Abstract
The tailings and fluorine-containing sludge were produced during the physical and chemical purification of natural crystalline graphite, containing heavy metals in different occurrence forms. To evaluate the threat of different heavy metals to the environment, this work uses the modified sequential extraction method (BCR) to study the presence of heavy metals in two solid wastes and their dissolution characteristics in different environments. The results show that the pollution risk of heavy metals in graphite tailings to the environment is ranked as Mn > Cr > Ni > Zn, and the pollution risk of Mn in fluorine-containing sludge is higher than that of Cr. This is because the Mn in the two solid wastes mainly exists in the form of weak acid extraction. The leaching number of heavy metals in the two solid wastes is directly proportional to the soaking time and soaking temperature, and inversely proportional to the pH value and the solid-to-liquid ratio. The number of heavy metals dissolved in solid waste landfills is significantly higher than that of acid rain and surface water environments. Based on the above results and the distribution of graphite solid waste, solidification agent was suggested to prevent heavy metal dissolution and reduce environmental risks.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lithium-ion batteries (LIBs) have a sharp rise in demand for electric vehicles, consumer electronics, new energy storage, medical and other fields due to their good cycle performance, low self-discharge rate, high energy density, and long life (Zhang et al. 2022),(Quan et al. 2022). The anode materials, which account for 30–40% of the mass of lithium-ion batteries, are mainly made of artificial and natural graphite (Yao et al. 2020). Compared with artificial graphite, natural graphite does not need to go through the high-temperature graphitization process, so the preparation process is green, low-cost, rich in reserves, and high conductivity (Jara et al. 2019). It has become an important anode electrode material in the field of new energy and energy storage. In addition, natural graphite can also be used to prepare graphene (Botas et al. 2013), fuel cell electrode materials (Dhakate et al. 2008), aerospace materials (Zhao et al. 2021), lubricants (Morstein and Dienwiebel 2021), water purification materials (Yin et al. 2021), refractory materials (Wang et al. 2019b), and so on.
The purification process is a necessary way for natural graphite to be used in high value-added applications. The grade of natural graphite ore is generally 8–12%, and the impurities mainly include silicate minerals, quartz, alumina, magnesium, calcium, etc. (Jara and Kim 2020). Graphite ore is floatable, and flotation is usually used as a common method of purification (Chehreh Chelgani et al. 2015). Through multi-stage grinding and multi-stage flotation, the purity of graphite can be increased to 94 ~ 96%. However, the impurities (3 ~ 6%) remaining in the graphite matrix after flotation are embedded with extremely fine particle size and stable phase. It needs hydrofluoric acid to dissolve and react to produce water-soluble substances (Jara and Kim 2020), which can be removed after multiple washings, and finally obtain high-purity graphite with a content of 99.9% (Kaya and Canbazoğlu 2009).
The complicated process of flotation results in 9 tons of graphite tailings for every ton of concentrate produced. The hydrofluoric acid method produces a large amount of acidic wastewater with fluoride ions due to the large amount of acid (purification of 1 ton of graphite requires 1 ton of 40% hydrofluoric acid) used to purify graphite. Add quicklime, slaked lime, or calcium salt to fluorine-containing wastewater to convert fluorine in the wastewater into fluoride precipitation or make fluoride adsorbed on the precipitate (Wang et al. 2015), and then achieve solid–liquid separation through natural sedimentation or filtration, and solid substances form fluorine-containing sludge. Graphite tailings and fluorine-containing sludge are usually stored in the form of tailings ponds, and some are even directly discharged in gullies. This not only caused a serious waste of land resources (Cheng et al. 2020; Das et al. 2019), but also the occurrence of dam breaches became a hidden danger to mine safety. Secondly, the chemical substances and heavy metal elements contained in it will seep out under the action of long-term wind, daily chemical, and rain, causing pollution of soil and groundwater sources (Brar et al. 2010; Wang et al. 2009). At the same time, the piled solid waste particles are extremely fine, which will become the source of dust storms during strong winds and generate a large amount of fugitive dust, which seriously pollutes the air (Wang et al. 2019a). Therefore, the treatment of solid waste generated during the graphite purification process has become one of the main problems faced by the new energy industry.
