Abstract
Green products such as plant pigments in all filed are gaining fame globally due to their excellent ayurvedic and biological characteristics. In this study, microwave rays have been employed for the isolation of colorants from Anar Phali while bio-mordant have been included to get color-fast shades. The colorant was isolated in an acidic medium before and after microwave rays for 2 min. For getting darker shades with different tints, sustainable chemical and plant-based extracts as bio-mordant have been employed before and after bio coloration of wool yarn at given conditions. CIE Lab system computed in Colori-spectrophotometer (CS-410) was used to observe the change in color depth and tonal variation of dyed fabrics, and ISO standard methods have been employed to rate the colorfastness to light, washing, and rubbing at grey scale. It is concluded that microwave rays have an excellent sustainable efficacy to isolate colorant from Anar Phali powder for wool dyeing, whereas the addition of bio-mordants has made the process more sustainable and eco-friendly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The world is beautiful as the people are. Lush green grasses, a variety of flowers, shady trees, herbal shrubs, a water ecosystem, etc. all are adding to the charm of the globe (Khan 2022). With the advancement of knowledge where every field is progressing, the color industry has also gained its share in all fields (Vogel et al. 2022). After the discovery of Mauvein by Perkin, the world of synthetic dyes has opened new horizons and the traditional art of natural dyes declined (Mahmoudpour et al. 2021). Although synthetic dyes are cheap and provide brilliant shades, yet their frequent uses for all fields have been observed keenly (Sonal and Mishra 2021). The majority of synthetic dyes contain such mediators that are carcinogenic, mutagenic, and nonrenewable (Patel et al. 2021). After their utilization, either by changing water quality parameters or by decreasing soil fertility, air quality index, and destruction of Agri-land (Worlanyo and Jiangfeng 2021) damage the eco-balance of the environment (Peter et al. 2021). It also affects the equilibrium of the environment by raising the water’s high pH. Due to such reasons, many synthetic dyes have been banned internationally by environmental organizations (Affat 2021). To protect the environment globally, they now promote green products such as either by forcing industries to include eco-label or by giving awareness through seminars, conferences, projects, etc. (Mungkung et al. 2021).
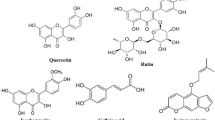
Natural dyes are functional bioactive dyeing molecules that extracted from natural sources (Zhang et al. 2021). These dyes are nontoxic, renewable, easily biodegradable, and possess pollution-free effluents (Singh et al. 2021). These dyes act as health remedies, and even their crude waste acts as fertilizers for the soil. These dyes can create distinctive and calming hues and even with time, the shades become better. Hence, the revival of bio colorants for all fields to make the process eco-friendly and to retain the traditional art of dyeing is on the way (Panda et al. 2022). For improving extraction yields, modern approaches have been adopted such as microwave treatment and for shade permanency, bio-mordants have been selected (Al-Sayed and Abdelrahman 2021). The use of microwave radiation (MW) as green heating technology has been included to get a high yield of colorant (Al-Sayed and Abdelrahman 2021). Microwave-aided extraction (MAE) is the approach that produces natural dyes with the highest yield of all by causing excellent mass transfer (colorant) into a solvent through a leveled and uniform heating process (Zin et al. 2022). Although natural dyes are classic dyes with numerous ayurvedic and biological characteristics, their isolation and shade permanency are the key drawbacks (Hamdy et al. 2021). Some conventional methods are used to extract the colorant. Still, these classic methods are time-, solvent-, economy-, and labor-consuming, and even if the heating process becomes prolonged, the functional moiety faces hydrolysis (Arabi et al. 2021; Adeel et al. 2023a). Hence their extraction require noval tools such as MW rays. For shade permanency, the salts of Cu, Cr, Co, Ni, Fe, and Al, etc., are used, but their effluents also pollute the water and, in turn, become dangerous for the ecosystem (Uddin et al. 2022). Bio-mordants are plant based functional molecules that have also a tendency to color substrate (Dulo et al. 2022; Barkaat et al. 2023). These bioactive through extra interaction with colorant and fabric produce variable gamutes onto various types of fibers used. In light of the benefits of cost-effective and eco-friendly nature of this isolation technique, the current study has been designed to explore the coloring potency of Opuntia ficus fruit (Fig. 1a) through microwave rays and to utilize sustainable mordants for getting permanent shades. Opuntia ficus is one of the members of the Cactaceae family that contain betalain as a potential coloring molecule (Fig. 1b) that impart color onto fibers (Barba et al. 2022). It is used for anti-inflammatory, hypocholesterolemic, pharmacologic, and antioxidant properties and as a medicine to treat diabetes (Sabtain et al. 2021).Wool is a well-known textile fabric because of its distinctive characteristics, which include smoothness and softness, resistance to wrinkles, heat and fire, high absorbency, flexibility, and resiliency (Salleh et al. 2021). The fiber is composed of keratin that has been joined together with peptide bonds (Shavandi et al. 2021). The amide linkage (Fig. 1c) is responsible for binding with dye and mordants (Hosseinnezhad et al. 2021a, b).
