Abstract
The objective of this study was to determine the associations between maternal exposure to PFASs and infant birth weight and to explore evidence for a possible dose-response relationship. Four databases including PubMed, Embase, Web of Science, and Medline before 20 September 2022 were systematically searched. A fixed-effect model was used to estimate the change in infant birth weight (g) associated with PFAS concentrations increasing by 10-fold. Dose-response meta-analyses were also conducted when possible. The study follows the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). A total of 21 studies were included. Among these studies, 18 studies examined the associations between PFOA and birth weight, 17 studies reported PFOS, and 11 studies discussed PFHxS. Associations between PFHxS (ES = −5.67, 95% CI: −33.92 to 22.59, P = 0.694) were weaker than those for PFOA and PFOS (ES = −58.62, 95% CI: −85.23 to −32.01, P < 0.001 for PFOA; ES = −54.75, 95% CI: −84.48 to −25.02, P < 0.001 for PFOS). The association was significantly stronger in the high median PFOS concentration group (ES = −107.23, 95% CI: −171.07 to −43.39, P < 0.001) than the lower one (ES = −29.15, 95% CI: −63.60 to −5.30, P = 0.097; meta-regression, P = 0.045). Limited evidence of a dose-response relationship was found. This study showed negative associations between maternal exposure to PFASs and infant birth weight. Limited evidence of a dose-response relationship between exposure to PFOS and infant birth weight was found. Further studies are needed to find more evidence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyfluoroalkyl substances (PFASs), first synthesized in the 1950s, are a wide family of synthetic organic compounds (Mokra 2021). The three most common and widely used are perfluorooctanoic acid (PFOA), perfluorooctane sulfonate (PFOS), and perfluorohexane sulfonate (PFHxS) (Deng et al. 2020; Lee et al. 2014). PFASs have fluorine atoms replacing all hydrogen atoms in their molecules, making them chemically stable and hydrophobic. PFASs are extensively used in a variety of industrial and household products, including fire-fighting foams, garments, and cookware (Lindstrom et al. 2011). They could be released into the environment by industrial sources or consumer products (Prevedouros et al. 2006). Diet, drinking water, vegetables, infant breast milk, food packaging, indoor dust, and outdoor aerosols have been identified as significant sources of PFAS exposure in humans (Haug et al. 2011; Herzke et al. 2013; Susmann et al. 2019; Yu et al. 2020). While PFOA, PFOS, and PFHxS were listed as persistent organic pollutants by the Stockholm Convention in 2009, 2019, and 2021, respectively (UNEP 2019a, 2019b), they are still present almost everywhere in the environment (Chen et al. 2022; Domingo and Nadal 2019; Lau et al. 2007; Pan et al. 2014; Yeung et al. 2006).
Unfortunately, as with most environmental chemicals, PFASs have a greater impact on infants than adults (Rappazzo et al. 2017). PFASs are causing concern due to reports of possible health effects in rodent studies (Fuentes et al. 2006; Lau et al. 2006; Lau et al. 2003; Ramhøj et al. 2018) and human observational studies (Starling et al. 2017), which suggested the fact that exposure to PFASs during pregnancy caused offspring growth and developmental delays as well as lower birth weight. The mechanism behind this may be related to alterations in corticosteroid and thyroid hormone homeostasis (Goudarzi et al. 2017; Shah-Kulkarni et al. 2016). As demonstrated in human observational studies (Cariou et al. 2015), PFASs can be transmitted to the fetus via the placental barrier after it accumulates in pregnant women, as well as to nursing infants through breast milk. This raises further concerns about PFASs’ potential health effects. It is imperative to emphasize that birth weight is an important predictor of neonatal and infant survival, a critical indicator of pregnancy outcome, and an important marker of fetal growth, development, and nutrition in utero exposure (Mathews and Driscoll 2017; Spracklen et al. 2014; Zhang et al. 2014). For these reasons, it is critical to evaluate the effect of maternal PFAS exposure on the infant birth weight.
A growing number of studies used epidemiological methods to analyze the associations between maternal PFAS exposure and infant birth weight. According to a recent analysis of the Danish National Birth Cohort, prenatal exposure to several PFASs was negatively associated with birth weight (Meng et al. 2018). Similarly, the Swedish pregnancy cohort study found that prenatal exposure to five different PFASs contributes to the decrease in infant birth weight, particularly in girls (Wikström et al. 2020). These findings were subsequently confirmed by a recent study of 504 Greenlandic women (Hjermitslev et al. 2020). However, the relationship between maternal PFAS exposure and infant birth weight was not statistically significant in some studies. According to a study in Beijing, no statistically significant association was found between PFAS exposure and infant birth weight in this study (Shi et al. 2017).
Since the connection between maternal PFAS exposure and infant birth weight is still debatable, a comprehensive meta-analysis is required to determine the relationship between maternal PFAS exposure and infant birth weight. This study’s primary objective was to assess the impact of prenatal exposure to the three forms of PFASs (PFOA, PFOS, and PFHxS) on the birth weight of the offspring. In addition, we investigated potential contributors to heterogeneity and conducted subgroup analyses based on the broad World Health Organization (WHO) geographical regions and median exposure concentration. As a final component of our study, we explored the dose-response relationship between PFOS exposure and infant birth weight.
Materials and methods
Search strategy
Our systematic review and meta-analysis followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Shamseer et al. 2015). On 20 September 2022, four databases including PubMed, Embase, Web of Science, and Cochrane Library were systematically searched using keywords such as perfluoroalkylated substances, maternal exposure, and infant birth weight (complete search terms in Table S1). In addition to this, references of all relevant studies were manually tracked by us to ensure that no study was omitted.
Inclusion and exclusion criteria
The inclusion criteria for the articles were as follows:
-
a.
Able to search the full text of literature.
-
b.
Epidemiological studies based on human observation were selected, including descriptive studies, case-control studies, cohort studies, and cross-sectional studies.
