Abstract
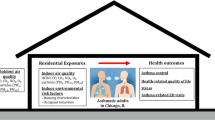
This investigation explored the association between indoor environmental factors and childhood asthma in Yancheng, China. Asthma case (201 children with recurrent asthma) and control cohorts (242 healthy subjects) were recruited from a Traditional Chinese Medical (TCM) Hospital in Yancheng city, based on the results of an ISAAC questionnaire. Questionnaires regarding environmental risk factors were completed by the child’s primary caregivers. To compare data on environmental VOCs and formaldehyde contents between asthma and control cohorts, we passively conducted a 10-day indoor and outdoor sampling. Breastfeeding was a major protective indoor environmental factor for recurrent asthma (adjusted odds ratio [aOR]: 0.368, 95% confidence interval [CI]: 0.216–0.627). Our analysis revealed that childhood recurrent asthma was intricately linked to a family history of asthma. Recurrent asthma was also associated with passive smoking [aOR2.115 (95%-CI 1.275–3.508)]. Analogous correlations were observed between household renovation or new furniture introduction and recurrent asthma [aOR3.129(95%-CI1.542–6.347)]. Benzene and formaldehyde were present in all examined homes. Enhanced benzene and formaldehyde concentrations were strongly evident among asthma versus control cohorts, and they were strongly correlated with augmented recurrent asthma risk. Home environment heavily regulates incidences of childhood recurrent asthma. Hence, actions against the indoor environmental risk factors described in this study may assist in the prevention of recurrent asthma among children.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Asthma is a common chronic respiratory condition marked with persistent airway inflammation and variable expiratory airflow limitation (Kuang et al. 2021). Over the past 20 years, there has been a steep global rise in the frequency of asthma and other diseases, affecting over 339 million people, particularly, children (Ambade et al. 2022; Kodate et al. 2016; Zhang et al. 2021). Asthma not only causes premature death, but also reduces quality of life of people involved. Relative to developed countries, low- and middle-income countries suffer disproportionally from the most severe asthma incidences and other diseases (Asher et al. 2006; Sankar et al. 2023). China is among the largest middle-income countries, and over the past few decades, there has been a substantial uptick in asthma cases in the country (Zhang et al. 2013).
In recent decades, numerous epidemiology investigations provided insight into the associations between asthma incidences and exposure to air pollutants. Some scholars reported that certain outdoor air pollution, such as living near major roads and particulate matter (PM), are potent asthma risk factors (Ambade and Ghosh 2013; Kumar et al. 2020). Alternately, children raised in urban and rural regions exhibit a reduced risk of allergies (Pennington et al. 2018). Additionally, house renovation and new furniture introduction were also reported as strong risk factors for childhood asthma during early childhood and pregnancy (Zhang et al. 2018). Several other factors, such as pets at home and family history of asthma, also contribute to childhood asthma (Arif 2018; Julia et al. 2020; Kansen et al. 2020; Zhang et al. 2020). Nevertheless, the most critical childhood asthma risk factors have not been sufficiently elucidated.
Indoor air quality, namely, temperature, humidity, and air pollutants, is critical regulator of most respiratory disease development. At present, a majority of individuals spend 90% of their time indoors (Athanasios 2019; Wang et al. 2021a). Volatile organic compounds (VOC) are commonly found in dwellings (Huang et al. 2021; Yang et al. 2020) and have already been linked to inflammatory response within airways of asthmatics and other respiratory challenges (Huang et al. 2017; Peel et al. 2020). Prior investigations revealed that reduced VOC household products potentially emit air pollutions, namely, VOCs and formaldehyde, which are potential risk factors for asthma, rhinitis, and eczema development among children (Chang et al. 2019; Franck et al. 2014). Nevertheless, the impact of VOCs and formaldehyde on childhood asthma remains undetermined.
To our knowledge, there are limited reports on the relationship between indoor environment and childhood asthma in China (Balram Ambade 2022, Luo et al. 2018, Sun et al. 2019). Moreover, all investigations did not confirm a link between the two (Hwang et al. 2011). This study is distinct from other asthma investigations in that it specifically investigated the association between indoor environment and recurrent asthma among children in Yancheng in Southeast of China. We utilized a hospital-based case-control design to identify long-term pediatric respiratory health impact factors, such as household decoration, living habits, dwelling characteristics, VOC and formaldehyde concentrations, indoor room temperature (RT), and relative air humidity (RAH). We also explored the influence of indoor microenvironments, such as breastfeeding, passive smoking, house decorations, family history of asthma, pets at home, and new furniture introduction on children with recurrent asthma versus healthy controls (Eassey et al. 2018; Gilbert et al. 2015). Additionally, we compared both indoor and outdoor VOC and formaldehyde contents to elucidate possible VOC- and formaldehyde-induced effects and quantify exposure risk levels. Enhanced understanding of the close relation between indoor environment and childhood asthma may benefit the prevention of childhood asthma caused by indoor environmental pollutants.
