Abstract
Endosymbiotic dinoflagellates provide the nutritional basis for marine invertebrates, especially reef–building corals. These dinoflagellates are sensitive to environmental changes, and understanding the factors that can increase the resistance of the symbionts is crucial for the elucidation of the mechanisms involved with coral bleaching. Here, we demonstrate how the endosymbiotic dinoflagellate Durusdinium glynnii is affected by concentration (1760 vs 440 µM) and source (sodium nitrate vs urea) of nitrogen after light and thermal stress exposure. The effectiveness in the use of the two nitrogen forms was proven by the nitrogen isotopic signature. Overall, high nitrogen concentrations, regardless of source, increased D. glynnii growth, chlorophyll–a, and peridinin levels. During the pre–stress period, the use of urea accelerated the growth of D. glynnii compared to cells grown using sodium nitrate. During the luminous stress, high nitrate conditions increased cell growth, but no changes in pigments composition was observed. On the other hand, during thermal stress, a steep and steady decline in cell densities over time was observed, except for high urea condition, where there is cellular division and peridinin accumulation 72 h after the thermal shock. Our findings suggest peridinin has a protective role during the thermal stress, and the uptake of urea by D. glynnii can alleviate thermal stress responses, eventually mitigating coral bleaching events.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dinoflagellates of the family Symbiodiniaceae play a crucial role in coral reef ecological balance providing chemical energy (in carbohydrate form) produced by photosynthetic pathways, enabling calcium carbonate accretion and growth. Furthermore, these dinoflagellates can produce several metabolites that can improve the coral reef health, and this resulted in recent interest to understand these host–Symbiodiniaceae relationships (Jiang et al. 2014). Additionally, these dinoflagellates have attracted great pharmacological attention, because many of these molecules have been proving as potential anti–inflammatory, analgesic, vasoconstrictor, cytotoxic, and antitumor compounds that can inspire new drugs (Bigham Soostani et al. 2021; Assunção et al. 2017). This has led to recent efforts to improve the cultivation techniques of these dinoflagellates (Sánchez-Suárez et al. 2021; Oliveira et al. 2020).
Endosymbiotic dinoflagellates have a complex life cycle composed by two stages: the motile mastigote stage and the non–motile coccoid one. In natural environment, Symbiodiniaceae cells grow as mastigotes during the light phase, and divide in the dark as coccoid cells. The coccoid cells of Symbiodiniaceae are spherical with an average diameter of 10 µm and, in this stage, they become intracellular symbionts inside of the coral and other hosts (Shah et al. 2020; Figueroa et al. 2021). On the other hand, motile mastigote cells (sometimes referred as free–living cells) have different dimensions of the epicone and hypocone among species, and they can be found in different marine ecosystems (Wham et al. 2017). The free–living mastigote cells are essential to about 80% of coral species that establish endosymbiotic relationships anew each generation or during an environmental change (e.g., salinity reduction and temperature rise) (Claar et al. 2020). In view of this, it is clear that exploring the diversity and coral–specificity, the nutritional strategies, the responses to stress of free–living Symbiodiniaceae is crucial to understand the functioning of coral reefs.
Nitrogen is an essential nutrient for microalgae growth and plays a fundamental role in biosynthesis of protein, lipid, and carbohydrate (Su 2021). Microalgae can assimilate nitrogen in the form of nitrate, nitrite, urea, and ammonium; nonetheless, although the latter is often most efficiently assimilated, at high concentrations (approxiamtely at 25 µM), it may exhibit toxicity to cells (Yaakob et al. 2021). Intracellularly, the excess nitrogen might be stored in chemical and biochemical forms, such as free amino acids, proteins (especially Rubisco), and chlorophylls (Guilherme et al. 2019; Walker et al. 2018). On the other hand, under nitrogen deplete condition, microalgae cells change their carbon storage patterns in favor of neutral lipids, generally by the degradation of polyunsaturated fatty acids for triacylglycerol (Rodolfi et al. 2009; Wang et al. 2019). Although there is a number of studies on the effects of nitrogen–replete and –deplete in microalgae cultivation, these studies mainly focus on lipid profile and yield of biomass produced, generally aiming a boosting in biofuels production (Tarazona Delgado et al. 2021; Wei et al. 2022), and almost never to assess the physiological state of cells and their susceptibility to stress factors.
Understanding nutritional strategies that can improve resistance of endosymbiont dinoflagellates can contribute for optimization of large–scale cultivation of the endosymbiotic dinoflagellates and elucidation of their susceptibility to environmental stress resulting in coral bleaching events. Here, we described the physiological mechanisms of Durusdinium glynnii cells cultured under high and low nitrogen supply, using sodium nitrate and urea as nitrogen source. Our approach is based on two main hypotheses: (1) nitrogen–replete condition increases the resistance of D. glynnii to stresses and; (2) urea is more efficient than nitrate to support cell growth and to improve the resistance to stresses.
