Abstract
A method of dehairing of goat skins using oxidative chemicals and protease enzymes has been attempted. The dehairing process is one of the important and essential steps in leather making, where hair is removed by lime and sodium sulphide in the conventional process. This conventional dehairing system generates a higher amount of pollution problem as compared to the other unit operations and unit processes. In this work, dehairing of the goat skins through oxidative agents namely magnesium peroxide and protease enzyme has been attempted. For this, protease has been produced from Bacillus sp. at the laboratory level and the activity was found. The dehairing of goat skins takes place for the duration of 14–16 h. The leather produced with the experimental sample showed comparable organoleptic and strength properties with the conventional sample. This method paved the way for the reduction of pollution loads especially BOD, COD, and TDS to the level of 59, 27, and 77%, respectively, in comparison with the control sample. The reaction kinetics for the formation of the ligand-macromolecular complex is found in the isothermal titration calorimetry (ITC) experiment and a mathematical model has been formulated. The dyed crust leather showed comparable colour properties. In addition to that, there is a reduction in processing time for leather making through skipping reliming and deliming processes which are said to be another advantage of this method. The physical strength properties of the experimental leather were also comparable with conventionally produced leather.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sustainable leather technology is the present-day need to cope with issues relating to environmental, societal, and economic factors (Sarkis 2003; Linton et al. 2007). The leather obtained from animal skin/hide possesses unique properties such as water-resistant, strength, abrasion resistance, long-lasting properties, etc., which cannot be matched by any synthetic materials. However, leather making is considered to be traditional process involving various unit operations. It is simply classified into pre-tanning, tanning, post-tanning, and finishing operations (Morera et al. 2011; Kanagaraj et al. 2020a, b; Kanagaraj et al. 2022). In the process of leather making, various chemicals such as sodium chloride, sodium sulphide, chromium, vegetable tannins, retanning agents, dyes, and fatliquors are used (Na Yang et al. 2022; Kamini et al. 1999). Some of these chemicals are not absorbed fully into leather matrix and released into the effluent stream causing environmental issues (Saran et al. 2013; Muthsubramanian and Mitra 2006; Kanagaraj and Mandal 2012). The leather-processing operations generate huge amount of liquid, solid, and gaseous wastes (Dixit et al. 2014; Jegannathan and Nielsen 2013; Kanagaraj et al. 2015a; Saravanan et al. 2014). In spite of that, several researchers are giving attention to develop sustainable leather technologies to counteract the conventional method of leather making, particularly dehairing where the hair is removed drastically using traditional ways (Dixit et al. 2014; Jegannathan and Nielsen 2013; Kanagaraj et al. 2015b). Dehairing process is the removal of hair using lime (10–20%) and sodium sulphide (2–5%) in the conventional liming method. This method dehairs the skin very satisfactorily and gives the expected leather quality (Saurabh et al. 2013; Kanagaraj et al. 2020a, b). However, on the other side, this process generates more pollution problems because of lime and sodium sulphide used in the process. This method generates huge quantity of pollution loads such as hair pulp, flesh, and interfibrillar proteins contributing BOD and COD to the level of 40 and 50% in leather processing operations (Qiang et al. 2016; Kanagaraj and Mandal 2012; Kanagaraj et al. 2016a). It also emits hydrogen sulphide gas which is said to be toxic in nature because of the application of sodium sulphide in the dehairing. Thus, researchers are giving enormous focus to develop technologies for dehairing process to make it sustainable with the conventional dehairing system (Cantera et al. 2004). The enzymatic system offers scope for an alternative to dehairing process that will eliminate or mitigate the above limitations in leather processing operations (Senthilvelan et al. 2012). Enzymes also play a significant role in the dehairing process where the depilation of keratin and conjugated protein especially glucosamine glycans and dermatan sulphate is taking place (Guo et al. 2006; Li et al. 2009; Kanth et al. 2009).
It has been found in the literature that enzymes were used exhaustively for the dehairing of skins and hides. Enzymatic dehairing and enzymatic-assisted dehairing systems have been developed to combat pollution problems in leather processing operations (Kanth et al. 2009; Ma et al. 2014; Hu et al. 2010). Keratinase obtained from bacteria was useful for dehairing, the cosmetic industry, detergents, and the development of biopolymers (Adriano 2008; Tian et al. 2019). Keratinases obtained from bacteria Bacillus subtilis P13 were responsible for feather degradation and hide depilation. This enzyme-exhibited depilation of goat hides with the recovery of intact animal hair. The same enzyme preparation could release peptides from ground feathers and bring about their weight reduction; however, similar action on hair was relatively weak (Priya and Archana 2008; Kanagaraj et al. 2016b). Besides, novel approaches to enzymatic unhairing and fibre opening of skin using enzymes immobilised on magnetite nanoparticles have also been attempted (Murugappan et al. 2016). Cleaner dehairing of skins using carbohydrase was also initiated by another researcher (Ranjithkumar et al. 2017).
The above-said initiatives have led to greener and environmentally friendly leather processing on the road to attain sustainability. In addition to these, enzymatic dehairing depends on various factors such as pH, temperature, duration, and concentration of enzymes. The above factors are very difficult to maintain in different countries because of prevailing cold climatic conditions. With a lowering of temperature, enzyme activity decreases due to the slow movement of molecules reducing the frequency of enzyme–substrate collisions. Hence, the alternative way is to use enzymes in combination with other chemicals to accelerate its activity. In this mode, oxidative reagents/chemicals in combination with enzymes are very useful for achieving complete dehairing in leather making.
Oxidative dehairing with various oxidative agents was initiated and attempted by many scientists (Gehring et al. 2003; Shi et al. 2003). In another approach, oxidative agents like calcium peroxide, sodium percarbonate (Kanagaraj et al. 2016a), alkaline peroxide (Marmer and Dudley 2005), magnesium peroxide, and potassium peroxymonosulfate have been used to develop dehairing systems for skins and hides (Marmer and Dudley 2005). Literature also reported that dehairing with oxidising agent namely calcium peroxide dehairs cattle hide very completely and satisfactorily as that of conventional method (Richardson et al. 2009). Satisfactory dehairing was also done through alkaline sodium perborate with alkaline hydrogen peroxide amended with either potassium cyanate or urea that produces comparable leather qualities (Wei et al. 2010).
Besides, some of the other methods of dehairing of skins and hides using urea and dicyandiamide have been reported. This method also produces better leather properties (Marshal et al. 2002; McKillop and Sanderson 1995). It has been found from the literature that hydrogen peroxide with enzyme had also been useful for dehairing of skins. In addition to this, several commercial enzyme formulations were available in combination with hydrosulphide and peroxides for dehairing purposes (Andrioli and Gutteres 2014). The limitations of using oxidative chemical as a solo dehairing agent are generation of unwanted gases, carrying out dehairing at slightly alkaline medium, pH, supply of oxygen, etc., whereas the combination of this oxidative chemical with enzyme reduces the limitations for these systems and leads to satisfactory dehairing and leather production.
