Abstract
To study the toxic effects of microcystin-LR (MC-LR) on crayfish, adult male Procambarus clarkii were exposed to different concentrations of MC-LR for 96 h. In the meantime, the accumulation characteristics of MC-LR and the alternations of antioxidant system, histopathology and intestinal flora of P. clarkii were investigated. The results demonstrated that the hepatopancreas, gills and intestines of P. clarkii could effectively accumulate MC-LR. Antioxidant-related genes such as Mn-sod, cat, gst, gpx, mt and hsp70 showed different expression trends in different organs to respond to MC-LR-induced oxidative stress. MC-LR led to histological changes in the hepatopancreas, gills and intestines, thus affecting their corresponding physiological functions. Additionally, the abundances of bacterial phyla including Firmicutes and Planctomycetes and genera including Dysgonomonas, Brevundimonas and Anaerorhabdus in the intestine were significantly changed after MC-LR exposure, and the disruption of intestinal flora might further cause abnormal intestinal microbial metabolism and genetics in P. clarkii. This study provides novel mechanistic insights into the toxic impacts of microcystins on aquatic crustaceans.
Highlights
• MC-LR was significantly accumulated in the hepatopancreas, gills and intestines of P. clarkii.
• MC-LR induced the differential expression of antioxidant-related genes of P. clarkii.
• MC-LR caused histological alterations in the hepatopancreas, gills and intestines of P. clarkii.
• MC-LR affected the intestinal microbial composition and function of P. clarkii.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microcystins (MCs), mainly produced by freshwater cyanobacteria species and released during harmful algal blooms, are among the most abundant cyanobacterial toxins (Ni et al. 2021). MCs have multiple organic toxicity, genetic toxicity, neurotoxicity, immunotoxicity and potential cancer-promoting effects. MCs exposure will cause the liver failure in wild animals, livestocks and aquatic animals, and even lead to human diseases and deaths (Schreidah et al. 2020). It has been reported that MCs can be accumulated in aquatic organisms, which will bring about the transmission of such environmental toxins to higher trophic levels along the food chain, thus becoming a potential threat to human health (Ibelings et al. 2015; Pham and Utsumi 2018). MC-LR, as one of the most toxic MCs, has gained extensive attention worldwide (Wu et al. 2015; Duan et al. 2020). MC-LR is capable of regulating the immune responses and expression of inflammatory cytokine, and has potent hepatotoxicity in different fish or shellfish species (Li et al. 2012; Rymuszka and Adaszek 2012; Qu et al. 2018; Duan et al. 2020). Due to its abundance and toxicity in the water environment, there have been growing concerns over the toxicological mechanisms in aquatic organisms.
The toxic impacts of MC-LR on aquatic crustaceans have been reported in several researches. MC-LR could inhibit the swimming speed and hopping frequency of water flea (Daphnia magna) (Pawlik-Skowrońska and Bownik et al. 2021). MC-LR could be accumulated in mysid crustacean (Neomysis awatschensis) (Min et al. 2018) and crab (Neohelice granulate) (Sabatini et al. 2015), and induce oxidative stress. Besides, Zhang et al. (2019c) documented that MC-LR was capable of entering the testis of prawn (Macrobrachium rosenbergii), injuring testicular germ cells, and exerting considerable inhibition on the development of testis. In the study by Sun et al. (2021), similar results were also found, in which MC-LR could inflict damage to the testis tissue of oriental river prawn (Macrobrachium nipponense), activate mitochondrial apoptosis, and induce cross-generational immunotoxicity. Qu et al. (2018) found a variety of significantly differentially expressed genes (DEGs) associated with toxicity and liver detoxification in silver carp (Hypophthalmichthys molitrix) following MC-LR exposure. In spite of these studies, the deep impacts of MC-LR on the antioxidant system, histopathology and intestinal flora of crustaceans deserve further investigations to clarify the toxicological mechanisms of MC-LR.
Hepatopancreas, as an organ with multiple functions for crustaceans, plays a significant role in metabolism, absorption, immunological defense and xenobiotic detoxification (Rőszer 2014; De Melo et al. 2019). Meanwhile, hepatopancreas acts as a key target organ for a variety of environmental stresses (Mazzei et al. 2014; Müller et al. 2020; Zhang et al. 2020a). Gills of crustaceans generally interface with the ambient environment directly and therefore have important functions in ionic regulation, ammonia excretion, acid–base balance and osmoregulation, which can accumulate toxic substances in a contaminated environment (Henry et al. 2012; Tang et al. 2020). Intestinal microbiota of crustaceans can regulate many crucial physiological functions, thus sustaining the health for the host (Zhang et al. 2020a). Notably, it can promote metabolism by synthesizing some enzymes that the host cannot produce (Rowland et al. 2018). It can also participate in energy storage and supply essential vitamins and amino acids for the host (Gill et al. 2006; Nayak 2010). Furthermore, as reported by Chen et al. (2017), intestinal flora is pivotal in regulating host immune system.
As a typical species of crustaceans, Procambarus clarkii can tolerate contaminated and extreme environments, accumulating toxins and pollutants. According to the study by Gherardi (2006), it is at the central location of aquatic food webs and acts a potential vector of pollutants to higher trophic levels. P. clarkii exhibits strong potential to indicate pollution and has acted as a typical test animal to evaluate the toxic impacts of pollutants in water environment (Vioque-Fernándeza et al. 2009; Zhang et al. 2019b). In the present work, adult male P. clarkii were exposed to different concentrations of MC-LR for 96 h, which were 0, 10, and 40 times the concentration of WHO-permitted maximum contaminant level (1 μg/L) in drinking water (WHO 2011). Quantitative real-time-PCR (qRT-PCR) was carried out to identify the expression levels of antioxidant-related genes. Meanwhile, the histological changes in the hepatopancreas, gills and intestines of crayfish were studied. Furthermore, the intestinal flora was examined through 16S rRNA Illumina sequencing to investigate the composition and function of intestinal flora. This study aimed to comprehensively evaluate the alterations of antioxidant system, histopathology and intestinal flora caused by MC-LR in crayfish, and reveal the toxicological mechanisms of MCs in aquatic crustaceans from different perspectives.
Materials and methods
Microcystin-LR, organisms and toxicity test
MC-LR was purchased from Agent Technology Co., Ltd. (Beijing, China). Adult male crayfish were bought from a market of Harbin, China. They were kept in glass tanks (0.40 m × 0.30 m × 0.25 m) containing eighteen liters of tap water after dichlorination (Table S1) for more than ten days with a photoperiod of 12 h light/12 h dark. Tubificid worms (Limnodrilus hoffmeisteri) were provided as foods for crayfish daily, and it was stopped during MC-LR exposure experiment.
