Abstract
NOx, SOx, and carbonaceous volatile organic compounds (VOCs) are extremely harmful to the environment, and their concentrations must be within the limits prescribed by the region-specific pollution control boards. Thus, NOx, SOx, and VOC abatement is essential to safeguard the environment. Considering the importance of NOx, SOx, and VOC abatement, the discussion on selective catalytic reduction, oxidation, redox methods, and adsorption using noble metal and non-noble metal-based catalytic approaches were elaborated. This article covers different thermal treatment techniques, category of materials as catalysts, and its structure–property insights along with the advanced oxidation processes and adsorption. The defect engineered catalysts with lattice oxygen vacancies, bi- and tri-metallic noble metal catalysts and non-noble metal catalysts, modified metal organic frameworks, mixed-metal oxide supports, and their mechanisms have been thoroughly reviewed. The main hurdles and potential achievements in developing novel simultaneous NOx, SOx, and VOC removal technologies are critically discussed to envisage the future directions. This review highlights the removal of NOx, SOx, and VOC through material selection, properties, and mechanisms to further improve the existing abatement methods in an efficient way.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Industrial revolution and growing global energy needs have been the prominent cause of air pollution. Uncontrolled release of pollutants and emissions contribute to global warming, climate change, acid rain, and other environmental changes (Munsif et al. 2021). These emissions include SOx, NOx, volatile organic compound (VOC), and CO emissions which are primary pollutants and are directly produced from industrial processes (Roberts 2021). VOCs react with NOx (mainly nitrogen dioxide) to form fine particles or secondary pollutants and accumulation of these secondary pollutants (ozone, fine particulates) causes smog. Apart from affecting the environment, adverse health effects are possible with emissions. According to a report by the economic consequences of outdoor air pollution, the worrying situation of air pollution is increasing premature deaths. The Organization for Economic Co-operation and Development’s (OECD) ENV-Linkages model, a computable general equilibrium (CGE) model, suggests the possibility of increase in deaths from 3 to 9 million in a span of 2010 to 2060. The number of premature deaths is unequally distributed across the world. The highest number of deaths takes place in non-OECD economies and particularly in China and India. Other major impacts of air pollution are as follows: reduced labor productivity, increased health expenditures and crop yields. Globally these impacts continue to increase significantly relative to gross domestic product (GDP). In contrast, agricultural impacts are relatively stable over time in percentage of GDP, i.e., in absolute terms these impacts grow more or less at the same speed as GDP. Taken together, the total annual market costs of outdoor air pollution are projected to rise from 0.3% in 2015 to 1.0% by 2060 (Highlights 1978).
Major pollutant like carbon monoxide emissions can directly cause poisoning when intake reaches high ppm levels and other emissions are responsible for respiratory problems like asthma, central nervous systems diseases, chronic pulmonary disease, and cardiovascular diseases occur in individuals with long-term exposure to air pollution. Repeated exposure to them can also lead to skin-related problems like melisma and photodamage. Prolonged exposure to NOx and SOx can cause breathing issue, bronchospasm, and pulmonary edema. Exposure to VOCs can cause headache, nausea, and along with respiratory issues. The longer exposure to the compound can affect liver and kidney and can even cause cancer (Puri et al. 2017; Manisalidis et al. 2020; Shi et al. 2021a, d). Damage up to this extent mainly occurs in urban and industrial areas due to industrial emissions and vehicular emissions (Ma et al. 2021b). To decrease such adverse effects, several countries have set up emission standards for industries and automobiles so that these emissions are limited. As of 2020, 124 countries (about two thirds) were found to have national ambient air quality standards which is 17% more than reported in 2016. However, only 9% of these adhere to the limits established by the World Health Organization (WHO) guidelines.
US Environmental Protection Agency implemented National Ambient Air Quality Standards where there are limits for each pollutant. Similarly, South Korea follows Clean Air Conservation Act and Japan follows Environmental Quality Standards for air pollution limits. Also, several countries have set up emission standards for industries and automobiles so that these emissions are limited. There is a standard for automobile emission where the current standard follows Euro 6 by most of the countries including South Korea, European Union, Thailand, Indonesia, etc. The Central Pollution Control Board (CPCB) of India has set emission standards for 31 categories of industries for control of air pollution. Different standards and concentration limits of the pollutants are explained in Table 1. Therefore, the need to render these pollutants harmless before releasing them into the environment is extremely important, for which several treatment technologies have to be implemented.
Types of emissions
NOx
Nitric acid and nitrogen dioxide (NO and NO2) are major contributors to air pollution and take a part in global warming and greenhouse effect. Industrial sources include power plants, industrial boilers, cement kilns, turbines, and other major sources are automobiles. Another oxide of nitrogen, N2O has 300 times higher greenhouse impact on a per molecular basis than carbon dioxide (Boningari and Smirniotis 2016; Skowron et al. 2021). They have adverse effects on human health too and therefore strict regulations and standards have been set up to limit NOx emissions which might have to be tightened further in future (Liu et al. 2018). Several methods have been found for NOx abatement but many of them are complex, produce undesirable products, and are not cost-effective (Roy et al. 2009; Martinez-Oviedo et al. 2021). Selective catalytic reduction (SCR) in presence of ammonia is one of the most effective ways for control of industrial NOx emissions (Tian et al. 2021a).
SOx
SOx emissions are majorly caused by combustion of fossil fuels at industries and power plants. SOx have acidifying effect and can harm both land and water by means of acid rain thereby reducing the growth of plants. Also, it can affect the respiratory tract and can cause lung infection (Jiang et al. 2011; Liu and Guo 2021). For SOx abatement, several pre-combustion and post-combustion techniques can be used like pressurized fluidized bed combustion which provides to about 98% SO2 reduction and sorbents can be used for removal of SOx (Rahmaninejad et al. 2012).
Carbon compound emissions
VOCs are all organic chemicals that include compounds of carbon. They also play a role in formation of secondary organic aerosols via photo-oxidation along with nitrogen oxides and sunlight which are found in airborne particulate matter and are the key precursors in formation of ground level ozone (Atkinson and Arey 2003). As far as human health is concerned, BTEX (group of VOCs of benzene, toluene, ethylbenzene, and xylenes) have carcinogenic effects and can lead to toxicity on inhalation even at low concentrations. Other health effects include headache, neurological diseases, eye irritation, etc. (Ueno et al. 2001; Kampa and Castanas 2008, 2018). Owing to stringent regulations, VOC abatement treatments have gained importance in the past few years and catalytic oxidation is regarded as one of the most efficient and promising approaches for VOC removal (Wu et al. 2016; Méausoone et al. 2019; Liu et al. 2021). Other major pollutant is carbon monoxide (CO) and carbon dioxide (CO2) which is present as a pollutant in troposphere and plays a significant role in global carbon cycle and greenhouse effect (Streets et al. 2006; Jiang et al. 2015). Catalytic oxidation and water–gas shift reactions are promising methods for CO abatement (Gokhale et al. 2008; Zhu and Wachs 2015; Chen and Chen 2020), where normally in water–gas shift reaction, CO reacts with water to form CO2 and H2. CO2 hydrogenation and reforming reactions are helpful to address CO2 abatement, where in reforming, CO2 reacts with CH4 to give syngas (Kathiraser et al. 2015; Cai and Hu 2019; Yang et al. 2019; Neha and Vir Singh 2020). However, in this review, our discussion is limited to NOx, SOx, and VOC abatement.
Treatment technologies
The research and adaptation of promising, cost-effective methods for industrial emission control have taken a faster pace in the past few years. A lot of technologies like photocatalysis (Roy et al. 2007b, a), catalytic ozonation (which is more cost effective in comparison to single ozonation), plasma catalysis, selective catalytic reduction/oxidation, storage and reduction (Roy and Baiker 2009; Roy et al. 2010, 2012), and adsorption are found to be effective methodologies. For automobile and vehicular emissions, control catalytic converters prove to be a promising method (Casagrande et al. 2020; Guo et al. 2020; Naveenkumar et al. 2020; Li et al. 2021c; Shang et al. 2021; Wang et al. 2021b; Tan et al. 2022).
Catalytic oxidation/reduction
Techniques such as catalytic oxidation, selective catalytic reduction, and selective catalytic oxidation occurs in presence of catalysts that decrease the activation energy, thereby increases the rate of reactions. Packed and fluidized bed catalytic reactors are found to be economically feasible, produces low concentrations of secondary pollutants at large scale, and can occur at low temperatures than other treatment methods (Zhao et al. 2020b; Guo et al. 2021b; Li et al. 2021b). As most of the catalysts require high temperatures for the emission removal, the obvious energy needs become greater and which significantly increases the operating cost of the process. Hence, research on the development of efficient catalysts is essential to bring down the operating costs. The catalytic efficiency can be increased in several ways such as tuning the catalyst preparative method, doping the active metal over other oxides/metals or by changing the support of the catalyst and modifying the catalyst morphology (Roy et al. 2007d, c, 2008c; Roy and Hegde 2008; Jurado et al. 2021; Zhang et al. 2021c). Recently, a lot of focus has been laid upon research of low temperature catalysts because of high return and low operational cost. Figure 1 shows the number of published research papers on low-temperature reactions from 2000 to 2021 as obtained from Scopus data. It indicates the growing scientific interest on pollution control through catalytic routes.
Adsorption
Adsorption is widely recognized as a highly effective and non-destructive method for the removal of volatile organic compounds (VOCs) and nitrogen oxides (NOx). It offers several advantages, including the ability to recover and reuse the adsorbed VOCs, making it an environmentally friendly approach. This has led to increased attention on commercially available adsorbents such as activated carbon, silica gel, biochar, and zeolites, which have proven to be highly efficient and cost-effective options for adsorption processes. Activated carbon is one of the most commonly used adsorbents due to its large surface area and high adsorption capacity. It exhibits excellent performance in removing a wide range of VOCs and NOx from various sources. Silica gel, another popular adsorbent, possesses a high affinity for polar molecules and is particularly effective in adsorbing moisture and certain types of VOCs. Biochar, derived from biomass pyrolysis, has emerged as a promising adsorbent in recent years. Its porous structure and surface chemistry make it suitable for VOC and NOx adsorption. Zeolites, with their well-defined pore structure and high thermal stability, are also extensively utilized for adsorption applications. They exhibit selective adsorption properties for specific VOCs and have shown remarkable performance in pollutant removal. Research studies conducted by Wu et al. (2019; Wu et al. 2021; Zhang et al. 2021a) have further highlighted the effectiveness of these adsorbents in VOC and NOx removal. Their findings demonstrate the successful application of these materials in various industries, emphasizing the potential of adsorption as a reliable and efficient technique.
Redox methods
Several advanced oxidation processes (AOPs) with good prospects for developing air cleaning technologies are UV/ozonation, H2O2/ozonation, or photocatalysis. The advantage of photocatalysis implementation in indoor air treatment is given by the absence of additional oxidizers (such as H2O2). The photocatalysis can occur at low temperatures and catalyst is used for longer hours (Sekiguchi et al. 2010; Challagulla et al. 2019).
The present comprehensive review aims to provide a consolidated overview of recent advancements in the field of NOx, SOx, and VOC abatement through catalysis. Though there are several review articles on NOx reduction and VOC abatements (He et al. 2019; Chen et al. 2021a; Zhao et al. 2022; Li et al. 2023), here we present a review which encompasses a wide range of topics, including NOx, SOx reduction, and VOC abatement by advanced catalysis techniques and adsorption. The development and optimization of catalytic materials play a vital role in achieving efficient and sustainable reduction of harmful emissions, contributing to improved air quality and a healthier environment. The performance characteristics of noble metal and non-noble metal catalysts are dealt in this study in the controlling mechanism point of view. The effect of mono-, bi-, and tri-metallic dopants on the reaction mechanism is also investigated. Further, redox and adsorption methods are also investigated for the removal of toxic gases. This will enable the researchers to choose a suitable material to abate the harmful gases in terms of energy efficiency, abundancy, and economics for catalytic/non-catalytic processes. By providing an amalgamated overview of the recent progress made in the field, this report aims to enhance understanding and knowledge regarding the significant role played by catalysis in the abatement of NOx, SOx, and VOCs, paving the way for further research and technological advancements in the field. A detailed schematic on the abatement technologies for NOx/SOx, VOC is shown in Fig. 2.
SCR for NOx removal
SCR is seen as an efficient method for NOx reduction to N2. Commercially used catalysts operate at 300–400 °C. But at higher operating temperature, sulfur contamination in the feed can cause sintering of the catalyst and affect its stability. Thus, sulfur-tolerant and low-temperature SCR is a welcoming strategy with efficient catalysts (Pappas et al. 2016; Yan et al. 2021). The demand for efficient NOx removal has led to the development of several classes of SCR catalysts. SCR mainly involves three reactions named as standard SCR, fast SCR, and NO2-SCR (Zhou et al. 2020). SCR can be categorized into noble metal based (Pt, Pd, Au, Ag) and non-noble or transition metal-based catalysts (Mn, Ce, Cu, Fe, V, W) (Roy et al. 2008a, b, d; Li et al. 2017b; Liu et al. 2017; Jiang et al. 2018a; Chang et al. 2020; Zhang et al. 2021b).
Noble metal-based catalysts for SCR: Pt, Ru, Rh, Au, Pd
Noble metals like Pt, Pd, Ru, Rh, etc. are used as the catalyst for SCR. Very high conversions to NH3 and good N2 selectivity are possible with noble metals like Pt, Ru, Rh, and Pd. Some of the noble metal-based catalyst systems for NOx conversion are shown in Table 2 (Sato et al. 2016), and Pt-based catalysts are also important for NOx reduction (García Cortés et al. 2007). Another important aspect emerged is the metal-support interaction. Synergistic effect of noble metal with other metals as metal-support increases the activity (Xue et al. 2018). Pt/La0.7Sr0.2Ce0.1FeO3 catalyst can convert 87% of 2500 ppm NO at 150 °C (Costa et al. 2002). Yang et al. has shown that doping with noble metals such as Pt, Pd, Rh, Ru on MnOx-CeO2 matrix has increased the catalytic activity compared to undoped structure (Yang et al. 2018).
Non-noble metal-based catalysts
Non-noble metal catalysts like manganese, cerium, and vanadium are widely accepted as SCR catalysts due to its abundancy, low cost, high activity towards NOx conversion, nitrogen selectivity, and sulfur resistance. Vanadium-based catalysts such as V2O5/TiO2 and V2O5/WO3/TiO2 are currently commercially used SCR catalysts that operate at temperatures above 350 °C (Cha et al. 2016; Wang et al. 2018a; Yang et al. 2020; Inomata et al. 2021; Wu et al. 2021; Zhang et al. 2021a). Furthermore, details on non-noble metal catalysts are elaborated in the following sections.
Non-noble metal oxides
Non-noble metal oxides generally constitute transition metals and rare earth metals. They have gained significant attention because of their decent catalytic performance for the potential applications in SCR. Yarong et Al. prepared a series of Co3O4 nanoparticles inlaid in porous carbon (CoOx@PC-T) using pyrolysis of ZIF-67 in nitrogen at different temperatures. For the sample of CoOx@PC-800, greater than 80% conversion was seen in the temperature range of 150–175 °C with high nitrogen selectivity as shown in Fig. 2d–f. The greater ratio of Co3+/Co2+, larger surface area, and oxidizing capability of the catalyst were responsible for its performance as observed from the XPS shown in Fig. 3a–c (Bai et al. 2019). Haoxi et al. (Jiang et al. 2018b) synthesized manganese-based catalysts by a solvo-thermal method with several capping agents of PVP, P123, HAc, and CTAB to expose crystal facets of α-MnO2. MnO2-PVP sample with exposed (200) crystalline facets showed 100% NO conversion at 200 °C which was better than other samples. These samples also had adsorption capabilities among which MnO2-PVP had the highest adsorption capacity. The (200) crystalline facet has maximum exposed acid sites for MnO2-PVP, and it helped the reactant leading to maximum conversion at 200 °C.

