Abstract
Previous studies have indicated that exposure to a single toxic metal can cause renal tubular damage, while evidence about the effects of multimetal exposure on renal tubular damage is relatively limited. We aimed to evaluate the relationships of multimetal coexposure with renal tubular damage in adults in heavy metal-polluted rural regions of China. A cross-sectional study of 1918 adults in China’s heavy metal-contaminated rural regions was conducted. Inductively coupled plasma–mass spectrometry (ICP–MS) was used to measure the plasma levels of 18 metals in participants, and immune turbidimetry was used to measure sensitive biological indicators, reflecting renal tubular damage (including retinol-binding protein and β2-microglobulin). Least absolute shrinkage and selection operator (LASSO) penalized regression analysis, logistic and linear regression analysis, restricted cubic spline (RCS) regression analysis and the Bayesian kernel machine regression (BKMR) method were used to explore associations of multimetal coexposure with renal tubular damage risk or renal tubular damage indicators. Plasma selenium, cadmium, arsenic, and iron were identified as the main plasma metals associated with renal tubular damage risk after dimensionality reduction. Multimetal regression models showed that selenium was positively associated, and iron was negatively associated with renal tubular damage risk or its biological indicators. Multimetal RCS analyses additionally revealed a non-linear relationship of selenium with renal tubular damage risk. The BKMR models showed that the metal mixtures were positively associated with biological indicators of renal tubular damage when the metal mixtures were above the 50th percentile of concentration. Our findings indicated that natural exposure to high levels of multimetal mixtures increases the risk of renal tubular damage. Under the conditions of multimetal exposure, selenium was positively associated, and iron was negatively associated with renal tubular damage risk or its biological indicators.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD), which has a high global prevalence and mortality (Bikbov et al. 2020), is a growing public health concern worldwide. China, which has the largest population and renal disease health care system, is facing an unprecedented economic and social burden of CKD (Liu 2013). In a nationwide survey conducted between 2009 and 2010, the prevalence of CKD in China has been reported to be 10.8% (Zhang et al. 2012). Risk factor identification coupled with early disease detection based on sensitive biomarkers has apparently become the core of CKD management.
Environmental factors were reported to account for 22% of the global burden of disease and 23% of deaths (Xu et al. 2018), and the hemofiltration function of human kidneys (almost 20% of the cardiac output of blood) makes kidneys especially vulnerable to environmental pollutants. Although some of the developing countries, including China, have achieved the rapid development of industrialization and urbanization during the past few decades, the accompanying environmental problems such as the release of metal pollutants, also posed some challenges to people’s health. Therefore, apart from traditionally known kidney health risk factors such as obesity, hypertension, and diabetes (Jha et al. 2013), increasing attention has been shifted to the associations between environmental pollutants represented by heavy metals and kidney health (Cui et al. 2018; Xu et al. 2018; Yang et al. 2019; Liu et al. 2020; Jalili et al. 2021).
Exposure to environmental metals, which can occur through air, food, drinking water, and soil (Soderland et al. 2010), is prevalent in daily life, especially in mineral-contaminated areas. Exposure to inorganic metal elements can adversely affect human health, and the kidney is one of the major target organs in metal exposure due to its characteristics of glomerular and tubular changes (Barbier et al. 2005; Orr and Bridges 2017). Renal tubular dysfunction is the most common manifestation of chronic cadmium and arsenic exposure, mainly through mechanisms including oxidative stress, DNA methylation, histone acetylation, impaired DNA repair, and apoptosis (Scott et al. 1993; Bork et al. 2010; Kim et al. 2015; Nair et al. 2015). The natural existence of heavy metals along with mining and smelting activities may increase both workers’ and nearby residents’ kidney damage risks, as metals accumulated in human kidneys are difficult to degrade (Soderland et al. 2010). People are naturally exposed to multiple metals, and synergistic or antagonistic effects caused by metal mixtures could be much more complex than previously thought (Braun et al. 2016; Shelley et al. 2012; Lin et al. 2014; Tsai et al. 2017). However, previous epidemiological studies have focused largely on the effects of exposure to a single or several metals on kidney health. Among these metals, major toxic metals, such as lead, cadmium, and arsenic, have received the most extensive attention (Ekong et al. 2006; Johri et al. 2010; Robles-Osorio et al. 2015). For other metals and trace elements, data are limited, and the effects of coexposure to multiple metals on kidney health are relatively unknown. In addition, although studies have previously explored the relationships between multiple metals and kidney function, in which the estimated glomerular filtration rate reflecting a severe decline in renal function was selected as the indicator, they may have ignored the broader population with early kidney damage and therefore underestimated the effects of metal elements on renal function (Yang et al. 2019; Liu et al. 2020). Considering that CKD is an irreversible disease, the identification of subclinical kidney damage (especially tubular damage) risk metals is of great preventive significance to patients with CKD.
Hunan province is located in the hilly areas of southern China, which is rich in mineral resources and dense population. The processing and refining of non-ferrous metals have caused serious heavy metal pollution to this region (Li et al. 2018; Du et al. 2019; Kan et al. 2021). In the current cross-sectional study, we analyzed the associations of coexposure to multiple plasma metals with local villagers’ renal tubular damage reflected by abnormal levels of retinol-binding protein (RBP) and β2-microglobulin (β2-MG). This is the first study of coexposure to 18 plasma metals and renal tubular damage in a Chinese population, and our findings will provide new clues and insights into the impact of exposure to metals on renal tubular damage.
