechanisms which can also be measured, in flow cytometry, by FSC, SSC, and fluorescence intensity of nuclei in G1 (IFG1) (Andrade-Vieira et al. 2012; Fioresi et al. 2020). Our findings show that pesticides and mixtures modified these parameters (Table 3). Imidacloprid at 0.14 μg/L (group 1), 2,4-D at 10 μg/L and imidacloprid at 1.13 μg/L (group 2), and 2,4-D at 30 μg/L, imidacloprid at 4.53 μg/L, and mixture M3 (group 3) reduced FSC. Furthermore, the intermediate concentrations (group 2) of all isolated pesticides significantly reduced the fluorescence intensity of G1, which is also evidenced in Fig. 5, where the displacement of the G1 peak to the left (lower fluorescence intensity) is shown. However, the effect of M2 did not differ statistically from that of the control. In group 3, this parameter was reduced by the glyphosate at 280 μg/L, the imidacloprid at 4.53 μg/L and the M3 mixture. SSC, which estimates nuclear complexity, was reduced after exposure to iprodione at 15.96 μg/L and imidacloprid at 0.14 μg/L (group 1), glyphosate at 65 μg/L, 2,4-D at 10 μg/L and imidacloprid at 1.13 μg/L (group 2), and 2,4-D at 30 μg/L, imidacloprid at 4.53 μg/L, and mixture M3 (group 3).
In flow cytometry, the light is deflected and refracted as the laser beam passes through the cell. This scattered light is collected by FSC and SSC photodiodes. FSC is associated with cell size. In contrast, SSC reflects cell granularity or internal complexity, for instance, organelle structure and cytoplasm or nucleus condensation (van der Meer et al. 2010; Wlodkowic et al. 2011). Cell shrinkage is characteristic of the initial stages of apoptosis, which results in decreased intensity of the FSC signal, followed by an increase in the SSC signal (condensation and nuclear fragmentation). When the apoptotic cascade advances, the cells become progressively smaller, and the intensity of the SSC signal also decreases. The development of apoptotic bodies is characterized by a decrease in both FSC and SSC signals (van der Meer et al. 2010; Wlodkowic et al. 2011). Nuclear condensation hinders the entry of propidium iodide and, consequently, the average fluorescence intensity of nuclei in G1 decreases (Andrade-Vieira et al. 2012; Souza et al. 2023).
All pesticides and all mixtures increased the CV concerning the control (p < 0.0001) (Table 3). Injured cells that have been unrepaired and escape the process of cell death contribute to an increase in the frequency of aberrant cells. In flow cytometry, these cells have been identified by an increase of the CV of the nuclei in G1, which can be associated with alterations that lead to an unequal distribution of genetic material among daughter cells, such as micronuclei and chromosomal adhesions (Bickham et al. 1992; Biradar and Rayburn 1995; Rayburn and Wetzel 2002; Fioresi et al. 2020; Souza et al. 2023). These were the main alterations induced by pesticides isolated and mixtures.
Final considerations
Our study showed that the maximum concentrations of 2,4-D and glyphosate allowed by Brazilian legislation caused toxicogenetic damage in A. epa. This result is an alert to other organisms. Brovini et al. (2021) performed an environmental risk assessment focused on three-bestseller pesticides in Brazil. Glyphosate presented the most significant environmental risk due to its elevated environmental concentrations and the toxic effects induced in aquatic organisms (Daphnia magna, Oncorhynchus mykiss, and Raphidocelis subcapitata). Only glyphosate concentrations above 1 μg/L would represent a high risk. In contrast, environmental concentrations of 2,4-D higher than 10 μg/L would cause significant risk. Based on these results, the authors concluded that the maximum values authorized by the legislation do not protect aquatic ecosystems and should be critically reviewed.
