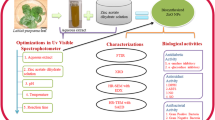
Abstract
During the present century, plant-based zinc oxide nanoparticles (ZnO-NPs) are exploited extensively for their vast biological properties due to their unique characteristic features and eco-friendly nature. Diabetes is one of the fast-growing human diseases/abnormalities worldwide, and the need for new/ novel antiglycation products is the need of the hour. The study deals with the phyto-fabrication of ZnO-NPs from Boerhaavia erecta, a medicinally important plant, and to evaluate their antioxidant and antiglycation ability in vitro. UV-visible spectroscopy (UV-Vis), X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), and energy dispersive spectroscopy (EDS) were used to characterize the phyto-fabricated ZnO-NPs. The characterization of nanoparticles revealed that the particles showed an absorption peak at 362 nm and band gap energy of 3.2 eV, approximately 20.55 nm in size, with a ZnO elemental purity of 96.61%. The synthesized particles were found agglomerated when observed under SEM, and the FT-IR studies proved that the phyto-constituents of the extract involved during the different stages (reduction, capping, and stabilization) of nanoparticles synthesis. The antioxidant and metal chelating activities confirmed that ZnO-NPs could inhibit the free radicals generated, which was dose-dependent with an IC50 value between 1.81 and 1.94 mg mL–1, respectively. In addition, the phyto-fabricated nanoparticles blocked the formation of advanced glycation end products (AGEs) as noticed through inhibition of Amadori products, trapping of reactive dicarbonyl intermediate and breaking the cross-link of glycated protein. It was also noted that the phyto-fabricated ZnO-NPs significantly prevented the damage of red blood corpuscles (RBCs) induced by MGO. The present study’s findings will provide an experimental basis for exploring ZnO-NPs in diabetes-related complications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The field of nanotechnology deals with the manipulation, production, and use of materials of ≥ 100 nm, which are synthesized either by top-down or bottom-up method irrespective of their synthesis mode (physical, chemical, or biological). These synthesized particles are grouped into organic or inorganic nanoparticles based on their nature of synthesis (Sharma et al. 2019). Nanoparticles have expressed significant advances in the present century owing to their wide range of applications in biomedical, sensors, antimicrobial, catalyst, and electronics, including agriculture (Basnet et al. 2018; Agarwal and Shanmugam 2020; Murali et al. 2021a). Both metal and metal oxide nanoparticles have been synthesized and employed in many industrial applications. However, zinc oxide nanoparticles have drawn the most attention because of their unique and superior biological properties, highlighting their use in the biomedical field. Zinc oxide is one of five zinc compounds that have been designated as “generally recognized as safe (GRAS)” materials by the Food and Drug Administration (FDA), USA (Gunalan et al. 2012; Anandan et al. 2019).
Among the different mode of synthesis, the biological route of ZnO nanoparticles synthesis by using plant extracts has been the study of interest as these are known to be highly effective in biomedical applications compared to other methods apart from reducing the risk of toxicity to the eco-system (Murali et al. 2021b). It is important to use plant resources for the synthesis of nanoparticles because they are known to possess various therapeutic compounds (such as alkaloids, terpenoids, phenols, and tannins) which have been exploited since ancient times as traditional medicine apart from the present-day application in industrial, pharmaceutical, and agricultural fields. During nanoparticle synthesis (zinc oxide), the metabolites of the plant act as reducing, capping, or stabilizing agents; as a result, biological qualities are better than those created through physical or chemical means (Stan et al. 2016; Kumar et al. 2020).
Glycation in humans has both physiological and pathophysiological significance that leads to diabetes condition and is one of the fast-growing diseases throughout the world, with an estimated number of victims to be raised to 642 million by 2040 (Ahmed 2005; International Diabetes Federation 2015). The process of sugar (carbohydrate) being covalently attached to a protein or lipid molecule without an enzyme is known as glycation. The glycation reaction is a slow process with the initial formation of Schiff’s base followed by dehydration, fragmentation, oxidative modification, etc., resulting in the formation of a mixed product called advanced glycation end products (AGEs) (Ahmed 2005). It has been well documented that the accumulation of these AGEs will result in secondary complications in the human body that result in oxidative stress, inflammation, etc., leading to neurodegenerative disorders (Singh et al. 2014; Lee et al. 2022). Numerous studies with various model systems have investigated the antiglycation effect of various chemical and natural antiglycation (Elosta et al. 2012). Various metal nanoparticles or their transformed forms (hybrids, composites, conjugates) are being explored as therapeutics for glycation-related disorders (Patil et al. 2022), but the gap in the effective management of AGEs is warranted. Recent studies have shown that the inhibition against AGE formation has led to the development of various AGEs inhibitors and AGE cross-link breakers from synthetic compounds and natural products. However, synthetic AGE inhibitors, along with their pharmacological efficiency, have adverse effects, expensive and are less available (Bolton et al. 2004; Anandan and Urooj 2019; Lee et al. 2022).
