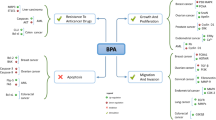
Abstract
Environmental pollution caused by persistent organic pollutants (POPs) has increased the challenge for the scientific communities. Polybrominated diphenyl ethers (PBDEs), classified as POPs, are widely applied in various materials as brominated flame retardants (BFRs). Because of the nature of these chemical compounds including toxicity, stability, and capability to bioaccumulate and biomagnify, PBDEs have posed a great challenge and risk to human health and wildlife. Therefore, the side effects of exposure to PBDEs as ubiquitous pollutants in the environment on cancer progression were investigated using a systematic review (SR) survey. To achieve this goal, forty studies were considered after defining the search terms and inclusion criteria, and/or exclusion criteria; the eligible records were collected from the international bibliographic databases. Based on the findings of the reviewed records, environmental exposure to the BFRs including PBDEs has a positive association with different mechanisms that induce cancer progression. However, the findings of the reviewed studies were not totally consistent with the mode of action and side effects are yet to be fully elucidated. Several articles have reported that BFRs can be carcinogenic and induce epithelial to mesenchymal transition via different mechanisms. The main mode of action involved in the environmental exposure to BFRs and the risk of cancer progression is endoplasmic reticulum and oxidative stress (OS). Generally, the imbalance of antioxidant mechanisms, reactive nitrogen species (RNSs) and reactive oxygen species (ROSs), during damage in cells, and stress caused OS, which increases tumorigenesis via multiple mechanisms, such as DNA damage, inflammation, and angiogenesis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, environmental pollution caused by persistent organic pollutants (POPs) has increased the challenge for scientific communities. POPs are defined as highly toxic chemical compounds, persistent in the environment, capable of bioaccumulating and biomagnifying. They are used in various applications such as manufacturing processes, agriculture, and industry. POPs are considered the endocrine disrupting compounds (EDCs) and, therefore, deteriorating the functional organs of the human body and wildlife (Zhao et al. 2009; Liu et al. 2017; Jaafarzadeh et al. 2019; Park et al. 2020; Mirzaee et al. 2021a, b).
Brominated flame retardants (BFRs) and their subgroup polybrominated diphenyl ethers (PBDEs) as organobrominated compounds are considered a broad group of “additive” materials to prevent the growth of fire in industrial, chemical, and household products, including construction materials, polyurethane foams, textiles, electronic devices, plastics, among others, during the last decades (Li et al. 2012; Liu et al. 2017; Wu et al. 2021). The PBDE compounds are additive materials; therefore, they are not link to their polymers by chemical reactions. Thus, they easily leach to various environmental media, and recently, these compounds have become important and widespread environmental pollutants (Costa and Giordano 2014; Huang et al. 2020). It is estimated that about 600,000 metric tons of flame retardants are produced in the globe annually. From this amount, 150,000 tons and 60,000 tons are brominated and chlorinated compounds, respectively (Darnerud et al. 2001).
The general characteristics of PBDEs or their congeners were depicted in Table 1 (Darnerud et al. 2001; Siddiqi et al. 2003; Costa and Giordano 2014; Huang et al. 2020; Wu et al. 2021). Due to their nature and physicochemical characteristics including their toxicity, capability of bioaccumulating and biomagnifying, and persistence in the environment, some of them such as hepta-, deca-, hexa-, tetra-, as well as penta-BDEs were categorized as POPs at the Stockholm Convention in 2009 and 2017 (Convention 2009, 2017). Despite the production and use of POPs, including PBDEs, were banned in the 1970s, environmental exposure to low concentrations of these chemical compounds continues. One of the most important environmental exposure to these type of compounds is the e-waste disassembly sites in the solid waste management section especially via solid waste incinerators (Agrell et al. 2004; Zhao et al. 2009; Wang et al. 2010). Regarding the characteristics of PBDEs, their use and their congeners were restricted; the evidence confirmed that due to the presence in different environmental media and products, the side effects of PBDEs or their congeners can be seen on the environment, human body, and wildlife (Table 2) (Siddiqi et al. 2003; Agrell et al. 2004; Kim et al. 2005; Wang et al. 2010; Ni et al. 2013; Park et al. 2020; Wu et al. 2021).
The mechanisms and mode of actions involved in the detrimental impacts of PBDEs are not completely known. Several pieces of evidence reveal that PBDEs or their congeners can disrupt endocrine system and induce toxic side effects, including neurotoxicity, estrogenicity, reproductive disorders, carcinogenicity, and teratogenicity. It is reported that the EDC potential of PBDEs may react as agonists or antagonists at estrogen, androgen, and progesterone receptors and change the reproductive function of rats (Meerts et al. 2001; Legler and Brouwer 2003; Li et al. 2012; Tian et al. 2016; Liu et al. 2017; Cao et al. 2018; Wei et al. 2018). Some of these compounds are responsible for DNA damage via reactive oxygen species (ROS) generation pathway (Pellacani et al. 2012; Montalbano et al. 2020). Recently, Leonetti et al. revealed that the sensitivity of total thyroid hormone sulfotransferases activity in placental cells exposed to brominated flame retardants occurs through unknown mechanisms (Leonetti et al. 2018). Hoffman et al. concluded that more studies are needed on exposure to some congeners of flame-retardant compounds, such as deca-BDE-209 could be related to progress of papillary thyroid cancer, not only its occurrence but also its severity (Hoffman et al. 2017). In another research, it was demonstrated that simultaneous exposure to some of the PBDEs (BDE-99 and BDE-47) can trigger synergistic OS-mediated neurotoxic effects in neuroblastoma cells in human body. It is worth noting that, in general in the environmental media, wildlife and even humans are exposed to mixtures of PBDEs (Tagliaferri et al. 2010).
To our knowledge, there is no evidence related to the assessment of side effects of environmental exposure to PBDEs, especially on cancer progression. Therefore, in the present work, a systematic literature search was performed to collect and evaluate all the evidence on the impacts of environmental contact to PBDEs in the risk of cancer progression.