-
c.
The research design had no defects and the literature quality was high.
-
d.
The study objects were pregnant women; not experimental animals, children, or postpartum exposure.
-
e.
Prenatal exposure factor was perfluoroalkylated substances.
-
f.
Changes in birth weight were measured as a result of the study.
-
g.
Regression coefficients (β) for infant birth weight and the 95% CI were provided in the primitive study.
-
h.
If the same population was used in different studies, we selected the recent one with a larger sample size.
The exclusion criteria for the articles were as follows:
-
1.
Not conform to the research topic.
-
2.
Animal research, conference summaries, editorial material, reviews, lecture literature or comments, and so on.
-
3.
The research objects’ exposure to multiple environmental endocrine disruptors.
-
4.
The level of maternal perfluoroalkylated substances besides PFOA, PFOS, or PFHxS was measured.
-
5.
The research has design defects or low-quality research.
-
6.
Raw data was unavailable.
-
7.
Unpublished studies.
Selection of studies and extraction of data
Data of all included studies were obtained from two independent researchers. In accordance with the standard format, we browsed the titles and abstracts of the articles according to the inclusion and exclusion criteria. Additionally, full-text articles were assessed for further confirmation. Relevant characteristics were extracted from selected original studies.
Quality assessment
Two independent reviewers evaluated the quality of the relevant literature using the Newcastle-Ottawa Scale (NOS), which consists of three basic components: selection of the study subjects, comparability, and outcome (Modesti et al. 2016; Stang 2010). There are eight identifiers to identify high-quality star picks. Up to one star will be awarded per item, except for the “comparability” category, which will receive up to two stars. Each article received up to nine stars, and more stars meant the study was of higher quality. Research of no less than seven stars is considered high quality.
Statistical analysis
Statistical analysis was performed using the meta-analysis module in Stata 12.0. Assessment of risk of bias was conducted in RevMan 5.4.1. Relevant data was gathered from qualified studies and entered into Excel. Adjusted β was used as the effect size (ES) for PFAS exposure during pregnancy. According to Liu et al. (2013), we standardized the logarithmic conversion to log10 to account for variations across studies (Table S2, Table S3, and Table S4).
The specific steps are as follows:
-
1.
Sensitivity analysis: to assess the credibility of included studies, we excluded each individual article one by one for sensitivity analysis.
-
2.
Heterogeneity test: if P < 0.05 and I2 ≥ 50%, the article is considered high heterogeneous, while the article is considered low heterogeneous. The statistical analysis method is determined by the magnitude of heterogeneity. For studies with high heterogeneity, the random-effect model was employed, while the fixed-effect model was utilized for studies with low heterogeneity (Dettori et al. 2022; Doi et al. 2015).
-
3.
Test of publication bias: to evaluate the publication bias in included studies, we considered funnel plots and Begg’s test for qualitative and quantitative testing. The included studies were not uniformly small, nor were they sponsored by industry. A comprehensive search was completed of the literature.
-
4.
Subgroup analysis: according to the results of our investigation, the median PFAS readings differed significantly between different geographical regions and spanned over a wide range of concentrations. Therefore, we separated the included research into three subgroup analyses based on the broad WHO geographical regions to investigate the impact of geographical factors on the outcomes of included studies. Additionally, we developed the cutoff values based on the average global serum PFAS content monitored by Fan et al. (2022) (2.3 ng/ml for PFOA; 6.6 ng/ml for PFOS; 1.3 ng/ml for PFHxS), and these values were utilized to conduct another subgroup to examine the effect of concentration on the results. The included studies were separated into high and low concentration subgroups based on the median exposure concentration in each research and cutoff value.
-
5.
Dose-response meta-analysis: the dose-response meta-analysis was examined using the reported mean of each category. In the category without the mean PFAS exposure, the midpoint was designated as the average level. In the unbounded category, we assumed the width of the category to be the same as the adjacent category.
Result
Study search and characteristic overview
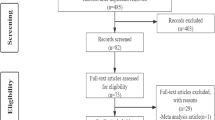
Totally, 617 studies were initially retrieved with the search strategy. A total of 365 records were obtained after eliminating duplicates (Fig. 1). By reading the titles and abstracts, 334 studies were excluded. Finally, after reviewing the full-text articles, we included 21 articles in our meta-analysis (Apelberg et al. 2007; Callan et al. 2016; Cao et al. 2018; Chang et al. 2022; Chen et al. 2021; Chen et al. 2012; Darrow et al. 2013; Hu et al. 2021; Kashino et al. 2020; Maisonet et al. 2012; Manzano-Salgado et al. 2017; Marks et al. 2019; Meng et al. 2018; Minatoya et al. 2017; Shi et al. 2017; Starling et al. 2017; Valvi et al. 2017; Wang et al. 2019; Washino et al. 2009; Wikström et al. 2020; Wu et al. 2012). The characteristics of these studies are illustrated in Table 1. The majority of the articles included were cohort studies, while only two were case-control studies (Apelberg et al. 2007; Callan et al. 2016). All included studies received a NOS score between 6 and 8. The detailed quality assessment of all included studies is shown in Table S5, Table S6, Figure S1, and Figure S2. Since the heterogeneity of PFOA, PFOS, and PFHxS was found to be low (I2 = 15.6% for PFOA; I2 = 44.0% for PFOS; I2 = 0.0% for PFHxS), the fixed-effect model was chosen to calculate the combined effect value. Forest map showed the effect size of each study. In most studies, potential confounders like maternal age, educational level, and body mass index (BMI) were adjusted. All 21 studies were classified into the Western Pacific, North American, and Europe regions based on broad WHO geographical regions. A total of 10 studies were from the Western Pacific region (Callan et al. 2016; Cao et al. 2018; Chen et al. 2021; Chen et al. 2012; Kashino et al. 2020; Minatoya et al. 2017; Shi et al. 2017; Wang et al. 2019; Washino et al. 2009; Wu et al. 2012), 5 studies from North America (Apelberg et al. 2007; Chang et al. 2022; Darrow et al. 2013; Hu et al. 2021; Starling et al. 2017), and the remaining 6 from Europe (Maisonet et al. 2012; Manzano-Salgado et al. 2017; Marks et al. 2019; Meng et al. 2018; Valvi et al. 2017; Wikström et al. 2020). We noted the median concentration of the three substances, the sample size, and the geographical regions of the research (Fig. 2 and Figure S3). One research did not offer data on median concentration (Chen et al. 2012). Consequently, the median concentrations of PFOA and PFOS differed by two orders of magnitude (0.71–16.95 ng/ml for PFOA; 0.65–30.1 ng/ml for PFOS). PFHxS varied by one order of magnitude (0.16–4.54ng/ml for PFHxS).