Crayfish were divided into three groups, with three replicates in each group. Besides, there were twelve specimens in each replicate. The crayfish of three groups were exposed to 0 μg/L (Con), 10 μg/L (M10) and 40 μg/L MC-LR (M40), respectively. Corresponding amounts of MC-LR were dissolved in methanol and added to dechlorinated tap water to make MC-LR solutions. It should be noted that no mortality was found during MC-LR exposure experiment.
Sampling
Three specimens from each group (one from each replicate) were taken after 96 h and dissected after anesthetization on ice for 15 min. Hepatopancreas (about 300 mg), gills and intestines were fixed in paraformaldehyde. Additionally, 300 mg of hepatopancreas was used for RNA extraction. Subsequently, nine specimens from each group (three ones from each replicate) were selected and dissected for collecting intestinal contents. The intestines were flushed with sterile phosphate solutions. In order to reduce inter-individual variation, the intestinal contents from three intestines in each replicate were pooled together. The resulted phosphate solutions were collected for DNA extraction. The remaining hepatopancreas, gills and intestines were washed using ultrapure water and kept for MC-LR extraction and quantification. All sampling procedures were accomplished under aseptic condition.
Histological examination of hepatopancreas, gills and intestines
Following fixation in paraformaldehyde, the samples of hepatopancreas, gills and intestines were dehydrated by ethanol and embedded in paraffin. The resulted paraffin blocks were sectioned at 4-μm thickness. After hematoxylin and eosin (H&E) staining, the sections were observed with an Olympus microscope (IX71).
MC-LR extraction and quantification
All the hepatopancreas, gills and intestines used for MC-LR extraction and quantification were lyophilized by a freeze drier (Labconco Corporation, USA). MC-LR was extracted and purified according the revised method of Xie et al. (2005). Specifically, lyophilized samples of hepatopancreas (0.25 g in dry weight (DW)), gills (0.15 g in DW), and intestines (0.05 g in DW) were respectively homogenized in a mortar and extracted three times with 10 mL of BuOH: MeOH: H2O (1: 4: 15) for 24 h while stirring. The extracts were centrifuged at 18,000 rpm and the supernatants were diluted with ultrapure water. The detailed procedures of MC-LR quantification are described in Supporting Information (SI) 1.1.
Quantitative real-time-PCR (qRT-PCR)
RNA of hepatopancreas was extracted by the Trizol reagent (Invitrogen, USA). The primer pairs of antioxidant-related genes of P. clarkii were listed in Table 1 and the 18S rRNA was used as a normalized control gene. The qRT-PCR was performed according to our previous report (Zhang et al. 2020b). The significant differences in expression level were tested by one-way ANOVA between Con group and M10 group or M40 group.
DNA extraction, PCR amplification and sequencing
The microbial genomic DNA (gDNA) of intestinal content samples was extracted by utilizing the E.Z.N.A.™ Mag-Bind Soil DNA Kit (Omega Bio-tek, USA). Subsequently, the V3-V4 regions of 16S rRNA genes were amplified using the primers in Table S2. Further detailed procedures are presented in SI 1.2 and SI 1.3, respectively. Raw data of 16S rRNA sequencing in the present study have been deposited in the NCBI SRA database (No. SRR 9,671,444).
Linear discriminant analysis (LDA) effect size (LEfSe) algorithm
Linear discriminant analysis (LDA) effect size (LEfSe) algorithm (Segata et al. 2011; Afgan et al. 2018) was used to identify the differences among groups at all taxonomic levels. As a suitable method used for discovering and interpreting multi-level biological markers and characteristics, it used Kruskal–Wallis (KW) sum-rank test with nonparametric coefficient to determine the significant differences in abundance between Con group and M10 group or M40 group (LDA score (log10) > 2).
Functional analysis of intestinal microbial community
According to the method described by Langille et al. (2013), the 16S rRNA gene sequencing data were compared with the Kyoto Encyclopedia of Genes and Genomes (KEGG) database, and microbial functions were predicted by PICRUSt (version 1.1.0). Statistical significance was evaluated by using Welch's t-test (P < 0.05).
Results
Accumulation of MC-LR in different organs
As shown in Table S3, the MC-LR concentrations in the hepatopancreas, gill and intestine of P. clarkii in Con group were not detectable. By contrast, MC-LR concentrations in three types of organs in M10 and M40 groups showed significantly higher levels (P < 0.01) compared those in Con group, which increased as the MC-LR concentration in water rose. In M10 group, the MC-LR concentrations were 0.021, 0.031 and 0.014 mg/kg in the hepatopancreas, gill and intestine, respectively. In M40 group, the MC-LR concentrations were 0.045, 0.046 and 0.023 mg/kg in the hepatopancreas, gill and intestine, respectively.
Expression profiles of antioxidant-related genes
In the hepatopancreas, the expression of Mn-sod was significantly up-regulated by 35.08% (P = 0.0047) in M10 group, but not significantly changed (P = 0.0865) in M40 group (Fig. 1a). In the gill, its expression was significantly up-regulated by 73.14% (P = 0.0093) and 262.35% (P = 1.25E-4) in M10 and M40 groups, respectively. In the intestine, its expression was remarkably up-regulated by 32.34% (P = 0.0136) and down-regulated by 11.66% (P = 0.0274) after exposure to 10 and 40 μg/L MC-LR, respectively.
Relative expression levels of (a) Mn-sod, (b) cat, (c) gst, (d) gpx, (e) mt and (f) hsp70 in the hepatopancreas, gills and intestines of crayfish exposed to different concentrations of MC-LR (n = 3). Data are analyzed by one-way ANOVA. Asterisks indicate statistically significant differences between control group and MC-LR exposure groups; *P ≤ 0.05, **P ≤ 0.01. Con: Control; M10: 10 μg/L MC-LR; M40: 40 μg/L MC-LR
As shown in Fig. 1b, in the hepatopancreas, the expression of cat was evidently up-regulated in M10 and M40 groups by 170.35% (P = 8.58E-5) and 232.29% (P = 1.53E-4), respectively. By contrast, its expression level in the gill and intestine did not change significantly in MC-LR exposure groups.