It has been reported that NPs improved cell survival, inhibited the glycation and consequent fibrillation of particular proteins, and drastically reduced the total production of reactive oxygen species (ROS). In addition, NPs have proven to have strong antiglycation, ROS scavenging capacity, blood-brain barrier permeability, and the capacity to inhibit protein aggregation and secondary structural modifications (Ashraf et al. 2016; Anandan et al. 2019). However, there is no clinical evidence that glycation targeting utilizing NP-based drug delivery systems might be employed as a therapy for T2DM and lower the risk of associated neurodegenerative problems; therefore, the clinical usefulness of these NPs is yet unknown (Patil et al. 2022). A proper investigation of NPs with antiglycation capabilities could be a very efficient way to treat AGEs-related disease and its disorder. The recent rise in the application of nanoparticles in medicine and their effectiveness as antiglycation agents has resulted in the exploration of these NPs (both chemical and biological) with various manipulations in the synthesis process. Due to the scope of these nanoparticles, the study was designed to phyto-fabricate ZnO-NPs from the aqueous leaf extract of Boerhaavia erecta L. (based on its medicinal importance and antidiabetic properties) (Surange and Pendse 1972; Nisha et al. 2018) and to assess its significance in preventing the formation of AGEs and its byproducts in vitro.
Materials and methods
Collection of plant material and phyto-fabrication of ZnO-NPs
The phyto-fabrication of ZnO-NPs was achieved using Boerhaavia erecta L. leaves (healthy) gathered from Manasagangotri, Mysuru, Karnataka, India (Murali et al. 2017) with minor changes. The collected fresh leaf material (25 g) was extracted with Milli-Q water [1:10 (w/v)] using a hand blender and subjected to filtration using Whatman No.1 filter paper. The aqueous leaf extract (ALE) (50 mL) was heated to ~ 80 °C over a magnetic stirrer, and upon reaching the said temperature, approximately 5 g of zinc nitrate hexahydrate was added with constant stirring and left until the solution became a paste/slurry. The resultant extract was moved to a silica crucible and calcinated at 300 °C for 2 h. The resulting powder (ZnO-NPs) was stored until further studies.
Physicochemical characterization of phyto-fabricated ZnO-NPs
By measuring the optical density between 200 and 800 nm and using Milli-Q water as a blank, the bio-reduction of ZnO was determined using a UV-Vis Spectrophotometer (Beckman Coulter-DU739, Germany) and band gap energy using Tauc’s plot equation. The X-Ray diffraction (XRD) study was performed with Miniflex II X-ray powder diffractometer using Cu k radiation as the energy source (recorded 20–80° at 2θ angles). The ZnO-NPs along with ALE were evaluated for their binding capacities between 4000 and 400 cm–1 using Perkin Elmer Spectrum 1000. Using a HITACHI S-3400N (Japan) scanning electron microscope (EEM), the morphology of ZnO-NPs was captured. An energy dispersive spectroscopy (EDS) of the particles was studied to determine the elemental properties of the ZnO-NPs using a HITACHI (Noran Equipment 7, USA) machine connected to an SEM.
Antioxidant properties of phyto-fabricated ZnO-NPs
Using the DPPH method that Kumar et al. (2020) developed, the free radical scavenging activity of phyto-fabricated ZnO-NPs was evaluated. The DPPH solution (0.1 mM) containing various doses of ALE and ZnO-NPs (0.25, 0.5, 1, 1.5, and 2 mg) (sonicated) was incubated for 30 min at 37 ± 2 °C in the dark. The percentage of RSA in the tested samples was calculated using spectroscopic measurement of the incubated samples at 517 nm.
Metal chelating properties of phyto-fabricated ZnO-NPs
The metal chelating (Fe2+) capabilities of ZnO-NPs were investigated according to Sajjad et al. (2021). Ferric chloride (2 mM) and ferrozine (5 mM) were added to 0.8 mL of each of the different quantities of ALE and ZnO-NPs (sonicated) (0.25, 0.5, 1, 1.5, and 2 mg) and thoroughly mixed. The reaction combination was incubated at 37 °C for 10 min in the dark before its absorbance was read spectrophotometrically at 562 nm. The reaction mixtures with and without EDTA served as the positive and negative controls, respectively.
Effect of phyto-fabricated ZnO-NPs on advanced glycation end products (AGEs)
Hemoglobin-δ-gluconolactone assay
The hemoglobin-δ-Gluconolactone (δ-Glu) assay was carried out to assess the inhibitory effect of ZnO-NPs on protein glycation as per the protocol of Losso et al. (2011) with a few modifications. About 800 µL of fresh human blood samples along with 0.1 M PBS (0.16 mL, pH 7.4) containing δ-Glu (50 mM) and ZnO-NPs (0.5, 1.0 and 2 mg) for a final volume of 1 mL was mixed thoroughly and subjected for incubation for 16 h at 37 °C. The fresh blood samples with PBS containing 50 mM δ-Glu alone and aminoguanidine (AG) (10 mM, final concentration) served as respective control. Using a hemoglobin A1C chromatographic-spectrophotometric ion-exchange kit, the incubated blood samples were then tested for the presence or absence of glycated hemoglobin (HbA1C) (Biosystems, India).