At present, the main research direction on the treatment of two types of purified solid waste is the recovery of valuable substances and the preparation of building materials. The rich SiO2, Al2O3, and alkali metals in graphite tailings can replace part of the sand and gravel when preparing concrete (Xue et al. 2021), and can also be used as raw materials for the preparation of ceramic tiles (Wu et al. 2021). The porous characteristics of graphite tailings can also be used as a catalyst carrier instead of activated carbon to treat pigment-containing waste water (Cuiping et al. 2012). In addition, graphite tailings are also found to contain a variety of trace elements necessary for plant growth (Hindersah 2018a), which can be used as a source of inorganic salt fertilizers for plants. At present, sludge solidification (Wang et al. 2021a), firing ceramics (Gao et al. 2020), and sludge making bricks (Wu et al. 2019) are the main treatment methods for fluorine-containing sludge. Calcium fluoride is the main form of fluorine in fluorine-containing sludge. The introduction of calcium fluoride in the cement calcination process can accelerate the formation of tricalcium silicate and improve the quality of cement (Da et al. 2021). Therefore, cement kiln is one of the main methods for curing fluorine-containing sludge. When the concentration of calcium fluoride in fluorine-containing sludge is high, ceramic products can also be prepared by high-temperature liquid-phase sintering technology (Wu et al. 2019).
However, one of the important factors limiting the resource utilization of solid waste is the leaching of heavy metals (Li et al. 2022). Materials prepared from solid waste, such as bricks and concrete, will be washed with rainwater during subsequent use, which will cause heavy metals to leach out and enter the environment with rainwater, causing secondary pollution (Wang et al. 2021b). The study pointed out that the dissolved number of heavy metals in bricks prepared from sludge was higher than that of commercial bricks under different pH conditions (Karius and Hamer 2001). The mixing amount of solid waste will also affect the leaching rate of heavy metals. When the sludge doping amount is increased from 15 to 20%, the leaching concentration of Cr is increased by 1.2 times (Karius and Hamer 2001). When materials prepared from solid waste fall off and break during use, the risk of heavy metal dissolution will also increase. This is because the dissolution rate of heavy metals will increase as the particle size decreases and the specific surface area increases. At the same time, the harmful elements in the solid waste will also adversely affect production equipment and the environment. Secondly, with the increase in demand for high-purity graphite, the output of purified solid waste has also increased significantly, resulting in a large amount of purified solid waste accumulating in the mining area (Peng et al. 2021). For example, in the Luobei Yunshan graphite mining area in Heilongjiang, China, the total amount of tailing has exceeded 7 million tons. The heavy metals in these accumulated solid wastes will be released through the physical and chemical effects of the interface and will continue to migrate and diffuse under the action of rainfall and wind, causing soil and water pollution and endangering human health (Silva et al. 2019). The pollution caused by the two types of solid waste in the production-utilization-use-storage process is formed by point-to-surface diffusion and discharge accumulation, and is closely related to the climate, soil properties, and water quality of the storage area. And the migration characteristics of heavy metals are different due to their different occurrence states. Therefore, it is necessary to systematically study the activity degree, migration ability, dissolution characteristics of various heavy metals in the two solid wastes in different environments, and the detoxification schemes and governance strategies should be suggested.
In this study, the physical and chemical properties and heavy metal content of graphite tailings and fluorine-containing sludge were first analyzed. Secondly, the modified BCR method was used to detect the occurrence status of heavy metals in the two solid wastes, thereby analyzing the influence of the existence form on the migration behavior and providing supporting data for the establishment of the element occurrence status database. In addition, the focus is on the dissolution characteristics of heavy metals under different conditions (pH, solid–liquid ratio, immersion temperature, immersion time). Finally, the dissolution of harmful elements in acid rain, landfills, and surface water environments were monitored. Provide a theoretical basis for establishing an environmental risk assessment system, realizing the green application of solid waste, and finding the most environmentally friendly solid waste disposal method.