In the same perspective, sustainable dehairing process through combination of oxidative and enzymatic chemicals was attempted. The main objective of this work is to establish dehairing system using oxidative and enzymatic chemicals with comparable organoleptic and strength properties of the leather. The established system shall be standardised to the next level for exploiting commercial purpose to achieve sustainable leather processing in the near future.
Materials and methods
Materials
Freshly flayed goat skins weighing 1 kg/skin were procured immediately after flaying from the local abattoir. Chemical namely magnesium peroxide for the experiments were purchased from Sigma-Aldrich, Mumbai, India, of laboratory reagent grade. Protease for the experiment was isolated from the Bacillus sp. strain and activity was found out. The other chemicals namely lime, sodium sulphide, and basic chromium sulphate (BCS) were purchased from a private chemical company, Chennai, India.
Methods
Leather processing
Leather is made from hides or skins which are putrescible materials in their raw stages and become durable after curing and treatment. There are three stages of treatments: (a) pre-tanning, (b) tanning, and (c) post-tanning. In the pre-tanning stage, hides are preserved under salts and glycolipid surfactants for 10–16 h. then they are soaked in water followed by liming (addition of sodium sulphide), de-fleshing (removal of extra flesh), de-liming (washing out of alkali), bating (addition of protease enzyme), and pickling (addition of H2SO4). In the tanning stage, pickled hides are treated with basic chromium sulphate in a tanning drum where the chromium gets liganded with active functional groups of hides/skins. At the end of the operation, the hides are taken out and the spent liquor may be discharged as effluent or reused for the next operation. These hides are then processed with neutraliser, dye, and then fat liquor for surface smoothening. In the third stage, the wet-blue leather is processed for samming, splitting, skiving, greasing, drying, and finishing. After every stage, hides are washed thoroughly to remove dirts, hairs, and excess chemicals. These wash waters constitute effluent.
Production of enzyme
A quantity of 250 ml of nutrient broth was dispensed in 500 ml of Erlen Meyer flask and sterilised in 121 °C at 1 atm for 15 min in autoclave and inoculated with the desired cultures and then incubated at 37 °C for 24–48 h. Then the broth was centrifuged at 12,000 rpm for 20 min at 4 °C and the supernatant containing the crude enzyme was assayed for its activity. The protein content present was estimated by Lowry’s method using bovine serum albumin (BSA) as standard.
Purification of enzyme
Purification of enzyme was carried out using ammonium sulphate precipitation method, because it was highly soluble in water and had no deleterious effect. This process was carried out at 0–10 °C to minimise the supernatant denaturation and was fractioned by adding 30% ammonium sulphate (Hi-pure fine chem, Industry) and incubated overnight at 4 °C. The precipitated biomass was separated and dried; after that protein was estimated by Lowry’s method. Further, the dried form of enzyme was used for application process.
Enzyme assay
The enzyme activity was determined by a modified method of Anson (Anson 1938). Using 1% w/v casein containing 2 mM CaCl2 in 0.02 M borate buffer, pH 8.0, and incubating the reaction mixture at 45 °C for 30 min. One unit of enzyme activity is defined as the amount of enzyme required to liberate 1 µg of tyrosine under standard assay conditions.
Dehairing studies on goat skins
Oxidative-enzymatic dehairing was carried out using magnesium peroxide and protease. For this, the salted goat skins were used for carrying out experiments. In this work, four dehairing experiments and three control experiments were carried out. The dehairing experiments were designed by keeping the protease at a constant level of 1% and altering the magnesium peroxide levels from 0.5, 1, 1.5, and 2%. Three control experiments namely using protease at the level of 5% (as a first control), magnesium peroxide at the level of 1.0% (as a second control), and lime at the level of 10% plus sodium sulphide at the level of 2% and water (20%) (as third control experiment) were carried out to assess the efficacy of the dehairing systems. In all the experiments, oxidative chemicals and enzymes were used and applied on the flesh side of the skin without adding water. The chemicals were applied on the flesh side of the skin and piled one over the other and cooled air was circulated over the skins through fans to enhance the oxidation process for a period of 14–16 h. Then the skins were dehaired manually by knife and the percent of dehairing was calculated based on the x factor. The experimental dehaired skins and the effluent streams were analysed for various factors such as pollution parameters of BOD, COD, chrome content, shrinkage temperature, colour properties, and physical strength properties of leather by using a standard procedure (Eaton et al. 1995; Thangam and Rajkumar 2002; IUP 162015). DSC and TGA analyses, UV–vis analysis, 1H NMR analyses, FT-IR analysis, chrome content and shrinkage temperature estimation, SEM analysis, colour properties of the leather, and physical testing properties of dyed leather were carried out using a standard procedure.
Experiments with ITC
Oxidative-enzymatic reactions are studied for finding out thermodynamic models. Macromolecular interactions between enzymes and substrates are important in the understanding mechanism of binding of complex species. The temperature and pressure of the reaction, and conditions of buffer, ligand, and substrates (macromolecule) are important to predict temperature and concentration profiles during the reaction. Stoichiometry, affinity, and enthalpy of the ligand-macromolecular complex reaction is found in the ITC experiment. Experiments have been conducted under different conditions of temperature to find the kinetics of the binding reaction (Alan Brown 2009).
DSC and TGA analyses
The experimental leather and effluent samples were analyzed for DSC and TGA in Dupont 2910 DSC instrument with a heating rate of 10 °C per minute in a nitrogen atmosphere.
UV–vis analysis
The effluent collected from dehaired skin for both experimental and control samples were analysed for UV–vis analysis. The samples were filtered initially and then scanned in the range of 200–800 nm using UV 1601 Spectrophotometer (Shimadzu, Japan) to observe the spectral shifts caused by dehaired samples.
1H NMR analyses
1H NMR spectra with water suppression using the watergate sequence 0.2 ml of sample and 0.4 ml of 99.9% D2O were taken in a standard 5.0-mm Wilmad NMR tube. All the samples were prepared in the same proportion. Since the samples contained 90% water and 10% D2O, obtaining a 1H spectrum with a standard single pulse sequence was not possible. In order to get water-suppressed 1H spectra, a double-pulsed field gradient spin echo water gate (DPFGSE) sequence was used to reveal the functional groups. Before applying this pulse sequence gradient, shimming was carried out with field gradient control. In all experiments, the DPFGSE watergate sequence was used to obtain high-resolution 1H spectra on a JEOL ECA 500-MHz NMR spectrometer.
