Abstract
Cadmium (Cd) pollution threatens food security and the environment. Willow species (Salix, Salicaceae) exhibit a remarkable potential to restore Cd-polluted sites due to their high biomass production and high Cd accumulation capacities. This study examined the Cd accumulation and tolerance in 31 genotypes of shrub willow in hydroponic conditions at varying Cd levels (0 μM Cd, 5 μM Cd, and 20 μM Cd). The root, stem, and leaf biomass of 31 shrub willow genotypes showed significant differences to Cd exposure. Among 31 willow genotypes, four patterns of biomass variation response to Cd were identified: insensitive to Cd; growth inhibition due to excessive Cd supply (high Cd inhibition); low Cd causing inhibited growth, whereas high Cd leading to increased biomass (U-shape); and growth increment with excessive Cd exposure (high Cd induction). The genotypes belonging to the “insensitive to Cd” and/or “high Cd induction” were candidates for the utilization of phytoremediation. Based on the analysis of Cd accumulation of 31 shrub willow genotypes at high and low Cd levels, genotypes 2372, 51–3, and 1052 obtained from a cross between S. albertii and S. argyracea grew well and accumulated relatively more Cd levels than other genotypes. In addition, for Cd-treated seedlings, root Cd accumulation was positively correlated with shoot Cd accumulation and total Cd uptake, demonstrating that Cd accumulation in roots could serve as a biomarker for evaluating the Cd extraction capacity of willows, especially in hydroponics screening. The results of this study screened out willow genotypes with high Cd uptake and translocation capacities, which will provide valuable approaches for restoring Cd-contaminated soils with willows.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over recent decades, anthropogenic activities have triggered catastrophic impacts on global environmental changes, one of which is heavy metals pollution (Ye et al. 2015; Yang et al. 2018). Plants are heavily threatened by high metal concentrations in soils. Heavy metal can be accumulated in the shoots and roots of plants and enter the food web, thus threatening human health and animals (Nascimento and Xing 2006; Wei et al. 2006; Clemens and Ma 2016; Yang et al. 2018; Vardhan et al. 2019).
Cadmium (Cd) is among the most dangerous environmental pollutants due to its high toxicity, strong migration, as well as widespread global contamination (Anjum et al. 2015; Vardhan et al. 2019). The use of pesticides and chemical fertilizers, irrigation with sewage, and atmospheric deposition that resulted from metal smelting and coal combustion are the three main sources of soil Cd (Zhao et al. 2015). Soil fertility may be impacted by the physical and chemical changes caused by Cd enrichment (Li et al. 2016). In addition, excessive Cd uptake by plants has direct effects on normal physiological function. Plants may experience metabolic blockage, leaf chlorosis, and even death as a result of the symptoms (Lunáčková et al. 2003; Sikka et al. 2009; Belkhadi et al. 2010; Anjum et al. 2015; Tauqeer et al. 2016). Additionally, other living organisms, such as humans at the top of the food chain, could be poisoned by the absorbed Cd (Clemens and Ma 2016). According to Matović et al. (2015), Cd enters the human body and selectively enriches in the kidney and liver, impairing organ function. Exposure to Cd led to the Itai-itai sickness that struck Toyama Prefecture in Japan (Inaba et al. 2005).
To prevent heavy metals from mobilizing into water bodies and subsequent bioaccumulation, the reclamation of polluted soil is necessary. However, traditional methods (e.g., excavation) or chemical treatment (e.g., electroremediation and chemical leaching) used for mitigating and repairing Cd pollution waste resources and time (Camargo et al. 2016; Domínguez-Garay et al. 2016). These techniques also run the risk of causing secondary contamination and destroying the original habitat of plants. In recent years, phytoremediation, also referred as botanical bioremediation, has developed into a novel, eco-friendly technique for remediating heavy metal-contaminated soil over the long term (Lunáčková et al. 2003; Rascio and Navari-Izzo 2011; Camargo et al. 2016; Mahar et al. 2016; Wei et al. 2021). This method can effectively remove, detoxify, or lock in pollutants in the growth matrix through physical, chemical, biological, or natural processes in plants. However, there are significant differences among species and cultivars within species in the capability of accumulating heavy metals, and thus, not all plants are applicable for phytoremediation. Hence, screening germplasms able to extract heavy metals is of great importance and value in phytoremediation.