Materials and methods
Study subjects
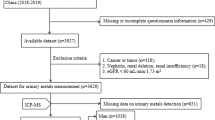
A total of 2764 villagers were recruited from August 2016 to July 2017 in three counties (Shimen, Qingshuitang, and Huayuan) of Hunan Province. The selected southern districts are heavy industrial bases and rich in non-ferrous metal resources. The local soil, air, and water are seriously polluted by heavy metals. Our inclusion criteria were as follows: (1) participants aged ≥ 18 years and (2) those who had been an area resident for at least 5 years. The exclusion criteria were as follows: (1) a lack of main data, such as plasma metal elements and biomarkers; (2) a lack of covariate data; (3) outliers with abnormal metal levels; and (4) serious diseases such as cancer and stroke. After screening, the number of participants ultimately included in this study was 1918. The study was approved by the Medical Ethics Committee of Xiangya Hospital, Central South University, and all participants signed informed consent forms.
Data collection and variable definitions
Each participant was required to complete a questionnaire. Trained investigators collected personal information, including age, sex, education level, and income level, as well as lifestyle information, including alcohol intake, smoking status, and exercise frequency. In addition, personal histories of diseases, including hypertension and hyperlipidemia, were also collected. In the questionnaire, education level was categorized as primary school or less, junior high school, high school, and college or more. Annual household income was classified as below ten thousand yuan, ten to thirty thousand yuan, thirty to fifty thousand yuan, and fifty thousand yuan or more. Drinking and smoking status were categorized as never, current, and past, while exercise in the last 6 months was categorized as yes or no. Clinical traits, including weight, height, systolic blood pressure, and diastolic blood pressure, were measured. Blood pressure measurements were taken in the sitting position after at least 5 min of rest. Body mass index (BMI) was calculated as the weight in kilograms divided by the square of the height in meters. Hypertension was defined as a blood pressure ≥ 140/90 mmHg or self-reported physician-diagnosed hypertension. All blood samples were collected the next morning after 8 h of fasting and used to detect total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglyceride, and metal element concentrations. Hyperlipidemia was defined as a triglyceride level ≥ 2.26 mmol/L, a total cholesterol level ≥ 6.22 mmol/L, an HDL-C level < 1.04 mmol/L, an LDL-C level ≥ 4.14 mmol/L, or a previous physician diagnosis of hyperlipidemia. Morning urine was used to measure the levels of creatinine, RBP, and β2-MG. Since a β2-MG level ≥ 300 μg/g creatinine or an RBP level ≥ 300 μg/g creatinine was deemed abnormal (Aitio et al. 2007); subclinical tubular impairment was defined as a β2-MG level ≥ 300 μg/g creatinine or an RBP level ≥ 300 μg/g creatinine.
Measurement of exposure and biological indicators
The measurement of plasma metal element concentrations has been described in a previous article (Huang et al. 2021). Peripheral venous heparin blood specimens were collected after overnight fasting for at least 8 h. All samples were sent to the local laboratory for centrifugation within 2 h and were stored at − 80℃. Plasma concentrations of 18 metal elements, including vanadium (V), nickel (Ni), chromium (Cr), zinc (Zn), manganese (Mn), rubidium (Rb), iron (Fe), cobalt (Co), copper (Cu), selenium (Se), arsenic (As), molybdenum (Mo), tin (Sn), lead (Pb), antimony (Sb), cadmium (Cd), tungsten (W), and uranium (U), were detected by an inductively coupled plasma mass spectrometer (ICP–MS; Agilent Technologies, Waldbronn, Germany). Quality control of plasma metal measurements was managed by spiked recovery of pooled plasma samples (random sample extraction of 200 samples), three replicate measurements, and analysis of certified reference agents provided by ClinChek®controls (no. 8883 and no. 8884). Approximately 30 mL of the first morning midstream urine was collected from each participant and was kept frozen (− 20 °C) before the analysis. The concentrations of urinary RBP and β2-MG were determined at the hospital laboratory by immune transmission turbidimetry and immune latex turbidimetry, respectively. Considering that the concentrations of biomarkers in urine samples vary according to the water content in the urine sample, a standardized method of dividing the urinary tubular marker concentration by the urinary creatinine concentration was applied in our study.
Statistical analysis
Descriptive data on continuous variables are reported as the means ± standard deviations or the median (25th and 75th), and categorical variables are summarized as counts (percentages). Student’s t tests or Wilcoxon rank-sum tests were used for the comparison of continuous variables according to the skewness of the data, while categorical variables were compared using chi-square analysis. In addition, Wilcoxon rank-sum tests were also used to validate the distinction of metal element concentrations between participants with and without renal tubular damage. Concentrations of plasma metals and renal tubular damage indicators were log10-transformed in the following analyses because of their skewed distributions. Considering the moderate or high correlations among some plasma metals (Huang et al. 2021), LASSO penalized regression analysis (Lv et al. 2021) was performed first to select the plasma metals with the greatest effect on renal tubular damage risk. All the covariates and 18 metals were included in our LASSO regression model. To evaluate the relationship between the selected metal elements and renal tubular damage, we further applied single- and multimetal logistic regression models to calculate the odds ratios (ORs) and 95% confidence intervals (CIs). Multimetal linear regression analyses were used to assess the associations between the selected metal elements and renal tubular damage indicators. In addition, an RCS regression model was employed to analyze the dose–response associations of multiple-metal exposure and renal tubular damage risk. The overall association of coexposure to the selected metals with renal tubular damage indicators was calculated by BKMR analysis (Bobb et al. 2018). Finally, subgroup analyses were performed to evaluate whether the associations of the selected metals with renal tubular damage varied by different disease statuses (hypertension and dyslipidemia). All data were statistically analyzed using Stata software version 16.0 (Stata Corp, College Station, Texas, USA) and R 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria). In all analyses, a two-sided value of P < 0.05 was considered statistically significant.