The expression levels of gst in different organs of P. clarkii under MC-LR exposure changed to varying degrees (Fig. 1c,). In the hepatopancreas, the expression of gst in M10 group was significantly up-regulated by 39.57% (P = 0.0114), while in M40 group it was down-regulated by 11.55% (P = 0.2153). In the gill, gst expression was obviously down-regulated (P = 0.0261) in M10 group, while significantly up-regulated (P = 0.0071) in M40 group. Unlike that in the hepatopancreas and gill, gst expression in the intestine did not change significantly (P = 0.5896) in M10 group, but decreased markedly (P = 0.0278) in M40 group.
The expression levels of gpx in different organs of P. clarkii exposed to different doses of MC-LR are shown in Fig. 1d. In the hepatopancreas, the expression level of gpx in M10 group was markedly increased by 210.36% (P = 1.12E-5), and decreased by 52.76% (P = 0.0011) in M40 group. In the gill, however, its expression was down-regulated (P = 0.0986) and up-regulated (P = 0.2429) under exposure to 10 and 40 μg/L MC-LR, respectively. Meanwhile, the expression of gpx in the intestine was significantly up-regulated (P = 0.0287) in M10 group, while the expression level in M40 group was close to normal (P = 0.1005).
In the hepatopancreas, gill and intestine of P. clarkii, mt expression changed significantly in M10 and M40 groups (Fig. 1e). The expression of mt in the hepatopancreas was significantly down-regulated by 23.14% (P = 0.0044) and 77.18% (P = 3.74E-5), respectively. Similarly, its expression in the intestine was markedly down-regulated by 36.40% (P = 3.31E-4) and 29.44% (P = 0.0024), respectively. By contrast, in the gill, the expression of mt in M10 group was significantly up-regulated by 17.12% (P = 0.0046), while it was significantly down-regulated by 59.39% (P = 3.92E-6) in M40 group.
As shown in Fig. 1f, the expression of hsp70 in the hepatopancreas was up-regulated in M10 group (P = 0.0079) and M40 group (P = 0.1722). However, in the gill, its expression was remarkably down-regulated (P = 0.0192) in M10 group and maintained at a normal level (P = 0.8461) in M40 group. Similar to that in the hepatopancreas, the expression of hsp70 in the intestine was significantly up-regulated in both M10 (P = 0.0014) and M40 (P = 0.0025) groups.
Histological analysis of hepatopancreas, gills and intestines
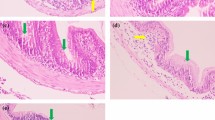
Histological analysis was performed to elaborate the morphological and histopathological effects of MC-LR on the main organs including hepatopancreas, gill and intestine of P. clarkii (Fig. 2). The hepatopancreas without MC-LR exposure had intact structures and the tubule lumens demonstrated asterisk-like shapes (Fig. 2a). By contrast, the hepatopancreas exposed to MC-LR exhibited histological changes and damage. Epithelium vacuolization and tubule lumen dilatation were observed in the hepatopancreas in M10 and M40 groups (Fig. 2b, c). The gill of P. clarkii in control group showed typical structures such as intact gill membrane, orderly inner respiratory epithelial cells (RECs), complete gill leaf and haemocytes in microvascular cavity (Fig. 2d). The corneum in the gill of P. clarkii in control group was holonomic and smooth (Fig. 2d), while that in M40 group was tortuous and partially ruptured (Fig. 2f). Large areas of RECs in the gills of P. clarkii exposed to both 10 and 40 μg/L MC-LR were scattered in microvascular lumen (Fig. 2e, f). Notably, the gill of crayfish after 40 μg/L MC-LR exposure showed hemolymph congestions in vessels, and the number of haemocytes was reduced under this condition. The intestine of crayfish in Con group exhibited a normal histological structure including epithelium, lumen, submucosa, lamina propria and muscularis, and neither injury nor inflammation was shown (Fig. 2g). Nevertheless, there appeared eosinophilic granule cells (EGCs) in both M10 and M40 groups (Fig. 2h, i). Particularly, abnormal muscularis and lamina propria infiltrated by lympaocytes were displayed in the intestine of crayfish in M40 group (Fig. 2i).
Histological photomicrographs of hepatopancreas, gills and intestines. (a) Hepatopancreas exposed to 0 μg/L MC-LR; (b) hepatopancreas exposed to 10 μg/L MC-LR; (c) hepatopancreas exposed to 40 μg/L MC-LR; (d) gill exposed to 0 μg/L MC-LR; (e) gill exposed to 10 μg/L MC-LR; (f) gill exposed to 40 μg/L MC-LR; (g) intestine exposed to 0 μg/L MC-LR; (h) intestine exposed to 10 μg/L MC-LR; (i) intestine exposed to 40 μg/L MC-LR; C: corneum; H: haemocytes; Rec: respiratory epithelium cells; Hc: hemolymph congestion in vessels; L: lumen; E: epithelium; Lp: lamina propria; Sm: submucosa; M: muscularis; Tubule lumen dilatation (marked by black solid arrows), vacuolization (marked by white solid arrows) and eosinophilic granule cells (marked by black hollow arrows) were observed. Hematoxylin and eosin (H&E) stain (100 ×)
Alteration in the taxa of intestinal microbiota
The most prevalent phyla included Proteobacteria, Bacteroidetes, Firmicutes and Fusobacteria, accounting for over 98% of total phyla among the intestinal microbiota (Fig. 3). According to the supervised comparison of the intestinal microbiota between Con group and M10/M40 group by utilizing the LEfSe algorithm and remarkable differences were identified. In this study, we particularly analyzed the differences at the levels of phylum and genus. It was characterized by a predominance of the phylum Planctomycetes and the genus Pseudorhodobacter in Con group (Con vs M10) (Fig. 4a and Fig. S1). The relative abundance of phylum Firmicute was higher in M40 group, whereas the relative abundances of phylum Planctomycetes and genera including Dysgonomonas, Brevundimonas and Anaerorhabdus were higher in Con group (Con vs M40) (Fig. 4b and Fig. S2).