Reproduced from Elsevier 2019 (Bai et al. 2019)
XPS spectra of Co 2p in CoOx@PC-600 (a), CoOx@PC-800 (b), and CoOx@PC-1000 (c). NH3-SCR activity of Co@PC-800 and CoOx@PC-800 (d). NH3-SCR activity (e) and N2 selectivity (f) of CoOx@PC-600, CoOx@PC-800, and CoOx@PC-1000. Reaction conditions: [NOx] = [NH3] = 500 ppm, [O2] = 5 vol%, Ar balance, and GHSV = 14,000 h.−1.
Ole et al. (Bjørkedal et al. 2022) used zirconia as support and developed Cu/ZrO2 by sol–gel method with different loading amounts of Cu (3%, 6%, 15%) which showed promising SCR performance, providing 75% NO conversion at 150 °C for 6 wt.% catalyst. Even though increase in copper loading (15%) increased the SCR activity at 150 °C, it was found to undergone sintering above 350 °C and showed poor selectivity. Therefore, 6% Cu on ZrO2 is found to be better catalyst among the others.
Table 3 lists the variety of other non-noble metals used for the NOx conversion and further emphasis on the role of mono-metallic and bi-metallic non-noble catalysts for the NOx reduction are briefly showcased.
Non-noble metal based bimetallic catalysts
Bimetallic catalysts are combination of two different metals that exhibits several new and improved catalytic properties. For instance, Liu et al. (2020b) prepared Sm-Mn/TiO2 by modifying Mn/TiO2 by Samarium using ultrasonic impregnation method which improved the surface dispersion of active metal cation and Mn/TiO2 were also prepared for comparison. Mn/TiO2 showed best catalytic activity with optimum Mn loading of 20% showing only 52% NOx conversion at 120 °C whereas 10 wt.% Sm modified Mn/TiO2 showed best low-temperature catalytic activity. The optimal interaction between Mn and Sm species was achieved with 10 wt.% Sm and 20 wt.% Mn loading forming 20Mn-10Sm/TiO2 which exhibited greater than 80% conversion in 110 − 250 °C. Similarly, Liu et al. (2020a) used urea instead of ammonia as a reductant and binary catalyst Cu-Mn/NUAC and Cu-Co/NUAC were synthesized by ultrasonic-assisted impregnation method for LTU-SCR, and it was found that catalytic performance of binary catalysts was better than unary catalyst. Their study shows the synergic effect between the two metals (Cu-Co/Mn) which will enhance the catalytic properties compared to the individual metals. One hundred percent NO conversion for Cu0.5Mn0.5/NUAC was observed as the temperature increased from 50 to 80 °C due to the synergy between the oxides of active metal increases in this range. Synergistic mechanism of a catalyst involves distinct catalytic sites acting on different substrates sites to enhance the catalytic activity.
Vanadium-based catalysts generally offer high SO2 resistance, high catalytic activity due to that these materials preferred commercially (Xu et al. 2019; Kwon et al. 2021). Zhu et al. (2018) looked for the effect of niobium oxide as a promoter on V/WT (vanadium-tungsten-titanium) catalyst with different contents of dopant. Out of different contents, 6 wt.% niobium oxide (3V6Nb/WTi) was found to be appropriate and catalyst deactivation was found to be negligible at around 250 °C. Similarly, the commercially used V2O5-WO3/TiO2 was mixed with several Fe2O3 samples (Fe(N), Fe(P), and Fe(C)). NOx conversion increased with 95% for VW/Ti + Fe at 300 °C. Addition of Fe2O3 inhibited the formation of ammonium sulphate thereby preventing catalyst deactivation (Zhu et al. 2018). In another study, Quanming et al. and Woojoon et al. (Cha et al. 2016; Liang et al. 2021) studied the effect of addition of cerium oxide on commercially used binary V2O5-WO3/TiO2 for NH3-SCR. CeO2-V2O5-WO3/TiO2 was prepared via one-step impregnation method using 0% and 3% amount of CeO2. It was observed that 3Ce-3VWT could achieve 99.3% NO conversion at 160 °C. V4+ and V5+ was interchanged by the addition of 3% CeO2, thereby increasing the NO2/NOx ratio in flue gas that improved the low temperature catalyst.
Yunfan et al. (Xu et al. 2019) studied about its effect of V2O5-MoO3/TiO2 and V2O5-WO3/TiO2 monolithic catalysts. NOx conversion was observed to increase from 70 to 88% at 220 °C for V2O5-MoO3/TiO2 while it decreased for V2O5-WO3/TiO2. Manganese based catalysts are seen to have excellent redox ability at low temperature and have various valence states due to that these materials are highly recommended as SCR catalysts (Fang et al. 2019; Tang et al. 2020). Specifically, the mixed metal oxides such as MnOx-CeO2 can reduce NOx even below 250 °C. Support materials also directly affect the catalytic performance and plays an important role in SCR. Its structure, size, and surface area have a critical role in catalytic performance (Patel and Sharma 2021). Bora Ye et al. prepared nitrogen-doped reduced graphene oxide as a support for Mn-Ce oxide catalyst (Mn-Ce/N-rGO). The catalytic activity was seen to provide 80% conversion at 200 °C. This could be achieved instead of using TiO2 as support as the nanoparticles were evenly distributed without any aggregation on N-rGO which remains thermally stable at temperatures lower than 300 °C (Ye et al. 2021). Carbon monoxide can also be used as a reductant for SCR instead of NH3. It has good reducing capability to convert NO into CO2 and N2 (Zhang et al. 2020b). Recently, metal organic frameworks (MOFs) have found to be capable for SCR due to high surface area, porosity, and synthetic tunability (Jiang et al. 2016, 2018a). Shi et al. (2021b) prepared bimetallic catalyst Ni1-xMnx-MOF-74 with different molar ratio of Mn/(Mn + Ni) (x = 0.1, 0.2, 0.35, and 0.5) via one-pot solvothermal method which all reported higher conversion percentages in comparison to monometallic Ni-MOF-74 and Mn-MOF-74. Among them, Ni0.65Mn0.35-MOF-74 achieved highest conversion of nearly 100% at 175 °C and 100% nitrogen selectivity at around 200 °C. Similarly, Zhong et al. (Wang et al. 2020) prepared a novel ball-flowerlike bimetallic CoMnOx-BF catalyst using hydrothermal method for NH3-SCR. It was observed that it was SO2 resistant and had better catalytic performance in terms of conversion (in range of 150 to 350 °C) and N2 selectivity than CoMnOx catalyst and was also more durable catalyst to provide high NOx conversion till 36 h. This is attributed to the large surface area of the CoMnOx-BF catalyst, also the strong interaction between Mn and Co.
Non-noble metal-based tri/poly-metallic catalysts
Some dopants like Co and Ce can be used to enhance the performance of bimetallic catalysts and resistance against SO2 deactivation (Wang et al. 2019a). Liu et al. (2019b) studied the effect Co doping onto Mn-Sm/Ti for NH3-SCR using ultrasonic-assisted impregnation method with various amounts of doping. For 5 wt.% Co loading, 5CoMnSm/Ti achieved greater than 90% conversion at 100–200 °C having a GHSV of 60,000 h−1. Qinghua et al. (Yan et al. 2018) prepared a Cu0.5Mg1.5Mn0.5Al0.5Ox synthesized from layered double hydroxides via co-precipitation method. It showed high conversion ranging from 87 to 96.6% in 100–250 °C. The highest NOx conversion was observed for Cu0.5Mg1.5Mn0.5Al0.5Ox providing 96.7% conversion at 150 °C in comparison to others Cu0.5Mg1.5Mn0.5Al0.5Ox (96.7%) > Mg2Mn0.5Al0.5Ox (93.3%) > Cu1Mg1- Mn0.5Al0.5Ox (92.2%) > Cu1.5Mg0.5Mn0.5Al0.5Ox (91.3%) > Cu2Mn0.5Al0.5Ox (91.2%). Similarly, Wei et al. (Wei et al. 2018) used co-precipitation method to synthesize a series of iron-samarium mixed oxide catalysts modified by zirconium, cobalt, and titanium. Ti0.1Sm0.075Fe0.825Ox-400 sample possessed the best activity up to 95% at 150 °C and N2 selectivity of Ti0.1Sm0.075Fe0.825Ox-400 and Zr0.1Sm0.075Fe0.825Ox-400 was above 95% which was higher than that of Sm0.075Fe0.825Ox-400 between 100 and 250 °C, suggesting that modification by transition metals can enhance the catalytic activity of iron-samarium mixed oxides. Tourmaline, a manganese-iron-cerium-oxide composite, plays an important role in enhancing the catalytic activity. Zhao et al. (Zhao et al. 2020a) observed the effect of tourmaline on CeMnFeOx catalyst. Manganese-iron composite catalyst and a manganese-iron-cerium composite catalyst were prepared and named as M7F3 and MFC-0.02. For 2% addition of tourmaline, NOx conversion for MFCT-2% was 100% at 170 °C which was highest among all the other samples. Addition of tourmaline promotes the uniform distribution of layered composite materials on the surface and even small quantities of tourmaline can disperse the particles more effectively. The structure of catalysts also plays a critical role in its activity. Zhou et al. (Zhou et al. 2020) synthesized a two-dimensional MnFeCo layered double oxide (MnFeLDO, MnCo-LDO, and MnFeCo-LDO), based on the transition metal chemical compositions. Layered double oxides have large specific surface area and various active sites that enhance the activity. It was found that at 100 °C, 100% NO conversion was achieved and even at 50 °C, 86% conversion was observed. In presence of 5% H2O vapor and 100 ppm SO2, NO conversion was seen to be 89% and this further shows layered double oxide materials as the highly effective catalysts even under these operating conditions.
Alkali metal promoted NOx reduction
The electro-positive nature of alkali metals as promoters are gaining interest in scientific community for NOx reduction. Matsouka et al. have studied the un-promoted and sodium promoted Pt catalyst on supports such as Al2O3, CeO2/La2O3 modified Al2O3. The study was conducted at under automobile exhaust conditions with CO, NO, C3H6, and O2. Pt on Al2O3 support mainly has formats and acetates, leading to carbonyl species formation. But when Na is added as a promoter 100% NO reduction was at 395 °C. Konosolakiss et al. studied two different promotions separately to observe the synergy effect on de-NOx properties. Pd/Al2O3-TiO2 system and K doped Pd(K)/Al2O3-TiO2 system was thoroughly studied and found that K doping improves NOx reduction (85% at 100–400 °C) and N2 selectivity. This indicates a synergy effect in the latter catalyst. This is because Na usually strengthens the metal-NO bond and that, in turn, weakens the N–O bond due to its electro-positive nature to adsorb the electron accepter molecule NO. This was confirmed by performing TPD over model Na on Pt (111) surface. Yentekasis et al. explored a comparative study by a promoted Pt/γ-Al2O3 for three reactions C3H6 + NO + O2, C3H6 + O2, and NO + O2 at similar conditions with excess oxygen. Their studies showed how Na affecting electron-donating hydrocarbon and electron-accepting NO at lower temperatures. At Na loading of 2.6%, the NO dissociation is found to be limited with significant NO2 formation. In another study by Consuega et al., Pt/K-γAl2O3/Pt was used as electrochemical catalyst for NOx storage/reduction properties. Here, the catalytic system allows for the electrochemical storage of NOx as potassium nitrates and simultaneously decomposes by the positively polarized film without changing the reaction atmosphere. The electro-positive nature of alkali metals supports NO decomposition and, thereby, NOx reduction. This happens via bond strengthening of NO on alkali metal-induced metal.
Hydrocarbons/H2 for NOx reduction
Currently, there is a significant focus on reducing NOx emissions using hydrocarbons or carbon monoxide, mainly by taking out gas mixtures from exhaust gases like CO. In NOx reduction with hydrocarbon, first NOx will be oxidizing the surface nitrates along with conversion of hydrocarbons to surface oxygenates. Further reaction between these intermediates leads to the formation of NCO−, CN− species and finally forms N2 and CO2. Boutros et al. has studied the NOx reduction by ethanol using Ag and Al supported mesoporous SBA-15. The first catalyst, Ag-SBA-15 showed a better catalytic activity as Ag was doped in the catalytic system efficiently because of incipient wetness impregnation method (Boutros et al. 2009). In another study by Oton et al. NOx reduction was done by CO by using Pt, Ni, Co, Fe, or Ni nanoparticles dispersed on porous alumina. The interaction and synergy between Pt/Ni nanoparticle and the support Al2O3 made Pt/Al2O3 and NiPt/Al2O3 as better catalysts among the other. The porous nature and Lewis-acidity sites also contributed to high performance of the catalyst. According to the kinetics based on based on Eley–Rideal and Langmuir–Hinshelwood models, here Pt0 receives an electron from NOx (or CO) and forms PtOx. PtOx then adsorbs CO (than NOx) leading to the formation of NCO intermediates on Pt sites. Further CO oxidation to CO2, along with N2 reduction from NOx monodentate occurs simultaneously leaving PtO2 for the next redox cycle (Oton et al. 2020). Seo et al. used H2 for the reduction of NOx and CO over 0.5Pt-2CeO2/TiO2/ZrO2 SCR catalyst at lower temperature. By the addition of ZrO2 at 100 °C, the catalyst showed highest NOx reduction. The presence of CO promoted water gas shift reaction which further improved NOx reduction at 100 °C. The structural property change was because of the following: the oxygen storage capacity due to doping Pt was high, and improvement in surface acidity in presence of CO (Choong-kil 2022).
N2O reduction
N2O is another class of NOx, which is a significant anthropogenic greenhouse gases and one of the major reason for ozone depletion (Zhang et al. 2019b). Thus, reduction of N2O to N2 is important reaction. Recently, hydrocarbons are used for the conversion of N2O to N2. Hevia et al. have studied Fe-ZSM-5 zeolite for the same. Different C1-C3 alkanes, alkenes, and alkynes are used as the reductant and thoroughly checked for the N2O reduction. Alkanes showed better catalytic activity compared to alkenes, and alkynes (very less reactive) because of their reactivity with oxygen and cost. Methane and ethane were best reductant for the N2O reaction (Hevia 2008). Similarly, Zhang et al. have explored Mans–van Krevelen mechanism on phosphotungstic acid supported single-atom catalysts for the N2O reduction by CO. Here the CO get oxidized by the surface oxygen present in the phosphotungstic acid, and N2O occupies the oxygen vacancy and converts to N2 (Zhang et al. 2019b).
SCO for NH3 emissions
Selective catalytic reduction with ammonia (NH3-SCR) is one of the most proficient techniques for NOx removal because of excellent removal efficiency and low maintenance cost in which NH3 is used as a reductant. Apart from high efficiency, this technique also has some shortcomings. For instance, high NH3/NOx ratio and catalyst deactivation by SO2/H2O poisoning leads to slipping of ammonia, initiating secondary pollution (Chen et al. 2019; Nakamura et al. 2021). NH3 emissions are harmful to both environment and human health. Excess ammonia leads to eutrophication and also contributes in creation of secondary particulate aerosols. NH3 emissions have adverse health effects, its high exposure can result in blindness and permanent lung damage (Gheorghe and Ion 2011). The techniques used for the removal of ammonia emissions are adsorption, catalytic decomposition and selective catalytic oxidation (Cardenas et al. 2021; Chen et al. 2021b; Pinzón et al. 2021). Selective catalytic oxidation (SCO) is one of the favorable approaches which can be used in complimenting to NH3-SCR system to convert ammonia into nitrogen directly because of high nitrogen selectivity, low cost, and excellent efficiency (Guo et al. 2019; Gao et al. 2021). Catalysts play a key role in selective catalytic oxidation of ammonia. V2O5-TiO2 oxide with addition of WO3 or MoO3 is used as commercial catalyst for SCO of ammonia. Several noble metals based, non-noble metal based, zeolites-based catalysts are used as active catalysts for selective catalytic oxidation of ammonia (Jabłońska and Palkovits 2016; Li et al. 2017a). Different categories of SCO catalysts have been elaborated below.