Materials and methods
Biological material
Durusdinium glynnii was maintained in filter–sterilized seawater (salinity of 30 psu) enriched with f/2 medium–Si (Guillard 1975) at 22 ± 1 °C, under continuous lighting at 150 μmol photons m−2 s–1. Cultures were kept in exponential growth by regular transfer to fresh medium to avoid nutrient limitation.
Experimental setup
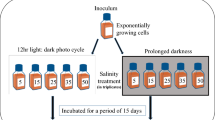
A methodological flowchart of the experimental setup is presented in Fig. 1. Experimental acclimated cultures of D. glynnii were performed in 250-mL Erlenmeyer flasks, under irradiance of 300 μmol photons m−2 s−1 provided by 36 W light–emitting diodes panels, and bubbled with atmospheric air. In order to assess the effects of concentration and source of nitrogen on the light– and heat–tolerance of D. glynnii, two concentrations, double and half the amount of nitrogen present in the f/2 culture medium (i.e., 1760 and 440 µM resulting in a N:P ratio of 28:1 and 7:1, respectively), in form of sodium nitrate (NaNO3) and urea (CH4N2O), normalized by nitrogen percentage in each form, were evaluated resulting in a bi–factorial (2 × 2) design with six independent replicates (n = 6) for each condition.
Initially, cultures grown for 96 h, and then, they were diluted to a mean cell concentration of 21.4 ± 2.6 × 104 cells mL−1. After dilution, the nitrogen concentration in each of the treatments was adjusted as close as possible to the time before dilution (after growth), when necessary. Then, two experimental units from each nitrogen condition were submitted for stress assays: (1) in the thermal stress, the cultures were transferred to a germination chamber with a thermostat adjusted to 30 ± 0.5 °C, under the same illumination regime (i.e., 300 μmol photons m−2 s−1); (2) in the light stress, the irradiance subjected to the cultures was increased to 600 μmol photons m−2 s−1, by adding a new LED panel, and adjusting using a quantameter and the distance of the cultures from the light source. The other cultures (control group) were maintained at the same conditions previously described (i.e., 22 ± 1 °C and 300 μmol photons m−2 s−1). The stress assays were maintained for 96 h. Stress levels were chosen based on previous photoacclimation (Oliveira et al. 2022) and thermal stress (Lin et al. 2019) studies with Symbiodiniaceae species.
Nitrogen quantification
For nitrogen quantification, samples from filtered medium were conducted following the Hach® colorimetric methods. For nitrate and urea determination, the NitraVer 5 (Hach method 8171) and Total Nitrogen Kit (Hach method 10071), respectively, were used according to the manufacturer's instructions.
Growth analysis
Cell concentration (c, cells mL−1) and cell type (mastigote and coccoid forms based on morphological characteristics reported in Kang et al. (2020)) were analyzed using a hemocytometer under an optical microscope (400 or 1000 × of magnification). An asymmetric logistic equation was used for fitting the cell concentration (C(t)) vs. time (t) data in order to accurately determine the specific growth rate (µ, day–1), before (µc), and after the stress (µs) according to equation elsewhere described (Oliveira et al. 2022).
Photosynthetic pigments
At the end of the stress assays, samples from each experimental unit were taken to analyze intracellular pigments. After centrifuging 100 mL of algal culture for 10 min at 2000 rpm, the remained biomass was subjected to pigment extraction using acetone 90% (Strickland and Parsons 1972). Chlorophylls–a (Chl–a) and c (Chl–c; c1 + c2) contents were calculated according to Jeffrey and Humphrey (1975), while carotenoid content (i.e., total carotenoids, β–carotene, and peridinin) were analyzed following the methods proposed by Carreto and Catoggio (1977) and Prézelin (1976). Values for all pigment concentrations were expressed as pg cell−1.
Stable isotope analysis
Sample preparation
At the end of the stress assays, 50 mL from each duplicate treatment were pooled together and vacuum filtrated (~ 200 mbar) onto pre–combusted (450 °C for 4 h) GF/C glass microfiber filters (pore size 1.2 μm), and stored in sterile glass Petri dishes. Filters were oven dried at 60 °C for 24 h and decarbonated with concentrated HCl (12 M) for 4 h in a desiccator (Lorrain et al. 2003). Afterwards, the filters were cut and encapsulated in high purity aluminum disc.