Salix (willow) species (Salicaceae), trees or shrubs, are well-known pioneer plants that can withstand high concentrations of heavy metals (Punshon and Dickinson 1997; van der Heijden 2001; Unterbrunner et al. 2007). There are ca. 520 species of willow, and most of them are widely distributed in cold and temperate regions of the N hemisphere and a few in the S hemisphere. In addition to stabilizing the soil surface and reducing wind and water erosion, willow species also lower the danger of pollutant leaching because of their extensive, perennial root systems (Sander and Ericsson 1998). Willow species have proven to be an effective part of decontamination plans for soils with elevated Cd contents (Hammer et al. 2003; Vaculík et al. 2012) and have been utilized to successfully re-vegetate extremely contaminated land (Dickinson and Pulford 2005). Willows are therefore regarded as being important for upcoming replanting in phytoremediation activities (Landberg and Greger 1996; Rosselli et al. 2003).
Compared with tree species, shrub willows, when planted in contaminated soils, exhibit more rapid development, high biomass, and the ability to collect and accumulate significant levels of heavy metals in their shoots without displaying any harmful effects. However, different willow species and cultivars exhibit different translocation abilities of Cd to the shoot (Moreno-Caselles et al. 2000; Hammer et al. 2003; Dos Santos Utmazian et al. 2007; Sankaran and Ebbs 2007; Amna et al. 2015). Therefore, screening genotypes with high Cd translocation ability is of vital importance and the first step in practice. To do so, cuttings of 31 willow genotypes were cultivated under high and low Cd concentrations in a hydroponic condition. Biomass and Cd accumulation and translocation of all genotypes were measured following 4 week of Cd uptake. The results of our study will not only improve the database on willow species’ accumulation and tolerance to Cd for phytoremediation, but also offer helpful methods for replanting willow species in Cd-contaminated soils.
Materials and methods
Plant materials and growth conditions
Thirty-one shrub Salix genotypes obtained from the Jiangsu Academy of Forestry, National Willow Germplasm Resources (Nanjing, China) were analyzed for comparison of Cd accumulation (Table 1). These genotypes were grown in Cd-free soil. Cuttings of 31 different Salix species, with each about 12 cm in height, were put into a plastic tank filled with 12 L of water. After 10 days, the roots had emerged and were well developed. Then, the cuttings were supplied with low Cd (5 µM), high Cd (20 µM), or null Cd (0 µM) for 4 weeks to evaluate the phenotype. The water with Cd or without Cd was exchanged every 3 days to maintain the Cd concentrations and each tank was equipped with adequate aeration. The cuttings were grown in a growth chamber for 12 h each day and exposed to temperatures of 26 °C during the day and 20 °C at night, with a humidity level of 70%.
Sample collection and biomass measurement
Roots of Salix species (see above) were thoroughly rinsed in 20 mmol/L EDTA-Na2 for 5 min and then washed with deionized water to remove Cd from their surface after 4 weeks of Cd treatment. All 31 species were harvested for biomass analysis. The collected roots, stems, and leaves were dried for 3 days at 70 °C to a consistent weight. To assess tissue dry weight for each genotype and treatment, ten replicates were examined.
Cd determination
Following the procedure outlined by Guo et al. (2022), the Cd in willow tissues was examined. Briefly, the roots, stems, and leaves of 31 Salix genotypes were ground into powder. Using a microwave system, samples (0.10 g) were digested with a solution of 3 mL deionized water, 5 mL nitric acid, and 3 drops of hydrogen peroxide (MARS5; CEM Corporation, USA). Cd concentration was determined using an inductively coupled plasma-atomic emission spectrometer. For quality assurance, a standard Cd solution (GBW(E)080119, National Institute of Metrology, China) was applied.