The main aims of the current study are:
-
1.
To find a sustainable solvent medium for extraction of betalain using selected time of microwave rays
-
2.
To find sustainable dyeing points through central composite design (CCD) as a statistical tool
-
3.
To use a low level of chemical and bio-mordants for getting permanent shades
Materials and methods
Materials required
Opuntia ficus as a source of bio-dye pomegranate peel and Acacia bark, harmal, and neem as a source of bio-mordants were purchased from the local market in Faisalabad, Pakistan. After being cleaned with distilled water, all the cleaned samples were dried, sliced, and ground finely. Woolen yarn samples were provided by the Carpet Department, University of Sistan and Baluchestan, Zahedan, Iran. For shade fastness, commercial-grade mordants such as iron sulfate (FeSO4), aluminum sulfate (Al2[SO4]3), chromium sulpfate (Cr2(SO4)3), and tannic acid (T.A.) were used.
Extraction process
The crude powder of dried Opuntia ficus fruit was sieved and subjected to isolation. The aqueous extract was prepared by heating 4-g crude powder with 100 mL of water at boiling for 45 min. The acidic extract was prepared by boiling 4 g of crude sieved powder with 100 mL of acidic solution (1% HCl). Extracts after filtration and woolen yarns were exposed to microwave (MW) using Dawlance-made domestic Irradiator at high power. The four different sets were designed and used to observe coloring efficiency of Opuntia ficus fruit. In one step, before treatment, the extract was used to dye yarn at 80 ºC for 45 min. In another set, after radiation up to 5 min, the radiated extracts were used to dye radiated yarns at given conditions.
Statistical analysis of dyeing variables
Central composite design (CCD) was formulated under response surface methodology for statistical optimization of the dyeing variable (Chaker et al. 2021). The range taken for the dyeing parameter was: temperature, 55–85 °C; time, 35–65 min; pH 3–7; salt amount, 1 g/100 mL; extract volume 35–65 mL. The trials made have been conducted under various conditions, and the results were assessed through computer simulation.
Mordanting
Using salts of Al, Fe, Cr, and tannic acid, 25 mL of their solutions was used to develop shades onto yarns before and after dyeing at optimized conditions. For comparative studies, plant extracts were obtained by boiling 0.5–2.5 g with 100 mL of water, and the extracts were used before and after the dyeing at given conditions. For this purpose harmal seeds, pomegranate peels, henna leaves, and acacia bark were dried, crushed, and ground finely before being used to extract bio-mordants. Pre- and post-mordanting were carried out at 85 °C for 35 min to improve the shade quality of yarns before and after dyeing (Table 1).
Assessment of dyed and undyed materials
The yarns were scanned under the scanning electron microscope to observe the surface modification of the yarn and under ATR-FTIR (Perkin Elmer) to take spectra between the ranges of 400 to 4000 cm−1 for confirmation of active functional groups. The dyed fabric was examined under color spectra photometer CS-410 (China) to get color quality value (K/S, L*, a*, b*). The dyed yarns before and after mordanting were assessed for fastness rating as per ISO standards for light fastness (ISO 105 B02), washing (ISO 105 C03), and rubbing (ISO 105 X-12) (Hosseinnezhad et al. 2021a, b).