Elemental and stable isotope analysis
Cellular particulate organic carbon (POC) content, carbon stable isotope ratio (δ13C), nitrogen stable isotope ratio (δ15N), and the stoichiometric ratio of particulate organic carbon to nitrogen (C:N) were determined with an elemental analyzer (EA, EuroVector, model EA3000 Single) coupled to an isotopic ratio mass spectrometer (IRMS, Thermo Scientific, model Delva V Advantage). The temperature in the EA furnace was maintained at 980 °C. Helium (purity: 99.99%) was used as a carrier gas at a flow rate of 93 mL min−1. A pulse of 15 mL of oxygen (purity: 99.99%) was introduced into the reactor to facilitate combustion of the sample. The gases generated in the reactor were separated in a chromatographic column maintained in an isothermal oven (70 °C), and then transferred to the IRMS. The analysis time of a sample totaled 5 min. The IRMS was routinely calibrated with reference gases (CO2 and N2) traceable to an international isotopic standard (Vienna Pee Dee Belemnite — VPDB). A certified reference material (casein, Elemental Microanalysis P/N B2155) was employed for quality control. Analytical precision for δ13C was 0.02‰. Results are presented according to the commonly used δ–notation (Eqs. (1) and (2)) expressed as per mil (‰) as follow:
Statistical analysis
One-way analysis of variance (ANOVA), followed by the Tukey’s test, when necessary, was applied, using a significance level of 0.05 for growth data before stress incubation (n = 6). Normality and homoscedasticity were evaluated by the Shapiro–Wilk and Levene tests, respectively.
Results
In the first 96 h of Durusdinium glynnii growth in control conditions, the high urea concentration accelerated cell growth (p < 0.01, n = 6), reaching 91.83 ± 16.83 × 104 cells mL−1 at 0.55 ± 0.05 day−1, while the other nitrogen conditions grown at nearly 0.24 day−1. In the 96 h after cultures dilution, without environmental changes, the high nitrogen concentration presented higher growth in comparison to the low one. During the light stress, in oppose to control conditions, the use of sodium nitrate showed a higher growth performance compared to urea, in both concentrations. In the temperature stress, a gradual reduction in cell concentration was observed for all nitrogen conditions, except the high urea concentration, resulting in negatives values of µs due to decline in cell densities (Fig. 2a–c; Table 1).
Growth curves (a–c) and cell type proportion (d–f) of Durusdinium glynnii subjected to the light (b and e) and temperature (c and f) stress. Figures a and d represent the control. Black arrows indicate the dilution of the culture and the start of stress assay. Yellow and red arrows indicate the approximate timing of nitrogen depletion at low and high concentrations, respectively. Points plotted in cell type figures represent mean values from each experimental unit during the 96 h of stress
The cell morphotype of Durusdinium glynnii was mostly (over 80% of the total population) mastigote cells in the control conditions (Fig. 2d) and in the light stress (Fig. 2e) assay. On the other hand, the percentage of mastigote cells was lower (below 90%) during the temperature stress (Fig. 2f), except for the use of high urea concentration — above 95% of the population was mastigote cells. In addition, the population in the low nitrate concentration condition at the temperature stress was composed mainly by coccoid cells.
The cellular δ13C values were relatively stable both with respect to the use of sodium nitrate and urea, their respective concentrations, and also when subjected to thermal and light stress, ranging from −16.45 to −13.85‰. For δ15N, a clear distinction between nitrogen sources was observed: the sodium nitrate source ranged from −29.44 to −27.95‰, while for urea one, ranged from −3.03 to −0.67. Furthermore, for the use of urea as nitrogen source, higher δ15N values were reported for both stress assays compared to control conditions. Finally, cells grown using sodium nitrate (5.27 ± 0.20) as nitrogen source showed higher C:N ratio (p < 0.05; n = 6) compared to those grown using urea (4.76 ± 0.22) (Table 1).
Pigments contents in D. glynnii subjected to light and thermal stress showed differences compared to the control (Fig. 3). Overall, cells grown under high nitrogen condition had higher contents of chlorophyll–a. Cells subjected to the light stress had higher content of total carotenoids when grown using urea as nitrogen source at the two concentrations — it was also reflected in the contents of β–carotene and peridinin. In the thermal stress, the contents of all pigments in cells grown at high urea concentration were higher than other treatments.
Pigments content and composition of Durusdinium glynnii grown using sodium nitrate and urea as nitrogen source under control (a), light (b), and temperature (c) stress. HU, high urea; LU, low urea; HN, high sodium nitrate; LN, low sodium nitrate; Chl–a, chlorophyll–a; Chl–c, chlorophyll–c; TC, total carotenoids; βc, β–carotene; Per, peridinin
Discussion
The dataset here analyzed suggests that nitrogen overaccumulation can increase the tolerance of the endosymbiotic dinoflagellate Durusdinium glynnii subjected to light and thermal stress. Moreover, the use of high urea concentration was the only condition that supported cell growth and division of D. glynnii subjected to thermal stress. This may be associated with the fact that urea is more rapidly converted intracellularly into amino acids compared to nitrate–based compounds, as demonstrated in Fig. 4. The use of urea as nitrogen source also has a less energetic cost during its assimilation (Su 2021).