LEfSe analysis revealing significant differences in intestinal microbiota. (a) Differences between control group (Con, positive score) and 10 μg/L MC-LR exposure group (M10, N.A.); (b) differences between control group (Con, negative score) and 40 μg/L MC-LR exposure group (M40, positive score). The LDA scores (log10) > 2 and P < 0.05 are listed
Functional characterization of intestinal microbiome
PICRUSt was used to analyze the main functions of the intestinal flora, and the functional categories were compared between Con group and M10 group or M40 group (Fig. 5). Microbial sequences annotated to ribosome biogenesis, DNA replication proteins, homologous recombination, DNA repair and recombination proteins, DNA replication and chaperones and folding catalysts were significantly more enriched in MC-LR exposure groups, and the enrichments in M40 group were more remarkable. Inversely, the abundances of sequences involved in the pathways including glycine, serine and threonine metabolism, porphyrin and chlorophyll metabolism, galactose metabolism, amino acid metabolism, valine, leucine and isoleucine biosynthesis, phenylalanine metabolism and mineral absorption were significantly decreased after MC-LR exposure.
Discussion
P. clarkii is a dominant economic species of crayfish in China and it frequently lives in water with cyanobacterial blooms producing MC-LR. Therefore, it is essential to analyze the accumulation characteristics of MC-LR in P. clarkii to assess its potential hazard to human health. In this study, the gills accumulated the most MC-LR in a unit mass, followed by the hepatopancreas and intestines, which might result from the fact that the gill of crayfish can interface with the water environment containing MC-LR and accumulate the toxin directly. It is worth noting that the intestine accumulated the least MC-LR in a unit mass, which was different from the results reported by Yuan et al. (2016), in which the intestine was the main accumulation organ of MC-LR in P. clarkii. Due to the fact that the crayfish were fed with commercial pellet foods once a day in the study by Yuan et al. (2016), it was inferred that the MC-LR could be transported to intestine along with foods. However, no food as transporter was provided in our study, which might cause the intestine accumulate less MC-LR.
There were evidences indicating that the increased activities of antioxidant enzymes could be realized by activating available enzymes or/and up-regulating enzyme synthesis, while their decreased activities might result from the secondary effects (e.g. substrate inhibition of existing molecules) or down-regulation of enzyme synthesis (Kaushik and Kaur 2003; Cazenave et al. 2006). Mn-SOD, as the only antioxidant enzyme in mitochondria (Al Kaddissi et al. 2012), has important antioxidant and protective effects, which can quickly scavenge the free radicals generated in the electron transfer process of mitochondrial respiratory chain, and eliminate or alleviate the oxidative stress in organisms. The results in this study indicated that MC-LR produced excessive ROS in the gill, and also induced antioxidant defense (Fig. 6a). Besides, 10 μg/L MC-LR exposure could induce intestinal antioxidant defense by increasing the expression level of Mn-sod. However, the expression levels of cat and gpx did not change significantly under 40 μg/L MC-LR exposure, suggesting that H2O2 might accumulate in large quantities and inhibit the expression of Mn-sod and the activity of Mn-SOD (Guecheva et al. 2003; Hernández et al. 2013). In the hepatopancreas, the inducement and inhibition reached a balance. Hou et al. (2015) also found a similar phenomenon when studying the toxic impacts of MC-LR on zebrafish (Danio rerio).
A schematic diagram showing the proposed pathways of how MC-LR leads to oxidative stress and affects antioxidant system by regulating the expression of antioxidant-related genes including (a) Mn-sod, (b) cat, (c) gst, (d) gpx, (e) mt and (f) hsp70 in different organs. The physiological functions are realized by corresponding enzymes including Mn-SOD, CAT, GST, GPx, MT and HSP70. Data are analyzed by one-way ANOVA. Asterisks indicate statistically significant differences between control group and MC-LR exposure groups; *P ≤ 0.05; **P ≤ 0.01. Con: Control; M10: 10 μg/L MC-LR; M40: 40 μg/L MC-LR
The up-regulation of cat indicated that redundant ROS were produced in the hepatopancreas of P. clarkii exposed to both concentrations of MC-LR, which contained excessive H2O2. Therefore, the expression of cat in the hepatopancreas is up-regulated, which promoted the increase of CAT content to cope with excessive H2O2 (Fig. 6b). By contrast, when exposed to two concentrations of MC-LR, the expression level of this gene in the gill and intestine did not change significantly. Some studies have shown that the accumulation of superoxide radical can inhibit the activity of CAT (Chance et al. 1952; Kono and Fridovich 1982). According to the results in the present study, it can be found that the inhibition was realized by restraining the expression of cat. Both 10 and 40 μg/L MC-LR resulted in the production of excessive superoxide anions in the gill and intestine of P. clarkii, and inhibited the expression of cat. Therefore, when the promotion from excessive H2O2 and the inhibition from excessive superoxide anions were in balance, the expression level of cat showed no significant change.
As a free radical scavenger, reduced glutathione (GSH) plays a crucial role in the metabolic pathways associated with the degradation of MCs through conjugation with those toxins (Goncalves-Soares et al. 2012). Under the catalysis of GST, GSH can be combined with many heterotypic biomasses to produce GSH-containing combined products. The results in this study demonstrated that two concentrations of MC-LR caused oxidative stress in the hepatopancreas, and the expression of gst was up-regulated under exposure to 10 μg/L MC-LR to realize the detoxification of P. clarkii (Fig. 6c). Meanwhile, it has been found that MC-LR exposure can reduce the level of GSH (Lin et al. 2018). Under 40 μg/L MC-LR exposure, the content of GSH was reduced, so the content of GST with GSH as substrate also decreased by down-regulating the expression of gst (Fig. 6c). The reduced expression level of gst in M10 group possibly resulted from that the gill produced more SOD under this condition, and its content was enough to reduce or eliminate excessive ROS, thus reducing the demand for GST (Fig. 6c). Similar to that in the hepatopancreas, excessive ROS induced by 40 μg/L MC-LR exposure in the intestine reduced the amount of GSH, so GST content also decreased through the down-regulation of gst expression. At the same time, based on the expression changes of Mn-sod, cat and gst in the intestine of crayfish in M40 group, it could be found that the antioxidant capacity of intestine decreased after exposure to 40 μg/L MC-LR.