FT-IR analysis
FT-IR analysis was carried out for the experimental and control samples of the dehaired skins. The samples were mixed with potassium bromide (1:20; 0.02 g of sample with KBr at a final weight of 0.4 g) and then ground, desorbed at 60 °C for 24 h, and made into IR-transparent pellets. Then the samples were subjected to FT-IR spectra using an FT-IR spectrum 2000 Perkin-Elmer spectrophotometer. The spectra were absorbed and calibrated within the scanning range of 400–4000 cm−1. The FT-IR was first calibrated for background scanning signal against a control sample of pure KBr. Further, the experimental samples were scanned in a similar way as above.
Analysis of pollution parameters in the dehairing process
The experimental and control samples were analyzed for pollution parameters in the dehairing process. For this, the spent liquor from control and experimental samples after the dehairing process was collected and filtered (Whatman 40), and filtrates were analysed for various pollution loads. The pollution loads such as BOD, COD, and TDS were estimated using the standard method (Eaton et al. 1995; Thangam and Rajkumar 2002). The results are expressed in parts per million (ppm) of the sample. While scaling up, these values will guide into consideration the capacity of the effluent treatment plant.
Chromium content and shrinkage temperature estimation
The chrome-tanned leather samples from the official butt portion of experimental and control-tanned leather were estimated for chromium content. A known weight of (~ 1 g) of the sample was digested using an acid mixture containing 11.5 ml of perchloric acid, 3.5 ml of sulphuric acid, and 5 ml of nitric acid. The chromium present in the digested sample was determined using the standard procedure and was expressed as % Cr2O3. (Hones et al. 1999). The tanned leather sample was also tested for shrinkage temperature using a Theis shrinkage meter (ISO 3380:2015 [IULTCS/IUP 16]). The leather sample of dimension 20 × 3 mm was taken and hooked in the shrinkage meter. Then the leather samples were immersed in a glycerol-water solution (70:30). From this, the temperature at which the specimen starts to shrink was noted as the shrinkage temperature of the particular leather.
SEM analysis
The control and experimental samples obtained from tanning experiments were dried and made into powder form, and then all specimens were then coated with gold using JEOL JFC-1100E ion-sputtering device. A JEOL JSM-5300 scanning electron microscope was used for the analysis. The micrographs for the sample were obtained by operating the SEM at an accelerating voltage of 20 kV with different lower and higher magnification levels. The sample that showed clear views was presented in the present investigation. Similarly, control and experimental samples were subjected to SEM studies to assess the effect of dehairing in the leather sample. Both control and experimental samples were gold coated for 3 s and magnified in different magnification ranges. A JOEL JSM 5300 scanning electron microscope was used to study the experimental samples.
Colour properties of the leather
The dehaired experimental tanned leathers were made into dyed crust leather. These leathers were subjected to colour properties and compared with control samples. All colour measurements were carried out using Gretag Macbeth Spectrolino spectrophotometer with the measurement geometry of 45°/0° and L, a, b, c, and H were found out using the standard procedure.
Physical testing properties of dyed leather
The dyed leather samples of both experimental and control were estimated for various physical strength properties as per IULTCS standard method. Specimens were conditioned at 80 ± 4 °C and 65 ± 2% RH. Over a period of 48 h, physical properties such as tensile strength, % elongation at break, tear strength, and grain crack were found for both experimental and control leather samples.
Isolation of protease and enzyme preparation for dehairing study
The fresh goat skin was dehaired and from this 100 g of skin samples (four numbers) was taken. These skins were cut into small pieces and soaked well in sterile water; taken in four different beakers. After soaking the skins, the skin samples were suspended in four different conical flasks containing 100 ml nutrient broth, incubated, and then serially diluted. This method was followed to isolate and enumerate different types of bacteria that are obtained from the skin samples. From the biochemical analysis, the species was identified as Bacillus sp. (Gehring et al. 2003). This strain was used for protease production. The organism was systematically checked for the presence of proteolytic activity and gelatinase activity by using skimmed milk agar and gelatin agar medium. The organism that showed protease activity was taken for enzyme production and tested for the dehairing of goat skin. The alkaline proteolytic activity was determined by hydrolysis of casein, where the centrifuged culture broth along with casein solution in Tris–HCl buffer was reacted and the mixture was subjected to UV tests (275 nm) for proteolytic activity. The supernatant from crude extract was precipitated with (NH4)2SO4 and further re-suspended in Tris–HCl buffer (with CaCl2) and dialysed for enzyme preparation. The precipitate was stored at 4 °C. One-unit protease (U/ml) activity is defined as the activity that liberates 1 µg of tyrosine per minute (µg Tyr × ml−1 min−1) under standard conditions. The dehairing study was performed in combination with cell-free supernatant.
Results and discussion
Dehairing is the removal of hair by means of employing chemicals namely lime and sodium sulphide in the conventional process. This method is said to be a traditional process and pragmatically feasible in spite of toxic chemicals generated in the liming process. Due to this, researchers are continuously giving attention to searching for alternate in the dehairing process. This is possible only through developing sustainable dehairing technology for mitigating/eliminating the pollution problem in the liming process.
Preparation of protease and its activity in various factors
The protease was isolated and tested for its activity in the dehairing of goat skins. Prior to this, the activity of the enzyme at the different parameters of pH, duration, and temperature was found and the results are presented in Fig. 1. It was seen from the optimization results that the bacteria grow well on pH 7 and at 37 °C. It was also observed from the trend that the enzyme activity was highest when the bacteria were incubated for 48 h at 37 °C. The maximum enzymatic activity for the crude enzyme was found to be 44 U/ml. Besides, the graph in Fig. 1 shows one more curve describing enzyme concentration profile where the activity increases with concentration.
Development of dehairing system
In this work, sustainable dehairing systems with the help of magnesium peroxide and protease were developed at CSIR-CLRI. The experimental outcomes are presented in Table 1. The table shows dehairing efficiency and % hair removal for the experiments and control samples at various pH values. In this work, four experiments and three controls were carried out. For the experiments, the protease at the constant level of 5% with varying levels of magnesium peroxide (0.5, 1.0, 1.5, and 2%) has been used to develop the dehairing systems. Similarly, in the control experiments protease at the level of 5% (as control-1), magnesium peroxide at the level of 1.0% (as control-2), and the conventional method of dehairing with the help of lime-10%, sodium sulphide-2%, and water-20% (as control-3) have been used for developing dehairing systems.
All the experiments were carried out in stationary conditions with the circulation of air from the fans. The skins were piled one over the other after applying the chemicals. It was proven factor that the stationary method was proved to be a better one for the dehairing experiments because of the availability of more contact time of skins with available chemicals (Kanagaraj et al. 2016b; Marmer and Dudley 2005). Magnesium peroxide was used in the dehairing process which has the molecular formula of MgO2 and possesses the character of fine powder form with off-white colour in nature and releases oxygen by breaking down at a controlled rate in contact with a hydrous fluid. It was also used as a bleaching, disinfecting, and deodorising agent and releases hydrogen peroxide on decomposition with water. Magnesium peroxide also has very low solubility and releases oxygen for a long time which was very well exploited for the present work because of the oxidation phenomenon that helps in enhancing the effect of dehairing of skin. If water is in excess quantity in the process, there is a possibility of the formation of H2O2 in the media under the presence of an acid:
Otherwise, when water is not in excess, there is a possibility of decomposition of MgO2 which is almost insoluble in water, finally liberating O2 (Neil 2006).