Experimental
Raw material
Heilongjiang Province, with 46.9% of natural crystalline graphite, has become the largest distribution area of mineral resources in China. Therefore, the samples in this paper are all selected from mining areas in Heilongjiang Province. Among them, the graphite tailings (GT) sample comes from the beneficiation plant in the graphite mining area of Luobei Yunshan, Heilongjiang Province, and is the fresh tailings on the surface of the tailings pond. The fluorine-containing sludge (FS) sample comes from a graphite deep processing enterprise in Heilongjiang Province. The company uses the hydrofluoric acid method to purify graphite, and the acidic fluorine-containing wastewater produced after purification is treated by adding lime and flocculants. The fluorine-containing sludge sample used in the experiment is the solid waste produced by the treatment of acidic fluorine-containing wastewater from the deep processing of graphite.
Methods
Water content, pH
The method of measuring the moisture content is to weigh 30 g of solid waste sample into the oven. After drying at a constant temperature of 70.5 °C to a constant weight, the moisture content of the two solid waste samples can be calculated by using the difference in mass before and after drying.
The method of pH measurement is to maintain a solid–liquid ratio of 1:2.5 and immerse the solid waste sample in deionized water. Let stand at room temperature for 1 h, and use a pH meter to measure the pH value of the supernatant after soaking. The measured pH value is the pH of the solid waste sample.
Characterization
ICP2060T type inductively coupled plasma emission spectrometer (ICP-OES) was used to analyze the element content of two solid wastes. An X-ray fluorescence spectrometer (XRF) model EDX1800B was used to analyze the chemical composition of the two solid wastes. Use D/max2200VPC X-ray diffractometer (XRD) to analyze the mineral composition of two solid wastes. Zeiss EVO MA1 scanning electron microscope (SEM) was used to analyze the microscopic morphology of the two solid wastes.
Improved BCR method
The occurrence state of the harmful elements in the two solid wastes is determined by the improved BCR method, and the operation method is as follows:
-
(1)
Weak acid extraction state: Weigh 1.0 g of solid waste sample into a 50 mL centrifuge tube, add 20 mL, 0.11 mol/L acetic acid. Oscillate at a constant temperature of 30 °C for 16 h. After that, it was centrifuged at 4000 r/min for 20 min in a centrifuge. The supernatant was filtered with a 0.45 μm microporous membrane to be tested. The residue will continue to be extracted at the next level.
-
(2)
Reducible state: Add 20 mL, 0.4 mol/L hydroxylamine hydrochloride solution to the weak acid extraction residue (Use HNO3 solution to adjust the pH to around 2), shake in a 30 °C water bath for 6 h, centrifuge. The supernatant obtained by filtration is measured, and the residue will continue to be extracted at the next stage.
-
(3)
Oxidizable state: Add 10 mL of water and 30% hydrogen peroxide to the reducible residue (Adjust the pH value to between 2 and 3 with HNO3 solution), shake at room temperature for 1 h, then shake in 85 °C water bath for 2 h. After cooling, add 10 mL of 1 mol/L ammonium acetate solution with a pH of about 2 (Also use HNO3 solution to acidify), and continue shaking for 1 h. After centrifugal separation, the supernatant obtained by filtration is tested, and the residue will be extracted in the next stage.
-
(4)
Residual state: Weigh 0.3 g solid waste sample, put it into a PTFE (polytetrafluoroethylene) crucible, add 10 mL of concentrated nitric acid, 5 mL of hydrofluoric acid, and 2.5 mL of perchloric acid. Heat for 30 min on a hot plate at 190 °C. When white smoke no longer comes out from the crucible and the acid liquid evaporates and thickens, add 2.5 mL of perchloric acid and continue heating. When the solid waste sample is completely dissolved and transparent, remove it and cool it, dilute it with a 2% dilute nitric acid solution, and finally use ICP-OES to determine the heavy metal content in the residue extract.