By broad WHO geographical regions, the median values for PFOS and PFHxS differed the most considerably. Western Pacific studies had the lowest median, while European studies had the highest median.
Associations between maternal exposure to PFOA and infant birth weight
We included a total of 18 articles to evaluate the association between maternal PFOA exposure and the birth weight of infants. Among them, only 1 article showed that PFOA exposure during pregnancy resulted in increased infant birth weight (Shi et al. 2017), while other articles showed that per 10-fold increase in PFOA concentration during pregnancy resulted in decreased birth weight. Specific results were follows: ES = −58.62, 95% CI: −85.23 to −32.01, P < 0.001, I2 = 15.60%, indicating that prenatal exposure to PFOA and infant birth weight were negatively correlated (Fig. 3a).
Associations between prenatal exposure to PFOS and infant birth weight
Totally, 17 studies investigating the relationship between prenatal exposure to PFOS and infant birth weight were included. A fixed-effect model was used in the meta-analysis (I2 = 44.0%). Specific results were follows: ES = −54.75, 95% CI: −84.48 to −25.02, P < 0.001, I2 = 44%. The difference was statistically significant, which showed a negative association between prenatal exposure to PFOS and infant birth weight (Fig. 3c).
Associations between maternal exposure to PFHxS and infant birth weight
There were 11 articles related to the relationship between prenatal PFHxS exposure and infant birth weight. Meta-analysis was performed using a fixed-effect model (I2 = 0.0%). Specific results were as follows: ES = −5.67, 95% CI: −33.92 to 22.59, P = 0.694, I2 = 0.0%. The results indicated that prenatal exposure to PFHxS has a statistically significant effect on the birth weight of the infant (Figure S4).
Subgroup analysis
The first subgroup analysis based on broad WHO geographical regions was undertaken as part of the meta-analysis. A total of 5 studies were conducted in North America (Apelberg et al. 2007; Chang et al. 2022; Darrow et al. 2013; Hu et al. 2021; Starling et al. 2017), 9 studies in the Western Pacific (Callan et al. 2016; Chen et al. 2021; Chen et al. 2012; Kashino et al. 2020; Minatoya et al. 2017; Shi et al. 2017; Wang et al. 2019; Washino et al. 2009; Wu et al. 2012), and the remaining 4 in Europe (Manzano-Salgado et al. 2017; Meng et al. 2018; Valvi et al. 2017; Wikström et al. 2020) for PFOA, There was only one study from the Western Pacific that did not report exposure to PFOS (Wu et al. 2012). AS for PFHxS, 3 studies were conducted in North America (Chang et al. 2022; Hu et al. 2021; Starling et al. 2017), 4 studies in the Western Pacific (Callan et al. 2016; Chen et al. 2021; Kashino et al. 2020; Shi et al. 2017), and the remaining 4 in Europe (Manzano-Salgado et al. 2017, Meng et al. 2018, Valvi et al. 2017, Wikström et al. 2020), but no data was available for Southeast Asia and Africa. In a broad WHO geographical region subgroup analysis, maternal PFOA exposure and infant birth weight were significantly negatively correlated in the Europe and North American subgroups (ES = −97.20, 95% CI: −153.70 to −40.49, P < 0.001 for Europe; ES = −52.76, 95% CI: −99.73 to −5.80, P = 0.028 for North American) (Fig. 4a). Furthermore, PFOS exposure was also associated with a significant negative association (ES = −95.25, 95% CI: −153.79 to −36.71, P < 0.001 for Europe; ES = −30.70, 95% CI: −78.68 to 17.27, P = 0.210 for North American) (Fig. 4b). As for PFHxS exposure, no significant negative association was found (ES = −0.14, 95% CI: −45.50 to 45.22, P = 0.995 for Europe; ES = −12.85, 95% CI: −64.46 to 38.76, P = 0.626 for North American) (Figure S5). In the Western Pacific subgroup, PFOA and PFOS exposures were both negatively associated with infant birth weight, but not statistically significant (ES = −44.03 95% CI: −83.37 to −4.69, P = 0.028 for PFOA; ES = −51.37, 95% CI: −101.06 to −1.67, P = 0.043 for PFOS). PFHxS exposure was positively associated with infant birth weight, but this association was not statistically significant (ES = −5.64, 95% CI: −56.20 to 44.92, P = 0.827 for PFHxS). The meta-regression found no statistical significance among all studies in this subgroup (Table S7).
