Abstract
Heavy metals such as lead, mercury, and cadmium have been identified to have negative impacts on human health. Although the individual effects of these metals have been extensively researched, the present study aims to explore their combined effects and their association with serum sex hormones among adults. Data for this study were obtained from the general adult population of the 2013-2016 National Health and Nutrition Survey (NHANES) and included five metal (mercury, cadmium, manganese, lead, and selenium) exposures and three sex hormones (total testosterone [TT], estradiol [E2], and sex hormone-binding globulin [SHBG]) levels. The free androgen index (FAI) and TT/E2 ratio were also calculated. The relationships between blood metals and serum sex hormones were analysed using linear regression and restricted cubic spline regression. The effect of blood metal mixtures on sex hormone levels was examined using the quantile g-computation (qgcomp) model. There were 3,499 participants in this study, including 1,940 males and 1,559 females. In males, positive relationships between blood cadmium and serum SHBG (β=0.049 [0.006, 0.093]), lead and SHBG (β=0.040 [0.002, 0.079]), manganese and FAI (β=0.080 [0.016, 0.144]), and selenium and FAI (β=0.278 [0.054, 0.502]) were observed. In contrast, manganese and SHBG (β=-0.137 [-0.237, -0.037]), selenium and SHBG (β=-0.281 [-0.533, -0.028]), and manganese and TT/E2 ratio (β=-0.094 [-0.158, -0.029]) were negative associations. In females, blood cadmium and serum TT (β=0.082 [0.023, 0.141]), manganese and E2 (β=0.282 [0.072, 0.493]), cadmium and SHBG (β=0.146 [0.089, 0.203]), lead and SHBG (β=0.163 [0.095, 0.231]), and lead and TT/E2 ratio (β=0.174 [0.056, 0.292]) were positive relationships, while lead and E2 (β=-0.168 [-0.315, -0.021]) and FAI (β=-0.157 [-0.228, -0.086]) were negative associations. This correlation was stronger among elderly women (>50 years old). The qgcomp analysis revealed that the positive effect of mixed metals on SHBG was mainly driven by cadmium, while the negative effect of mixed metals on FAI was mainly driven by lead. Our findings indicate that exposure to heavy metals may disrupt hormonal homeostasis in adults, particularly in older women.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are ubiquitous environmental pollutants found in air, soil, water, and food (Clemens & Ma 2016). Exposure to heavy metals has been linked to several human diseases, such as cardiovascular disease, renal insufficiency, diabetes, and cancer (Rehman et al. 2018). Furthermore, heavy metals are known endocrine-disrupting chemicals, which can interfere with the normal functioning of the endocrine system (Iavicoli et al. 2009). However, the relationship between metal exposure and sex hormone levels remains poorly understood (Rami et al. 2022). Tao et al. addressed this knowledge gap by investigating the association between fourteen urinary heavy metals and three serum sex steroid hormones (Tao et al. 2021). They employed a subject component analysis-weighted quantile and regression (PCA-WQSR) model and found that the mixture of industrial metal pollutants was negatively associated with oestradiol (E2) in females, while the mixture of water metal pollutants was negatively related to sex hormone binding globulin (SHBG) in males.
Sex steroid hormones, including androgens and estrogens, are crucial components of the hypothalamic-pituitary-gonadal (HPG) axis and have a significant impact on human health (Heck & Handa 2019, Pillerova et al. 2021). Total testosterone (TT) plays a crucial role in testicular endocrinology and spermatogenesis (Finkelstein et al. 2013). E2 is closely related to human sexual development and reproductive function (Kumar et al. 2018). SHBG is a plasma glycoprotein that regulates plasma-free sex hormone levels (Bond & Davis 1987). In addition, sex hormones play a vital role in bone metabolism (Riggs 2000), muscle strength (Cigarran et al. 2013) and metabolism (Kelly & Jones 2013). However, disruption of sex steroid hormones can have adverse effects on the human body, such as diabetes (Gianatti & Grossmann 2020), metabolic syndrome (Banos et al. 2011), cardiovascular disease (Cunningham 2015) and cancer (Flores-Ramirez et al. 2019).