International law immersion test
The three types of environments where solid waste is located are simulated using the national standard method. Specific operation: Take 20 g of solid waste samples with a particle size of less than 149 µm, put it into a 250 mL glass reagent bottle with a lid, and add 200 mL of the extract (Deionized water: simulated surface water environment; acetic acid solution with pH 2.64 ± 0.05: simulated landfill environment; sulfuric acid-nitric acid solution with pH 3.20 ± 0.05: simulated acid rain environment). Keep the oscillation frequency of 200 r/min on the horizontal oscillator and oscillate for 20 h. The obtained supernatant was filtered, and the leaching number of heavy metals was measured by ICP-OES.
Results and discussion
The physical and chemical properties
Water content and pH analysis
Firstly, the moisture content and pH of graphite tailings and fluorine-containing sludge were measured. The water content of fluorine-containing sludge is as high as 47.02%, and the pH is 7.227. The water content of graphite tailings is relatively small, accounting for 10.45%, and the pH is 7.866. According to the pH value, it can be seen that both solid wastes are weakly alkaline.
Chemical composition analysis
The element and chemical composition analysis of graphite tailings is shown in Fig. 1a–b. According to the elemental composition analysis, it can be known that the O element with the highest content in the graphite tailings accounts for 46.702%, and the Si element takes up 23.583%. At the same time, the content of Ca, Fe, and Al is relatively high, which is consistent with the results of Hindersah et al. (Hindersah et al. 2018b). Since manganese, chromium, nickel, and zinc all have a certain amount of occurrence, there is a potential hazard of leaching of heavy metal ions. Therefore, the subsequent detection of harmful elements in graphite tailings focuses on the four elements of Mn, Cr, Ni, and Zn.
According to the chemical composition analysis (Fig. 1b), the main substance contained in graphite tailings is SiO2 (Jara et al. 2019), with a content of up to 50.453%, which is consistent with the quartz sand that the main component of graphite tailings is pointed out in the literature. Followed by Al2O3, SO3, CaO, Fe2O3, their content is about 10%.
Figure 1c–d shows the elemental and chemical composition analysis of fluorine-containing sludge. The elemental composition of fluorine-containing sludge and graphite tailings is different in that the content of Ca element in fluorine-containing sludge is higher than that of O element, accounting for 41.511%. These Ca elements are mainly caused by excessive lime input and CaF2 generated by the reaction of F− and Ca2+ when treated with acidic fluorine-containing wastewater (Aldaco et al. 2007).
According to the analysis of chemical composition (Fig. 1d), the proportion of CaO in fluorine-containing sludge is the highest. Like graphite tailings, fluorine-containing sludge contains high SiO2. It is speculated that the addition of HF acid causes the SiO2 in the graphite crystal gaps to be dissolved out. The heavy metal elements in fluorine-containing sludge are mainly Mn and Cr. Ni has a small amount of occurrence, and its proportion is only 0.011%, and the presence of Zn is not detected. Therefore, subsequent investigations on harmful elements in fluorine-containing sludge focused on Mn and Cr elements.
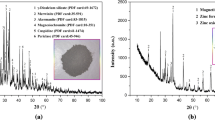
Mineral composition analysis
The mineral composition of graphite tailings and fluorine-containing sludge is shown in Fig. 2. According to the characteristic peak signal in Fig. 2a, the main minerals contained in graphite tailings are quartz (SiO2), microcline, biotite, which are consistent with the chemical composition analysis results of graphite tailings in Fig. 1. It can be seen that the diffraction peak of calcium fluoride in Fig. 2b is obvious, indicating that the main mineral contained in fluorine-containing sludge is calcium fluoride. When lime is used as a precipitant to treat fluorine-containing wastewater, it can not only adjust the pH value of the wastewater, but also dissolve and react in the water to form calcium hydroxide. After that, the Ca2+ in the solution will react with F− to form CaF2 precipitation. The chemical equations are shown in formulas (1–1) ~ (1–3). In addition, the presence of SiO2 and CaCO3 is also found in the spectrum. The existence of CaCO3 is due to the long-term exposure of graphite tailings to the air, and the Ca(OH)2 in the tailings will react with CO2 in the air, as shown in formula (1–4).
