Abstract
The growth response and incorporation of As into the Sargassum horneri was evaluated for up to 7 days using either arsenate (As(V)), arsenite (As(III)) or methylarsonate (MMAA(V) and DMAA(V)) at 0, 0.25, 0.5, 1, 2, and 4 μM with various phosphate (P) levels (0, 2.5, 5 and 10 μM). Except As(III), algal chlorophyll fluorescence was almost similar and insignificant, regardless of whether different concentrations of P or As(V) or MMAA(V) or DMAA(V) were provided (p > 0.05). As(III) at higher concentrations negatively affected algal growth rate, though concentrations of all As species had significant effects on growth rate (p < 0.01). Growth studies indicated that toxicity and sensitivity of As species to the algae followed the trend: As(III) > As(V) > MMAA(V) ~ DMAA(V). As bioaccumulation was varied significantly depending on the increasing concentrations of all As species and increasing P levels considerably affected As(V) uptake but no other As species uptake (p < 0.01). The algae accumulated As(V) and As(III) more efficiently than MMAA(V) and DMAA(V). At equal concentrations of As (4 μM) and P (0 μM), the alga was able to accumulate 638.2 ± 71.3, 404.1 ± 70.6, 176.7 ± 19.6, and 205.6 ± 33.2 nM g-1 dry weight of As from As(V), As(III), MMAA(V), and DMAA(V), respectively. The influence of low P levels with increased As(V) concentrations more steeply increased As uptake, but P on other As species did not display similar trends. The algae also showed passive modes for As adsorption of all As species. The maximum adsorption of As (63.7 ± 6.1 nM g-1 dry weight) was found due to 4 μM As(V) exposure, which was 2.5, 7.3, and 6.9 times higher than the adsorption amounts for the same concentration of As(III), MMAA(V), and DMAA(V) exposure, respectively. The bioavailability and accumulation behaviors of As were significantly influenced by P and As species, and this information is essential for As research on marine ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The metalloid arsenic (As) shows no well-known biological function (Edmonds and Francesconi 2003), but has been ranked number one among hazardous substances of highest priority (ATSDR 2017). As is the most widely dispersed food chain pollutants in nature and can form a rich variety of chemical species (usually known as arsenicals) with their inconsistent toxicological behavior. As reaches the marine ecosystem from both natural and anthropogenic points and diffuse sources (Cullen and Reimer 1989; Francesconi 2010), and is effectively transformed by biological processes, creating an As biogeocycle (Bhattacharjee and Rosen 2007). The primary bioavailable and inorganic forms of As in aqueous systems are arsenate (As(V)) and arsenite (As(III)), while As(V) is the most important and relevant inorganic forms in oxic seawater. As(V) and As(III) are interconvertible, depending on the redox state of the aquatic ecosystem (Ozturk et al. 2010). Inorganic and organoarsenic compounds (methylated and other organic As species) account for about 80% and 20% of the total dissolved As in the marine environment, respectively (Anninou and Cave 2009). Marine algae are the key contributors to methylated As species (monomethyl arsinate, MMAA(V) and/or dimethylarsinic acid, DMAA(V)) in seawater; they thus play a crucial role in As biogeochemical cycle (Duncan et al. 2014; Zhang et al. 2014). The latest discoveries of more than 50 arsenicals have broadened the research area. Therefore, a renewed interest has been shown in investigating how marine algae grow, accumulate, detoxify, and produce As species in the marine food web and in identifying the interaction between algae and arsenicals in the context of As cycling.
Various biota uses a variety of contaminant uptake routes from multiple and complex aqueous phases. In general, cellular absorption of toxic ions occurs through selective ion channels or carriers. Macroalgae can take dissolved ion over their entire surfaces. Algae absorb As in the highest oxidation state As(V) through phosphate transporters, because of its structural and physicochemical analogous properties with P (H2PO4− versus H2AsO4−) (Taylor and Jackson 2016), whereas As(III), MMAA(V) and DMAA(V) are believed to be transported through the aquaglyceroporin channels (Rahman and Hasegawa 2011; Zhao et al. 2009). Following the uptake of As species, several organisms including algae detoxify As by employing different accumulation and biotransformation mechanisms either outside or within cells to resist the adverse effect of As. These processes are essential in controlling the mobilization and subsequent distribution of arsenicals in the ecosystem. Wang et al. (2015) thoroughly reviewed the microalgae (both freshwater and marine) laboratory studies and focused on the contribution of these organisms to the speciation, toxicity, and metabolism of different As species. However, there are preliminary researches, and a limited number of laboratory-based culture studies exist concerning macroalgae, but they have only begun to be studied in the sense of As metabolism.
Algae are the primary producers in the aquatic food web and display tremendous capacity in their cells to concentrate As. Marine macroalgae can accumulate 1000 to 50000-fold higher As from seawater (Ma et al. 2018), and can play a critical role between As circulating in the water column and other organisms. P is an essential macronutrient for algal growth, but its absence or presence complicates As to aquatic organisms’ toxicity (Rahman et al. 2014). P regulates the bioavailability of As species in the aquatic system and governs the uptake and subsequent processes of transformation. The higher amount of P in both microalgal and macroalgal cultures has been well known to inhibit the cellular accumulation of As(V) (Mamun et al. 2019c). Many studies have also documented the P-independent uptake of As(V) (Duncan et al. 2013; Foster et al. 2008), As(III) (Mamun et al. 2019b), and MMAA(V), and DMAA(V) (Rahman et al. 2008a). The effects of P on As uptake by the aquatic organisms have been concentrated almost entirely on inorganic species (As(V) and/or As(III)). Studies of organoarsenic species such as MMAA(V) and DMAA(V) either by plants or algae are few in comparison to inorganic species (Rahman et al. 2011), and the transport mechanism of organic species with or without P remains unexplored in the marine macroalgal culture system. On a similar note, consideration of both organic and inorganic species of metalloids As for environmental impact assessment and ecological risks is also important (Filella et al. 2009). Besides, species-specific macroalgal and P-dependent toxicity disparity on both organic and inorganic As species remain mostly unknown. It would thus be interesting to systematically explore the toxicity of As species corresponding to the growth and accumulation behavior together with the underlying mechanism under conditions of laboratory culture.