Several metals, such as lead, arsenic, mercury, and cadmium, are known to be toxic even at low concentrations (Singh et al. 2011). These metals frequently co-occur in the natural environment, and their interactions can be synergistic or antagonistic. Moreover, the individual effects of each metal cannot fully explain the onset and progression of diseases associated with metal exposure. In light of these factors, the present study aimed to investigate the relationship between exposure to a mixture of blood metals and serum sex hormone levels using data from the US National Health and Nutrition Examination Survey (NHANES) of adults. Specifically, we examined the association of five blood metals, namely mercury, manganese, lead, cadmium, and selenium, and their mixtures to better understand the potential impact of metal exposure on serum sex hormone levels.
Materials and methods
Study population
The NHANES aimed to assess the health and nutritional status of adults and children in the United States. This study included extensive interviews, physical examinations, and laboratory tests. All data can be downloaded from the official website (https://www.cdc.gov/nchs/nhanes). The Centers for Disease Control and Prevention (CDC) Research Ethics Review Board approved the project, and all participants gave informed consent.
The representative data from NHANES 2013-2016 were analyzed in this study. We enrolled eligible participants aged >18 who had complete data on blood metals and serum sex hormones. Women who had their ovaries removed, were pregnant, or were breastfeeding were also not eligible for this study. Participants who used sex hormones were likewise excluded, both for male and female.
Assessment of blood metals
The CDC mainly used inductively coupled plasma dynamic reaction cell mass spectrometry (ICP-DRC-MS) to analyse the metal levels of the participants (Duan et al. 2020). Mercury, cadmium, manganese, lead, and selenium were among the five heavy metals that were the focus of our research. We determined the level of concentration as well as the distribution of these heavy metals throughout the blood. The lower limit of detection (LOD) of blood mercury, cadmium, manganese, lead, and selenium was 0.28 μg/L, 0.10 μg/L, 0.99 μg/L, 0.07 μg/L, and 24.48 μg/L, respectively. All concentrations below the LODmax were substituted with a value of LODmax divided by a square root of two.
Assessment of serum sex hormones
Serum samples were collected in a Mobile Examination Centre (MEC) and stored at -20°C until analysis to avoid degradation of the specimen. Three typical sex hormones, including TT, E2 and SHBG, were quantified in the serum samples. TT and E2 levels in serum were measured using isotope dilution liquid chromatography-tandem mass spectrometry (ID-LC-MS/MS). SHBG levels in serum were measured using an immunoassay. The LOD of TT, E2 and SHBG was 0.75 ng/ml, 2.99 pg/ml and 0.80 nmol/L, respectively. The free androgen index (FAI) was calculated as [(TT/SHBG) x 100], representing the serum-free testosterone level that can act in combination with the receptor (Wei et al. 2020). The TT/E2 ratio was calculated as total testosterone/total oestrogen and represented aromatase activity.
Covariates
Information on baseline data through the NHANES was collected, including age (≤50 or >50 years old), race/ethnicity (Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, or other race), education level (below high school, high school, or above high school). Body mass index (BMI) status was divided into normal or underweight (<25.0 kg/m2), overweight (25.0-29.9 kg/m2), and obese (>29.9 kg/m2). Poverty was assessed using the poverty income ratio (PIR) and defined according to a cutoff PIR of <1 for a given family (Hu et al. 2021). Never smokers were classified as those who reported smoking <100 cigarettes during their lifetime. Those who smoked >100 cigarettes in their lifetime were considered as current smokers, and those who smoked >100 cigarettes and had quit smoking were considered as former smokers (Qiu et al. 2022). Drinking status was classified as nondrinker, low-to-moderate drinker (<2 drinks/day in men and <1 drink/day in women), or heavy drinker (≥2 drinks/day in men and ≥1 drinks/day in women) (Qiu et al. 2022). To control for circadian and seasonal effects on hormone secretion, we included a six-month time period (November 1 through April 30, or May 1 through October 31), and time of blood draw (morning, afternoon, or evening) as covariates.
Statistical analysis
The categorical variables were presented as numbers (percentages) and compared using the chi-square test. Taking into account the considerable disparities between male and female sex hormone levels, participants were divided by gender. The blood metals and serum sex hormones were ln-transformed to normalize their distributions. The concentrations and the distributions of blood metals and serum sex hormones were analysed. Spearman correlation was used to calculate the correlation coefficients among blood metals and serum sex hormones.