The analysis of the adverse effects of the combinations of pesticides is a more realistic scenario of exposure to the isolated pesticides. The results of this study showed that the M3 mixture caused the greatest deleterious effects on A. cepa. Therefore, taking M3 as an example, we suggest an association between the induced changes when all parameters used are analyzed together. Thus, radicular growth inhibition can be explained by the cell cycle blockage in G1 and induction of cell death. In turn, this cytotoxic action was confirmed by (a) inhibition of the mitotic index, with the consequent decrease in the frequency of cells in G2/M; (b) increased frequency of cells in the G1 sub-phase; (c) complexity and size of nucleus; and (d) reduction in fluorescence intensity. In general, the interaction of pesticides in the M3 mixture resulted in an additive or synergistic effect for cytotoxicity parameter. Due to it, the total frequency of aberrant cells did not differ from the control. However, micronuclei and chromosomal adhesions were significantly induced, contributing to an increase in CV.
Attention should be given to the M1 and M2 mixtures with the lowest concentrations of pesticides, which triggered a positive response to genotoxicity. Their effect was equivalent to those of pesticides alone, indicating that the activation of checkpoints for DNA repair and cell death, demonstrated in flow cytometry, were not sufficient to maintain cell integrity.
Considering the toxic action of pesticide mixtures investigated in this study, we only found studies on binary combinations in the scientific literature. Finkler et al. (2022) investigated the effects of 2,4-D mixed with glyphosate in two different combinations: environmental concentrations (average concentrations applied in the field) and doses relevant to human health. Both associations caused a significant decrease in the mitotic index of A. cepa and a possible additive or synergistic effect due to a considerable increase in aberrant cells with respect to the herbicides applied in isolation. Carvalho et al. (2020) showed, through the comet assay, that glyphosate associated with different acid formulations 2,4-dichlorophenoxyacetic acid (2,4-D, 2,4-D-amine and 2,4-D-ister) increased the genotoxicity index on the erythrocytes of the fish species Cnesterodon decemmaculatus. The mixtures glyphosate + 2,4-D and glyphosate + 2,4-D-amine showed a synergistic effect and the mixture glyphosate + 2,4-D-ester had an antagonistic effect.
Fioresi et al. (2020) found that the mixture of iprodione and imidacloprid in field concentrations also induced cytogenotoxic damage in A. cepa. We highlight that Brazil’s laws do not define the levels of iprodione and imidacloprid allowed in the water. However, non-target organisms can be harmed by these pesticides either alone or when associated with other ones.
Finally, EU directive 2020/2184 sets a limit of 5 µg/L for pesticide mixtures in drinking water. Meanwhile, Brazilian legislation only establishes maximum values for some isolated pesticides. Hence, the experiments with A. cepa aided in comprehending the impacts of pesticide combinations, taking into account significant levels in the water ecosystem.
Data availability
The data that support the funding of this study are available from the corresponding author, upon reasonable request.