The bioaccumulation of As by marine organisms largely depends on the bioavailability of As species (Casado-Martinez et al. 2010; Maher et al. 2011). Consequently, the abundance and sources of As species as either inorganic or organic are potentially important to clarify the difference among marine algae species for As transportation and bioaccumulation. Current literature lists 615 species, forms, and varieties of Sargassum. Many Sargassum species can be employed for environmental management, including heavy metal remediation, irrespective of their diverse industrial and biotechnological potential (Stiger-Pouvreau et al. 2023; Stiger-Pouvreau and Zubia 2020). Some studies have highlighted the hidden danger posed by the high As content of beach-deposited Sargassum sp., necessitating further research in the context of environmental sanitary and reusing of this algae (Devault et al. 2022; Devault et al. 2021). We selected Sargassum horneri as our study organism because of its higher As accumulation capacity in laboratory culture (Mamun et al. 2017). The research has been designed for a set of macroalgal culture experiments in seawaters with the following objectives: (i) to explore the discrepancy between the bioavailability of biogeochemically important and dominant As species—As(V), As(III), MMAA(V), and DMAA(V)—to Sargassum horneri, in terms of growth efficiency; (ii) to investigate how As bioaccumulation is controlled in the presence P ions in the growth medium; and (iii) to investigate the surface adsorption of As due to the differences in As species as well as the presence of coexisting ions like iron (Fe) in the culture medium.
Materials and Methods
Pre-culture of macroalgae
The collection of large macroalgae samples S. horneri, an average length of 50 cm (n = 10), were done through the sites along the coast of Noto Peninsula (facing the Sea of Japan), Ishikawa, Japan. Samples were vigorously washed with on-site seawater and shaken to remove the water. No signs of desiccation were detected during the sample processing. The cooled box was used to move samples in the laboratory and instantly cleaned with filtered natural seawater (NSW) for removing adhering sands, debris, and epiphytic organisms. Individual large samples were then kept in plastic buckets comprising 20 L NSW with 1% Provasoli enriched seawater (PES) medium (pH: 8.1 ± 0.2). The PES medium was prepared in distilled water containing salts and antibiotics. The composition of PES medium is provided in Table S1 in Appendix A. The buckets were placed in a chamber (RZ-2S, Oriental Giken Industry Co., Ltd., Tokyo, Japan) with constant temperature (20 ± 2°C), light (12/12 h light/dark period), and aeration. The NSW and PES medium were renewed once a couple of days and retained up to one week for maintaining healthy and stable growth conditions of the algae. For the main experiment, small shoots (length of 4–5 cm) were excised and isolated from each alga. Shoots were also stored in glass flasks (10 L) containing NSW and 25% PES medium for 48 h to minimize the harmful effects of excision (Endo et al. 2013). Shoots were also maintained for another 48 h in NSW and 1% PES medium (P-free) before the start of the culture experiment. The optimal macroalgal cultivation in an incubator was done under 90 μmol photons m-2 s-1 photon flux density (from the white, fluorescent tube), 18 ± 1.0 °C temperature along with a 12/12 h light/dark photoperiod, and the culture bottles were continuously aerated via tube and silicon plug attached with an air pump (e-Air 4000 WB, GEX) for stirring the medium.
Experimental setup
The algae were cultivated using polycarbonate bottles (500 mL). The culture solution was prepared with 1% PES medium, and 250 mL sterilized NSW. The shoots were washed three times with deionized water before being inoculated. Four randomly selected shoots (average weight of 1.5–2.0 g) were introduced into each culture bottle. A series of culture experiments were carried out by incubating the algae with four different As forms as As(V)/As(III)/MMAA(V)/DMAA(V) at concentrations of 0.25, 0.5, 1.0, 2.0 and 4.0 μM and with another three test concentrations of P at 2.5, 5 and 10 μM. The cultures where no P and As were exposed to algae served as a control. A set of cultures of the tested As species with 1 μM were done without algae and regarded as chemical control. A total of 24 treatment combinations for each As species were replicated three times following a randomized design. The sources of As(III), As(V), MMAA(V), and DMAA(V), P were NaAs2O3, Na2HAsO4.7H2O, CH3AsO3, and (CH3)2AsO2Na.3H2O, and KH2PO4 respectively. A clean bench (MCV-711ATS, Sanyo Electric Co. Ltd., Osaka, Japan) was used to maintain the sterile condition during inoculation. The algal and chemically controlled culture bottles were incubated for seven days in a constant growth chamber at a temperature of 18°C ± 1°C; with fluorescent lights under 12/12 h light/dark photocycle at an irradiance level of 45 μmol photons m-2 S-1. Culture solutions were aerated by air pumps connecting with silicon plug and the plastic tubes (~4 mm diameter). At the end of incubation, algal samples were collected and stored at 4 °C in a refrigerator for freeze-drying till required for further analysis.
A separate experiment was carried out to determine the quantity of As and Fe adsorbed on the surface of algae. Aquatic organisms, including algae either growing in the laboratory or occurring naturally, showed to have an external pool of Fe (Fe-plaque) on their surface and can sequester As and other trace metals (David et al. 1999; Mamun et al. 2019c). For this purpose, algae were grown similarly as above for 7 d in NSW enriched with 1% PES medium. The specified standard concentration of Fe and P in PES medium were not modified, and the algae were inoculated with 4 μM of As either as As(V) or As(III), or MMAA(V) or DMAA(V). Control cultures were also maintained where none of the As were exposed to algae.
Analytical procedures
The total As (TAs) concentration in the algal tissues and CBE extracts were measured by an inductively coupled plasma mass spectrometer (ICP-MS, SPQ 9000, Seiko, Japan) after the digestion of samples using a heat decomposition microwave system (Multiwave 3000, Anton Paar GmbH, Graz, Austria). Concentrated HNO3 (65%) and optimized digestive operating conditions were chosen according to the recommendations of the manufacturer. Following the instrumental reactions, the digested solution was transmitted into heat-resistant plastic containers (DigiTUBEs, SCP Science, Japan). Approximately 5.0 mL of purified water was added into each container and placed them into a heat-block type thermal decomposition system (DigiPREP Jr, SCP Science, Japan) at 100 °C for about 5 h, until the samples were dry. The contents were then redissolved and diluted by 10 mL of deionized water and filtered via 0.45 μm filters (Advantec, Tokyo, Japan). A standard reference material (National Metrology Institute of Japan (NMIJ) CRM 7405-a, No. 265: Trace Elements and Arsenic Compounds in Seaweed (Hijiki)) (Ibaraki, Japan) was also digested and analyzed using the same procedure for determining the accuracy of the analytical method. A good recovery efficiency (96.7 ± 3.8%) was observed for the certified values.
The adsorbed As and Fe from the algal surfaces were extracted using the citrate-bicarbonate ethylenediaminetetraacetate (CBE) (Rahman et al. 2008b). Sodium citrate, sodium bicarbonate, and EDTA at a concentration of 0.03, 0.125, and 0.05 M respectively, were used for preparing CBE solution. The CBE solution also included 0.025 M NaCl and 0.05 M KCl (pH was adjusted to 8.0 using NaHCO3) because of the seawater originated samples. The algal samples after incubation were treated at room temperature (25 °C) with 30 mL of CBE solution for 30 min. The samples were then washed and rinsed three times with deionized water, and rinsed water was added to the CBE extracts to a volume of 50 mL. As contents in CBE extracts and tissues were regarded as adsorbed and intracellular contents, respectively.