Methods
Ethical statement
This work received ethical approval from the Chinese TCM Hospital and obtained informed consents from all participants or guardians prior to the commencement of the study.
Study area and period
This hospital-based case-control prospective study was conducted in the TCM-based district hospital in Yancheng, China, between July 2021 and February 2023.
Study design
The present prospective study (hospital-based case-control study) was conducted at the age of 3 to 6 years.
Source and study population
Our sample population included all children between 3 and 6 years who lived in the Yancheng city, who also sought care at the TCM Hospital during our data collection time. The TCM Hospital selected in this study is famous for treatment with children asthma, especially with childhood recurrent asthma in Yancheng City and other neighboring cities. The number of TCM hospital visitors was significantly more than other hospitals in preschool-age children (Huang et al. 2013). This specific age group was selected due to a lack of existing research on this age group, and due to this population spending more time inside the bedroom.
Inclusion and exclusion criteria
Included patients
Children between the ages of 3 and 6 years visited the participating hospital. Children with a history of respiratory symptoms (for example, wheezing, shortness of breath, chest tightness, or orthopnea) and recurrent asthma diagnosis by pediatricians based on the Global Initiative for Asthma criteria. Control children were selected from general outpatient pediatrics; they were from the same age group as the asthmatic children, they had no respiratory disease diagnosis, and they, much like the asthmatic children, were recruited from regions away from industrial and traffic roads, thereby avoiding enhanced VOCs exposure. A team of physicians and two highly trained interviewers obtained patient information using standard questionnaire. The average ages of the asthmatic and non-asthmatic children were 5.02 ± 1.4 and 5.11 ± 1.3 (mean ± standard deviation), respectively.
Excluded patients
Among those excluded from analysis were children with acute bronchiolitis, pneumonia, stridor, and other respiratory diseases. We also eliminated healthy children who suffered from cold or cough within 6 months of study. Asthmatic children were eliminated from controls. Also excluded were children or primary caregivers who were severely ill or suffered from hearing or speech impairments.
Sampling
Sample size
We employed the single population equation to determine the sample population needed for this study. A prior epidemiological investigation indicated that the recurrence asthma incidence among children < 5 years of age was between 9.8 and 33.1%.(Ibrahim et al. 2021; Stanford et al. 2012) Herein, we selected the intermediate value 16% as the expected prevalence and maintained a margin of error of 5%. Our equation was as follows:
whereby n represented the minimum required sample size.
p denoted an estimate of the recurrent asthma prevalence.
Z represented the standard normal variable at (1−α)% confidence level, with mostly 0.05 α, i.e., with 95% CI (z = 1.96).
D denoted tolerated error margin (%):
Sampling procedures
Among the 5 Class III Grade A hospitals within our district, we selected 3 using simple lottery. Subsequently, we acquired prospective eligible patient information until the aforementioned sample population was reached.
Study variables
Dependent variables: recurrent asthmatic children (Yes/No) between 3 and 6 years of age.
Questionnaires
This investigation employed a questionnaire to evaluate children health based on the International Study of Asthma and Allergy in Childhood (ISAAC) criteria. The questionnaire was provided to the parents of 443 children (Madureira et al. 2015), and it included a set of questions related to the potential risk factors for asthma recurrence, namely, gender (boy or girl), age, birth weight (kg), premature delivery, breastfeeding period, parent education, family asthma history (Yes/No), passive smoking (Yes/No), and so on. We also collected data on the residential environment, such as house location, traffic exposure (Yes/No), quantity of household members (n), household redecoration/new furniture introduction (Yes/No), presence of potted plants (Yes/No), and presence of pets (dog, cat, hamster) (Yes/No).
The respiratory health was assessed using the following medical questions: “ever been told you have asthma” and “still have asthma”. A “yes” answer to both questions was regarded as having asthma. Conversely, “don’t know” and “refused” answers were regarded as missing data.
Environmental exposure measurements
We collected indoor air samples from 443 households for exposure evaluation. Our analytical approach, as well as quality control and assurance evaluations, was done as follows:
Air samples and exposure assessment for VOCs and formaldehyde
We obtained indoor and outdoor air samples from 443 households for exposure evaluation. In brief, two professional building examiners visited each child’s bedroom and other rooms where child spent the most time for equipment installation. Prior to obtaining an air sample, windows and doors were left open for 30 min to facilitate air circulation. Then, they were kept closed for at least 5 h. The air sample of VOCs and formaldehyde were collected by activated charcoal (DDY-1.5, Xingyu, China) in the living room at 150 cm above floor level and at least 30 cm from the wall. All samplings were conducted for 7 consecutive days at 0.5 L/min. The VOCs concentration was measured by a gas chromatograph with a mass spectrometer equipped a flame ionization detector (GC-MS/FID). The formaldehyde contents were determined by a visible spectrophotometry using the 3-methyl-2-benzothiazolinone hydrazine method. Moreover, reviewers were blinded to the children disease condition (asthma vs. control). The outdoor sampler was positioned within a plastic box that was hung outside a house window, away from exhaust ducts and heat sources. It was also protected from rain and direct solar irradiation. We also measured children bedroom temperature (°C) and RAH (%) once every hour over a 1-week period, using a hygro-anemometer (Model: HHC261, Omega Engineering, Inc., Norwalk, CT, USA) (MitecSatelite-TH, Mitec Instrument AB, Säffle, Sweden).