The generation of intermediate H2O2 is the key factor behind the dehairing of skin because of the availability of a higher quantity of H2O2 that diffuses through the porous nature of the flesh side of the skin and helps for loosening of conjugated proteins in the skin. In addition, the H2O2 helps to transport the enzyme to the keratin basement or hair bulb that enhances the depilation of keratin in a rapid manner.
On the contrary, several researchers have also carried out the dehairing process using protease enzymes. Sometimes, alkali pre-treatment before enzymatic dehairing was also effective in the depilation of skin. This would result in the cleavage of proteoglycans and protein denaturation in strong alkaline conditions that would result in the cleavage of more peptide bonds, facilitating proteases with narrow ranges of specificities to disrupt the integrity of proteins (Cantera et al. 2004). The same concept was used to develop a dehairing system with the oxidative-enzymatic approach for better results. The oxidative chemical not only helps to release the hair but also makes the enzyme more active and effective in achieving complete depilation of skin. Dehairing with protease on cow hides gave reasonable qualities with a conventional sample that has been reported (Abraham et al. 2014). The protease from P. Aeruginosa was very helpful in soaking. This method reduces soaking time and assisted hair loosening by minimising the use of chemicals.
Alkaline protease from Vibrio metschnikovii NG 155 was tested for the dehairing on goat skins and buffalo hides. The dehairing was effective and the hair was removed, completely eliminating lime and sulphide with a duration of 8 h. This dehairing system showed leather with comparable leather qualities and reduced pollution loads in the dehairing stage (George et al. 2014; Subbarao et al. 2009).
Similarly, proteases isolated from Pseudomonas fluorescens and Vibrio metschnikovii NG155 applied for dehairing yielded eco-friendly leathers with comparable properties (Kandasamy et al. 2012; Detimer et al. 2013). In another attempt of using enzymes to replace lime and sulphide for achieving better dehairing with the release of more proteoglycans and carbohydrates, have been reported (Saravanan et al. 2014). In a similar attempt, an enzyme that was capable of dehairing the sheepskins in 7 h has been reported (Ramesh et al. 2018). The dehairing process carried out on goat skins using protease isolated from Aspergillus tamarii gave satisfactory results. The protease at the level of 1% w/v (or, 0.4 IU) and for a duration of 18–24 h was the optimal condition for better dehairing of skin. The dehairing process gave better leather quality with a reduction of BOD (50%), COD (40%), TDS (60%), and TSS (20%) in the dehairing process (Dayanadan et al. 2003).
Protease enzyme isolated from Bacillus subtillis can be utilised in poultry processing industry as well as enzymatic dehairing of skin in tannery industry to control the environment from pollution, which is a prerequisite for biotechnological applications (Uddin et al. 2017). Application of nanozyme for dehairing and fibre opening yielded satisfactory results. Besides the enzyme gave comparable organoleptic properties of leather with conventional ones (Murugappan et al. 2016). The other works carried out using carbohydrases namely galactosidases from Aspergillus terreus were also tested for fibre opening of pelts (Durga et al. 2016).
It is evident from the table that experiment carried out with the help of magnesium peroxide at the level of 0.5% and protease at the level of 5% showed moderate dehairing with 80% hair removal. It does not show complete dehairing, whereas the other experiments carried out with magnesium peroxide at the levels of 1.0%, 1.5, and 2% and protease at the level of 5% showed complete dehairing as compared with the earlier experiment. It was confirmed that the experiment carried out using magnesium peroxide at the level of 1.0% and protease at the level of 5% was the optimum one for dehairing the skins. This experiment was further extended to confirm the organoleptic properties of leather and strength qualities. The control experiment carried out with the help of magnesium peroxide at the level of 1% (control-1) and protease at the level of 5% (control-2) did not give fruitful results in dehairing. The dehairing experiments carried out on the skins showed only 60% of hair removal and the hair was intact and very difficult to remove for these auxiliaries. On the other hand, the control experiment (control-3) carried out with the help of lime and sodium sulphide showed complete hair removal. The mechanism of conventional dehairing may be due to the redox potential of chemicals which was due to the breakdown of the di-sulphide bond of cysteine to cystine amino acids. In a similar approach, a combination of 4% hydrogen peroxide with enzyme was able to dehair the skins in a better way to replace lime and sulphide in the dehairing process (Andrioli and Gutteres 2014). The literature is also available on the dehairing of skins using enzymes in combination with sodium hydroxide, sodium sulphide, and disodium pyrophosphate that produced better leather quality (Valeika et al. 2009).
Several reports are available on dehairing systems using various auxiliaries. Among these, enzymatic dehairing plays an important role in mitigating the pollution load of BOD and COD in leather processing operations (Akram et al. 2021). Enzymatic dehairing carried out with protease isolated from Bacillus species was very effective for fresh goat skins at the level of 1–3%. The enzyme preparation was mixed with kaolin at 10% and applied on raw goat skins. The tough hair present in the neck region could be easily removed without any difficulty for this composition. Further to that, the physical strength properties of the leather were comparable to the conventionally produced ones (Annapurna et al. 1996).
The enzymes isolated from Bacillus species and Pseudomonas species were capable of dehairing the skins at both aerobic and anaerobic conditions, but the hydrolysis of skin collagen was found less when the organisms were grown anaerobically studied on jawasee protease, microbial protease, and lime for dehairing system (George et al. 2014; Subbarao et al. 2009). Some of these experiments gave satisfactory results but it was more temperature dependent. The enzymatic dehairing with alcalase (homogenous mixture), bacterial proteinase by Novo industry, Denmark, showed that enzyme with proteolytic activity of broad specificity was necessary to bring about depilation (Kandasamy et al. 2012; Verma et al. 2011; Arunachalam and Saritha 2009). Dehairing of skin with oxidative chemicals has also been reported in the literature.
The dehairing experiment carried out on skins with 5% perborate along with 5% sodium hydroxide (w/v in 200% water) resulted in better dehairing (Marmer et al. 2005). The other dehairing systems established with the help of calcium peroxide, alkaline sodium perborate, with alkaline hydrogen peroxide modified with either potassium cyanate or urea dehairs the skin completely (Wei et al. 2010). Dehairing experiment carried out with sodium percarbonate and sodium hydroxide was also initiated (Kanagaraj et al. 2016a). All the above said dehairing systems were influenced by various parameters; hence, the efficacy of dehairing and the quality of the leather were not consistent. Earlier reports and research have shown that sustainable dehairing could be achieved only through a combination of oxidative-enzymatic methodology.