P and Fe content in the digested samples were determined in an inductively coupled plasma atomic emission spectrometer (ICP-AES, iCAP 6300, Thermo Scientific, Waltham, MA). Arium Pro water purification system from Sartorius Stedium Biotech GmbH (Gottingen, Germany) was used to produce the purified water (resistivity > 18.2 MΩ cm).
Chemicals and standards
During the entire study, analytical reagent grade chemicals were used without further purification unless otherwise stated. Stock solutions and/or working standards were prepared on the day of analysis as nM and/or μM levels. For pH adjustment, either HCl or NaOH (1 M) was used. Standard chemicals of Na2HAsO4.7H2O (for As(V)), CH3AsO3 (for MMAA(V)), and KH2PO4 (for P) were purchased from Wako Pure Chemical Ind. Ltd. (Tokyo, Japan); NaAs2O3 (for As(III)) was purchased from Merck (Tokyo, Japan); and (CH3)2AsO2Na.3H2O (for DMAA(V)) was purchased from Nacalai Tesque (Kyoto, Japan). Deionized water was used for preparing standard solutions. However, Fe standard solution was prepared by dissolving FeCl3.6H2O (Wako Pure Chemical Ind. Ltd.) in 1 M HCl and deionized water.
Low-density polyethylene bottles and micropipettes (Nichiryo, Tokyo, Japan) and other laboratory items used in the experiments were cleaned as specified by Hasegawa et al. (2017). Glassware was autoclaved before stored standard solutions or used for algal culture.
Measurement of chlorophyll fluorescence
Chlorophyll fluorescence was measured by quantifying the quantum yield (maximum photochemical efficiency) of open RCIIs using pulse amplitude modulation fluorometry (PAM, OS1p, Opti-Sciences, USA). Replicated samples were adapted to the dark for about 15 min before measurements were made. The following equation was used to convey the maximum quantum yield stated elsewhere (Cosgrove and Borowitzka 2010):
Where, Fv/Fm = maximum quantum yield; F0 = minimum fluorescence yield (dark adapted, all RCIIs open); Fm = maximum fluorescence yield (dark adapted, all RCIIs colsed with no NPQ); and Fv = maximum variable fluorescence yield, (Fm – F0).
Measurement of growth rate
The fresh weights of the shoots before incubation and after incubation were measured using an electrical balance (0.10 mg accuracy) after drying blotting them dry. The mean fresh weights were calculated for each replicate, and daily growth rates (GRs) were calculated with the following equation (Loureiro et al. 2012):
Where, Wi is the initial weight, Wt is the weight after 7 days, and t is the experimental time in days.
Statistical analysis
Data were expressed as the mean value ± standard deviation (SD) (n = 3). The IBM SPSS 22.0 for Windows (IBM Co., NY, USA) and Graph Pad Prism 6.0 (GraphPad Software Inc., San Diego, CA, USA) were used to perform statistical analyses and graphical works, respectively. Analysis of variance (ANOVA) was performed for determining the significance (p < 0.05) of the results, and post-hoc multiple comparisons (Tukey’s test) were used to define the significant differences among treatments. The correlation between As accumulation with studied parameters was also done using the same statistical package.
Results and Discussion
Chlorophyll fluorescence of S. horneri
By measuring the chlorophyll fluorescence using PAM devices, the photosynthetic function of PSII of algae and seagrasses can be estimated. A number of environmental and abiotic stresses such as temperature, light, radiation, salinity, drought, metals, pesticides, salts, etc. can affect the PSII activity. The maximum photochemical efficiency (Fv/Fm), an important chlorophyll fluorescence parameter, is often used in field and laboratory tests and is rich evidence of photosynthetic efficiency for specific stresses on plants (Suggett et al. 2010). After 7 d incubation with As(V), MMAA(V), and DMAA(V), the Fv/Fm of the algae was not significantly affected by the main effects of different As and P levels or their interaction (p > 0.05). However, the effect of different concentrations of As(III) and P had a significant effect on Fv/Fm (p < 0.01), whereas their interaction effects were insignificant (p > 0.05) (Table S2 in Appendix A). Prior to exposure to As, the Fv/Fm of the algae ranged from 0.713 to 0.732 (average of 0.722 ± 0.01), 0.710 to 0.733 (average of 0.722 ± 0.01), 0.716 to 0.731 (average of 0.723 ± 0.01), and 0.711 to 0.737 (average of 0.722 ± 0.01) under As(V), As(III), MMAA(V), and DMAA(V) treatments, respectively.
Algae showed a slightly decreasing Fv/Fm trend after 7 d incubation with all As treated cultures compared to their respective control or initial values (Fig. 1 and 2). The decreasing trend is more marked with the cultures receiving initial low P levels (0 to 2.5 μM) and higher As levels (2 and 4 μM) as the substrate. It was also noted that Fv/Fm decreased as the concentration of As species increased in the medium. At 4 μM As and 10 μM P exposure level, Fv/Fm decreased by 1.7, 9.6, 0.8 and 2.4% from their initial values, respectively in As(V), As(III), MMAA(V), and DMAA(V) treated cultures. The Fv/Fm changes in controlled algae (no As) did not correspond with Fv/Fm changes in the As treated algae. It was also found that Fv/Fm increased from the initial values when algae received increasing levels of P without As. When As concentrations rose from 0.25 to 4 μM without the addition of P in cultures, the decreasing trend of Fv/Fm were: 1.1 to 5.3% in As(V), 3.7 to 23.9% in As(III), 1.2 to 4.4% in MMAA(V), and 2.1 to 4.9 in DMAA(V) compared to As free cultured algae. The chlorophyll fluorescence of the tested algae was more susceptible because of the effects of As(III) than As(V), indicating increasing toxicity with inorganic As though there was no sign of discoloration of the shoots. However, there have been hardly any toxic effects of MMAA(V) and DMAA(V), and both species almost had the same effect on the chlorophyll fluorescence response. These findings indicated that the tested concentration of As species may inhibit the chlorophyll fluorescence partly, and the inhibitory effect follows the order: As(III) > As(V) > MMAA(V) ~ DMAA(V). Healthy and non-stressed vascular plants and macroalgae were claimed to be ideal Fv/Fm by 0.832 ± 0.004, whereas the value of dead material would be below 0.1 (Chaloub et al. 2010; Maxwell and Johnson 2000). As toxicity in plants can cause damage to the membrane of chloroplast and disturb the membrane structure leading to lowered chlorophyll biosynthesis (Miteva and Merakchiyska 2002; Stoeva et al. 2005). It was reported that algae could display stress or photoinhibition, and photosynthesis may often be downregulated at lower Fv/Fm values (Velez-Ramirez et al. 2017). Fv/Fm in brown algae ranged from 0.7 to 0.8, which is consistent with our initial Fv/Fm results in the tested algae (Büchel and Wilhelm 1993). The possible toxicity of As depends on its speciation, and it has been reported that 1 mg L-1 of soluble As causes damage to plants and algae. As(III) and As(V) are highly toxic to plants and can inhibit P uptake by detaching the process of phosphorylation (Vithanage et al. 2012). Lemna gibba, an aquatic macrophyte, has shown greater toxicity to As(III) concentration between 300 to 800 μg L-1. However, As(V) was more toxic than As(III) when the cultures received As(V) concentration above 800 μg L-1 (Mkandawire et al. 2004). It was also evidenced that freshwater microalgae are more sensitive to As(V), while marine microalgae are more sensitive to As(III) (Levy et al. 2005).