The linear regression model was used to calculate adjusted β-coefficients and 95% confidence intervals (CIs) to assess the serum sex hormones associated with blood metals. We also grouped the participants with blood metals based on tertiles, and the reference category was considered the lowest tertile. Restricted cubic spline (RCS) was used to investigate potential dose-response relationships with three knots (10th, 50th, and 90th). Because sex hormone levels differed between young and older women, we stratified the female participants according to age (≤50 or >50 years old).
The quantile g-computation model combines the inferential simplicity of weighted quantile sum (WQS) regression with the flexibility of g-computation and allows inference on unbiased mixture effects with appropriate CI coverage (Scinicariello & Buser 2016). The quantile g-computation model allows the weights to move in either direction, suggesting that some exposures may be beneficial and some may be harmful (Schmidt 2020). The parameter q, which stands for the supplied quantile, was set to 10. We used 1000 bootstrap iterations to compute the confidence intervals (CIs). The R package "qgcomp" was utilized to obtain each mixture component's positive and negative weight coefficients.
All models were adjusted for age (≤50 or >50), race/ethnicity (Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, or other race), education level (below high school, high school, or above high school), family poverty income ratio (<1.0 or ≥1.0), smoking status (never smoker, former smoker, or current smoker), drinking status (nondrinker, low-to-moderate drinker, or heavy drinker), BMI (<25.0, 25.0-29.9, or >29.9), six-month time period (November 1 through April 30, or May 1 through October 31), time of blood draw (morning, afternoon, or evening). All statistical analyses were performed using R software (version 4.2.0), and two-sided P<0.05 was considered statistically significant.
Results
Baseline characteristics of participants
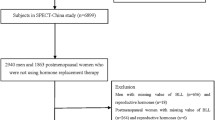
There was a total of 20,146 participants from NHANES 2013–2016. Among them, those with missing data on serum sex hormones and five blood metals (n=12,696), age < 18 (n=2,516), ovary removed (n=246), sex hormone usage (n=171), pregnant women (n=20), breastfeeding women (n=30), and missing data on any covariate (n=968) were excluded (Figure S1).
A total of 3,449 adults were included in this study, and their baseline characteristics are shown in Table 1. 53.87% of participants were ≤50 years old. Non-Hispanic White accounted for 39.5% of the total study group. 54.76% of participants completed more than high school education. 78.14% of participants reported a family poverty income ratio ≥1.0. 56.13% of participants never reported smoking and 71.16% reported low-to-moderate drinking. 39.01% of participants were obese (>29.9 kg/m2). The participants included 1,940 males and 1,559 females. The distribution of the two groups did not differ among age, race, education level, six-month time period, and time of blood draw. The male population had higher rates of poverty, non-smoking, non-drinking and obesity (P<0.001).
Distribution of blood metals and serum sex hormones
The frequency of detection was above 70% for all blood metals (Table S1), including mercury (74.4%), cadmium (72.7%), manganese (100%), lead (100%), and selenium (100%). The geometric mean (GM) of blood mercury, cadmium, manganese, lead, and selenium in males was 0.81 μg/L, 0.25 μg/L, 8.66 μg/L, 1.06 μg/L, and 198.03 μg/L, respectively. The GM of blood mercury, cadmium, manganese, lead, and selenium in females was 0.79 μg/L, 0.34 μg/L, 10.03 μg/L, 0.82 μg/L, and 192.45 μg/L, respectively (Table S2). No strong correlation was found among the blood metals (Figure S2), except that a relatively weak correlation was observed between lead and cadmium (r = 0.36).
We also analyzed the distribution of serum sex hormones in all participants (Table S2). The GM of TT, E2, SHBG, FAI, and TT/E2 ratio in males was 384.35 ng/dl, 23.04 pg/ml, 37.58 nmol/L, 1022.64, and 16.68, respectively. The mean concentration of TT, E2, SHBG, FAI, and TT/E2 ratio in females was 20.35 ng/dl, 23.81 pg/ml, 58.31 nmol/L, 34.91, and 0.86, respectively. The Spearman correlation of serum sex hormones is shown in Figure S2. We observed a strong correlation between TT and FAI (r=0.84) and a significant correlation between TT and TT/E2 (r=0.83). There was a negative correlation between SHBG and FAI (r=-0.58), and a significant positive correlation between FAI and TT/E2 (r=0.73).