Statistical analysis
Microsoft Excel 2010 and SPSS 20.0 were used for statistical analysis. The translocation factors (TFs) of Cd from roots to leaves or shoots in plants were expressed as TF = [Cd]root/[Cd]leaf follows by Dos Santos Utmazian et al. (2007). Analysis of variance (ANOVA) was used to test the significant difference of Cd response among different willow clones. Probability level of p < 0.05 is considered significant.
Results
Growth of 31 shrub willows to Cd exposure
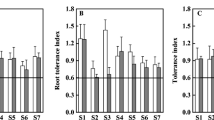
To investigate the response of shrub willows to Cd, 31 genotypes with representative genetic backgrounds were fed with 0 μM, 5 μM, and 20 μM Cd for 4 weeks (Table 1). Great variations in the root, stem, and leaf biomass of 31 willow genotypes were observed with or without Cd supply (Fig. S1). The ratios of the total dry weight of the genotypes under different Cd treatments were calculated to excavate willows which could be used as the candidates for Cd phytoextraction. The results showed that genotype 22 and 2372 grew better with higher biomass under low and/or high Cd treatments (Fig. 1A, and B).
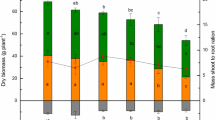
Based on the above data, we found four patterns to describe the responses of willow growth variations to Cd, including insensitive to Cd, high Cd inhibition, the U-shape, and high Cd induction with 58–2, 9–6, 2324, and 22 genotypes as representative, respectively (Fig. 2). It has been revealed that seventeen genotypes were insensitive to Cd exposure, indicating that most willows were ideal species for soil remediation (Fig. 2A–C). Excessive Cd supply led to the growth inhibition of six genotypes (Fig. 2D-F). Interestingly, for 35–9 and 2324 genotypes, low Cd caused inhibited growth, whereas high Cd caused increasing biomass, indicating that the two genotypes displayed the divergence from different Cd treatments (Fig. 2G–I). The definition of “low Cd” or “high Cd” in the U-shape pattern referred to different Cd concentrations in the nutrient solutions. In addition, among thirty-one genotypes, the total dry weight of five genotypes was increased with the increment of Cd concentration (Fig. 2J–L). The results showed that willows could take up Cd efficiently and the biomass variation in response to Cd was genotype-dependent. The willow genotypes belonging to the “insensitive to Cd” and/or “high Cd induction” were candidates for the utilization of phytoremediation. For example, P1024, 1050, and 2372 genotypes grew well at both low and high Cd concentrations (Fig. 2).
Four types of willow biomass responses to different Cd treatments. 31 genotypes were grown in hydroponic solution without Cd or containing 5 and 20 μM Cd for 4 weeks. A, D, G, J Models of four types. 58–2, 9–6, 2324, and 22 genotypes represent different types. Cd0, Cd5, Cd20: 0, 5, 20 μM Cd concentrations. Scale bar: 5 cm. B, E, H, K Root, stem, leaf, and total dry weight of 58–2, 9–6, 2324, and 22 genotypes. C, F, I, L The total dry weight of 18 genotypes in type I, 6 clones in type II, 2 clones in type III, and 5 clones in type IV. Values are mean ± SD (n = 10). Different letters above bars indicate significant differences by one-way ANOVA followed by Tukey’s test (p < 0.05)
Cd accumulation and translocation among 31 willow genotypes
To investigate the amounts of Cd in willows, Cd concentrations in different tissues were first measured. In our hydroponic experiments, Cd concentrations in roots of all 31 willow genotypes were much higher than that of stems or leaves when exposed to 5 μM or 20 μM Cd for 4 weeks (Fig. 3). For 5 μM Cd-treated plants, the Cd concentrations in roots ranked from 186.45 to 671.07 μg/g, while it ranged from 218.32 to 958.35 μg/g at the concentration of 20 μM Cd (Fig. 3A, and B). However, the changes in the stem or leaf Cd concentrations were confined within narrow margins compared with that in roots (Fig. 3C–F). The results showed that the stable levels of Cd taken up by roots could be transported to the shoots. In addition, in the low Cd environment, the genotypes (58–2, P605) with greater Cd concentration in roots essentially matched the genotypes with higher Cd concentration in the stems and leaves, demonstrating that while Cd concentration increased in the roots, it also increased in the shoots (Fig. 3A, C, and E). However, under a high Cd environment, Salix genotypes did not display the inherent attributes in the ability of Cd transport from roots to shoots (Fig. 3B, D, and F). Furthermore, total Cd uptake also showed a large variation. For 5 μM and 20 μM Cd-treated seedlings, 2357 and 2358 obtained from a cross between S. albertii and S. aurita showed the lower Cd accumulation capacity, while hybrid 2372, 51–3, and 1052 (S. albertii × S. argyracea) accumulated higher Cd per plant (Fig. S2; Fig. 4). Considering that most portion of Cd was accumulated in stems and leaves, these materials had the potential to be used as candidate materials for remediation of high Cd pollution environment (Fig. 5).