Noble metal-based catalysts
Many noble metals like Pt, Pd, Rh, Ru, and Ag show high catalytic activity for selective oxidation of ammonia in terms of high NH3 conversion and N2 selectivity (Zhang and He 2009; Hung 2010; Hung 2012; Ma and Schneider 2020). Similarly, some transition metal oxides have gained more attention than noble-metal based catalysts due to high nitrogen selectivity (Wang et al. 2009; Zhang et al. 2017a). Wang et al. (2019b) utilized sol–gel method to synthesize RuO2-Fe2O3 composite oxide catalysts with different contents of RuO2 (0.5%, 1%, 1.5%, and 2%) for selective catalytic oxidation of ammonia and results were compared with pure Fe2O3. At 100 °C, pure Fe2O3 achieved 100% NH3 conversion with 79% N2 selectivity which is less in comparison to RuO2-Fe2O3 composite oxide catalyst. The sample prepared with 1.5% of RuO2 content showed best catalytic activity with 100% NH3 conversion and 89% N2 selectivity at 225 °C. Addition of RuO2 had a significant effect on surface acidity of catalyst and also increased surface area. Pt/V/TiO2 can even over-oxidize NH3 to NOx in spite of showing excellent catalytic activity at low temperatures (Kim et al. 2018; Dann et al. 2019). Therefore, support plays a vital role in activity of SCO catalysts. For instance, Liu et al. (2019a) studied the promotional effects of ethylenediamine over Pt/SiAlOx (Pt/SiAl-E) catalysts using co-impregnation method. Pt/SiAl-E showed better low-temperature activity than Pt/SiAl for NH3-SCO. For 90% NH3 conversion, the operating temperature was lower for Pt/SiAl-E (219 °C) in comparison to Pt/SiAl (235 °C) with almost similar nitrogen selectivity as shown in Fig. 4. Addition of ethylenediamine led to reduction of particle size which in turn led to larger surface/bulk atomic ratio increasing the active sites for SCO of ammonia.
a The NH3 conversion of catalysts. b The N2 yield of catalysts. Experimental condition: 200 ppm NH3, 10% O2, 8% CO2, 5% H2O and balance N2. The space velocity was 100,000 h−1 (Liu et al. 2019a).
Similarly, Sun et al. (2017a) modified Pt/ZrO2 catalyst by the addition of W using co-impregnation method and loadings of Pt and W were 1.5 wt.% and 5 wt.%, respectively. With decrease in activation energy from 113.4 to 96.2 kJ∙mol−1, the light off temperature for ammonia also shifted from 284 to 249 °C with same nitrogen selectivity for both of them. Redox properties and acidic nature of the catalysts are improved due to the addition of W and it also resulted in electron transfer from W species to Pt species increasing the electron density of Pt. Pt-WO3/ZrO2 had larger number of surface acid sites which contributed to improved catalytic activity. Zhang et al. (2009) studied the role of silver species on Al2O3 using incipient wetness impregnation and sol–gel methods to prepare 10 wt.% Ag/Al2O3 catalyst and then were pre-treated by H2. Pre-treatment of Ag/Al2O3 prepared by impregnation method resulted in excellent catalytic activity of 100% conversion at 160 °C. However, for fresh Ag/Al2O3 showed complete conversion at 220 °C. Ag0 was concluded as the most active species at low temperatures.
Metal oxide and zeolites-based SCO catalysts
Zeolite-based materials are another class of catalysts that show promising results and have been extensively used for selective catalytic oxidation of ammonia. Their activity can be increased by addition of transition metals (e.g., Cu, Fe, Co) or noble metals (e.g., Pt/Ag/Au). The shape selectivity and high surface area often attributes to excellent SCO activity (Qi et al. 2004; Jabłońska et al. 2014; Li et al. 2017a; Rutkowska et al. 2017, 2019; Wang et al. 2021c). Therefore, many scientific studies are being done for low temperature zeolites that operate below 200 °C. With respect to low operating temperature, Wang et al. (2019c) synthesized hollow ZSM-5 encapsulating Ag particles (Ag/ZSM-5-OH) in Al2O3, ZSM-5 and hollow ZSM-5. For 100% NH3 conversion, operating temperature for Ag/ZSM-5 catalyst was 110 °C. By modification of Ag nanoparticles, activation energy for Ag/ZSM-5 was found to be the lowest measuring 16.4 kJ∙mol−1. High content of Ag0 species contributed to higher catalytic activity. Leaching of Ag particles was prevented via hollow structure of ZSM-5 zeolite.
Similarly, Sun et al. (2017b) modified Pt/ZSM-5 by adding Cu via co-impregnation method with loading of 1.5 wt.% of both Pt and Cu. Catalytic performance of PtCu/ZSM-5 was better than original Pt/ZSM-5. It was observed that Cu acted as promoter lowering T90% temperature from 250 to 245 °C as shown in Fig. 5. It also promoted N2 selectivity (79% at 200 °C) which was 64% for Pt/ZSM-5. It was observed that Cu species adjusted the states of Pt species thereby increasing the electron density. Dealuminated zeolites are having higher Brønsted as well as Lewis acidity, and this contributed to higher nitrogen selectivity. Metal oxide-based SCO catalysts (MnO2, CuO, Fe2O3, Co3O4) have higher nitrogen selectivity than noble metal-based catalysts but, they generally have lower catalytic activity. CuO/Fe2O3 catalysts for SCO have higher nitrogen selectivity than noble metal-based catalysts, however, majority of the catalysts offered lower catalytic activity. CuO/CeO2 metal oxides can provide excellent catalytic activity as well as nitrogen selectivity (Wang et al. 2013; Zhang et al. 2017a). Further modification to the metal oxide catalysts enhances the catalytic activity. Preparation method, support and structure of catalyst plays an important role in catalytic activity and further to favorable results (Nassos et al. 2007; Jabłońska et al. 2017b, a; Wang et al. 2021a). For example, Song and Jiang (2012) prepared CuO/CNTs (carbon nanotubes) for SCO of ammonia. Nanocomposites were named as CuO/CNTs-80, CuO/CNTs-100, CuO/CNTs-120, CuO/CNTs-140, and CuO/CNTs-180. Higher the defect density of CNTs, higher catalytic activity was observed. Highest nitrogen selectivity of 98.7% was achieved on CuO/CNTs-140 with 100% NH3 conversion at 189 °C. By these carbon nanotube defects, electron transfer was promoted and copper oxide was activated by these nanotubes. Similarly, Qu et al. (2015) used urea assisted hydrothermal method. MnO2(UH) catalyst showed outstanding catalytic activity of 90% NH3 conversion at 140 °C while 100% conversion at 170 °C which was better in contrast to MnO2(H) (200 °C) and commercial MnO2 (260 °C). However, some by-products like N2O, NO, and NO2 were obtained that led to reduced nitrogen selectivity. Urea-assisted synthesis successfully changed the surface properties of the catalyst by imparting more adsorption sites for NH3 adsorption and activation. Similarly, Duan et al. (2010) improved Cu-Mn compounds catalysts with trivalent rare earth oxide Ce2O3 and La2O3 respectively by incipient wet impregnation method. Also, comparative analysis was done for different preparation methods of 2.5%Ce-5%Cu-5%Mn/TiO2 namely incipient wet impregnation, co-precipitation and sol–gel as follows. By addition of La and Ce, better distribution of Cu and Mn species was observed along with increased interaction between supporter and Cu-Mn, better oxidation ability. Selective catalytic oxidation for NH3 is discussed in Table 4 in detail.
a NH3 conversion, b N2 selectivity, c Arrhenius plots of NH3 oxidation, and d TOF profiles versus reaction temperature over the Ag/ZSM-5, Ag/Al2O3, and Ag/ZSM-5-OH catalyst. Reaction conditions: [NH3] = 1000 ppm, [O2] = 10 vol%, He balance, and GHSV = 35,000 h−1 (Wang et al. 2019c).
Different mechanistic pathways of NOx abatement are explained in Fig. 6. Mechanisms such as Langmuir–Hinshelwood and Eley–Rideal are explained in the schematics.
In the Langmuir–Hinshelwood mechanism, NH3 adsorbs on the acid site of the catalyst to form NH4+. Further, NO will be physically adsorbed on the metallic surface. The metal with higher oxidation state undergoes reduction in order to oxidize NO to NOx species. Then, the NOx species and the adsorbed NH4+ species react to form NH4NOx. Further, NH4NOx decomposes to N2 or N2O. Similarly, in Eley–Rideal mechanism, activation of NH3 species by metallic site takes place forming NH2−. This NH2− reduces NO species to N2 or N2O.
Photo/electro catalysts for the NOx removal
Numerous studies have explored the application of electro- and photocatalysis for the removal of NOx, SOx, and VOCs, highlighting the diverse catalyst materials and reaction mechanisms involved. Electrocatalytic reduction has been extensively studied for the removal of NOx. Tan et al. (2016) explored the electrochemical reduction of NOx using various electrode materials, including metal oxides, metal alloys, and conductive polymers. The study revealed that the electrochemical reduction of NOx can effectively convert nitrogen oxides into harmless nitrogen under specific reaction conditions, presenting a viable pathway for NOx abatement One such catalyst category is using graphene-based materials. Lee et al. (2019) studied the electrocatalytic reduction of NOx using graphene-based catalysts. They reported enhanced catalytic activity and stability, suggesting graphene as a promising material for NOx removal. The utilization of visible light-responsive catalysts, such as carbon-based materials and metal–organic frameworks, was discussed as a promising avenue for improved NOx degradation. Si et al. (2021) investigated the photocatalytic reduction of NOx using TiO2-based catalysts, highlighting the influence of factors such as crystalline structure and surface area on the catalytic performance. They reported high conversion rates under UV irradiation than visible light.
Conjugate polymers and their composites are renowned for their metallic conductivity and impressive physical properties subjected to doping. Foreign elements inclusion can greatly enhance the photocatalytic activity under visible light by improving the separation of charge carriers and expanding the range of light absorption. Additionally, combining conductive polymers with oxide semiconductor photocatalysts creates a synergistic effect, resulting in highly efficient photocatalytic degradation. Ajmal et al. (2023) critically evaluates the previously unexplored utilization of conjugated polymers (CPs) in combination with common inorganic semiconductors as innovative photocatalysts. The distinctive characteristics of CPs, such as conductivity, exceptional light responsiveness, effective sorption capability, superior redox charge generation, and separation abilities facilitated by a delocalized π-electron system, differentiate them from inorganic semiconductors. Ajmal et al. (2022) discussed the advancements in photocatalytic removal of three major air pollutants, namely CO2, NOx, and VOCs, using Conjugate Polymer based photocatalysts. Additionally, the synergistic effects observed when conjugated polymers are combined with inorganic semiconductors are comprehensively summarized. Notably, the combined system enhances charge generation and separation, potentially activating the adsorb and shuttle process, wherein conjugate polymers may play a crucial role in the sorption process. Semiconductor-based catalysts are studied for the photochemical removal of NOx, such as WO3 and Bi2WO6 (Dong et al. 2016; Zhang et al. 2019a) These catalysts facilitated the efficient photodecomposition of NOx species, offering potential solutions for NOx abatement. Photo catalysis has shown promise for the removal of SOx pollutants. The research demonstrates the potential of various catalyst materials, such as TiO2-based catalysts, graphene, MOFs, and semiconductor-based catalysts, for efficient pollutant degradation. Additionally, the combination of photo and electrocatalysis has shown synergistic effects, leading to enhanced removal efficiency. However, further research is required to optimize catalyst design, improve stability, and scale up these catalytic systems for practical applications.
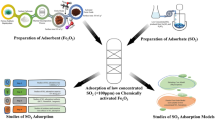
Adsorption techniques for the NOx/SOx removal
Air pollution caused by nitrogen oxides (NOx) and sulfur oxides (SOx) has serious environmental and health implications. Adsorption is another effective method for the removal of these pollutants from industrial exhaust gases. Several studies have investigated the adsorption of NOx, SOx, and VOCs using various adsorbents and process conditions. Mergbi et al. (2023) emphasizes the relationship between the synthesis method or surface modification of carbon materials derived from biomass waste and their effectiveness in removing organic, NOx, SOx, and heavy metal pollutants by photocatalysis. Surface modification of activated carbon is a technique that can enhance the presence of immobilized functional groups, leading to improved removal of NOx from the air. Activated carbon is used for the removal of NOx and SOx. The primary role of activated carbon is to initially convert NO to NO2, which can be easily adsorbed. Catalytically oxidizing NO to NO2 could be a practical approach for removing NOx from fuel gas, as NO2 can be readily adsorbed.
The adsorption process for NOx, SOx, and VOC removal typically involves passing the gas stream through an adsorbent bed. The adsorbent materials, such as activated carbon, zeolites, and metal oxides, are selected based on their specific affinity for the target pollutants as shown in Fig. 7. The efficiency of the adsorption process depends on factors such as temperature, pressure, contact time, and adsorbent characteristics (Zhu et al. 2020; Sabzehmeidani et al. 2021; Zhu and Xu 2022). Despite the promising results, challenges persist in adsorption-based pollutant removal. Saturation of adsorbents, competition between different pollutants, and the regeneration of spent adsorbents are major hurdles that need to be addressed. The development of efficient regeneration methods and the treatment of complex gas streams are areas of ongoing research.
VOC abatement
Volatile organic compounds (VOCs) comprise organic compounds formed from burning fossil fuels, chlorination in water treatment, components of petroleum, dry cleaning agents, paint industries, etc. VOCs such as volatile benzene, toluene, ethylbenzene, and xylenes (BTEX) levels are usually higher in both industrial areas and cities (Montero-Montoya et al. 2018). Exposure to these pollutants can cause both minor and major health effects including nose, eye irritation, headache, nausea and extreme effects like kidney or liver failure, damage to nervous system and can be even carcinogenic in some cases. VOCs have adverse effect on environment and also generates secondary pollutants. Considering the toxicity and long-term adverse effects on human health and environment, strict regulations have been set up for release of VOCs into the environment; therefore, industries need to render them harmless before releasing them into the environment (Tsimpidi et al. 2012; Manisalidis et al. 2020). Several treatment technologies for VOC abatement are being currently used such as thermal oxidation, catalytic oxidation, adsorption, photocatalysis, bio-treatments including bio trickling filters, bio-scrubbers and membrane separation (Abou Saoud et al. 2020; Zhang et al. 2020a; Cheng et al. 2021; Tian et al. 2021b; Wantz et al. 2021). Among all the possible techniques, the focus has been given to low-temperature catalytic techniques for VOC removal as it possesses low operating costs compared to other techniques.
Low-temperature catalysts for VOC oxidation
Noble metal-based catalysts
Wu et. al. (2016) synthesized three-dimensionally mesoporous silica (KIT-6) was studied for toluene oxidation as shown in Fig. 8. The three-dimensionally ordered mesoporous Cr2O3 (meso-Cr2O3) was fabricated using the ultrasound-assisted strategy with KIT-6. The meso-Cr2O3-supported Au − Pd samples were prepared and Au and Pd loading (x) of 0.90 and 1.00 wt.% were loaded and indicated as xAu/meso-Cr2O3 and xPd/meso-Cr2O3 samples. The bimetallic 1.95Au1Pd2/meso-Cr2O3 sample performed exceptionally for toluene decomposition and its T90% was 165 °C. Small particle size of the noble metals, high concentration of adsorbed oxygen species, low-temperature reducibility, and dispersion of noble metals contributed to excellent catalytic activity.
A Toluene conversion as a function of reaction temperature of the (filled squares) meso-Cr2O3, (filled circles) 0.50Au1Pd2/meso-Cr2O3, (filled triangles) 0.97Au1Pd2/meso-Cr2O3, (filled diamonds) 1.95Au1Pd2/meso-Cr2O3, (empty triangles) 0.90Au/meso-Cr2O3, and (empty diamonds) 1.00Pd/meso-Cr2O3 samples at SV = 20,000 mL g−1 h−1. B Effect of SV on the catalytic activity of 1.95Au1Pd2/meso-Cr2O3 for toluene oxidation (Wu et al. 2016).