Associations between blood metals and serum sex hormones
Table 2 shows the results of the linear regression analysis of blood metals with serum sex hormones. In males, we found that blood cadmium and serum SHBG (β=0.049 [0.006, 0.093]; P=0.028), lead and SHBG (β=0.040 [0.002, 0.079]; P=0.043), manganese and FAI (β=0.080 [0.016, 0.144]; P=0.018), and selenium and FAI (β=0.278 [0.054, 0.502]; P=0.008) were positive relationships, whereas manganese and SHBG (β=-0.137 [-0.237, -0.037]; P=0.011), selenium and SHBG (β=-0.281 [-0.533, -0.028]; P=0.032), and manganese and TT/E2 ratio (β=-0.094 [-0.158, -0.029]; P=0.008) were negative associations. In females, blood cadmium and serum TT (β=0.082 [0.023, 0.141]; P=0.010), manganese and E2 (β=0.282 [0.072, 0.493]; P=0.013), cadmium and SHBG (β=0.146 [0.089, 0.203]; P<0.001), lead and SHBG (β=0.163 [0.095, 0.231]; P<0.001), and lead and TT/E2 ratio (β=0.174 [0.056, 0.292]; P=0.007) were positive relationships, while lead and E2 (β=-0.168 [-0.315, -0.021]; P=0.029) and FAI (β=-0.157 [-0.228, -0.086]; P<0.001) were negative associations. Similarly, consistent associations of blood metals with sex hormones were also detected across tertiles of exposure (Table S3).
To explore the dose-response relationship between metal exposure and serum sex hormones, the exposure-response curves of each serum sex hormone stratified by sex were shown graphically in Fig. 1. In males, RCS analysis showed that blood cadmium and lead were linearly and positively correlated with E2, and manganese was linearly and negatively correlated with TT/E2 ratio. In addition, blood cadmium levels and serum E2 (P for nonlinearity [PNL]=0.035), blood manganese levels and serum SHBG (PNL = 0.008) and FAI (PNL<0.001), blood selenium levels and serum SHBG (PNL<0.001) and FAI (PNL<0.001) were non-linear relationships, with inflection points of 0.33, 8.85, 8.85, 197.16, and 197.16 μg/L, respectively. In females, blood cadmium and TT, manganese and E2, lead and SHBG were linear and positive associations, while lead was linearly and negatively correlated with FAI. Furthermore, blood cadmium levels and serum SHBG (PNL=0.029), blood manganese levels and serum SHBG (PNL=0.030) and TT/E2 ratio (PNL=0.009), blood lead levels and serum E2 (PNL=0.041) and TT/E2 ratio (PNL=0.003), and blood selenium levels and TT/E2 ratio (PNL=0.015) were non-linear relationships, with inflection points of 0.38, 10.23, 10.23, 0.87, 0.87, and 193.06 μg/L, respectively.
The exposure-response relationships between blood metals and serum sex hormones in males and females by restricted cubic spline (RCS). Adjusted for age (≤50 or >50), race/ethnicity (Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, or other race), education level (below high school, high school, or above high school), family poverty income ratio (<1.0 or ≥1.0), smoking status (never smoker, former smoker, or current smoker), drinking status (nondrinker, low-to-moderate drinker, or heavy drinker), BMI (<25.0, 25.0-29.9, or >29.9), six-month time period (November 1 through April 30, or May 1 through October 31), time of blood draw (Morning, Afternoon, or Evening)
As female reproductive function typically declines significantly with age, we further investigated whether the association between metal exposure and sex hormones differed by age group (Table 3). In both age groups, cadmium and lead were positively associated with SHBG and lead was negatively associated with FAI. We also found a positive association between cadmium and TT (β=0.186 [0.083, 0.290]; P=0.002) and a negative association between lead and E2 (β=-0.242 [-0.374, -0.109]; P=0.002) in females >50 years old only. In addition, in older women, cadmium was positively associated with TT/E2 ratio (β=0.271 [0.073, 0.470]; P=0.011), and lead was also positively associated with TT/E2 ratio (β=0.321 [0.180, 0.462]; P<0.001).
The adverse association of the mixture of blood metals with serum sex hormones.