Some authors suggest that the preferred order of nitrogen utilization by eukaryote microalgae is ammonium > nitrate > nitrite > urea (Perez-Garcia et al. 2011; Su 2021). However, some recent reports (e.g., Ou et al. 2019; Huang et al. 2020) suggest that urea is the most preferred nitrogen source by dinoflagellates, as reported by Matantseva et al. (2016) that the addition of urea to the nitrate–acclimated culture of the marine dinoflagellate Prorocentrum minimum led to noticeable suppression of the nitrate–nitrogen uptake. Thus, probably, the preferred order of nitrogen assimilation for dinoflagellates is “ammonium > urea > nitrate > nitrite” or “urea > ammonium > nitrate > nitrite,” depending on species (Burford 2005). For decades, the use of urea as nitrogen–based fertilizer by farmers has pointed out as one of the main contributing factors to coastal eutrophication (Glibert et al. 2006). The escalating use of urea has been associated with harmful dinoflagellate blooms, due to the higher urease activity compared to other phytoplankton groups (Solomon and Glibert 2008; Jing et al. 2017). The findings of the present study corroborate this information, since D. glynnii presented high cell division when using urea as nitrogen source.
Regarding the isotopic signatures, no major changes were observed in the δ13C values, and these values were within the range reported for D. glynnii under control conditions (Müller et al. 2021) and other C4–photosynthetic microalgae (Raven et al. 2020). On the other hand, a clear difference in the δ15N signature was observed between the nitrogen sources, providing the effectiveness in the uptake of the different nitrogen source in the medium. The δ15N signatures for D. glynnii cultured using urea as nitrogen source were similar to those reported by Bateman and Kelly (2007) for urea fertilizers from different manufacturers. Similarly, Freyer and Aly (1974) reported that the isotopic composition of sodium nitrate is much lower than that of other sources of inorganic nitrogen due to residual nitrogen oxides from the nitric acid process, resulting in δ15N signatures close to –22 ‰. Although stable isotope analysis is routinely used in ecological studies of phytoplankton (e.g., Cai et al. 2019; Yang et al. 2020; Sabadel et al. 2022), it is rarely used to prove the effectiveness in absorption of dissolved compounds in microalgae cultures. Thus, this analysis can be successfully used to effectively track the uptake of inorganic and organic compounds by microalgae.
Contrary to our second hypothesis, the use of sodium nitrate as nitrogen source was more efficient than urea in terms of growth performance of D. glynnii subject to the light stress. This can be associated with the fact that light can stimulate the enzymatic activity of nitrate reductase and glucose–6–phosphate dehydrogenase, which may have increased the rate of nitrate uptake and assimilation to protein (Tischner and Hüttermann 1978; Wang et al. 2022). Moreover, it may also be associated to increased nitrogen accumulation during the 96 h before the stress. At this moment, the cells cultured with high urea concentration presented a faster metabolism and this resulted in an intense cell division in the first days of cultivation, while the cultures with nitrate may be accumulating nitrate during this first moment.
The high content of peridinin in high nitrogen cultures can be linked to the fact that this carotenoid is associated with proteins, in the form of peridinin–chlorophyll–protein complex, and thus, the nitrogen limitation may inhibit the peridinin biosynthesis (Di Valentin et al. 2016; Dorrell et al. 2019). During the thermal stress, the higher peridinin content in high urea condition may explain greater cellular health at this condition, while in the other conditions, the peridinin may have oxidized. The oxidation of intracellular metabolites (such as fatty acids and pigments) in Symbiodiniaceae subjected to thermal stress has also been previously reported. For example, Botana et al. (2022) reported an increase in oxy–polyunsaturated fatty acids in Breviolum minutum cells after heat shock (at 34 °C). Here, we also provide evidence that peridinin may act as a protector against oxidative stress resulting from a temperature rise. In a previous study (Oliveira et al. 2022), we reported that under optimal irradiance for growth D. glynnii maintained a peridinin to chlorophyll–a ratio of approximately 1 (at 3.5 pg cell−1), while under high–light exposure, this ratio increased to approximately 4, before the photoinhibition zone. Here, this ratio was close to 1, in the high nitrate condition, but at a content of 1.5 pg cell−1. These differences can be attributed to the cell concentration, since higher number of cells increases the light attenuation (Pruvost et al. 2015), reducing potential photo–oxidative stress.