Similarly, Hu et al. (2018) synthesized ruthenium (Ru) nanoparticles with mass loading ranging from 1.5 to 3.2 wt.% supported on cerium dioxide (CeO2) for application in the catalytic combustion of propane. Once Ru was loaded on the CeO2 or Al2O3 support, the catalytic activities improved drastically, which clearly suggests that Ru has intrinsic catalytic properties for propane oxidation, which is due to the interface between Ru and CeO2 or Al2O3 forms oxygen defects and attributes to strong metal support interaction, that can help in the propane oxidation. These findings confirmed that the Ru/CeO2-X catalysts exhibited higher catalytic activities than the Ru/Al2O3-X catalysts in the total oxidation of propane. Also, CeO2 support can act as a reservoir for oxygen and provide additional sites for propane adsorption. Shi et al. (2021c) prepared a series of Pt/CeO2-TiO2 with 0.5 wt% Pt via a modified ethylene glycol reduction method and their activity for catalytic oxidation for benzene and 1,2-dichloroethane (DCE) were tested. It was observed that Pt/CeTi-11 (where 11 represents the pH value of the synthesis solution) with the smallest average Pt particle size (1.53 nm) showed 90% conversion of benzene at 152 °C. The high performance was seen due to stronger interaction between PtOx and CeO2-TiO2 support results in both the formation of more Pt2+ species and strong low-temperature redox properties of Pt/CeO2-TiO2 catalyst. Zeng et al. (2020) prepared Fe-doped Mn3O4 hexagonal plates with reactive (1 1 2) facet in one step and 4 nm Pd particles were loaded on FexMn3−xO4-HP. The methyl acetate conversion as a function of temperature is shown in Fig. 9. The stability of the catalyst was tested for 50 h and methyl acetate conversion found to be slightly decreased during the 50-h time on stream.
a Catalytic activity of FexMn3−xO4-HP, Pd/Mn3O4-HP, and Pd/FexMn3−xO4-HP for methyl acetate combustion. b Stability test of Pd/Fe0.135Mn2.865O4-HP at 190 °C with an initial conversion of 75% (Zeng et al. 2020).
It was found that Fe doping increases the oxidation state of the Pd that supports the formation of highly active oxygen species in greater extent. The targeted VOC was methyl acetate and Pd/Fe0.135Mn2.865O4 exhibited best catalytic performance of the lowest T100% of 200 °C. Zhao et al. (2020b) prepared Ru/TixSn1-x catalysts via incipient-wetness with aqueous solution of RuCl3 and co-precipitation method for catalytic oxidation of chlorobenzene and dichloromethane. As a result of the interaction between Ru and TiO2/SnO2, higher concentration of surface oxygen was observed. This is because of Ru–O-Ti and Ru–O-Sn interactions where they enhance strong basic surface lattice oxygen is possible and it further increase surface oxygen mobility. Moreover, the interface Ru–O-Sn has high Lewis acidity which enhances the transfer of surface oxygen to active site making suitable for dichloromethane oxidation.
Non-noble metal-based catalysts
Similarly, Zhenxuan Zhao et al. prepared three-dimensionally ordered macro-porous La0.6Sr0.4FeO3-α (LSF) for oxidation of toluene. Among the prepared LSF catalysts, LSFPEG (PEG-polyethylene glycol) has high catalytic performance for toluene combustions T10%, T50%, and T90% of the LSFPEG were 54, 225, and 280 °C, respectively. Low-temperature reducibility, high specific surface area, and high oxygen ad-species concentration of LSFPEG contributed to achieve improvement in catalytic activity.
Peng et al. (2020) performed low-temperature oxidative degradation of formaldehyde (HCHO) via novel manganese dioxide (MnO2)/N-doped carbon nanotubes (NCNT) composites having varying MnO2 content and the catalyst was defined as 70% MnO2/CNTs (40% MnO2/CNTs, 20% MnO2/CNTs). When the temperature increased above 100 °C, the composite with the highest MnO2 content (70% MnO2/CNTs) showed the best activity with 100% HCHO removal efficiency at 150 °C. A complete 100% conversion of HCHO to CO2 on 40% MnO2/NCNT was obtained at as low as 100 °C, which was about 50 °C lower than the equivalent MnO2/CNT catalyst. Synergy between well-formed MnO2-CNT interfaces and strong electron transfer contributed to excellent catalytic activity. The detailed mechanism proposed for the formaldehyde oxidation is shown in Fig. 10.
a Proposed formaldehyde oxidation mechanism on MnO2-CNTs/NCNT. b Catalytic cycle of formaldehyde oxidation on MnO2-based on DFT calculations and the oxygen molecule activation on CNT and NCNT. Where red = O, white = H, grey = C, blue = N and purple = Mn (Peng et al. 2020).
Li et al. (2021a) prepared amorphous Co-Mn binary oxides with defects for catalytic oxidation of propane. Highly defective Co1Mn3Ox showed highest catalytic activity of T90% at 207 °C and high space velocity of 18,000 mL g−1 h−1. It possesses numerous oxygen vacancy defects that weaken the Mn–O bond and improve the mobility of surface lattice oxygen which in turn leads to activation of C-H bond and helps in oxidation. Zhao et al. (2020c) synthesized monolithic Co3O4 and Co3O4@MnOx by a hydrothermal method and among the different catalysts synthesized bamboo leaf like Co3O4-NF-10 showed T90% at 193 °C. The catalyst prepared with NH4F as template agent and gave stronger adherence onto the Ni foam. Then, another catalyst Co3O4@MnOx–NF was prepared by secondary hydrothermal method which showed T90% at 177 °C which was 16 °C lower than Co3O4-NF-10. The improved performance is attributed to abundant Co3+ and increased the concentration of surface adsorbed oxygen species.
VOC removal using AOPs
VOC can be removed by using other AOPs such as photocatalysis and catalytic ozonation. Catalytic ozonation is predominantly focused by Ikhlaq et al. and Fujita et al. in presence of zeolites, TiO2, and Al2O3 (Fujita et al. 2004; Ikhlaq et al. 2014). Manganese oxide-based catalysts are preferred for catalytic ozonation of VOCs and manganese addition can improve the decomposition rates of certain VOC like toluene. These metal-oxide catalysts are cost effective and have high redox potential (Xu et al. 2017; Shao et al. 2021; Gan et al. 2021).
Catalytic ozonation can degrade pollutants at lower temperature in comparison to catalytic oxidation which makes the process to be more feasible and safer for industrial use (Shao et al. 2021). In a study, Chen et al. (2020) performed catalytic ozonation of chlorobenzene over some MnOx-based catalysts using different supports namely Al2O3, TiO2, SiO2, CeO2, and ZrO2. The manganese oxide-based catalysts were synthesized via an impregnation method and were tested at 120 °C. Mn/Al2O3 showed highest chlorobenzene conversion efficiency of 89.2% owing to its excellent textual properties, O2 desorption, redox ability, its desirable surface adsorbed oxygen species and acidity. The other catalyst performance was in the following order: Mn/TiO2 > Mn/SiO2 > Mn/CeO2 > Mn/ZrO2. Another study by Amir et al. (Ikhlaq and Kasprzyk-Hordern 2017) revealed that as opposed to alumina, zeolites promoted decomposition of VOCs and catalytic ozonation of volatile organic compounds on zeolites takes a non-radical path which involves reactions of molecular ozone with pollutants adsorbed on the surface of zeolites since the presence of hydroxyl radical did not have significant effects on removal rates of VOCs.
Photocatalysis for VOC removal
Heterogenous photocatalysis in combination with catalytic ozonation also provides better results for VOC removal. The combination of these techniques overpowers the limitations of individual techniques and provides a high synergistic effect (Ebrahimi et al. 2017; Gérardin et al. 2021; Saqlain et al. 2021). But photocatalytic ozonation is difficult to scale-up therefore design optimization is required in order to make it industrially feasible. The advantage of photocatalytic route is the low operating cost as it occurs at room temperature with sun light or artificial light source that contain UV/Visible light spectrum.
The photocatalytic process involves adsorption of VOC on the surface sites of adsorbents followed by chemical reactions that convert harmful VOCs into carbon dioxide and water. As shown in Fig. 11, the semiconductors like titanium dioxide, ZnO are highly recommended which get activated by absorbing a photon via UV irradiation source which in turn leads to release of an electron from the valance band to the conduction band causing the oxidation of VOC absorbed on the surface (Zou et al. 2006; Hu et al. 2020). The photocatalysis mechanism proceeds via absorption of visible light by the catalyst, thereby creating electron hole pair in the conduction and valance band, respectively. These pairs can either recombine or can participate in the surface redox reaction. In order to maximise the charge separation and minimising the recombination many modification by doping, heterojunction and morphology enhancement taken care of (Zhao et al. 2022). Fiorenza et al. studied noble metal free co-catalyst for VOC removal. MnOx-ZrO2 mixed oxides used for removal of toluene and ethanol in the gas phase with the multi-catalytic solar photothermal approach. Among the photocatalytic, thermo-catalytic and the photo-thermo-catalytic methods for removal of VOCs, first method has the advantage of working at room temperature and that with the MnOx-5% ZrO2, it reached a similar activity of the most used TiO2-based materials, showing the importance of the material (Fiorenza et al. 2022). Similarly, Enesca et.al. worked on a titania-free heterostructure based on CuS/SnO2/WO3 material and compared with single WO3 for the removal of formaldehyde and acetaldehyde. While WO3 showed 41% and 52%, the CuS/SnO2/WO3 material exhibits a superior photocatalytic activity of 62.9% and 78.5% for acetaldehyde and formaldehyde respectively (Alexandru Enesca and Viorel Sisman 2022). Detailed comparison of the catalyst for VOC abatement is explained in Table 5.
Schematic of the basic mechanism of photocatalysis (Almaie et al. 2022).
Adsorbents for VOC abatement
Adsorption is a simple and cost effective regenerative method for removal of volatile organic compounds at low temperature (Zhang et al. 2017b; Ma et al. 2021a). For the industrial application of VOC removal, wide variety of materials are used as adsorbents, namely, activated carbon, CNT, zeolites, metal organic frameworks, carbon nanofibers, hyper cross-linked polymer, microporous polymer, and silica gel (Wang et al. 2018b; Ojstršek et al. 2020; Zhang et al. 2020c; Anand et al. 2021; Kutluay and Temel 2021). MOFs are known to have high adsorption capacity towards VOCs due to its peculiar properties of high specific surface area, large porous volume, and numerous metal sites which can interact with gaseous molecules of VOCs. MOFs consist of metal clusters that are connected by organic ligands and can form many structures (Britt et al. 2008; Bahri et al. 2017; Ongari et al. 2017). Therefore, metal organic frameworks make excellent adsorbents. For instance, Rui Ou et al. found that structural defects in UiO-666 can actually lead to improved performance. The modified UiO-66 by adding acetic acid (HAc) via a hydrothermal method has increased the surface area and pore volume of the initial adsorbent. The modified adsorbent UiO-66–2.0 HAc has been studied for adsorption of benzene and toluene at various HAc concentrations. The adsorbent UiO-66–1.0HAc has tuning concentration of HAc/Tac = 24, and it showed highest adsorption capacity of 367.13 mg g−1 for benzene which was 41.9% higher than non-defected UiO-66. When the concentration of Hac/Tac was increased to 48 molar ratios, the adsorption capacity of UiO-66–2.0Hac was achieved with the highest capture values of 410.21 mg g−1 of toluene at 25 °C which was 93% greater than original UiO-66. The high adsorption capacities can be attributed to addition of acetic acid which controllably modulated the number of defects by missing linkers (Ou et al. 2021). Similarly, Yang et al. carried out pyrolysis of Zn based MOF(ZIF-8) to synthesize Zn-containing graphite carbon (Zn-GC) which targeted the adsorption of formaldehyde. The adsorption capacity of it was found to be 736 times greater than commercial activated carbon and 5.6 times that of ZSM-5 adsorbents. The adsorption capacities of Zn-GC-550, Zn-GC-650, Zn-GC-750, and Zn-GC-850 for formaldehyde adsorption were 16.67, 17.57, 13.49, and 11.32 mg g−1 of formaldehyde, respectively, among which Zn-GC-650 exhibited the best performance. This excellent adsorption capacity is attributed to the hierarchical porous structure of the framework which enhanced the capture of formaldehyde (having a strong affinity for formaldehyde molecules) through hydrogen bonding as the porous structure provided abundant space to pass through and hold the water vapor in the air (Yang et al. 2021a).
Kutluay (2021) modified magnetic Fe3O4/AC@SiO2 nanoparticles with 8-hydroxyquinoline-5-sulfonic acid via the co-precipitation and sol–gel methods to form Fe3O4/AC@SiO2@8HQ5SA as effective adsorbent for BTX (benzene, toluene, xylene) vapors. The maximum adsorption capacities of the BTX vapours by Fe3O4/AC@SiO2@8HQ5SA were seen to be 555.85, 620.80, and 745.54 mg g−1, respectively. After five consecutive adsorption–desorption cycle tests, Fe3O4/AC@SiO2@8HQ5SA maintained the reuse efficiencies of 91.92%, 91.17%, and 90.65% for the BTX vapors which makes them cost-effective (Kutluay 2021). The dynamic adsorption capacities of Fe3O4, Fe3O4/AC, Fe3O4/AC@SiO2, and Fe3O4/AC@SiO2@8HQ5SA for the removal of the BTX vapors is shown in Fig. 12. Activated hydrochars derived hickory wood and peanut hull with H3PO4 and KOH using hydrothermal carbonization method have also improved the VOCs of acetone and cyclohexane removal efficacy. Hydrochar derived from hickory wood and peanut hull were abbreviated as HH and PH. Those modified by KOH and H3PO4 were abbreviated as HHK, PHK, HHP, and PHP. The VOC adsorption (acetone) of the activated hydrochars (50.57–159.66 mg g−1) were greater than that of the nonactivated hydrochars (15.98–25.36 mg g−1), which was mainly due to increase in surface area. The adsorption capacities for acetone on HHP and PHP were 147.77 mg g−1 and 113.94 mg g−1, which were higher than that on HHK and PHK. After several adsorption and desorption cycles, the reusability decreased slightly which means that they can be used as effective adsorbents (Yu et al. 2020) as shown in Fig. 13.
The comparison of dynamic adsorption capacities of Fe3O4, Fe3O4/AC, Fe3O4/AC@SiO2, and Fe3O4/AC@SiO2@8HQ5SA for the removal of the BTX vapors (Kutluay 2021).
Breakthrough curves of a acetone, b toluene, c acetic ether on PCs, and d adsorption capacities of PCs; 300 mg of PC at 300 K. Total adsorption flow rate-500 mL min−1, with 1000 ppmv VOCs and N2 as balance gas (Yu et al. 2020).
Plata-Gryl et al. made chemical modifications to nitrated asphaltenes and used these as an active layer coated on a surface of diatomaceous earth. The AsfNitro adsorbent was prepared using the methods of coating of stationary phases onto solid support. The adsorption capacities for benzene, pyridine, and 1-nitropropane increased to 26, 12, and 8 times respectively by addition of AsfNitro. Comparing to commercial carbotrap adsorbent, twice adsorption capacity was observed for benzene. However, in high humidity, there was a decrease in performance due to competitive adsorption of water molecules (Plata-Gryl et al. 2022). A schematic explaining VOC abatement mechanism on carbon surface to form CO2 and H2O as the final products is shown in Fig. 14. Here, L–H model is Langmuir–Hinshelwood model, where the interaction of two molecules m adsorbed at the metal surface reacts to form CO2 and H2O. In the second model, Eley–Rideal mechanism, reaction between gaseous O2 and reactant adsorbed at the surface is explained. In the third mechanism, Mars-Van-Krevelen model, the catalyst surface is assumed to be an oxygen surface and thus explains the interaction of oxygen surface of the catalyst and the reactant molecules.
Catalytic converters, adsorption systems, and filtration minimize VOC emissions. These abatement procedures help meet regulatory criteria and make living situations cleaner and healthier. To design the large-scale systems, the mechanism and reaction pathway investigations are critical.
Challenges and opportunities
Abatement technologies offer unique challenges and opportunities for the abatement of nitrogen oxides (NOx), sulfur oxides (SOx), and volatile organic compounds (VOCs).
Challenges
Catalyst-reactant interaction
Achieving efficient catalytic conversion of NOx, SOx, and VOCs requires a strong interaction between the catalyst surface and the pollutants. However, the adsorption and activation of these molecules on the catalyst surface can be challenging due to their diverse chemical nature and the presence of interfering species in the reaction mixture.
Reaction kinetics
The kinetics of the catalytic reactions for NOx, SOx, and VOC abatement can be complex. Multiple reactions, including oxidation, reduction, and acid–base chemistry, may co-occur leading to challenges in controlling reaction selectivity and optimizing conversion efficiency.