Air samples and exposure assessment in relation to RT, RAH, and CO2 content
We recorded the RT (°C), RAH (%), and CO2 contents of bedrooms during a 90-min period when the children were typically present in the room and computed the averages. These measurements were simultaneously taken using Q-track, a direct reading apparatus, accompanied with a data logger (TSI Incorporated, ST Paul, MN, USA). The machine was positioned at 1.2 m above the floor, and at least 1.5 m from the children breathing zone and continuously at every hour for a week.
Data extraction and analysis
All data analyses employed the Statistical Package for Social Sciences (SPSSversion20.0 Ltd., USA). Categorical data was assessed using the chi-square test. Continuous data with normal (Gaussian) distribution employed the Student’s t test, and those with non-normal distribution (for example, temperature, relative humidity, VOCs levels, and formaldehyde concentration) employed the Mann Whitney U test. Recurrent asthma risk factors were also assessed using the aforementioned methods. Multiple logistic regression was used to adjust potential confounders, namely, gender, age, birth weight, premature delivery, breastfeeding duration, and family asthma history. Adjusted odds ratios (ORs) for recurrent childhood asthma experiencing a 10 mg/m3 rise in VOC exposure were computed with 95% confidence intervals (CIs). P-value < 0.05 was set as the significance threshold.
Results
Demographic profiles of analyzed children
In all, 1960 (91.1%), out of 2150 care-givers, responded to our residential air and asthma incidence survey. Among them, 201 (9.75%) corresponding children had asthma. Table 1 summarizes the personal profiles of all examined children with an average age of 5.11 years (SD1.6). Most (86%) investigated children were between 3 and 6 years of age, and some (12%) were older (maximum7 years). Approximately 41.4% were girls. Among asthmatics and controls, the male-to-female ratio was 1.1:1 among asthmatic, and 0.8:1 among controls. Among the examined children, 10.7% had a history of pediatrician-identified asthma, 20.1% suffered from allergic rhinitis, and 36.7% from pneumonia.
Environmental risks or protective factors-related to asthma
Based on our analysis, asthma was strongly correlated with breastfeeding (P=0.001), family asthma history (P=0.004), passive smoking (P=0.000), redecoration or new furniture addition (P=0.005), pets at home (P=0.008), and potted plants at home (P=0.019). In contrast, it was not associated with sex, age, birth weight, premature delivery, education of parents, house location, home size, household dampness, and quantity of household members. Table 2
The average RT and RAH at 6- and 24-h
A total of 434 children bedrooms provided valid RT and RAH information (Table 3). Our analyses revealed elevated 6- and 24-h mean RTs in the bedrooms of asthmatic children versus controls; however, the values did not reach significance. The 6- and 24-h mean RAHs were not obviously different between the asthmatic and control children (Table 2).
The 6- and 24-h mean formaldehyde and CO2 levels
We retrieved valid formaldehyde and CO2 information from 417 child-bedrooms (Table 3). The formaldehyde concentrations ranged between 7.1 and 30.0 mg/m3 (mean: 21.9mg/m3) in the 6-h period and between 6.7 and 29.4 mg/m3 (mean: 21.5mg/m3) in the 24-h time period, which were lower than the Chinese test standard and other climate zones. The 6- and 24-h mean formaldehyde levels were substantially elevated among asthmatics, relative to controls (p-value <0.001). The 6- and 24-h mean CO2 concentrations were generally less than 1000 ppm and were close to the 6-h concentration of 910.7 ppm (Table 4). The association between CO2 level and asthma did not reach statistical significance.
Measurements of indoor and outdoor air pollution
VOC concentrations, namely, benzene, toluene, ethylbenzene, and xylene, were measured in both the indoor and outdoor environments of asthmatic and healthy children (Table 5). Among all the VOC concentrations, toluene had the highest GM indoor concentration (128.31 μg/m3), followed by benzene (6.91 μg/m3) and xylene (6.41 μg/m3). Ethylbenzene had the lowest GM concentration (4.68 μg/m3). In Yancheng, toluene was the most abundant indoor air environment as for other cities (Huang et al. 2021). In case of outdoor pollution, the asthmatic children exhibited a much higher exposure to benzene, toluene, and xylene concentrations than healthy children. Similarly, benzene concentration (2.9 μg/m3) was considerably higher among asthmatic children than healthy ones (1.67 μg/m3, p = 0.000). Lastly, toluene concentrations were also elevated among asthmatic children (32.48 ± 7.93μg/m3), relative to healthy ones (29.77 ± 8.24, p = 0.003). While comparing outdoor pollution, we revealed that the benzene concentration was markedly elevated among asthmatic children, relative to controls. Moreover, the benzene concentration was considerably high among asthmatic children (1.92 ± 0.56μg/m3), compared to healthy ones (1.52 ± 0.54μg/m3, p = 0.000). Lastly, the average ΣVOCs concentrations were 81.37 μg/m3 indoor and 32.00 μg/m3 outdoor, thereby showing significant difference.
