Abstract
In the present study, distribution characteristics of ten typical phthalic acid esters (PAEs) were investigated in 90 air samples collected from the urban areas in Hanoi, Vietnam from May to August 2022. The total concentrations of PAEs in indoor and ambient air samples were in the range of 320–4770 ng/m3 and 35.9–133 ng/m3, respectively. Total concentrations of PAEs in indoor air were about one order of magnitude higher than those in ambient air. Among PAEs studied, di-(2-ethyl)hexyl phthalate (DEHP) was measured at the highest levels in all air samples, followed by di-n-octyl phthalate (DnOP) and di-n-butyl phthalate (DnBP). The PAEs concentrations in air samples collected from laboratories at nighttime were significantly higher than those during daytime (p < 0.05). Meanwhile, the distributions of PAEs in various micro-environments in the same house are no statistically significant difference. The median exposure doses of PAEs through inhalation for adults and children were 248 and 725 ng/kg-bw/d, respectively. These exposure levels were still lower than the respective reference doses (RfD) proposed by the US EPA for selected compounds such as diethyl phthalate (DEP), DnBP, and DEHP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phthalic acid esters (PAEs) are common plasticizers added to polymeric materials to improve their flexibility and workability (Zhang et al. 2015; Jeddi, et al. 2016; Blessinger et al. 2020; Du et al. 2020). Therefore, PAEs are contained in many commercial products such as building materials, household products, medical items, and personal care products (PCPs) (Guo et al. 2014; Orecchio et al. 2014; Salaudeen et al. 2018; Le et al. 2022). In 2015, the amount of PAEs used globally ranged from 6 to 8 million tons per year, with about 2.2 million tons used in China (Niu et al. 2014; Wang et al. 2015).
Due to PAEs are widely used in large amounts in various commercial products, they have been released into different environmental matrices (soil, sediment, air, dust, water, and food) (Pei et al. 2013; Guo et al. 2014; Wang et al. 2014; Tran et al. 2017; Ouyang et al. 2019; Amorello et al. 2022; Orecchio et al. 2022). A study reported concentrations of PAEs found in several types of soil samples collected in China to be up to tens of mg/g (Lü et al. 2018). The levels of PAEs in indoor dust collected from workplaces and homes in Vietnam were up to 153,000 ng/g and 83,700 ng/g, respectively (Anh et al. 2022). Interestingly, a high level of PAEs was found in bottled and tap water collected from Tehran, Iran (mean: 3.55 µg/L, Abtahi et al. 2019). Recently, the mean total concentrations of 10 PAEs found in water samples collected from Hanoi, Vietnam was 6,400 ng/L (bottled water), 11,200 ng/L (tap water), 51,800 ng/L (lake water), and 121,000 ng/L (wastewater) (Le et al. 2021). In addition, the measured levels in indoor air samples collected from homes in Vietnam ranged from 57.0 to 14,900 ng/g (the gas phase) and from 95.2 to 13,100 ng/g (the particulate phase) (Tran et al. 2017). Even, PAEs metabolites were found in marine organisms (Hu et al. 2020) and tissue samples such as urine, blood, and sweat samples (Genuis et al. 2012; Choi et al. 2014; Liao, et al 2017; Babu-Rajendran et al. 2018).
PAEs were known as one of the organic substances causing endocrine-disrupting chemicals (EDCs) in the human body (EC 2020). The United States Environmental Protection Agency (EPA) classification of di-(2-ethyl)hexyl phthalate (DEHP) as a B2 "probable human" carcinogen (US EPA 1987c; Doull et al. 1999; Chen et al. 2017). Lehraiki et al. (2009) indicated that PAEs can reduce fertility and impair germ cell number in a mouse fetal testis by an Androgen—and Estrogen-independent mechanism. Several studies on PAEs in the human body have shown that PAEs can cause neurotoxicity, affecting fertility, and developmental delay (Zarean et al. 2016; Radke et al. 2020).
There have been several air sampling methods for PAEs analysis using polyurethane foam (PUF) (Tran et al. 2017; Anh et al. 2019; Szewczyńska et al. 2020; 2021), XAD-2 resin adsorbent, Tenax TA tubes (Zhou et al. 2021). In general, these methods required a long sampling time and used a large amount of extraction solvent. Meanwhile, octadecylsilane (C18) was widely used in sampling procedures for the determination of toxic organic compounds, and volatile organic compounds in air samples (US EPA 1999; Vera et al. 2022). Therefore, C18 in glass tubes was selected as a sorbent for sampling air for PAEs analysis in this investigation. The purpose of this study is to: (1) develop the analysis procedure for PAEs in air samples; (2) monitor the distributions of PAEs in the air from various micro-environments in Hanoi, Vietnam; (3) assess the risk of human exposure to PAEs through inhalation according to age groups.