Results
Baseline characteristics and plasma metal levels of the study participants
A total of 1918 participants were recruited at three heavy industrial bases in Hunan Province, South China, from August 2016 to July 2017. The general characteristics of the participants are summarized in Table 1. The mean age of the participants was 55.5 years, and 60.0% of participants were women. Approximately 52.4% of the participants had hypertension, and 86.4% suffered from dyslipidemia. Among the total population, 56% of the participants had less than a primary school education, and approximately a quarter of the participants were smokers. The clinical values of urinary β2-MG and RBP were 78.5 (29.0 and 208.3) μg/g creatinine and 44.2 (20.7 and 197.8) μg/g creatinine, respectively. The concentrations of the 18 plasma metals are presented in Table 2. The levels of Cu, As, Se, Cd, and Sn were significantly higher (all P < 0.05) in the participants with renal tubular damage than in the participants without renal tubular damage.
Selection of main plasma metals associated with renal tubular damage by the LASSO regression method
We conducted LASSO penalized regression models to reduce the dimensionality, and a total of 9 variables were selected within the optimal value of λ (the largest log (λ) with the minimum binomial deviance) based on tenfold cross-validation. Among the selected 9 variables, Se, Cd, As, and Fe were identified as the main metals associated with the risk of renal tubular damage (Fig. S.1).
Logistic regression analyses
Single-metal logistic regression analyses and multiple-metal logistic regression analyses were performed to evaluate the relationship of metal element concentrations with renal tubular damage (Table 3). In the covariate-adjusted single-metal model (Model 2), compared with those in the lowest quartile, participants in the highest quartile of As, Cd, and Se were at 1.46-fold (95% CI 1.09, 1.96), 1.53-fold (95% CI 1.14, 2.05), and 2.71-fold (95% CI 2.01, 3.67) higher risks for renal tubular damage, respectively. In the covariate-adjusted multimetal model (Model 3), compared with the first quartile, no significant associations were found between the fourth quartile of As or Cd and renal tubular damage, and the adjusted ORs (95% CIs) in the fourth quartile were 3.14 (2.27 and 4.34) for Se and 0.60 (0.44 and 0.83) for Fe.
Dose–response relationships between the selected metals and renal tubular damage
In the multimetal adjusted RCS regression analyses, log-transformed plasma Fe (Poverall < 0.001, Pnon-linearity = 0.900), As (Poverall < 0.001, Pnon-linearity = 0.094), and Cd (Poverall < 0.001, Pnon-linearity = 0.066) showed linear associations with renal tubular damage risk. However, a significantly non-linear association was observed between log-transformed plasma Se and renal tubular damage risk (Poverall < 0.001, Pnon-linearity = 0.002), and the slope increased at the log10-transformed concentration of 1.79 μg/L (Fig. 1).
Multimetal-adjusted dose-response relationships of selected plasma metal levels with renal tubular damage risk. Models were adjusted for age, sex, education level, income level, drinking status, smoking status, exercise level, BMI, and the four metals (log-transformed). The black line and blue shadow represent the OR and 95% CI, respectively
Linear regression analyses
To assess the associations of the selected metals with RBP and β2-MG, multiple-metal linear regression analyses were further performed (Table 4). The covariate-adjusted multimetal linear regression model (Model 3) showed a significant positive association of Se levels with the two indicators (β2-MG, β 0.61, 95% CI 0.43, 0.80, P < 0.001; RBP, β 0.67, 95% CI 0.46, 0.88, P < 0.001). Fe levels were found to be negatively associated with β2-MG (β − 0.32, 95% CI − 0.49, − 0.16, P < 0.001) and RBP levels (β − 0.29, 95% CI − 0.48, − 0.11, P = 0.002). However, no associations were observed between plasma As or Cd and renal tubular damage indicators in our model.
Bayesian kernel machine regression analyses
The BKMR model was first used to assess the overall effects of exposure to metal mixtures on RBP (Fig. 2A) or β2-MG levels (Fig. 3A). Compared to when the four metals were at the 50th percentile of concentration, a significantly positive relationship of the metal mixtures with RBP or β2-MG levels was observed when the four metals were above the 50th percentile of concentration, and the positive correlation increased with increasing metal mixture levels. In contrast, the metal mixture presented a negative relationship with RBP or β2-MG levels when the four metals were below the 50th percentile of concentration, although not all levels had statistically significant differences, and the negative correlation decreased with increasing metal mixture levels. When the metals were fixed at the 25th, 50th, and 75th percentiles of concentration, Fe was significantly negatively associated with RBP (Fig. 2B) or β2-MG levels (Fig. 3B) at the 25th, 50th, and 75th percentiles of concentration, while Se was significantly positively associated with the two indicators. In addition, Cd was significantly positively associated with only RBP levels, while the association of As with the two indicators showed no statistically significant difference. In the analyses of RBP, increased estimates of Fe, Cd, and Se were observed (from the 25th to 75th percentile of concentration) when the levels of metals were set from the 25th to the 75th percentile of concentration. However, the estimates of Fe increased and Se decreased (from the 25th to the 75th percentile of concentration) when the levels of metals were set at the 25th to the 75th percentiles of concentration in the analyses of β2-MG.