Table 5 illustrates the adjusted ORs of multiple logistic regression models for associations between asthma and benzene and formaldehyde exposures. Potential confounding factors, namely, pets at home, family allergic history, new furniture addition, kitchen ventilation condition, potted plants at home, and toluene and xylene presence, were considered in our analysis.
We demonstrated that breastfeeding over 6 months was protective against asthma [OR: 0.368 (95% CI: 0.216~0.627)]. Both family asthma history and passive smoking were strong risk factors for asthma [OR: 2.076(95% CI: 1.226~3.515)] and 2.115 (95% CI: 1.275~3.508), respectively]. Redecoration/new furniture addition was also a significant risk factor for asthma [OR3.129 (95% CI: 1.542~6.347)]. Additionally, benzene [OR6.819 (95% CI: 3.436~13.531)] and formaldehyde [OR11.985 (95% CI: 4.983~28.826)] exposures were large contributors to recurrent asthma among children (Table 6).
Discussion
This hospital-based case-control study was in agreement with prior investigations. We demonstrated that breastfeeding was highly protective against childhood asthma during the first 3–6 years of life. In cross-sectional investigations, prolonged exclusive breastfeeding (> 6 months), relative to never breastfeeding, was shown to protect children against asthma in their first 2–9 years of life (Abarca et al. 2018; Harvey et al. 2020; Huo et al. 2018). A hospital-based case-control investigation in China reported that exclusive breastfeeding (> 6 months) dramatically diminished asthma risk, compared to children who were never breastfed. A population-based childbirth cohort from 2017, examined by Annika et al., exhibited a direct link between breastfeeding and protection against asthma, relative to formula feeding at 3 months, 3 years, and 9 years of age (Annika Klopp et al. 2017). However, a Chinese study reported that a longer breastfeeding duration (> 6 months) was negatively correlated with an enhanced childhood asthma/allergy risk (Hu et al. 2021). Given this controversy, the true effect of breastfeeding on asthma remains undetermined. This is also reflected in the conflicting data produced by earlier research, which sometimes employed methods with considerable limitations and was influenced by the wide physiological variability in human milk. As such, additional epidemiologic and biomedical investigations are warranted to clarify the link between breastfeeding and asthma occurrence, to establish causality, and to elucidate the underlying mechanism(s).
Among the well-established contributors to wheezing and asthma are mothers’ history of asthmatic diseases and children’s respiratory infections (Arif 2018; Hallit et al. 2021). Herein, we verified that a family asthma history was intricately linked to enhanced asthma OR. Our results corroborated with the work of Mirzakhani et al., who revealed that, among a large American cohort of asthmatic and non-asthmatic mothers and their children, asthma risk is elevated among children whose mothers have uncontrolled asthma (Mirzakhani et al. 2019; Morales 2019). This suggests that both genetic and genomic factors contribute to the pathobiology of asthma (Morales 2019).
Passive smoking is another risk factor for asthma in children, even after confounder adjustment. However, there are reports that show either inverse or no correlation. A strong direct association between passive smoking and wheeze exists among 0–36-month-old Arco Ribeirinho region-born children, and 86.7% of these children present a minimum of 1 episode in the past 12 months (Rodrigues dos Santos et al. 2020). In a case-control investigation, children exposure to maternal smoking at 8–21 years of age was strongly correlated with pediatric and adult asthma (Neophytou et al. 2019). In a secondary cross-sectional investigation, the author demonstrated a strong link between passive smoking exposure and asthma among US adolescents (Merianos et al. 2018). Nevertheless, findings from an extensive prospective population-based lifelines cohort investigation in the Netherlands, involving 4–18-year-old children at baseline examinations, who were followed up for about 7 years, revealed that passive smoking exposure at home at baseline and during follow up was not associated with wheezing and asthma incidences (Mahon et al. 2021). Hence, our results reinforce the significance of avoiding indoor smoking for parents of young children. It is up to the public health authorities now to tailor the message for parents to avoid such behavior.