Protein glycation and aggregation by BSA-MGO model
Sample preparation
BSA (10 mg mL–1) was incubated with methylglyoxal (MGO) (10 mM, final concentration) as a glycating agent in PBS (0.1 mM, pH 7.4) following the method of Prasanna and Saraswathi (2016) under sterile conditions. The phyto-fabricated ZnO-NPs (0, 0.5, 1.0, and 2 mg) was added to the reaction mixture, mixed thoroughly, and subjected to incubation for 30 min. Only BSA was used as native and the reaction mixtures with and without AG (10 mM, final concentration) served as the positive and negative controls, respectively. The incubated samples were dialyzed (with PBS) to remove unbound MGO.
Thioflavin-T (ThT) assay and fluorescence microscopy
Thioflavin-T, a marker for amyloid cross β-structure, was used to determine the inhibitory activity of phyto-fabricated ZnO-NPs for β-aggregation. The glycated and control MGO-BSA samples were incubated for 60 min with ThT (32 µM). The incubated sample was subjected to fluorescence measurements by their characteristic excitation (435 nm) and emission (485 nm) fluorescence wavelengths. The aggregation of AGEs formation was detected by fluorescence microscopy (LeVine 1999). The dyed samples (10 µL) were put on glass slides and a fluorescence microscope was used to capture the pictures.
Protective effect of ZnO-NPs on red blood cells
A healthy individual blood sample was taken (2 mL) in citrate-containing centrifuge tubes and centrifuged (1000 rpm, 20 min) to remove the white blood corpuscles and other plasma proteins (Prasanna and Saraswathi 2016). The pellet was washed three times with phosphate buffer saline (PBS), and MGO (5 µM, final concentration) was added with or without ZnO-NPs (2 mg mL–1) and allowed to react for 60 min at 37 °C. After incubation, the samples were centrifuged and the pellet (RBCs) was repeatedly washed with PBS before fixing with 2.5% glutaraldehyde for 30 min. The fixed samples were centrifuged (1000 rpm for 30 min), and the washing step was repeated to remove glutaraldehyde adhered to RBCs. The obtained suspension (5 µL) of RBCs was placed on an aluminum foil and dried aseptically overnight, which was later observed under SEM after gold coating.
GK-peptide ribose assay
The G.K. peptide-ribose experiment was carried out using the method outlined in Nagaraj et al. (1996). Under sterile conditions, a reaction mixture including 0.5 M sodium phosphate buffer (pH 7.4), G.K. peptide (80 mg mL–1), and ribose (0.1 M), with or without ZnO-NPs (2 mg), was made in an amount of around 1 mL. The resulting sample was kept at 37 °C for roughly 24 h, and each sample was examined for its unique fluorescence at 340 nm (excitation) and 420 nm (emission) after incubation. The positive and negative controls were the reaction mixtures containing or omitting AM (10 mM).
Statistical analysis
Each experiment was run three times, and the mean results were subjected to a one-way ANOVA using SPSS Inc. 16.0. The magnitude of the F value (p ≤ 0.05) and Tukey’s HSD test were used to differentiate the significant effects of the treatments.