Materials and methods
Sample collection
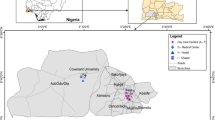
Indoor and ambient air samples were collected in several laboratories (Lab, n = 36), private homes and apartments (H, n = 48), nail and hair salons (SL, n = 6), university campus, and residential campus (Ambient air–AA, n = 12), in the urban areas of Hanoi, Vietnam from May to August 2022 (Table S1, Supplementary data). The sampler was deployed at a height of 1.0 to 1.5 m from backgrounds (i.e., room and ground floors). The area of rooms sampled is larger than 15 m2 and usually has at least 1 person inside. The sampling protocol generally followed those reported in our previous works (Tran and Kannan 2015; Tran et al. 2017), with some modifications (absorbent-C18 was used instead of PUF). In brief, the air samples were collected by using a low-volume air sampler (Buck Libra Plus LP-7 230 V Pump Kit; AP Buck Inc., USA) for 10–12 h at a flow rate of 4 L/min. The air samples were collected by a glass tube (7 cm length × 0.6 cm ID) using 150 mg of octadecylsilane (C18: 17%C, 40–63 µm, 60 Å) (SiliCycle, Quebec City, Canada) as the sorbent (Fig. 1). Before collecting samples, the adsorbent glass tubes were preserved in a desiccator at room temperature for no more than 48 h. After the sample collection, the glass adsorbent tubes were immediately removed from a pump sampler, resealed, and transported to the laboratory. Then, the samples have stored for a maximum of one week at -4 oC until analysis. Additional information about the sampling is provided in Table S1.
Analysis procedure
Before elution, 200 ng (200 µL of 1000 ng/mL solution) of each surrogate standard (Text S1, Supplementary data) was spiked into the samples (glass sorbent tubes). Fifteen mL of n-hexane/DCM (1:1, v/v) were flowed through the glass sorbent tubes by using a vacuum pump (Rocker 300, Rocker Scientific, Taiwan). Then, the eluted solution was concentrated under a gentle stream of nitrogen to 1 mL and transferred into a GC vial for instrumental analysis.
In this study, 10 PAEs (including DMP, DEP, DPP, DnBP, DiBP, DnHP, BzBP, DCHP, DEHP, and DnOP) (Text S1) were quantified by using a gas chromatograph (GC Trace 1310, Thermo Scientific, USA) interfaced with a mass spectrometer (ISQ7000, Thermo Scientific, USA). A fused silica capillary column (TG–5MS, 30 m × 0.25 mm × 0.25 μm; Thermo Scientific, USA) was used for chromatographic separation of analytes. Detailed instrumental parameters for PAEs analysis were reported in previous studies (Tran and Kannan 2015; Tran et al. 2017; Anh et al. 2021) and are provided in Table S2.
QA/QC
Previous studies have reported significant contamination of PAEs from solvents, glassware, and laboratory equipment (Tran et al. 2017; Le et al. 2021; Anh et al. 2019). Therefore, several attempts have been done to reduce the background levels of PAEs in this work. In particular, all glasses were rinsed respectively with double-distilled deionized water, acetone, and n-hexane before baking at 400 °C for 8 h. Then, the glassware was kept in an oven at 100 °C until use. Solvents and chemicals were used directly from the new glass vessels. Plastic items were restricted for use in the laboratory. n-hexane was injected before every sample as a check for background contamination. The calibration curves for individual PAEs were linear over a concentration range of 1–1000 ng/mL (with a regression coefficient R2 > 0.995). The recoveries of the surrogate standards in blank and real samples ranged from 90.0% to 105% (a relative standard deviation: RSD < 15%). The recoveries of the native standards in the blank procedure (matrix spiked) ranged from 81.0% to 110% with the RSD < 15%. The method detection limits (MDLs) of PAEs ranged from 0.08 to 0.30 ng/m3. This study defined them as shown peaks with signal-to-noise (S/N) ratios > 3. Further QA/QC data (e.g. blank levels and MDLs of all target compounds) are detailed in Table S3. For the concentrations below the MDLs (not detected–ND), a value of one-half of the MDLs was assigned in statistical analysis.
Statistical analysis
Statistical analysis was conducted by using Microsoft Excel (Microsoft Office 2016) and Minitab 18® Statistical Software (Minitab Inc.). Pearson correlation analysis and principal component analysis (PCA) were applied to the whole dataset to assess potential relationships and emission sources of PAEs.
Risk assessment
Daily intake doses (ID) were estimated based on concentrations measured in air samples, the mean inhalation rate, and body weights for age groups including adults and children. The intake doses of selected compounds such as DEP, DnBP, and DEHP were compared with respective reference doses (RfD) proposed by the US Environmental Protection Agency (US EPA 1987a, b, c) to estimate hazard quotients (HQ). Equations for ID estimation and exposure parameters were generally adopted from the US EPA (US EPA 2011) and quite similar to those applied in our previous studies (Tran et al. 2017; IARC 2018; Anh et al. 2020). Detailed risk assessment equations and related parameters are tabulated in Table S5.