Nano-DSC analyses
The dehaired samples of experiment and control were subjected to DSC study and the results are shown in Fig. 2. The experiment sample (that has been carried out with the help of magnesium peroxide-1 and enzyme-5%) showed DSC curve at 128.98 °C and the control sample (lime and sulphide) exhibited a DSC curve at 130.48 °C. The results indicated that these figures showed a depletion trend indicating that the process was endothermic. Further, the experiment sample showed a generation of peroxide resulting in oxidation phenomenon. The curves show total liberated enthalpy due to several injected amount of enzyme. MgO2 induces the formation of H2O2 which has also been determined/confirmed by ferrous thiocyanate assay. The presence of Fe contaminant/supplement in process water increases the formation of hydroxyl ions (OH– or radicals. The process follows as
It was confirmed that the reason for dehairing in this system is due to formation of the said radical. This radical fastens oxidation reaction and was helpful in achieving better dehairing of skins. On the other hand, the control sample showed slower reaction rate compared to experiment sample, thereby taking longer reaction time for the completion of dehairing process.
UV and TGA analyses
Figures 3a and b show UV analyses of the experiment and control samples. The UV curve from the experiment showed a wider one as compared to the control sample. This may be due to the fact that experimental skins that were dehaired and subjected to UV spectra indicates, that removal of lower amount of non-fibrous protein such as albumin, globulin, mucous; and also the fibrous proteins such as reticulin and elastin in the sample whereas complete removal of hair took place indicating an efficient method for dehairing. The reason was that the experimental dehaired process of oxidative-enzymatic approach was based on hair-saving process which would not account for the removal of non-fibrous/interfibrillary proteins namely albumin, globulin, and mucoids in the skin; hence, the spectrum is wider in the case of experiment sample. The required keratinase activity for the dehairing process has been reported as 67.2 U/mg (Malay and Rajnish 2017). The control dehairing process was based on the hair pulp method and accounts for the removal of the larger quantity of proteins as compared to the experimental sample; hence, the curve is narrower one.
Figures 3c and d show TGA curves for the experimental and control samples. It was inferred from the curves that it was a diffusion-controlled reaction, indicating that the reactant magnesium peroxide-protease dehairing phenomenon helps to transport the peroxide and protease to the skin in several steps. The figure shows that loss in weight percent in the control sample is more than that of the experimental sample, revealing that more amount of peroxide has been transported to the leather matrix giving rise to better dehairing compared to sulphide-led control sample. Though both the samples show almost similar thermal stability, the residue is higher in case of experimental sample.
1H NMR analyses
The dehaired sample was analysed for 1H NMR and the results are presented in Figs. 4a–d. The spent liquors containing peroxide were examined for 1H -NMR test using D2O at 400 MHz. There were two samples, one experimental containing magnesium peroxide (1%) with protease (5%) and another control (lime and sulphide only). The 1H NMR reveals resonance at frequency 4 due to the presence of H of hydration H2O. This may be due to carboxylic-ester. But at peak 41, it may be due to the allylic C–CH group or a ketone group. It indicates the presence of an allylic group in the ester in the collagen structure of the skin. Further, it confirms the presence of hydration H2O inside the enzyme matrix. These functional groups favour the easy removal of keratin components in the skin during the dehairing process.
Besides, the main purpose of the analysis is to find if there are any traces of hydrogen, due to the presence of H2O/H2O2, left in the sample. The entire H2O2 evolved in the intermediate reactions have been converted to H2O and no other protons are left out after dehairing, which clearly indicates the reason for removal of hair in this process.
FTIR spectra analysis
The FTIR spectra give the details of functional groups present in the spent liquor. Hence, the FTIR analysis has been carried out and the results are presented in Fig. 5, respectively. It is seen from the curve that the peak in the range of 3300–2500 cm–1 represents the functional groups O–H, N–H, and C–H. The experimental sample showed slightly larger peaks due to the breakdown of some hydrogen bonds in the collagen, and this may be due to the fact that some non-fibrous proteins and glycoproteins were generated in the dehairing process. This was also due to the peroxide generated (process water containing contaminated Fe) in the experimental sample that helps for penetration of the compound inside the collagen matrix, favouring removal of keratin and other proteins as compared to the conventional method of dehairing. The control curve showed peaks in the region of 1760–1720 cm–1, confirming the presence of carboxylic acid groups, but was almost nil in the experimental curve, confirming the process is a hair-saving one. The curve showed a peak of C–O–H in the range of 1440–1400 cm–1 which may be due to deformation vibration, and the peak of C–O in the range of 1320–1210 cm–1 is responsible for valence vibration. An intense peak for the valence vibration is present in the range of 1650–1550 cm–1 while a weak peak for the symmetric valence vibration is found at ~ 1400 cm–1, confirming the carboxylate anion. The peaks visible in the ranges of 1620–1629 cm–1, 1538–1540 cm–1, and 1235 cm–1 for the control and experimental samples correspond to deformation vibration of N–H (respectively, I, II, and III amide bands) which indicates the removal of hair and interfibrillary proteins present in the skins. The experiment sample showed an almost small peak in those functional groups clearly confirming that the process is hair-saving system where the presence of interfibrillary proteins and collagen is minimal.
Pollution reduction in the dehairing process
The present work aims for a sustainable dehairing process where an oxidative-enzymatic approach has been attempted. This method is a very efficient method that enhances hair removal by eliminating the limitations in the dehairing process, at the same time, abates a considerable amount of pollution load. The pollution parameters such as BOD, COD, TDS, and TSS were estimated and presented in Table 2.
The experiment showed promising results of dehairing that were carried out using magnesium peroxide at the level of 1% and enzyme at the level of 5%. The spent liquor analysed for BOD, COD, and TDS for the experiment sample was at the level of 1176, 2475, and 2687, respectively. Similarly, the conventional lime and sulphide experiment showed BOD at the level of 2823, COD at the level of 3380, and TDS at the level of 11,236 ppm. The experimental sample showed a BOD reduction of 59% in comparison with the control sample of the lime and sodium sulphide dehairing process. Similarly, the COD and TDS reduction for the experiment sample was 27% and 77% over the control samples. The reduction in pollution load may be due to the fact that more quantities of chemicals such as lime at the level of 10% and sodium sulphide at the level of 2% were used in the conventional process of dehairing. In addition to these, the chemicals used in the process convert the hair into hair pulp, thereby increasing the sludge in the effluent stream that contributes a huge amount of pollution load in the conventional dehairing process. The dehairing by the enzymatic method also reduces pollution loads to a similar level, but the efficacy at various parameters over the period has to be reviewed thoroughly to understand the process. Literature also reports that there was a reduction in pollution load in oxidative dehairing because of the elimination of sodium sulphide and lime. Both of these dehairing systems are said to be hair-saving systems. It was reported that dehairing using protease also gave a similar type of pollution reduction because of the hair-saving systems (Kanagaraj et al. 2016b; Andrioli and Gutteres 2014; Richardson et al. 2009; Nashy et al. 2005; Wang et al. 2009).