Ecological implications
Our findings cannot be directly applied to natural environments for the obvious reason that artificial nitrogen enrichment would result in an invaluable environmental imbalance. Furthermore, the physiological responses of non–motile coccoid cells in endosymbiosis may be different from free–living mastigote cells. However, it is worth noting that the presence of free–living cells of Symbiodiniaceae in the environment represents important pools for coral symbiont acquisition (Claar et al. 2020). Additionally, the results herein presented may help for understanding the Symbiodiniaceae susceptibility to changing environmental conditions, particularly those linked to global warming and increased solar irradiance due to intensified stratification of the upper ocean layer. Thus, a schematic drawing was constructed to summarize the response of D. glynnii related to a rise temperature or an exposure to high light (Fig. 5). Overall, our findings support the idea that corals from nutrient–poor waters (particularly in nitrogen) are more susceptible to bleaching events in a situation of temperature rise. Similarly, Symbiodiniaceae cells when using urea tend to accumulate the carotenoid peridinin to prevent photo–oxidation — these results may be related to the shallow water reefs.
Undoubtedly, the temperature rise is one of the main stressors for endosymbiotic dinoflagellates. Here, we show that a well–established culture of D. glynnii gradually reduced its population over time after increasing temperature — except for the high urea condition. In the natural environment, a temperature rise may occur in combination with another environmental stressors, such as the presence of an emerging pollutant or a change in salinity, resulting in even more severe impacts (Coles and Jokiel 1978; Camp et al. 2016; Stien et al. 2020), and these multiple disturbances of the host–symbiont relationship can rapidly impact the ecological balance of reef systems. Therefore, the synergism between various environmental stressors should be a priority issue, given the modern changing world.
Several factors, such as geographic (e.g., river inputs on coastal), anthropogenic (e.g., industrial wastewater disposal and eutrophication), temporal (e.g., seasonality of inorganic fertilization of agricultural land in coastal regions), and oceanographic (e.g., remineralization and lateral transport of nutrients), can influence the sources and dynamics of nitrogen in the oceans (Zehr and Ward 2002; Howarth 2008). This causes seas to be diversified in terms of nitrogen sources and concentrations, resulting in different coral susceptibilities depending among other factors, on main sources of nitrogen input (Roff and Mumby 2012; Cannon et al. 2021). However, it is worth noting that due to the diversity of Symbiodiniaceae taxa, the behavior reported here for D. glynnii may not be the same for other Symbiodiniaceae species.
Conclusions
Our approach showed nitrogen as a key nutrient involved with resistance to light- and thermal-stressors for D. glynnii. The availability of reduced nitrogen form, such as urea, can accelerate intracellular metabolism and alleviate environmental stressors (i.e., thermal stress). Additionally, our findings provide initial evidence that the carotenoid peridinin may have a thermal protective role for endosymbiont dinoflagellates. However, future interspecific and molecular investigations (assessing the regulation of correlated genes) still need to be conducted.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Assunção J, Guedes AC, Malcata FX (2017) Biotechnological and pharmacological applications of biotoxins and other bioactive molecules from dinoflagellates. Mar Drugs 15(12):393. https://doi.org/10.3390/md9040625
Bateman AS, Kelly SD (2007) Fertilizer nitrogen isotope signatures. Isot Environ Healt Stud 43(3):237–247. https://doi.org/10.1080/10256010701550732
Bigham Soostani S, Yousefzadi M, Zarei Darki B, Ranjbar MS (2021) Evaluation of cytotoxic and antibacterial properties of Symbiodinium sp. isolated and purified from Stichodactyla haddoni in the Persian Gulf and Gulf of Oman. Aquat Physiol Biotechnol 9(2):125–144