References
Albuquerque AF, Ribeiro JS, Kummrow F, Nogueira AJA, Montagner CC, Umbuzeiro GA (2016) Pesticides in Brazilian freshwaters: a critical review. Environ Sci J Integr Environ Res: Process Impacts 18:779–787. https://doi.org/10.1039/c6em00268d
Alves TA, Pinheiro PF, Praça-Fontes MM, Andrade-Vieira LF, Lourenço MP, Lage MR, Alves TA, Cruz FA, Carneiro JWM, Ferreira A, Soares TCB (2021a) Bioactivity and molecular properties of phenoxyacetic acids derived from eugenol and guaiacol compared to the herbicide 2,4-D. An Acad Bras Cienc 93:e20191368. https://doi.org/10.1590/0001-3765202120191368
Alves TA, Roberto CEO, Pinheiro PF, Alves TA, Henrique MKC, Ferreira A, Clarindo WR, Praça-Fontes MM (2021b) Searching an auxinic herbicide to use as positive control in toxicity assays. An Acad Bras Cienc 93:e20181262. https://doi.org/10.1590/0001-3765202120181262
Andrade-Vieira LF, Campos JMS, Davide LC (2012) Effects of spent pot liner on mitotic activity and nuclear DNA content in meristematic cells of Allium cepa. J Environ Manag 107:140–146. https://doi.org/10.1016/j.jenvman.2012.04.008
Aragão FB, Queiroz VT, Ferreira A, Costa AV, Pinheiro PF, Carrijo TT, Vieira LFA (2017) Phytotoxicity and cytotoxicity of Lepidaploa rufogrisea (Asteraceae) extracts in the plant model Lactuca sativa (Asteraceae). Rev Biol Trop 65:435–443. https://doi.org/10.15517/rbt.v65i2.25696
Aragão FB, Bernardes PM, Ferreira A, Ferreira MFS, Andrade-Vieira LF (2019) Cyto(geno)toxicity of commercial fungicides based on the active compounds tebuconazole, difenoconazole, procymidone, and iprodione in Lactuca sativa L. meristematic cells. Water Air Soil Pollut 230:25. https://doi.org/10.1007/s11270-019-4080-6
Aranha A, Rocha L (2019) “Coquetel” com 27 agrotóxicos foi achado na água de 1 em cada 4 municípios. Agência Pública/ Repórter Brasil. Avaliable in https://portrasdoalimento.info/2019/04/15/coquetel-com-27-agrotoxicos-foi-achado-na-agua-de-1-em-cada-4-municipios/. Accessed August 2022
Araújo IMM, Oliveira AGRC (2017) Agronegócio e agrotóxicos: impactos à saúde dos trabalhadores agrícolas no Nordeste brasileiro. Trab Educ Saúde 15:117–129. https://doi.org/10.1590/1981-7746-sol00043
Barbosa AMC, Solano MLM, Umbuzeiro GA (2015) Pesticides in drinking water — the Brazilian monitoring program. Front Public Health 3:246. https://doi.org/10.3389/fpubh.2015.00246
Battaglin WA, Meyer MT, Kuivila KM, Dietze JE (2014) Glyphosate and its degradation product AMPA occur frequently and widely in U.S. soils, surface water, groundwater, and precipitation. J Am Water Resour Assoc 50:275–290. https://doi.org/10.1111/jawr.12159
Bernardes PM, Andrade-Vieira LF, Aragão FB, Ferreira A, Ferreira MFS (2019) Toxicological effects of commercial formulations of fungicides based on procymidone and iprodione in seedlings and root tip cells of Allium cepa. Environ Sci Pollut Res 26:21013–21021. https://doi.org/10.1007/s11356-019-04636-x
Bianchi J, Cabral-de-Mello DC, Marin-Morales MA (2015) Toxicogenetic effects of low concentrations of the pesticides imidacloprid and sulfentrazone individually and in combination in vitro tests with HepG2 cells and Salmonella typhimurium. Ecotoxicol Environ Safet 120:174–183. https://doi.org/10.1016/j.ecoenv.2015.05.040
Bianchi J, Fernandes TCC, Marin-Morales MA (2016) Induction of mitotic and chromosomal abnormalities on Allium cepa cells by pesticides imidacloprid and sulfentrazone and the mixture of them. Chemosphere 144:475–483. https://doi.org/10.1016/j.chemosphere.2015.09.021