SEM analyses
The experimental leather prepared using magnesium peroxide and proteolytic enzyme sample is considered for SEM experimental study. Leather samples were subjected to this auxiliary recipe. The control leather test sample has been made using lime and sodium sulphide. Both the samples (experimental and control) were made into crust leather which was finally subjected to SEM analysis. The results are shown in Fig. 6a–f. In this figure, the top three pictures represent SEM for the test sample and the bottom three pictures depict the control sample. It can be visualised that the experimental sample (Fig. 6a–c), which was subjected to the present auxiliary, is clear of hair particles, indicating complete removal of hair. This is otherwise referring that hair is removed with intact hair-bulb surrounding portions which are mainly composed of glycoproteins. The hair root and shaft along with hair-bulb surrounding portions are removed in this type of dehairing which leads to a commendable way of hair removal in the experimental leather. Thus, the efficiency of the enzyme-based-unhairing is good. Whereas in the control sample (Fig. 6d–f), traces of hair (black marks near the small patches/pits) can be found, indicating the inefficiency of the conventional dehairing process. This is because the top portion of hair is removed, only (called shaft) leaving the hair root and hair bulb intact in the leather matrix itself. The control process does not remove the hair completely and small hairs remain intact which affects the penetration of the chemicals in the tanning and post-tanning processes of leather making. The results can be seen clearly in Figs. 6a–c that hair removal is complete. The control sample indicates that some portion of hair or maybe hair root is still visible, confirming that the process is still not complete or satisfactory. The hair portion can be seen clearly in the form of holes/patches. It can also be observed that with the increase of concentrations of oxidative-enzymatic dehairing agent in the recipe, the removal of hair becomes cleaner and clear (as it is evident that Fig. 6c indicates a cleaner view compared to that of Fig. 6a.
Impact of dehairing on leather properties
The experimental leathers were processed into leather and the leathers were analysed for shrinkage temperature and a chrome content of leather. The results are tabulated in Table 3. The experimental sample showed a shrinkage temperature of 105 ± 1.0 as compared to the leather made from the control sample of 102 ± 1.0 °C. The shrinkage temperature of the leather made in the experiment was comparable to the control sample. Similarly, the chromium content of the experimental samples exhibited to a level of 4.12 ± 0.5 in comparison with conventional control lime and sulphide sample of chromium level of 3.95 ± 0.6. The increase in chromium content was due to peroxide-assisting creation of more functional groups. The results agree with earlier reports (Marmer et al. 2005; Song et al. 2011; Kilic et al. 2011; Amir et al. 2008; Kanagaraj et al. 2020a, b). The present system also shows a reduction of cost of 30% over enzymatic dehairing and a slight increase of cost (25%) over conventional lime and sulphide system but overall has the advantage over the reduction of pollution load as compared to the conventional system.
Colour measurement properties
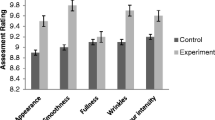
The tanned leathers were dyed into the crust leather and the dye uptake was measured in colour data equipment to know the effect of dehairing process. The colour measurement results of the experimental and control samples are presented in Table 4. The various colour details representing L, a, b, and c are colour coordinates indicating lightness (L), colour differences for red-green (a), and yellow-blue (b). It is evident from the results that experimental crust leather showed L value of 35.40 as compared L value of 28.98 for the control sample. It is therefore inferred that the experimental sample was of slightly lighter in shade and towards the yellow component. It is confirmed that the magnitude of the overall colour difference for the experiment sample was relatively comparable. The overall colour properties of the experimental leather were comparable according to the colour data values. The auxiliaries that increased the colour uptake were investigated by various researchers and found that protein-based products yield better colour properties for the leather (Kanagaraj et al. 2015a, 2008; Kanagaraj and Panda 2011).
Physical strength properties of the dehaired leather
The physical strength properties of the leather are very important to assess the impact of the experiment. The crust leathers were estimated for various physical strength properties and the results are presented in Table 5. It is seen from the table that physical strength properties such as tensile strength, % elongation at break, tear strength, load at grain crack, and distension at grain crack for the experimental samples were comparable to the conventionally manufactured leathers. The results indicate that the auxiliaries used for dehairing do not damage the collagen matrix at the fibre level, and therefore this method could be well practiced without any hesitation. Similarly, the conventionally produced leather also exhibited very good physical strength properties.
Conclusions
Sustainable leather technologies are the need of the hour to overcome environmental and societal issues. In this perspective, dehairing using an oxidative-enzymatic system was established which would obviate the problems associated with conventional dehairing system. In this work, dehairing of goat skins using magnesium peroxide and protease has been attempted. The results showed that dehairing using magnesium peroxide at the level of 1% and protease at the level of 5% were able to provide satisfactory dehairing results. This dehairing process was able to reduce pollution problem of BOD and COD at the levels of 59 and 27%, respectively, over conventional lime and sulphide system. It also eliminates the toxic sodium sulphide in the dehairing process. The shrinkage temperature and chromium content of the experimental leather samples were comparable with control samples. Macromolecular interactions between oxidative-enzyme and substrate are considered in the understanding mechanism of binding of complex species and the complex reaction is found from the ITC experiment. The dehaired skins were further processed into the crust leather which showed comparable colour properties and physical strength properties of the leather with conventional samples. Overall, the present work of the dehairing system would certainly bring sustainability in the leather processing along with alteration of in-process control measures for attaining the goals of present and future one. The present system would also be very well suited for carrying out the dehairing process with other range of raw materials of leather industries with an aim to achieve a cleaner and greener environment of the leather industry.
Data availability
All the authors have given all the data and materials for publishing the manuscript in this journal. If any supplementary material is required, all the authors abide to journal to provide the same.