Growth rate of S. horneri
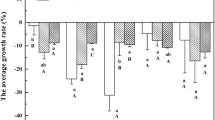
The algal shoots displayed no detectable sign of toxicity, no death tissue sections, color, and visual damage after 7 d exposure to various As species and P at different concentrations. The shoots looked healthy, remaining intact throughout the incubation period. The growth rate of algae was significantly impacted by the different concentrations of the As species studied. The different P levels also caused a significant difference in growth rate with the exception of DMAA(V) (p < 0.05). However, there were statistically insignificant variations for the interaction between As species and P concentrations (p > 0.05) (Table S2 in Appendix A). The fresh weights of the algal shoot were substantially increased over their initial weight after 7 d under most of the cultures treated with As(V), MMAA(V) or DMAA(V), but under As(III) treated cultures at above 1 μM concentration, the weights of the shoots were decreased. Noteworthy and negative growth rates were observed at higher As(III) levels, especially when the substrate began from 1 μM, whereas algae continued to generate growth rate even with a higher level of As(V), MMAA(V), and DMAA(V). Irrespective of P and As concentration, the growth rate (% d-1) of the different cultures enriched with As(V), As(III), MMAA(V), and DMAA(V) treatments ranged from – 0.11 to 1.06 (average 0.33 ± 0.09), – 2.23 to 1.02 (average – 0.29 ± 0.26), 0.12 to 0.81 (average 0.43 ± 0.09), and 0.18 to 0.88 (average 0.52 ± 0.11), respectively (Fig. 3 and 4). Increased levels of all As species were observed to decrease the growth rate. Significant negative correlation was determined between growth rate and As accumulation in the algae (r = − 0.64, − 0.88, − 0.72, and − 0.83 for As(V), As(III), MMAA(V), and DMAA(V) respectively, p < 0.01). As toxicity caused a decrease of growth rate and growth rate of algae was decreased by 81.5% when As(V) in the cultures increased from 0 to 4 μM under 10 μM P. Similarly, the decrease was 58.1% and 56% for cultures treated with MMAA(V) and DMAA(V), respectively. The increasing As(III) levels (1 to 4 μM) showed a negative growth rate regardless of the addition of P in cultures. P is vital for controlling the As toxicity, metabolism, and macroalgae growth.
It was observed that with increasing P concentration from 2.5 to 10 μM, both algal biomass and daily growth rate were increased in all As treated cultures. It was found that As(III)-treated algal shoots in P controlled culture (0 μM) had a relatively lower growth rate than P-added treatments regardless of As(III) concentration.
Reduced synthesis and substrate regeneration in the Calvin-Benson cycle, along with a decrease in the light utilization rate needed for carbon fixation, are correlated with the immediate effects of P limitation on algae (Chisti 2013). The P deficiency was also reported to enhance astaxanthin build-up and decrease chlorophyll a and protein content, leading to a reduced overall growth rate of microalgae (Juneja et al. 2013; Kobayashi et al. 1993). The overall results from the present study indicate that the organic As species (MMAA(V)/DMAA(V)) had a less severe effect on the growth rate than the inorganic species As(V), but a stronger effect than As(III). It is not known why As(V) and other two organic As species lead to an increase in algal biomass. Chlorella vulgaris has been reported to absorb both inorganic and methylated As species. Methylated species have been suggested to be further biomethylated into the cells that cause less stress for As (Maeda et al. 1992). The increased sensitivity of As toxicity to algae leading to interference with P metabolism or inhibition of the production of adenosine triphosphates (ATP) (Bhattacharya et al. 2015; Levy et al. 2005).
As accumulation by S. horneri
During a week (7 d) incubation period, the concentrations of TAs in the algae increased significantly with increasing external dosage of all concentrations of As species (p < 0.01). However, the effect of different P levels and the interaction effect between levels of P and As on the accumulation of TAs were not significantly varied when the cultures enriched with As(III), MMAA(V), and DMAA(V) (p > 0.05). Only the significant difference was found due to increasing concentrations of As(V) and P as well as their interaction (p < 0.01) (Table S3 in Appendix A). The changes in the content of TAs due to the effect of various species of As and P concentration are shown in Fig. 5 and 6. When the external As(V), As(III), MMAA(V), and DMAA(V) concentration was up to 4 μM together with 0 μM P, the alga was able to accumulate 638.2 ± 71.3, 404.1 ± 70.6, 176.7 ± 19.6, and 205.6 ± 33.2 nM g-1 dry weight TAs, respectively. The amounts of TAs taken up by the algae followed the trend: MMAA(V) < DMAA(V) < As(III) < As(V), regardless of the rate of addition of As and P. Such high affinity for different species of As at higher concentrations by S. horneri indicated that this alga was equipped with detoxification mechanisms that enabled it to accumulate additional As. Similar to the findings from previous macrophytes, microalgae, and macroalgae studies (Duncan et al. 2010; Mamun et al. 2019b; Rahman et al. 2008a), the present study also found concentration-dependent uptake of As from all As species.