Joint effect of the metal mixture on RBP levels. Data were estimated by the BKMR model adjusted for age, sex, education level, income level, drinking status, smoking status, exercise level, and BMI. The concentrations of RBP and metals were log-transformed. A Overall effect of the metal mixture on RBP levels (estimates and 95% CI). The metal mixture at different percentiles of concentration was compared to the metal mixture at the 50th percentile of concentration. B Single-exposure effect of the individual metals on RBP levels (estimates and 95% CI). The individual metal effects on RBP levels at the 25th, 50th, and 75th percentiles of concentration when the metals were fixed at the 25th, 50th, or 75th percentiles of concentration
Joint effect of the metal mixture on β2-MG levels. Data were estimated by the BKMR model adjusted for age, sex, education level, income level, drinking status, smoking status, exercise level, and BMI. The concentrations of β2-MG and metals were log-transformed. A Overall effect of the metal mixture on β2-MG levels (estimates and 95% CI). The metal mixture at different percentiles of concentration was compared to the metal mixture at the 50th percentile of concentration. B Single-exposure effect of the individual metal on β2-MG levels (estimates and 95% CI). The individual metal effects on β2-MG levels at the 25th, 50th, and 75th percentiles of concentration when the metals were fixed at the 25th, 50th, or 75th percentiles of concentration
Subgroup analysis
The associations of Se and Fe with renal tubular damage were further assessed by multimetal-adjusted subgroup analyses according to different disease statuses (Table S.1). The significantly harmful effect of plasma Se on renal tubules was consistently observed in all subgroups and was not modified by disease status. However, in participants with hypertension or dyslipidemia, we found a significantly protective effect of plasma Fe on renal tubules (hypertension: adjusted OR for Q4 versus Q1 = 0.59, 95% CI 0.39, 0.89; dyslipidemia: adjusted OR for Q4 versus Q1 = 0.55, 95% CI 0.39, 0.78). However, among individuals without hypertension or dyslipidemia, no significant associations of plasma Fe with renal tubular damage were observed (Fig. S.2).
Discussion
It is well known that RBP and β2-MG in urine are sensitive biological indicators of renal tubular damage. Under normal conditions, low molecular weight proteins such as RBP and β2-MG can be effectively reabsorbed by proximal tubules after free filtration from the glomerulus. When metals cause injury to the epithelial cells of tubules, tubule reabsorption dysfunction causes these low molecular weight proteins to appear in urine (Prozialeck and Edwards 2010; Klotz et al. 2013). Although early tubular functional impairment is considered a benign condition, and its significance is often overlooked, previous studies have indicated that renal tubular damage caused by heavy metals is irreversible or not completely recovered and that subclinical tubular dysfunction may be associated with long-term renal function decline (Piscator 1984; Iwata et al. 1993; Liang et al. 2012). In the current study, we explored the associations of plasma As, Cd, Se, and Fe with renal tubular damage and the two sensitive biological indicators after dimensionality reduction by LASSO regression. Single-metal models showed that exposure to Se, Cd, and As was a risk factor for renal tubular damage, while multiple-metal models indicated that Se was positively associated, and Fe was negatively associated with renal tubular damage risk or the two sensitive biological indicators. In addition, RCS analysis revealed a non-linear relationship of selenium with renal tubular damage risk. BKMR analyses further verified the positive associations of metal mixtures with RBP and β2-MG levels when they were above the 50th percentile of concentration. Last, according to the subgroup analyses, the effects of Fe exposure on renal tubular damage presented a discrepancy between participants with and without hypertension or dyslipidemia.
Toxic heavy metals
Cd and As are widely known as toxic heavy metals, and their kidney damage effects have been extensively explored in previous epidemiological and animal experiments. According to the experimental findings, As and Cd can activate the caspase family and p-53 apoptotic pathway in proximal tubules, which leads to the overproduction of free radicals and inflammatory factors, further promoting oxidative stress and inflammation of renal tubular epithelial cells and ultimately causing pyroptosis or apoptosis of cells (Robles-Osorio et al. 2015; Zhang et al. 2021; Chou et al. 2019). Our single-metal models showed that plasma As and Cd were positively associated with renal tubular damage, which was in accordance with previous epidemiological studies in humans (Liang et al. 2012; Akesson et al. 2005; Hong et al. 2004; Nordberg et al. 2005). However, interestingly, the hazardous effects of traditional toxic metals on renal tubules in our study were modified by multimetal exposure. One possible explanation for this finding is that the hazardous effects of As or Cd exposure might be attenuated because of the complex interactions of other metals. For example, previous experiments indicated that the absorption of Cd was negatively associated with the concentration of Fe and that a sufficient Fe level could protect against the toxicity of Cd (Jamakala and Rani 2015). Notably, Fe was included in our multimetal models, and therefore, the current findings may inspire the exploration and understanding of traditional toxic metals.
Iron
Although no significant association between Fe and renal tubular damage was found in single-metal models, a protective effect of Fe on renal tubules was observed for the first time in our multimetal models, and they seemed to have greater effects on populations with hypertension and dyslipidemia. Fe, an essential element for human energy metabolism, oxygen transport and enzymatic activity, is mainly stored in hemoglobin and myoglobin. The renal tubular epithelial system has been identified as one of the important places for Fe handling. The maintenance of Fe homeostasis plays an important role in renal health because both Fe overload and Fe deficiency can negatively affect renal function and may lead to renal tubular epithelial cell injury (Van Swelm et al. 2020). Since the relationship of plasma ionic Fe with the actual iron load of the human body is relatively unknown, and there has been no previous population-based epidemiological research focused on Fe ion exposure and renal tubular damage risk, our results warrant further investigation.