Results and discussion
PAEs concentrations in air samples
In this investigation, 10 PAEs were found in 90 air samples collected in the Hanoi urban areas including 78 indoor air samples (laboratories, homes, and salons) and 12 ambient air samples (Table 1). DEHP was detected in 100% of all samples, followed by DnBP (90.0%) > DEP (98.0%) > DiBP (96.0%) > DnOP (94.0%) > DMP (93.0%) > DCHP (77.0%)) > DPP (68.0%) > BzBP (64.0%) > DnHP (60.0%). For indoor air samples, the total concentrations of 10 PAEs (Σ10PAE) were in the ranges of 402–1380 ng/m3 (laboratories); 320–3030 ng/m3 (homes), and 2570–4470 ng/m3 (salons). The levels of PAEs in ambient air were significantly lower than those in indoor air (range: 35.9–133 ng/m3). These results reinforce the assumption that the origin of PAEs is from indoor environments (Afshari et al. 2004; Koniecki et al. 2011; Pei et al. 2013; Tran and Kannan 2015). Moreover, environmental factors and photochemical degradation may be responsible for the decrease of PAEs levels in the ambient air (Markiewicz et al. 2017; Chen et al. 2018; Anh et al. 2021a, b; Zhou, et al. 2021).
Among PAEs, DEHP was found at the higher concentration in air samples from various micro-environments: laboratories (mean: 456 ng/m3, range: 217–858 ng/m3), homes (mean: 651 ng/m3, range: 134–1770 ng/m3), salons (mean: 2350 ng/m3, range: 1670–3040 ng/m3), and ambient air (mean: 31.3 ng/m3, range: 15.1–70.6 ng/m3). DnOP was also commonly found in various micro-environments: laboratories (mean: 145 ng/m3), homes (143 ng/m3), salons (668 ng/m3), and ambient air (2.98 ng/m3), followed by DnBP: laboratories (mean: 46.2 ng/m3), homes (156 ng/m3), salons (350 ng/m3), and ambient air (1.95 ng/m3). This is consistent with previous reports indicating that DEHP and DnBP were more commonly used in commercial products (US EPA 1987b; 1987c; Koniecki et al. 2011; Guo et al. 2014). Furthermore, these two compounds were also found at higher levels in various environments (Net et al. 2015; Li et al. 2020; Anh et al. 2021a, b).
The PAEs concentrations in indoor air samples collected from the salons were higher than those from the laboratories and homes (p < 0.05). Meanwhile, Σ10PAE in ambient air samples was the lowest level. Previous studies have reported that PAEs found at high levels in commercial products such as building materials, polyvinyl chloride (PVC) (Afshari et al. 2004), and vinyl flooring (Liang and Xu 2015), which are all used in homes, salons, and laboratories. Moreover, most consumer products used in households and salons contain large amounts of PAEs such as plastic materials (Orecchio et al. 2022), personal care products (EC 2009; Guo et al. 2014; Pagoni et al. 2022), and cosmetics (Koniecki et al. 2011). Such commercial products are the main sources of PAEs released into the indoor air, whereas the distribution of PAEs in the ambient air is attributed to diffusion through the indoor environment (Ouyang et al. 2019; Szewczyńska et al. 2020; 2021; Anh et al. 2021a, b).
The distribution of PAEs in indoor air from various categories (bedrooms, n = 22; living rooms, n = 7; and kitchen, n = 7) (p > 0.05) in the same house was compared (Table 2). The highest levels of PAEs were found in kitchens (mean: 1230 ng/m3, range: 824–2330 ng/m3), followed by bedrooms (mean = 1200 ng/m3, range = 432–3030 ng/m3), and living room (mean = 854 ng/m3, range = 320–1770 ng/m3). The most predominant compounds were DEHP (55.0–59.0%), DPP (13.0–18.0%), and DnOP (6.00–16.0%) (Fig. 2). The results showed that there was no statistically significant difference in PAEs concentrations between the pairs of micro-environments. Very few studies reported the distribution of PAEs in indoor air from different categories with documented data. The concentrations in the air from bedrooms and living rooms from this study were much lower than those from a previous report in Hangzhou, China (mean: 9740 ng/m3 and 17,400 ng/m3, respectively) (Pei et al. 2013). To our knowledge, the levels of PAEs in categories in the same house may be depended on various factors to which this study is limited such as the number of items in each room capable of spreading PAEs, connections between rooms, ventilation, temperature, etc. Further studies need to be done to elucidate this issue.
In comparison, the PAEs levels found in air samples from Vietnam are similar to the air samples in the center of Paris, France (Σ7PAE: 207 ± 150 to 918 ± 443 ng/m3) (Moreau-Guigon et al. 2016), in New York, USA (Σ9PAE; mean: 390 ng/m3; range: 53.6–4850 ng/m3) (Tran and Kannan 2015), and in Poland (Σ3PAE: mean = 2860 ng/m3, median = 3030 ng/m3) (Szewczyńska et al. 2020). Besides, the results in our study are higher than the air samples collected in the center of Beijing, China with air samples in dormitory (Σ6PAE: mean = 468 ng/m3; SD = 353 ng/m3), office (Σ6PAE: mean = 280 ng/m3; SD = 264 ng/m3), and ambient air (Σ6PAE: mean = 125 ng/m3; SD = 99.8 ng/m3) (Chen et al. 2018). Meanwhile, Σ10PAE levels in air from this study are much lower than in similar studies in China such as newly decorated indoor air samples in Hangzhou (Pei et al. 2013) and office areas in Hangzhou, China (Σ15PAE: 3620–25,500 ng/m3; Ouyang et al. 2019). These results show that the distribution of PAEs in the air is very different by geographical area (Fig. S2). This can be explained by various habits in the use of commercial products containing PAEs. Environmental factors can also influence the release of PAEs into the environment and need to be further investigated.