Correlation analysis showed that the concentration of As in the tissues of algae was positively related to the addition rates of As in the cultures regardless of P addition (r = 0.96, 0.95, 0.98, and 0.98 for As(V), As(III), MMAA(V), and DMAA(V), respectively; p < 0.01). In comparison with the maximum TAs accumulation (454.1 ± 28.2, 200.0 ± 22.2, and 214.1 ± 34.0 nM g-1 dry weight from 4 μM of As(III), MMAA(V), and DMAA(V) with 10 μM P, respectively), the TAs concentration in alga was higher when exposed to the same level of As(V) with 0 μM P, reaching up to 638.2 ± 71.3 nM g-1 dry weight. Following exposure to different As species, our results show that S. horneri accumulate As in the form of As(V) and As(III) more efficiently compared to MMAA(V) and DMAA(V), which represent the bioavailable forms of inorganic As in seawater. At equal As and P concentrations in solution (4 μM As and 0 μM P), the concentrations of TAs in the algae were almost similar between the MMAA(V) and DMAA(V) treatments and were 3.6 and 3.1 times lower than As(V) and 2.3 and 1.9 times lower than As(III) treatment, respectively. Many studies have reported that inorganic As (As(III) and As(V)) were more easily accumulated in organisms, including algae, than organic As (MMAA(V)/DMAA(V)) due to the high transfer rate of inorganic As inside the living organisms (Cullen et al. 1994; Duncan et al. 2010; Rahman et al. 2008a). The methylated species showed relatively lower or decreasing uptake rates, and possibly it might be due to the increased hydrophobicity of methylated species compared with the inorganic As species (Zhao et al. 2013).
Irrespective of P concentration, when algal shoots were exposed to different concentration of As species, TAs concentrations ranged from 5.1 ± 0.6 to 638.2 ± 71.3, 5.6 ± 0.4 to 454.1 ± 28.2, 5.1 ± 0.8 to 199.9 ± 22.2, and 6.0 ± 0.9 to 214.1 ± 33.9 nM g-1 dry weight under As(V), As(III), MMAA(V), and DMAA(V) treatments, respectively. Such differences in accumulation might be the possible detoxification mechanisms due to different sensitivities and bioavailabilities towards As species of the algae (Rahman and Hassler 2014). The accumulation of As in previous algal study also suggested the varied accumulation of As mainly based on algae’s different susceptibility to different As-forms and algae-specific accumulation behavior (Bahar et al. 2013).
The relatively higher tolerance and accumulative capacity of As(V) by the algae are compatible with other observations of higher background As levels in the macrophyte and algae growing media (Mamun et al. 2019b; Rahman et al. 2008a; Wang et al. 2017). Also, As(V) may be more mobile and bioavailable forms than other As species for marine macroalgae when exposed to high As concentrations. It was noted that increased P in As(V) treatments showed significantly lower TAs absorption, while the opposite was observed for As(III), MMAA(V), and DMAA(V). This could have been due to competitive behavior between As(V) and P for similar routes. The presence of P could also increase the accumulation of TAs from the solutions of As(III) and organic As species.
When 10 μM P was amended with 4 μM As(V), TAs accumulation was significantly (p < 0.01) reduced by 43.9%, with respect to control (0 μM P). This observation was further supported statistically by obtaining significant (p < 0.05) negative correlation coefficient values between the TAs accumulation versus total P content tissues (Table S6 in Appendix A). However, there were nonsignificant and positive correlation (p > 0.05) observed between P content and As accumulation from As(III), MMAA(V), and DMAA(V). As(III), MMAA(V) and DMAA(V) are mainly absorbed in the aquaporin plasma membrane channel. Such uptake channel could be independent of P absorption, and as a consequence of soluble P in the medium, intracellular As accumulation had no effect (Li et al. 2009; Rahman and Hassler 2014). A significant reduction of uptake and toxicity of As(V) and As(III) were reported in Chlorella salina when P concentration in the seawater was 1.3 mg L-1. However, the presence of P didn’t cause any changes in the toxicity as well as intracellular accumulation of MMAA(V) and DMAA(V) (Karadjova et al. 2008). Studies showed that higher P in algal culture inhibits the As(V) uptake. The underlying fact might be the uptake regulation due to decline in the number of As(V) transporters or affinity on the cell membrane or competition for binding sites of cytoplasmic As(V) reductase, which ultimately lowering the intracellular content (Castro et al. 2015; Karadjova et al. 2008; Wang et al. 2013). It has been suggested that As(III) is more bioavailable and more toxic than As(V) to marine phytoplankton, particularly in the presence of high concentrations of dissolved inorganic P. The wide variability in sensitivities to As may be due to differences in biotic factors such as uptake and metabolic pathways, detoxification mechanisms, as well as abiotic factors such as the effects of P concentrations and exposure period (Levy et al. 2005).
P accumulation by S. horneri
The different concentrations of P showed a significant difference among the As species used in this study. Increasing As(V) concentration in the solution decreased the concentration of P in the algal tissues significantly (p < 0.01). In contrast, the concentration of other As species such as As(III), MMAA(V), and DMAA(V) treatments and their interaction effects with increasing P levels had a lower or nonsignificant effect on the P concentration in the algae (p > 0.05) (Table S3 in Appendix A). P concentration in the algal tissues generally increased with increasing levels of applied P, with the maximum P content found from high levels of P (10 μM) exposures such as 43.5 ± 3.8, 48.0 ± 5.7, 42.9 ± 8.4, and 46.5 ± 8.3 mg kg-1 from As(V), As(III), MMAA(V), and DMAA(V), respectively and the minimum from low levels of P (0 μM) exposures such as 26.9 ± 2.5, 28.5 ± 2.4, 30.7 ± 4.7, and 29.2 ± 2.0 mg kg-1 from As(V), As(III), MMAA(V), and DMAA(V), respectively (Table S4 in Appendix A). It is well established that P plays a vital role in living cells and can reduce the toxic effects of As(V) in plant tissues (Fayiga and Ma 2006). The highest P concentration in algae was found in 0 μM As(V) and 10 μM P treatment and the lowest in the 4 μM As(V) and 0 μM P. However, the pattern of P distribution under other As species treatments were markedly different from that of As(V) and not consistent with the increasing levels of As concentration. It was noticed that P addition to the media suppressed the As(V) uptake and its bioaccumulation. The possible mechanism towards this fact is that both ions share the same transporter for their uptake. As(V) and P are relatively similar in terms of geochemical behavior, and the absorption of As(V) through the P uptake system is well documented for different organisms, including algae. As(V) could compete with P for P transporters due to their structural similarity (Wang et al. 2016). The correlation results presented in Table S6 showed that the P accumulation by S. horneri decreased negatively with the accumulation of As(V) (r = − 0.48, p < 0.05). On the contrary, As(III), MMAA(V), and DMAA(V) treatments had a nonsignificant positive relationship on P uptake (r = 0.25, 0.10, and 0.002, p < 0.05 for As(III), MMAA(V), DMAA(V) respectively). The results indicated that the P accumulation into the algae might be inhibited by As(V) in the culture solution while its accumulation was not influenced by As(III) or organic As species like MMAA(V)/DMAA(V). In previous studies of macrophytes and macroalgae, similar findings were also reported (Mamun et al. 2019b; Rahman et al. 2008a).