Methods
Strategy of search and extraction of main data
This research does not require patient consent or ethical approval because the present research was a study on previously published papers. This SR was done according to the PRISMA guideline (www.prisma-statement.org) (Liberati et al. 2009; Moher et al. 2015; Mirzaee et al. 2021a, b; Noorimotlagh et al. 2021). We identified articles that describe the relationship between the effects of the environmental contact to BFRs and the threat of cancer progress. A systematic search was performed from May 1, 1980 to June 1, 2022 in four international bibliographic databases, including Web of Science, Scopus, PubMed, and Google scholar engines for Medical subject Heading (MeSH) terms. Search terms with a medical subject heading were used in all possible combinations (Supplementary section). After completing the article search, two independent reviewers (SAM, SM) imported all articles into Endnote and Mendeley software to remove duplicate articles. Finally, a data list was applied to extract relevant findings from full texts of all remaining studies. This form contains information such as study ID, country, study type, pollutant(s) and cancer type, study model, number of cases, main finding, mechanism (mode of action), and biological end-point. Figure 1 shows a summary of the PRISMA protocol in terms of the record selection method.
Inclusion and exclusion criteria
The reviewers then screened titles and abstracts of all articles for inclusion criteria, including article language based on English, original articles, and studies on the effect of BFRs and the risk of cancer progress. Articles were excluded if they were review articles, short communications, letter to the editor, book chapter, and articles on other chemicals.
Evaluation of the quality of reviewed records
In order to assess of the quality of reviewed records, the ARRIVE checklist was applied (Moher et al. 2001; Kilkenny et al. 2010). This guideline has been fulfilled and developed based on CONSORT statement. The ARRIVE checklist was applied to evaluate observational studies such as case control studies. It has 20 questions according to the study sections and evaluates the results of the studies and, finally, represents a quantitative scale to assessing the overall quality of the studies. Herein, the obtained results of the checklist for the quality of the studies are shown in the Supplementary section (Tables S1 and S2).
Results
Based on systematic searching of electronic databases, this is the first work on the impacts of the environmental contact to BFRs and the risk of cancer progression. Of 244 articles collected from the initial search on ISI, Scopus, PubMed, and Google scholar, 45, 31, 11, and 93 papers were removed because they were duplicates, review articles, short communication, and not related to the effect of BFRs and the risk of cancer progression, respectively. Then, in the next step, we studied the full text of all 64 articles and 24 studies were removed from this research because they did not meet our inclusion criteria. Finally, in this SR, forty studies were reviewed and included (Jensen et al. 1982, 1984; Gupta et al. 1983; Jensen et al. 1983; Williams et al. 1984; Kavanagh et al. 1985; Rezabek et al. 1989; Rangga-Tabbu and Sleight 1992; Chhabra et al. 1993; Meerts et al. 2001; Madia et al. 2004; Hu et al. 2007; He et al. 2008; Song et al. 2009; Zhao et al. 2009, 2022; Tagliaferri et al. 2010; Man et al. 2011; Li et al. 2012, 2019; Pellacani et al. 2012; Sakamoto et al. 2013; Zhang et al. 2013, 2017, 2019; Harvey et al. 2015; Qu et al. 2015; Wang et al. 2015; Terrell et al. 2016; Tian et al. 2016; Hoffman et al. 2017; Liu et al. 2017; He et al. 2018; Leonetti et al. 2018; Wei et al. 2018; Han et al. 2019; Hurley et al. 2019; Kanaya et al. 2019; Tang et al. 2019; Huang et al. 2020). The main information, mechanism, and biological end-points of forty reviewed and included studies are provided in Tables 3 and 4.
According to the results depicted in Table 3, of the 40 reviewed and included studies, nineteen studies, seventeen studies, one study, two studies, and one study were carried out at USA, China, Sweden, Italy, and Japan, respectively.
It is worth noting that most of the reviewed studies were case–control study. This systematic review based on the available articles provides different mechanisms to show the association of BFRs and the risk of cancer incidence. The findings of the included studies were not fully consistent. However, among 40 reviewed studies, seventeen studies examined BFR-induced human neoplasia (Zhao et al. 2009; Terrell et al. 2016; Hoffman et al. 2017; Liu et al. 2017; He et al. 2018; Li et al. 2019; Huang et al. 2020), ten studies are associated to animal-related neoplasia (Jensen et al. 1982, 1983, 1984; Gupta et al. 1983; Williams et al. 1984; Rezabek et al. 1989; Rangga-Tabbu and Sleight 1992; Chhabra et al. 1993; Sakamoto et al. 2013; Harvey et al. 2015), while twenty studies performed neoplasia by cell line (according to results depicted in Table 3), as well as some studies showed that BFRs induced the proliferation and growth of different tumor cells through a variety of mechanisms (according to results shown in Table 3). Eventually, some of the reviewed records reported that BFRs had no impacts to induce neoplasia (according to results shown in Table 3).
Quality assessment of the reviewed studies
ARRIVE guideline checklist with different items was used, and the percentage of the all included studies was classified in the Supplementary Section (Tables S1 and S2). Based on the finding depicted in Tables S1 and S2. According to the finding shown in Table S1 and S2. most of the reviewed studies obtained high rate; therefore, they were reviewed in the present study.
Discussion
Cancer mortality continues in the industrialized world despite the extensive research and quick progress shown in the last 10 years that appears to be due to changing patterns of cancer risk factors. Thus, a key way to identify cancer control opportunities is to learn about the causes and risk factors of common cancers that provide a foundation for understanding the potential to prevent and reduce cancer incidence (Anand et al. 2008). BFRs are commonly applied in various products, such as electronics, textiles and clothing, toys, automobiles, and plastics to alleviate flammability and have become ubiquitous and persistent environmental pollutants (Hoffman et al. 2017). Because laboratory and epidemiological studies have shown that BFR exposure can result in cancer, developmental and neurobehavioral disorders, reproductive and behavioral abnormalities, and immune system dysfunctions, there is increasing worry about BFR’s global distribution and influence on life (He et al. 2010). Human exposure to BRFs occurs through the environment via water (Yang et al. 2015), air (Deng et al. 2007; Wu et al. 2021), dust inhalation as well as food ingestion (Ni et al. 2013), and especially E-waste (contain plastic waste) municipal solid waste incinerators (Kim et al. 2005; Zhao et al. 2009; Wang et al. 2010; Ni et al. 2013). Infants could also be exposed through ingestion of breast milk, and fetal exposure can occur through the placenta, leading to the highest body burden in infants and young children (Chen et al. 2014). Therefore, as the levels of BFRs in the environment and human body tissues are rising year after year, BRFs have posed a serious threat to both human health and safety due to their toxicity and widespread use in the environment (Chhabra et al. 1993; Zhao et al. 2009).