Distribution of PAEs in air samples between daytime and night
In this study, a total of 36 samples at 2 different times in each of 6 laboratories (Table S4) were collected from 7–8 am to 6–7 pm (during laboratory staff hours/office hours) and 7–8 pm to 6–7 am (outside office hours). Four PAEs including DIBP, DnBP, DEHP, and DnOP were detected with a frequency of 100% in all samples. DEHP was found at the highest levels (mean = 456 ng/m3, median = 430 ng/m3), followed by DnOP (mean = 145 ng/m3, median = 120 ng/m3), and DnBP (mean = 46.2 ng/m3, median = 39.5 ng/m3). Whereas, other PAEs were measured at the low concentrations: DnHP (mean/median: 4.41/2.09 ng/m3); BzBP (mean/median: 3.48/1.02 ng/m3); and DPP (mean/median: 2.91/1.90 ng/m3) (Table 3).
Overall, the total concentrations of PAEs in the night air samples were significantly higher than in the daytime air samples (p < 0.05) (Fig. 3). These results suggest that several factors may affect the distribution of PAEs in indoor air such as temperature, pressure, wind speed, or weather but according to our observations in the night conditions, the laboratories are always closed so the circulation of air to the outside is lower than during the daytime. An earlier study revealed that the use of air purifiers with windows kept closed (natural ventilation) can lead to a significant reduction in terms of indoor-to-outdoor concentration ratios and a decrease in organic gaseous pollutants (Pacitto et al. 2019). The other study concluded that temperature, humidity, air ventilation, and seasonality all affected the concentrations of volatile organic compounds (Huo et al. 2020).
Potential sources of PAEs in air
The principle component analysis (PCA) was used to find the potential sources of PAEs in the air samples (Table S6 and Fig. S1, Supplementary data). From the analysis of the correlation matrix (Table S6), only PC1 to PC5, the data sets have achieved over 80% of the cumulative variance. PC1, PC2, PC3, PC4, and PC5 accounted for 35.0%, 15.0%, 12.0%, 9.50%, and 9.00% of the cumulative variance, respectively. Particularly, PCs with the highest percentage of cumulative variance tended to correlate with high-molecular-weight PAEs including PC1 (DEHP, DnOP), PC2 (DnHP, DCHP), and PC3 (BzBP, DEHP). BzBP has been used as a plasticizer in various indoor and outdoor items such as vinyl tiles, vinyl gloves, adhesives, caulks, food conveyor belts, carpet tiles, artificial leather, tarps, automotive trim, and traffic cones (Calvo-Flores et al. 2018). DEHP and DnOP are mainly used as plasticizers in household plastic products and cosmetics (Rudel and Perovich 2009; Net et al. 2015). Additionally, DnHP and DCHP are widely used as a binder in ink or coating products (NTP-CERHR 2003; US CPCS 2011). In general, these data suggest that high-molecular-weight PAEs were the main pollutant components and they had a strong impact on the accumulation of PAEs in the air samples.
To assess the origin of each pair of individual PAEs, the Pearson correlation matrix was used for our data sets in this study (Table S7). Some pairs of PAEs have moderate correlation (with p < 0.05) such as DMP versus BzBP (r = 0.569); DnBP versus DCHP (r = 0.507); DiBP versus DEHP (r = 0.544). Especially, two pairs of high-molecular-weight PAEs have a high correlation: BzBP versus DnOP (r = 0.644; p < 0.05) and DEHP versus DnOP (r = 0.672; p < 0.05). However, some pairs of individual PAEs have a lower correlation and the rest have no or very weak correlation relationships with the others. Some high-molecular-weight PAEs such as BzBP, DEHP, DnOP were used a lot as a plasticizer in common indoor or outdoor products such as vinyl tiles, vinyl gloves, adhesives, caulks, food conveyor belts, carpet tiles, artificial leather, tarps, automotive trim, and traffic cones (Calvo-Flores et al. 2018; Rudel and Perovich 2009; Net et al. 2015). Conclusively, these results propose that high-molecular-weight PAEs in air samples collected in Hanoi urban areas could have the same origin because of their strong correlations.
Risk assessment of human exposure to PAEs through inhalation
In this study, daily intake doses (ID) of individual PAEs through inhalation and hazard quotients (HQ) for non-cancer endpoints of DEP, DnBP, and DEHP were calculated and the results are shown in Table 4 and Fig. 3. ID values ranged from a few ng/kg-bw/d to several hundred ng/kg-bw/d. Overall, ID values in this study were much lower than the reference dose of some PAEs such as DEP (800,000 ng/kg-bw/d; US EPA 1987a), DnBP (100,000 ng/kg-bw/d; US EPA 1987b), and DEHP (20,000 ng/kg-bw/d; US EPA, 1987c). This is more evident when evaluating Hazard Quotients values (HQ), the mean of HQ values (including DEP, DnBP, and DEHP) were in the range of 10–5–10–3 for children and adults (Fig. 4).