As and Fe adsorption by S. horneri
Adsorption phenomena are common in aquatic organisms where suspended Fe oxides adsorb As on the surface of organisms (Robinson et al. 2006). Often because the culture media Fe was present in the form of a very stable Fe-EDTA chelate, the importance of the biological surface-bound Fe has been overlooked. However, even in the algal medium containing excess EDTA, the possibility and existence of surface binding have persisted (Miller et al. 2013). The mechanism involved with surface adsorption of As in algae species depends on the algae-specific surface properties of the cell wall. The critical processes driven for passive surface adsorption are electrostatic attraction, ion exchange, co-ordination, complexation, chelation, and micro-precipitation, etc (Dönmez et al. 1999). The Fe concentration in the intracellular parts as well as surface adsorbed parts (Fe-plaque) of algae were not significantly varied due to the different As species (p > 0.05). The intracellular and adsorbed Fe in algal tissues ranged from 87.2 ± 7.1 to 90.1 ± 9.4 and 19.1 ± 2.5 to 21.1 ± 2.9 nM g-1, respectively. The content of TAs in the tissues of algae (intracellular) at 4 μM of As was nearly similar and in line with the main experimental results in Fig. 5 and 6. A significant quantity of As was adsorbed onto the surface of algae when exposed to the same concentration of different species of As at 4 μM. It was observed that the adsorbed As was significantly different among the As species (p < 0.01) (Table 1).
Passive surface adsorption was found negligible for algal shoots exposed to As(III), MMAA(V), and DMAA(V). As(V) adsorption was found to be an active process in S. horneri because the surface of shoots showed higher amounts of As(V) adsorbed than other As forms. The amounts of adsorbed As due to exposure of As(V) was 63.7 ± 6.1 nM g-1 dry weight, which was 2.5, 7.3, and 6.9 times higher than the amounts of adsorbed As due to exposure of As(III, MMAA(V), and DMAA(V), respectively. Adsorption was found to be consistently lower in algae surface-mediated with As(III) as well as organic As species (MMAA(V)/DMAA(V)) and comparable to As(V). This may be because of the low adsorption affinities of As(III) in comparison with As(V) at the adsorption site (Chen et al. 2005). It was reported that certain algae species Dunaliella teritolecta (microalgae), Undaria pinnatifida and Sargassum patens, Sargassum horneri, Pyropia yezoensis (macroalgae) could bind inorganic As species (As(V) and As(III)) extracellularly (Duncan et al. 2010). However, there is no evidence of extracellularly surface adsorption of organic species like MMAA(V) and AB by Dunaliella teritolecta (Duncan et al. 2010; Mamun et al. 2019a; Mamun et al. 2019b; Mamun et al. 2019c). Though the significance of this external pool of As species adsorption is uncertain but necessitates further research in this area (Duncan et al. 2014).
Conclusion
The pattern of sensitivity to As species is important to link between environmental concentrations and macroalgal assemblage. The pronounced decreasing trend of chlorophyll fluorescence coupled with negative growth rate indicates the more toxicity of inorganic As(III) than As(V) and corresponding organic As species to the S. horneri. The study has demonstrated that tolerance of this algae to organic species (MMAA(V) and DMAA(V)) was greater compared to inorganic species (As(III) and As(V)), and the tolerance to As was significantly decreased with increasing exposure concentration. The increasing P levels showed to have a positive influence on ameliorating As stress. Irrespective of P levels, TAs bioaccumulation in 4 μM As(V) exposure was higher than 4 μM As(III) or MMAA(V) or DMAA(V) exposure, indicating that As(V) might be more bioavailable than other As species to the studied algae when faced with high As exposure levels. Although the tissue concentration of TAs in the algae increased more steeply in low P feeding algae with increased As(V) concentrations, the impact of P on other As species did not display similar trends. As(V) adsorption was found to be easier compared to any other organic and inorganic species. The accumulation behaviors of this alga may be exploited in a plant-based treatment system for remediation of As contaminated water. Also, it is possible to utilize this alga for algae-mediated biomonitoring tool on As toxicity. An additional benefit from this study is the direct comparison of different As species which are firmly connected by their chemistry. However, more research is required to explore the marine macroalgal differential sensitivity mechanisms of As species on a molecular basis.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Anninou P, Cave RR (2009) How conservative is arsenic in coastal marine environments? A study in Irish coastal waters. Estuar Coast Shelf Sci 82:515–524
ATSDR (2017) The ATSDR 2017 Substance Priority List. U.S.A.: Agency for Toxic Substances and Disease Registry, U.S. Public Health Service
Bahar MM, Megharaj M, Naidu R (2013) Toxicity, transformation and accumulation of inorganic arsenic species in a microalga Scenedesmus sp. isolated from soil. J Appl Phycol 25:913–917
Bhattacharjee H, Rosen BP (2007) Arsenic Metabolism in Prokaryotic and Eukaryotic Microbes. In: Nies DH, Silver S (Editors), Molecular Microbiology of Heavy Metals. Springer Berlin Heidelberg, Berlin, Heidelberg, pp. 371-406
Bhattacharya P, Chakraborty N, Pal R (2015) Bioremediation of toxic metals using algae. In: Das D (ed) Algal Biorefinery: An Integrated Approach. Springer International Publishing, Cham, pp 439–462
Büchel C, Wilhelm C (1993) In vivo analysis of slow chlorophyll fluorescence induction kinetics in algae: progress, problems and perspectives. Photochem Photobiol 58:137–148
Casado-Martinez M, Smith B, Luoma S, Rainbow P (2010) Bioaccumulation of arsenic from water and sediment by a deposit-feeding polychaete (Arenicola marina): A biodynamic modelling approach. Aquat Toxicol 98:34–43