Cd accumulation in roots as a biomarker for evaluating the Cd extraction capacity of willows
Correlation analysis showed that for 5 μM Cd-treated seedlings, root Cd accumulation was positively correlated with total Cd uptake and shoot Cd accumulation, and there was also a positive correlation between shoot Cd accumulation and total Cd accumulation (Fig. 6A–C). When exposed at 20 μM Cd, root Cd accumulation was positively correlated with Cd accumulation per plant but no significant correlation with shoot Cd accumulation. Likewise, shoot Cd accumulation showed no significant correlation with total Cd accumulation in seedlings (Fig. 6D–F). It was noted that the relationship between Cd concentration in roots and leaves was obvious when the genotypes were treated with 5 μM Cd, whereas the relationship was not significant at 20 μM Cd supply (Fig. 6G, and H). In contrast, the correlation analysis of the Cd translocation factor between root, shoot, or whole plant Cd accumulation indicated that there was a negative or no relationship between most of them (Fig. S3). The higher the root concentration was, the lower Cd was transferred to the shoot.
Discussion
Cd pollution is a major hazard to human health and food security and is a global environmental issue. Several plants appear retarded growth at 5–10 μM Cd application in hydroponic experiments (Benáková et al. 2017). Some willow species are known to have a high Cd remediation capacity and may thrive in conditions with a 50-μM Cd stress (Tőzsér et al. 2017). Nevertheless, the remediation potential of Cd for most willows is still unclear. In this study, we discovered that willow species and genotypes responded differently to Cd concentration, with responses ranging from a significant reduction to a major stimulation of root and shoot biomass production in comparison to the control. We also found four patterns to describe the responses of willows growth variations to Cd, including insensitive to Cd, high Cd inhibition, the U-shape, and high Cd induction, with genotype 58–2, 9–6, 2324, and 22 as representative, respectively. In addition, our study indicated that Cd accumulation in roots served as a biomarker for evaluating the Cd extraction capacity of willows.
Different willow genotypes exhibited different biomass accumulations in response to Cd exposure. These results were in line with previous studies (Yang et al. 2015; Tőzsér et al. 2017) and indicated that biomass variation in response to Cd is genotype-dependent. Excessive Cd supply only led to growth inhibition of six genotypes (Fig. 2D–F), indicating that most willows were ideal species for soil remediation. Excess Cd causes several direct and indirect harmful effects. For instance, excessive Cd affects a variety of physical and biological processes in the plant by producing free radicals and reactive oxygen species (ROS) that damage proteins, DNA, lipids, and carbohydrates (Gratão et al. 2009; Belkhadi et al. 2010; Fernández and Brown 2013; Hashem et al. 2016). In contrast, the biomass of 17 genotypes was insensitive to Cd exposure and five genotypes were increased with the increment of Cd concentration. Such Cd hormesis and insensitive effects have also been found in other plants, such as Hordeum vulgare (Aery and Rana 2003), Brassica juncea (Singh and Tewari 2003), and Pennisetum ssp. (Zhang et al. 2010). The Cd stimulus indicated the presence of several detoxification mechanisms in plants against metal effects on plant physiology, for example, to prevent poisoning, the Zn/Cd hyperaccumulator Thlaspi praecox Wulfen accumulated Zn and Cd in the vacuoles of epidermal and mesophyll cells (Vogel-Mikuš et al. 2008; Liu et al. 2010). In addition, for two genotypes, 35–9 and 2324 genotypes, low Cd caused inhibited growth, whereas high Cd caused increasing biomass, indicating those two genotypes displayed the divergent response to different Cd treatments (Fig. 2G–I). Their mechanisms need further investigation. Nevertheless, our results indicated significant differences among species and cultivars within species in the capability of accumulating heavy metals, and most species of Salix spp. can tolerate Cd stress.