References
Abraham J, Gea T, Sanchez A (2014) Substitution of chemical dehairing by proteases from solid-state fermentation of hair wastes. J Clean Prod 74:191–198
Adriano B (2008) Bacterial Keratinases: Useful enzymes for bioprocessing agroindustrial wastes and beyond. Food Bioprocess Technol 1:105–116
Akram F, Haq IU, Hayat AK, Ahmed Z, Jabbar Z, Baig IM, Akram R (2021) Keratinolytic enzyme from a thermotolerant isolate Bacillus sp. NDS-10: an efficient green biocatalyst for poultry waste management, laundry and hide-dehairing applications. Waste Biomass Valor 12:5001–5018
Amir S, Benlboukht F, Cancian N, Winterton P, Hafidi M (2008) Physico-chemical analysis of tannery solid waste and structural characterization of its isolated humic acids after composting. J Haz Mat 160:448–445
Andrioli E, Gutteres M (2014) Associated use of enzymes and hydrogen peroxide for cowhide hair removal. J Am Leat Chem Assoc 109(2):35–69
Annapurna Raju A, Chandrababu NK, Samivelu N, Rose C, Muralidhara Rao N (1996) Eco-friendly enzymatic dehairing using extra-cellular proteases from a Bacillus species isolate. J Am Leath Chem Ass 91:115–118
Arunachalam C, Saritha K (2009) Protease enzyme an eco-friendly alternative for leather industry. Ind J Sci Technol 2(12):29–32
Brown A (2009) Analysis of cooperativity by isothermal titration calorimetry. Int J Mol Sci 10:3457–3477
Cantera CS, Garro ML, Goya L, Babeito C, Galarza B (2004) Hair saving unhairing process. Part 6. Stratum corneum as a diffusion barrier: chemical- mechanical injury of epidermis. J Soc Leather Technol Chem 88:121–130
Dayanadan A, Kanagaraj J, Sunderraj L, Govindaraju R, Suseela RG (2003) Application of alkaline protease in leather processing - an eco-friendly approach. J Clean Prod 11:533–536
Detimer A, Patricia SDA, Maritiz G (2013) Special review paper: enzymes in the leather industry. J Am Leat Chem Assoc 10:146–155
Dixit S, Yadav A, Dwivedi PD, Das M (2014) Toxic hazards of leather industry and technologies to combat threat: a review. J Clean Prod 87(1):39–49
Durga J, Ranjithkumar A, Ramesh R, Girivasan KT, Rose C, Muralidharan C (2016) Replacement of lime with carbohydrases – a successful cleaner process for leather making. J Clean Prod 112(1):1122–1127
Eaton AD, Clesceri, LS, Greenberg AE (1995) Standard methods of the examination of water and waste water. The American Public Health Association (APHA), Washington
Gehring AG, Bailey DG, Dimaio GL (2003) Rapid oxidative unhairing with alkaline calcium peroxide. J Am Leat Chem Assoc 98(6):216–223
George N, Chauhan PS, Kumar V, Puri N, Gupta N (2014) Approach to eco-friendly leather: charactersistics and application of an alkaline protease for chemical free dehairing of skins and hides at pilot scale. J Clean Prod 7:249–257
Guo ZR, Zhang G, Fang J, Dou X (2006) Enhanced chromium recovery from tanning waste water. J Clean Prod 14:75–79
Hones P, Diserens M, Levy F (1999) Characterization of sputter-deposited chromium oxide thin films. Surf Coat Technol 120–121:277–283
Hu J, Xiao Z, Zhou R, Deng W, Wang M, Ma S (2010) Ecological utilization of leather tannery waste with circular economy model. J Clean Prod 18:221–228
IUP 16 (2015) ISO 3380: 2015. Leather - physical and mechanical tests - determination of shrinkage temperature up to 100 °C
Jegannathan KR, Nielsen PH (2013) Environmental assessment of enzyme use in industrial production– a literature review. J Clean Prod 42:228–240
Kamini NR, Hemachander C, Mala SM, Puvanakrishnan R (1999) Microbial enzyme technology as an alternative to conventional chemicals in leather industry. Current Scie 77:80–86
Kanagaraj J, Mandal AB (2012) Combined biodegradation and ozonation for removal of tannins and dyes for the reduction of pollution loads. Environ Scie Poll Res 19:42–52
Kanagaraj J, Panda RC (2011) Modelling of dye uptake rate, related interactions, and binding energy estimation in leather matrix using protein based nanoparticle polymer. Ind Eng Chem Res 50(22):12400–12408
Kanagaraj J, Chandra Babu NK, Mandal AB (2008) Recovery and reuse of chromium from chrome tanning waste water aiming towards zero discharge of pollution. J Clean Prod 16:1807
Kanagaraj J, Panda RC, Sumathi V (2015a) Water soluble graft copolymer synthesized from collagenous waste and PEG with functional carboxylic chains: A highly efficient adsorbent for chromium (III) with continuous recycling and molecular docking studies. Ind Eng Chem Res 54:7401–7414
Kanagaraj J, Senthilvelan T, Panda RC, Kavitha S (2015b) Eco-friendly waste management strategies for greener environment towards sustainable development in leather industry: a comprehensive review. J Clean Prod 89:1–17
Kanagaraj J, Senthilvelan T, Panda RC (2016a) Remediation of sulfide based dehairing by oxidative dehairing of skin and correlation by mathematical model: an eco-friendly approach. Proc Safety Environ Protection 100:36–48
Kanagaraj J, Panda RC, Senthilvelan T, Gupta S (2016b) Cleaner approach in leather dyeing using graft copolymer adsorbent: related kinetics and mechanism. J Clean Prod 112:4863–4878
Kanagaraj J, Panda RC, Vinodh Kumar M (2020a) Trends and advancements in sustainable leather processing: future directions and challenges—a review. J Env Chem Eng 8:104379
Kanagaraj J, Panda RC, Jayakumar JC (2020b) Interaction of glyoxal with collagenous matrix and its behavioral aspects for non-toxic and sustainable tanning system. Int J Environ Scie Technol 17(2):879–890
Kanagaraj J, Panda RC, Prasanna R (2022) Sustainable chrome tanning system using protein based product developed from leather waste: wealth from waste. Polym Bull 79:10201–10228
Kandasamy N, Velmurugan P, Sundarvel A, Raghava RJ, Bangaru C, Palanisamy T (2012) Eco-benign enzymatic dehairing of goatskins utilizing a protease from a Pseudomonas fluorescens species isolated from fish visceral waste. J Clean Prod 25:27–33
Kanth SV, Venba R, Madhan B, Chandrababu NK, Sadulla S (2009) Cleaner tanning practices for tannery pollution abatement: role of enzymes in eco-friendly vegetable tanning. J Clean Prod 17(5):507–515
Kilic E, Puig R, Baquero G, Font J, Colak S, Gurler D (2011) Environmental optimization of chromium recovery from tannery sludge using a life cycle assessment approach. J Hazard Mat 192(1):393–401
Li K, Chen H, Wang Y, Shan Z, Yang J, Brutto PA (2009) Salt free pickling regime for hides and skins using oxazolidine. J Clean Prod 17(17):1603–1606
Linton DJ, Klassen R, Jayaraman V (2007) Sustainable supply chains: an introduction. J Oper Management 25:1075–1082
Ma J, Hou X, Gao D, Lv B, Zhang J (2014) Greener approach to efficient leather soaking process: role of enzymes and their synergistic effect. J Clean Prod 78:226–232