Microscopic morphology analysis
Figure 3 shows the microscopic morphology of the two solid wastes. Figure 3a–c shows that the graphite tailings present scaly irregular crystals, which are speculated to be quartz, corundum, and flake graphite remaining after beneficiation. According to Fig. 3d–f, it can be seen that the surface of the fluorine-containing sludge has no obvious crystal characteristics, and the surface is in a granular state and a large number of particles are agglomerate together. This shows that there are amorphous minerals in fluorine-containing sludge, and organic matter may adhere to the surface of crystalline minerals, resulting in insufficient crystal morphology characteristics (He et al. 2020).
The SEM–EDS analysis of graphite tailings and fluorine-containing sludge is shown in Fig. 4. According to the EDS results in Fig. 4a, it can be seen that there are elements such as C, O, Si, Al, Mg, etc. on the surface of the graphite tailings, and the content of C is 31.43%. Figure 4b shows the presence of C, O, F, Si, Al, Mg, and other elements on the surface of fluorine-containing sludge, and the content of F is only lower than that of C and O. This is because fluorine-containing sludge is produced in the process of purifying natural graphite with hydrofluoric acid. The EDS results of the two solid wastes were consistent with the above ICP results, but EDS did not detect the presence of Mn, Cr, Ni, and Zn, which was caused by the relatively low relative content of heavy metal elements in the two solid wastes.
Occurrence status of harmful elements
According to the analysis of the two solid waste elements and chemical composition in Sect. 2.1.2, it can be known that the harmful elements in graphite tailings are mainly Mn, Ni, Cr, Zn, and the fluorine-containing sludge is mainly Mn and Cr. The occurrence form of harmful heavy metal elements is directly related to their behavior on the environment. Therefore, in order to better understand the activity and migration capacity of the harmful elements in the two solid wastes, it is necessary to study the occurrence state of harmful elements.
Figure 5a shows the occurrence state of various elements in graphite tailings. It can be seen that the residue state is the main occurrence form of the four elements. It is worth noting that the weak acid extraction state of Mn accounts for 12.88%, second only to the residue state. Moreover, the proportion of Mn element in the weak acid extraction state is much higher than that of the other three elements, which shows that Mn has the greatest environmental risk and can be dissolved in a large amount in a neutral and acidic environment. The weak acid extraction state of Ni element accounts for 3.40%, second only to Mn element, but its oxidizable state accounts for 27.15%. Therefore, when the graphite tailings are in an oxidizing environment, a large amount of Ni will dissolve out. The study by Han et al. also pointed out that Ni is the main heavy metal element that causes soil pollution around the tailings pond (Han 2011). Both Cr and Zn have a relatively low proportion of the weak acid extraction state, and the weak acid extraction state of Zn element even accounts for only 0.7%, so the two elements pose a low threat to the environment.
Figure 5b shows the occurrence state of Mn and Cr elements in fluorine-containing sludge. The main forms of the two elements are in the residue state, and the proportion of the Mn element in the residue state is as high as 91.65%, which is relatively stable. However, the proportion of the weak acid extraction state of the two elements is higher than that of the graphite tailings, so it is preliminarily judged that there is still a risk of dissolution. Although the proportion of Cr element in the weak acid extraction state is relatively high, but the content is relatively small, it is considered that its harm to the environment is less than that of Mn element.