Castro MCR, Urrea G, Guasch H (2015) Influence of the interaction between phosphate and arsenate on periphyton's growth and its nutrient uptake capacity. Sci Total Environ 503:122–132
Chaloub RM, Reinert F, Nassar CA, Fleury BG, Mantuano DG, Larkum AW (2010) Photosynthetic properties of three Brazilian seaweeds. Braz J Bot 33:371–374
Chen Z, Zhu YG, Liu WJ, Meharg AA (2005) Direct evidence showing the effect of root surface iron plaque on arsenite and arsenate uptake into rice (Oryza sativa) roots. New Phytol 165:91–97
Chisti Y (2013) Constraints to commercialization of algal fuels. J Biotechnol 167:201–214
Cosgrove J, Borowitzka MA (2010): Chlorophyll Fluorescence Terminology: An Introduction. In: Suggett DJ, Prášil O , Borowitzka MA (Editors), Chlorophyll A Fluorescence in Aquatic Sciences: Methods and Applications. Developments in Applied Phycology, vol 4. Springer, Dordrecht, pp. 1-17
Cullen WR, Reimer KJ (1989) Arsenic speciation in the environment. Chem Rev 89:713–764
Cullen WR, Harrison LG, Li H, Hewitt G (1994) Bioaccumulation and excretion of arsenic compounds by a marine unicellular alga, Polyphysa peniculus. Appl Organomet Chem 8:313–324
David AH, Wen XW, Mark AS, Nicholas SF (1999) Dual-labeling techniques for trace metal biogeochemical investigations in aquatic plankton communities. Aquat Microb Ecol 19:129–138
Devault DA, Massat F, Baylet A, Dolique F, Lopez P-J (2022) Arsenic and chlordecone contamination and decontamination toxicokinetics in Sargassum sp. Environ Sci Pollut Res 29:6–16
Devault DA, Modestin E, Cottereau V, Fabien V, Stiger-Pouvreau V, Ronan P, Alexandra C (2021) The silent spring of Sargassum. Environ Sci Pollut Res 28:15580–15583
Dönmez GÇ, Aksu Z, Öztürk A, Kutsal T (1999) A comparative study on heavy metal biosorption characteristics of some algae. Process Biochem 34:885–892
Duncan E, Foster S, Maher W (2010) Uptake and metabolism of arsenate, methylarsonate and arsenobetaine by axenic cultures of the phytoplankton Dunaliella tertiolecta. Bot Mar 53:377–386
Duncan EG, Maher WA, Foster SD, Krikowa F (2013) The influence of arsenate and phosphate exposure on arsenic uptake, metabolism and species formation in the marine phytoplankton Dunaliella tertiolecta. Mar Chem 157:78–85
Duncan EG, Maher WA, Foster SD (2014) Contribution of arsenic species in unicellular algae to the cycling of arsenic in marine ecosystems. Environ Sci Technol 49:33–50
Edmonds J, Francesconi K (2003) Organoarsenic Compounds in the Marine Environment. In: Craig P (ed) Organometallic Compounds in the Environment. England, Wiley, West Sussex, pp 196–222
Endo H, Suehiro K, Kinoshita J, Gao X, Agatsuma Y (2013) Combined effects of temperature and nutrient availability on growth and phlorotannin concentration of the brown alga Sargassum patens (Fucales; Phaeophyceae). Am J Plant Sci 4:14–20
Fayiga AO, Ma LQ (2006) Using phosphate rock to immobilize metals in soil and increase arsenic uptake by hyperaccumulator Pteris vittata. Sci Total Environ 359:17–25
Filella M, Williams PA, Belzile N (2009) Antimony in the environment: knowns and unknowns. Environ Chem 6:95–105
Foster S, Thomson D, Maher W (2008) Uptake and metabolism of arsenate by anexic cultures of the microalgae Dunaliella tertiolecta and Phaeodactylum tricornutum. Mar Chem 108:172–183
Francesconi KA (2010) Arsenic species in seafood: origin and human health implications. Pure Appl Chem 82:373–381
Hasegawa H, Tate Y, Ogino M, Maki T, Begum ZA, Ichijo T, Rahman IMM (2017) Laboratory culture experiments to study the effect of lignite humic acid fractions on iron solubility and iron uptake rates in phytoplankton. J Appl Phycol 29:903–915
Juneja A, Ceballos R, Murthy G (2013) Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: a review. Energies 6:4607–4638
Karadjova IB, Slaveykova VI, Tsalev DL (2008) The biouptake and toxicity of arsenic species on the green microalga Chlorella salina in seawater. Aquat Toxicol 87:264–271
Kobayashi M, Kakizono T, Nagai S (1993) Enhanced carotenoid biosynthesis by oxidative stress in acetate-induced cyst cells of a green unicellular alga, Haematococcus pluvialis. Appl Environ Microbiol 59:867–873
Levy JL, Stauber JL, Adams MS, Maher WA, Kirby JK, Jolley DF (2005) Toxicity, biotransformation, and mode of action of arsenic in two freshwater microalgae (Chlorella sp. and Monoraphidium arcuatum). Environ Toxicol Chem 24:2630–2639
Li R-Y, Ago Y, Liu W-J, Mitani N, Feldmann J, McGrath SP, Ma JF, Zhao F-J (2009) The rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiol 150:2071–2080
Loureiro RR, Reis RP, Berrogain FD, Critchley AT (2012) Extract powder from the brown alga Ascophyllum nodosum (Linnaeus) Le Jolis (AMPEP): a “vaccine-like” effect on Kappaphycus alvarezii (Doty) Doty ex P.C. Silva. J Appl Phycol 24:427–432
Ma Z, Lin L, Wu M, Yu H, Shang T, Zhang T, Zhao M (2018) Total and inorganic arsenic contents in seaweeds: Absorption, accumulation, transformation and toxicity. Aquaculture 497:49–55
Maeda S, Ohki A, Kusadome K, Kuroiwa T, Yoshifuku I, Naka K (1992) Bioaccumulation of arsenic and its fate in a freshwater food chain. Appl Organomet Chem 6:213–219
Maher WA, Foster SD, Taylor AM, Krikowa F, Duncan EG, Chariton AA (2011) Arsenic distribution and species in two Zostera capricorni seagrass ecosystems, New South Wales, Australia. Environ Chem 8:9–18
Mamun MAA, Datta RR, Kosugi C, Miki O, Oura M, Rahman IMM, Maki T, Hasegawa H (2017) Arsenic speciation and biotransformation by marine macroalgae in seawater, Asia/CJK Symposium on Analytical Chemistry. The Japan Society for Analytical Chemistry, Tokyo University of Science, Tokyo, Japan, pp. 62-63
Mamun MAA, Omori Y, Miki O, Rahman IMM, Mashio AS, Maki T, Hasegawa H (2019a) Comparative biotransformation and detoxification potential of arsenic by three macroalgae species in seawater: Evidence from laboratory culture studies. Chemosphere 228:117–127
Mamun MAA, Omori Y, Papry RI, Kosugi C, Miki O, Rahman IMM, Mashio AS, Maki T, Hasegawa H (2019b) Bioaccumulation and biotransformation of arsenic by the brown macroalga Sargassum patens C. Agardh in seawater: effects of phosphate and iron ions. J Appl Phycol 31:2669–2685