Selenium
In our study, Se was significantly associated with renal tubular damage in both single-metal models and multimetal models. Se is one of the major antioxidant trace elements (Battin and Brumaghim 2009) due to its role as a cofactor of glutathione peroxidase. The primary pathway for Se exposure is food, followed by polluted water and air, and the toxicity of Se depends on its different chemical forms (Barceloux 1999; Ullah et al. 2019). The relationship between Se and the kidney has rarely been explored in epidemiological investigations. In a 2-year prospective study aimed at investigating the etiology of endemic nephropathy, the association of Se with the kidney was first reported in humans (Karmaus et al. 2008). A positive correlation was found between serum Se and renal biomarkers (including creatinine clearance and β2-MG levels) in adult offspring, which supported Se exposure as a risk factor for renal tubular injury. In addition, animal experiments have shown that the nephrotoxicity of Se varies greatly in different chemical forms (Nagy et al. 2015). Exposure to excess Se (usually much higher than normal human intake) may be harmful to the kidney (Eckhert et al. 1992; Johnson et al. 2000) and cause lesions of proximal curved tubules (Cukierski et al. 1989). Although excessive Se exposure is considered harmful, the general intake of Se in the population is often considered beneficial. Early studies suggested that Se intake cannot only ameliorate oxidative stress-related diseases, such as cardiovascular diseases, diabetes, cancer, and CKD but also moderate the toxicity of metals such as Cd and As, which has made it widely used as a dietary and medicinal supplement for a long time (Faure 2003; Zachara 2015; Zhang et al. 2016; Cai et al. 2016; Zwolak 2020). However, it is worth noting that an increasing number of recent studies have shown that the protective effects of Se may be uncertain or even harmful (Vinceti et al. 2017, 2018a). Observational epidemiological studies in seleniferous areas have provided the most powerful evidence for the assessment of Se toxicity, which found a positive correlation of environmental Se exposure with CKD risk factors such as blood pressure and blood glucose (Kuruppu et al. 2014; Li et al. 2017; Vinceti et al. 2018b, 2019). These studies suggested that we need to take Se species, exposure times, concentrations, and confounding factors into consideration, especially in cross-sectional studies based on various countries and population groups, when evaluating the health effects of Se exposure (Rayman and Stranges 2013). The lowest observable adverse effect level (LOAEL) for blood Se set by the EPA is 1350 μg/L (Lemire et al. 2012). Although the concentration of Se in our study was much lower than this standard, it should be noted that the upper safe limit of Se levels may be lower than previously thought (Vinceti et al. 2009). Moreover, the total Se levels measured in our participants’ plasma do not reflect the species of Se and therefore cannot fully assess its toxicity. Although available evidence that directly support our results is limited, we suspect that long-term environmental Se exposure may have adverse effects on the kidneys. However, more research is needed to verify our conclusions.
This is the first study of coexposure to 18 plasma metals and renal tubular damage in a Chinese population. The multimetal models and BKMR models used in our study helped to assess the association of multimetal coexposure with renal tubular damage, which better reflected the real exposure situation. Therefore, our findings will provide new clues and insights into the impact of exposure to metals on renal tubular damage. Nevertheless, several limitations remain in our study. First, this was a cross-sectional study and causal inferences cannot be made. There are no conclusions regarding whether early renal injuries in our study population are reversible and whether they can predict the occurrence of CKD in the future; thus, a prospective follow-up is needed to confirm our findings. Second, due to the substantial missing of data for some covariates during the questionnaire survey, potentially important covariates such as daily dietary intake, years of residence at the study site, family history, and family inheritance were not considered in our models and therefore may influence our results. Third, except for RBP and β2MG, renal tubular damage indicators such as Kim Injury Molecule 1 (KIM-1), alpha-1 microglobulin (α1-MG) and N-acetyl-β-D-glucosaminidase (NAG) were not included in this study. Therefore, the effects of exposure to heavy metals on renal tubular damage may be underestimated.
Conclusion
Our findings indicated that exposure to high levels of multimetal mixtures may increase the risk of renal tubular damage and that plasma Se and Fe were the main metals related to renal tubular damage when coexposure to multiple metals occurred. Higher levels of Se might increase the risk of renal tubular damage, while higher levels of Fe appeared to attenuate the risk of renal tubular damage. To further confirm these relationships and explore the specific mechanisms, large prospective studies coupled with animal experiments are needed.
Data availability
Not applicable.