The associations between the mixed metal exposures and sex hormones are presented in Table 4. In the qgcomp model, we found that mixed metal exposure was positively associated with SHBG (β=0.044 [0.025, 0.063]; P<0.001) and inversely associated with FAI (β=-0.027 [-0.053, -0.002]; P=0.034) in females. The estimated weights of the blood metal-sex hormone associations for the male and female groups were shown in Figure S3 and Figure S4. Cadmium was the primary metal associated with positive SHBG in females (weight=0.506). At the same time, lead was the key factor associated with lower FAI in females (weight=0.778), which is consistent with the results of multivariate linear regression analysis.
Discussion
This cross-sectional study of an adult population provides evidence of a link between exposure to heavy metals and sex hormones. Our study included 3,449 adults. Among males, we observed positive associations between blood cadmium and SHBG, lead and SHBG, manganese and FAI, and selenium and FAI. In contrast, we found negative associations between manganese and SHBG, selenium and SHBG, and manganese and TT/E2. Among females, we found positive associations between blood cadmium and serum TT, manganese and E2, cadmium and SHBG, lead and SHBG, and lead and TT/E2. However, we found negative associations between lead and E2, and between lead and FAI. These associations were more pronounced in women over the age of 50. Our qgcomp analysis revealed that cadmium was the primary contributor to the positive correlation between the metal mixture and SHBG in females, while lead was the primary contributor to the negative correlation between the metal mixture and FAI.
Testosterone plays a crucial role in human health, not only in reproduction but also in cardiovascular, physical, and cognitive function (Morgentaler et al. 2019). The majority of testosterone in the bloodstream is bound to SHBG, and only free testosterone can bind to androgen receptors to exert biological effects (Goldman et al. 2017). Telisman et al. investigated the relationship between metal exposure and sex hormones in males and demonstrated that cadmium and lead could synergistically increase serum testosterone levels (Telisman et al. 2007). This observation was consistent with that of Lewis and Meeker (Lewis & Meeker 2015). However, our study found only a positive association between cadmium and TT in females. This finding is consistent with that of Ali et al., who reported a similar positive association in postmenopausal females from Sweden (Ali et al. 2014). Rotter et al. suggested that cadmium and lead have a negative impact on bioavailable testosterone levels in males (Rotter et al. 2016). However, our study only found a negative association between lead and FAI in females. Maggio et al. observed a correlation between TT and manganese in older males, and our study also found a positive correlation between FAI and manganese in females (Maggio et al. 2011). Furthermore, a clinical trial showed that magnesium supplementation increased TT and FAI levels in humans (Cinar et al. 2011).
There is mounting evidence that E2 plays a crucial role in reproductive function, bone growth, glucose metabolism, and vasodilation (Russell & Grossmann 2019). Our study only found a positive association between E2 and manganese and a negative association between E2 and lead in females. Agusa et al. reported a positive association between blood mercury and E2, which was not observed in our study (Agusa et al. 2007). Furthermore, studies have suggested that E2 dysregulation may be a risk factor for metabolic diseases in postmenopausal females (Wang et al. 2021). Urinary cadmium in postmenopausal females was significantly negatively associated with E2, and similar results were found in animal studies (Ali et al. 2014, Nasiadek et al. 2018). However, in our analysis, lead and manganese were identified as disruptors of E2. Lead could regulate E2 in female rats by altering the synthesis or secretion of serum insulin-like growth factor-1 (IGF-1) (Dearth et al. 2002). Additionally, lead could interfere with E2 synthesis in mice by downregulating the expression of steroid acute regulatory protein (StAR) (Srivastava et al. 2004). Moreover, our study found that lead was positively correlated with TT/E2 ratio in females, while manganese was negatively correlated with TT/E2 ratio in males. This suggests that lead and manganese may alter serum E2 levels by affecting aromatase activity.
SHBG, a liver-secreted protein, is involved in the regulation of sex steroid hormone bioavailability (Simo et al. 2015) and plays a significant role in the metabolic clearance of TT and E2 (Selby 1990). SHBG serum concentrations are influenced by various factors and display substantial inter-individual variability (Allen et al. 2002). Our study observed positive correlations between SHBG and cadmium and lead levels in males and females, respectively, and negative correlations between SHBG and manganese and selenium levels in males. Kresovich et al. also reported positive associations between SHBG and lead and cadmium exposure in males (Kresovich et al. 2015) , which aligns with our findings. Exposure to cadmium and lead has been shown to interfere with human endocrine function in several studies (Takiguchi & Yoshihara 2006). However, a cross-sectional study of pre- and postmenopausal women reported no association between blood or urinary cadmium levels and serum SHBG concentrations (Nagata et al. 2016). These discrepancies in outcomes may be linked to racial differences.