The second subgroup analysis was conducted depending on the concentration of media exposure. In the high median PFOS concentration group (>6.6 ng/ml, n = 3), PFOS exposure showed a statistically significant negative association with infant birth weight (ES = −107.23, 95% CI: −171.07 to −43.39, P < 0.001), while studies with low median PFOS concentration did not observe such a significant negative association (ES = −29.15, 95% CI: −63.61 to 5.30, P = 0.097) (Fig. 5). Similarly, such a significant negative association was not found in both the high (>1.3 ng/ml, n = 1) or low (<1.3 ng/ml, n = 11) median PFHxS concentration groups (ES = 49.83, 95% CI: −58.13 to 157.79, P = 0.366 for high median PFHxS concentration group; ES = −9.75, 95% CI: −39.02 to 19.53, P = 0.514 for low median PFHxS concentration group) (Figure S6). In contrast, a significant negative association was found in both the high (>2.3 ng/ml, n = 11) and low (<2.3 ng/ml, n = 6) median PFOA concentration groups (ES = −57.53, 95% CI: −103.25 to −11.81, P = 0.014 for high median PFOA concentration group; ES = −60.90, 95% CI: −95.44 to −26.35, P < 0.001 for low median PFOA concentration group) (Figure S7). Additionally, comparing studies with low and high median levels of exposure to PFOS, the meta-regression found statistical significance (difference in associations β = −80.93; 95% CI: −159.88 to −1.98, P = 0.045). The results indicated a potential inverse relationship between PFOS concentrations and infant birth weight. In addition, the results also suggested a possible dose-response relationship between PFOS and birth weight. While comparing studies with low and high median levels of exposure to PFOA and PFHxS, statistical significance was not found in the meta-regression (Table S8). Consequently, to find additional evidence of a dose-response relationship between PFOS and birth weight, additional analyses under distinct PFOS dose concentration exposure groups were required. To further explore the dose-response relationship between PFOS and infant birth weight. Eight articles reported the associations between categories of maternal PFOS concentrations and infant birth weight relative to the lowest category of maternal PFOS concentrations (reference group). Each independent study represents the dose-response for each independent study. The monotonic link between various levels of maternal PFOS concentration and regression coefficients (β) for infant birth weight revealed evidence of a dose-response relationship (Fig. 6).
The regression coefficient (β) of infant birth weight decreased with increasing PFOS concentration in most studies, suggesting that the concentration of maternal PFOS and the birth weight of infants may be related to a dose-response relationship.
Sensitivity analyses
To determine the credibility of the included articles, sensitivity analysis was performed by eliminating each article individually. There were no significant differences (the results remained unchanged) when we removed any study (Fig. 7 and Figure S4). The consistency of the findings indicates that the studies included in the meta-analysis are reliable.
Publication bias
The funnel plots do suggest that there may be some cases of bias, such that at the base of the plot, there is less symmetry than toward the tip of the plot, and for PFOA and PFOS outcome, some studies fall outside the funnel itself (Fig. 3b and d and Figure S8). Note that results from funnel plots should be evaluated with caution, as we were also not able to obtain results from unpublished studies that demonstrated different results than the published studies that were included. For quantitative testing, Begg’s tests revealed that there was no evidence of material publication bias (P = 0.150 for PFOA; P = 0.387 for PFOS; P = 0.755 for PFHxS).
Discussion
Our meta-analysis included 21 studies and 18,700 participants to investigate the association between maternal PFAS exposure and infant birth weight. Fixed-effect models were used in all cases. In accordance with the meta-analysis of Cao et al. (2021), maternal exposure to three forms of PFASs (PFOA, PFOS, and PFHxS) was found to be negatively associated with the birth weight of offspring. The observed negative association between PFHxS exposure and infant birth weight was not statistically significant. Moreover, the impact values were lower than those for PFOA and PFOS. According to the result, it seems that PFHxS, as an alternative to PFOA and PFOS, has a less detrimental effect on infant birth weight. To reduce potential heterogeneity between studies, subgroup analyses based on broad WHO geographic regions and median concentration were performed. In the subgroup based on median concentrations, a significantly stronger negative association was found in the high PFOS exposure group than in the low PFOS exposure group. To further investigate the dose-response relationship, we extracted the quartiles of PFOS exposure and the related impact values and examined the correlation between PFOS impact values corresponding to each quartile. Consequently, our results corroborate the findings of several previous studies indicating that maternal exposure to PFASs is negatively associated with birth weight.
Subgroup analysis was conducted to decrease considerable heterogeneity. Several factors including geographic regions, the concentration level of PFAS exposure, the management of relevant confounders, and the timing of PFAS content measurements might contribute to the heterogeneity. In subgroup based on WHO geographical regions, the heterogeneity of PFOS exposure was mostly attributable to research conducted in the Western Pacific. This heterogeneity may be explained by the large number of studies attributed to the Western Pacific group, five of which were conducted in developing country (Chen et al. 2021; Chen et al. 2012; Shi et al. 2017; Wang et al. 2019; Wu et al. 2012) and another from a developed country (Callan et al. 2016; Kashino et al. 2020; Minatoya et al. 2017; Washino et al. 2009). There is also the potential that this high heterogeneity is the consequence of random mistakes across studies and factors such as the climate, nutrition, lifestyle, and ethnicity of respondents in the Western Pacific region. However, we suggest further research from other locations to evaluate the influence of geographic location on the connection between PFASs and the birth weight of infants. Additionally, the European studies showed a stronger negative association between both maternal PFOA and PFOS exposure and infant birth weight than in North American and the Western Pacific studies. The sensitivity of pregnant women to PFOA and PFOS during pregnancy may be ethnically related. To identify populations vulnerable to PFOA and PFOS, additional research simulating toxicokinetic variability diversity is required (Ring et al. 2017). For both high and low groups of exposure to the three PFASs, another subgroup analysis based on PFAS concentration revealed a reduction in heterogeneity. This indicates that exposure level may have a significant impact on determining heterogeneity.
There are also variations in potential confounders across articles, such as maternal age and BMI. Even though the statistics have been adjusted, several studies still miss crucial confounders. According to Washino et al. (2009), maternal age and maternal education were adjusted, while Maisonet et al. (2012) study did not consider these crucial covariables. This may have implications for the association between maternal exposure to PFASs during pregnancy and the birth weight of the offspring.