GPx plays an important role in eliminating redundant ROS that cause oxidative stress in aquatic organisms to protect them from oxidative damage. The expression patterns of gpx and gst were similar, which might be on account of the fact that both GST and GPx encoded by these two genes use GSH as substrate to realize antioxidant and detoxification functions (Fig. 6d). Both CAT and GPx can degrade H2O2, thus eliminating or alleviating oxidative stress (Zhang et al. 2019a). Under exposure to 10 μg/L MC-LR, excessive ROS, especially H2O2, were produced in the hepatopancreas, so the expression of gpx was up-regulated to generate more GPx to decompose H2O2. In addition, it could be inferred that under 40 μg/L MC-LR exposure, large quantities of CAT were produced, thus inhibiting the expression of gpx. This phenomenon might also be related to the decrease of GSH content and the increase of oxidized glutathione (GSSG) content under 40 μg/L MC-LR exposure (Serafini et al. 2019). It should be noted that the expression of this gene in the gill didn′t change significantly, which indicated that ROS induced by two concentrations of MC-LR balanced the promotion and inhibition of gpx expression. According to the results of gpx expression in the intestine, it could be inferred that it produced more GPx as a response to10 μg/L MC-LR-induced stress to compensate for CAT shortage by elevating the gpx expression level, while the effects of promotion by compensation and inhibition by GSH deficiency on gpx expression were at equilibrium under 40 μg/L MC-LR exposure.
As a low-molecular-weight, cysteine-rich and metal-binding protein, metallothionein (MT) widely exists in various organisms and can effectively combine with ROS (Fang et al. 2010), so it can also participate in antioxidant defense system, especially in the tissues under stresses (Fig. 6e). It could be concluded that the up-regulation of mt in the gill was related to MC-LR-induced oxidative stress (Al Kaddissi et al. 2012). There were studies reporting that MC-LR could cause cytotoxicity in organisms (Rozman et al. 2017), which could further inhibit the expression of mt (Roesijadi et al. 1997). From the experimental results in the present study, it could be concluded that the inhibition of mt expression in different organs was caused by the cytotoxicity induced by MC-LR, and the degree of inhibition varied with different organ types and MC-LR concentrations. At the same time, the statistical significance of mt expression changes certificated that it could act as a biomarker indicating MC-LR toxicity in aquatic crustaceans.
Heat shock proteins (HSPs) can protect the body from apoptosis caused by oxidative stress by acting on multiple sites of apoptosis pathway (Garrido et al. 2001). Meanwhile, as reported by Molina et al. (2002), HSPs can protect cells from oxidative stress by obstructing irreversible loss of important proteins and promoting their subsequent regeneration. Generally, environmental stresses can up-regulate the expression of hsp70, and then maintain homeostasis in vivo by inhibiting protein denaturation (Morimoto and Santoro 1998; Christians et al. 2002). In addition, HSP70 is also related to innate immunity and adaptive immunity of organisms (Srivastava 2002). The expression results suggested that 10 and 40 μg/L MC-LR exposure induced obvious oxidative stress in the hepatopancreas and intestine, while the body maintained the homeostasis in vivo by up-regulating the expression level of hsp70 (Fig. 6f). Similar to this study, it has been found that the expression level of hsp70 in the liver of tilapia (Oreochromis niloticus) exposed to MC-LR was also up-regulated (He et al. 2010). In addition, it was found by immunohistochemical staining that MC-LR exposure could induce the expression of hsp70 in the liver of common carp (Cyprinus carpio L.) (Jiang et al. 2012). Therefore, the significant up-regulation of its expression could result from MC-LR-induced oxidative stress, which enhanced the translation of HSP70 as cell defense (Fig. 6f). The expression level of hsp70 in the gill in M10 group indicated that this concentration of MC-LR would reduce the content of HSP70 by inhibiting its expression. The reason for this change might be that the gill produced more SOD under this condition, which was enough to reduce or eliminate excessive ROS produced in the gill, thus reducing the demand for HSP70. However, when MC-LR concentration was 40 μg/L, the promotion and inhibition of this gene expression caused by environmental stress just reached a balance, so the expression level of this gene in the gill showed no significant change.
There were studies (Lin et al. 2018; Duan et al. 2022) documenting that MC-LR could cause severe histological injuries in the liver or hepatopancreas of D. rerio and Pacific white shrimp (Litopenaeus vannamei). Based on the result in this study and above reports, it was found that MC-LR could destroy the normal physiological structure of hepatopancreas at the histological level, and might bring adverse effects on the crucial functions including detoxification, metabolism and immunity. Additionally, the gills of P. clarkii exhibited similar pathological changes and damaged characteristics of zebrafish caused by MC-LR (Chen et al. 2016). The pathological changes of gills in this study suggested that MC-LR exposure at environmental concentrations could pose a hazard to the processes of gas exchange and ionic regulation of crayfish. As for intestines, there were reports supporting the viewpoints that EGCs function as inflammatory cells (Reite 1997) and participate in host defense (Da Silva et al. 2017). The elevated quantity of lymphocytes could be attributed to immunological reaction to produce larger numbers of antibodies as responding to MC-LR-induced stress. Hence, the showing up of abnormal muscularis, EGCs and lymphocyte infiltration in the intestine demonstrated that MC-LR caused intestinal histological damage and induced inflammatory and immune responses of P. clarkii.
In the intestinal flora of P. clarkii, Proteobacteria, Firmicutes, Fusobacteria and Bacteroidetes were predominant phyla, which was consistent with some other studies on the intestinal flora of P. clarkii under different stresses (Chen et al. 2021; Huang et al. 2021; Xue et al. 2022). The dominance of those phyla indicated that they could play significant roles in the intestinal functions including immunity, digestion and absorption of crayfish. Firmicutes can enhance the fatty acid absorption in the host intestine (Semova et al. 2012), and its increased abundance in our research demonstrated that 40 μg/L MC-LR exposure could accelerate the absorption process of fatty acid. NH4+ and NO3− can be used by Planctomycetes as substrates to generate nitrogen through anaerobic oxidation. Its abundance showed a positive correlation with the concentration of organic nitrogen (Tal et al. 2003), and its dominance in Con group (Con vs M10 and Con vs M40) suggested both concentrations of MC-LR might reduce the amount of organic nitrogen in the intestine of crayfish. Pseudorhodobacter is a genus capable of generating diketopiperazine and alloxazine alkaloids (Youn et al. 2019). Its enrichment in Con group (Con vs M10) suggested 10 μg/L MC-LR exposure could inhibit the production of those compounds by the intestinal microbiota. The genus Anaerorhabdus can generate a high level of acetate (Parte et al. 2011), while adding acetate as a feed could exert inhibition on pathogens in marine shrimps (Da Silva et al. 2013) and decrease inflammatory variations in intestine (Maslowski et al. 2009). Dysgonomonas has been reported by Sun et al. (2015) to be mainly involved in decomposing lignocellulose and offering nutrients to the host. The decreased abundances of Anaerorhabdus and Dysgonomonas indicated that those corresponding physiological functions were disturbed by 40 μg/L MC-LR. It has been found by Tao et al. (2006) that Brevundimonas can generate hydroxylated astaxanthins, and its enrichment in Con group (Con vs M40) suggested that the production of this type of compound was potentially restricted by 40 μg/L MC-LR. Therefore, it could be concluded that MC-LR exposure disrupted the composition of intestinal flora, which could further hazard the intestinal health status of P. clarkii.