In our hydroponic experiments, Cd concentrations in roots of all 31 willow genotypes were significantly higher than stems or leaves when exposed to 5 μM or 20 μM Cd for 4 weeks (Fig. 3). The root Cd content ranged from 186.45 to 958.35 μg/g. Similarly, the highest Cd concentrations in stems and leaves were also 3–4 times more than the lowest (Fig. 3). Similar results have also been observed in other plant species. For example, Soudek et al. (2014) found that the highest Cd concentration in roots or shoots of five sorghum cultivars was subjected to 200 μM Cd in a hydroponic environment for 28 days, with the highest cultivar being about two times higher than the lowest. Sorghum genotypes had Cd uptake and accumulation concentrations ranging from 19.0 to 202.4 mg/kg in shoots and 277.0–898.3 mg/kg in roots (Jia et al. 2017). Compared with roots, the Cd concentrations in stems and leaves were confined within narrow margins compared with that in roots (Fig. 3). Those results indicated that stable levels of Cd taken up by roots could be transported to the shoots. However, for phytoextraction, genotypes with high accumulation in shoots are needed. Thus, to increase the effectiveness of phytoremediation using willow species, a technique to promote Cd transport from the root to the shoot will be used. In this regard, hybrid 2372, 51–3, and 1052 (S. albertii × S. argyracea) had the potential to be used as candidate materials for remediation of high Cd pollution environment (Figs. 4 and 5).
Hydroponics screening provided a preliminary assessment of Cd phytoextraction capacity for willows (Watson et al. 2003). The results of correlation analysis showed a positive relationship between Cd accumulation per plant and root Cd accumulation (Fig. 6). In hydroponics screening, the dry weight of ground tissues was much higher than the root biomass (Fig. S1). Additionally, because root Cd levels are easier to be tested, it could serve as a more significant indicator of willow's ability to extract Cd compared to other variables and could be used to forecast willow's capability for phytoremediation. Results of correlation analysis of Cd translocation factor between root, shoot, or whole plant Cd accumulation indicated that there was a negative or no relationship between most of them (Fig. S3). The poor uptake of Cd to shoots in the majority of species and genotypes was consistent with earlier studies on metal uptake in hydroponically grown willows (Dickinson et al. 1994; Punshon and Dickinson 1997; Kuzovkina et al. 2004). Metal tolerance in these species/clones appeared to be linked to a restricted transfer to shoots to protect photosynthesis-related plant organs (Landberg and Greger 1996). Those results also indicated that the Cd translocation factor is not a good indicator of Cd accumulation in willow species.
Implications and limitations
Based on our studies, the patterns of willow biomass variation response to Cd were generalized, which laid the foundation for understanding the physiological mechanisms of Cd accumulation in woody plants. Meanwhile, genotype 2372, 51–3, and 1052 obtained from a cross between S. albertii and S. argyracea exhibited both large biomass and high Cd accumulation capacities, which could be utilized in phytoremediation of Cd-contaminated soils. Additionally, root Cd levels served as a more significant indicator of willow’s ability to extract Cd, which provided guidance for the hydroponics screening of high Cd-accumulating and tolerant willow genotypes. Our results can facilitate the restoring of Cd contaminated soil by willows. Nevertheless, our study clearly has limitations.
Hydroponics has been recognized as an efficient and rapid approach to evaluating considerable plants for phytoremediation (Watson et al. 2003). However, the plants screened in hydroponics or in the field were not well correlated. Meanwhile, in our research, the performance of Cd accumulation among 31 willow genotypes was evaluated in 4 weeks. Considering the limited time of Cd treatment, the results of screening needed to be confirmed in the long term and in Cd-contaminated soil/field. In addition, our results, which indicate that Cd accumulation in roots, rather than in leaves and/or stems, served as a biomarker for evaluating the Cd extraction capacity, should be further tested among a diverse range of willow species in Cd-contaminated field.