Results and discussion
Physicochemical characterization of phyto-fabricated ZnO-NPs
The physicochemical characteristics of the ZnO-NPs were evaluated by various techniques to confirm the size, morphology and elements of the said particles. The UV-visible study of the ZnO-NPs revealed an absorption peak at 362 nm (Fig. 1A), which falls in long wave (UV-A region: 315–400 nm) of the spectra with a band gap energy of 3.2 eV (Fig. 1B). The spectral outcome confirms that the particle possesses high optical absorbance, which is characteristic of ZnO and is also considered one of the main reasons for their enhanced biological applications (Sirelkhatim et al. 2015; Udayashankar et al. 2021; Ramesh et al. 2022). Well-defined narrow peaks were observed during the XRD studies of the ZnO-NPs at Bragg's angle (2θ) = 31.78, 34.45 and 36.28 that correspond to the planes with miller indices (100), (002) and (101) (Fig. 1C) and are considered to be obtained of products of pure and high crystalline nature with hexagonal wurtzite (JCPDS File No. 01-079-2205) (Venu Gopal and Kamila 2017). The average size of the phyto-fabricated particles was 20.55 nm, which was calculated with Scherrer’s formula (Supplementary Table 1). The XRD results of the study are in agreement with Nagajyothi et al. (2015) and Dinga et al. (2022), wherein the planes that correspond to the miller indices at (100), (002), and (101) indicate the pure and crystalline nature in the ZnO-NPs. FT-IR spectra of ALE and the phyto-fabricated ZnO-NPs were comparatively evaluated to identify the functional groups that contributed to metal oxide (ZnO) bonding during the study (Fig. 1D). Likewise, in the ALE of B. erecta, spectrum bands were observed at 3288.24 cm–1 [alcohol/phenol (O‒H)], 2974.95 cm–1 [aliphatic stretch (C-H)], 1731.78 cm–1 [aldehyde aliphatic (C = O)], 1628.00 cm–1 [(CO-NH) amide stretch], 1546.16 cm–1 (NH bend), 1371.19 cm–1 [alkane group (C-H)], 1240.08 cm–1 [nitro compound group (N-O)], and 1014.89 cm–1 [aliphatic amine group (C-N)]. Besides, from the FT-IR studies of the ZnO-NPs, it was noted that a shift or change in the intensity peak position directly correlates to the interaction of functional groups with the nanoparticles through the donation of electrons (capping, reducing, or stabilizing) that reduces the zinc ions from Zn2+ to Zn+1 and finally leading to Zn0 (Alamdari et al. 2020). Additionally, a spectral band was observed at 521.53 cm–1 during the study that agrees with the findings of Udhayan et al. (2021), wherein the spectral band observed between 600 and 450 cm−1 is designated for the presence of the Zn–O bond. The morphology of the Zno-NPs evaluated through SEM studies revealed that the particles were agglomerated (Fig. 2A). According to Zhang et al. (2002), the agglomeration was mainly due to the polarity and electrostatic attraction of ZnO-NPs. Numerous other researchers’ observations support the study’s results wherein ZnO-NP agglomeration was observed during the green-route synthesis (Udayashankar et al. 2021; Murali et al. 2021b). Besides, high-quality ZnO-NPs were identified through quantitative and qualitative elemental analysis using EDS (Fig. 2B), which demonstrated a high zinc content of 63.53% and the presence of oxygen at 33.08%. Similarly, high amounts of ZnO have been observed when analyzed through EDS in the ZnO-NPs synthesized using the plant extracts (Ali et al. 2021; Ramesh et al. 2022).
Antioxidant properties of phyto-fabricated ZnO-NPs
The free RSA evaluated for ALE and phyto-fabricated ZnO-NPs are depicted in Table 1. The study’s results noted that the phyto-fabricated nanoparticles showed an RSA of 63.38% at 2 mg mL–1, which was dose-dependent with an IC50 of 1.62 mg mL–1. Similarly, in the above-said concentrations of the ALE of B. erecta, a maximum of 29.26% RSA was noticed. The study’s findings are in line with many other researchers wherein concentration-dependent antioxidant potential was noticed in ZnO-NPs synthesized using plants irrespective of their parts used (Stan et al. 2016; Basnet et al. 2018; Murali et al. 2021b). The enhanced antioxidant ability of the nanoparticles phyto-fabricated using the plant source is ascribed to the presence of Zn ions in the structure or the capacity of the particles to transfer an electron from an oxygen atom (Khan et al. 2019; Alamdari et al. 2020). Besides, the literature suggests that the capping of phyto-constituents on the surface of nanoparticles helps in the donation of extra electrons on a nitrogen atom, thereby ensuring the creation of a stable DPPH molecule which results in their better antioxidant potential (Murali et al. 2022).
Metal chelating properties of phyto-fabricated ZnO-NPs
The evaluation of phyto-fabricated ZnO-NPs for the metal chelating properties indicated that the synthesized nanoparticles had the potential to reduce the concentration of metal catalyzing transition, i.e., from ferric ion (Fe3+) to ferrous ion (Fe2+) in oxidative degradation which is in agreement with the findings of Sajjad et al. (2021). There was an increase in the metal chelating properties from 5.51% to 57.32%, which was dose-dependent (from 0.25 to 2 mg mL–1) (Table 1). The IC50 value of the particles’ metal chelating ability was noted at 1.69 mg mL–1. The increase in the metal chelation properties compared to ALE is allied to the capping of phyto-constituents during fabrication (Arumugam et al. 2021). According to the reports of Younus and Anwar (2016), the property of bioactive constituents to chelate metal ions is believed to prevent the formation of AGEs. It has been well noted that ZnO-NPs effectively blocked the formation of ferrous and ferrozine complexes in our study, indicating that they had a chelating effect on the ferrous ion prior to the combination with ferrozine being formed and antioxidant ability is believed to be one of the main variables that determine the metal ion chelation from our ZnO-NPs (Soren et al. 2018; Khan et al. 2019).