Multiple studies suggest exposure to various congener of BFRs (such as PBBs, PBDEs, and PCBs) can lead to tumors (Wu et al. 2020) and the greater tissue concentrations of FRs may contribute to the elevated cancer incidence in the disassembly locations (Zhao et al. 2009). Hoffman et al., in a case–control study, demonstrated that some FRs in the indoor environment of home such as TCEP and BDE-209 could be related with the severity and occurrence of papillary thyroid cancer (PTC) (Hoffman et al. 2017). Furthermore, in a cohort study of 33 patients with thyroid cancer, body burdens of the serum thyroid status and 11-OH-PBDEs and 7-PBDEs demonstrated that OH-PBDEs and PBDEs were widely distributed in the population with thyroid cancer, and their concentration is comparable to those in the general population in China (Liu et al. 2017). According to a review study of cohort information from the US Department of Defense from 2000 to 2013, an increment in BDE-28 concentrations considerably raised the risk of papillary thyroid cancer (Huang et al. 2020). The effect of BFRs also has been confirmed to induce gastrointestinal neoplasm (Gupta et al. 1983). Several studies in different animal models such as rats, mice, or hamsters have shown that short and chronic (long-term) exposure to PBB and PCB have the potential to enhance the development of foci of enzymatic alteration, so these components act as promoters of experimental hepatocarcinogenesis (Jensen et al. 1982, 1983, 1984; Williams et al. 1984; Rezabek et al. 1989; Smith et al. 1990; Chhabra et al. 1993). For example, in a single oral dose study of FM (a commercial combination of PBBs), the development of changed hepatocellular loci in rats challenged with N-nitrosodimethylamine and N-nitrosopyrrolidine (NPYR) was significantly enhanced (Rangga-Tabbu and Sleight 1992). Furthermore, it has been reported that FM directly through inhibition of cell–cell communication may be involved in the promotion of hepatocellular neoplasms (Kavanagh et al. 1985). Other results also reported that exposure to PBDEs may influence the development and occurrence of breast cancer. In addition, Kwieciñska et al. demonstrated that in the presence of 17-estradiol, BDE-47 and BDE-209 can increase MCF-7 cell proliferation and decrease cell apoptosis in vitro (Kwiecińska et al. 2011). Additionally, researchers in China measured the concentrations of 14 distinct PBDEs in the adipose tissue of women without and with breast cancer. In comparison to controls, breast cancer cases had higher concentrations of the total PBDEs. Breast cancer risk factors have been proposed for some of the specific PBDE congeners (He et al. 2018). Although these studies have reported that exposure to these components could increase the incidence of cancer, the main mechanisms that could contribute to the tumorigenesis remain to be clarified.
In our study, several articles reported that BFRs may be carcinogenic through different mechanisms. Most studies have introduced endoplasmic reticulum stress (ERS) and OS as the main mechanism of these compounds in cancer induction. In general, the agglomeration of toxic compounds in the ER destroys the basic function of the organelles and increases the accumulation of misfolded proteins in the lumen of ER leading to ERS. Decreased protein synthesis, increased protein folding mechanism, and removal of terminal misfolded protein are multiple mechanisms that are induced by the unfolded protein response (UPR) after ERS to maintain homeostasis in cell (Lin et al. 2019). However, in a chronic ERS due to persistence of the risk factor, the UPR fails to restore ER homeostasis and UPR response promotes the production of ROS in the endoplasmic reticulum (Victor et al. 2021). Meanwhile, mitochondrial function is disrupted by ERS, and this caused increased mitochondrial ROS production (Cao and Kaufman 2014; Arfin et al. 2021). Thus, during cellular stress and injury, imbalance of antioxidant systems and ROS along with nitrogen reactive species (RNSs) led to OS which increases tumorigenesis via multiple mechanisms such as DNA damage, inflammation, angiogenesis, immune response evasion, and drug resistance (Hayes et al. 2020). In agreement with this issue, Pellacani et al., in their study, showed that two PBDEs flame retardants such as BDE-209 and BDE-47 could damage DNA in neuroblastoma cells of human, which is mainly mediated by induction of OS (Pellacani et al. 2012). It has also been shown that low concentrations of BFRs can induce OS in neuroblastoma cells (Tagliaferri et al. 2010). Furthermore, according to many research, PBDE-47 is cytotoxic and genotoxic and can cause LDH leakage, ROS production, cell death, and as well as DNA damage via ERS and OS (He et al. 2008; Zhang et al. 2017). Additionally, it was noted that PBDE-99, PBDE-47, and PBDE-209 may affect the function of the respiratory epithelium of human by causing OS and DNA damage in an in vitro and/ or ex vivo experimental model of bronchial epithelial cells, thereby encouraging inflammation, tissue damage, genetic anomalies, and oncogenesis (Montalbano et al. 2020). Wei et al. investigated the toxicity mechanism of BDE-47 in MCF-7 breast cancer cells in a study and found that exposure to BDE-47 hindered the production of NADPH in the PPP. Multiple ROS scavenging system pathways may be impacted by the absence of NADPH. Because antioxidases were unable to remove ROS in time without NADPH, OS was produced and eventually resulted in cell damage (Wei et al. 2018). Therefore, ROS produced after exposure to BFRs through oxidative stress, such as strand breaks, DNA base alterations, expression of proto-oncogene, and damage to tumor suppressor genes resulting in the alteration of normal cells in malignant (virulent) cells and the positive regulation of growth factors and cytokines, exerts its effect. Figure 2 shows a summary of the mechanisms mentioned above.