Conclusion
In this study, Cd accumulation and tolerance in 31 Salix genotypes were investigated at different Cd levels. According to the response of 31 shrub Salix genotypes to Cd, we found four models: insensitive to Cd, high Cd inhibition, high Cd induction, and the U-shape. Moreover, the root Cd levels had a linear relationship with the shoot Cd accumulation and total Cd accumulation of the whole plant, which could be used for reference in the screening of phytoremediation materials.
Data availability
All relevant data can be found within the manuscript and its supporting information.
References
Aery NC, Rana DK (2003) Growth and cadmium uptake in barley under cadmium stress. J Environ Biol 24(2):117–123
Amna AN, Masood S, Mukhtar T, Kamran MA, Rafique M, Munis MFH, Chaudhary HJ (2015) Differential effects of cadmium and chromium on growth, photosynthetic activity, and metal uptake of Linumusitatissimum in association with Glomusintraradices. Environ Monit Assess 187(6):311
Anjum SA, Tanveer M, Hussain S, Bao M, Wang L, Khan L, Ullah E, Tung SA, Samad RA, Shahzad B (2015) Cadmium toxicity in Maize (Zea mays L.): consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ Sci Pollut Res Int 22(21):17022–17030
Belkhadi A, Hediji H, Abbes Z, Nouairi I, Barhoumi Z, Zarrouk M, Chaïbi W, Djebali W (2010) Effects of exogenous salicylic acid pre-treatment on cadmium toxicity and leaf lipid content in Linumusitatissimum L. Ecotoxicol Environ Saf 73(5):1004–1011
Benáková M, Ahmadi H, Dučaiová Z, Tylová E, Clemens S, Tůma J (2017) Effects of Cd and Zn on physiological and anatomical properties of hydroponically grown Brassicanapus plants. Environ Sci Pollut Res Int 24(25):20705–20716
Camargo FP, Tonello PS, dos Santos ACA, Duarte ICS (2016) Removal of toxic metals from sewage sludge through chemical, physical, and biological treatments—a review. Water Air Soil Poll 227(12):1–11
Clemens S, Ma JF (2016) Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu Rev Plant Biol 67(1):489–512
Dickinson NM, Pulford ID (2005) Cadmium phytoextraction using short-rotation coppice Salix: the evidence trail. Environ Int 31(4):609–613
Dickinson NM, Punshon T, Hodkinson RB, Lepp NW (1994) Metal tolerance and accumulation in willows. Swedish University of Agricultural Sciences, Uppsala, Willow Vegetation Filters for Municipal Wastewater and Sludges, pp 121–127
Domínguez-Garay A, Boltes K, Esteve-Núñez A (2016) Cleaning-up atrazine-polluted soil by using microbial electroremediating cells. Chemosphere 161:365–371
Dos Santos Utmazian MN, Wieshammer G, Vega R, Wenzel WW (2007) Hydroponic screening for metal resistance and accumulation of cadmium and zinc in twenty clones of willows and poplars. Environ Pollut 148(1):155–165
Fernández V, Brown PH (2013) From plant surface to plant metabolism: the uncertain fate of foliar-applied nutrients. Front Plant Sci 4:289
Gratão PL, Monteiro CC, Rossi ML, Martinelli AP, Peres LE, Medici LO, Lea PJ, Azevedo RA (2009) Differential ultrastructural changes in tomato hormonal mutants exposed to cadmium. Environ Exp Bot 67(2):387–394
Guo N, Fan LY, Cao Y, Ling H, Xu GH, Zhou J, Chen QS, Tao J (2022) Comparison of two willow genotypes reveals potential roles of iron-regulated transporter 9 and heavy-metal ATPase 1 in cadmium accumulation and resistance in Salixsuchowensis. Ecotoxicol Environ Saf 244:114065
Hammer D, Kayser A, Keller C (2003) Phytoextraction of Cd and Zn with Salixviminalis in field trials. Soil Use Manage 19(3):187–192