PICRUSt analysis indicated that the altered intestinal microbiota was involved in a variety of metabolic and genetic pathways at KEGG level 3. The decreased abundances of sequences involved in the pathways related to the metabolism of glycine, serine, threonine, galactose, porphyrin, chlorophyll and mineral absorption indicated that those corresponding metabolic functions of intestinal flora could be inhibited by MC-LR exposure. Similarly, Duan et al. (2020) also found that mineral absorption of intestinal flora was significantly inhibited in L. vannamei exposed to MC-LR. Meanwhile, the remarkably increased proportion of functional genes related to DNA replication, repair and recombination in the intestinal flora of P. clarkii exposed to MC-LR demonstrated that MC-LR exposure had the potential to accelerate intestinal microbial genetic information transmission. The findings on the KEGG pathways suggested that disruption of intestinal flora caused by MC-LR exposure might further cause abnormal intestinal microbial metabolism and genetics in P. clarkii.
Conclusion
Overall, MC-LR exposure could pose adverse effects on P. clarkii at the histological and molecular levels. The hepatopancreas, gills and intestines of P. clarkii could effectively accumulate MC-LR. Meanwhile, antioxidant-related genes including Mn-sod, cat, gst, gpx, mt and hsp70 showed different expression trends in different organs to respond to MC-LR-induced oxidative stress. MC-LR could damage the histological structures of hepatopancreas, gills and intestines, thus affecting their corresponding physiological functions. Additionally, MC-LR could result in the disruption of intestinal flora, which might further cause abnormal intestinal microbial metabolism and genetics in P. clarkii. This study can extend existing knowledge regarding the toxic effects of MCs on aquatic crustaceans and elucidate the deep mechanisms.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Afgan E, Baker D, Batut B, van den Beek M, Bouvier D, Cech M, Chilton J, Clements D, Coraor N, Grüning BA, Guerler A, Hillman-Jackson J, Hiltemann S, Jalili V, Rasche H, Soranzo N, Goecks J, Taylor J, Nekrutenko A, Blankenberg D (2018) The galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res 46(W1):W537–W544
Al Kaddissi S, Legeay A, Elia AC, Gonzalez P, Camilleri V, Gilbin R, Simon O (2012) Effects of uranium on crayfish Procambarus clarkii mitochondria and antioxidants responses after chronic exposure: What have we learned? Ecotoxicol Environ Saf 781:218–224
Cazenave J, Bistoni D, Pesce SE, Wunderlin DA (2006) Differential detoxification and antioxidant response in diverse organs of Corydoras paleatus experimentally exposed to microcystin-RR. Aquat Toxicol 76:1–12
Chance B, Greenstein DS, Roughton FJW (1952) The mechanism of catalase action. I. Steady-state analysis. Arch Biochem Biophys 37:301–321
Chen C, Liu W, Wang L, Li J, Chen Y, Jin J, Kawan A, Zhang X (2016) Pathological damage and immunomodulatory effects of zebrafish exposed to microcystin-LR. Toxicon 118:13–20
Chen CJ, Wu GH, Kuo RL, Shih SR (2017) Role of the intestinal microbiota in the immunomodulation of influenza virus infection. Microbes Infect 19:570–579
Chen H, Wang Y, Zhang J, Bao J (2021) Intestinal microbiota in white spot syndrome virus infected red swamp crayfish (Procambarus clarkii) at different health statuses. Aquaculture 542:736826
Christians ES, Yan LJ, Benjamin IJ (2002) Heat shock factor 1 and heat shock proteins: critical partners in protection against acute cell injury. Crit Care Med 30:S43–S50
Da Silva BC, Do Vieira FN, Mourino JLP, Ferreira GS, Seiffert WQ (2013) Salts of organic acids selection by multiple characteristics for marine shrimp nutrition. Aquaculture 384:104–110
Da Silva WF, Simões MJ, Gutierre RC, Egami MI, Santos AA, Antoniazz MM, Sasso GR, Ranzani-Paiva MJT (2017) Special dyeing, histochemistry, immunohistochemistry and ultrastructure: A study of mast cells/eosinophilic granules cells (MCs/EGC) from Centropomus parallelus intestine. Fish Shellfish Immunol 60:502–508
De Melo MS, Dos Santos TPG, Jaramillo M, Nezzi L, Muller YMR, Nazari EM (2019) Histopathological and ultrastructural indices for the assessment of glyphosate-based herbicide cytotoxicity in decapod crustacean hepatopancreas. Aquat Toxicol 210:207–214
Duan Y, Xiong D, Wang Y, Dong H, Huang J, Zhang J (2020) Effects of Microcystis aeruginosa and microcystin-LR on intestinal histology, immune response, and microbial community in Litopenaeus vannamei. Environ Pollut 265:114774
Duan Y, Zeng S, Lu Z, Dan X, Mo Z, Xing Y, Zhang J, Li Y (2022) Responses of lipid metabolism and lipidomics in the hepatopancreas of Pacific white shrimp Litopenaeus vannamei to microcystin-LR exposure. Sci Total Environ 820:153245
Fang Y, Yang H, Wang T, Liu B, Zhao H, Chen M (2010) Metallothionein and superoxide dismutase responses to sublethal cadmium exposure in the clam Mactra veneriformis. Comp Biochem Physiol C Toxicol Pharmacol 151(3):325–333
Garrido C, Gurbuxani S, Ravagnan L, Kroemer G (2001) Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Bioph Res Co 286:433–442
Gherardi F (2006) Crayfish invading Europe: the case study of Procambarus clarkii. Mar Freshw Behav Phy 39:175–191
Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE (2006) Metagenomic analysis of the human distal gut microbiome. Science 312:1355–1359
Goncalves-Soares D, Zanette J, Yepiz-Plascencia GM, Bainy ACD (2012) Expression and activity of glutathione S-transferases and catalase in the shrimp Litopenaeus vannamei inoculated with a toxic Microcystis aeruginosa strain. Marine Environ Res 17:54–61