Main processes that influence the risk of cancer progression and environmental exposure to BFRs. BFR accumulation impairs fundamental organelle function in cells and accelerates the aggregation of misfolded proteins in the ER lumen that cause ERS. Decreased protein synthesis, enhanced protein folding mechanism, and removal of terminal misfolded protein are multiple mechanisms induced by UPR response following ERS. In a chronic ERS due to persistence of the risk factor, the UPR fails to restore ER homeostasis and UPR response promotes the production of reactive oxygen species (ROS) in the endoplasmic reticulum. Meanwhile, mitochondrial function is disrupted by ERS, and this caused mitochondrial ROS production to increase. As a result, OS, which enhances carcinogenesis through a variety of mechanisms including DNA damage and inflammation, is induced by an imbalance between antioxidant systems and ROS during stress and injury in cells. Furthermore, BFRs induced the growth and proliferation of different tumor cells via the PI3K/Akt/Snail signaling pathway
The impacts of 11-OH-PBDE on the trigger of one of the UPR arms, namely the PERK pathway, have also been confirmed, and it appears that its bioaccumulation may disrupt adrenocortical secretory pathways via UPR pathways and ERS. The possible endocrine-disrupting effects of BFRs and their derivatives are mediated by ERS (Song et al. 2009). Other studies also reported that some of the BFRs resemble BDE-47 through disturbance in the metabolism of acidic amines, such as alanine, aspartate, and glutamate, and the metabolism of pyrimidines and purines after OS may have an adverse effect on human health (Wei et al. 2018; Tang et al. 2019).
Moreover, other mechanisms have been proposed to show that BFRs can exert their carcinogenic effects. Li et al. reported that PBDE-209 through activated protein kinase C (PKCα) and extracellular signal-regulated kinases (ERK1/2) phosphorylation has proliferative effects and antiapoptotic effects in the female reproductive system and normal ovarian CHO cells. They also treated breast cancer cells with PBDE-209 and showed that PBDE-209 neutralized the effects of the cancer drug tamoxifen (Li et al. 2012). Additionally, Zhang et al. observed that endometrial cancer (EC) has been linked to prolonged exposure to BDE-47, which can exacerbate malignant phenotypes and chemoresistance by triggering estrogen receptor (ER)/G protein-coupled receptor (GPR30) and epidermal growth factor receptor (EGFR)/ERK signaling pathways in EC cells both in vivo and in vitro (Zhang, Peng et al. 2019). Several pure PBDE congeners have been proved that activate the estrogen receptor signal transduction pathway in vitro because they are agonists of both the ERα and ERβ receptors and increased growth in breast cancer cells because they acted like estrogen (Meerts et al. 2001). An included study by Kanaya et al. also revealed that PBDEs such as BDE-47, -100, and -153 stimulated an estrogen-dependent proliferation in the cell line of breast cancer (MCF-7aroERE) by modulation of ERα, ERRα, progesterone receptor (PR)/AhR pathways, so they can exert their effect on human breast cancer cells (Kanaya et al. 2019). A study performed on wild-type and CARKO mice as well noted that DBDE (a brominated flame retardant) may act via constitutive androstane receptor (CAR)-independent pathways during hepatocarcinogenesis because it can increase the multiplicity of basophilic altered foci/adenomas in wild-type and CARKO mice (Sakamoto et al. 2013). The role of tetrabromobisphenol A (TBBPA), a widely used BFR, in the induction of uterine carcinomas was confirmed. Results have reported that TBBPA-induced uterine carcinomas in Wistar Han rats exhibited greater rates of proliferation, Tp53 mutation, a tendency for reduced PR expression, and enhanced human epidermal growth factor receptor 2 expression compared to spontaneous uterine cancers (Harvey et al. 2015). In another study, BDE-47 was utilized to examine how steroidogenic activity was affected in mouse Leydig tumor cells (mLTC-1). Results demonstrated that BDE-47 lowered progesterone production via decreasing cAMP generation and cholesterol side-chain cleavage enzyme (P450scc) activity (Han et al. 2012). In a related study, Han et al. demonstrated that deca-brominated diphenyl ether (BDE-209) can reduce progesterone secretion in mouse Leydig tumor cells, mostly by inhibiting the expression of the mRNA for P450scc and 3β hydroxysteroid dehydrogenase (3β-HSD) (Han et al. 2019). PBDEs as well may influence the production of thyroid hormone and estrogen in ways that raise the risk of breast and other cancers (Wu et al. 2020). In this regard, the two congeners of PBDE, such as BDE-99 and BDE-47 which are poisonous and persistent, attract the most public worry in this area because they may interfere with thyroid hormone function and neurobehavioral development (Man et al. 2011; Leonetti et al. 2018). It has also been suggested that by acting as epigenetic agents, PBDEs may have the same ability to promote cancer as PCBs (He et al. 2008).
The connection between PBDEs and the promotion of the epithelial to mesenchymal transition (EMT) has been validated by certain experimental studies of BFRs on various cancer cells. Qu et al. have discovered that the most prevalent OH-PBDE congener in human serum, 6-OH-BDE-47, increased the in vitro migration of lung cancer A549 and H358 cells through controlling the expression of epithelial markers E-cadherin (E-Cad), zona occludin-1 (ZO-1), mesenchymal markers vimentin (Vim), and N-cadherin (N-Cad). 6-OH- 6-OH-BDE-47 also upregulated the protein of expression Snail and increased the phosphorylation of AKT and ERK, so this component induced EMT via AKT/Snail signals and has effects of tumorigenesis and development of lung cancer (Qu et al. 2015). The role of BDE-47 in the induction of metastasis in another study, done in human neuroblastoma SH-SY5Y cells, has been reported. This study showed that BDE-47 can cause metastasis in human neuroblastoma by upregulating MMP-9 and downregulating the epithelial makers E-Cad and ZO-1 through the GPER/PI3K/Akt signal pathway in a dose- and time-dependent manner (Tian et al. 2016). It was also shown that BDE-99 boosted cell migration and invasion in colon cancer HCT-116 cells and caused the epithelial to mesenchymal transition through activating the PI3K/Akt/Snail signaling pathway (Wang et al. 2015).