In comparison, the ID value of PAEs through inhalation for children was 338 ng/kg-bw/d (Anh et al. 2020) and was lower than in this study (724 ng/kg-bw/d). The mean/median exposure doses of Σ10PAE calculated for adults and children through the consumption of bottled drinking water were 254/231 and 256/233 ng/kg-bw/d, respectively (Le et al. 2021). Furthermore, the estimated exposure doses to PAEs through dust ingestion (based on the median concentrations) were in the ranges of 19.4 ng/kg-bw/d (for adults) to 90.4 ng/kg-bw/d (for toddlers) (Tran et al. 2016). In a recent study on PAEs in house and workplace dust samples, the ID of DEHP (0.29–44.0 ng/kg-bw/d) were about one order of magnitude higher than those estimated for DnBP, DiBP, and BzBP (0.013–18.0 ng/kg-bw/d) (Anh et al. 2021a, b). Overall, the ID value in this study is assessed as average because the contributions of many other sources have not been taken into calculation.
Conclusions
In this work, 10 PAEs were investigated in indoor and outdoor air samples collected from Hanoi, Vietnam. The PAEs patterns in the air samples were dominated by DEHP, followed by DnOP, DiBP, and DEP. The high concentration correlations existed between some PAEs pairs such as DMP/BzBP, DnBP/DCHP, DiBP/DEHP, and two pairs of high-molecular-weight PAEs: BzBP/DnOP, DEHP/DnOP. The concentrations in air samples from laboratories at the night were significantly higher than in the daytime. Human exposure doses to PAEs via inhalation were higher than other pathways such as dust ingestion and drinking water but did not exhibit serious health risks. Overall, the results of this study add baseline data needed for nationwide monitoring surveys of PAEs and may help the authority in terms of issuing the policy related to these chemicals in the future.
Data availability
Not applicable.
References
Abtahi M, Dobaradaranc S, Torabbeigi M, Jorfig M, Gholamniai R, Koolivand A, Darabi H, Kavousi A, Saeedi R (2019) Health risk of PAE in water environment: Occurrence in water resources, bottled water, and tap water, and burden of disease from exposure through drinking water in Tehran. Iran Environ Res 173:469–479. https://doi.org/10.1016/j.envres.2019.03.071
Afshari A, Gunnarsen L, Clausen PA, Hansen V (2004) Emission of phthalates from PVC and other materials. Indoor Air 14:120–128. https://doi.org/10.1046/j.1600-0668.2003.00220.x
Amorello D, Indelicato R, Barreca S, Orecchio S, Orecchio S (2022) Analytical method for quantification of several phthalate acid esters by gas chromatograpgy-mass spectrometry in coffee brew samples. ChemitryOpen. https://doi.org/10.1002/open.202200082
Anh QH, Le TM, Nguyen NMH, Le HQ, Vu ND, Chu NC, Dang MGH, Minh TB, Takahashi S, Tran TM (2022) Phthalic acid esters (PAE) in workplace and house dust from Vietnam: concentrations, profiles, emission sources, and exposure risk. Environ Sci Pollut Res 10:14046–14057. https://doi.org/10.1007/s11356-021-16851-6
Anh QH, Nguyen NMH, Do QT, Tran QK, Minh BT, Tran MT (2021) Air pollution caused by PAE and cyclic siloxanes in Hanoi, Vietnam: Levels, distribution characteristics, and implications for inhalation exposure. Sci Total Environ 760:143380. https://doi.org/10.1016/j.scitotenv.2020.143380
Anh QH, Tomioka K, Tue NM, Tuyen LH, Chi NK, Minh TB, Viet PH, Takahashi S (2019) A preliminary investigation of 942 organic micro-pollutants in the atmosphere in waste processing and urban areas, northern Vietnam: levels, potential sources, and risk assessment. Ecotoxicol Environ Saf 167:354–364. https://doi.org/10.1016/j.ecoenv.2018.10.026
Babu-Rajendran R, Preethi G, Poopal RK, Nikhil NP, Vimalkumar K, Subramanian A, Krishna-Kumar S (2018) GC–MS determination of phthalate esters in human urine: A potential biomarker for phthalate bio-monitoring. J Chromatogr B 1079:15–24. https://doi.org/10.1016/j.jchromb.2018.01.039
Blessinger TD, Euling SY, Wang L, Hogan KA, Cai C, Klinefelter G, Sailenfait AM (2020) Ordinal dose-response modeling approach for the phthalate syndrome. Environ Int 134:105287. https://doi.org/10.1016/j.envint.2019.105287
Calvo-Flores FG, Isac-Garcia J, Dobado JA (2018) Industrial Chemicals as Emerginh Pollutant. In: Calvo-Flores, FG, Isac-Garcia J, Dobado JA (Eds.) Emerging Pollutants: Origin, Structure, and Properties. Wiley-VCH, Weinheim, 265–340. https://doi.org/10.1002/9783527691203.ch9.