Guecheva TN, Erdtmann B, Benfato MS, Henriques JA (2003) Stress protein response and catalase activity in freshwater planarian Dugesia (Girardia) schubarti exposed to copper. Ecotoxicol Environ Saf 3:351–357
He S, Liang XF, Li RQ, Li GG, Wang L, Shen D (2010) Molecular characterization of heat shock protein 70 genes in the liver of three warm freshwater fishes with differential tolerance to microcystin-LR. J Biochem Mol Toxic 24:293–302
Henry RP, Čedomil L, Onken H, Weihrauch D (2012) Multiple functions of the crustacean gill: osmotic/ionic regulation, acid-base balance, ammonia excretion, and bioaccumulation of toxic metals. Front Physiol 3:431–431
Hernández AF, Lacasaña M, Gil F, Rodríguez-Barranco M, Pla A, López-Guarnido O (2013) Evaluation of pesticide-induced oxidative stress from a gene-environment interaction perspective. Toxicology 307:95–102
Hou J, Li L, Xue T, Long M, Su Y, Wu N (2015) Hepatic positive and negative antioxidant responses in zebrafish after intraperitoneal administration of toxic microcystin-LR. Chemosphere 120:729–736
Huang Y, Hong Y, Yin H, Yan G, Huang Q, Li Z, Huang Z (2021) Imidacloprid induces locomotion impairment of the freshwater crayfish, Procambarus clarkii via neurotoxicity and oxidative stress in digestive system. Aquat Toxicol 238:105913
Ibelings BW, Backer LC, Kardinaal WEA, Chorus I (2015) Current approaches to cyanotoxin risk assessment and risk management around the globe. Harmful Algae 49:63–74
Jiang J, Shi Y, Shan Z, Yang L, Wang X, Shi L (2012) Bioaccumulation, oxidative stress and HSP70 expression in Cyprinus carpio L. exposed to microcystin-LR under laboratory conditions. Comp Biochem Physiol C Toxicol Pharmacol 155:483–490
Kaushik S, Kaur J (2003) Chronic cold exposure affects the antioxidant defense system in various rat tissues. Clin Chim Acta 333:69–77
Kono Y, Fridovich I (1982) Superoxide radical inhibits catalase. J Biol Chem 257:5751–5754
Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Thurber RLV, Knight R, Beiko RG, Huttenhower C (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31:814–821
Li H, Cai Y, Xie P, Chen J, Hao L, Li G, Xiong Q (2012) Identification and expression profile of Id1 in bighead carp in response to microcystin-LR. Environ Toxicol Pharmacol 34:324–333
Lin W, Hou J, Guo H, Li L, Wang L, Zhang D, Li D, Tang R (2018) The synergistic effects of waterborne microcystin-LR and nitrite on hepatic pathological damage, lipid peroxidation and antioxidant responses of male zebrafish. Environ Pollut 235:197–206
Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR (2009) Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461:1282–1286
Mazzei V, Longo G, Brundo MV, Sinatra F, Copat C, Conti GO, Ferrante M (2014) Bioaccumulation of cadmium and lead and its effects on hepatopancreas morphology in three terrestrial isopod crustacean species. Ecotoxicol Environ Saf 110:269–279
Min BH, Ravikumar Y, Lee DH, Choi KS, Kim BM, Rhee JS (2018) Age-dependent antioxidant responses to the bioconcentration of microcystin-LR in the mysid crustacean, Neomysis awatschensis. Environ Pollut 232:282–294
Molina A, Carpeaux R, Martial JA, Muller M (2002) A transformed fish cell line expressing a green fluorescent protein-luciferase fusion gene responding to cellular stress. Toxicol in Vitro 16:201–207
Morimoto RI, Santoro MG (1998) Stress-inducible responses and heat shock proteins: New pharmacologic targets for cytoprotection. Nat Biotechnol 16(9):833–838
Müller YMR, de Melo MS, Weiss VMC, de Quadros T, Ammar D, Nazari EM (2020) Ultraviolet B radiation affects epithelial cell morphology and ultrastructure in the hepatopancreas of the freshwater decapod Macrobrachium olfersii. Ecotoxicol Environ Saf 204:111096
Nayak SK (2010) Role of gastrointestinal microbiota in fish. Aquacult Res 41(11):1553–1573
Ni L, Wu H, Du C, Li X, Li Y, Xu C, Wang P, Li S, Zhang J, Chen X (2021) Effects of allelochemical artemisinin in Artemisia annua on Microcystis aeruginosa: growth, death mode, and microcystin-LR changes. Environ Sci Pollut Res 28:45253–45265
Parte A, Krieg NR, Ludwig W, Whitman W, Hedlund BP, Paster BJ, Staley JT, Ward N, Brown D (2011) Bergey's manual of systematic bacteriology. Volume 4: The Bacteroidetes, Spirochaetes, Tenericutes (Mollicutes), Acidobacteria, Fibrobacteres, Fusobacteria, Dictyoglomi, Gemmatimonadetes, Lentisphaerae, Verrucomicrobia, Chlamydiae, and Planctomycetes. Springer, New York
Pawlik-Skowrońska B, Bownik A (2021) Cyanobacterial anabaenopeptin-B, microcystins and their mixture cause toxic effects on the behavior of the freshwater crustacean Daphnia magna (Cladocera). Toxicon 198:1–11
Pham TL, Utsumi M (2018) An overview of the accumulation of microcystins in aquatic ecosystems. J Environ Manage 213:520–529
Qu X, Hu M, Shang Y, Pan L, Jia P, Fu C, Liu Q, Wang Y (2018) Liver transcriptome and miRNA analysis of silver carp (Hypophthalmichthys molitrix) intraperitoneally injected with microcystin-LR. Front Physiol 9:381
Reite OB (1997) Mast cells/eosinophilic granule cells of salmonids: staining properties and responses to noxious agents. Fish Shellfish Immunol 7(8):567–584
Rőszer T (2014) The invertebrate midintestinal gland (“hepatopancreas”) isan evolutionary forerunner in the integration of immunity and metabolism. Cell Tissue Res 358:685–695
Roesijadi G, Brubacher LL, Unger ME, Anderson RS (1997) Metallothionein mRNA induction and generation of reactive oxygen species in molluscan hemocytes exposed to cadmium in vitro. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 118(2):171–176
Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, Tuohy K (2018) Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr 57:1–24
Rozman KB, Jurič DM, Šuput D (2017) Selective cytotoxicity of microcystins LR, LW and LF in rat astrocytes. Toxicol Lett 265:1–8