References
Aitio A, Bernard A, Fowler BA, Nordberg GF (2007) Chapter 4: biological monitoring and biomarkers. In: Nordberg GF, Fowler BA, Nordberg M, Friberg LT (eds) Handbook on the toxicology of metals, 3rd edn. Elsevier, New York, NY, USA, p 75
Akesson A, Lundh T, Vahter M, Bjellerup P, Lidfeldt J, Nerbrand C et al (2005) Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environ Health Perspect 113:1627–1631. https://doi.org/10.1289/ehp.8033
Barbier O, Jacquillet G, Tauc M, Cougnon M, Poujeol P (2005) Effect of heavy metals on, and handling by, the kidney. Nephron Physiol 99:105–110. https://doi.org/10.1159/000083981
Barceloux DG (1999) Selenium. J Toxicol Clin Toxicol 37:145–172. https://doi.org/10.1081/clt-100102417
Battin EE, Brumaghim JL (2009) Antioxidant activity of sulfur and selenium: a review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochem Biophys 55:1–23. https://doi.org/10.1007/s12013-009-9054-7
Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M et al (2020) Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 395:709–733. https://doi.org/10.1016/S0140-6736(20)30045-3
Bobb JF, Henn BC, Valeri L, Coull BA (2018) Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Health 17:67. https://doi.org/10.1186/s12940-018-0413-y
Bork U, Lee WK, Kuchler A, Dittmar T, Thévenod F (2010) Cadmium-induced DNA damage triggers G2/M arrest via chk1/2 and cdc2 in p53-deficient kidney proximal tubule cells. Am J Physiol Renal Physiol 298:255–265. https://doi.org/10.1152/ajprenal.00273.2009
Braun JM, Gennings C, Hauser R, Webster TF (2016) What can epidemiological studies tell us about the impact of chemical mixtures on human health? Environ Health Perspect 124:A6–A9. https://doi.org/10.1289/ehp.1510569
Cai X, Wang C, Yu W, Fan W, Wang S, Shen N et al (2016) Selenium exposure and cancer risk: an updated meta-analysis and meta-regression. Sci Rep 6:19213. https://doi.org/10.1038/srep19213
Chou X, Ding F, Zhang X, Ding X, Gao H, Wu Q et al (2019) Sirtuin-1 ameliorates cadmium-induced endoplasmic reticulum stress and pyroptosis through XBP-1s deacetylation in human renal tubular epithelial cells. Arch Toxicol 93:965–986. https://doi.org/10.1007/s00204-019-02415-8
Cui X, Cheng H, Liu X, Giubilato E, Critto A, Sun H et al (2018) Cadmium exposure and early renal effects in the children and adults living in a tungsten-molybdenum mining areas of South China. Environ Sci Pollut Res Int 25:15089–15101. https://doi.org/10.1007/s11356-018-1631-0
Cukierski MJ, Willhite CC, Lasley BL, Hendrie TA, Book SA, Cox DN et al (1989) 30-day oral toxicity study of L-selenomethionine in female long-tailed macaques (Macaca fascicularis). Fundam Appl Toxicol 13:26–39. https://doi.org/10.1016/0272-0590(89)90304-7
Du Y, Chen L, Ding P, Liu L, He Q, Chen B et al (2019) Different exposure profile of heavy metal and health risk between residents near a Pb-Zn mine and a Mn mine in Huayuan county, South China. Chemosphere 216:352–364. https://doi.org/10.1016/j.chemosphere.2018.10.142
Eckhert CD, Lockwood MK, Hsu MH, Ho J, Kang R (1992) Microvascular changes in rat glomeruli as a consequence of small differences in selenium exposure. Exp Mol Pathol 57:222–234. https://doi.org/10.1016/0014-4800(92)90013-2
Ekong EB, Jaar BG, Weaver VM (2006) Lead-related nephrotoxicity: a review of the epidemiologic evidence. Kidney Int 70:2074–2084. https://doi.org/10.1038/sj.ki.5001809
Faure P (2003) Protective effects of antioxidant micronutrients (vitamin E, zinc and selenium) in type 2 diabetes mellitus. Clin Chem Lab Med 41:995–998. https://doi.org/10.1515/cclm.2003.152
Hong F, Jin T, Zhang A (2004) Risk assessment on renal dysfunction caused by co-exposure to arsenic and cadmium using benchmark dose calculation in a Chinese population. Biometals 17:573–580. https://doi.org/10.1023/b:biom.0000045741.22924.d8
Huang M, Chen J, Yan G, Yang Y, Luo D, Chen X et al (2021) Plasma titanium level is positively associated with metabolic syndrome: a survey in China’s heavy metal polluted regions. Ecotoxicol Environ Saf 208:111435. https://doi.org/10.1016/j.ecoenv.2020.111435
Iwata K, Saito H, Moriyama M, Nakano A (1993) Renal tubular function after reduction of environmental cadmium exposure: a ten-year follow-up. Arch Environ Health 48:157–163. https://doi.org/10.1080/00039896.1993.9940814
Jalili C, Kazemi M, Cheng H, Mohammadi H, Babaei A, Taheri E et al (2021) Associations between exposure to heavy metals and the risk of chronic kidney disease: a systematic review and meta-analysis. Crit Rev Toxicol 51:165–182. https://doi.org/10.1080/10408444.2021.1891196
Jamakala O, Rani UA (2015) Amelioration effect of zinc and iron supplementation on selected oxidative stress enzymes in liver and kidney of cadmium-treated male albino rat. Toxicol Int 22:1–9. https://doi.org/10.4103/0971-6580.172289
Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B et al (2013) Chronic kidney disease: global dimension and perspectives. Lancet 382:260–72. https://doi.org/10.1016/s0140-6736(13)60687-x
Johnson VJ, Tsunoda M, Sharma RP (2000) Increased production of proinflammatory cytokines by murine macrophages following oral exposure to sodium selenite but not to seleno-L-methionine. Arch Environ Contam Toxicol 39:243–250. https://doi.org/10.1007/s002440010101