Emerging evidence revealed that home renovations, including indoor painting or buying new furniture and other sources, can potentially enhance VOCs and formaldehyde emissions, which remain for 6 months (Wag et al. 2020). Based on a report involving a self-administered questionnaire on indoor paint-based chemical emissions and asthmatic children, certain VOCs aggravate airway inflammation. Moreover, new furniture introduction strongly associated with pneumonia among asthmatic children (Wang et al. 2021a; Wang et al. 2021b; Zhang et al. 2018). In a retrospective cohort investigation, involving 3–6-year-old children from 7 cities in northern and southern China, it was revealed that household renovation and new large furniture addition during pregnancy strongly correlated with childhood doctor-diagnosed lifetime and parent-reported asthmatic symptoms and current cough (Zhang et al. 2018). Given the aforementioned evidences, we speculated whether both household renovation and new furniture addition potentially influence the male sperm, which, in turn, negatively affects offspring health later in life. One case-control study involving 242 congenital malformation cases and 270 controls reported that exposure of the father to solvents strongly enhanced the risk of congenital malformations within the child (El-Helaly et al. 2011). Thus, it is better to avoid renovation during pregnancy.
Herein, we demonstrated that total VOCs exposure had a significant influence on pediatric asthma. Our model was also adjusted for breastfeeding, passive smoking, pets at home, house decorations, family asthma history, family allergic history, new furniture addition, and potted plants at home. A prior study conducted in Iran revealed a strong link between asthma and benzene (Shakerkhatibi et al. 2021). Thus, both benzene and formaldehyde can be considered as specific contaminants within the indoor environment. Moreover, the wide variety of indoor formaldehyde exposures may be due to the presence of several and distinct formaldehyde sources, emitting pollutants over a variable period of time. At this point, there are no data to confirm this. The inconsistencies associated with previous studies may be due to the study design, outcome classification, differing living conditions, differences in geographic locations, and population characteristics.
Since the total VOC indoor exposure had a significant influence on pediatric asthma, comprehensive asthma programs with multicomponent interventions that include indoor air quality improvement and indoor environmental asthma trigger avoidance are necessary to achieve better health outcomes for childhood asthma.
There are a few noteworthy limitations of this research. Firstly, this study only examined children between the ages of 3 and 6 years from select preschools in Yancheng. Our sample population was relatively low, which negatively affected our statistical power of analysis, and considerably increased our confidence interval. Owing to this selection process, our conclusions may not fully represent the general population, which may be subject to select bias. Secondly, despite stating that the “asthma” must be physician-diagnosed in the questionnaire, it is possible that the reported data may have error and/or bias. Thirdly, it is possible that some children may have had asthma-related airway diseases/symptoms, but no doctor-diagnosis. Finally, in China, since most of children aged 3 to 6 always spend a certain amount of time in kindergarten, the indoor air quality of kindergarten classrooms should also be considered. Hence, some bias may have been introduced during the asthma-control classification in this study.
Conclusion
This study was the first to report a strong link between indoor environmental pollution and recurrent childhood asthma risk in China’s third-tier cities. We also provided the average RT and RAH between asthmatics and controls. Given our evidences, the indoor environment has a significant effect on asthmatic children, and passive smoking, redecoration within 1 year before pregnancy, and family asthma history substantially increase both the risk and severity of benzene and formaldehyde-induced asthmatic attacks in children.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abarca NE, Garro AC, Pearlman DN (2018) Relationship between breastfeeding and asthma prevalence in young children exposed to adverse childhood experiences. J Asthma 56(2):142–151. https://doi.org/10.1080/02770903.2018.1441869