Chen Q, Yang H, Zhou N, Sun L, Bao H, Cao J et al (2017) Phthalate exposure, even below US EPA reference doses, was associated with semen quality and reproductive hormones: Prospective MARHCS study in general population. Environ Int 104:58–68. https://doi.org/10.1016/j.envint.2017.04.005
Chen Y, Li D, Li X, Zhu T (2018) PM2.5 -bound PAE in indoor and outdoor air in Beijing: Seasonal distributions and human exposure via inhalation. Environ Pollut 241:369–377. https://doi.org/10.1016/j.envpol.2018.05.081
Choi J, Eom J, Kim J, Lee S, Kim Y (2014) Association between some endocrine-disrupting chemicals and childhood obesity in biological samples of young girls: A cross-sectional study. Environ Toxicol Pharmacol 38:51–57. https://doi.org/10.1016/j.etap.2014.04.004
Doull J, Cattley R, Elcombe C, Lake B, Swenberg J, Wilkinson C, Williams G, Gemert MV (1999) A cancer risk assessment of Di(2-ethylhexyl) PAE: Application of the new US EPA risk assessment guidelines. Regul Toxicol Pharmacol 29:327–357. https://doi.org/10.1006/rtph.1999.1296
Du PP, Huang YH, Lü H, Xiang L, Li YW, Li H, Mo CH, Cai QY, Li QX (2020) Rice root exudates enhance desorption and bioavailability of phthalic acid esters (PAE) in soil associating with cultivar variation in PAE accumulation. Environ Res 186:109611. https://doi.org/10.1016/j.envres.2020.109611
EC [Health Canada] (2009) Cosmetics and Personal Care. Health Canada (EC), Consumer Product Safety. Available at https://www.canada.ca/en/health-canada/services/cosmetics. Accessed 20 Aug 2022
EC (2020) Chemicals strategy for sustainability towards a toxic-free environment. Available at https://ec.europa.eu/environment/pdf/chemicals/2020/10/SWD_on_Endocrines_disruptors.pdf. Accessed 17 Jan 2023.
Genuis SJ, Beesoon S, Lobo RA, Birkholz D (2012) Human elimination of phthalate compounds: blood, urine, and sweat (BUS) study. Sci World J 615086. https://doi.org/10.1100/2012/615068
Guo Y, Wang L, Kannan K (2014) Phthalates and parabens in personal care products from China: concentrations and human exposure. Arch Environ Contam Toxicol 66:113–119. https://doi.org/10.1007/s00244-013-9937-x
Hu H, Mao L, Fang S, Xie J, Zhao M, Jin H (2020) Occurrence of phthalic acid esters in marine organisms from Hangzhou Bay, China: Implications for human exposure. Sci Total Environ 721:137605. https://doi.org/10.1016/j.scitotenv.2020.137605
Huo CY, Liu LY, Hung H, Sun Y, Guo JQ, Wu YK, Sverko E, Li WL (2020) Accumulations and equilibrium conditions of organophosphate esters (OPEs) in the indoor window film and the estimation of concentrations in air. Sci Total Environ 848:157724. https://doi.org/10.1016/j.scitotenv.2022.157724
IARC (International Agency for Research on Cancer) (2018) Agents Classified by the IARC Monographs, Volumes 1–123. https://monographs.iarc.who.int/wp-content/uploads/2018/09/List_of_Classifications.pdf. Accessed 20 Aug 2022
Jeddi MZ, Janani L, Memari AH, Akhondzadeh S, Yunesian M (2016) The role of phthalate esters in autism development: A systematic review. Environ Res 151:493–504. https://doi.org/10.1016/j.envres.2016.08.021
Koniecki D, Wang R, Moody RP, Zhu J (2011) Phthalates in cosmetic and personal care products: Concentrations and possible dermal exposure. Environ Res 111:329–336. https://doi.org/10.1016/j.envres.2011.01.013
Le MT, Nguyen NMH, Nguyen VK, Nguyen VA, Vu DN, Yen NTH, Anh QH, Minh TB, Kannan K, Tran MT (2021) Profiles of phthalic acid esters (PAE) in bottled water, tap water, lake water, and wastewater samples collected from Hanoi Vietnam. Sci Total Environ 788:147831. https://doi.org/10.1016/j.scitotenv.2021.147831
Le MT, Pham TLC, Nguyen NMH, Duong TT, Le TPQ, Nguyen DT, Vu DN, Minh TB, Tran MT (2022) Distribution and ecological risk assessment of phthalic acid esters in surface sediments of three rivers in Northern Vietnam. Environ Res 209:112843. https://doi.org/10.1016/j.envres.2022.112843
Lehraiki A, Racine C, Krust A, Habert R, Levacher C (2009) Phthalates impair germ cell number in the mouse fetal testis by an Androgen- and Estrogen- independent mechanism. Toxicol Sci 111:372–382. https://doi.org/10.1093/toxsci/kfp153
Li B, Zhao ZB, Thapa S, Sun SJ, Ma LX, Geng JL, Wang K, Qi H (2020) Occurrence, distribution and human exposure of phthalic esters in road dust samples across China. Environ Res 191:110222. https://doi.org/10.1016/j.envres.2020.110222
Liang YR, Xu Y (2015) The influence of surface sorption and air flow rate on phthalate emissions from vinyl flooring: measurement and modeling. Atmos Environ 103:147–155. https://doi.org/10.1016/j.atmosenv.2014.12.029