Results and discussion
For the isolation of colorants, new modern tools such as microwave irradiations (MWI) are gaining popularity (Kumar et al. 2021). This is because these rays, on interacting with the isolation system, penetrate the solvent and transfer its energy to rupture the plant boundary (Špadina et al. 2021; Nasreen et al. 2023). The boundary, after rupture, evolves functional molecules, and colorant through mass transfer kinetics, and by this powder-liquid match up without consuming more time and solvent, a high yield is observed (Adeel et al. 2023b). Upon dyeing, the yield obtained shows the maximum extent onto surface-tuned fabric (Hassan and Saifullah 2021). The same is the case has been observed in our studies where microwave treatment to both woolen yarn (WY) and the acidic extract was given up to 2 min which has furnished high color depth (K/S = 40.29). Before microwave irradiation (MWI), the acidic extract (NRE) obtained shows less yield onto woolen yarn (K/S = 30.15). The color coordinates show that the shades obtained were draker (L* = 23.87), redder (a* = 22.95), and yellow in hue (b* = 23.87). After 2 min. of microwave treatment (MWI), the shade obtained was much darker (L* = 17.57), more redder (a* = 28.61) but less yellower in hue (b* = 14.14). For the aqueous extract, when employed before radiation, the yield was low (K/S = 8.83) and the shades were brighter (L* = 46.14), with less red (a* = 12.96) but more yellow (b* = 29.87) tone. After radiation of fabric, up to 5 min., the shades were high in strength (K/S = 20. 01), darker (L* = 30.79), and much redder (a* = 24.23), and less yellower in hue (b* = 20.43). The acidic extract (Fig. 2b) has shown an overall high yield on yarn when treated for up to 2 min. as compared to the aqueous extract without radiation used for irradiated wool yarn (Fig. 2a).
The role of microwave radiation was observed through spectral outlines by FTIR analysis (Figs. 3, 4, and 5) and scanned images (Fig. 4(a, b)) through SEM. The role of MW can be seen in physical changes occured in woolen yarn through scanned images. The results reveal that before radiation, the fiber is smooth (Fig. 3(a)), whereas after radiation, the yarn surface has been peeled (Fig. 3(b)). The peeled surface upon dyeing has sorbed dye significantly. Hence, this uniform and leveled source of heating has modified the fiber physically. Similarly dyed woolen yarn (Fig. 3) also reveals that after radiation, the peeled surface was covered with dye. Previous studies show that these rays tune the fiber physically without causing any change in its chemistry. The chemical nature of yarn has been evaluated by FTIR before and after radiation (Fig. 4(a and b)). The peaks are shown at 3300–3400 cm−1, 1500–1700 cm−1, and 1200 cm−1 representing the presence of amino, carbonyl, and a methyl group. Even after treatment, the spectral lines did not alter their position, which shows that MW treatment does not change the chemical nature of the fiber (Adeel et al. 2023b). The presence of betalain from O. ficus extract before and after radiation was also analyzed by FTIR (Fig. 5 (a and b)). The functional peaks present at 3400 and 3000 cm−1 represented the presence of amino and carboxylic groups. Similarly, in addition to these peaks, the sharp lines present at 1500–1700 cm−1 and 1000–1100 cm−1 represent the presence of CH and OH groups. Similarly, the presence of –C≡H and –C = 0 has been observed at 1500 and 1000 cm−1. Hence, these peaks did not alter their position even after MW treatment. Similarly, the narrow band peaks (Fig. 6 a and b) show that the fiber dyed with betalain extracted from O. ficus has developed some interaction through chemical binding. Then, overall, it can be narrated that MW ray treatment is useful in the modification of fiber without altering the chemical nature of stuff (dye and fiber).