Ambade B, Ghosh S (2013) Characterization of PM10 in the ambient air during Deepawali festival of Rajnandgaon district India. Natural Hazards 69(1):589–598. https://doi.org/10.1007/s11069-013-0725-8
Ambade B, Sethi SS, Kurwadkar S, Mishra P, Tripathee L (2022) Accumulation of polycyclic aromatic hydrocarbons (PAHs) in surface sediment residues of Mahanadi River Estuary: Abundance, source, and risk assessment. Mar Pollut Bull 183:114073. https://doi.org/10.1016/j.marpolbul.2022.114073
Annika Klopp LV, Becker AB, Subbarao P, Mandhane PJ, Stuart E, Diana L, Lefebvre Malcolm BA (2017) Modes of infant feeding and the risk of childhood asthma: a prospective birth cohort study. J Pediatr 190:192–199.e192. https://doi.org/10.1016/j.jpeds.2017.07.012
Arif AA (2018) The association of prenatal risk factors with childhood asthma. J Asthma 56(10):1056–1061. https://doi.org/10.1080/02770903.2018.1515224
Asher MI, Montefort S, Björkstén B, Lai CKW, Strachan DP, Weiland SK, Williams H (2006) Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet 368(9537):733–743. https://doi.org/10.1016/s0140-6736(06)69283-0
Athanasios K (2019) ‘Cocktails and dreams’: the indoor air quality that people are exposed to while sleeping. Curr Opin Environ Sci Health 8:6–9. https://doi.org/10.1016/j.coesh.2018.12.005
Chang T, Wang J, Lu J, Shen Z, Huang Y, Sun J, Xu H, Wang X, Ren D, Cao J (2019) Evaluation of indoor air pollution during decorating process and inhalation health risks in Xi’an, China: a case study. Aerosol and Air Quality Res 19(4):854–864. https://doi.org/10.4209/aaqr.2018.07.0261
Eassey D, Reddel HK, Foster JM, Kirkpatrick S, Locock L, Ryan K, Smith L (2018) “…I've said I wish I was dead, you'd be better off without me”: a systematic review of people's experiences of living with severe asthma. J Asthma 56(3):311–322. https://doi.org/10.1080/02770903.2018.1452034
El-Helaly M, Abdel-Elah K, Haussein A, Shalaby H (2011) Paternal occupational exposures and the risk of congenital malformations — a case-control study. Int J Occupat Med Environ Health 24(2). https://doi.org/10.2478/s13382-011-0019-x
Franck U, Weller A, Röder SW, Herberth G, Junge KM, Kohajda T, von Bergen M, Rolle-Kampczyk U, Diez U, Borte M, Lehmann I (2014) Prenatal VOC exposure and redecoration are related to wheezing in early infancy. Environ Int 73:393–401. https://doi.org/10.1016/j.envint.2014.08.013
Gilbert KC, Mirabelli MC, Ragan K, Gower EW, Ashburn NP, Gower WA (2015) Pediatric asthma deaths in North Carolina, 1999-2012. J Allerg Clin Immunol 135(2):AB86. https://doi.org/10.1016/j.jaci.2014.12.1212
Hallit S, Sacre H, Kheir N, Hallit R, Waked M, Salameh P (2021) Prevalence of asthma, its correlates, and validation of the Pre-School Asthma Risk Factors Scale (PS-ARFS) among preschool children in Lebanon. Allergologia et Immunopathologia 49(1):40–49. https://doi.org/10.15586/aei.v49i1.25
Harvey SM, Murphy VE, Gibson PG, Collison A, Robinson P, Sly PD, Mattes J, Jensen ME (2020) Maternal asthma, breastfeeding, and respiratory outcomes in the first year of life. Pediatr Pulmonol 55(7):1690–1696. https://doi.org/10.1002/ppul.24756
Hu Y, Chen Y, Liu S, Jiang F, Wu M, Yan C, Tan J, Yu G, Hu Y, Yin Y, Qu J, Li S, Tong S (2021) Breastfeeding duration modified the effects of neonatal and familial risk factors on childhood asthma and allergy: a population-based study. Respirator Res 22(1). https://doi.org/10.1186/s12931-021-01644-9
Huang L, Wei Y, Zhang L, Ma Z, Zhao W (2021) Estimates of emission strengths of 43 VOCs in wintertime residential indoor environments Beijing. Sci Total Environ 793:148623. https://doi.org/10.1016/j.scitotenv.2021.148623
Huang S, Wei W, Weschler LB, Salthammer T, Kan H, Bu Z, Zhang Y (2017) Indoor formaldehyde concentrations in urban China: preliminary study of some important influencing factors. Sci Total Environ 590-591:394–405. https://doi.org/10.1016/j.scitotenv.2017.02.187
Huang TP, Liu PH, Lien ASY, Yang SL, Chang HH, Yen HR (2013) Characteristics of traditional Chinese medicine use in children with asthma: a nationwide population-based study. Allergy 68(12):1610–1613. https://doi.org/10.1111/all.12273
Huo X, Chu S, Hua L, Bao Y, Du L, Xu J, Zhang J (2018) The effect of breastfeeding on the risk of asthma in high-risk children: a case-control study in Shanghai China. BMC Preg Childbirth 18(1). https://doi.org/10.1186/s12884-018-1936-5
Hwang G, Yoon C, Choi J (2011) A case-control study: exposure assessment of VOCs and formaldehyde for asthma in children. Aerosol and Air Quality Res 11(7):908–914. https://doi.org/10.4209/aaqr.2011.05.0072