Bickham JW, Sawin VL, Burton DW, McBee K (1992) Flow cytometric analysis of the effects of triethylenemelamine on somatic and testicular tissues of the rat. Cytom 13:368–373. https://doi.org/10.1002/cyto.990130406
Biradar DP, Rayburn AL (1995) Flow cytogenetic analysis of whole cell clastogenicity of herbicides found in groundwater. Arch Environ Contam Toxicol 28:13–17. https://doi.org/10.1007/BF00213963
Bortoluzzi EC, Rheinheimer DS, Gonçalves CS, Pellegrini JBR, Maroneze AM, Kurz MHS, Bacar NM, Zanella R (2007) Investigation of the occurrence of pesticides residues in rural wells and surface water following application to tobacco. Quím Nova 30:1872–1876. https://doi.org/10.1590/S0100-40422007000800014
Brovini EM, Deus BCT, Vilas-Boas JA, Quadra GR, Carvalho L, Mendonça RF, Pereira RO, Cardoso SJ (2021) Three-bestseller pesticides in Brazil: Freshwater concentrations and potential environmental risks. Sci Total Environ 771–144754. https://doi.org/10.1016/j.scitotenv.2020.144754
Bukowska (2006) Toxicity of 2,4-dichlorophenoxyacetic acid — molecular mechanisms. Polish J Environ Stud 15:365–374
Carneiro LS, Martínez LC, Gonçalves WG, Santana LM, Serrão JM (2020) The fungicide iprodione affects midgut cells of non-target honey bee Apis mellifera workers. Ecotoxicol Environ Saf 189:109991. https://doi.org/10.1016/j.ecoenv.2019.109991
Carvalho WF, Arcaute CR, Torres L, Silva DM, Soloneski S, Larramendy ML (2020) Genotoxicity of mixtures of glyphosate with 2,4-dichlorophenoxyacetic acid chemical forms towards Cnesterodon decemmaculatus (Pisces, Poeciliidae). Environ Sci Pollut Res 27:6515–6525. https://doi.org/10.1007/s11356-019-07379-x
Castro Berman M, Marino DJG, Quiroga MV, Zagarese H (2018) Occurrence and levels of glyphosate and AMPA in shallow lakes from the Pampean and Patagonian regions of Argentina. Chemosphere 200:513. https://doi.org/10.1016/j.chemosphere.2018.02.103
Chaufan G, Coalova I, Molina MCR (2014) Glyphosate commercial formulation causes cytotoxicity, oxidative ffects, and apoptosis on human cells: differences with its active ingredient. Int J Toxicol 33:29–38. https://doi.org/10.1177/1091581813517906
Chaufan G, Galvano C, Nieves M, Mudry MD, Molina MCR, Andrioli NB (2019) Oxidative response and micronucleus centromere assay in HEp-2 cells exposed to fungicide iprodione. Chem Res Toxicol 32:745–752. https://doi.org/10.1021/acs.chemrestox.8b00405
CONAMA – Conselho Nacional do Meio Ambiente. Resolução nº 357, de 17 de março de 2005. “Dispõe sobre a classificação dos corpos d’água e as diretrizes ambientais para sua classificação, bem como estabelece as condições e padrões de lançamento de efluentes e dá outras providências.” Diário Oficial da União, seção 1, Brasília, DF, n. 053, pp. 58–63, 18 mar. 2005 <http://www.siam.mg.gov.br/sla/download.pdf?idNorma=2747>. Accessed 10 June 2021
Demsia G, Vlastos D, Goumenou M, Matthopoulos DP (2007) Assessment of the genotoxicity of imidacloprid and metalaxyl in cultured human lymphocytes and rat bonemarrow. Mutat Res 634:32–39. https://doi.org/10.1016/j.mrgentox.2007.05.018
Directive (EU) (2020) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption. Off J Eur Union 435:1–62. http://data.europa.eu/eli/dir/2020/2184/oj. Accessed July 2023