Effect of phyto-fabricated ZnO-NPs on advanced glycation end products (AGEs)
Hemoglobin-δ-gluconolactone assay
It has been well noted from the literature that an increase in the hemoglobin level in RBCs results in a significant increase in the HbA1c level (Anandan et al. 2019). Similarly, during the study, the increase or decrease in the HbA1c level in the Amadori product (resulted in RBCs reacting with δ-gluconolactone) upon treatment with different concentrations of ZnO-NPs was measured using ion-exchange HPLC (BIOSYSTEMS). From the results of the HDG assay, the HbA1c level was higher in the HDG reaction mixture alone compared to non-glycated ones (fresh blood). In addition, it was noted that the glycated hemoglobin (HbA1c) level decreased in the presence of nanoparticles which was concentration-dependent after the incubation period (Fig. 3). Due to ZnO-NPs’ ability as an antioxidant, this observation can be explained by the fact that they have a greater impact on the suppression of the production of early AGEs, because, during the Amadori glycation process, the generation of free radicals is noticed (Ahmad et al. 2011; Prasanna et al. 2018). Hence, it may be accomplished that during the glycation process, our ZnO-NPs might have impacted on inhibiting the glycated analogue of hemoglobin as observed from the results of the present study.
Protein glycation and aggregation by BSA-MGO model
BSA-MGO glycation model determines the antiglycation property of ZnO-NPs towards MGO mediated glycation, and the results are represented in Fig. 4A. The results specify that phyto-fabricated ZnO-NPs could inhibit the MGO mediated glycation of albumin, as evidenced by low fluorescence intensity. The results might corroborate that the ZnO-NPs blocked the development of AGEs products derived from methylglyoxal in a BSA-MGO model and inhibited the conversion of dicarbonyl intermediates to AGEs (Prasanna et al. 2018). Our findings further reveal that ZnO-NPs can interact with carbonyl groups of reducing sugars, Amadori products, and dicarbonyl intermediates, inhibiting them from converting to AGEs. Dicarbonyl intermediates like MGO have risen in popularity as mediators of AGEs synthesis and are known to generate glycosylamine protein cross-links when they react with lysine, arginine, and cysteine residues in proteins (Monnier 2003). However, there is no significant difference when comparing the intensity of fluorescence in BSA incubated with ZnO-NPs and AG to that in native BSA protein.
Further, utilizing amyloid-specific Th-T, which specifically binds to fibrous structure, it was able to observe the degree of β-amyloid cross-structure in albumin (see Fig. 4B). Since the Th-T fluorescence intensity was higher in glycated albumin than in albumin without MGO, it seems that protein glycation eventually caused the formation of amyloid structure in BSA. Th-T is a dye that reacts with the fibrillar structure of proteins to increase their fluorescence but is weakly fluorescent in its native/free form. Emission of high Th-T fluorescence indicates that glycation protein induced the formation of the fibrillar state in protein (Biancalana and Koide 2010). This observation can be explained due to the existence of antioxidant properties of ZnO-NPs that might have helped in the inhibitory activity at different stages of protein glycation, interrupting the cascade of events and preventing glycation.
Protective effect of ZnO-NPs on red blood cells
The abnormal function of RBCs is disrupted by high levels of MGO, leading to acanthocyte formation (Awasthi et al. 2015). In our study, in the absence of MGO, RBCs had normal biconcave morphology (Fig. 5A), while the same was completely lost, distorted, and ruptured in the presence of MGO (Fig. 5B). It was noted that RBCs incubated with AG (Fig. 5C) and ZnO-NPs (Fig. 5D) exhibited a protective effect against MGO-induced damage. The disruption of RBCs causes the oxygen transport protein hemoglobin to lose function, resulting in oxidative stress. It is important to note that AGEs inhibition and dicarbonyl scavenging reduce oxidative stress and stop proteins from binding to the AGEs receptor (RAGE) (Prasanna et al. 2018). RBCs lose their normal form due to hyperglycemia, and the presence of dicarbonyl intermediates like MGO, but when treated with ZnO-NPs, the morphological abnormalities brought on by MGO were stopped, and the results were comparable to AG. Similar observations have also been noticed in the RBCs upon incubation with the nanoparticles (Anandan et al. 2019).
GK-peptide ribose assay
As AGEs are also directly correlated to protein-crosslinking, a study was conducted to know whether the phyto-fabricated ZnO-NPs can break the cross-linking by GK-peptide–ribose assay. The literature reported that co-incubating GK peptide with ribose led to the rise in protein glycation end products due to protein cross-linking, also known as the Maillard reaction product (Rahbar et al. 2000). Similarly, in the present study, incubation of phyto-fabricated ZnO-NPs (2 mg) along with GK peptide and ribose, a decreased fluorescence intensity was noted, which is directly attributed to the inhibition of protein cross-linking or AGEs formation (Fig. 6). It was also noted that the co-incubation of AG with GK peptide + ribose and ZnO-NPs with GK peptide + ribose resulted in the inhibition of 84.8% and 60.92% of AGEs formation, respectively. The results are confirmed with the study of Sengani and Rajeswari (2017), wherein the gold nano-supplements were found to have a strong antioxidant effect, lower blood glucose levels, and prevent the interaction between methylglyoxal (MGO) and lysine.