Unlike the mentioned above, studies reported that PCBs and PBDE concentrations in the breast fat tissues of fifty patients of breast cancer and control cases were not substantially different (Li et al. 2019), and there was also no link between exposure to PBDEs and thyroid cancer (Aschebrook-Kilfoy et al. 2015). Similarly, the serum of Californian women showed no correlation between BDE-100, -47, or -153 levels and the risk of developing breast cancer, especially when they are present alone (Kanaya et al. 2019). Another study, with a smaller sample size, indicated an elevated risk of breast cancer with increased PBB exposure, but did not discover statistically significant connections between the incidence of breast cancer and higher serum PBB concentrations (Terrell et al. 2016). Furthermore, the above results are not in agreement with some of the studies that reported BFRs as an inducer of apoptosis. In this regard, it was shown that PBDE-209 has the harmful effect of inhibiting proliferation and inducing apoptosis in tumor cells in vitro at different doses. Kwieciska et al. have demonstrated that no PBDEs, including 47, 99, 100, and 209 congener, had an impact on cell proliferation (Hu et al. 2007; Kwiecińska et al. 2011). Another investigation found that exposure to PBDE-47 can cause apoptosis in SH-SY5Y cells by upregulating p53 and Bax, downregulating Bcl-2 and the Bcl-2/Bax ratio, enhancing Cyt c production, and activating caspase-3 (Zhang et al. 2013). The role of PBDE-99 in inducing cell cycle arrest and/or apoptotic cell death in human astroglial cells through increased p53 expression has also been reported (Madia et al. 2004). Overall, the conflicting findings suggested that more investigations are required to show the exact side effects of BFRs, and differences between previous studies may attribute dependence to the type of BFRs, concentration, route and time of exposure, cell type, sensitivity of several cell lines exposed to various PBDEs isomers, as well as to genetic polymorphisms and levels of endogenous estrogen in humans (Hurley et al. 2019).
Conclusion and suggestions
In this study, the role of environmental exposure to PBDEs as ubiquitous pollutants in the environment on cancer progression was analyzed. According to the findings of the reviewed investigations, environmental exposure to BFRs was positively associated with cancer progression. However, the findings of the reviewed studies were not entirely consistent. Several articles have reported that BFRs can be carcinogenic through different mechanism. The connection between PBDE and the induction of the epithelial to mesenchymal transition has been verified by some experimental studies of BFRs in various cancer cells. The main mechanisms involved in environmental exposure to BFRs and the risk of cancer progression are endoplasmic reticulum stress and OS. Overall, the accumulation of toxic substances in the ER destroys the basic function of the organelle and increases the accumulation of misfolded proteins in the lumen of ER leading to ERS. UPR response under chronic stress promotes the generation of ROS in the endoplasmic reticulum. Mitochondrial function is disrupted by ERS, and this caused mitochondrial ROS production to increase. Thus, the imbalance of antioxidant mechanisms and ROS and nitrogen reactive species during stress and injury in cells caused OS which increases tumorigenesis via multiple mechanisms such as DNA damage, inflammation, angiogenesis, evading immune response, and drug resistance. Eventually, the conflicting finding of the reviewed studies suggested that more investigations are required to show the exact side effects of BFRs, and differences between reported studies may attribute dependence to type of BFRs, concentration, pathway and time of exposure, type of cell, sensitivity of different cell lines exposed to different PBDEs isomers, as well as to genetic polymorphisms and endogenous estrogen levels in humans.
The evidence reported that because of the voluntary and mandatory flammability standards for electronic devices, house furnishings, and construction made of plastic and other plastic-related materials, the use of the BFRs during the last decades increased, and, therefore, the exposure to these chemical contaminants may be increased (Alaee et al. 2003; van der Veen and de Boer 2012). Keep this in mind, it is suggested to increase monitoring of e-waste contaminated site including municipal solid waste for human and wildlife exposure, comprehensive study of join human exposure to the various types of BPRs (PBDEs), and upgrade the e-waste recycling system and strengthen incinerators for e-waste or plastic material in the integrated solid waste management system.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Agrell C, ter Schure AFH, Sveder J, Bokenstrand A, Larsson P, Zegers BN (2004) Polybrominated diphenyl ethers (PBDES) at a solid waste incineration plant I: Atmospheric concentrations. Atmos Environ 38(30):5139–5148
Alaee M, Arias P, Sjödin A, Bergman Å (2003) An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ Int 29(6):683–689
Anand P, Kunnumakara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, Sung B, Aggarwal BB (2008) Cancer is a preventable disease that requires major lifestyle changes. Pharm Res 25(9):2097–2116
Arfin S, Jha NK, Jha SK, Kesari KK, Ruokolainen J, Roychoudhury S, Rathi B, Kumar D (2021) Oxidative stress in cancer cell metabolism. Antioxidants (basel, Switzerland) 10(5):642