Dissolution characteristics of harmful elements
The influence of pH
Under different pH values and redox conditions, harmful heavy metals will precipitate or hydrolyze, which will lead to the dissolution of elements. The influence of pH value on the leaching amount of Mn and Ni in graphite tailings and Mn in fluorine-containing sludge is shown in Fig. 6a. It can be seen that with the increase of the pH value of the extract, the dissolved number of harmful elements in the two solid wastes shows a downward trend. Among them, regarding the elution of Mn element, graphite tailings are much higher than fluorine-containing sludge. When the pH value is 2.5, the maximum amount of Mn dissolved in graphite tailings reaches 1.643 mg/kg. This is due to the high content of Mn in graphite tailings, and the weak acid extraction state is the main occurrence state. Therefore, it should be noted that when processing graphite tailings, the pH value in the tailings pond should not be too low. Otherwise, the amount of Mn dissolved out will exceed the standard.
The influence of soaking time
The influence of soaking time value on the leaching amount of Mn and Ni in graphite tailings and Mn in fluorine-containing sludge is shown in Fig. 6b. It can be seen that as the soaking time increases, the dissolution amount of element presents an increasing trend. Among them, the soaking time has the greatest impact on the amount of Mn dissolved in graphite tailings, followed by the Mn element in fluorine-containing sludge. At the same time, it can be seen that the dissolution rate of Mn element in fluorine-containing sludge is faster; it can reach the maximum dissolution rate of 6.86 mg/kg when the soaking time is 12 h. Soaking time has little effect on the dissolution of Ni in graphite tailings, and the dissolution of Ni is basically about 0.5 mg/kg.
All in all, the dissolution rate of the harmful elements in the two solid wastes is faster, and the higher the dissolution rate is reached after 6 h of soaking. At the same time, it is believed that the decomposition of harmful elements is controlled by the surface chemical process, so the number of harmful elements dissolved is proportional to the soaking time.
The influence of solid–liquid ratio
One of the important factors affecting the dissolution of harmful elements is the solid–liquid ratio. Among them, the liquid phase environment provides a reaction space for the release of harmful elements; the dissolution of harmful elements will experience from the solid phase to the liquid phase. This stage is caused by the interaction of the liquid–solid interface. The liquid–solid ratio is an important factor that determines the concentration and release rate of harmful elements released to the environment (Li 2001).
The influence of solid–liquid ratio on the leaching amount of Mn and Ni in graphite tailings and Mn in fluorine-containing sludge is shown in Fig. 6c. It can be seen that the smaller the solid-to-liquid ratio, the more beneficial the dissolution of harmful elements. Among them, the Mn element in the graphite tailings has a large amount of dissolution at different solid-to-liquid ratios, so beware of excessive dissolution. The solid–liquid ratio has relatively little effect on the Ni element in graphite tailings, but it is still necessary to pay attention to drying the graphite tailings as far as possible before discharging the graphite tailings to the tailings pond. The solid–liquid ratio has a great influence on the amount of Mn dissolved in fluorine-containing sludge. When the solid–liquid ratio is 1:10, the dissolution amount can reach 6 times that of 1:2. It shows that Mn in fluorine-containing sludge is more sensitive to the change of solid–liquid ratio than Mn and Ni in graphite tailings.
In summary, the solid-to-liquid ratio has a greater impact on the dissolution of harmful elements. The smaller the solid-to-liquid ratio, the more beneficial the dissolution of harmful elements, and the greater the environmental risk. This is because as the solid–liquid ratio decreases, the ratio of the leaching liquid increases, and the contact area between the solid waste and the leaching liquid increases, resulting in an increase in the number of harmful elements leached. Therefore, before discharging the two solid wastes, try to use dry discharge technology.