Conclusions
The present study is the first report on the phyto-fabrication of ZnO-NPs from the medicinally important plant Boerhaavia erecta. The phyto-fabricated nanoparticles showed a band gap energy of 3.2 eV with a particle size of 20.55 nm. From the XRD results, it was noted that the particles were crystalline with a hexagonal wurtzite shape. The ZnO-NPs were agglomerated as observed in SEM and EDS confirmed that the phyto-fabricated particles were of high purity (96.61%). Besides, FT-IR proved that the phyto-constituents of the plant extract were involved during the fabrication of nanoparticles resulting in the formation of a metal oxide bond. It was noted that the phyto-fabricated ZnO-NPs possessed efficient antioxidant and metal chelating properties with an IC50 value of ~ 1.6 mg mL–1. Further, during the antiglycation studies, it was observed that the ZnO-NPs were effective in all the stages of evaluation, as noted through the prevention of the advanced glycation end products (AGEs) and formation of Amadori products, trapping the reactive dicarbonyl intermediate and breaking the cross-link of glycated protein along with offering damage prevention in RBCs induced by MGO. The study’s findings will provide an experimental basis for exploring ZnO-NPs from B. erecta in diabetes-related complications.
Data availability
Not applicable.
References
Agarwal H, Shanmugam V (2020) A review on anti-inflammatory activity of green synthesized zinc oxide nanoparticle: mechanism-based approach. Bioorg Chem 94:103423
Ahmad S, Dixit K, Shahab U, Alam K, Ali A (2011) Genotoxicity and immunogenicity of DNA-advanced glycation end products formed by methylglyoxal and lysine in presence of Cu2+. Biochem Biophys Res Commun 407(3):568–574
Ahmed N (2005) Advanced glycation endproducts - role in pathology of diabetic complications. Diabetes Res Clin Pract 67(1):3–21
Alamdari S, Sasani Ghamsari M, Lee C, Han W, Park HH, Tafreshi MJ, Afarideh H, Ara MHM (2020) Preparation and characterization of zinc oxide nanoparticles using leaf extract of Sambucus ebulus. Appl Sci 10(10):3620
Ali SG, Ansari MA, Jamal QMS, Almatroudi A, Alzohairy MA, Alomary MN, Rehman S, Murali M, Jalal M, Khan HM, Adil SF (2021) Butea monosperma seed extract mediated biosynthesis of ZnO NPs and their antibacterial, antibiofilm and anti-quorum sensing potentialities. Arab J Chem 14(4):103044
Anandan S, Urooj A (2019) Bioactive compounds from Morus indica as inhibitors of advanced glycation end products. Ind J Pharm Sci 81(2):282–292
Anandan S, Murali M, Ansari MA, Alzohairy MA, Alomary MN, Farha Siraj S, HaluguddeNagaraja S, Mahendra C, Lakshmeesha TR, Hemanth Kumar NK, Ledesma AE, Amruthesh KN, Asna U (2019) Biosynthesized ZnO-NPs from Morus indica attenuates methylglyoxal-induced protein glycation and RBC damage: in-vitro, In-vivo and molecular docking study. Biomolecules 9:882
Arumugam M, Manikandan DB, Dhandapani E, Sridhar A, Balakrishnan K, Markandan M, Ramasamy T (2021) Green synthesis of zinc oxide nanoparticles (ZnO NPs) using Syzygium cumini: potential multifaceted applications on antioxidants, cytotoxic and as nanonutrient for the growth of Sesamum indicum. Environ Technol Innov 23:101653
Ashraf JM, Ansari MA, Khan HM, Alzohairy MA, Choi I (2016) Green synthesis of silver nanoparticles and characterization of their inhibitory effects on AGEs formation using biophysical techniques. Sci Rep 6:20414
Awasthi S, Gayathiri SK, Ramya R, Duraichelvan R, Dhason A, Saraswathi NT (2015) Advanced glycation-modified human serum albumin evokes alterations in membrane and eryptosis in erythrocytes. Appl Biochem Biotechnol 177(5):1013–1024
Basnet P, Chanu TI, Samanta D, Chatterjee S (2018) A review on bio-synthesized zinc oxide nanoparticles using plant extracts as reductants and stabilizing agents. J Photochem Photobiol B Biol 183:201–221
Biancalana M, Koide S (2010) Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim Biophys Acta 7:1405–1412
Bolton WK, Cattran DC, Williams ME, Adler SG, Appel GB, Cartwright K, Foiles PG, Freedman BI, Raskin P, Ratner RE, Spinowitz BS (2004) Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Ame J Nephrol 24(1):32–40
Dinga E, Mthiyane DMN, Marume U, Botha TL, Horn S, Pieters R, Wepener V, Ekennia A, Onwudiwe DC (2022) Biosynthesis of ZnO nanoparticles using Melia azedarach seed extract: evaluation of the cytotoxic and antimicrobial potency. OpenNano 8:100068