The strengths of this study are the large and representative sample size, allowing us to generalize our results to the non-institutionalized U.S. civilian population. Second, as different age groups show different sensitivities to environmental chemical toxicity and sex hormone levels in females are strongly influenced by age, we also analysed the relationship between metal exposure and sex hormone levels in different age groups of females, which will assist to identify the population of females who are susceptible to heavy metals. Third, this study used a novel and sophisticated method to estimate the effects of metal mixtures on sex hormones. The qgcomp regression is a recently developed model for analysing the health effects of chemical mixtures that identify the components that contribute most to the observed associations. The qgcomp statistical method guarantees that our results are not confused by exposure collinearity difficulties, allowing the statistical significance of a particular metal to be independent of the effect of co-exposure to other metals. Fourth, the robustness of the estimate of the link between exposure and outcome was improved by adjusting for potential biases attributable to varied seasons and times of blood collecting.
This study has several limitations. First, we could not demonstrate a causal association as a cross-sectional study. Second, sex hormone levels were highly dependent on the menstrual cycle of females, but the sample was collected randomly and we could not identify the menstrual period. However, in our study, TT and FAI were less affected by the menstrual cycle and were found to be associated with metal exposure in females. Third, our study did not describe other markers of sex steroid hormone measurement, such as free testosterone (FT) and bioavailable testosterone (BAT), which may provide a more comprehensive endocrine profile. This study, however, suggests that exposure to heavy metals may pose a risk for altered sex hormone levels in adults, particularly in older women. The positive effect of mixed metals on SHBG was primarily attributable to cadmium, while the negative effect of mixed metals on FAI was primarily attributable to lead.
Conclusion
In this population-based cross-sectional study, we found metals linked to changes in human sex hormones, particularly in older females. These findings provide important insights into the potential impact of environmental exposures on hormonal balance and suggest the need for further research to investigate the long-term consequences of these changes.
Data availability
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/.
References
Agusa T, Kunito T, Iwata H, Monirith I, Chamnan C, Tana TS, Subramanian A, Tanabe S (2007) Mercury in hair and blood from residents of Phnom Penh (Cambodia) and possible effect on serum hormone levels. Chemosphere 68:590–596
Ali I, Engstrom A, Vahter M, Skerfving S, Lundh T, Lidfeldt J, Samsioe G, Halldin K, Akesson A (2014) Associations between cadmium exposure and circulating levels of sex hormones in postmenopausal women. Environ Res 134:265–269
Allen NE, Appleby PN, Davey GK, Key TJ (2002) Lifestyle and nutritional determinants of bioavailable androgens and related hormones in British men. Cancer Causes Control 13:353–363
Banos G, Guarner V, Perez-Torres I (2011) Sex steroid hormones, cardiovascular diseases and the metabolic syndrome. Cardiovasc Hematol Agents Med Chem 9:137–146
Bond A, Davis C (1987) Sex hormone binding globulin in clinical perspective. Acta Obstet Gynecol Scand 66:255–262
Cigarran S, Pousa M, Castro MJ, Gonzalez B, Martinez A, Barril G, Aguilera A, Coronel F, Stenvinkel P, Carrero JJ (2013) Endogenous testosterone, muscle strength, and fat-free mass in men with chronic kidney disease. J Ren Nutr 23:e89–e95
Cinar V, Polat Y, Baltaci AK, Mogulkoc R (2011) Effects of magnesium supplementation on testosterone levels of athletes and sedentary subjects at rest and after exhaustion. Biol Trace Elem Res 140:18–23