Furthermore, heterogeneity may also be due to the timing of the measurements of PFAS concentrations. Among the 21 studies, 15 used maternal serum as the sample, and only 6 used cord blood. Nevertheless, this could still result in heterogeneity. In the studies where the sample was maternal serum, Callan et al. (2016) and Kashino et al. (2020) measured PFAS concentrations in the third trimester of pregnancy. Another study from British (Maisonet et al. 2012) measured PFASs throughout pregnancy, with median times of measurement at 30 weeks. Therefore, we urge that additional research be conducted to investigate the effect of various measurement periods on the concentration of PFASs in pregnant women and the birth weight of their progeny.
Pregnancy is well known to be a vulnerable period for chemical exposure (Mallozzi et al. 2016). Several animal and cellular investigations have identified potential mechanisms for the effects of exposure to PFASs during this time on infant birth weights. PFASs may affect infant birth weight through a reduction in IGF2 methylation, disruption of maternal and neonatal thyroid hormone function, activation of peroxisome proliferator-activated receptor-α, and changes in epigenetic composition. Maternal PFAS exposure led to a reduction in IGF2 methylation in cord blood, which was associated with a lower ponderal index at birth (Kobayashi et al. 2017). In addition, by changing thyroid hormone signaling and interfering with thyroid hormone function and homeostasis, PFASs may be able to influence fetal birth weight (Kim et al. 2021; Long et al. 2013). Wolf et al. (2014) found that PFASs could also activate peroxisome proliferator-activated receptor-α, which was also a potential mechanism of effect on fetal growth. According to the findings of Kaijser et al.’s (2000) study, there was a significant positive relation between estriol levels and birth weight. Regrettable PFASs could cause a change in estrogen synthesis by affecting the expression of the estrogen response gene and interfering with the estrogen receptor of the human body (Benninghoff et al. 2011; Kjeldsen and Bonefeld-Jørgensen 2013). Moreover, human data from a cohort study further confirmed that estrogens might mediate the association between exposure to PFASs and fetal growth (Wang et al. 2019). These findings might help to explain the potential mechanisms by which maternal exposure to PFASs might decrease the birth weight of infants.
According to the subgroup analysis of studies based on median PFOS exposure levels, the high median concentration of PFOS was associated with significantly greater effect values than the low median concentration. Each quartile of a single trial and a subgroup analysis based on the median concentration of many studies revealed evidence of a dose-response relationship. However, since we included only a limited number of articles, the dose-response relationship between the two requires further investigation.
In our study, a total of 16,843 participants were included in the PFOA study, 16,685 participants in the PFOS study, and another 12,139 participants in the PFHxS study. It was possible to conduct a meta-analysis with adequate statistical validity. Since most of the PFAS analysis techniques in relevant studies were LC/MS/MS, their accuracy and sensitivity further contribute to the reliability of the results. In addition, we observed evidence of a dose-effect relationship between PFOA exposure and the birth weight of offspring. Finally, the majority of the articles included in the study were prospective studies of high quality, which may strengthen the credibility of our research.
However, our study still has some limitations. First, various potential confounding factors such as other environmental chemicals, maternal nutrition, and race may affect infant birth weight. Despite the fact that many studies have adjusted for confounding factors, it is impossible to eliminate potential confounding factors. A second drawback is that the majority of the included studies were from the Western Pacific, Europe, and North America, with no representation from Southeast Asia, Africa, or Eastern Mediterranean. The lack of geographical diversity among the included studies limits the extrapolation of results to all regions. Finally, exposure to PFASs was measured at different times during pregnancy in the included studies, which may have introduced bias into the findings.
Notably, the findings of some previous meta-analyses demonstrated the fact that maternal PFAS exposure showed a significant negative association with infant birth weight, but the majority of meta-analyses only contained a limited number of studies and only focused on the association between maternal exposure to PFOA and PFOS and infant birth weight (Cao et al. 2021). However, a biomonitoring study conducted by Fan et al. (2022) based on 2325 publications revealed that PFOS exposure was the highest in the world, followed by PFHxS and PFOA exposure. Multiple human and animal investigations have revealed that PFHxS can diminish thyroid hormone levels, which may result in birth weight variations in offspring (Preston et al. 2020; Ramhøj et al. 2018; Ramhøj et al. 2020). In addition, the half-life of PFHxS in humans is 4.7–35 years longer than that of PFOA and PFOS, which range from 2.1 to 10.1 years and 3.3 to 27 years, respectively (ATSDR) 2021). Therefore, exposure to PFHxS during pregnancy cannot be dismissed. In our meta-analysis, we included a larger number of articles and a wider variety of PFASs. In addition, our study provides the first limited evidence of a dose-response relationship between maternal exposure to PFOS and the birth weight of the offspring.
Consequently, our findings contribute to a greater understanding of the detrimental effects of PFAS exposure during pregnancy. To improve birth outcomes and preserve the health of future generations, the government authorities should continue to implement further the Stockholm Convention to restrict PFAS-related environmental pollution and minimize prenatal exposure to PFASs. It would be appropriate to conduct further studies to examine potential sources of heterogeneity, such as geographical location, the timing of exposure measurements, and exposure levels.
Conclusion
In our meta-analysis, the findings indicated an adverse association between maternal exposure to PFASs during pregnancy and infant birth weight. Additionally, the result varied primarily according to the type of PFAS exposure and the geographical region. Moreover, evidence of a dose-response relationship was found between PFOS exposure and infant birth weight for the first time.
Data availability
All data supporting the funding of the research are presented.
References
Apelberg BJ, Witter FR, Herbstman JB, Calafat AM, Halden RU, Needham LL, Goldman LR (2007) Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ Health Persp 115:1670–1676
(ATSDR)AfTSaDR (2021): Toxicological profile for perfluoroalkyls. Atlanta, GA: U.S.