Felisbino K, Santos-Filho R, Piancini LDS, Cestari MM, Leme DM (2018) Mesotrione herbicide does not cause genotoxicity, but modulates the genotoxic effects of atrazine when assessed in mixture using a plant test system (Allium cepa). Pestic Biochem Physiol 150:83–88. https://doi.org/10.1016/j.pestbp.2018.07.009
Fenech M, Knasmueller S, Bolognesi C, Holland N, Bonassi S, Kirsch-Volders M (2020) Micronuclei as biomarkers of DNA damage, aneuploidy, inducers of chromosomal hypermutation and as sources of pro-inflammatory DNA in humans. Mutat Res/Rev Mutat Res 786:108342. https://doi.org/10.1016/j.mrrev.2020.108342
Finkler M, Rodrigues GZP, Kayser JM, Ziulkoski AL, Gehlen G (2022) Cytotoxic and genotoxic effects induced by associated commercial glyphosate and 2,4-D formulations using the Allium cepa bioassay. J Environ Sci Health Part B. https://doi.org/10.1080/03601234.2022.2034432
Fioresi VS, Vieira BCR, Campos JMS, Souza TS (2020) Cytogenotoxic activity of the pesticides imidacloprid and iprodione on Allium cepa root meristem. Environ Sci Pollut Res 27:28066–28076. https://doi.org/10.1007/s11356-020-09201-5
Fiskesjö G (1985) The Allium test as a standard in environmental monitoring. Hereditas 102:99–112. https://doi.org/10.1111/j.1601-5223.1985.tb00471.x
Flores-Céspedes F, Figueredo-Flores CI, Daza-Fernández I, Vidal-Peña F, Villafranca-Sánchez M, Fernández-Pérez M (2012) Preparation and characterization of imidacloprid lignin–polyethylene glycol matrices coated with ethylcellulose. J Agric Food Chem 60:1042–1051. https://doi.org/10.1021/jf2037483
Fossen M (2006) Environmental fate of imidacloprid. Environmental Monitoring. Department of Pesticide Regulation. Available Available in http://cues.cfans.umn.edu/old/pollinators/pdf-pesticides/2006CAImidclprdfate.pdf
Gadeva P, Dimitrov B (2008) Genotoxic effects of the pesticides Rubigan, Omite and Rovral in root-meristem cells of Crepis capillaris L. Mutat Res 652:191–197. https://doi.org/10.1016/j.mrgentox.2008.02.007
Galbraith DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabady E (1983) Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220:1049–1051. https://doi.org/10.1126/science.220.4601.1049
George J, Shukla Y (2013) Emptying of intracellular calcium pool and oxidative stress imbalance are associated with the glyphosate-induced proliferation in human skin keratinocytes HaCaT cells. ISRN Dermatol 2013:25180. https://doi.org/10.1155/2013/825180
Ghosh M, Bhadra S, Adegoke A, Bandyopadhyay M, Mukherjee A (2015) MWCNT uptake in Allium cepa root cells induces cytotoxic and genotoxic responses and results in DNA hyper-methylation. Mutat Res/Fundam Mol Mech Mutagen 774:49–58. https://doi.org/10.1016/j.mrfmmm.2015.03.004
Ghosh M, Jana A, Sinha S, Jothiramajayam M, Nag A, Chakraborty A, Mukherjee A, Mukherjee A (2016) Effects of ZnO nanoparticles in plants: cytotoxicity, genotoxicity, deregulation of antioxidant defenses, and cell-cycle arrest. Mutat Res/Genet Toxicol Environ Mutagen 807:25–32. https://doi.org/10.1016/j.mrgentox.2016.07.006
Grabinska-Sota E, Wisniowska E, Kalka (2003) Toxicity of selected synthetic auxines—2,4-D and MCPA derivatives to broad-leaved and cereal plants. Crop Prot 22:355–360. https://doi.org/10.1016/S0261-2194(02)00178-3
Hanson B, Bond C, Buhl K, Stone D (2015) Pesticide half-life fact sheet. National Pesticide Information Center, Oregon State University Extension Services. Available in https://npic.orst.edu/factsheets/half-life.html. Accessed July 2023