Clemens S, Ma JF (2016) Toxic Heavy Metal and Metalloid Accumulation in Crop Plants and Foods. Annu Rev Plant Biol 67:489–512
Cunningham GR (2015) Testosterone and metabolic syndrome. Asian J Androl 17:192–196
Dearth RK, Hiney JK, Srivastava V, Burdick SB, Bratton GR, Dees WL (2002) Effects of lead (Pb) exposure during gestation and lactation on female pubertal development in the rat. Reprod Toxicol 16:343–352
Duan W, Xu C, Liu Q, Xu J, Weng Z, Zhang X, Basnet TB, Dahal M, Gu A (2020) Levels of a mixture of heavy metals in blood and urine and all-cause, cardiovascular disease and cancer mortality: A population-based cohort study. Environ Pollut 263:114630
Finkelstein JS, Lee H, Burnett-Bowie SA, Pallais JC, Yu EW, Borges LF, Jones BF, Barry CV, Wulczyn KE, Thomas BJ, Leder BZ (2013) Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med 369:1011–1022
Flores-Ramirez I, Baranda-Avila N, Langley E (2019) Breast Cancer Stem Cells and Sex Steroid Hormones. Curr Stem Cell Res Ther 14:398–404
Gianatti EJ, Grossmann M (2020) Testosterone deficiency in men with Type 2 diabetes: pathophysiology and treatment. Diabet Med 37:174–186
Goldman AL, Bhasin S, Wu FCW, Krishna M, Matsumoto AM, Jasuja R (2017) A Reappraisal of Testosterone's Binding in Circulation: Physiological and Clinical Implications. Endocr Rev 38:302–324
Heck AL, Handa RJ (2019) Sex differences in the hypothalamic-pituitary-adrenal axis' response to stress: an important role for gonadal hormones. Neuropsychopharmacology 44:45–58
Hu P, Su W, Vinturache A, Gu H, Cai C, Lu M, Ding G (2021) Urinary 3-phenoxybenzoic acid (3-PBA) concentration and pulmonary function in children: A National Health and Nutrition Examination Survey (NHANES) 2007-2012 analysis. Environ Pollut 270:116178
Iavicoli I, Fontana L, Bergamaschi A (2009) The effects of metals as endocrine disruptors. J Toxicol Environ Health B Crit Rev 12:206–223
Kelly DM, Jones TH (2013) Testosterone: a metabolic hormone in health and disease. J Endocrinol 217:R25–R45
Kresovich JK, Argos M, Turyk ME (2015) Associations of lead and cadmium with sex hormones in adult males. Environ Res 142:25–33
Kumar A, Banerjee A, Singh D, Thakur G, Kasarpalkar N, Gavali S, Gadkar S, Madan T, Mahale SD, Balasinor NH, Sachdeva G (2018) Estradiol: A Steroid with Multiple Facets. Horm Metab Res 50:359–374
Lewis RC, Meeker JD (2015) Biomarkers of exposure to molybdenum and other metals in relation to testosterone among men from the United States National Health and Nutrition Examination Survey 2011-2012. Fertil Steril 103:172–178
Maggio M, Ceda GP, Lauretani F, Cattabiani C, Avantaggiato E, Morganti S, Ablondi F, Bandinelli S, Dominguez LJ, Barbagallo M, Paolisso G, Semba RD, Ferrucci L (2011) Magnesium and anabolic hormones in older men. Int J Androl 34:e594–e600
Morgentaler A, Traish A, Hackett G, Jones TH, Ramasamy R (2019) Diagnosis and Treatment of Testosterone Deficiency: Updated Recommendations From the Lisbon 2018 International Consultation for Sexual Medicine. Sex Med Rev 7:636–649
Nagata C, Konishi K, Goto Y, Tamura T, Wada K, Hayashi M, Takeda N, Yasuda K (2016) Associations of urinary cadmium with circulating sex hormone levels in pre- and postmenopausal Japanese women. Environ Res 150:82–87
Nasiadek M, Danilewicz M, Sitarek K, Swiatkowska E, Darago A, Stragierowicz J, Kilanowicz A (2018) The effect of repeated cadmium oral exposure on the level of sex hormones, estrous cyclicity, and endometrium morphometry in female rats. Environ Sci Pollut Res Int 25:28025–28038
Pillerova M, Borbelyova V, Hodosy J, Riljak V, Renczes E, Frick KM, Tothova L (2021) On the role of sex steroids in biological functions by classical and non-classical pathways. An update. Front Neuroendocrinol 62:100926
Qiu Z, Chen X, Geng T, Wan Z, Lu Q, Li L, Zhu K, Zhang X, Liu Y, Lin X, Chen L, Shan Z, Liu L, Pan A, Liu G (2022) Associations of Serum Carotenoids With Risk of Cardiovascular Mortality Among Individuals With Type 2 Diabetes: Results From NHANES. Diabetes Care 45:1453–1461