Aschebrook-Kilfoy B, DellaValle CT, Purdue M, Kim C, Zhang Y, Sjodin A, Ward MH (2015) Polybrominated diphenyl ethers and thyroid cancer risk in the Prostate, Colorectal, Lung, and Ovarian Cancer Screening Trial cohort. Am J Epidemiol 181(11):883–888
Cao L-Y, Zheng Z, Ren X-M, Andersson PL, Guo L-H (2018) Structure-dependent activity of polybrominated diphenyl ethers and their hydroxylated metabolites on estrogen related receptor γ: in vitro and in silico study. Environ Sci Technol 52(15):8894–8902
Cao SS, Kaufman RJ (2014) Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal 21(3):396–413
Chen Z-J, Liu H-Y, Cheng Z, Man Y-B, Zhang K-S, Wei W, Du J, Wong M-H, Wang H-S (2014) Polybrominated diphenyl ethers (PBDEs) in human samples of mother–newborn pairs in South China and their placental transfer characteristics. Environ Int 73:77–84
Chhabra R, Bucher J, Haseman J, Elwell M, Kurtz P, Carlton B (1993) Comparative carcinogenicity of polybrominated biphenyls with or without perinatal exposure in rats and mice. Fundam Appl Toxicol 21(4):451–460
Convention S (2009) http://chm.pops.int/TheConvention/ThePOPs/AllPOPs/tabid/2509/Default.aspx. Accessed June 2022
Convention S (2017) http://chm.pops.int/TheConvention/ThePOPs/AllPOPs/tabid/2509/Default.aspx. Accessed June 2022
Costa LG, Giordano G (2014) Polybrominated diphenyl ethers. Encyclopedia of Toxicology (Third Edition). P. Wexler.Academic Press, Oxford. 1032–1034
Darnerud PO, Eriksen GS, Jóhannesson T, Larsen PB, Viluksela M (2001) Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ Health Perspect 109(suppl 1):49–68
Deng W, Zheng J, Bi X, Fu J, Wong MH (2007) Distribution of PBDEs in air particles from an electronic waste recycling site compared with Guangzhou and Hong Kong, South China. Environ Int 33(8):1063–1069
Gupta B, McConnell E, Moore J, Haseman J (1983) Effects of a polybrominated biphenyl mixture in the rat and mouse: II. Lifetime study. Toxicol Appl Pharmacol 68(1):19–35
Han X, Tang R, Chen X, Xu B, Qin Y, Wu W, Hu Y, Xu B, Song L, Xia Y (2012) 2, 2′, 4, 4′-Tetrabromodiphenyl ether (BDE-47) decreases progesterone synthesis through cAMP-PKA pathway and P450scc downregulation in mouse Leydig tumor cells. Toxicology 302(1):44–50
Han X, Wang Y, Chen T, Wilson MJ, Pan F, Wu X, Rui C, Chen D, Tang Q, Wu W (2019) Inhibition of progesterone biosynthesis induced by deca-brominated diphenyl ether (BDE-209) in mouse Leydig tumor cell (MLTC-1). Toxicol in Vitro 60:383–388
Harvey JB, Osborne TS, Hong H-HL, Bhusari S, Ton T-V, Pandiri AR, Masinde T, Dunnick J, Peddada S, Elmore S (2015) Uterine carcinomas in tetrabromobisphenol A–exposed Wistar Han rats harbor increased Tp53 mutations and mimic high-grade type I endometrial carcinomas in women. Toxicol Pathol 43(8):1103–1113
Hayes JD, Dinkova-Kostova AT, Tew KD (2020) Oxidative stress in cancer. Cancer Cell 38(2):167–197
He W, He P, Wang A, Xia T, Xu B, Chen X (2008) Effects of PBDE-47 on cytotoxicity and genotoxicity in human neuroblastoma cells in vitro. Mutat Res Genet Toxicol Environ Mutagen 649(1–2):62–70
He W, Wang A, Xia T, Gao P, Xu B, Xu Z, He P, Chen X (2010) Cytogenotoxicity induced by PBDE-47 combined with PCB153 treatment in SH-SY5Y cells. Environ Toxicol 25(6):564–572
He Y, Peng L, Zhang W, Liu C, Yang Q, Zheng S, Bao M, Huang Y, Wu K (2018) Adipose tissue levels of polybrominated diphenyl ethers and breast cancer risk in Chinese women: a case–control study. Environ Res 167:160–168
Hoffman K, Lorenzo A, Butt CM, Hammel SC, Henderson BB, Roman SA, Scheri RP, Stapleton HM, Sosa JA (2017) Exposure to flame retardant chemicals and occurrence and severity of papillary thyroid cancer: a case-control study. Environ Int 107:235–242
Hu X-Z, Xu Y, Hu D-C, Hui Y, Yang F-X (2007) Apoptosis induction on human hepatoma cells Hep G2 of decabrominated diphenyl ether (PBDE-209). Toxicol Lett 171(1–2):19–28
Huang H, Sjodin A, Chen Y, Ni X, Ma S, Yu H, Ward MH, Udelsman R, Rusiecki J, Zhang Y (2020) Polybrominated diphenyl ethers, polybrominated biphenyls, and risk of papillary thyroid cancer: a nested case-control study. Am J Epidemiol 189(2):120–132
Hurley S, Goldberg D, Park J-S, Petreas M, Bernstein L, Anton-Culver H, Neuhausen SL, Nelson DO, Reynolds P (2019) A breast cancer case-control study of polybrominated diphenyl ether (PBDE) serum levels among California women. Environ Int 127:412–419
Jaafarzadeh N, Baboli Z, Noorimotlagh Z, Martínez SS, Ahmadi M, Alavi S, Mirzaee S (2019) Efficient adsorption of bisphenol A from aqueous solutions using low-cost activated carbons produced from natural and synthetic carbonaceous materials. Desalin Water Treat 154:177–187
Jensen R, Sleight S, Aust S, Goodman J, Trosko J (1983) Hepatic tumor-promoting ability of 3, 3′, 4, 4′, 5, 5′-hexabromobiphenyl: the interrelationship between toxicity, induction of hepatic microsomal drug metabolizing enzymes, and tumor-promoting ability. Toxicol Appl Pharmacol 71(2):163–176
Jensen RK, Sleight SD, Aust SD (1984) Effect of varying the length of exposure to polybrominated biphenyls on the development of gamma-glutamyl transpeptidase enzymealtered foci. Carcinogenesis 5(1):63–66
Jensen RK, Sleight SD, Goodman JI, Aust SD, Trosko JE (1982) Polybrominated biphenyls as promoters in experimental hepatocarcinogenesis in rats. Carcinogenesis 3(10):1183–1186
Kanaya N, Bernal L, Chang G, Yamamoto T, Nguyen D, Wang Y-Z, Park J-S, Warden C, Wang J, Wu X (2019) Molecular mechanisms of polybrominated diphenyl ethers (BDE-47, BDE-100, and BDE-153) in human breast cancer cells and patient-derived xenografts. Toxicol Sci 169(2):380–398