The role of these dyeing variables has been analyzed statistically. For this purpose, a central composite design (CCD) was formulated by varying dyeing patterns through response surface methodology (RSM). A total of 32 experiments were done as briefed in Table 2, and the results obtained were analyzed. The result given in the table shows that 55 mL of acidic extract has 2 g/100 mL of salt after microwave treatment (MWI). Up to 2 min when employed onto irradiated yarn at 85 ºC and 4 pH for 35 min has shown maximum yield (K/S = 34.20). Statistically obtained results show that the model used for analysis is fit and linear (P = 0. 00). Also, the role of pH (P = 0.00), volume (P = 0.06), time (P = 0.00), and salt (P = 0.00) has been highly significant as the individual parameter. The two-way interaction, statistically for the table, reveals that the role of pH, along with volume and time, is highly significant. The role of salt with time, salt with temperature, and salt with volume are also systematically significant. The data shows that the maximum capacity for dye adsorption onto woolen yarn occurs in the pH range of 3–7, with dye adsorption increasing with decreasing pH. This is mostly caused by the protonation of wool amino groups in an acidic environment which is advantageous for ion–dipole interactions with the hydroxyl group of the chemical constituents of Opuntia ficus. Another major element that is frequently studied in the dyeing of textile fibers is temperature. Temperature causes the fiber structure to swell, makes it easier for dye composites to break down, and promotes the diffusion of dye molecules inside the fiber structure (Amesimeku et al. 2021). A large number of functional groups (amine and hydroxyl groups) added to the fiber surface in modified wool not only completely alter the surface morphology compared to that of untreated wool (Al Faruque et al. 2021), but also make them easily accessible to dye molecules, enhancing wool’s ability to absorb dye through ionic interactions, hydrogen bonds, van der Waals forces, and hydrophobic interactions (Lei et al. 2021). Large amounts of energy and dye material can be saved as a result (Allam et al. 2022).
In this study, 0.5–2.5 g/100 mL of Al, Cr, Fe, and tannic acid were used before and after the dyeing of 1 g of woolen yarn. Chemical mordanting in plant dyeing always needs an additive called mordant to get color-fastness properties (Hosseinnezhad et al. 2023). The mordants are metal electrolytes or tannic acid, which form complexes with fabric through coordinated covalent bonds before and after dyeing (Fig. 7a). Tannic acid forms additional hydrogen bonding with functional groups and dye-binding sites (Birniwa et al. 2022). The dyeing of natural or synthetic fabric with plant pigments or dyes always depends upon chemcial or biomordanting (Loum et al. 2021). The problem of poor fastness can be overcome by metal salts or tannic acid or by plant pigments (Aggarwal 2021; Adeel et al. 2023b). In the present studies, the results show in Fig. 8 (a and b) that before the dyeing of yarn, 2.0% Al, 1.5% of Fe, 2.5% of Cr, and 2.0% tannic acid gave a high yield. Similarly, after dyeing, 2.0% of Al, 1.5% of Fe, 0.5% of Cr, and 2.0% tannic acid developed shades of high strength onto the yarn. The proposed mechanism of plants mordant with yarn and colorant has been given in Fig. 7b. The selected amount of bio and chemical mordants applied before and after dyeing at selected conditions have shown shades of variable tones (Table 3).
Bio mordanting, also known as dual bio dyeing, is the new art for developing darker shades with new gamutes (Samant et al. 2022). These sources from herbal plants have functional molecules with excellent biological activities due to –OH, –C = O, and –OH groups (Adeel et al. 2023c; Özomay and Akalın 2022). Mostly, these extracts have an excellent place and are well known to treat fetal and seasonal diseases. In this study, four sources of bio-mordant have been introduced in combination with (Opuntia ficus) colorant to develop new gamutes of high strength (Fig. 8(a, b)). The shade quality penetrates given in Table 3 for using bio-mordants before and after dyeing reveal that dual dyeing has resulted in darker shades with more or less reddish yellow hue depending upon the nature of bio-mordants used. Overall, usually before dyeing, the functional group represents in prickly pear on interaction with betalain and woolen yarn as furnished excellent shade depth with darker hue having reddish yellow hue. The presence of bio-mordants in harmal on interaction with betalain and woolen yarn amido linkage has furnished a brighter shade with a less reddish yellow hue. The data shows that the sample washing fastness was excellent (4–5 to 5). The colored samples’ staining showed good fastness with grade. The colored yarns had good light fastness when mordants were used. However, all dyed yarns have seen a one-rating increase in light fastness.