Johri N, Jacquillet G, Unwin R (2010) Heavy metal poisoning: the effects of cadmium on the kidney. Biometals 23:783–792. https://doi.org/10.1007/s10534-010-9328-y
Kan X, Dong Y, Feng L, Zhou M, Hou H (2021) Contamination and health risk assessment of heavy metals in China’s lead–zinc mine tailings: a meta–analysis. Chemosphere 267:128909. https://doi.org/10.1016/j.chemosphere.2020.128909
Karmaus W, Dimitrov P, Simeonov V, Tsolova S, Bonev A, Georgieva R (2008) Metals and kidney markers in adult offspring of endemic nephropathy patients and controls: a two-year follow-up study. Environ Health 7:11. https://doi.org/10.1186/1476-069x-7-11
Kim HR, Lee KY, Ahn SG, Lee BH, Jung KT, Yoon JH et al (2015) Transcriptional regulation, stabilization, and subcellular redistribution of multidrug resistance-associated protein 1 (MRP1) by glycogen synthase kinase 3αβ: novel insights on modes of cadmium-induced cell death stimulated by MRP1. Arch Toxicol 89:1271–1284. https://doi.org/10.1007/s00204-014-1381-9
Klotz K, Weistenhöfer W, Drexler H (2013) Determination of cadmium in biological samples. Met Ions Life Sci 11:85–98. https://doi.org/10.1007/978-94-007-5179-8_4
Kuruppu D, Hendrie HC, Yang L, Gao S (2014) Selenium levels and hypertension: a systematic review of the literature. Public Health Nutr 17:1342–1352. https://doi.org/10.1017/s1368980013000992
Lemire M, Philibert A, Fillion M, Passos CJS, Guimarães JRD, Barbosa FJ, Mergler D (2012) No evidence of selenosis from a selenium-rich diet in the Brazilian Amazon. Environ Int 40:128–136. https://doi.org/10.1016/j.envint.2011.07.005
Li XT, Yu PF, Gao Y, Guo WH, Wang J, Liu X et al (2017) Association between plasma metal levels and diabetes risk: a case-control study in China. Biomed Environ Sci 30:482–491. https://doi.org/10.3967/bes2017.064
Li X, Zhao Z, Ye Y, Xiang W, Li X (2018) Heavy metal accumulation and its spatial distribution in agricultural soils: evidence from Hunan Province, China. RSC Adv 8(19):10665–10672. https://doi.org/10.1039/c7ra12435j
Liang Y, Lei L, Nilsson J, Li H, Nordberg M, Bernard A et al (2012) Renal function after reduction in cadmium exposure: an 8 year follow-up of residents in cadmium-polluted areas. Environ Health Perspect 120:223–228. https://doi.org/10.1289/ehp.1103699
Lin YS, Ho WC, Caffrey JL, Sonawane B (2014) Low serum zinc is associated with elevated risk of cadmium nephrotoxicity. Environ Res 134:33–38. https://doi.org/10.1016/j.envres.2014.06.013
Liu ZH (2013) Nephrology in China. Nat Rev Nephrol 9:523–528. https://doi.org/10.1038/nrneph.2013.146
Liu Y, Yuan Y, Xiao Y, Li Y, Yu Y, Mo T et al (2020) Associations of plasma metal concentrations with the decline in kidney function: a longitudinal study of Chinese adults. Ecotoxicol Environ Saf 189:110006. https://doi.org/10.1016/j.ecoenv.2019.110006
Lv Y, Xie L, Dong C, Yang R, Long T, Yang H et al (2021) Co-exposure of serum calcium, selenium and vanadium is nonlinearly associated with increased risk of type 2 diabetes mellitus in a Chinese population. Chemosphere 263:128021. https://doi.org/10.1016/j.chemosphere.2020.128021
Nagy G, Benko I, Kiraly G, Voros O, Tanczos B, Sztrik A et al (2015) Cellular and nephrotoxicity of selenium species. J Trace Elem Med Biol 30:160–170. https://doi.org/10.1016/j.jtemb.2014.12.011
Nair AR, Lee WK, Smeets K, Swennen Q, Sanchez A, Thévenod F et al (2015) Glutathione and mitochondria determine acute defense responses and adaptive processes in cadmium-induced oxidative stress and toxicity of the kidney. Arch Toxicol 89:2273–2289. https://doi.org/10.1007/s00204-014-1401-9
Nordberg GF, Jin T, Hong F, Zhang A, Buchet JP, Bernard A (2005) Biomarkers of cadmium and arsenic interactions. Toxicol Appl Pharmacol 206:191–197. https://doi.org/10.1016/j.taap.2004.11.028
Orr SE, Bridges CC (2017) Chronic kidney disease and exposure to nephrotoxic metals. Int J Mol Sci 18:1039. https://doi.org/10.3390/ijms18051039
Piscator M (1984) Long-term observations on tubular and glomerular function in cadmium-exposed persons. Environ Health Perspect 54:175–179. https://doi.org/10.1289/ehp.8454175
Prozialeck WC, Edwards JR (2010) Early biomarkers of cadmium exposure and nephrotoxicity. Biometals 23:793–809. https://doi.org/10.1007/s10534-010-9288-2
Rayman MP, Stranges S (2013) Epidemiology of selenium and type 2 diabetes: can we make sense of it? Free Radic. Biol Med 65:1557–1564. https://doi.org/10.1016/j.freeradbiomed.2013.04.003
Robles-Osorio ML, Sabath-Silva E, Sabath E (2015) Arsenic-mediated nephrotoxicity. Ren Fail 37:542–547. https://doi.org/10.3109/0886022x.2015.1013419
Scott N, Hatlelid KM, MacKenzie NE, Carter DE (1993) Reactions of arsenic (III) and arsenic(V) species with glutathione. Chem Res Toxicol 6:102–106. https://doi.org/10.1021/tx00031a016
Shelley R, Kim NS, Parsons P, Lee BK, Jaar B, Fadrowski J et al (2012) Associations of multiple metals with kidney outcomes in lead workers. Occup Environ Med 69:727–735. https://doi.org/10.1136/oemed-2012-100765