Catalyst stability
Heterogeneous catalysts used for NOx, SOx, and VOC abatement must withstand harsh reaction conditions, such as high temperatures, corrosive environments, and exposure to water and other reactants. Maintaining catalyst stability and preventing deactivation or degradation over time is a significant challenge.
Cost and energy efficiency
Implementing abatement technologies can be costly, particularly for large-scale industrial applications. Ensuring cost-effectiveness and energy efficiency in NOx, SOx, and VOC abatement is crucial for widespread adoption.
Opportunities
Catalyst design and development
Heterogeneous catalysis offers opportunities for tailored catalyst design. Catalyst composition, structure, and active sites can be optimized to enhance catalytic activity, selectivity, and stability. Incorporating promoters, modifiers, or nano-structuring the catalyst surface can improve NOx, SOx, and VOC abatement performance.
NOx abatement in hydrogen combustion engines
Optimizing lean-burn combustion, employing advanced combustion techniques, utilizing exhaust gas after treatment systems like SCR and LNT (Lean NOx Trap-NOx adsorber to control NOx emission from gasoline or lean burn engines), developing advanced catalyst materials, implementing engine optimization and control strategies, and establishing a robust hydrogen infrastructure are to be considered in designing efficient H2 combustion engines. These efforts aim to minimize NOx emissions and make hydrogen combustion engines more environmentally friendly and sustainable for transportation.
Novel catalyst materials
Exploration and development of novel catalytic materials can lead to NOx, SOx, and VOC abatement breakthroughs. For example, advanced metal oxides, zeolites, MOFs, or hybrid catalysts combining different materials can provide higher activity, selectivity, and resistance to deactivation.
Reaction engineering
Understanding and optimizing the reaction parameters and reactor design can improve catalytic performance. Strategies such as reactor configuration, flow pattern, catalyst bed geometry, and inert additives can enhance reactant/product distribution. These strategies will also facilitate better contact time with the catalyst, and minimize mass transfer limitations, and on overall improve the abatement efficiency.
Synergistic catalytic systems
Combining multiple catalysts or employing multifunctional catalysts can lead to synergistic effects and improved NOx, SOx, and VOC abatement performance. The overall abatement process can be enhanced by integrating complementary catalytic functions, such as oxidation and reduction.
Catalyst regeneration and recycling
Developing efficient regeneration techniques or catalyst recycling methods is crucial for the economic viability of heterogeneous catalytic processes. Designing catalysts with self-cleaning properties or developing regeneration strategies, such as controlled oxidation or reduction, can extend the catalyst’s lifespan and reduce the operational costs.
Process integration and scale-up
Integrating heterogeneous catalytic systems into industrial processes, such as power plants, refineries, or chemical manufacturing facilities, offers opportunities for large-scale NOx, SOx, and VOC abatement. Catalytic technologies can be effectively implemented by optimizing process parameters, considering the overall energy efficiency, and addressing safety and economic aspects.
Innovative adsorbents and absorbents
Developing novel adsorbents and absorbents with high selectivity and capacity for NOx, SOx, and VOC removal presents opportunities for efficient abatement. Materials such as activated carbon, zeolites, and MOFs show promise in this regard.
Conclusion and future aspects
The gradual increase in the emission of hazardous gases like NOx, SOx, and VOCs must be addressed on priority to safeguard the environment. Even though a large number of treatment technologies for reducing these gases are available, making these highly efficient is essential to reduce the adverse effect of the gaseous pollutant mixtures before releasing them into the atmosphere. The industries and R&D laboratories need to take radical steps in implementing technology transfer at a quicker pace. Low-temperature technologies like catalytic oxidation/reduction, non-destructive adsorption technology, redox methods like photo- and electrocatalytic ozonization are of great use with low operating cost and easily scalable for large-scale application, which are indeed required globally.
-
Noble metal catalysts for NOx reduction are preferred due to its low-temperature selective catalytic reduction, and non-noble metals like Mn, Ce, and V were started to use because of their high conversion, sulfur resistance, and nitrogen selectivity. However, cost and availability of the materials is the paramount concern for the scalability. A few transition metal oxides, zeolites, MOFs, and their modifications also have led to achieve excellent NOx conversion and N2 selectivity.
-
Recently flue gases containing hydrocarbon, CO are mostly highlighted for the NOx reduction. Redox reactions such as NOx to N2 and hydrocarbon to CO2 are extremely important for environment safety. The catalyst can be selected so as to have high surface area, surface acidity and oxygen storage capacity.
Oxidation of NH3 can be considered as another important step in NOx reduction, mainly in exhaust treatment. Four-way catalytic convertors with combination of oxidation and reduction units are getting attention nowadays for the oxidation of unreacted and excess NH3. Thus, selective oxidation of NH3 has its own importance.
-
High conversions of NH3 selective reduction were possible because of the increased synergic effect of non-noble metal-based bimetallic catalysts. Promoters such as NbO inhibits the catalyst deactivation even at elevated temperature.
-
Zeolite-based catalysts because of their shape selectivity and high surface area attributes to excellent SCO activity. Preparation method, support, and structure of catalyst plays an important role in catalytic activity and tuning these aspects can lead to favourable results.
-
Promoters such as V and W that can increase the Brønsted acidity and catalyst that has high oxygen vacancy are important for the SCO of NH3. There are promoters like W that decreases the activation energy there by increasing the activity.
It is well known that small particle size of noble metals, high adsorbed oxygen species concentration, and low-temperature reducibility contributed to excellent catalytic activity for the VOC removal. Non-noble metal-based catalysts with large surface area, high oxygen ad-species concentration is used for VOC abatement.
-
Catalytic ozonation and photocatalysis are used to overpower the limitation of VOC abatements to convert CO2 and H2O. Further, less-expensive adsorption technique is also efficient method to eliminate VOCs. With the help of activation, the surface nature and porosity of MOF and its derivatives can be tuned for the VOC removal.
-
Inexpensive activated hydrochars can attributed to increased surface area there by increased adsorption of VOCs. By introducing linkers, desired pore tunability can be achieved to enhance the adsorption capacity and thereby limiting the emission of pollutant gases.
Through this review, we have tried to combine the technologies for removing the gases like NOx, SOx, and VOC. The main application for the abatement of these gases requires in the automobile industries. Along with conventional catalytic technologies like selective catalytic reduction, advanced technologies such as advanced oxidation for the removal, adsorption, and photo-electro catalysis for the NOx/SOx removal have also been explored in the review. Similarly, VOC removal technologies have included low-temperature oxidation, advanced catalytic oxidation, adsorption, and other catalytic technologies. We have included how the different structures of the catalyst by modifying their surface properties have assisted in improving the catalytic properties. Dispersing active metal species like platinum, palladium, and rhodium as nanoparticles or clusters on the catalyst surface is an effective technique. This increases catalytic reaction surface area and catalyst-reactant contact, enhancing NOx reduction efficiency. Similarly, maximizing catalyst surface area with porous or high-surface-area supports (e.g., zeolites, mesoporous materials) can boost catalytic activity. These structures increase NOx adsorption and reaction, enhancing NOx reduction. Likewise, functional groups or additions on the catalyst surface improve NOx reduction selectivity and stability. Alkaline-earth metals like barium and strontium can help catalysts store and release NOx as well as VOC species, enhancing pollutants conversion efficiency. Similarly, cerium and other transition metal oxide catalysts can alter oxidation states during catalysis. Redox behaviour helps convert NOx as well as VOC species by transferring oxygen atoms between catalyst and reactants. Also, optimising catalyst support material affects NOx reduction efficiency. Support acidity/basicity, thermal stability, and oxygen storage capacity can optimise NOx reduction reaction catalytic activity and stability.
The future aspects of the abatement of gaseous pollutants involve low-cost technology. As several transition metals/carbon-based materials are abundant, relying on transition metal modified/CNT and other carbon-based supports as catalysts is a viable option from an economic viewpoint. A comprehensive understanding of the structure–activity relation, kinetic studies, and other related reaction engineering studies is yet to be explored. It is challenging to scale up the process and establish the removal in real-life applications. As discussed in the review, exploring and understanding the challenges and gaps and investigating future opportunities would be tremendous.
Data availability
Available upon request.
References
Abou Saoud W, Assadi AA, Kane A et al (2020) Integrated process for the removal of indoor VOCs from food industry manufacturing: elimination of butane-2,3-dione and heptan-2-one by cold plasma-photocatalysis combination. J Photochem Photobiol A Chem 386:112071. https://doi.org/10.1016/j.jphotochem.2019.112071
Ajmal Z, Haq M, ul, Naciri Y, et al (2022) Recent advancement in conjugated polymers based photocatalytic technology for air pollutants abatement: cases of CO2, NOx, and VOCs. Chemosphere 308:136358. https://doi.org/10.1016/j.chemosphere.2022.136358
Ajmal Z, Naciri Y, Ahmad M et al (2023) Use of conductive polymer-supported oxide-based photocatalysts for efficient VOCs & SVOCs removal in gas/liquid phase. J Environ Chem Eng 11:108935. https://doi.org/10.1016/j.jece.2022.108935
Almaie S, Vatanpour V, Rasoulifard MH, Koyuncu I (2022) Volatile organic compounds (VOCs) removal by photocatalysts: a review. Chemosphere 306:135655. https://doi.org/10.1016/J.CHEMOSPHERE.2022.135655
Anand B, Szulejko JE, Kim KH, Younis SA (2021) Proof of concept for CUK family metal-organic frameworks as environmentally-friendly adsorbents for benzene vapor. Environ Pollut 285:117491. https://doi.org/10.1016/j.envpol.2021.117491
Atkinson R, Arey J (2003) Atmospheric degradation of volatile organic compounds. Chem Rev 103:4605–4638. https://doi.org/10.1021/cr0206420
Bahri M, Haghighat F, Kazemian H, Rohani S (2017) A comparative study on metal organic frameworks for indoor environment application: adsorption evaluation. Chem Eng J 313:711–723. https://doi.org/10.1016/j.cej.2016.10.004
Bai Y, Dong J, Hou Y et al (2019) Co3O4@PC derived from ZIF-67 as an efficient catalyst for the selective catalytic reduction of NOx with NH3 at low temperature. Chem Eng J 361:703–712. https://doi.org/10.1016/j.cej.2018.12.109
Bedi U, Chauhan S (2020) Mathematical modeling of automotive catalytic converter for catalytic combustion of the volatile organic compound (voc) methane. J Phys Conf Ser 1706:012035. https://doi.org/10.1088/1742-6596/1706/1/012035
Bjørkedal OH, Regli SK, Nuguid RJG et al (2022) One-pot synthesis of highly dispersed mesoporous Cu/ZrO2 catalysts for NH3-SCR. Catal Today 384–386:113–121. https://doi.org/10.1016/j.cattod.2021.05.010
Boningari T, Smirniotis PG (2016) Impact of nitrogen oxides on the environment and human health: Mn-based materials for the NOx abatement. Curr Opin Chem Eng 13:133–141. https://doi.org/10.1016/j.coche.2016.09.004
Boutros M, Trichard JM, Da Costa P (2009) Silver supported mesoporous SBA-15 as potential catalysts for SCR NOx by ethanol. Appl Catal B Environ 91:640–648. https://doi.org/10.1016/J.APCATB.2009.07.004
Britt D, Tranchemontagne D, Yaghi OM (2008) Metal-organic frameworks with high capacity and selectivity for harmful gases. Proc Nat Acad Sci USA 105:11623–11627. https://doi.org/10.1073/pnas.0804900105
Brummer V, Jecha D, Martinec J et al (2015) Impact of catalytic oxidation operating conditions on VOC and Co conversions on the Pt-Pd/Al2O3 catalyst. Chem Eng Trans 45:1009–1014. https://doi.org/10.3303/CET1545169
Cai X, Hu YH (2019) Advances in catalytic conversion of methane and carbon dioxide to highly valuable products. Energy Sci Eng 7:4–29. https://doi.org/10.1002/ese3.278
Cardenas C, Latifi AM, Vallières C et al (2021) Analysis of an industrial adsorption process based on ammonia chemisorption: Modeling and simulation. Comput Chem Eng 154:107474. https://doi.org/10.1016/j.compchemeng.2021.107474
Carrillo AM, Carriazo JG (2015) Cu and Co oxides supported on halloysite for the total oxidation of toluene. Appl Catal B Environ 164:443–452. https://doi.org/10.1016/J.APCATB.2014.09.027
Casagrande CA, Repette WL, Hotza D (2020) Effect of environmental conditions on degradation of NOx gases by photocatalytic nanotitania-based cement mortars after long-term hydration. J Clean Prod 274:123067. https://doi.org/10.1016/j.jclepro.2020.123067
Cha W, Ehrman SH, Jurng J (2016) CeO2 added V2O5/TiO2 catalyst prepared by chemical vapor condensation (CVC) and impregnation method for enhanced NH3-SCR of NOx at low temperature. J Environ Chem Eng 4:556–563. https://doi.org/10.1016/j.jece.2015.10.033
Challagulla S, Payra S, Chakraborty C et al (2019) Understanding the role of catalytic active sites for heterogeneous photocatalytic oxidation of methanol and thermal reduction of NOx. Mol Catal 476:110505. https://doi.org/10.1016/J.MCAT.2019.110505
Chang S, Harle G, Ma J, Yi J (2020) The effect of textural properties of CeO2-SiO2 mixed oxides on NH3-SCO activity of Pt/CeO2-SiO2 catalyst. Appl Catal A Gen 604:117775. https://doi.org/10.1016/j.apcata.2020.117775