Conclusion
Using natural colorants in the modern world is the recent demand by the globe as an alternative to toxic synthetic colorants. The studies reveal that 55 mL of acidic extract from 4 g of prickly pear (Opuntia ficus) powder of 4 pH when employed onto wool yarn at 85 °C for 35 min has given excellent results. The results reveal that using optimum conditions, the particular amount of chemical and bio-mordants has not only given good color characteristics to wool yarns but also enhanced their fastness rating to light, washing, crocking, dry cleaning, and perspiration. Conclusively, it is recommended that microwave rays should be used to isolate colorant from prickly pear (Opuntia ficus) in acidic, medium, and bio-mordants should be preferred instead of chemical anchors at selected conditions for developing shades of good colorfastness onto wool yarn.
Data availability
The work is from M. Phil studies.
References
Adeel S, Kiran S, Alam M, Farooq T, Amin N, Gulzar T (2023a) Alkanna tinctoria-based sustainable alkanin natural colorant for eco-dyeing of wool. Environ Sci Pollut Res 30(10):27073–27083
Adeel S, Rehman FU, Amin A, Amin N, Batool F, Hassan A, Ozomay M (2023b) Sustainable exploration of coffee extracts (Coffea arabica L.) for dyeing of microwave treated bio-mordanted cotton fabric. Pigm Resin Technol 52(3):331–340
Adeel S, Kiran S, Alam M, Farooq T, Amin N, Gulzar T (2023c) Alkanna tinctoria-based sustainable alkanin natural colorant for eco-dyeing of wool. Environ Sci Pollut Res 30(10):27073–27083
Affat SS (2021) Classifications, advantages, disadvantages, toxicity effects of natural and synthetic dyes: a review. Uni Thi-Qar J Sci 1:130–135
Aggarwal S (2021) Indian dye yielding plants: efforts and opportunities. Nat Res Forum 45(1):63–86. https://doi.org/10.1111/1477-8947.12214
Al Faruque MA, Remadevi R, Guirguis A, Kiziltas A, Mielewski D, Naebe M (2021) Graphene oxide incorporated waste wool/PAN hybrid fibres. Sci Rep 11:1–12
Allam O, Elshemy N, El-Sayed H (2022) Simple and easily applicable method for reducing freshwater consumption in dyeing of wool fabric. J Nat Fiber 19:895–904
Al-Sayed W, Abdelrahman SH (2021) Sustainable chemistry in textile processes (pretreatment, coloration, and chemical finishing). In: Ibrahim N (ed) Green chemistry for sustainable textiles: modern design and approaches, 1st edn. Woodhead Publishing, pp. 93-111
Amesimeku J, Fan L, Jakpa W, Wang C (2021) Dyeing properties of meta-aramid fabric dyed with basic dye using ultrasonic-microwave irradiation. J Clean Prod 285:124844
Arabi M, Ostovan A, Li J, Wang X, Zhang Z, Choo J, Chen L (2021) Molecular imprinting: green perspectives and strategies. Adv Mater 33:2100543
Barba FJ, Garcia C, Fessard A, Munekata PE, Lorenzo JM, Aboudia A, Remize F (2022) Opuntia ficus indica edible parts: a food and nutritional security perspective. Food Rev Int 38:930–952
Barkaat S, Mehboob M, Adeel S, Rehman FU, Amin N, Habib N, Hosseinnezhad M (2023) Sustainable microwave-assisted extraction of santalin from red sandal wood powder (Ptrecarpus santalinus) for bio-coloration of mordanted silk fabric. Sep 10(2):118, 1–14
Birniwa AH, Abubakar AS, Mahmud HNME, Kutty SRM, Jagaba AH, Abdullahi SSA, Zango ZU (2022) Application of agricultural wastes for cationic dyes removal from wastewater. In: Muthu SS (ed) Textile Wastewater Treatment: sustainable bio-nano materials and macromolecules, 1st edn. Springer Singapore, 239–274
Chaker H, Attar AE, Djennas M, Fourmentin S (2021) A statistical modeling-optimization approach for efficiency photocatalytic degradation of textile azo dye using cerium-doped mesoporous ZnO: a central composite design in response surface methodology. Chem Engin Res Design 171:198–212