Botana MT, Chaves-Filho AB, Inague A, Güth AZ, Saldanha-Corrêa F, Müller MN, Sumida PYG, Miyamoto S, Kellermann MY, Valentine RC, Yoshinaga MY (2022) Thermal plasticity of coral reef symbionts is linked to major alterations in their lipidome composition. Limnol Oceanogr 67:1456–1469. https://doi.org/10.1002/lno.12094
Burford MA (2005) Relative uptake of urea and ammonium by dinoflagellates or cyanobacteria in shrimp mesocosms. Hydrobiologia 549(1):297–303. https://doi.org/10.1007/s10750-005-1702-3
Cai Y, Cao Y, Tang C (2019) Evidence for the primary role of phytoplankton on nitrogen cycle in a subtropical reservoir: reflected by the stable isotope ratios of particulate nitrogen and total dissolved nitrogen. Front Microbiol 10:2202. https://doi.org/10.3389/fmicb.2019.02202
Camp EF, Smith DJ, Evenhuis C, Enochs I, Manzello D, Woodcock S, Suggett DJ (2016) Acclimatization to high-variance habitats does not enhance physiological tolerance of two key Caribbean corals to future temperature and pH. Proc Royal Soc B Biol Sci 283:20160442. https://doi.org/10.1098/rspb.2016.0442
Cannon SE, Aram E, Beiateuea T, Kiareti A, Peter M, Donner SD (2021) Coral reefs in the Gilbert Islands of Kiribati: resistance, resilience, and recovery after more than a decade of multiple stressors. PloS ONE 16(8):e0255304. https://doi.org/10.1371/journal.pone.0255304
Carreto JI, Catoggio JA (1977) An indirect method for the rapid estimation of carotenoid contents in Phaeodactylum tricornutum: possible application to other marine algae. Mar Biol 40:109–116. https://doi.org/10.1007/BF00396255
Claar DC, Tietjen KL, Cox KD, Gates RD, Baum JK (2020) Chronic disturbance modulates symbiont (Symbiodiniaceae) beta diversity on a coral reef. Sci Rep 10(1):4492. https://doi.org/10.1038/s41598-020-60929-z
Coles SL, Jokiel PL (1978) Synergistic effects of temperature, salinity and light on the hermatypic coral Montipora verrucosa. Mar Biol 49(3):187–195. https://doi.org/10.1007/BF00391130
Di Valentin M, Dal Farra MG, Galazzo L, Albertini M, Schulte T, Hofmann E, Carbonera D (2016) Distance measurements in peridinin-chlorophyll a-protein by light-induced PELDOR spectroscopy. Analysis of triplet state localization. Biochimm Biophys Acta Bioenerg 12:1909–1916. https://doi.org/10.1016/j.bbabio.2016.09.008
Dorrell RG, Nisbet RER, Barbrook AC, Rowden SJ, Howe CJ (2019) Integrated genomic and transcriptomic analysis of the peridinin dinoflagellate Amphidinium carterae plastid. Protist 170(4):358–373. https://doi.org/10.1016/j.protis.2019.06.001
Figueroa RI, Howe-Kerr LI, Correa AMS (2021) Direct evidence of sex and a hypothesis about meiosis in Symbiodiniaceae. Sci Rep 11(1):1–17. https://doi.org/10.1038/s41598-021-98148-9
Freyer HD, Aly AIM (1974) Nitrogen‐15 variations in fertilizer nitrogen (Vol. 3, No. 4, pp. 405–406). American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America
Glibert PM, Harrison J, Heil C, Seitzinger S (2006) Escalating worldwide use of urea–a global change contributing to coastal eutrophication. Biogeochemistry 77(3):441–463. https://doi.org/10.1007/s10533-005-3070-5
Guilherme EA, Nascimento CS, Lobo AK, Carvalho FE, Silveira JA (2019) Nitrogen-utilization efficiency during early deficiency after a luxury consumption is improved by sustaining nitrate reductase activity and photosynthesis in cotton plants. Plant Soil 443(1):185–198. https://doi.org/10.1007/s11104-019-04214-7
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Culture of marine invertebrate animals, Springer US, Boston, pp. 29–60. https://doi.org/10.1007/978-1-4615-8714-9_3
Howarth RW (2008) Coastal nitrogen pollution: a review of sources and trends globally and regionally. Harmful Algae 8(1):14–20. https://doi.org/10.1016/j.hal.2008.08.015
Huang K, Feng Q, Zhang Y, Ou L, Cen J, Lu S, Qi Y (2020) Comparative uptake and assimilation of nitrate, ammonium, and urea by dinoflagellate Karenia mikimotoi and diatom Skeletonema costatum sl in the coastal waters of the East China Sea. Mar Pollut Bull 155:111200. https://doi.org/10.1016/j.marpolbul.2020.111200
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz 167:191–194
Jiang PL, Pasaribu B, Chen CS (2014) Nitrogen-deprivation elevates lipid levels in Symbiodinium spp. by lipid droplet accumulation: morphological and compositional analyses. PLoS ONE 9(1):e87416. https://doi.org/10.1371/journal.pone.0087416