Chen WH, Chen CY (2020) Water gas shift reaction for hydrogen production and carbon dioxide capture: a review. Appl Energy 258:114078. https://doi.org/10.1016/J.APENERGY.2019.114078
Chen C, Cao Y, Liu S et al (2019) The catalytic properties of Cu modified attapulgite in NH3–SCO and NH3–SCR reactions. Appl Surf Sci 480:537–547. https://doi.org/10.1016/J.APSUSC.2019.03.024
Chen G, Wang Z, Lin F et al (2020) Comparative investigation on catalytic ozonation of VOCs in different types over supported MnOx catalysts. J Hazard Mater 391:122218. https://doi.org/10.1016/j.jhazmat.2020.122218
Chen R, Zhang T, Guo Y et al (2021a) Recent advances in simultaneous removal of SO2 and NOx from exhaust gases: removal process, mechanism and kinetics. Chem Eng J 420:127588. https://doi.org/10.1016/j.cej.2020.127588
Chen Y, Chen X, Ma X et al (2021b) Selective catalytic oxidation of ammonia over AMn2O5 (A = Sm, Y, Gd) and reaction selectivity promotion through Nb decoration. J Catal 402:10–21. https://doi.org/10.1016/j.jcat.2021.07.027
Cheng HH, Lu IC, Huang PW et al (2021) Biological treatment of volatile organic compounds (VOCs)-containing wastewaters from wet scrubbers in semiconductor industry. Chemosphere 282:131137. https://doi.org/10.1016/j.chemosphere.2021.131137
Choong-kil, (2022) Improvement of NOx and CO reduction performance at low temperature of H2-SCR catalyst. Int J Automot Technol 23:1509–1515. https://doi.org/10.1007/s12239
Costa CN, Savva PG, Andronikou C et al (2002) An investigation of the NO/H2/O2 (Lean De-NOx) reaction on a highly active and selective Pt/La0.7Sr0.2Ce0.1FeO3 catalyst at low temperatures. J Catal 209:456–471. https://doi.org/10.1006/JCAT.2002.3645
Dann EK, Gibson EK, Blackmore RH et al (2019) (2019) Structural selectivity of supported Pd nanoparticles for catalytic NH3 oxidation resolved using combined operando spectroscopy. Nat Catal 22(2):157–163. https://doi.org/10.1038/s41929-018-0213-3
Dong P, Xu N, Xu Y, Wang X (2016) A study of Pt/WO3-carrier catalysts for photocatalytic purification of NO gas. Catal Commun 84:142–146. https://doi.org/10.1016/j.catcom.2016.06.027
Duan K, Tang X, Yi H et al (2010) Rare earth oxide modified Cu-Mn compounds supported on TiO2 catalysts for low temperature selective catalytic oxidation of ammonia and in lean oxygen. J Rare Earths 28:338–342. https://doi.org/10.1016/S1002-0721(10)60277-3
Ebrahimi H, Shahna FG, Bahrami A et al (2017) Photocatalytic degradation of volatile chlorinated organic compounds with ozone addition. Arch Environ Prot 43:65–72. https://doi.org/10.1515/aep-2017-0006
Enesca A, Sisman V (2022) Indoor air photocatalytic decontamination by UV–Vis activated CuS/SnO2/WO3 heterostructure. Catalysts 12:728. https://doi.org/10.3390/catal12070728
Fang D, Li D, He F et al (2019) Experimental and DFT study of the adsorption and activation of NH3 and NO on Mn-based spinels supported on TiO2 catalysts for SCR of NOx. Comput Mater Sci 160:374–381. https://doi.org/10.1016/J.COMMATSCI.2019.01.025
Fiorenza R, Farina RA, Malannata EM et al (2022) VOCs photothermo-catalytic removal on MnOx-ZrO2 catalysts. Catalysts 12:1–17. https://doi.org/10.3390/CATAL12010085
Fujita H, Izumi J, Sagehashi M et al (2004) Decomposition of trichloroethene on ozone-adsorbed high silica zeolites. Water Res 38:166–172. https://doi.org/10.1016/S0043-1354(03)00392-0
Gan Q, Fu M, Zhang Y et al (2021) Synergistic catalytic ozonation of toluene with manganese and cerium varies at low temperature. Chinese Chem Lett 33:2726–2730. https://doi.org/10.1016/J.CCLET.2021.09.006
Gao F, Liu Y, Sani Z et al (2021) Advances in selective catalytic oxidation of ammonia (NH3-SCO) to dinitrogen in excess oxygen: a review on typical catalysts, catalytic performances and reaction mechanisms. J Environ Chem Eng 9:104575. https://doi.org/10.1016/j.jece.2020.104575
García Cortés JM, Illán Gómez MJ, Martínez S, de Lecea C (2007) The selective reduction of NOx with propene on Pt-beta catalyst: a transient study. Appl Catal B Environ 74:313–323. https://doi.org/10.1016/J.APCATB.2007.03.004
Gérardin F, Cloteaux A, Simard J, Favre É (2021) A photodriven energy efficient membrane process for trace VOC removal from air: first step to a smart approach. Chem Eng J 419:129566. https://doi.org/10.1016/j.cej.2021.129566
Gheorghe IF, Ion B (2011) The effects of air pollutants on vegetation and the role of vegetation in reducing atmospheric pollution. Impact Air Pollut Heal Econ Environ Agric Sources. https://doi.org/10.5772/17660
Gokhale AA, Dumesic JA, Mavrikakis M (2008) On the mechanism of low-temperature water gas shift reaction on copper. J Am Chem Soc 130:1402–1414. https://doi.org/10.1021/ja0768237
Guo J, Peng Y, Zhang Y et al (2019) Comparison of NH3-SCO performance over CuOx/H-SSZ-13 and CuOx/H-SAPO-34 catalysts. Appl Catal A Gen 585:117–119. https://doi.org/10.1016/j.apcata.2019.117119
Guo M, Liu Q, Liu C et al (2021a) Rational design of novel CrZrOx catalysts for efficient low temperature SCR of NOx. Chem Eng J 413:127554. https://doi.org/10.1016/J.CEJ.2020.127554
Guo Y, Wen M, Li G, An T (2021b) Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: a critical review. Appl Catal B Environ 281:119447. https://doi.org/10.1016/j.apcatb.2020.119447
Guo L, Meng F, Zeng Y et al (2020) Catalytic ozonation of NOx into HNO3 with low concentration ozone over MnOx-CeO2/TiO2: two-phase synergistic effect of TiO2. Mol Catal 493:111095. https://doi.org/10.1016/j.mcat.2020.111095
Hahn C, Endisch M, Schott FJP, Kureti S (2015) Kinetic modelling of the NOx reduction by H2 on Pt/WO3/ZrO2 catalyst in excess of O2. Appl Catal B Environ 168–169:429–440. https://doi.org/10.1016/J.APCATB.2014.12.033
He C, Cheng J, Zhang X et al (2019) Recent advances in the catalytic oxidation of volatile organic compounds: a review based on pollutant sorts and sources. Chem Rev 119:4471–4568. https://doi.org/10.1021/acs.chemrev.8b00408
Hevia MAG (2008) Optimal hydrocarbon selection for catalytic N2O reduction over. Environ Sci Technol 42:8896–8900
Highlights P (1978) The economic consequences of air pollution. Environ Pollut 15:313. https://doi.org/10.1016/0013-9327(78)90018-6
Hu C, Zhu Q, Jiang Z et al (2008) Preparation and formation mechanism of mesoporous CuO–CeO2 mixed oxides with excellent catalytic performance for removal of VOCs. Microporous Mesoporous Mater 113:427–434. https://doi.org/10.1016/J.MICROMESO.2007.11.043
Hu Z, Wang Z, Guo Y et al (2018) Total oxidation of propane over a Ru/CeO2 catalyst at low temperature. Environ Sci Technol 52:9531–9541. https://doi.org/10.1021/ACS.EST.8B03448
Hu X, Li C, Sun Z et al (2020) Enhanced photocatalytic removal of indoor formaldehyde by ternary heterogeneous BiOCl/TiO2/sepiolite composite under solar and visible light. Build Environ 168:106481. https://doi.org/10.1016/j.buildenv.2019.106481
Hung CM (2010) Characterization and performance of Pt-Pd-Rh cordierite monolith catalyst for selectivity catalytic oxidation of ammonia. J Hazard Mater 180:561–565. https://doi.org/10.1016/j.jhazmat.2010.04.070
Hung C-M (2012) Application of Pt-Rh complex catalyst: feasibility study on the removal of gaseous ammonia. Int J Phys Sci 7:2166–2173. https://doi.org/10.5897/ijps12.024
Ikhlaq A, Kasprzyk-Hordern B (2017) Catalytic ozonation of chlorinated VOCs on ZSM-5 zeolites and alumina: Formation of chlorides. Appl Catal B Environ 200:274–282. https://doi.org/10.1016/j.apcatb.2016.07.019
Ikhlaq A, Brown DR, Kasprzyk-Hordern B (2014) Catalytic ozonation for the removal of organic contaminants in water on ZSM-5 zeolites. Appl Catal B Environ 154–155:110–122. https://doi.org/10.1016/j.apcatb.2014.02.010
Inomata Y, Hata S, Kiyonaga E et al (2021) Synthesis of bulk vanadium oxide with a large surface area using organic acids and its low-temperature NH3-SCR activity. Catal Today 376:188–196. https://doi.org/10.1016/j.cattod.2020.06.041
Jabłońska M, Palkovits R (2016) Copper based catalysts for the selective ammonia oxidation into nitrogen and water vapour-Recent trends and open challenges. Appl Catal B Environ 181:332–351. https://doi.org/10.1016/j.apcatb.2015.07.017
Jabłońska M, Król A, Kukulska-Zajac E et al (2014) Zeolite y modified with palladium as effective catalyst for selective catalytic oxidation of ammonia to nitrogen. J Catal 316:36–46. https://doi.org/10.1016/j.jcat.2014.04.022
Jabłońska M, Ciptonugroho W, Góra-Marek K et al (2017a) Preparation, characterization and catalytic performance of Ag-modified mesoporous TiO2 in low-temperature selective ammonia oxidation into nitrogen and water vapour. Microporous Mesoporous Mater 245:31–44. https://doi.org/10.1016/j.micromeso.2017.02.070
Jabłońska M, Nocuń M, Gołąbek K, Palkovits R (2017b) Effect of preparation procedures on catalytic activity and selectivity of copper-based mixed oxides in selective catalytic oxidation of ammonia into nitrogen and water vapour. Appl Surf Sci 423:498–508. https://doi.org/10.1016/j.apsusc.2017.06.144
Jiang R, Shan H, Li C, Yang C (2011) Preparation and characterization of Mn/MgAlFe as transfer catalyst for SOx abatement. J Nat Gas Chem 20:191–197. https://doi.org/10.1016/S1003-9953(10)60171-5
Jiang Z, Jones DBA, Worden HM, Henze DK (2015) Sensitivity of top-down CO source estimates to the modeled vertical structure in atmospheric CO. Atmos Chem Phys 15:1521–1537. https://doi.org/10.5194/acp-15-1521-2015
Jiang H, Wang C, Wang H, Zhang M (2016) Synthesis of highly efficient MnOx catalyst for low-temperature NH3-SCR prepared from Mn-MOF-74 template. Mater Lett 168:17–19. https://doi.org/10.1016/j.matlet.2015.12.150
Jiang H, Niu Y, Wang Q et al (2018a) Single-phase SO2-resistant to poisoning Co/Mn-MOF-74 catalysts for NH3-SCR. Catal Commun 113:46–50. https://doi.org/10.1016/j.catcom.2018.05.017
Jiang H, Wang Y, Zhou J et al (2018b) Morphology control of manganese-based catalysts for low-temperature selective catalytic reduction of NOx. Mater Lett 233:250–253. https://doi.org/10.1016/j.matlet.2018.09.004
Jurado L, Papaefthimiou V, Thomas S, Roger AC (2021) Low temperature toluene and phenol abatement as tar model molecules over Ni-based catalysts: influence of the support and the synthesis method. Appl Catal B Environ 297:120479. https://doi.org/10.1016/j.apcatb.2021.120479
Kampa M, Castanas E (2008) Human health effects of air pollution. Environ Pollut 151:362–367. https://doi.org/10.1016/j.envpol.2007.06.012
Kathiraser Y, Oemar U, Saw ET et al (2015) Kinetic and mechanistic aspects for CO2 reforming of methane over Ni based catalysts. Chem Eng J 278:62–78. https://doi.org/10.1016/j.cej.2014.11.143
Kim GJ, Kwon DW, Shin JH et al (2018) Influence of the addition of vanadium to Pt/TiO2 catalyst on the selective catalytic oxidation of NH3 to N2. Environ Technol 40:2588–2600. https://doi.org/10.1080/0959333020181554004
Kutluay S (2021) Excellent adsorptive performance of novel magnetic nano-adsorbent functionalized with 8-hydroxyquinoline-5-sulfonic acid for the removal of volatile organic compounds (BTX) vapors. Fuel 287:119691. https://doi.org/10.1016/j.fuel.2020.119691
Kutluay S, Temel F (2021) Silica gel based new adsorbent having enhanced VOC dynamic adsorption/desorption performance. Colloids Surfaces A Physicochem Eng Asp 609:125848. https://doi.org/10.1016/j.colsurfa.2020.125848
Kwon DW, Kim DH, Lee S et al (2021) A dual catalytic strategy by the nature of the functionalization effect as well as active species on vanadium-based catalyst for enhanced low temperature SCR. Appl Catal B Environ 289:120032. https://doi.org/10.1016/j.apcatb.2021.120032
Lee JC, Gopalan AI, Sai-Anand G et al (2019) Preparation of visible light photocatalytic graphene embedded rutile titanium(IV) oxide composite nanowires and enhanced NOx removal. Catalysts 9:170. https://doi.org/10.3390/catal9020170
Levasseur B, Kaliaguine S (2009) Effects of iron and cerium in La1−yCeyCo1−xFexO3 perovskites as catalysts for VOC oxidation. Appl Catal B Environ 88:305–314. https://doi.org/10.1016/J.APCATB.2008.11.007
Li L, Wu P, Yu Q et al (2010) Low temperature H2-SCR over platinum catalysts supported on Ti-containing MCM-41. Appl Catal B Environ 94:254–262. https://doi.org/10.1016/J.APCATB.2009.11.016
Li P, Zhang R, Liu N, Royer S (2017a) Efficiency of Cu and Pd substitution in Fe-based perovskites to promote N2 formation during NH3 selective catalytic oxidation (NH3-SCO). Appl Catal B Environ 203:174–188. https://doi.org/10.1016/j.apcatb.2016.10.021
Li X, Li X, Yang RT et al (2017b) The poisoning effects of calcium on V2O5-WO3/TiO2 catalyst for the SCR reaction: comparison of different forms of calcium. Mol Catal 434:16–24. https://doi.org/10.1016/j.mcat.2017.01.010
Li S, Song L, Zhan Z et al (2021b) Redox and acid properties of MnV2Ox/TiO2 catalysts synthesized by assistance of microwave for NO selective catalytic reduction by ammonia. Chem Eng J Adv 8:100156. https://doi.org/10.1016/j.ceja.2021.100156
Li Z, Jin Y, Chen T et al (2021c) Trimethylchlorosilane modified activated carbon for the adsorption of VOCs at high humidity. Sep Purif Technol 272:118659. https://doi.org/10.1016/j.seppur.2021.118659
Li G, Li N, Sun Y et al (2021a) Efficient defect engineering in Co-Mn binary oxides for low-temperature propane oxidation. Appl Catal B Environ 282:119512. https://doi.org/10.1016/j.apcatb.2020.119512
Li S, Lin Y, Liu G, Shi C (2023) Research status of volatile organic compound (VOC) removal technology and prospect of new strategies: a review. Environ Sci Process Impacts 25:727–740. https://doi.org/10.1039/d2em00436d
Liang Q, Li J, Yue T (2021) Promotional effect of CeO2 on low-temperature selective catalytic reduction of NO by NH3 over V2O5-WO3/TiO2 catalysts. Environ Technol Innov :. Article in press https://doi.org/10.1016/j.eti.2020.101209
Liu Y, Guo W (2021) SOx factors as cell-state regulators in the mammary gland and breast cancer. Semin Cell Dev Biol 114:126–133. https://doi.org/10.1016/j.semcdb.2021.01.002
Liu SM, Guo RT, Sun P et al (2017) The enhancement of Zn resistance of Mn/TiO2 catalyst for NH3-SCR reaction by the modification with Al2(SO4)3. J Taiwan Inst Chem Eng 78:370–377. https://doi.org/10.1016/j.jtice.2017.06.039
Liu Y, Zhao J, Lee JM (2018) Conventional and new materials for Selective Catalytic Reduction (SCR) of NOx. ChemCatChem 10:1499–1511. https://doi.org/10.1002/cctc.201701414
Liu J, Sun M, Lin Q et al (2019a) Promotional effects of ethylenediamine on the low-temperature catalytic activity of selective catalytic oxidation of ammonia over Pt/SiAlOx: states and particle sizes of Pt. Appl Surf Sci 481:1344–1351. https://doi.org/10.1016/j.apsusc.2019.03.199