Mamun MAA, Rahman IMM, Datta RR, Kosugi C, Mashio AS, Maki T, Hasegawa H (2019c) Arsenic speciation and biotransformation by the marine macroalga Undaria pinnatifida in seawater: A culture medium study. Chemosphere 222:705–713
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
Miller EP, Böttger LH, Weerasinghe AJ, Crumbliss AL, Matzanke BF, Meyer-Klaucke W, Küpper FC, Carrano CJ (2013) Surface-bound iron: a metal ion buffer in the marine brown alga Ectocarpus siliculosus? J Exp Bot 65:585–594
Miteva E, Merakchiyska M (2002) Response of chloroplasts and photosynthetic mechanism of bean plants to excess arsenic in soil. Bulg J Agric Sci 8:151–156
Mkandawire M, Lyubun YV, Kosterin PV, Dudel EG (2004) Toxicity of arsenic species to Lemna gibba L. and the influence of phosphate on arsenic bioavailability. Environ Toxicol 19:26–34
Ozturk F, Duman F, Leblebici Z, Temizgul R (2010) Arsenic accumulation and biological responses of watercress (Nasturtium officinale R. Br.) exposed to arsenite. Environ Exp Bot 69:167–174
Rahman MA, Hasegawa H, Ueda K, Maki T, Rahman MM (2008a) Arsenic uptake by aquatic macrophyte Spirodela polyrhiza L.: Interactions with phosphate and iron. J Hazard Mater 160:356–361
Rahman MA, Hasegawa H, Ueda K, Maki T, Rahman MM (2008b) Influence of phosphate and iron ions in selective uptake of arsenic species by water fern (Salvinia natans L.). Chem Eng J 145:179–184
Rahman MA, Hasegawa H (2011) Aquatic arsenic: Phytoremediation using floating macrophytes. Chemosphere 83:633–646
Rahman MA, Kadohashi K, Maki T, Hasegawa H (2011) Transport of DMAA and MMAA into rice (Oryza sativa L.) roots. Environ Exp Bot 72:41–46
Rahman MA, Hassler C (2014) Is arsenic biotransformation a detoxification mechanism for microorganisms? Aquat Toxicol 146:212–219
Rahman MA, Hogan B, Duncan E, Doyle C, Krassoi R, Rahman MM, Naidu R, Lim RP, Maher W, Hassler C (2014) Toxicity of arsenic species to three freshwater organisms and biotransformation of inorganic arsenic by freshwater phytoplankton (Chlorella sp. CE-35). Ecotoxicol Environ Saf 106:126–135
Robinson B, Kim N, Marchetti M, Moni C, Schroeter L, van den Dijssel C, Milne G, Clothier B (2006) Arsenic hyperaccumulation by aquatic macrophytes in the Taupo Volcanic Zone, New Zealand. Environ Exp Bot 58:206–215
Stiger-Pouvreau V, Mattio L, De Ramon N’Yeurt A, Uwai S, Dominguez H, Flórez-Fernández N, Connan S, Critchley AT (2023) A concise review of the highly diverse genus Sargassum C. Agardh with wide industrial potential. J. Appl. Phycol. https://doi.org/10.1007/s10811-023-02959-4
Stiger-Pouvreau V, Zubia M (2020) Macroalgal diversity for sustainable biotechnological development in French tropical overseas territories. Bot Mar 63:17–41
Stoeva N, Berova M, Zlatev Z (2005) Effect of arsenic on some physiological parameters in bean plants. Biol Plant 49:293–296
Suggett DJ, Prášil O, Borowitzka MA (2010) Chlorophyll a fluorescence in aquatic sciences: methods and applications, 4. Springer
Taylor VF, Jackson BP (2016) Concentrations and speciation of arsenic in New England seaweed species harvested for food and agriculture. Chemosphere 163:6–13
Velez-Ramirez AI, Carreño-Quintero N, Vreugdenhil D, Millenaar FF, van Ieperen W (2017) Sucrose and starch content negatively correlates with PSII maximum quantum efficiency in tomato (Solanum lycopersicum) exposed to abnormal light/dark cycles and continuous light. Plant Cell Physiol 58:1339–1349
Vithanage M, Dabrowska BB, Mukherjee AB, Sandhi A, Bhattacharya P (2012) Arsenic uptake by plants and possible phytoremediation applications: a brief overview. Environ Chem Lett 10:217–224
Wang NX, Li Y, Deng XH, Miao AJ, Ji R, Yang LY (2013) Toxicity and bioaccumulation kinetics of arsenate in two freshwater green algae under different phosphate regimes. Water Res 47:2497–2506
Wang Y, Wang S, Xu P, Liu C, Liu M, Wang Y, Wang C, Zhang C, Ge Y (2015) Review of arsenic speciation, toxicity and metabolism in microalgae. Rev Environ Sci Biotechnol 14:427–451
Wang Y, Zhang CH, Lin MM, Ge Y (2016) A symbiotic bacterium differentially influences arsenate absorption and transformation in Dunaliella salina under different phosphate regimes. J Hazard Mater 318:443–451
Wang Y, Zhang C, Zheng Y, Ge Y (2017) Bioaccumulation kinetics of arsenite and arsenate in Dunaliella salina under different phosphate regimes. Environ Sci Pollut Res 24:21213–21221
Zhang S, Rensing C, Zhu YG (2014) Cyanobacteria-mediated arsenic redox dynamics is regulated by phosphate in aquatic environments. Environ Sci Technol 48:994–1000
Zhao F-J, Zhu Y-G, Meharg AA (2013) Methylated arsenic species in rice: geographical variation, origin, and uptake mechanisms. Environ Sci Technol 47:3957–3966
Zhao FJ, Ma JF, Meharg AA, McGrath SP (2009) Arsenic uptake and metabolism in plants. New Phytol 181:777–794
Acknowledgements
The study has been partially supported by Grants-in-Aid for Scientific Research (18H03399 and 19K21897) from the Japan Society for the Promotion of Science.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors participated in the conception and design of the study. M. Abdullah Al Mamun, Shuhei Hayashi, and Rimana Islam Papry prepared the materials, collected the data, and conducted the chemical analysis. M. Abdullah Al Mamun performed the statistical analysis and wrote the first version of the manuscript. Osamu Miki, Ismail M. M. Rahman, and Asami S. Mashio discussed the results. Hiroshi Hasegawa supervised the study. All authors commented on and reviewed initial drafts of the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: V.V.S.S. Sarma
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 42 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mamun, M.A., Hayashi, S., Papry, R.I. et al. Influence of Different Arsenic Species on the Bioavailability and Bioaccumulation of Arsenic by Sargassum horneri C. Agardh: Effects under Different Phosphate Conditions. Environ Sci Pollut Res 30, 98246–98260 (2023). https://doi.org/10.1007/s11356-023-29371-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-29371-2