Kavanagh T, Rubinstein C, Liu P, Chang C-C, Trosko J, Sleight S (1985) Failure to induce mutations in Chinese hamster V79 cells and WB rat liver cells by the polybrominated biphenyls, firemaster BP-6, 2, 2′, 4, 4′, 5, 5′-hexabromobiphenyl, 3, 3′, 4, 4′, 5, 5′-hexabromobiphenyl, and 3, 3′, 4, 4′, 5, 5′-tetrabromobiphenyl. Toxicol Appl Pharmacol 79(1):91–98
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother 1(2):94–99
Kim B-H, Ikonomou MG, Lee S-J, Kim H-S, Chang Y-S (2005) Concentrations of polybrominated diphenyl ethers, polychlorinated dibenzo-p-dioxins and dibenzofurans, and polychlorinated biphenyls in human blood samples from Korea. Sci Total Environ 336(1):45–56
Kwiecińska P, Wróbel A, Gregoraszczuk EŁ (2011) Combinatory effects of PBDEs and 17β-estradiol on MCF-7 cell proliferation and apoptosis. Pharmacol Rep 63(1):189–194
Legler J, Brouwer A (2003) Are brominated flame retardants endocrine disruptors? Environ Int 29(6):879–885
Leonetti CP, Butt CM, Stapleton HM (2018) Disruption of thyroid hormone sulfotransferase activity by brominated flame retardant chemicals in the human choriocarcinoma placenta cell line, BeWo. Chemosphere 197:81–88
Li AJ, Feldman SM, McNally RK, Kannan K (2019) Distribution of organohalogen and synthetic musk compounds in breast adipose tissue of breast cancer patients in Ulster County, New York, USA. Arch Environ Contam Toxicol 77(1):68–78
Li Z-H, Liu X-Y, Wang N, Chen J-S, Chen Y-H, Huang J-T, Su C-H, Xie F, Yu B, Chen D-J (2012) Effects of decabrominated diphenyl ether (PBDE-209) in regulation of growth and apoptosis of breast, ovarian, and cervical cancer cells. Environ Health Perspect 120(4):541–546
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62(10):e1–e34
Lin Y, Jiang M, Chen W, Zhao T, Wei Y (2019) Cancer and ER stress: mutual crosstalk between autophagy, oxidative stress and inflammatory response. Biomed Pharmacother 118:109249
Liu S, Zhao G, Li J, Zhao H, Wang Y, Chen J, Zhao H (2017) Association of polybrominated diphenylethers (PBDEs) and hydroxylated metabolites (OH-PBDEs) serum levels with thyroid function in thyroid cancer patients. Environ Res 159:1–8
Madia F, Giordano G, Fattori V, Vitalone A, Branchi I, Capone F, Costa LG (2004) Differential in vitro neurotoxicity of the flame retardant PBDE-99 and of the PCB Aroclor 1254 in human astrocytoma cells. Toxicol Lett 154(1–2):11–21
Man YB, Lopez BN, Wang HS, Leung AOW, Chow KL, Wong MH (2011) Cancer risk assessment of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in former agricultural soils of Hong Kong. J Hazard Mater 195:92–99
Meerts I, Letcher RJ, Hoving S, Marsh G, Bergman A, Lemmen JG, van der Burg B, Brouwer A (2001) In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PDBEs, and polybrominated bisphenol A compounds. Environ Health Perspect 109(4):399–407
Mirzaee SA, Bayati B, Valizadeh MR, Gomes HT, Noorimotlagh Z (2021a) Adsorption of diclofenac on mesoporous activated carbons: physical and chemical activation, modeling with genetic programming and molecular dynamic simulation. Chem Eng Res Des 167:116–128
Mirzaee SA, Noorimotlagh Z, Ahmadi M, Rahim F, Martinez SS, Nourmohammadi A, Jaafarzadeh N (2021b) The possible oxidative stress and DNA damage induced in Diclofenac-exposed Non-target organisms in the aquatic environment: A systematic review. Ecol Ind 131:108172
Moher D, Schulz KF, Altman DG, Group C (2001) The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med 134(8):657–662
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4(1):1–9
Montalbano AM, Albano GD, Anzalone G, Moscato M, Gagliardo R, Di Sano C, Bonanno A, Ruggieri S, Cibella F, Profita M (2020) Cytotoxic and genotoxic effects of the flame retardants (PBDE-47, PBDE-99 and PBDE-209) in human bronchial epithelial cells. Chemosphere 245:125600
Ni K, Lu Y, Wang T, Shi Y, Kannan K, Xu L, Li Q, Liu S (2013) Polybrominated diphenyl ethers (PBDEs) in China: policies and recommendations for sound management of plastics from electronic wastes. J Environ Manage 115:114–123
Noorimotlagh Z, Azizi M, Pan H-F, Mami S, Mirzaee SA (2021) Association between air pollution and Multiple Sclerosis: a systematic review. Environ Res 196:110386
Park EY, Park E, Kim J, Oh J-K, Kim B, Hong Y-C, Lim MK (2020) Impact of environmental exposure to persistent organic pollutants on lung cancer risk. Environ Int 143:105925
Pellacani C, Buschini A, Galati S, Mussi F, Franzoni S, Costa LG (2012) Evaluation of DNA damage induced by 2 polybrominated diphenyl ether flame retardants (BDE-47 and BDE-209) in SK-N-MC cells. Int J Toxicol 31(4):372–379
Qu B-L, Yu W, Huang Y-R, Cai B-N, Du L-H, Liu F (2015) 6-OH-BDE-47 promotes human lung cancer cells epithelial mesenchymal transition via the AKT/Snail signal pathway. Environ Toxicol Pharmacol 39(1):271–279
Rangga-Tabbu C, Sleight S (1992) Development of preneoplastic lesions in the liver and nasal epithelium of rats initiated with N-nitrosodimethylamine or N-nitrosopyrrolidine and promoted with polybrominated biphenyls. Food Chem Toxicol 30(11):921–926
Rezabek M, Sleight S, Jensen R, Aust S (1989) Effects of dietary retinyl acetate on the promotion of hepatic enzyme-altered foci by polybrominated biphenyls in initiated rats. Food Chem Toxicol 27(8):539–544
Sakamoto Y, Inoue K, Takahashi M, Taketa Y, Kodama Y, Nemoto K, Degawa M, Gamou T, Ozawa S, Nishikawa A (2013) Different pathways of constitutive androstane receptor–mediated liver hypertrophy and hepatocarcinogenesis in mice treated with piperonyl butoxide or decabromodiphenyl ether. Toxicol Pathol 41(8):1078–1092