Elosta A, Ghous T, Ahmed N (2012) Natural products as anti-glycation agents: possible therapeutic potential for diabetic complications. Curr Diabetes Rev 8(2):92–108
Gunalan S, Sivaraj R, Rajendran V (2012) Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog Nat Sci: Mater Inter 22(6):693–700
IDF Diabetes Atlas (2015) International Diabetes Federation, 7th edn. IDF, Brussels, Belgium, pp 33
Khan ZUH, Sadiq HM, Shah NS, Khan AU, Muhammad N, Hassan SU, Tahir K, Zamnsafi S, Khan FU, Imran M, Ahmad N, Ullah F, Ahmad A, Sayed M, Khalid MS, Qaisrani SA, Ali M, Zakir A (2019) Greener synthesis of zinc oxide nanoparticles using Trianthema portulacastrum extract and evaluation of its photocatalytic and biological applications. J Photochem Photobiol B Biol 192:147–157
Kumar NKH, Murali M, Satish A, Singh SB, Gowtham HG, Mahesh HM, Lakshmeesha TR, Amruthesh KN, Jagannath S (2020) Bioactive and biocompatible nature of green synthesized zinc oxide nanoparticles from Simarouba glauca DC.: an endemic plant to Western Ghats. India J Clust Sci 31:523–534
Lee J, Yun JS, Ko SH (2022) Advanced glycation fend products and their effect on vascular complications in Type 2 diabetes mellitus. Nutrients 14(15):3086
LeVine H (1999) Quantification of β-sheet amyloid fibril structures with thioflavin T. Methods Enzymol 309:274–284
Losso JN, Bawadi HA, Chintalapati M (2011) Inhibition of the formation of advanced glycation end products by thymoquinone. Food Chem 128:55–61
Monnier VM (2003) Intervention against the Maillard reaction in vivo. Archiv Biochem Biophy 419(1):1–5
Murali M, Mahendra C, Rajashekar N, Sudarshana MS, Raveesha KA, Amruthesh KN (2017) Antibacterial and antioxidant properties of biosynthesized zinc oxide nanoparticles from Ceropegia candelabrum L.– An endemic species. Spectrochim Acta Part A Mol Biomol Spectrosc 179:104–109
Murali M, Anandan S, Ansari MA, Alzohairy MA, Alomary MN, Asiri SMM, Almatroudi A, Thrivveni MC, Brijesh Singh S, Gowtham HG, Aiyaz M, Chandrashekar S, Urooj A, Amruthesh KN (2021a) Genotoxic and cytotoxic properties of zinc oxide nanoparticles phyto-fabricated from the obscure morning glory plant Ipomoea obscura (L.) Ker Gawl. Molecules 26(4):891
Murali M, Kalegowda N, Gowtham HG, Ansari MA, Alomary MN, Alghamdi S, Shilpa N, Singh SB, Thriveni MC, Aiyaz M, Angaswamy N, Lakshmidevi N, Adil SF, Mohammad RH, Amruthesh KN (2021b) Plant-mediated zinc oxide nanoparticles: advances in the new millennium towards understanding their therapeutic role in biomedical applications. Pharmaceutics 13(10):1662
Murali M, Manjula S, Shilpa N, Ravishankar DK, Shivakumara CS, Thampy A, Ayeshamariam A, Pandey S, Anandan S, Amruthesh KN, Al-Mekhlafi FA, Kaviyarasu K (2022) Facile synthesis of ZnO-NPs from yellow creeping daisy (Sphagneticola trilobata L.) attenuates cell proliferation by inducing cellular level apoptosis against colon cancer. J King Saud Univ Sci 34(5):102084
Nagajyothi P, Cha SJ, Yang IJ, Sreekanth T, Kim KJ, Shin HM (2015) Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J Photochem Photobiol B Biol 146:10–17
Nagaraj RH, Shipanova IN, Faust FM (1996) Protein cross-linking by the Maillard reaction: isolation, characterization, and in vivo detection of a lysine-lysine cross-link derived from methylglyoxal. J Biol Chem 271(32):19338–19345
Nisha M, Vinod BN, Sunil C (2018) Evaluation of Boerhavia erecta L. for potential antidiabetic and antihyperlipidemic activities in streptozotocin-induced diabetic Wistar rats. Future J Pharm Sci 4(2):150–155
Patil N, Kelkar A, Sivaram A (2022) Prevention of protein glycation by nanoparticles: potential applications in T2DM and associated neurodegenerative diseases. BioNanoScience 18:1–3
Prasanna G, Saraswathi N (2016) Aspartic acid functions as carbonyl trapper to inhibit the formation of advanced glycation end products by chemical chaperone activity. J Biomol Struct Dyn 34:943–951
Prasanna G, Hari N, Saraswathi NT (2018) Hydroxymethoxybenzaldehyde from Sesbania grandilfora inhibits the advanced glycation end products (AGEs)-mediated fibrillation in hemoglobin. J Biomol Struct Dyn 36(4):819–829
Rahbar S, Yerneni KK, Scott S, Gonzales N, Lalezari I (2000) Novel inhibitors of advanced glycation endproducts (part II). Mol Cell Biol Res Commun 3:360–366