Liu L, Su S, Xu K et al (2019b) Insights into the highly efficient Co modified MnSm/Ti catalyst for selective catalytic reduction of NOx with NH3 at low temperature. Fuel 255:115798. https://doi.org/10.1016/j.fuel.2019.115798
Liu K, Yu Q, Wang B et al (2020a) Binary copper-manganese based catalysts with urea for low-temperature selective catalytic reduction of NO: performance, characterization and mechanism. Appl Surf Sci 508:144755. https://doi.org/10.1016/J.APSUSC.2019.144755
Liu R, Zhou B, Liu L et al (2021) Enhanced catalytic oxidation of VOCs over porous Mn-based mullite synthesized by in-situ dismutation. J Colloid Interface Sci 585:302–311. https://doi.org/10.1016/j.jcis.2020.11.096
Liu L, Xu K, Su S, et al (2020b) Efficient Sm modified Mn/TiO2 catalysts for selective catalytic reduction of NO with NH3 at low temperature. Appl Catal A Gen 592:. https://doi.org/10.1016/j.apcata.2020.117413
Ma H, Schneider WF (2020) DFT and microkinetic comparison of Pt, Pd and Rh-catalyzed ammonia oxidation. J Catal 383:322–330. https://doi.org/10.1016/j.jcat.2020.01.029
Ma X, Hou Y, Yang L, Lv H (2021a) Adsorption behaviors of VOCs under coal-combustion flue gas environment using activated carbon injection coupled with bag filtering system. Colloids Surfaces A Physicochem Eng Asp 627:127158. https://doi.org/10.1016/j.colsurfa.2021.127158
Ma X, Zhang T, Ji C et al (2021b) Threats to human health and ecosystem: looking for air-pollution related damage since 1990. Renew Sustain Energy Rev 145:111146. https://doi.org/10.1016/j.rser.2021.111146
Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E (2020) Environmental and health impacts of air pollution: a review. Front Public Health 0:14. https://doi.org/10.3389/FPUBH.2020.00014
Martinez-Oviedo A, Kshetri YK, Joshi B, Lee SW (2021) Surface modification of blue TiO2 with silane coupling agent for NOx abatement. Prog Nat Sci Mater Int 31:230–238. https://doi.org/10.1016/j.pnsc.2021.02.001
Méausoone C, El Khawaja R, Tremolet G et al (2019) In vitro toxicological evaluation of emissions from catalytic oxidation removal of industrial VOCs by air/liquid interface (ALI) exposure system in repeated mode. Toxicol Vitr 58:110–117. https://doi.org/10.1016/j.tiv.2019.03.030
Mergbi M, Galloni MG, Aboagye D et al (2023) Valorization of lignocellulosic biomass into sustainable materials for adsorption and photocatalytic applications in water and air remediation. Springer, Berlin Heidelberg
Montero-Montoya R, López-Vargas R, Arellano-Aguilar O (2018) Volatile organic compounds in air: sources, distribution, exposure and associated illnesses in children. Ann Glob Heal 84:225–238. https://doi.org/10.29024/aogh.910
Munsif R, Zubair M, Aziz A, Zafar MN (2021) Industrial air emission pollution: potential sources and sustainable mitigation. Environ Emiss. https://doi.org/10.5772/INTECHOPEN.93104
Nakamura K, Muramatsu T, Ogawa T, Nakagaki T (2021) Prediction of de-NOx performance using monolithic SCR catalyst under load following operation of natural gas-fired combined cycle power plants. Energy 227:120383. https://doi.org/10.1016/j.energy.2021.120383
Nassos S, Elm Svensson E, Boutonnet M, Järås SG (2007) The influence of Ni load and support material on catalysts for the selective catalytic oxidation of ammonia in gasified biomass. Appl Catal B Environ 74:92–102. https://doi.org/10.1016/j.apcatb.2007.01.015
Naveenkumar R, Ramesh Kumar S, Pushyanthkumar G, Senthil Kumaran S (2020) NOx, CO & HC control by adopting activated charcoal enriched filter in catalytic converter of diesel engine. Mater Today Proc 22:2283–2290. https://doi.org/10.1016/j.matpr.2020.03.349
Neha PR, Vir Singh S (2020) Catalytic abatement of CO, HCs and soot emissions over spinel-based catalysts from diesel engines: an overview. J Environ Chem Eng 8:103627. https://doi.org/10.1016/j.jece.2019.103627
Ojstršek A, Fakin D, Hribernik S, et al (2020) Electrospun nanofibrous composites from cellulose acetate / ultra-high silica zeolites and their potential for VOC adsorption from air. Carbohydr Polym 236:. https://doi.org/10.1016/j.carbpol.2020.116071
Ongari D, Tiana D, Stoneburner SJ et al (2017) Origin of the strong interaction between polar molecules and copper(II) paddle-wheels in metal organic frameworks. J Phys Chem C 121:15135–15144. https://doi.org/10.1021/ACS.JPCC.7B02302
Oton LF, Oliveira AC, de Araujo JCS et al (2020) Selective catalytic reduction of NOx by CO (CO-SCR) over metal-supported nanoparticles dispersed on porous alumina. Adv Powder Technol 31:464–476. https://doi.org/10.1016/J.APT.2019.11.003
Ou R, Zhu W, Li L, et al (2021) Boosted capture of volatile organic compounds in adsorption capacity and selectivity by rationally exploiting defect-engineering of UiO-66(Zr). Sep Purif Technol 266:. https://doi.org/10.1016/j.seppur.2020.118087
Pappas DK, Boningari T, Boolchand P, Smirniotis PG (2016) Novel manganese oxide confined interweaved titania nanotubes for the lowerature selective catalytic reduction (SCR) of NOx by NH3. J Catal 334:1–13. https://doi.org/10.1016/j.jcat.2015.11.013
Patel VK, Sharma S (2021) Effect of oxide supports on palladium based catalysts for NO reduction by H2-SCR. Catal Today 375:591–600. https://doi.org/10.1016/j.cattod.2020.04.006
Peng S, Yang X, Strong J et al (2020) MnO2-decorated N-doped carbon nanotube with boosted activity for low-temperature oxidation of formaldehyde. J Hazard Mater 396:122750. https://doi.org/10.1016/j.jhazmat.2020.122750
Pinzón M, Ruiz-López E, Romero A et al (2021) Electrochemical activation of Ru catalyst with alkaline ion conductors for the catalytic decomposition of ammonia. Mol Catal 511:111721. https://doi.org/10.1016/j.mcat.2021.111721
Piumetti M, Fino D, Russo N (2015) Mesoporous manganese oxides prepared by solution combustion synthesis as catalysts for the total oxidation of VOCs. Appl Catal B Environ 163:277–287. https://doi.org/10.1016/J.APCATB.2014.08.012
Plata-Gryl M, Momotko M, Makowiec S, Boczkaj G (2022) Characterization of diatomaceous earth coated with nitrated asphaltenes as superior adsorbent for removal of VOCs from gas phase in fixed bed column. Chem Eng J 427:130653. https://doi.org/10.1016/j.cej.2021.130653
Puri P, Nandar SK, Kathuria S, Ramesh V (2017) Effects of air pollution on the skin: a review. Indian J Dermatol Venereol Leprol 83:415–423. https://doi.org/10.4103/0378-6323.199579
Qi G, Gatt JE, Yang RT (2004) Selective catalytic oxidation (SCO) of ammonia to nitrogen over Fe-exchanged zeolites prepared by sublimation of FeCl3. J Catal 226:120–128. https://doi.org/10.1016/j.jcat.2004.05.023
Qu Z, Fan R, Wang Z et al (2015) Selective catalytic oxidation of ammonia to nitrogen over MnO2 prepared by urea-assisted hydrothermal method. Appl Surf Sci 351:573–579. https://doi.org/10.1016/j.apsusc.2015.05.154
Rahmaninejad F, Gavaskar VS, Abbasian J (2012) Dry regenerable CuO/γ-Al2O3 catalyst for simultaneous removal of SOx and NOx from flue gas. Appl Catal B Environ 119–120:297–303. https://doi.org/10.1016/j.apcatb.2012.03.005
Roberts W (2021) Air pollution and skin disorders. Int J Women’s Dermatology 7:91–97. https://doi.org/10.1016/j.ijwd.2020.11.001
Roy S, Baiker A (2009) NOx Storage-reduction catalysis: from mechanism and materials properties to storage-reduction performance. Chem Rev 109:4054–4091. https://doi.org/10.1021/cr800496f
Roy S, Hegde MS (2008) Pd ion substituted CeO2: a superior de-NOx catalyst to Pt or Rh metal ion doped ceria. Catal Commun 9:811–815. https://doi.org/10.1016/J.CATCOM.2007.09.019
Roy S, Aarthi T, Hegde MS, Madras G (2007a) Kinetics of photocatalytic reduction of NO by CO with Pd 2+-ion-substituted nano-TiO2. Ind Eng Chem Res 46:5798–5802. https://doi.org/10.1021/ie0704593
Roy S, Hegde MS, Ravishankar N, Madras G (2007b) Creation of redox adsorption sites by Pd 2+ ion substitution in nanoTiO2 for high photocatalytic activity of CO oxidation, NO reduction, and NO decomposition. J Phys Chem C 111:8153–8160. https://doi.org/10.1021/jp066145v
Roy S, Marimuthu A, Hegde MS, Madras G (2007c) High rates of CO and hydrocarbon oxidation and NO reduction by CO over Ti0.99Pd0.01O1.99. Appl Catal B Environ 73:300–310. https://doi.org/10.1016/J.APCATB.2007.01.003
Roy S, Marimuthu A, Hegde MS, Madras G (2007d) High rates of NO and N2O reduction by CO, CO and hydrocarbon oxidation by O2 over nano crystalline Ce0.98Pd0.02O2−δ: Catalytic and kinetic studies. Appl Catal B Environ 71:23–31. https://doi.org/10.1016/J.APCATB.2006.08.005
Roy S, Hegde MS, Sharma S et al (2008a) Low temperature NOx and N2O reduction by H2: mechanism and development of new nano-catalysts. Appl Catal B Environ 84:341–350. https://doi.org/10.1016/J.APCATB.2008.04.008
Roy S, Marimuthu A, Deshpande PA et al (2008b) Selective catalytic reduction of NOx: mechanistic perspectives on the role of base metal and noble metal ion substitution. Ind Eng Chem Res 47:9240–9247. https://doi.org/10.1021/ie8010879
Roy S, Marimuthu A, Hegde MS, Madras G (2008c) NO reduction by H2 over nano-Ce0.98Pd0.02O2−δ. Catal Commun 9:101–105. https://doi.org/10.1016/J.CATCOM.2007.05.031
Roy S, Viswanath B, Hegde MS, Madras G (2008d) Low-temperature selective catalytic reduction of NO with NH3 over Ti0.9M0.1O2-δ (M ) Cr, Mn, Fe Co, Cu). J Phys Chem C 112:6002–6012
Roy S, Hegde MS, Madras G (2009) Catalysis for NOx abatement. Appl Energy 86:2283–2297. https://doi.org/10.1016/J.APENERGY.2009.03.022
Roy S, van Vegten N, Baiker A (2010) Single-step flame-made Pt/MgAl2O4 – A NOx storage-reduction catalyst with unprecedented dynamic behavior and high thermal stability. J Catal 271:125–131. https://doi.org/10.1016/J.JCAT.2010.02.017
Roy S, van Vegten N, Maeda N, Baiker A (2012) NOx storage and reduction over flame-made M/MgAl2O4 (M = Pt, Pd, and Rh): a comparative study. Appl Catal B Environ 119–120:279–286. https://doi.org/10.1016/J.APCATB.2012.03.008
Rutkowska M, Pacia I, Basąg S et al (2017) Catalytic performance of commercial Cu-ZSM-5 zeolite modified by desilication in NH3-SCR and NH3-SCO processes. Microporous Mesoporous Mater 246:193–206. https://doi.org/10.1016/j.micromeso.2017.03.017
Rutkowska M, Duda M, Macina D et al (2019) Mesoporous Beta zeolite functionalisation with FexCry oligocations; catalytic activity in the NH3–SCO process. Microporous Mesoporous Mater 278:1–13. https://doi.org/10.1016/j.micromeso.2018.11.003
Sabzehmeidani MM, Mahnaee S, Ghaedi M et al (2021) Carbon based materials: a review of adsorbents for inorganic and organic compounds. Mater Adv 2:598–627. https://doi.org/10.1039/d0ma00087f
Saqlain S, Cha BJ, Kim SY et al (2021) Impact of humidity on the removal of volatile organic compounds over Fe loaded TiO2 under visible light irradiation: insight into photocatalysis mechanism by operando DRIFTS. Mater Today Commun 26:102119. https://doi.org/10.1016/j.mtcomm.2021.102119
Sato K, Tomonaga H, Yamamoto T et al (2016) A synthetic pseudo-Rh: NOx reduction activity and electronic structure of Pd-Ru solid-solution alloy nanoparticles. Sci Rep 6:28265. https://doi.org/10.1038/srep28265
Sekiguchi K, Morinaga W, Sakamoto K et al (2010) Degradation of VOC gases in liquid phase by photocatalysis at the bubble interface. Appl Catal B Environ 97:190–197. https://doi.org/10.1016/j.apcatb.2010.03.039
Shang K, Ren J, Zhang Q et al (2021) Successive treatment of benzene and derived byproducts by a novel plasma catalysis-adsorption process. J Environ Chem Eng 9:105767. https://doi.org/10.1038/srep28265
Shao Q, Dong H, Zhang J et al (2021) Manganese supported on controlled dealumination Y-zeolite for ozone catalytic oxidation of low concentration toluene at low temperature. Chemosphere 271:129604. https://doi.org/10.1016/j.chemosphere.2021.129604
Shi W, Bi J, Liu R et al (2021a) Decrease in the chronic health effects from PM2.5 during the 13th Five-Year Plan in China: impacts of air pollution control policies. J Clean Prod 317:128433. https://doi.org/10.1016/j.jclepro.2021.128433
Shi Y, Chu Q, Xiong W et al (2021b) A new type bimetallic NiMn-MOF-74 as an efficient low-temperatures catalyst for selective catalytic reduction of NO by CO. Chem Eng Process - Process Intensif 159:108232. https://doi.org/10.1016/j.cep.2020.108232
Shi Y, Wang J, Zhou R (2021c) Pt-support interaction and nanoparticle size effect in Pt/CeO2–TiO2 catalysts for low temperature VOCs removal. Chemosphere 265:129127. https://doi.org/10.1016/j.chemosphere.2020.129127
Shi Y, Zhang L, Li W et al (2021) Association between long-term exposure to ambient air pollution and clinical outcomes among patients with heart failure: findings from the China PEACE Prospective Heart Failure Study. Ecotoxicol Environ Saf 222:112517. https://doi.org/10.1016/j.ecoenv.2021.112517
Si H, Zhou M, Fang Y et al (2021) Photocatalytic concrete for NOx degradation: influence factors and durability. Constr Build Mater 298:123835. https://doi.org/10.1016/j.conbuildmat.2021.123835
Skowron A, Lee DS, De LRR et al (2021) (2021) Greater fuel efficiency is potentially preferable to reducing NOx emissions for aviation’s climate impacts. Nat Commun 121(12):1–8. https://doi.org/10.1038/s41467-020-20771-3
Song S, Jiang S (2012) Selective catalytic oxidation of ammonia to nitrogen over CuO/CNTs: the promoting effect of the defects of CNTs on the catalytic activity and selectivity. Appl Catal B Environ 117–118:346–350. https://doi.org/10.1016/j.apcatb.2012.01.030
Streets DG, Zhang Q, Wang L et al (2006) Revisiting China’s CO emissions after the Transport and Chemical Evolution over the Pacific (TRACE-P) mission: synthesis of inventories, atmospheric modeling, and observations. J Geophys Res Atmos 111:D14. https://doi.org/10.1029/2006JD007118
Sun M, Wang S, Li Y et al (2017a) Promotion of catalytic performance by adding W into Pt/ZrO2 catalyst for selective catalytic oxidation of ammonia. Appl Surf Sci 402:323–329. https://doi.org/10.1016/j.apsusc.2016.12.241
Sun M, Wang S, Li Y et al (2017b) Promotion of catalytic performance by adding Cu into Pt/ZSM-5 catalyst for selective catalytic oxidation of ammonia. J Taiwan Inst Chem Eng 78:401–408. https://doi.org/10.1016/j.jtice.2017.06.045
Tan W, Chen F, Gong L et al (2016) The role of ceria in LSM-GDC composite cathode for electrochemical reduction of nitric oxide. Appl Catal B Environ 197:244–253. https://doi.org/10.1016/j.apcatb.2016.02.029
Tan W, Wang J, Cai Y et al (2022) Molybdenum oxide as an efficient promoter to enhance the NH3-SCR performance of CeO2-SiO2 catalyst for NOx removal. Catal Today 397–399:475–483. https://doi.org/10.1016/j.cattod.2021.07.007