Dulo B, De Somer T, Phan K, Roosen M, Githaiga J, Raes K, De Meester S (2022) Evaluating the potential of natural dyes from nutshell wastes: sustainable coloration and functional finishing of wool fabric. Sust Mater Technol 34:e00518. https://doi.org/10.1016/j.susmat.2022.e00518
Hamdy D, Hassabo AG, Othman HA (2021) Various natural dyes using plant palette in coloration of natural fabrics. J Text Color Poly Sci 18:121–141
Hassan MM, Saifullah K (2021) Sustainable dyeing and functionalization of jute fabric with a Chinese sumac gall-derived gallotannin using eco-friendly mordanting agents. Cellul 28:5055–5070
Hosseinnezhad M, Gharanjig K, Iamin H, Rouhani S, Adeel S (2023) Environmentally dyeing of wool yarns using of combination of myrobalan and walnut husk as bio-mordants. Prog Color Color Coat 16:197–205
Hosseinnezhad M, Gharanjig K, Jafari R, Imani H (2021a) Green dyeing of woolen yarns with weld and madder natural dyes in the presences of bio-mordant. Prog Color Color Coat 14:35–45
Hosseinnezhad M, Gharanjig K, Jafari R, Imani H, Razani N (2021b) Cleaner colorant extraction and environmentally wool dyeing using oak as eco-friendly mordant. Environ Sci Pollut Res 28:7249–7260
Khan BA (2022) Ecotourism source of poverty alleviation and natural conservation in kashmir india. Americ J Environ Econom 1(1):19–39
Kumar H, Das R, Choithramani A, Gupta A, Khude D, Bothra G, Shard A (2021) Efficient green protocols for the preparation of pyrazolopyrimidines. Chem Sel 6:5807–5837
Lei M, Yang L, Shen Y, Yang L, Sun J (2021) Efficient adsorption of anionic dyes by ammoniated waste polyacrylonitrile fiber: mechanism and practicability. ACS Omega 6:19506–19516
Loum J, Byamukama R, Wanyama PAG (2021) Efficient extraction of natural dyes from selected plant species. Chem Africa 4:677–689
Mahmoudpour M, Ding S, Lyu Z, Ebrahimi G, Du D, Dolatabadi JEN, Lin Y (2021) Aptamer functionalized nanomaterials for biomedical applications: recent advances and new horizons. Nano Today 39:101177
Mungkung R, Sorakon K, Sitthikitpanya S, Gheewala SH (2021) Analysis of green product procurement and ecolabels towards sustainable consumption and production in Thailand. Sustain Prod Consum 28:11–20
Nasreen H, Adeel S, Yameen M, Amin N, Ozomay M, Qayyum MA (2023) Green application of ultrasonic waves for extraction of yellow colorant from haar singhar and its colouring behaviour in cotton dyeing. Text Leather Rev 6(1):18–36
Özomay M, Akalın M (2022) Optimization of fastness properties with gray relational analysis method in dyeing of hemp fabric with natural and classic mordant. J Nat Fibers 19:2914–2928
Panda A, Maiti S, Madiwale P, Adivarekar R (2022) Natural dyes-a way forward. In: Pandit P(ed) Textile dyes and pigments: a green chemistry approach, 1st edn. Scrivener Publishing LLC, US, 323–343
Patel Y, Chhaya U, Rudakiya DM, Joshi S (2021) Biological decolorization and degradation of synthetic dyes: a green step toward a sustainable environment. In: Deepak G (edn) Microbial rejuvenation of polluted environment, 1st edn. Springer, New York, pp 77-110
Peter AP, Khoo KS, Chew KW, Ling TC, Ho SH, Chang JS, Show PL (2021) Microalgae for biofuels, wastewater treatment and environmental monitoring. Environ Chem Lett 19(4):2891–2904
Sabtain B, Farooq R, Shafique B, Modassar M, Ranjha AN (2021) A narrative review on the phytochemistry, nutritional profile and properties of prickly pear fruit. Open Acc J Biog Sci Res (7):1–11. https://doi.org/10.46718/JBGSR.2021.07.000164
Salleh KM, Armir NAZ, Mazlan NSN, Wang C, Zakaria S (2021) Cellulose and its derivatives in textiles: primitive application to the current trend. In: Ibrahim MH (ed) Fundamental of natural fibre and textile, 1st edn. Woodhead Publishing, UK, pp 33–63