Ramesh AM, Pal K, Kodandaram A, Manjula BL, Ravishankar DK, Gowtham HG, Murali M, Rahdar A, Kyzas GZ (2022) Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta. Green Process Synth 11(1):857–867
Sajjad A, Bhatti SH, Ali Z, Jaffari GH, Khan NA, Rizvi ZF, Zia M (2021) Photoinduced fabrication of zinc oxide nanoparticles: transformation of morphological and biological response on light irradiance. ACS Omega 6(17):11783–11793
Sengani M, Rajeswari D (2017) Gold nanosupplement in selective inhibition of methylglyoxal and key enzymes linked to diabetes. IET Nanobiotechnol 11(7):861–865
Sharma D, Kanchi S, Bisetty K (2019) Biogenic synthesis of nanoparticles: a review. Arab J Chem 12(8):3576–3600
Singh VP, Bali A, Singh N, Jaggi AS (2014) Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol 18(1):1–14
Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, Bakhori SKM, Hasan H, Mohamad D (2015) Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Lett 7:219–242
Soren S, Kumar S, Mishra S, Jena PK, Verma SK, Parhi P (2018) Evaluation of antibacterial and antioxidant potential of the zinc oxide nanoparticles synthesized by aqueous and polyol method. Microb Pathog 119:145–151
Stan M, Popa A, Toloman D, Silipas TD, Vodnar DC (2016) Antibacterial and antioxidant activities of ZnO nanoparticles synthesized using extracts of Allium sativum, Rosmarinus officinalis and Ocimum basilicum. Acta Met Sin Engl Lett 29:228–236
Surange SR, Pendse GS (1972) Pharmacognostical studies of Boerhaavia Gaert. and its comparison with Boerhaavia diffusa Linn. Quarterly journal. Crude Drug Res 12(3):1937–1950
Udayashankar AC, Shivaram AB, Murali M, Ramachndrappa LT, Lalitha SG, Krishnappa HKN, Anandan S, Sudarshana BS, Chanappa EG, Ramachandrappa NS (2021) Biosynthesis of zinc oxide nanoparticles using leaf extract of Passiflora subpeltata: characterization and antibacterial activity against Escherichia coli isolated from poultry faeces. J Clust Sci 32(6):1663–1672
Udhayan S, Udayakumar R, Sagayaraj R, Gurusamy K (2021) Evaluation of bioactive potential of a Tragia involucrata healthy leaf extract @ ZnO nanoparticles. BioNanoScience 11:703–719
Venu Gopal VR, Kamila S (2017) Effect of temperature on the morphology of ZnO nanoparticles: a comparative study. Appl Nanosci 7(3):75–82
Younus H, Anwar S (2016) Prevention of non-enzymatic glycosylation (glycation): implication in the treatment of diabetic complication. Inter J Health Sci 10(2):261
Zhang J, Sun LD, Lin YJ, Su H, Liao C, Yan C (2002) Control of ZnO morphology via a simple solution route. Chem Mater 14:4172–4177
Acknowledgements
The authors thank the Department of Studies in Botany, Biotechnology and Microbiology of the University of Mysore, Mysuru, for providing facilities to carry out research. The authors would also like to thank the University with Potential of Excellence (UPE) Authorities University of Mysore, Mysuru, for instrumentation facilities.
Funding
The author M. Murali likes to acknowledge the University Grants Commission (UGC), New Delhi, India, for providing financial support by awarding a Post-Doctoral Fellowship (No. F/PDFSS-2015-17-KAR-11846).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The authors indicated in parentheses made substantial contributions to the following tasks of research: conceptualization (Mahadevamurthy Murali, Anjana Thampy, Satish Anandan, Ashween Bilagi, Mohammed Aiyaz, Natarajamurthy Shilpa, Sudarshana Brijesh Singh, Hittanahallikoppal Gajendramurthy Gowtham, Abhilash Mavinakere Ramesh, Abbas Rahdar, George Z. Kyzas); writing-original draft, writing-revised, investigation, and methodology (Mahadevamurthy Murali, Anjana Thampy, Satish Anandan, Mohammed Aiyaz, Natarajamurthy Shilpa, Sudarshana Brijesh Singh, Hittanahallikoppal Gajendramurthy Gowtham, Abhilash Mavinakere Ramesh, Abbas Rahdar, George Z. Kyzas); and supervision (Satish Anandan). All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Murali, M., Thampy, A., Anandan, S. et al. Competent antioxidant and antiglycation properties of zinc oxide nanoparticles (ZnO-NPs) phyto-fabricated from aqueous leaf extract of Boerhaavia erecta L.. Environ Sci Pollut Res 30, 56731–56742 (2023). https://doi.org/10.1007/s11356-023-26331-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26331-8