Malay S, Rajnish V (2017) Partial characterization of keratinase from Stenotrophomonas maltophilia K279a and study of its dehairing potential. Int J Biotechnol Biochem 13(1):95–110
Marmer WN, Dudley RL (2005) Oxidative dehairing by sodium percarborate. J Am Leat Chem Assoc 100:427–431
Marshal A, Cot J, Bartoli E (2002) Oxidising unhairing process with hair recovery. J Soc Leat Technol Chem 86:30–35
McKillop A, Sanderson WR (1995) Sodium perborate and sodium percarbonate: cheap, safe and versatile oxidizing agents for organic synthesis. Tetrahedron 55:6145–6166
Morera JM, Bartolí E, Chico R, Solé C, Cabeza LF (2011) Minimization of the environmental impact of chrome tanning: a new process reusing the tanning floats. J Clean Prod 19(17–18):2128–2132
Murugappan G, Zakir MJ, Jayakumar GC, Khambhaty Y, Sreeram KJ, Rao JR (2016) A novel approach to enzymatic unhairing and fiber opening of skin using enzymes immobilized on magnetite nanoparticles. ACS Sust Chem Engg 4(3):828–834
Muthsubramanian L, Mitra RB (2006) A cleaner production method for the synthesis of bronopol- a bactericide that is useful in leather making. J Clean Prod 14:536–538
Nashy EHA, Ismail SA, Ahmady AM, El-Fadaly H, El-Sayed NH (2005) Enzymatic bacterial dehairing of bovine hide by a locally isolated strain of Bacillus lichniformis. J Soc Leat Technol Chem 89:242–249
Neil MJ (ed) (2006) The Merck index - an encyclopedia of chemicals, drugs, and biologicals. Merck and Co., Inc., Whitehouse Station, NJ
Priya P, Archana G (2008) Hide depilation and feather disintegration studies with keratinolytic serine protease from a novel Bacillus subtilis isolate. Appl Microbiol Biotechnol 78:643–650
Qiang T, Xin G, Jing R, Xiaoke C, Xuechuan W (2016) A chrome-free and chrome-less tanning system based on the hyperbranched polymer. ACS Sust Chem Eng 4(3):701–707
Ramesh RR, Muralidharan V, Palanivel S (2018) Preparation and application of unhairing enzyme using solid wastes from the leather industry-an attempt toward internalization of solid wastes within the leather industry. Environ Sci Poll Res 25(3):2121–2136
Ranjithkumar A, Durga J, Ramesh R, Rose C (2017) Cleaner processing: a sulphide-free approach for depilation of skins. Env Scie Poll Res 24(1):1–9
Richardson Gavin D, Fantauzzo KA, Bazzi H, Maatta A, Jahoda CAB (2009) Dynamic expression of syndecan-1 during hair follicle morphogenesis. Gene Expr Patterns 9(6):454–460
Saran S, Mahajan RV, Kaushik R, Isar J, Saxena RK (2013) Enzyme mediated beam house operations of leather industry: a needed step towards greener technology. J Clean Prod 54(1):315–322
Saravanan P, Shiny Renitha T, Gowthaman MK, Kamini NR (2014) Understanding the chemical free enzyme based cleaner unhairing process in leather manufacturing. J Clean Prod 79(15):258–264
Sarkis J (2003) A strategic decision framework for green supply chain management. J Clean Prod 11:397–409
Saurabh S, Richi VM, Rekha K, Jasmine I, Rajendra KS (2013) Enzyme mediated beam house operations of leather industry: a needed step towards greener technology. J Clean Prod 54(1):315–322
Senthilvelan T, Kanagaraj J, Mandal AB (2012) Application of enzymes for dehairing of skins: cleaner leather processing. Clean Techn Environ Policy 14:889–897
Shi B, Lu X, Sun D (2003) The mechanism of oxidative unhairing using hydrogen peroxide. J Am Leat Chem Ass 98(5):185–192
Song S, Tao W, Chen W (2011) Kinetics of enzymatic unhairing by protease in leather industry. J Clean Prod 119(4):325–331
Subbarao CH, Sathish T, Ravichandra P, Prakasam RS (2009) Characterization of thermo and detergent stable serine protease from isolated bacillus circulans and evaluation of eco-friendly applications. Proc Biochem 44:262–268
Thangam EB, Rajkumar GS (2002) Purification and characterization of alkaline protease from Alcaligenes faecalis. Biotechnol Appl Biochem 35(2):149–154
Tian J, Xu Z, Long X, Tian Y, Shi B (2019) High-expression keratinase by Bacillus subtilis SCK6 for enzymatic dehairing of goatskins. Int J Biol Macromol 15(135):119–126
Uddin ME, Ahmad T, Ajam MM, Moniruzzaman M, Mandol D, Ray SK, Sufian A, Rahman MA, Hossain E, Ahammed T (2017) Thermotolerant extracellular proteases produced by bacillus subtilis isolated from local soil that representing industrial applications. J Pure Appl Microbiol 11(2):733–741
Valeika V, Beleska K, Valeikiene V, Kolodzeiskis V (2009) An approach to cleaner production: from hair burning to hair saving using a lime-free unhairing system. J Clean Prod 17(2):214–221
Verma A, Pal HS, Singh R, Agarwal S (2011) Pottential of alkaline protease isolated from thermoactinomyces sp. RM4 as an alternative to conventional chemicals in leather industry dehairing process. Int J Agril Env Biotech 4(2):173–178
Wang R, Min C, Haiming C, Li Z (2009) Enzyme unhairing an eco-friendly biotechnological process. J Soc Leat Technol Chem 93:51–55
Wei X, Lifen H, Lei Z (2010) Cleaner dehairing technology for goatskins: effects of hydrosulfide and peroxide on enzyme unhearing. Adv Mat Res 113–114:1726–1731
Yang Na, Ma J, Shi J, Yang X, Jun Lu (2022) Manipulate the nano-structure of layered double hydroxides via calcination for enhancing immobilization of anionic dyes on collagen fibers. J Coll Int Scie 610:182–193
Acknowledgements
All the authors would like to acknowledge and thank CSIR, New Delhi for approving the FBR project with code MLP-2004 for carrying out this work.
Funding
The authors thank Council of Scientific & Industrial Research (CSIR), New Delhi, India for sponsoring and permitting to carry out this work under Focused Basic Research (FBR) project with code: MLP 2004.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Dr. James Kanagaraj, Dr. Rames C. Panda, Ramakrishna Prasanna, and Alagamuthu Tamilselvi. The first draft of the manuscript was written by Dr. James Kanagaraj and all authors commented on previous versions of the manuscript. Dr. Alagamuthu Tamilselvi has contributed in analysis of results. All authors read and approved the final manuscript. All authors agree to publish and participate as per the norms of the journal.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving dehairing systems were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
All authors who have contributed to the study have agreed to participate as per the journal directions.
Consent for publication
All authors who have contributed to the study have agreed to publish as per the journal directions.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kanagaraj, J., Panda, R.C., Prasanna, R. et al. An efficient dehairing system supported by oxidative-enzymatic auxiliary towards sustainability. Environ Sci Pollut Res 30, 43817–43832 (2023). https://doi.org/10.1007/s11356-023-25380-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25380-3