Hashem A, Abd Allah EF, Alqarawi AA, Al Hugail AA, Egamberdieva D, Wirth S (2016) Alleviation of cadmium stress in Solanumlycopersicum L. by arbuscular mycorrhizal fungi via induction of acquired systemic tolerance. Saudi J Biol Sci 23(2):272–281
Inaba T, Kobayashi E, Suwazono Y, Uetani M, Oishi M, Nakagawa H, Nogawa K (2005) Estimation of cumulative cadmium intake causing Itai-itai disease. Toxicol Lett 159(2):192–201
Jia W, Miao F, Lv S, Feng J, Zhou S, Zhang X, Wang D, Li SZ, Li YX (2017) Identification for the capability of Cd-tolerance, accumulation and translocation of 96 sorghum genotypes. Ecotoxicol Environ Saf 145:391–397
Kuzovkina YA, Knee M, Quigley MF (2004) Cadmium and copper uptake and translocation in five willow (Salix L.) species. Int J Phytoremediation 6(3):269–287
Landberg T, Greger M (1996) Differences in uptake and tolerance to heavy metals in Salix from unpolluted and polluted areas. Appl Geochem 11(1–2):175–180
Li X, Zhang XM, Yang Y, Li BQ, Wu YS, Sun H, Yang YP (2016) Cadmium accumulation characteristics in turnip landraces from China and assessment of their phytoremediation potential for contaminated soils. Front Plant Sci 7:1862
Liu XQ, Peng KJ, Wang AG, Lian CL, Shen ZG (2010) Cadmium accumulation and distribution in populations of Phytolacca Americana L. and the role of transpiration. Chemosphere 78(9):1136–1141
Lunáčková L, Masarovičová E, Kral’Ova K, Streško V (2003) Response of fast growing woody plants from family Salicaceae to cadmium treatment. Bull Environ Contam Toxicol 70(3):576–585
Mahar A, Wang P, Ali A, Awasthi MK, Lahori AH, Wang Q, Li RH, Zhang ZQ (2016) Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: a review. Ecotox Environ Safe 126:111–121
Matović V, Buha A, Ðukić-Ćosić D, Bulat Z (2015) Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem Toxicol 78:130–140
Moreno-Caselles J, Moral R, Pérez-Espinosa A, Pérez-Murcia MD (2000) Cadmium accumulation and distribution in cucumber plant. J Plant Nutr 23(2):243–250
Nascimento CWAD, Xing BS (2006) Phytoextraction: a review on enhanced metal availability and plant accumulation. Sci Agr 63(3):299–311
Punshon T, Dickinson NM (1997) Acclimation of Salix to metal stress. New Phytol 137(2):303–314
Rascio N, Navari-Izzo F (2011) Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci 180(2):169–181
Rosselli W, Keller C, Boschi K (2003) Phytoextraction capacity of trees growing on a metal contaminated soil. Plant Soil 256(2):265–272
Sander ML, Ericsson T (1998) Vertical distributions of plant nutrients and heavy metals in Salixviminalis stems and their implications for sampling. Biomass Bioenerg 14(1):57–66
Sankaran RP, Ebbs SD (2007) Cadmium accumulation in deer tongue grass (Panicumclandestinum L.) and potential for trophic transfer to microtine rodents. Environ Pollut 148(2):580–9
Sikka R, Nayyar V, Sidhu SS (2009) Monitoring of Cd pollution in soils and plants irrigated with untreated sewage water in some industrialized cities of Punjab. India Environ Monit Assess 154(1):53–64
Singh PK, Tewari RK (2003) Cadmium toxicity induced changes in plant water relations and oxidative metabolism of Brassicajuncea L. plants. J Environ Biol 24(1):107–12
Soudek P, Petrová Š, Vaňková R, Song J, Vaněk T (2014) Accumulation of heavy metals using Sorghum sp. Chemosphere 104:15–24
Tauqeer HM, Ali S, Rizwan M, Ali Q, Saeed R, Iftikhar U, Ahmad R, Farid M, Abbasi GH (2016) Phytoremediation of heavy metals by Alternantherabettzickiana: growth and physiological response. Ecotoxicol Environ Saf 126:138–146
Tőzsér D, Magura T, Simon E (2017) Heavy metal uptake by plant parts of willow species: a meta-analysis. J Hazard Mater 336:101–109