Liao C, Liu W, Zhang J, Shi W, Wang X, Cai Z, Zou Z, Lu R, Sun C, Wang H, Huang C, Zhao Z (2017) Urine metabolites of phthalate esters in 434 Shanghai children and their associations with ventilation habits. Procedia Eng 205:1146–1151. https://doi.org/10.1016/j.proeng.2017.10.183
Lü H, Mo CH, Zhao HM, Xiang L, Katsoyiannis A, Li YW, Cai QY, Wong MH (2018) Soil contamination and sources of phthalates and its health risk in China: A review. Environ Res 164:417–429. https://doi.org/10.1016/j.envres.2018.03.013
Markiewicz A, Bjorklund K, Eriksson E, Kalmykova Y, Stromvall AM, Siopi A (2017) Emissions of organic pollutants from traffic and roads: priority pollutants selection and substance flow analysis. Sci Total Environ 580:1162–1174. https://doi.org/10.1016/j.scitotenv.2016.12.074
Moreau-Guigon E, Alliot F, Gasperi J, Blanchard M, Teil MJ, Mandin C, Chevreuil M (2016) Seasonal fate and gas/particle partitioning of semi-volatile organic compounds in indoor and outdoor air. Atmos Environ 147:423–433. https://doi.org/10.1016/j.atmosenv.2016.10.006
National Toxicology Program - US Department of Health and Human Services - Center for the evaluation of risks to human reproduction (NTP-CERHR) (2003) Monograph on the potential human reproductive and developmental effects of Di-n -hexyl PAE (DnHP). NIH Publication, No, pp 03–4489
Net S, Sempere R, Delmont A, Paluselli A, Ouddane B (2015) Occurrence, fate, behavior and ecotoxicological state of PAE in different environmental matrices. Environ Sci Technol 49:4019–4035. https://doi.org/10.1021/es505233b
Niu L, Xu Y, Xu C, Yun L, Liu W (2014) Status of phthalate esters contamination in agricultural soils across China and associated health risks. Environ Pollut 195:16–23. https://doi.org/10.1016/j.envpol.2014.08.014
Orecchio S, Amorello D, Indelicato R, Barreca S, Orrechio S (2022) A short review of simple analytical methods for the evaluation of PAHs and PAEs as indoor pollutants in house dust samples. Atmosphere 13:1799. https://doi.org/10.3390/atmos13111799
Orecchio S, Indelicato R, Barreca S (2014) Determination of selected phthalates by gas chromatography-mass spectrometry in mural paintings from Palermo (Italy). Microchem J 114:187–191. https://doi.org/10.1016/j.microc.2013.11.015
National Toxicology Program - US Department of Health and Human Services - Center for the evaluation of risks to human reproduction (NTP-CERHR) (2003) Monograph on the potential human reproductive and developmental effects of Di-n -hexyl PAE (DnHP). NIH Publication, No, pp 03–4489
Pacitto A, Amoto F, Moreno T, Pandolfi M, Fonseca M, Mazaheri M, Stabile L, Buananno G, Querol X (2019) Effect of ventilation strategies and air purifiers on the children’s exposure to airborne particles and gaseous pollutants in school gyms. Sci Total Environ 712:135673. https://doi.org/10.1016/j.scitotenv.2019.135673
Pagoni A, Arvaniti OS, Klantzi OL (2022) Exposure to phthalates from personal care products: Urinary levels and predictors of exposure. Environ. Res. 212(Part A):113194. https://doi.org/10.1016/j.envres.2022.113194
Pei XQ, Song M, Guo M, Mo FF, Shen XY (2013) Concentration and risk assessment of PAE present in indoor air from newly decorated apartments. Atmos Environ 68:17–23. https://doi.org/10.1016/j.atmosenv.2012.11.039
Radke EG, Braun JM, Nachman RM, Cooper GS (2020) Phthalate exposure and neurodevelopment: a systematic review and meta-analysis of human epidemiological evidence. Environ Int 137:105408. https://doi.org/10.1016/j.envint.2019.105408
Rudel RA, Perovich LJ (2009) Endocrine disrupting chemicals in indoor and outdoor air. Atmos Environ 43:170–181. https://doi.org/10.1016/j.atmosenv.2008.09.025
Salaudeen T, Okoh O, Agunbiade F, Okoh A (2018) Fate and impact of PAE in activated sludge treated municipal wastewater on the water bodies in the Eastern Cape, South Africa. Chemosphere 203:336–344. https://doi.org/10.1016/j.chemosphere.2018.03.176
Szewczyńska M, Dobrzyńska E, Pośniak M (2021) Determination of phthalates in particulate matter and gaseous phase emitted in indoor air of offices. Environ Sci Pollut Res 28:59319–59327. https://doi.org/10.1007/s11356-020-10195-3
Szewczyńska M, Pośniak M, Dobrzyńska E (2020) Determination of phthalates in particulate matter and gaseous phase emitted into the air of the working environment. Int J Environ Sci Technol 17:175–186. https://doi.org/10.1007/s13762-019-02435-y
Tran TM, Kannan K (2015) Occurrence of phthalates diesters in particulate and vapor phases in indoor air and implications for human exposure in Albany, New York USA. Arch Environ Contam Toxicol 68:489–499. https://doi.org/10.1007/s00244-015-0140-0