IARC – International Agency for Research Cancer (2017) World Health Organization. Monographs on the identification of carcinogenesis hazards to humans. 112. https://publications.iarc.fr/549. Accessed 10 June 2022
IARC – International Agency for Research Cancer (2018) World Health Organization. Monographs on the identification of carcinogenesis hazards to humans. DDT, Lindane, and 2,4-D. 113. https://publications.iarc.fr/550. Accessed 10 June 2022
IBAMA – Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis, Relatórios de Comercialização de Agrotóxicos – Boletim Anual de Produção, Importação, Exportação e Vendas de Agrotóxicos no Brasil. https://www.gov.br/ibama/pt-br/assuntos/quimicos-e-biologicos/agrotoxicos/relatorios-de-comercializacao-de-agrotoxicos#boletinsanuais. Accessed 12 November 2022
Iturburu FG, Simoniello MF, Medici S, Panzeri AM, Menone ML (2018) Imidacloprid causes DNA damage in fish: clastogenesis as a mechanism of genotoxicity. Bull Environ Contam Toxicol 100:760–764. https://doi.org/10.1007/s00128-018-2338-0
Karabay NU, Oguz MG (2005) Cytogenetic and genotoxic effects of the insecticides, imidacloprid and methamidophos. Genet Mol Res 4:653–662
Kier LD, Kirkland DJ (2013) Review of genotoxicity studies of glyphosate and glyphosate-based formulations. Crit Rev Toxicol 43:283–315. https://doi.org/10.3109/10408444.2013.770820
MAPA - Ministério da Agricultura, Pecuária e Abastecimento. Sistema de Agrotóxicos Fitossanitários. https://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons. Accessed 16 July 2022
Marc J, Bellé R, Morales J, Cormier P, Mulner-Lorillon O (2004) Formulated glyphosate activates the dna-response checkpoint of the cell cycle leading to the prevention of G2/M transition. Toxicol Sci 82:436–442. https://doi.org/10.1093/toxsci/kfh281
Marcato ACC, Souza CP, Fontanetti CS (2017) Herbicide 2,4-D: a review of toxicity on non-target organisms. Water Air Soil Pollut 228:1–12. https://doi.org/10.1007/s11270-017-3301-0
Mazzeo DEC, Fernandes TCC, Levy CE, Fontanetti CS, Marin-Morales MA (2015) Monitoring the natural attenuation of a sewage sludge toxicity using the Allium cepa test. Esological Indic 56:60–69. https://doi.org/10.1016/j.ecolind.2015.03.026
Mercado SAS, Caleño JDQ (2020) Cytotoxic evaluation of glyphosate, using Allium cepa L. as bioindicator. Sci Total Environ 700:134452. https://doi.org/10.1016/j.scitotenv.2019.134452
Mondal S, Kumar M, Haque S, Kundu D (2017) Phytotoxicity of glyphosate in the germination of Pisum sativum and its effect on germinated seedlings. Environ Health Toxicol 32:e2017011. https://doi.org/10.5620/eht.e2017011
Monteiro MS, Rodriguez E, Loureiro J, Mann RM, Soares AMVM, Santos C (2010) Flow cytometric assessment of Cd genotoxicity in three plants with different metal accumulation and detoxification capacities. Ecotoxicol Environ Saf 73:1231–1237. https://doi.org/10.1016/j.ecoenv.2010.06.020
Moreira NCS, Lima JEBF, Sakamoto-Hojo ET (2021) Citometria de fluxo – fundamentos, aplicações e análise do ciclo celular e apoptose. In: Salvadori DMF, Takahashi CS, Grisolia CK (eds) Da Toxicogenética à Toxicogenômica. Santos, R.A. 1 ed – Rio de Janeiro: Atheneu, p 388
MS - Ministério da Saúde. Portaria no. 2.914 de 12 de dezembro de (2011) Dispõe sobre os procedimentos de controle e de vigilância da qualidade da água para consumo humano e seu padrão de potabilidade. Diário Oficial da União, Brasília, 14 de dezembro de 2011. https://bvsms.saude.gov.br/bvs/saudelegis/gm/2011/prt2914_12_12_2011.html. Accessed 10 April 2020