Benninghoff AD, Bisson WH, Koch DC, Ehresman DJ, Kolluri SK, Williams DE (2011) Estrogen-like activity of perfluoroalkyl acids in vivo and interaction with human and rainbow trout estrogen receptors in vitro. Toxicol Sci An Official J The Soc Toxicol 120:42–58
Callan AC, Rotander A, Thompson K, Heyworth J, Mueller JF, Odland J, Hinwood AL (2016) Maternal exposure to perfluoroalkyl acids measured in whole blood and birth outcomes in offspring. Sci Total Environ 569-570:1107–1113
Cao W, Liu X, Liu X, Zhou Y, Zhang X, Tian H, Wang J, Feng S, Wu Y, Bhatti P, Wen S, Sun X (2018) Perfluoroalkyl substances in umbilical cord serum and gestational and postnatal growth in a Chinese birth cohort. Environ Int 116:197–205
Cao T, Qu A, Li Z, Wang W, Liu R, Wang X, Nie Y, Sun S, Zhang X, Liu X (2021) The relationship between maternal perfluoroalkylated substances exposure and low birth weight of offspring: a systematic review and meta-analysis. Environ Sci Pollut Res Int 28:67053–67065
Cariou R, Veyrand B, Yamada A, Berrebi A, Zalko D, Durand S, Pollono C, Marchand P, Leblanc JC, Antignac JP, Le Bizec B (2015) Perfluoroalkyl acid (PFAA) levels and profiles in breast milk, maternal and cord serum of French women and their newborns. Environ Int 84:71–81
Chang CJ, Barr DB, Ryan PB, Panuwet P, Smarr MM, Liu K, Kannan K, Yakimavets V, Tan Y, Ly V, Marsit CJ, Jones DP, Corwin EJ, Dunlop AL, Liang D (2022) Per- and polyfluoroalkyl substance (PFAS) exposure, maternal metabolomic perturbation, and fetal growth in African American women: a meet-in-the-middle approach. Environ Int 158:106964
Chen MH, Ha EH, Wen TW, Su YN, Lien GW, Chen CY, Chen PC, Hsieh WS (2012) Perfluorinated compounds in umbilical cord blood and adverse birth outcomes. PLoS One 7:e42474
Chen L, Tong C, Huo X, Zhang J, Tian Y (2021) Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances and birth outcomes: a longitudinal cohort with repeated measurements. Chemosphere 267:128899
Chen S, Yan M, Chen Y, Zhou Y, Li Z, Pang Y (2022) Perfluoroalkyl substances in the surface water and fishes in Chaohu Lake, China. Environ Sci Pollut Res Int 29:75907–75920
Darrow LA, Stein CR, Steenland K (2013) Serum perfluorooctanoic acid and perfluorooctane sulfonate concentrations in relation to birth outcomes in the Mid-Ohio Valley, 2005-2010. Environ Health Persp 121:1207–1213
Deng S, Bao Y, Cagnetta G, Huang J, Yu G (2020) Mechanochemical degradation of perfluorohexane sulfonate: aynergistic effect of ferrate(VI) and zero-valent iron. Environ Pollut (Barking, Essex 1987) 264:114789
Dettori JR, Norvell DC, Chapman JR (2022) Fixed-effect vs random-effects models for meta-analysis: 3 points to consider. Glob Spine J 12:1624–1626
Doi SA, Barendregt JJ, Khan S, Thalib L, Williams GM (2015) Advances in the meta-analysis of heterogeneous clinical trials I: the inverse variance heterogeneity model. Contemp Clin Trials 45:130-8
Domingo JL, Nadal M (2019) Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: a review of the recent scientific literature. Environ Res 177:108648
Fan X, Tang S, Wang Y, Fan W, Ben Y, Naidu R, Dong Z (2022) Global exposure to per- and polyfluoroalkyl substances and associated burden of low birthweight. Environ Sci Technol 56:4282–4294
Fuentes S, Colomina MT, Rodriguez J, Vicens P, Domingo JL (2006) Interactions in developmental toxicology: concurrent exposure to perfluorooctane sulfonate (PFOS) and stress in pregnant mice. Toxicol Lett 164:81–89
Goudarzi H, Araki A, Itoh S, Sasaki S, Miyashita C, Mitsui T, Nakazawa H, Nonomura K, Kishi R (2017) The association of prenatal exposure to perfluorinated chemicals with glucocorticoid and androgenic hormones in cord blood samples: the Hokkaido study. Environ Health Persp 125:111–118
Haug LS, Huber S, Becher G, Thomsen C (2011) Characterisation of human exposure pathways to perfluorinated compounds--comparing exposure estimates with biomarkers of exposure. Environ Int 37:687–693
Herzke D, Huber S, Bervoets L, D'Hollander W, Hajslova J, Pulkrabova J, Brambilla G, De Filippis SP, Klenow S, Heinemeyer G, de Voogt P (2013) Perfluorinated alkylated substances in vegetables collected in four European countries; occurrence and human exposure estimations. Environ Sci Pollut Res Int 20:7930–7939
Hjermitslev MH, Long M, Wielsøe M, Bonefeld-Jørgensen EC (2020) Persistent organic pollutants in Greenlandic pregnant women and indices of foetal growth: The ACCEPT study. Sci Total Environ 698:134118
Hu JMY, Arbuckle TE, Janssen P, Lanphear BP, Zhuang LH, Braun JM, Chen A, McCandless LC (2021) Prenatal exposure to endocrine disrupting chemical mixtures and infant birth weight: a Bayesian analysis using kernel machine regression. Environ Res 195:110749
Kaijser M, Granath F, Jacobsen G, Cnattingius S, Ekbom A (2000) Maternal pregnancy estriol levels in relation to anamnestic and fetal anthropometric data. Epidemiology (Cambridge, Mass.) 11:315–319
Kashino I, Sasaki S, Okada E, Matsuura H, Goudarzi H, Miyashita C, Okada E, Ito YM, Araki A, Kishi R (2020) Prenatal exposure to 11 perfluoroalkyl substances and fetal growth: a large-scale, prospective birth cohort study. Environ Int 136:105355