Rami Y, Ebrahimpour K, Maghami M, Shoshtari-Yeganeh B, Kelishadi R (2022) The Association Between Heavy Metals Exposure and Sex Hormones: a Systematic Review on Current Evidence. Biol Trace Elem Res 200:3491–3510
Rehman K, Fatima F, Waheed I, Akash MSH (2018) Prevalence of exposure of heavy metals and their impact on health consequences. J Cell Biochem 119:157–184
Riggs BL (2000) The mechanisms of estrogen regulation of bone resorption. J Clin Invest 106:1203–1204
Rotter I, Kosik-Bogacka DI, Dolegowska B, Safranow K, Kuczynska M, Laszczynska M (2016) Analysis of the relationship between the blood concentration of several metals, macro- and micronutrients and endocrine disorders associated with male aging. Environ Geochem Health 38:749–761
Russell N, Grossmann M (2019) MECHANISMS IN ENDOCRINOLOGY: Estradiol as a male hormone. Eur J Endocrinol 181:R23–R43
Schmidt S (2020) Quantile g-Computation: A New Method for Analyzing Mixtures of Environmental Exposures. Environ Health Perspect 128:104004
Scinicariello F, Buser MC (2016) Serum Testosterone Concentrations and Urinary Bisphenol A, Benzophenone-3, Triclosan, and Paraben Levels in Male and Female Children and Adolescents: NHANES 2011-2012. Environ Health Perspect 124:1898–1904
Selby C (1990) Sex hormone binding globulin: origin, function and clinical significance. Ann Clin Biochem 27(Pt 6):532–541
Simo R, Saez-Lopez C, Barbosa-Desongles A, Hernandez C, Selva DM (2015) Novel insights in SHBG regulation and clinical implications. Trends Endocrinol Metab 26:376–383
Singh R, Gautam N, Mishra A, Gupta R (2011) Heavy metals and living systems: An overview. Indian J Pharm 43:246–253
Srivastava V, Dearth RK, Hiney JK, Ramirez LM, Bratton GR, Dees WL (2004) The effects of low-level Pb on steroidogenic acute regulatory protein (StAR) in the prepubertal rat ovary. Toxicol Sci 77:35–40
Takiguchi M, Yoshihara S (2006) New aspects of cadmium as endocrine disruptor. Environ Sci 13:107–116
Tao C, Li Z, Fan Y, Li X, Qian H, Yu H, Xu Q, Lu C (2021) Independent and combined associations of urinary heavy metals exposure and serum sex hormones among adults in NHANES 2013-2016. Environ Pollut 281:117097
Telisman S, Colak B, Pizent A, Jurasovic J, Cvitkovic P (2007) Reproductive toxicity of low-level lead exposure in men. Environ Res 105:256–266
Wang Y, Aimuzi R, Nian M, Zhang Y, Luo K, Zhang J (2021) Perfluoroalkyl substances and sex hormones in postmenopausal women: NHANES 2013-2016. Environ Int 149:106408
Wei B, O'Connor R, Goniewicz M, Hyland A (2020) Association between Urinary Metabolite Levels of Organophosphorus Flame Retardants and Serum Sex Hormone Levels Measured in a Reference Sample of the US General Population. Expo Health 12:905–916
Acknowledgments
We appreciate the people who contributed to the NHANES data we studied.
Funding
No specific funding was received for this work.
Author information
Authors and Affiliations
Contributions
Qiongshan Liu: Conceptualization, Methodology, Software, Formal analysis, Writing-original draft, Visualization. Shijian Hu: Conceptualization, Methodology, Writing-original draft. Fufang Fan: Writing-review and editing. Zhixiang Zhen: Project administration, Supervision. Xinye Zhou: Conceptualization, Methodology, Supervision. Yuanfeng Zhang: Conceptualization, Methodology, Project administration, Writing-review and editing, Supervision.
Corresponding author
Ethics declarations
Ethics approval and consent to participant
All participants provided written informed consent and study procedures were approved by the National Center for Health Statistics Research Ethics Review Board.
Consent to publish
The manuscript is approved by all authors for publication.
Competing interests
The authors declare no or competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Q., Hu, S., Fan, F. et al. Association of blood metals with serum sex hormones in adults: A cross-sectional study. Environ Sci Pollut Res 30, 69628–69638 (2023). https://doi.org/10.1007/s11356-023-27384-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-27384-5