Tang X, Wang C, Gao F et al (2020) Effect of hierarchical element doping on the low-temperature activity of manganese-based catalysts for NH3-SCR. J Environ Chem Eng 8:104399. https://doi.org/10.1016/j.jece.2020.104399
Tian B, Ma S, Zhan Y et al (2021a) Stability and catalytic activity to NOx and NH3 of single-atom manganese catalyst with graphene-based substrate: a DFT study. Appl Surf Sci 541:148460. https://doi.org/10.1016/j.apsusc.2020.148460
Tian S, Zhan S, Lou Z et al (2021b) Electrodeposition synthesis of 3D-NiO1-δ flowers grown on Ni foam monolithic catalysts for efficient catalytic ozonation of VOCs. J Catal 398:1–13. https://doi.org/10.1016/j.jcat.2021.04.011
Tsimpidi AP, Trail M, Hu Y et al (2012) Modeling an air pollution episode in northwestern United States: identifying the effect of nitrogen oxide and volatile organic compound emission changes on air pollutants formation using direct sensitivity analysis. J Air Waste Manag Assoc 62:1150–1165. https://doi.org/10.1080/10962247.2012.697093
Ueno Y, Horiuchi T, Morimoto T, Niwa O (2001) Microfluidic device for airborne BTEX detection. Anal Chem 73:4688–4693. https://doi.org/10.1021/ac010210+
Wang C-C, Yang Y-J, Jiang J-C (2009) DFT study of NHx (x = 1–3) adsorption on RuO2(110) surfaces. J Phys Chem C 113:2816–2821. https://doi.org/10.1021/JP8062355
Wang Z, Qu Z, Quan X et al (2013) Selective catalytic oxidation of ammonia to nitrogen over CuO-CeO2 mixed oxides prepared by surfactant-templated method. Appl Catal B Environ 134–135:153–166. https://doi.org/10.1016/j.apcatb.2013.01.029
Wang T, Zhu C, Liu H et al (2018a) Performance of selective catalytic reduction of NO with NH3 over natural manganese ore catalysts at low temperature. Environ Technol 39:317–326. https://doi.org/10.1080/09593330.2017.1300190
Wang Y, Yang D, Li S et al (2018b) Ru/hierarchical HZSM-5 zeolite as efficient bi-functional adsorbent/catalyst for bulky aromatic VOCs elimination. Microporous Mesoporous Mater 258:17–25. https://doi.org/10.1016/j.micromeso.2017.08.052
Wang F, Shen B, Zhu S, Wang Z (2019a) Promotion of Fe and Co doped Mn-Ce/TiO2 catalysts for low temperature NH3-SCR with SO2 tolerance. Fuel 249:54–60. https://doi.org/10.1016/j.fuel.2019.02.113
Wang HM, Ning P, Zhang QL et al (2019b) Effect of different RuO2 contents on selective catalytic oxidation of ammonia over RuO2-Fe2O3 catalysts. Ranliao Huaxue Xuebao/journal Fuel Chem Technol 47:215–223. https://doi.org/10.1016/s1872-5813(19)30011-8
Wang Z, Sun Q, Wang D et al (2019c) Hollow ZSM-5 zeolite encapsulated Ag nanoparticles for SO2-resistant selective catalytic oxidation of ammonia to nitrogen. Sep Purif Technol 209:1016–1026. https://doi.org/10.1016/j.seppur.2018.09.045
Wang ZY, Guo RT, Shi X et al (2020) The superior performance of CoMnOx catalyst with ball-flowerlike structure for low-temperature selective catalytic reduction of NOx by NH3. Chem Eng J 381:122753. https://doi.org/10.1016/j.cej.2019.122753
Wang C, Ren D, Harle G et al (2021a) Ammonia removal in selective catalytic oxidation: influence of catalyst structure on the nitrogen selectivity. J Hazard Mater 416:125782. https://doi.org/10.1016/j.jhazmat.2021.125782
Wang F, Zhu Y, Li Z et al (2021b) Promoting effect of acid sites on NH3-SCO activity with water vapor participation for Pt-Fe/ZSM-5 catalyst. Catal Today 376:311–317. https://doi.org/10.1016/j.cattod.2020.06.039
Wang H, Lin M, Murayama T et al (2021c) Selective catalytic oxidation of ammonia to nitrogen over zeolite-supported Pt-Au catalysts: effects of alloy formation and acid sites. J Catal 402:101–113. https://doi.org/10.1016/j.jcat.2021.08.002
Wang X, Tan W, Guo K et al (2021d) Evaluation of manganese oxide octahedral molecular sieves for CO and C 3 H 6 oxidation at diesel exhaust conditions. Front Environ Chem 2:72250. https://doi.org/10.3389/fenvc.2021.672250
Wantz E, Kane A, Lhuissier M et al (2021) A mathematical model for VOCs removal in a treatment process coupling absorption and biodegradation. Chem Eng J 423:130106. https://doi.org/10.1016/j.cej.2021.130106
Wei Y, Fan H, Wang R (2018) Transition metals (Co, Zr, Ti) modified iron-samarium oxide as efficient catalysts for selective catalytic reduction of NOx at low-temperature. Appl Surf Sci 459:63–73. https://doi.org/10.1016/j.apsusc.2018.07.151
Wu Z, Deng J, Xie S et al (2016) Mesoporous Cr2O3-supported Au–Pd nanoparticles: high-performance catalysts for the oxidation of toluene. Microporous Mesoporous Mater 224:311–322. https://doi.org/10.1016/J.MICROMESO.2015.11.061
Wu R, Li L, Zhang N et al (2021) Enhancement of low-temperature NH3-SCR catalytic activity and H2O & SO2 resistance over commercial V2O5-MoO3/TiO2 catalyst by high shear-induced doping of expanded graphite. Catal Today 376:302–310. https://doi.org/10.1016/j.cattod.2020.04.051
Xu H, Yan N, Qu Z et al (2017) Gaseous heterogeneous catalytic reactions over Mn-based oxides for environmental applications: a critical review. Environ Sci Technol 51:8879–8892. https://doi.org/10.1021/ACS.EST.6B06079
Xu Y, Wu X, Lin Q et al (2019) SO2 promoted V2O5-MoO3/TiO2 catalyst for NH3-SCR of NOx at low temperatures. Appl Catal A Gen 570:42–50. https://doi.org/10.1016/j.apcata.2018.10.040
Xue Y, Sun W, Wang Q et al (2018) Sparsely loaded Pt/MIL-96(Al) MOFs catalyst with enhanced activity for H2-SCR in a gas diffusion reactor under 80 °C. Chem Eng J 335:612–620. https://doi.org/10.1016/J.CEJ.2017.11.011
Yan Q, Chen S, Zhang C et al (2018) Synthesis of Cu0.5Mg1.5Mn0.5Al0.5Ox mixed oxide from layered double hydroxide precursor as highly efficient catalyst for low-temperature selective catalytic reduction of NOx with NH3. J Colloid Interface Sci 526:63–74. https://doi.org/10.1016/j.jcis.2018.04.099
Yan DJ, Guo T, Yu Y, Chen ZH (2021) Lead poisoning and regeneration of Mn-Ce/TiO2 catalysts for NH3-SCR of NOx at low temperature. Ranliao Huaxue Xuebao/journal Fuel Chem Technol 49:113–120. https://doi.org/10.1016/S1872-5813(21)60003-8
Yang L, You X, Sheng Z et al (2018) The promoting effect of noble metal (Rh, Ru, Pt, Pd) doping on the performances of MnOx-CeO2 / graphene catalysts for the selective catalytic reduction of NO with NH3 at low temperatures. New J Chem 42:11673. https://doi.org/10.1039/c8nj01417e
Yang W, Wang X, Song S, Zhang H (2019) Syntheses and applications of noble-metal-free CeO2-based mixed-oxide nanocatalysts. Chem 5:1743–1774. https://doi.org/10.1016/j.chempr.2019.04.009
Yang B, Zhou J, Wang W et al (2020) Extraction and separation of tungsten and vanadium from spent V2O5–WO3/TiO2 SCR catalysts and recovery of TiO2 and sodium titanate nanorods as adsorbent for heavy metal ions. Colloids Surfaces A Physicochem Eng Asp 601:124963. https://doi.org/10.1016/j.colsurfa.2020.124963
Yang J, Qin J, Guo Z et al (2021a) Zn-based metal organic framework derivative with uniform metal sites and hierarchical pores for efficient adsorption of formaldehyde. Chinese Chem Lett 32:1819–1822. https://doi.org/10.1016/j.cclet.2020.11.023
Yang M, Shen G, Wang Q et al (2021b) Roles of oxygen vacancies of CeO2 and Mn-doped CeO2 with the same morphology in benzene catalytic oxidation. Mol 26:6363. https://doi.org/10.3390/molecules26216363
Ye B, Kim J, Lee MJ et al (2021) Mn-Ce oxide nanoparticles supported on nitrogen-doped reduced graphene oxide as low-temperature catalysts for selective catalytic reduction of nitrogen oxides. Microporous Mesoporous Mater 310:110588. https://doi.org/10.1016/j.micromeso.2020.110588
Yu Q, Richter M, Li L et al (2010) The promotional effect of Cr on catalytic activity of Pt/ZSM-35 for H2-SCR in excess oxygen. Catal Commun 11:955–959. https://doi.org/10.1016/J.CATCOM.2010.03.021
Yu X, Liu S, Lin G et al (2020) KOH-activated hydrochar with engineered porosity as sustainable adsorbent for volatile organic compounds. Colloids Surfaces A Physicochem Eng Asp 588:124372. https://doi.org/10.1016/j.colsurfa.2019.124372
Zeng Y, Gu L, Feng Y et al (2020) Morphologically uniform Pd/FexMn3−xO4-HP interfaces as the high-performance model catalysts for catalytic combustion of volatile organic compound. Appl Surf Sci 528:147006. https://doi.org/10.1016/j.apsusc.2020.147006
Zhang L, He H (2009) Mechanism of selective catalytic oxidation of ammonia to nitrogen over Ag/Al2O3. J Catal 268:18–25. https://doi.org/10.1016/j.jcat.2009.08.011
Zhang L, Zhang C, He H (2009) The role of silver species on Ag/Al2O3 catalysts for the selective catalytic oxidation of ammonia to nitrogen. J Catal 261:101–109. https://doi.org/10.1016/j.jcat.2008.11.004
Zhang Q, Wang H, Ning P et al (2017a) In situ DRIFTS studies on CuO-Fe2O3 catalysts for low temperature selective catalytic oxidation of ammonia to nitrogen. Appl Surf Sci 419:733–743. https://doi.org/10.1016/j.apsusc.2017.05.056
Zhang X, Gao B, Creamer AE et al (2017b) Adsorption of VOCs onto engineered carbon materials: a review. J Hazard Mater 338:102–123. https://doi.org/10.1016/j.jhazmat.2017.05.013
Zhang L, Yang C, Lv K et al (2019a) SPR effect of bismuth enhanced visible photoreactivity of Bi2WO6 for NO abatement. Cuihua Xuebao/chinese J Catal 40:755–764. https://doi.org/10.1016/s1872-2067(19)63320-6
Zhang LL, Chen XM, Liu CG (2019b) Reduction of N2O by CO via Mans-van Krevelen mechanism over phosphotungstic acid supported single-atom catalysts: a density functional theory study. Inorg Chem 58:5221–5229. https://doi.org/10.1021/acs.inorgchem.9b00290
Zhang Y, Liu J, Xing H, Li J (2020a) Performance and fungal diversity of bio-trickling filters packed with composite media of polydimethylsiloxane and foam ceramics for hydrophobic VOC removal. Chemosphere 256:127093. https://doi.org/10.1016/j.chemosphere.2020.127093
Zhang Y, Zhao L, Duan J, Bi S (2020b) Insights into deNOx processing over Ce-modified Cu-BTC catalysts for the CO-SCR reaction at low temperature by in situ DRIFTS. Sep Purif Technol 234:116081. https://doi.org/10.1016/j.seppur.2019.116081
Zhang Z, Jiang C, Li D et al (2020c) Micro-mesoporous activated carbon simultaneously possessing large surface area and ultra-high pore volume for efficiently adsorbing various VOCs. Carbon N Y 170:567–579. https://doi.org/10.1016/j.carbon.2020.08.033
Zhang X, Lv S, Zhang X et al (2021a) Improvement of the activity and SO2 tolerance of Sb-modified Mn/PG catalysts for NH3-SCR at a low temperature. J Environ Sci (China) 101:1–15. https://doi.org/10.1016/j.jes.2020.07.027
Zhang Z, Li R, Wang M, et al (2021b) Two steps synthesis of CeTiOx oxides nanotube catalyst: enhanced activity, resistance of SO2 and H2O for low temperature NH3-SCR of NOx. Appl Catal B Environ 282:. https://doi.org/10.1016/j.apcatb.2020.119542
Zhang Z, Li Y, Yang P et al (2021c) Improved NH3-SCR deNOx activity and tolerance to H2O & SO2 at low temperature over the NbmCu0.1-mCe0.9Ox catalysts: role of acidity by niobium doping. Fuel 303:121239. https://doi.org/10.1016/j.fuel.2021.121239
Zhao Z, Dai H, Deng J et al (2012) Three-dimensionally ordered macroporous La0.6Sr0.4FeO3−δ: high-efficiency catalysts for the oxidative removal of toluene. Microporous Mesoporous Mater 163:131–139. https://doi.org/10.1016/J.MICROMESO.2012.07.006
Zhao D, Gao Z, Xian H et al (2018) Addition of Pd on La0.7Sr0.3CoO3 perovskite to enhance catalytic removal of NOx. I&EC Res 57:521–531. https://doi.org/10.1021/acs.iecr.7b04399
Zhao G, Li M, Wang L et al (2020a) Environmentally-friendly tourmaline modified CeMnFeOx catalysts for low-temperature selective catalytic reduction of NOx with NH3. Catal Today 355:385–396. https://doi.org/10.1016/j.cattod.2019.08.018
Zhao J, Xi W, Tu C et al (2020b) Catalytic oxidation of chlorinated VOCs over Ru/TixSn1-x catalysts. Appl Catal B Environ 263:118237. https://doi.org/10.1016/j.apcatb.2019.118237
Zhao W, Adeel M, Zhang P et al (2022) A critical review on surface-modified nano-catalyst application for the photocatalytic degradation of volatile organic compounds. Environ Sci Nano 9:61–80. https://doi.org/10.1039/d1en00955a
Zhao Q, Zheng Y, Song C et al (2020) Novel monolithic catalysts derived from in-situ decoration of Co3O4 and hierarchical Co3O4@MnOx on Ni foam for VOC oxidation. Appl Catal B Environ 265:118552. https://doi.org/10.1016/j.apcatb.2019.118552
Zhou G, Lan H, Wang H et al (2014) Catalytic combustion of PVOCs on MnOx catalysts. J Mol Catal A Chem 393:279–288. https://doi.org/10.1016/J.MOLCATA.2014.06.028
Zhou X, Yu F, Sun R et al (2020) Two-dimensional MnFeCo layered double oxide as catalyst for enhanced selective catalytic reduction of NOx with NH3 at low temperature (25–150 °C). Appl Catal A Gen 592:117432. https://doi.org/10.1016/j.apcata.2020.117432
Zhu M, Wachs IE (2015) Iron-based catalysts for the high-temperature water−gas shift (HT-WGS) reaction: a review. ACS Catal 6:722–732. https://doi.org/10.1021/acscatal.5b02594
Zhu Z, Xu B (2022) Purification technologies for NOx removal from flue gas: a review. Separations 9:1–27. https://doi.org/10.3390/separations9100307
Zhu L, Zhong Z, Xue J et al (2018) NH3-SCR performance and the resistance to SO2 for Nb doped vanadium based catalyst at low temperatures. J Environ Sci (China) 65:306–316. https://doi.org/10.1016/j.jes.2017.06.033
Zhu H, Song S, Wang R (2020) Removal of nox by adsorption/decomposition on H3PW12O406·H2O/CeO2 supported on ceria. Aerosol Air Qual Res 20:2273–2279. https://doi.org/10.4209/aaqr.2020.03.0110
Zou L, Luo Y, Hooper M, Hu E (2006) Removal of VOCs by photocatalysis process using adsorption enhanced TiO2-SiO2 catalyst. Chem Eng Process Process Intensif 45:959–964. https://doi.org/10.1016/j.cep.2006.01.014
Funding
Dr. S.A. Singh is financially supported by the Science & Engineering Research Board (SERB), Department of Science and Technology (Grant No. CRG/2021/000333). The BITS-Pilani Hyderabad campus provided institute fellowship to Aathira Bhaskaran.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Aathira Bhaskaran and Deepika Sharma. The first draft of the manuscript was written by Aathira Bhaskaran, Deepika Sharma, Sounak Roy, and Satyapaul A. Singh, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
All the authors are consented to participate in the drafting of the review article.
Consent for publication
All authors are consented to publish the review article.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: George Z. Kyzas
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhaskaran, A., Sharma, D., Roy, S. et al. Technological solutions for NOx, SOx, and VOC abatement: recent breakthroughs and future directions. Environ Sci Pollut Res 30, 91501–91533 (2023). https://doi.org/10.1007/s11356-023-28840-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-28840-y