Jing X, Lin S, Zhang H, Koerting C, Yu Z (2017) Utilization of urea and expression profiles of related genes in the dinoflagellate Prorocentrum donghaiense. PLoS One 12(11):e0187837. https://doi.org/10.1371/journal.pone.0187837
Kang NS, Kim ES, Lee JA, Kim KM, Kwak MS, Yoon M, Hong JW (2020) First report of the dinoflagellate genus Effrenium in the east sea of Korea: morphological, genetic, and fatty acid characteristics. Sustainability 12(9):3928. https://doi.org/10.3390/su12093928
Lin S, Yu L, Zhang H (2019) Transcriptomic responses to thermal stress and varied phosphorus conditions in Fugacium kawagutii. Microorganisms 7(4):96. https://doi.org/10.3390/microorganisms7040096
Lorrain A, Savoye N, Chauvaud L, Paulet YM, Naulet N (2003) Decarbonation and preservation method for the analysis of organic C and N contents and stable isotope ratios of low-carbonated suspended particulate material. Anal Chim Acta 491(2):125–133. https://doi.org/10.1016/S0003-2670(03)00815-8
Matantseva O, Skarlato S, Vogts A, Pozdnyakov I, Liskow I, Schubert H, Voss M (2016) Superposition of individual activities: urea-mediated suppression of nitrate uptake in the dinoflagellate Prorocentrum minimum revealed at the population and single-cell levels. Front Microbiol 7:1310. https://doi.org/10.3389/fmicb.2016.01310
Müller MN, Yogui GT, Gálvez AO, de Sales Jannuzzi LG, de Souza Filho JF, Montes MDJF, Melo PAMC, Neumann-Leitão S, Zanardi-Lamardo E (2021) Cellular accumulation of crude oil compounds reduces the competitive fitness of the coral symbiont Symbiodinium glynnii. Environ Pollut 289:117938. https://doi.org/10.1016/j.envpol.2021.117938
Oliveira CYB, Oliveira CDL, Müller MN, Santos EP, Dantas DM, Gálvez AO (2020) A scientometric overview of global dinoflagellate research. Publications 8(4):50. https://doi.org/10.1155/2021/1983589
Oliveira CYB, Abreu JL, Santos EP, Matos ÂP, Tribuzi G, Oliveira CDL, Veras BO, Bezerra RS, Müller MN, Gálvez AO (2022) Light induces peridinin and docosahexaenoic acid accumulation in the dinoflagellate Durusdinium glynnii. Appl Microbiol Biotechnol 1–14. https://doi.org/10.1016/j.biortech.2022.127387
Ou LJ, Huang KX, Li JJ, Jing WY, Dong HP (2019) Transcriptomic responses of harmful dinoflagellate Prorocentrum donghaiense to nitrogen and light. Mar Pollut Bull 149:110617. https://doi.org/10.1016/j.marpolbul.2019.110617
Perez-Garcia O, Escalante FM, De-Bashan LE, Bashan Y (2011) Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 45(1):11–36. https://doi.org/10.1016/j.watres.2010.08.037
Prézelin BB (1976) The role of peridinin-chlorophyll a-proteins in the photosynthetic light adaption of the marine dinoflagellate Glenodinium sp. Planta 1303(130):225–233. https://doi.org/10.1007/BF00387826
Pruvost J, Cornet JF, Le Borgne F, Goetz V, Legrand J (2015) Theoretical investigation of microalgae culture in the light changing conditions of solar photobioreactor production and comparison with cyanobacteria. Algal Res 10:87–99. https://doi.org/10.1016/j.algal.2015.04.005
Raven JA, Suggett DJ, Giordano M (2020) Inorganic carbon concentrating mechanisms in free-living and symbiotic dinoflagellates and chromerids. J Phycol 56(6):1377–1397. https://doi.org/10.1111/jpy.13050
Rodolfi L, Chini Zittelli G, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102(1):100–112. https://doi.org/10.1002/bit.22033
Roff G, Mumby PJ (2012) Global disparity in the resilience of coral reefs. Trends Ecol Evol 27(7):404–413. https://doi.org/10.1016/j.tree.2012.04.007
Sabadel AJM, Décima M, McComb K, Meyers M, Barr N, Gall M, Safi K, Law CS (2022) Amino acid nitrogen stable isotopes as biomarkers of coastal phytoplankton assemblages and food web interactions. Mar Ecol Prog Ser 690:1–13. https://doi.org/10.3354/meps14046
Sánchez-Suárez J, Garnica-Agudelo M, Villamil L, Díaz L, Coy-Barrera E (2021) Bioactivity and biotechnological overview of naturally occurring compounds from the dinoflagellate family Symbiodiniaceae: a systematic review. Sci World J 2021:1983589. https://doi.org/10.1155/2021/1983589
Shah S, Chen Y, Bhattacharya D, Chan CX (2020) Sex in Symbiodiniaceae dinoflagellates: genomic evidence for independent loss of the canonical synaptonemal complex. Sci Rep 10(1):9297. https://doi.org/10.1038/s41598-020-66429-4
Solomon CM, Glibert PM (2008) Urease activity in five phytoplankton species. Aquat Microb Ecol 52(2):149–157. https://doi.org/10.3354/ame01213