Kim J, Lee G, Lee YM, Zoh KD, Choi K (2021) Thyroid disrupting effects of perfluoroundecanoic acid and perfluorotridecanoic acid in zebrafish (Danio rerio) and rat pituitary (GH3) cell line. Chemosphere 262:128012
Kjeldsen LS, Bonefeld-Jørgensen EC (2013) Perfluorinated compounds affect the function of sex hormone receptors. Environ Sci Pollut Res Int 20:8031–8044
Kobayashi S, Azumi K, Goudarzi H, Araki A, Miyashita C, Kobayashi S, Itoh S, Sasaki S, Ishizuka M, Nakazawa H, Ikeno T, Kishi R (2017) Effects of prenatal perfluoroalkyl acid exposure on cord blood IGF2/H19 methylation and ponderal index: the Hokkaido study. J Exp Sci Environ Epidemiol 27:251–259
Lau C, Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Stanton ME, Butenhoff JL, Stevenson LA (2003) Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: postnatal evaluation. Toxicol Sci Official J Soc Toxicol 74:382–392
Lau C, Thibodeaux JR, Hanson RG, Narotsky MG, Rogers JM, Lindstrom AB, Strynar MJ (2006) Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol Sci Official J Soc Toxicol 90:510–518
Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J (2007) Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci Official J Soc Toxicol 99:366–394
Lee YJ, Choi SY, Yang JH (2014) NMDA receptor-mediated ERK 1/2 pathway is involved in PFHxS-induced apoptosis of PC12 cells. Sci Total Environ 491-492:227–234
Lindstrom AB, Strynar MJ, Libelo EL (2011) Polyfluorinated compounds: past, present, and future. Environ Sci Technol 45:7954–7961
Liu J, Zeng FF, Liu ZM, Zhang CX, Ling WH, Chen YM (2013) Effects of blood triglycerides on cardiovascular and all-cause mortality: a systematic review and meta-analysis of 61 prospective studies. Lipids Health Dis 12:159
Long M, Ghisari M, Bonefeld-Jørgensen EC (2013) Effects of perfluoroalkyl acids on the function of the thyroid hormone and the aryl hydrocarbon receptor. Environ Sci Pollut Res Int 20:8045–8056
Maisonet M, Terrell ML, McGeehin MA, Christensen KY, Holmes A, Calafat AM, Marcus M (2012) Maternal concentrations of polyfluoroalkyl compounds during pregnancy and fetal and postnatal growth in British girls. Environ Health Persp 120:1432–1437
Mallozzi M, Bordi G, Garo C, Caserta D (2016) The effect of maternal exposure to endocrine disrupting chemicals on fetal and neonatal development: a review on the major concerns. Birth defects research. Part C, Embryo Today : Rev 108:224–242
Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, Ballester F, Iñiguez C, Martinez D, Costa O, Santa-Marina L, Pereda-Pereda E, Schettgen T, Sunyer J, Vrijheid M (2017) Prenatal exposure to perfluoroalkyl substances and birth outcomes in a Spanish birth cohort. Environ Int 108:278–284
Marks KJ, Cutler AJ, Jeddy Z, Northstone K, Kato K, Hartman TJ (2019) Maternal serum concentrations of perfluoroalkyl substances and birth size in British boys. Int J Hygiene Environ Health 222:889–895
Mathews TJ, Driscoll AK (2017): Trends in infant mortality in the United States, 2005-2014. NCHS data brief, 1-8
Meng Q, Inoue K, Ritz B, Olsen J, Liew Z (2018) Prenatal exposure to perfluoroalkyl substances and birth outcomes; an updated analysis from the Danish national birth cohort. Int J Environ Res Public Health 15:1832
Minatoya M, Itoh S, Miyashita C, Araki A, Sasaki S, Miura R, Goudarzi H, Iwasaki Y, Kishi R (2017) Association of prenatal exposure to perfluoroalkyl substances with cord blood adipokines and birth size: the Hokkaido study on environment and children's health. Environ Res 156:175–182
Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, Perruolo E, Parati G (2016) Panethnic Differences in blood pressure in europe: a systematic review and meta-analysis. PLoS One 11:e0147601
Mokra K (2021) Endocrine disruptor potential of short- and long-chain perfluoroalkyl substances (PFASs)-a synthesis of current knowledge with proposal of molecular mechanism. Int J Mol Sci 22:2148
Pan CG, Ying GG, Liu YS, Zhang QQ, Chen ZF, Peng FJ, Huang GY (2014) Contamination profiles of perfluoroalkyl substances in five typical rivers of the Pearl River Delta region, South China. Chemosphere 114:16–25
Preston EV, Webster TF, Claus Henn B, McClean MD, Gennings C, Oken E, Rifas-Shiman SL, Pearce EN, Calafat AM, Fleisch AF, Sagiv SK (2020) Prenatal exposure to per- and polyfluoroalkyl substances and maternal and neonatal thyroid function in the Project Viva Cohort: a mixtures approach. Environ Int 139:105728
Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH (2006) Sources, fate and transport of perfluorocarboxylates. Environ Sci Technol 40:32–44
Ramhøj L, Hass U, Boberg J, Scholze M, Christiansen S, Nielsen F, Axelstad M (2018) Perfluorohexane sulfonate (PFHxS) and a mixture of endocrine disrupters reduce thyroxine levels and cause antiandrogenic effects in rats. Toxicol Sci official J Soc Toxicol 163:579–591
Ramhøj L, Hass U, Gilbert ME, Wood C, Svingen T, Usai D, Vinggaard AM, Mandrup K, Axelstad M (2020) Evaluating thyroid hormone disruption: investigations of long-term neurodevelopmental effects in rats after perinatal exposure to perfluorohexane sulfonate (PFHxS). Sci Rep 10:2672