NPCI - National Pesticide Information Center (2011) Oregon University State. 2,4-D Technical Fact Sheet. Available in http://npic.orst.edu/factsheets/archive/2,4-DTech.html. Accessed July 2023
Piotrowicz-Cieślak AI, Adomas B, Michalczyk DJ (2010) Different glyphosate phytotoxicity to seeds and seedlings of selected plant species. Pol J Environ Stud 19:123–129
Queiroz VT, Azevedo MM, Quadros IPS, Costa AV, Amaral AA, Amaral GM, Santos DA, Juvanhol RS, Telles LAA, Santos AR (2018) Environmental risk assessment for sustainable pesticide use in coffee production. J Contam Hydrol 219:18–27. https://doi.org/10.1016/j.jconhyd.2018.08.008
Rank J, Nielsen MH (1994) Evaluation of the Allium anaphase-telophase test in relation to genotoxicity screening of industrial wastewater. Mutat Res 312:17–24. https://doi.org/10.1016/0165-1161(94)90004-3
Rayburn AL, Wetzel JB (2002) Flow cytometric analyses of intraplant nuclear DNA content variation induced by sticky chromosomes. Cytometry 49:36–41. https://doi.org/10.1002/cyto.10135
Rodrigues LB, Costa GG, Thá EL, Silva LR, Oliveira R, Leme DM, Cestari MM, Grisolia CK, Valadares MC, Oliveira GAR (2019) Impact of the glyphosate-based commercial herbicide, its components and its metabolite AMPA on non-target aquatic organisms. Mutat Res 842:94–101. https://doi.org/10.1016/j.mrgentox.2019.05.002
Rodríguez YA, Christofoletti CA, Pedro J, Bueno OC, Fontanetti CS (2015) Allium cepa and Tradescantia pallida bioassays to evaluate effects of the insecticide imidacloprid. Chemosphere 120:438–442. https://doi.org/10.1016/j.chemosphere.2014.08.022
Samojeden CG, Pavan FA, Rutkoski CF, Folador A, Fré SP, Müller C, Hartmann PA, Hartmann M (2022) Toxicity and genotoxicity of imidacloprid in the tadpoles of Leptodactylus luctator and Physalaemus cuvieri (Anura: Leptodactylidae). Sci Rep 12:11926. https://doi.org/10.1038/s41598-022-16039-z
Sequinatto L, Reichert JM, Santos DR, Reinert DJ, Copetti ACC (2013) Occurrence of agrochemicals in surface waters of shallow soils and steep slopes cropped to tobacco. Quím Nova 36:768–772
Shuma JM, Quick WA, Raju MV, Hsiao AI (1995) Germination of seeds from plants of Avenafatua L. treated with glyphosate. Weed Res 35:249–255. https://doi.org/10.1111/j.1365-3180.1995.tb01787.x
Silva LCM, Moreira RA, Pinto TJS, Ogura AP, Yoshii MPC, Lopes LFP, Montagner CC, Goulart BV, Daam MA, Espíndola ELG (2020) Acute and chronic toxicity of 2,4-D and fipronil formulations (individually and in mixture) to the Neotropical cladoceran Ceriodaphnia silvestrii. Ecotoxicology 29:1462–1475. https://doi.org/10.1007/s10646-020-02275-4
Souza TS, Barone LSF, Lacerda D, Vergilio CS, Oliveira BCV, Almeida MG, Thompson F, Rezende C (2021) Cytogenotoxicity of the water and sediment of the Paraopeba River immediately after the iron ore mining dam disaster (Brumadinho, Minas Gerais, Brazil). Sci Total Environ 775:145193. https://doi.org/10.1016/j.scitotenv.2021.145193
Souza VV, Silva Souza T, Campos JMS, Oliveira LA, Ribeiro YM, Melo Hoyos DC, Xavier RMP, Charlie-Silva I, Lacerda SMSN (2023) Ecogenotoxicity of environmentally relevant atrazine concentrations: a threat to aquatic bioindicators. Pestic Biochem Physiol 189:105297. https://doi.org/10.1016/j.pestbp.2022.105297