Ibrahim NM, Almarzouqi FI, Al Melaih FA, Farouk H, MAL A, Jassim FM (2021) Prevalence of asthma and allergies among children in the United Arab Emirates: a cross-sectional study. World Allerg Org J 14(10):100588. https://doi.org/10.1016/j.waojou.2021.100588
Julia A, Santigao P, Ricardo B, Amat A, Ruiz I, Mora A, Escolano S, Chofre L (2020) Asthma prevalence and risk factors in school children: The RESPIR longitudinal study. Allergologia et Immunopathol 48(3):223–231. https://doi.org/10.1016/j.aller.2019.06.003
Kansen HM, Le TM, Uiterwaal C, van Ewijk BE, Balemans WAF, Gorissen DMW, de Vries E, van Velzen MF, Slabbers G, Meijer Y, Knulst AC, van der Ent CK, van Erp FC (2020) Prevalence and predictors of uncontrolled asthma in children referred for asthma and other atopic diseases. J Asthma Allerg 13:67–75. https://doi.org/10.2147/jaa.s231907
Kodate J, Marganwar R, Dhurvey V, Dhawas S, Urkude R (2016) Assessment of groundwater quality with special emphasison fluoride contamination in some villages of Chandrapur district of Maharashtra,India. IOSR J Environ Sci , Toxicol Food Technol 10(3):15–26. https://doi.org/10.9790/2402-10311526
Kuang H, Li Z, Lv X, Wu P, Tan J, Wu Q, Li Y, Jiang W, Pang Q, Wang Y, Fan R (2021) Exposure to volatile organic compounds may be associated with oxidative DNA damage-mediated childhood asthma. Ecotoxicol Environ Safety 210:111864. https://doi.org/10.1016/j.ecoenv.2020.111864
Kumar A, Ambade B, Sankar TK, Sethi SS, Kurwadkar S (2020) Source identification and health risk assessment of atmospheric PM2.5-bound polycyclic aromatic hydrocarbons in Jamshedpur, India. Sustain Cities Soc 52:101801. https://doi.org/10.1016/j.scs.2019.101801
Luo S, Sun Y, Hou J, Kong X, Wang P, Zhang Q, Sundell J (2018) Pet keeping in childhood and asthma and allergy among children in Tianjin area China. Plos One 13(5):e0197274. https://doi.org/10.1371/journal.pone.0197274
Madureira J, Paciência I, Ramos E, Barros H, Pereira C, Teixeira J P, Fernandes E d O,2015. Children’s health and indoor air quality in primary schools and homes in Portugal—study design. J Toxicol Environ Health, Part A 78(13-14), 915-930. https://doi.org/10.1080/15287394.2015.1048926
Mahon GM, Koppelman GH, Vonk JM (2021) Grandmaternal smoking, asthma and lung function in the offspring: the Lifelines cohort study. Thorax 76(5):441–447. https://doi.org/10.1136/thoraxjnl-2020-215232
Merianos AL, Jandarov RA, Mahabee-Gittens EM (2018) Association of secondhand smoke exposure with asthma symptoms, medication use, and healthcare utilization among asthmatic adolescents. J Asthma 56(4):369–379. https://doi.org/10.1080/02770903.2018.1463379
Mirzakhani H, Carey VJ, Zeiger R, Bacharier LB, O'Connor GT, Schatz MX, Laranjo N, Weiss ST, Litonjua AA (2019) Impact of parental asthma, prenatal maternal asthma control, and vitamin D status on risk of asthma and recurrent wheeze in 3-year-old children. Clin Exp Allergy 49(4):419–429. https://doi.org/10.1111/cea.13320
Morales E (2019) Genetics and gene-environment interactions in childhood and adult onset asthma. Front Pediatr 7. https://doi.org/10.3389/fped.2019.00499
Neophytou AM, Oh SS, Hu D, Huntsman S, Eng C, Rodríguez-Santana JR, Kumar R, Balmes JR, Eisen EA, Burchard EG (2019) In utero tobacco smoke exposure, DNA methylation, and asthma in Latino children. Environ Epidemiol 3(3):e048. https://doi.org/10.1097/ee9.0000000000000048
Peel AM, Wilkinson M, Sinha A, Loke YK, Fowler SJ, Wilson AM (2020) Volatile organic compounds associated with diagnosis and disease characteristics in asthma – a systematic review. Respiratory Medicine 169:105984. https://doi.org/10.1016/j.rmed.2020.105984
Pennington AF, Strickland MJ, Klein M, Zhai X, Bates JT, Drews-Botsch C, Hansen C, Russell AG, Tolbert PE, Darrow LA (2018) Exposure to mobile source air pollution in early-life and childhood asthma incidence. Epidemiology 29(1):22–30. https://doi.org/10.1097/ede.0000000000000754
Rodrigues dos Santos R, Gregório J, Castanheira L, Fernandes AS (2020) Exploring volatile organic compound exposure and its association with wheezing in children under 36 months: a cross-sectional study in South Lisbon, Portugal. Int J Environ Res Public Health 17(18):6929. https://doi.org/10.3390/ijerph17186929
Sankar TK, Kumar A, Mahto DK, Das KC, Narayan P, Fukate M, Awachat P, Padghan D, Mohammad F, Al-Lohedan HA, Soleiman AA, Ambade B (2023) The health risk and source assessment of polycyclic aromatic hydrocarbons (PAHs) in the soil of industrial cities in India. Toxics 11(6):515. https://doi.org/10.3390/toxics11060515