Rappazzo KM, Coffman E, Hines EP (2017) Exposure to perfluorinated alkyl substances and health outcomes in children: a systematic review of the epidemiologic literature. Int J Environ Res Public Health 14:691
Ring CL, Pearce RG, Setzer RW, Wetmore BA, Wambaugh JF (2017) Identifying populations sensitive to environmental chemicals by simulating toxicokinetic variability. Environ Int 106:105–118
Shah-Kulkarni S, Kim BM, Hong YC, Kim HS, Kwon EJ, Park H, Kim YJ, Ha EH (2016) Prenatal exposure to perfluorinated compounds affects thyroid hormone levels in newborn girls. Environ Int 94:607–613
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ (Clinical research ed.) 350:g7647
Shi Y, Yang L, Li J, Lai J, Wang Y, Zhao Y, Wu Y (2017) Occurrence of perfluoroalkyl substances in cord serum and association with growth indicators in newborns from Beijing. Chemosphere 169:396–402
Spracklen CN, Wallace RB, Sealy-Jefferson S, Robinson JG, Freudenheim JL, Wellons MF, Saftlas AF, Snetselaar LG, Manson JE, Hou L, Qi L, Chlebowski RT, Ryckman KK (2014) Birth weight and subsequent risk of cancer. Cancer Epidemiol 38:538–543
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605
Starling AP, Adgate JL, Hamman RF, Kechris K, Calafat AM, Ye X, Dabelea D (2017) Perfluoroalkyl substances during pregnancy and offspring weight and adiposity at birth: examining mediation by maternal fasting glucose in the healthy start study. Environ Health Persp 125:067016
Susmann HP, Schaider LA, Rodgers KM, Rudel RA (2019) Dietary habits related to food packaging and population exposure to PFASs. Environ Health Persp 127:107003
UNEP D (2019a): SC-9/12. Listing of perfluorooctanoic acid (PFOA), its salts and PFOA-related compounds. UNEP-POPS-COP. 9-SC-9-12, Conference of the Parties to the Stockholm …
UNEP D (2019b): SC-4/17. Listing of perfluorooctane sulfonic acid, its salts and perfluorooctane sulfonyl fluoride. UNEP-POPS-COP. 4-SC-4-17, Conference of the Parties to the Stockholm …
Valvi D, Oulhote Y, Weihe P, Dalgård C, Bjerve KS, Steuerwald U, Grandjean P (2017) Gestational diabetes and offspring birth size at elevated environmental pollutant exposures. Environ Int 107:205–215
Wang H, Du H, Yang J, Jiang H, Karmin O, Xu L, Liu S, Yi J, Qian X, Chen Y, Jiang Q (2019) PFOS, PFOA, estrogen homeostasis, and birth size in Chinese infants. Chemosphere 221:349–355
Washino N, Saijo Y, Sasaki S, Kato S, Ban S, Konishi K, Ito R, Nakata A, Iwasaki Y, Saito K, Nakazawa H, Kishi R (2009) Correlations between prenatal exposure to perfluorinated chemicals and reduced fetal growth. Environ Health Persp 117:660–667
Wikström S, Lin PI, Lindh CH, Shu H, Bornehag CG (2020) Maternal serum levels of perfluoroalkyl substances in early pregnancy and offspring birth weight. Pediatr Res 87:1093–1099
Wolf CJ, Rider CV, Lau C, Abbott BD (2014) Evaluating the additivity of perfluoroalkyl acids in binary combinations on peroxisome proliferator-activated receptor-α activation. Toxicology 316:43–54
Wu K, Xu X, Peng L, Liu J, Guo Y, Huo X (2012) Association between maternal exposure to perfluorooctanoic acid (PFOA) from electronic waste recycling and neonatal health outcomes. Environ Int 48:1–8
Yeung LW, So MK, Jiang G, Taniyasu S, Yamashita N, Song M, Wu Y, Li J, Giesy JP, Guruge KS, Lam PK (2006) Perfluorooctanesulfonate and related fluorochemicals in human blood samples from China. Environ Sci Technol 40:715–720
Yu N, Wen H, Wang X, Yamazaki E, Taniyasu S, Yamashita N, Yu H, Wei S (2020) Nontarget discovery of per- and polyfluoroalkyl substances in atmospheric particulate matter and gaseous phase using cryogenic air sampler. Environ Sci Technol 54:3103–3113
Zhang Z, Kris-Etherton PM, Hartman TJ (2014) Birth weight and risk factors for cardiovascular disease and type 2 diabetes in US children and adolescents: 10 year results from NHANES. Mater Child Health J 18:1423–1432
Acknowledgements
The authors appreciate the valuable comments of the anonymous referees.
Funding
This work was supported by the National Natural Science Foundation of China (82273662, 81971405), Jiangsu Natural Science Foundation (BK20221307), Medical Research Project of Jiangsu Health and Health Commission (Z2019010), and the Priority Academic Program for the Development of Jiangsu Higher Education Institutions (Public Health and Preventive Medicine).
Author information
Authors and Affiliations
Contributions
Conceptualization: L.L. and W.W.; data curation: L.L., H.W., and D.C.; formal analysis: L.L.; funding acquisition: W.W. and Q.T.; investigation: L.L., H.W., and D.C.; methodology: L.L., H.W., and D.C.; project administration: W.W. and C.L.; resources: W.W.; supervision: W.W. and J.L.; writing-original draft: L.L. and L.P.; writing-review and editing: L.P., Q.X., Q.T., and W.W.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lan, L., Wei, H., Chen, D. et al. Associations between maternal exposure to perfluoroalkylated substances (PFASs) and infant birth weight: a meta-analysis. Environ Sci Pollut Res 30, 89805–89822 (2023). https://doi.org/10.1007/s11356-023-28458-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-28458-0