Siddiqi MA, Laessig RH, Reed KD (2003) Polybrominated diphenyl ethers (PBDEs): new pollutants–old diseases. Clin Med Res 1(4):281–290
Smith AG, Francis JE, Carthew P (1990) Iron as a synergist for hepatocellular carcinoma induced by polychlorinated biphenyls in Ah-responsive C57BL/10ScSn mice. Carcinogenesis 11(3):437–444
Song R, Duarte TL, Almeida GM, Farmer PB, Cooke MS, Zhang W, Sheng G, Fu J, Jones GD (2009) Cytotoxicity and gene expression profiling of two hydroxylated polybrominated diphenyl ethers in human H295R adrenocortical carcinoma cells. Toxicol Lett 185(1):23–31
Tagliaferri S, Caglieri A, Goldoni M, Pinelli S, Alinovi R, Poli D, Pellacani C, Giordano G, Mutti A, Costa LG (2010) Low concentrations of the brominated flame retardants BDE-47 and BDE-99 induce synergistic oxidative stress-mediated neurotoxicity in human neuroblastoma cells. Toxicol in Vitro 24(1):116–122
Tang Z, Li Y, Jiang Y, Cheng J, Xu S, Zhang J (2019) Cellular metabolomics reveals glutamate and pyrimidine metabolism pathway alterations induced by BDE-47 in human neuroblastoma SK-N-SH cells. Ecotoxicol Environ Saf 182:109427
Terrell ML, Rosenblatt KA, Wirth J, Cameron LL, Marcus M (2016) Breast cancer among women in Michigan following exposure to brominated flame retardants. Occup Environ Med 73(8):564–567
Tian P, Wang H, Chen G, Luo Q, Chen Z, Wang Y, Liu Y (2016) 2, 2′, 4, 4′-Tetrabromodiphenyl ether promotes human neuroblastoma SH-SY5Y cells migration via the GPER/PI3K/Akt signal pathway. Hum Exp Toxicol 35(2):124–134
van der Veen I, de Boer J (2012) Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88(10):1119–1153
Victor P, Sarada D, Ramkumar KM (2021) Crosstalk between endoplasmic reticulum stress and oxidative stress: Focus on protein disulfide isomerase and endoplasmic reticulum oxidase 1. Eur J Pharmacol 892:173749
Wang F, Ruan X-J, Zhang H-Y (2015) BDE-99 (2, 2′, 4, 4′, 5-pentabromodiphenyl ether) triggers epithelial-mesenchymal transition in colorectal cancer cells via PI3K/Akt/Snail signaling pathway. Tumori J 101(2):238–245
Wang M-S, Chen S-J, Lai Y-C, Huang K-L, Chang-Chien G-P (2010) Characterization of persistent organic pollutants in ash collected from different facilities of a municipal solid waste incinerator. Aerosol Air Qual Res 10(4):391–402
Wei J, Xiang L, Yuan Z, Li S, Yang C, Liu H, Jiang Y, Cai Z (2018) Metabolic profiling on the effect of 2, 2′, 4, 4′-tetrabromodiphenyl ether (BDE-47) in MCF-7 cells. Chemosphere 192:297–304
Williams G, Tong C, Telang S (1984) Polybrominated biphenyls are nongenotoxic and produce an epigenetic membrane effect in cultured liver cells. Environ Res 34(2):310–320
Wu Z, He C, Han W, Song J, Li H, Zhang Y, Jing X, Wu W (2020) Exposure pathways, levels and toxicity of polybrominated diphenyl ethers in humans: a review. Environ Res 187:109531
Wu Z, Liu Y, Lyu H, Chen Y, Han T, Zhang Y, Xu P, Song J, Wu W (2021) Polybrominated diphenyl ethers in indoor dusts from university dormitories and printing shops in Xinxiang, China. Build Environ 187:107416
Yang Y, Xie Q, Liu X, Wang J (2015) Occurrence, distribution and risk assessment of polychlorinated biphenyls and polybrominated diphenyl ethers in nine water sources. Ecotoxicol Environ Saf 115:55–61
Zhang C, Li P, Zhang S, Lei R, Li B, Wu X, Jiang C, Zhang X, Ma R, Yang L (2017) Oxidative stress-elicited autophagosome accumulation contributes to human neuroblastoma SH-SY5Y cell death induced by PBDE-47. Environ Toxicol Pharmacol 56:322–328
Zhang F, Peng L, Huang Y, Lin X, Zhou L, Chen J (2019) Chronic BDE-47 exposure aggravates malignant phenotypes and chemoresistance by activating ERK through ERα and GPR30 in endometrial carcinoma. Front Oncol 1079
Zhang S, Kuang G, Zhao G, Wu X, Zhang C, Lei R, Xia T, Chen J, Wang Z, Ma R (2013) Involvement of the mitochondrial p53 pathway in PBDE-47-induced SH-SY5Y cells apoptosis and its underlying activation mechanism. Food Chem Toxicol 62:699–706
Zhao G, Wang Z, Zhou H, Zhao Q (2009) Burdens of PBBs, PBDEs, and PCBs in tissues of the cancer patients in the e-waste disassembly sites in Zhejiang, China. The Science of the Total Environment 407(17):4831–4837
Zhao T, Tang X, Li D, Zhao J, Zhou R, Shu F, Jia W, Fu W, Xia H, Liu G (2022) Prenatal exposure to environmentally relevant levels of PBDE-99 leads to testicular dysgenesis with steroidogenesis disorders. J Hazard Mater 424:127547
Funding
The authors would like to thank the Health and Environment Research Center, Ilam University of Medical Sciences, Ilam, Iran for its support (Grant No:401H003/22).
Author information
Authors and Affiliations
Contributions
MA, SM, and NB: methodology, validation, writing—review and editing. SSM: conceptualization, validation, writing—review and editing. ZN: conceptualization, methodology, validation, formal analysis, investigation, supervision. SAM: conceptualization, methodology, validation, resources, writing—original draft, writing—review and editing, project administration.
Corresponding author
Ethics declarations
Ethical approval
The authors would like to thank the Health and Environment Research Center, Ilam University of Medical Sciences, Ilam.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ludek Blaha
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azizi, M., Mami, S., Noorimotlagh, Z. et al. The role of polybrominated diphenyl ethers in the induction of cancer: a systematic review of insight into their mechanisms. Environ Sci Pollut Res 30, 9271–9289 (2023). https://doi.org/10.1007/s11356-022-24538-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-24538-9