Truta E, Vochita G, Rosu C, Zamfirache M-M, Olteanu Z (2011) Evaluation of roundup-induced toxicity on genetic material and on length growth of barley seedlings. Acta Biol Hung 62:290–301. https://doi.org/10.1556/ABiol.62.2011.3.8
Türkoğlu S (2012) Determination of genotoxic effects of chlorfenvinphos and fenbuconazole in Allium cepa root cells by mitotic activity, chromosome aberration, DNA content, and comet assay. Pestic Biochem Physiol 103:224–230. https://doi.org/10.1016/j.pestbp.2012.06.001
US Environmental Protection Agency (USEPA) (1996) Seed germination/root elongation toxicity test, OPPTS 850.4200, EPA 712/C-96/154. Ecological effects test guidelines. EPA 712-C-96-154. Washington, DC
US Environmental Protection Agency (EPA) (2007) Office of Prevention, Pesticides and Toxic Substances. Iprodione drinking water assessment, including proposed new use on pistachio (IR4) and label revisions affecting rates for strawberries, stone fruits, and grapes. DP Barcode: D285550. p 22. Available in https://ordspub.epa.gov/ords/pesticides/f?p=CHEMICALSEARCH:7::::1,3,31,7,12,25:P3_XCHEMICAL_ID:2597. Accessed July 2023
van der Meer FJ, Faber DJ, Aalders MCG, Poot AA, Vermes I, van Leeuwen TG (2010) Apoptosis- and necrosis-induced changes in light attenuation measured by optical coherence tomography. Lasers Med Sci 25:259–267. https://doi.org/10.1007/s10103-009-0723-y
Vieira C, Marcon C, Droste A (2022) Phytotoxic and cytogenotoxic assessment of glyphosate on Lactuca sativa L. Braz J Biol 84. https://doi.org/10.1590/1519-6984.257039
WHO - World Health Organization. Environmental Health Criteria 5 (1985) Guide to short-term tests for detecting mutagenic and carcinogenic chemicals. Geneva, p 208. Accessed in https://wedocs.unep.org/handle/20.500.11822/29495
Wlodkowic D, Telford W, Skommer J, Darzynkiewicz Z (2011) Apoptosis and beyond: cytometry in studies of programmed cell death. Methods Cell Biol 103:55–98. https://doi.org/10.1016/B978-0-12-385493-3.00004-8
Funding
This work was supported by the Fundação de Amparo à Pesquisa do Espírito Santo — FAPES (No. 14/2019-PROCAP 2020). Luanna Alves Miranda has received from FAPES Master fellowship 096/2020.
Author information
Authors and Affiliations
Contributions
Conceptualization was performed by Tatiana da Silva Souza and Victor Ventura de Souza. Material preparation, data collection, and analysis were performed by Luanna Alves Miranda, Renata Alice Campos, and José Marcello Salabert de Campos. The first draft of the manuscript was written by Luanna Alves Miranda. Writing — review and editing: Tatiana da Silva Souza. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Supervision: Tatiana da Silva Souza.
Corresponding author
Ethics declarations
Ethics approval
This is an original article that did not use other information that requires ethical approval.
Consent to participate
All authors participated in this article.
Consent for publication
All authors have given consent to the publication of this article.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Miranda, L.A., de Souza, V.V., Campos, R.A. et al. Phytotoxicity and cytogenotoxicity of pesticide mixtures: analysis of the effects of environmentally relevant concentrations on the aquatic environment. Environ Sci Pollut Res 30, 112117–112131 (2023). https://doi.org/10.1007/s11356-023-30100-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30100-y