Samant L, Jose S, Rose NM, Shakyawar DB (2022) Antimicrobial and UV protection properties of cotton fabric using enzymatic pretreatment and dyeing with Acacia catechu. J Nat Fiber 19:2243–2253
Shavandi A, Jafari H, Zago E, Hobbi P, Nie L, De Laet N (2021) A sustainable solvent based on lactic acid and l-cysteine for the regeneration of keratin from waste wool. Green Chem 23:1171–1174
Singh M, Vajpayee M, Ledwani L (2021) Eco-friendly surface modification of natural fibres to improve dye uptake using natural dyes and application of natural dyes in fabric finishing: A review. Mate Today Proc 43:2868–2871
Sonal S, Mishra BK (2021) A comprehensive review of the synthesis and performance of different zirconium-based adsorbents for the removal of various water contaminants. Chem Eng J 424:130509
Špadina M, Dufrêche JF, Pellet-Rostaing S, Marčelja S, Zemb T (2021) Molecular forces in liquid–liquid extraction. Lang 37:10637–10656
Uddin MA, Rahman MM, Haque ANMA, Smriti SA, Datta E, Farzana N, Sayem ASM (2022) Textile colouration with natural colourants: a review. J Clean Prod 131489. https://doi.org/10.1016/j.jclepro.2022.131489
Vogel R, Göbel M, Grewe-Salfeld M, Herbert B, Matsuo Y, Weber C (2022) Cross-sector partnerships: mapping the field and advancing an institutional approach. Int J Manag Revi 24(3):394–414
Worlanyo AS, Jiangfeng L (2021) Evaluating the environmental and economic impact of mining for post-mined land restoration and land-use: a review. J Environ Manag 279:111623
Zhang Y, Zhou Q, Rather LJ, Li Q (2021) Agricultural waste of Eriobotrya japonica L.(Loquat) seeds and flora leaves as source of natural dye and bio-mordant for coloration and bio-functional finishing of wool textile. Ind Crop Prod 169:113633
Zin MM, Nagy K, Bánvölgyi S, Abrankó L, Nath A (2022) Effect of microwave pretreatment on the extraction of antioxidant-rich red color betacyanin, phenolic, and flavonoid from the crown of Cylindra-type beetroot (Beta vulgaris L.). J Food Proc Engineering, e14175. https://doi.org/10.1111/jfpe.14175
Acknowledgements
The authors are highly grateful to Department of Chemistry, Riphah International University Faisalabad Campus, Faisalabad, for providing the necessary glassware, chemicals, and apparatus for smooth running of experiments for this M.Phil. Studies. The authors are also thankful to the Department of Chemistry and to the Department of Applied Chemistry, Government College University Faisalabad for giving expertise to facilitate this work.
Funding
This join research work has been funded in form of glassware, chemicals, and apparatus used by Department of Chemistry, Riphah International University Faisalabad Campus, Faisalabad, 44000, Pakistan, and the expertise for analysis has been provided by Department of Applied Chemistry and Department of Chemistry jointly.
Author information
Authors and Affiliations
Contributions
The whole experiments have been conducted by M. Phil student; Yousra Riaz, Dr. Samra Barkaat, and Dr. Shahid Adeel have supervised and co-supervised the work. And Dr. Muhammad Zuber and Dr. Muhammad Ibrahim and Dr. Shahnaz Parveen Khattak helped in manuscript writing, whereas Dr. Meral Ozomay and Dr. Fazal-ur-Rehman have helped in characterization of dyed samples.
Corresponding author
Ethics declarations
Ethical approval
N/A.
Consent to participate and for publication
We give consent to publish our work of M.Phil studies and is jointly contributed by all authors.
Competing interests
N/A.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Riaz, Y., Barkaat, S., Adeel, S. et al. Anar Phali (Opuntia ficus) juice extract as a novel pollution-free source of natural betalain dye for wool yarn. Environ Sci Pollut Res 30, 92084–92094 (2023). https://doi.org/10.1007/s11356-023-28470-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-28470-4