Shakerkhatibi M, Benis KZ, Asghari-Jafarabadi M, Sadeghi-Bazarghani H, Allahverdipour H, Oskouei DS, Fatehifar E, Farajzadeh M, Yadeghari A, Ansarin K, Jafari R, Zakeri A, Moshashaei P, Behnami A (2021) Air pollution-related asthma profiles among children/adolescents: a multi-group latent class analysis. Ecotoxicol Environ Safety 219:112344. https://doi.org/10.1016/j.ecoenv.2021.112344
Stanford RH, Buikema AR, Riedel AA, Camargo CA, Rey GG, Chapman KR (2012) Asthma controller delay and recurrence risk after an emergency department visit or hospitalization. Respirator Med 106(12):1631–1638. https://doi.org/10.1016/j.rmed.2012.08.017
Sun Y, Hou J, Sheng Y, Kong X, Weschler LB, Sundell J (2019) Modern life makes children allergic. A cross-sectional study: associations of home environment and lifestyles with asthma and allergy among children in Tianjin region, China. Int Arch Occupational Environ Health 92(4):587–598. https://doi.org/10.1007/s00420-018-1395-3
Wag J, Huang K, Feng G, Song J (2020) Analysis of winter formaldehyde and volatile organic compound pollution characteristics of residential kitchens in severe cold regions of northeast China. Indoor Built Environ 30(8):1226–1243. https://doi.org/10.1177/1420326x20937462
Wang J, Janson C, Jogi R, Forsberg B, Gislason T, Holm M, Torén K, Malinovschi A, Sigsgaard T, Schlünssen V, Svanes C, Johannessen A, Bertelsen RJ, Franklin KA, Norbäck D (2021a) A prospective study on the role of smoking, environmental tobacco smoke, indoor painting and living in old or new buildings on asthma, rhinitis and respiratory symptoms. Environ Res 192:110269. https://doi.org/10.1016/j.envres.2020.110269
Wang J, Zhang Y, Li B, Zhao Z, Huang C, Zhang X, Deng Q, Lu C, Qian H, Yang X, Sun Y, Sundell J, Norbäck D (2021b) Asthma and allergic rhinitis among young parents in China in relation to outdoor air pollution, climate and home environment. Sci Total Environ 751:141734. https://doi.org/10.1016/j.scitotenv.2020.141734
Yang S, Perret V, Hager Jörin C, Niculita-Hirzel H, Goyette Pernot J, Licina D (2020) Volatile organic compounds in 169 energy-efficient dwellings in Switzerland. Indoor Air 30(3):481–491. https://doi.org/10.1111/ina.12667
Zhang H-l, Wang B-y, Luo Y, Li Y, Cai C-s, Huang L-l, He B-h, Cai J, Li Z-y, Mai A-d, Guo Y (2020) Association of pet-keeping in home with self-reported asthma and asthma-related symptoms in 11611 school children from China. J Asthma 58(12):1555–1564. https://doi.org/10.1080/02770903.2020.1818772
Zhang J, Sun C, Liu W, Zou Z, Zhang Y, Li B, Zhao Z, Deng Q, Yang X, Zhang X, Qian H, Sun Y, Sundell J, Huang C (2018) Associations of household renovation materials and periods with childhood asthma, in China: a retrospective cohort study. Environ Int 113:240–248. https://doi.org/10.1016/j.envint.2018.02.001
Zhang Y, Li B, Huang C, Yang X, Qian H, Deng Q, Zhao Z, Li A, Zhao J, Zhang X, Qu F, Hu Y, Yang Q, Wang J, Zhang M, Wang F, Zheng X, Lu C, Liu Z et al (2013) Ten cities cross-sectional questionnaire survey of children asthma and other allergies in China. Chinese Sci Bull 58(34):4182–4189. https://doi.org/10.1007/s11434-013-5914-z
Zhang Z, Weichenthal S, Kwong JC, Burnett RT, Hatzopoulou M, Jerrett M, van Donkelaar A, Bai L, Martin RV, Copes R, Lu H, Lakey P, Shiraiwa M, Chen H (2021) A population-based cohort study of respiratory disease and long-term exposure to iron and copper in fine particulate air pollution and their combined impact on reactive oxygen species generation in human lungs. Environ Sci Technol 55(6):3807–3818. https://doi.org/10.1021/acs.est.0c05931
Acknowledgements
The authors would like to thank all the reviewers who participated in the review and MJEditor (http://www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.
Funding
This study was supported in part by grants from the Natural Science Foundation of the Jiangsu Higher Education Institutions of China [20KJA610002] and the “Qing Lan Project” of Colleges and Universities in JiangSu Province.
Author information
Authors and Affiliations
Contributions
Tianming Chen conceived and designed the studies. Baoping Zhang performed the studies and drafted the manuscript. Chuntao Yin, Yang Yuan, and Zhibin Xia contributed data collection. Xu Jiang contributed data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, B., Xia, Z., Jiang, X. et al. Indoor environment in relation to recurrent childhood asthma in Yancheng, China: a hospital-based case-control study. Environ Sci Pollut Res 30, 102212–102221 (2023). https://doi.org/10.1007/s11356-023-29631-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-29631-1