Rymuszka A, Adaszek L (2012) Pro- and anti-inflammatory cytokine expression in carp blood and head kidney leukocytes exposed to cyanotoxin stress–an in vitro study. Fish Shellfish Immunol 33:382–388
Sabatini SE, Brena BM, Pirez M, de Molina MCR, Luquet CM (2015) Oxidative effects and toxin bioaccumulation after dietary microcystin intoxication in the hepatopancreas of the crab Neohelice (Chasmagnathus) granulata. Ecotoxicol Environ Saf 120:136–141
Schreidah C, Ratnayake K, Senarath K, Karunarathne A (2020) Microcystins: Biogenesis, toxicity, analysis, and control. Chem Res Toxicol 33:2225–2246
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12(6):R60
Semova I, Carten JD, Stombaugh J, Mackey LC, Knight R, Farber SA, Rawls JF (2012) Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe 12:277–288
Serafini S, De Freitas SC, Baldissera MD, Baldisserotto B, Picoli F, Segat JC, Baretta D, Da Silva AS (2019) Fish exposed to eprinomectin show hepatic oxidative stress and impairment in enzymes of the phosphotransfer network. Aquaculture 508:199–205
Srivastava P (2002) Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol 2:185–194
Sun S, Zheng C, Shi X (2021) Effect of paternal exposure to microcystin-LR on testicular dysfunction, reproduction, and offspring immune response in the oriental river prawn (Macrobrachium nipponense). Aquaculture 534:736332
Sun X, Yang Y, Zhang N, Shen Y, Ni J (2015) Draft genome sequence of Dysgonomonas macrotermitis strain JCM 19375T, isolated from the gut of a termite. Genome Announc 3(4):e00963-e1015
Tal Y, Watt JEM, Schreier SB, Sowers KR, Schreier HJ (2003) Characterization of the microbial community and nitrogen transformation processes associated with moving bed bioreactors in a closed recirculated mariculture system. Aquaculture 215(1–4):187–202
Tang D, Shi X, Guo H, Bai Y, Shen C, Zhang Y, Wang Z (2020) Comparative transcriptome analysis of the gills of Procambarus clarkii provides novel insights into the immune-related mechanism of copper stress tolerance. Fish Shellfish Immunol 96:32–40
Tao L, Rouvière PE, Cheng QA (2006) Carotenoid synthesis gene cluster from a non-marine Brevundimonas that synthesizes hydroxylated astaxanthin. Gene 379:101–108
Vioque-Fernándeza A, Alves de Almeidab E, López-Barea J (2009) Assessment of Doñana National Park contamination in Procambarus clarkii: integration of conventional biomarkers and proteomic approaches. Sci Total Environ 407:1784–1797
WHO (2011) Guidelines for Drinking Water Quality, 4th edn. World Health Organization, Geneva, Switzerland
Wu J, Yuan M, Song Y, Sun F, Han X (2015) MC-LR exposure leads to subfertility of female mice and induces oxidative stress in granulosa cells. Toxins 7(12):5212–5223
Xie L, Xie P, Guo L, Li L, Miyabara Y, Park H (2005) Organ distribution and bioaccumulation of microcystins in freshwater fish at different trophic levels from the eutrophic Lake Chaohu, China. Environ Toxicol 20:293–300
Xue M, Jiang N, Fan Y, Yang T, Li M, Liu W, Li Y, Li B, Zeng L, Zhou Y (2022) White spot syndrome virus (WSSV) infection alters gut histopathology and microbiota composition in crayfish (Procambarus clarkii). Aquacult Rep 22:101006
Yuan J, Gu Z, Zheng Y, Zhang Y, Gao J, Chen S, Wang Z (2016) Accumulation and detoxification dynamics of microcystin-LR and antioxidant responses in male red swamp crayfish Procambarus clarkii. Aquat Toxicol 177:8–18
Youn UJ, Lee JH, Han SJ (2019) Diketopiperazine and alloxazine alkaloids from the antarctic bacteria, Pseudorhodobacter psychrotolerans sp. nov. Biochem Syst Ecol 85:21–23
Zhang W, Li J, Chen Y, Si Q, Tian J, Jiang Q, Yang J (2019a) Exposure time relevance of response to nitrite exposure: Insight from transcriptional responses of immune and antioxidant defense in the crayfish, Procambarus clarkii. Aquat Toxicol 214:105262
Zhang Y, Li Z, Kholodkevich S, Sharov A, Feng Y, Ren N, Sun K (2019b) Cadmium-induced oxidative stress, histopathology, and transcriptome changes in the hepatopancreas of freshwater crayfish (Procambarus clarkii). Sci Total Environ 666:944–955
Zhang Y, Li Z, Kholodkevich S, Sharov A, Chen C, Feng Y, Ren N, Sun K (2020a) Effects of cadmium on intestinal histology and microbiota in freshwater crayfish (Procambarus clarkii). Chemosphere 242:125105
Zhang Y, Li Z, Kholodkevich S, Sharov A, Feng Y, Ren N, Sun K (2020b) Microcystin-LR-induced changes of hepatopancreatic transcriptome, intestinal microbiota, and histopathology of freshwater crayfish (Procambarus clarkii). Sci Total Environ 711:134549
Zhang Y, Zhuang H, Yang H, Xue W, Wang L, Wei W (2019c) Microcystin-LR disturbs testicular development of giant freshwater prawn Macrobrachium rosenbergii. Chemosphere 222:584–592
Funding
This work was financially supported by the Scientific Research Project of the Education Department of Jilin Province (No. JJKH20220345KJ) and the Research Start-up Fund Project of Jilin Agricultural University (No. 201020742).
Author information
Authors and Affiliations
Contributions
Yu Zhang: Conceptualization, Investigation, Methodology, Writing—original draft, Funding acquisition. Zheyu Li: Data curation, Investigation, Methodology, Writing—review and editing. Xing Tian: Data curation, Investigation, Methodology. Pianpian Xu: Data curation, Investigation, Methodology. Kai Sun: Funding acquisition, Investigation, Supervision. Nanqi Ren: Investigation, Project administration, Supervision.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Li, Z., Tian, X. et al. Acute toxic effects of microcystin-LR on crayfish (Procambarus clarkii): Insights from antioxidant system, histopathology and intestinal flora. Environ Sci Pollut Res 30, 56608–56619 (2023). https://doi.org/10.1007/s11356-023-26171-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26171-6