Soderland P, Lovekar S, Weiner DE, Brooks DR, Kaufman JS (2010) Chronic kidney disease associated with environmental toxins and exposures. Adv Chronic Kidney Dis 17:254–264. https://doi.org/10.1053/j.ackd.2010.03.011
Tsai TL, Kuo CC, Pan WH, Chung YT, Chen CY, Wu TN et al (2017) The decline in kidney function with chromium exposure is exacerbated with co-exposure to lead and cadmium. Kidney Int 92:710–720. https://doi.org/10.1016/j.kint.2017.03.013
Ullah H, Liu G, Yousaf B, Ali MU, Irshad S, Abbas Q et al (2019) A comprehensive review on environmental transformation of selenium: recent advances and research perspectives. Environ Geochem Health 41:1003–1035. https://doi.org/10.1007/s10653-018-0195-8
van Swelm RPL, Wetzels JFM, Swinkels DW (2020) The multifaceted role of iron in renal health and disease. Nat Rev Nephrol 16:77–98. https://doi.org/10.1038/s41581-019-0197-5
Vinceti M, Maraldi T, Bergomi M, Malagoli C (2009) Risk of chronic low-dose selenium overexposure in humans: insights from epidemiology and biochemistry. Rev Environ Health 24:231–248. https://doi.org/10.1515/reveh.2009.24.3.231
Vinceti M, Filippini T, Cilloni S, Bargellini A, Vergoni AV, Tsatsakis A et al (2017) Health risk assessment of environmental selenium: emerging evidence and challenges (Review). Mol Med Rep 15:3323–3335. https://doi.org/10.3892/mmr.2017.6377
Vinceti M, Filippini T, Wise LA (2018) Environmental selenium and human health: an update. Curr Environ Health Rep 5:464–485. https://doi.org/10.1007/s40572-018-0213-0
Vinceti M, Filippini T, Rothman KJ (2018) Selenium exposure and the risk of type 2 diabetes: a systematic review and meta-analysis. Eur J Epidemiol 33:789–810. https://doi.org/10.1007/s10654-018-0422-8
Vinceti M, Chawla R, Filippini T, Dutt C, Cilloni S, Loomba R et al (2019) Blood pressure levels and hypertension prevalence in a high selenium environment: results from a cross-sectional study. Nutr Metab Cardiovasc Dis 29:398–408. https://doi.org/10.1016/j.numecd.2019.01.004
Xu X, Nie S, Ding H, Hou FF et al (2018) Environmental pollution and kidney diseases. Nat Rev Nephrol 14:313–324. https://doi.org/10.1038/nrneph.2018.11
Yang F, Yi X, Guo J, Xu S, Xiao Y, Huang X et al (2019) Association of plasma and urine metals levels with kidney function: a population-based cross-sectional study in China. Chemosphere 226(321–328):2019. https://doi.org/10.1016/j.chemosphere.2019.03.171
Zachara BA (2015) Selenium and selenium-dependent antioxidants in chronic kidney disease. Adv Clin Chem 68:131–151. https://doi.org/10.1016/bs.acc.2014.11.006
Zhang L, Wang F, Wang L et al (2012) Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 379:815–822. https://doi.org/10.1016/S0140-6736(12)60033-6
Zhang X, Liu C, Guo J, Song Y (2016) Selenium status and cardiovascular diseases: meta-analysis of prospective observational studies and randomized controlled trials. Eur J Clin Nutr 70:162–169. https://doi.org/10.1038/ejcn.2015.78
Zhang C, Lin T, Nie G, Hu R, Pi S, Wei Z et al (2021) Cadmium and molybdenum co-induce pyroptosis via ROS/PTEN/PI3K/ AKT axis in duck renal tubular epithelial cells. Environ Pollut 272:116403. https://doi.org/10.1016/j.envpol.2020.116403
Zwolak I (2020) The role of selenium in arsenic and cadmium toxicity: an updated review of scientific literature. Biol Trace Elem Res 193:44–63. https://doi.org/10.1007/s12011-019-01691-w
Acknowledgements
The authors thank all the participants for their invaluable contributions.
Funding
This work was supported by the National Natural Science Foundation of China (82173905 and 82070759) and the Ministry of Science and Technology of China (2015FY111100).
Author information
Authors and Affiliations
Contributions
Jingjing Quan: conceptualization, methodology, data curation, writing — original draft, writing — review and editing, visualization, and project administration. Yan Li: conceptualization, methodology, data curation, writing — original draft, writing — review and editing, and project administration. Hong Yuan: conceptualization, resources, writing — review and editing. Yao Lu: conceptualization, methodology, resources, writing — review and editing, and project administration. Bin Yi: conceptualization, methodology, resources, writing — review and editing, and project administration. Xiang Chen: conceptualization, methodology, resources, writing — review and editing, and project administration. Zhijun Huang: conceptualization, methodology, resources, writing — review and editing, and project administration.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The study was approved by the Medical Ethics Committee of the Xiangya Hospital, Central South University, and all the participants have signed the informed consent.
Consent for publication
All of the authors have read and approved the paper.
Competing interests
The authors declared no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.


Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Quan, J., Li, Y., Shen, M. et al. Coexposure to multiple metals and renal tubular damage: a population-based cross-sectional study in China’s rural regions. Environ Sci Pollut Res 30, 52421–52432 (2023). https://doi.org/10.1007/s11356-023-25909-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25909-6