Tran TM, Le HT, Minh TB, Kannan K (2017) Occurrence of phthalates diesters in indoor air from several Northern cities in Vietnam, and its implication for human exposure. Sci Total Environ 601–602:1695–1701. https://doi.org/10.1016/j.scitotenv.2017.06.016
Tran TM, Minh TB, Kumosani TA, Kannan K (2016) Occurrence of phthalate diesters (phthalates), p-hydroxybenzoic acid esters (parabens), bisphenol A diglycidyl ether (BADGE) and their derivatives in indoor dust from Vietnam: Implications for exposure. Chemosphere 144:1553–1559. https://doi.org/10.1016/j.chemosphere.2015.10.028
US CPSC (US Consumer Product Safety Commission) (2011) Toxicity Review for Dicyclohexyl PAE (DCHP, CASRN 84–61–7). https://www.cpsc.gov/s3fs-public/ToxicityReviewOfDCHP.pdf. Accessed 20 Aug 2022
US EPA (US Environmental Protection Agency) (1987a) Diethyl PAE; CASRN 84–66–2. https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0226_summary.pdf#nameddest=rfd. Accessed 20 Aug 2022
US EPA (1987b) Dibutyl PAE; CASRN 84–74–2. https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0038_summary.pdf. Accessed 20 Aug 2022
US EPA (1987c) Di(2-ethylhexyl)PAE (DEHP); CASRN 117–81–7. https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0014_summary.pdf#nameddest=rfd. Accessed 20 Aug 2022
US EPA Compendium Method TO–11A (1999) https://www.epa.gov/sites/default/files/2019-11/documents/to-11ar.pdf. Accessed 20 Aug 2022
US EPA (2011) Exposure Factors Handbook 2011 Edition (Final Report). https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=236252. Accessed 20 Aug 2022
Vera T, Villanueva F, Wimmerová L, Tolis ET (2022) An overview of methodologies for the determination of volatile organic compounds in indoor air. Appl Spectrosc Rev 57:625–674. https://doi.org/10.1080/05704928.2022.2085735
Wang J, Chen G, Christie P, Zhang M, Luo Y, Teng Y (2015) Occurrence and risk assessment of phthalate esters (PAE) in vegetables and soils of suburban plastic film greenhouses. Sci Total Environ 523:129–137. https://doi.org/10.1016/j.scitotenv.2015.02.101
Wang X, Tao W, Xu Y, Feng J, Wang F (2014) Indoor phthalate concentration and exposure in residential and office buildings in Xi’an, China. Atmos Environ 87:146–152. https://doi.org/10.1016/j.atmosenv.2014.01.018
Zarean M, Keikha M, Poursafa P, Khalighinejad P, Amin M, Kelishadi R (2016) A systematic review on the adverse health effects of di-2-ethylhexyl phthalate. Environ Sci Pollut Res 23:24642–24693. https://doi.org/10.1007/s11356-016-7648-3
Zhang Y, Wang P, Wang L, Sun G, Zhao J, Zhang H, Du N (2015) The influence of facility agriculture production on phthalate esters distribution in black soils of northeast China. Sci Total Environ 506–507:118–125. https://doi.org/10.1016/j.scitotenv.2014.10.075
Zhou X, Lian J, Cheng Y, Wang X (2021) The gas/particle partitioning behavior of phthalate esters in indoor environment: Effects of temperature and humidity. Environ. Res. 194:110681. https://doi.org/10.1016/j.envres.2020.110681
Acknowledgements
We thank Nhai Thanh Thi Vu and Oanh Kim Thi Nguyen for their help with sampling.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Anh Viet Nguyen (Conceptualization, Methodology, Formal analysis, Writing–Original draft preparation); daicavietanh98@gmail.com.
Tu Van Vu (Conceptualization, Methodology, Formal analysis); vvtuiet@gmail.com.
Chi Linh Thi Pham (Methodology, Formal analysis); phamthilinhchi_t64@hus.edu.vn.
Viet Ngoc Nguyen (Conceptualization, Resources); ngocviet.hus@gmail.com.
Nguyen Thuy Ta (Methodology, Formal analysis); ptud1976@gmail.com.
Anh Quoc Hoang (Conceptualization, Writing–Review and editing); hoangquocanh1990@gmail.com.
Tu Binh Minh (Conceptualization, Writing–Review and editing); tubinhminh@gmail.com.
Tri Manh Tran (Conceptualization, Methodology, Resources, Supervision, Writing–Review and editing, Project investigator); manhtri0908@gmail.com.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Before entering any homes, salons, or laboratories, we have to receive permission from the owner.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Constantini Samara
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nguyen, A.V., Van Vu, T., Pham, C.L.T. et al. Widespread distribution of phthalic acid esters in indoor and ambient air samples collected from Hanoi, Vietnam. Environ Sci Pollut Res 30, 63175–63184 (2023). https://doi.org/10.1007/s11356-023-26558-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26558-5