Stien D, Suzuki M, Rodrigues A, Yvin M, Clergeaud F, Thorel E, Lebaron P (2020) A unique approach to monitor stress in coral exposed to emerging pollutants. Sci Rep 10(1):9601. https://doi.org/10.1038/s41598-020-66117-3
Strickland J, Parsons T (1972) A practical handbook of seawater analysis, 2nd. Fisheries research board of Canada, Ottawa
Su Y (2021) Revisiting carbon, nitrogen, and phosphorus metabolisms in microalgae for wastewater treatment. Sci Total Environ 762:144590. https://doi.org/10.1016/j.scitotenv.2020.144590
Tarazona Delgado R, Guarieiro MDS, Antunes PW, Cassini ST, Terreros HM, Fernandes VDO (2021) Effect of nitrogen limitation on growth, biochemical composition, and cell ultrastructure of the microalga Picocystis salinarum. J Appl Phycol 33(4):2083–2092. https://doi.org/10.1007/s10811-021-02462-8
Tischner R, Hüttermann A (1978) Light-mediated activation of nitrate reductase in synchronous Chlorella. Plant Physiol 62(2):284–286. https://doi.org/10.1104/pp.62.2.284
Walker RP, Benincasa P, Battistelli A, Moscatello S, Técsi L, Leegood RC, Famiani F (2018) Gluconeogenesis and nitrogen metabolism in maize. Plant Physiol Biochem 130:324–333. https://doi.org/10.1016/j.plaphy.2018.07.009
Wang X, Fosse HK, Li K, Chauton MS, Vadstein O, Reitan KI (2019) Influence of nitrogen limitation on lipid accumulation and EPA and DHA content in four marine microalgae for possible use in aquafeed. Front Mar Sci 6:95. https://doi.org/10.3389/fmars.2019.00095
Wang Q, Wei D, Luo X, Zhu J, Rong J (2022) Ultrahigh recovery rate of nitrate from synthetic wastewater by Chlorella-based photo-fermentation with optimal light-emitting diode illumination: from laboratory to pilot plant. Bioresour Technol 348:126779. https://doi.org/10.1016/j.biortech.2022.126779
Wei Q, Yao J, Chen R, Yang S, Tang Y, Ma X (2022) Low-frequency ultrasound and nitrogen limitation induced enhancement in biomass production and lipid accumulation of Tetradesmus obliquus FACHB-12. Bioresour Technol 358:127387. https://doi.org/10.1016/j.biortech.2022.127387
Wham DC, Ning G, LaJeunesse TC (2017) Symbiodinium glynnii sp. nov., a species of stress-tolerant symbiotic dinoflagellates from pocilloporid and montiporid corals in the Pacific Ocean. Phycologia 56(4):396–409. https://doi.org/10.2216/16-86.1
Yaakob MA, Mohamed RMSR, Al-Gheethi A, Aswathnarayana Gokare R, Ambati RR (2021) Influence of nitrogen and phosphorus on microalgal growth, biomass, lipid, and fatty acid production: an overview. Cells 10(2):393. https://doi.org/10.3390/cells10020393
Yang SC, Hawco NJ, Pinedo-González P, Bian X, Huang KF, Zhang R, John SG (2020) A new purification method for Ni and Cu stable isotopes in seawater provides evidence for widespread Ni isotope fractionation by phytoplankton in the North Pacific. Chem Geol 547:119662. https://doi.org/10.1016/j.chemgeo.2020.119662
Zehr JP, Ward BB (2002) Nitrogen cycling in the ocean: new perspectives on processes and paradigms. Appl Environ Microbiol 68(3):1015–1024
Funding
This research was funded in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) — Finance Code 001. MNM and AOG received research fellowships PQ 305467/2020–4 and PQ 308063/2019−8, respectively, from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Contributions
Carlos Yure B. Oliveira: conceptualization, data curation, formal analysis, investigation, methodology, writing — original draft. Barbara Cássia S. Brandão: formal analysis, investigation. Luiz Gustavo de S. Jannuzzi: data curation, formal analysis. Deyvid Willame S. Oliveira: formal analysis. Gilvan T. Yogui: data curation, formal analysis, resources. Marius N. Müller: resources, supervision, writing — review and editing. Alfredo O. Gálvez: project administration, resources, supervision, writing — review and editing.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Robert Duran
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oliveira, C.Y.B., de Cássia S. Brandão, B., de S. Jannuzzi, L.G. et al. New insights on the role of nitrogen in the resistance to environmental stress in an endosymbiotic dinoflagellate. Environ Sci Pollut Res 30, 82142–82151 (2023). https://doi.org/10.1007/s11356-023-28228-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-28228-y