Unterbrunner R, Puschenreiter M, Sommer P, Wieshammer G, Tlustoš P, Zupan M, Wenzel WW (2007) Heavy metal accumulation in trees growing on contaminated sites in Central Europe. Environ Pollut 148(1):107–114
Vaculík M, Konlechner C, Langer I, Adlassnig W, Puschenreiter M, Lux A, Hauser M (2012) Root anatomy and element distribution vary between two Salix caprea isolates with different Cd accumulation capacities. Environ Pollut 163:117–126
Van der Heijden EW (2001) Differential benefits of arbuscular mycorrhizal and ectomycorrhizal infection of Salix repens. Mycorrhiza 10:185–193
Vardhan KH, Kumar PS, Panda RC (2019) A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J Mol Liq 290:111197
Vogel-Mikuš K, Simčič J, Pelicon P, Budnar M, Kump P, Necemer M, Mesjasz-Przybyłowicz J, Przybyłowicz WJ, Regvar M (2008) Comparison of essential and non-essential element distribution in leaves of the Cd/Zn hyperaccumulator Thlaspi praecox as revealed by micro-PIXE. Plant Cell Environ 31(10):1484–1496
Watson C, Pulford ID, Riddell-Black D (2003) Screening of willow species for resistance to heavy metals: comparison of performance in a hydroponics system and field trials. Int J Phytoremediation 5(4):351–365
Wei SH, Zhou QX, Koval PV (2006) Flowering stage characteristics of cadmium hyperaccumulator Solanum nigrum L. and their significance to phytoremediation. Sci Total Environ 369(1–3):441–446
Wei ZH, Le VQ, Peng WX, Yang YF, Yang H, Gu HP, Lam SS, Sonne C (2021) A review on phytoremediation of contaminants in air, water and soil. J Hazard Mater 403:123658
Yang WD, Zhao FL, Zhang XC, Ding ZL, Wang YY, Zhu ZQ, Yang XE (2015) Variations of cadmium tolerance and accumulation among 39 Salix clones: implications for phytoextraction. Environ Earth Sci 73:3263–3274
Yang QQ, Li ZY, Lu XN, Duan QN, Huang L, Bi J (2018) A review of soil heavy metal pollution from industrial and agricultural regions in China: pollution and risk assessment. Sci Total Environ 642:690–700
Ye XZ, Xiao WD, Zhang YZ, Zhao SP, Wang GJ, Zhang Q, Wang Q (2015) Assessment of heavy metal pollution in vegetables and relationships with soil heavy metal distribution in Zhejiang province, China. Environ Monit Assess 187(6):1–9
Zhang XF, Xia HP, Li ZA, Zhuang P, Gao B (2010) Potential of four forage grasses in remediation of Cd and Zn contaminated soils. Bioresour Technol 101(6):2063–2066
Zhao FJ, Ma YB, Zhu YG, Tang Z, McGrath SP (2015) Soil contamination in China: current status and mitigation strategies. Environ Sci Technol 49(2):750–759
Funding
This work was supported by the National Natural Science Foundation of China (grant number 42207001), the Jiangsu Province Innovation and Extension Project of Forestry Science and Technology of China (grant number LYKJ[2020]03), the Yangzhou Science and Technology Planning Social Development Project of China (grant number YZ2022082), and the Jiangsu Province Qing-lan Project of China.
Author information
Authors and Affiliations
Contributions
JT and NG designed and supervised the study; XS, RY, and NG conducted the research; RH, KZ, QC, and NG wrote the first draft and various revisions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Elena Maestri
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, X., Guo, N., Yu, R. et al. Assessment of the capability of cadmium accumulation and translocation among 31 willows: four patterns of willow biomass variation response to cadmium. Environ Sci Pollut Res 30, 76735–76745 (2023). https://doi.org/10.1007/s11356-023-27393-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-27393-4