The influence of soaking temperature
The influence of soaking temperature on the leaching amount of Mn and Ni in graphite tailings and Mn in fluorine-containing sludge is shown in Fig. 6d. It can be seen from the figure that as the soaking temperature increases, the dissolved amount of Mn in the graphite tailings slightly increases. This is because the Mn element is more active, and the dissolution amount reaches 4.195 mg/kg at 25 °C. The dissolved amount of Ni in graphite tailings and Mn in fluorine-containing sludge both increased first and then slightly decreased with the increase of temperature. Among them, the dissolution of Ni in graphite tailings reaches a maximum of 0.6725 mg/kg at 35 °C; the amount of Mn dissolved in fluorine-containing sludge reaches the maximum value of 0.713 mg/kg at 45 °C.
All in all, the number of harmful elements dissolved in the two solid wastes is proportional to the temperature. First of all, this is because the movement activity of molecules or ions inside a substance is directly affected by temperature; when the temperature is higher, the energy of the molecules will increase, and their mobility will increase, which will cause the molecules to change easily and accelerate the release of harmful elements. Secondly, under the influence of water and other media, the harmful elements in the solid waste will undergo a dissolution reaction and dissolve out. At the same time, when the temperature increases, the chemical equilibrium will move in the direction of the dissolution reaction, resulting increase in the number of harmful elements dissolved at higher temperatures. The conclusions of this study show that temperature has a relatively small effect on the number of harmful elements released. In the range of 25 to 55 °C, the dissolution of the elements has been kept in a relatively stable range.
Dissolution of harmful elements in different environments
In order to understand the dissolution of harmful elements in the two solid wastes in the real environment, three common environments such as surface water, acid rain environment, and landfill were simulated using the national standard method. In addition, the dissolution of harmful elements in the three environments is shown in Fig. 7. It can be seen that the Mn element in the graphite tailings is the most active, and it has a certain amount of dissolution in the three environments. The dissolution amount is higher than the other three elements, and the Mn element in the fluorine-containing sludge and the dissolution rate is as high as 10.64 mg/kg in the simulated landfill environment. In the landfill environment, the leaching amount of Cr element in graphite tailings is lower than that of Mn element, but the leaching amount is still 7.36 mg/kg. The dissolution amounts of Ni and Zn in graphite tailings and Mn in fluorine-containing sludge are low in the three environments, even lower than the lower limit of instrument detection, so the harm to the environment is relatively small.
In summary, it can be seen that there are differences in the migration ability of elements in solid waste in different environments. Among them, the Mn and Cr elements in graphite tailings are the most harmful, and the dissolution of all harmful elements in the landfill environment is much higher than the surface water and acid rain environment, indicating that landfilling solid waste is the least environmentally friendly measure.
Governance methods
According to the above analysis, it is clear that heavy metals have the risk of dissolution in any environment, and preventing the dissolution of heavy metals in solid waste is an effective means to reduce environmental pollution. In view of the characteristics of wide distribution, large amount, low secondary utilization rate, and non-degradable metal of the generated solid waste, the solidification of heavy metals has become the most feasible method to limit the dissolution. One of the methods of heavy metal solidification is to use a solidifying agent (clay or cement) to encapsulate and solidify the heavy metal in the structure of the hydration to change the easy migration characteristics. However, the increased solid waste volume and land footprint after solidifying agent treatment limit the implementation of this method. However, the solid waste volume and land occupied area increase after curing agent treatment, thus limiting the implementation of this method. Another method is to chemically react with heavy metals by adding chemical reagents; it is converted into a mineral phase with low solubility and high stability, thereby reducing the dissolution of heavy metals. This method of treating solid waste not only has small volume change, simple operation, and high efficiency, but also meets the needs of industrial development and application (Zhao et al. 2022). Therefore, it is widely used in the treatment of fly ash produced by waste incineration.
Typically, the agents used to solidification heavy metals include organic and inorganic chemical agents. Organic reagents mainly refer to macromolecular organic stabilizers, such as heavy metal chelators. These chelators and heavy metals form complexes with two-dimensional or three-dimensional structures. Therefore, the heavy metals treated with chelators can be stably solidified in solid waste in a wide pH range. Table 1 summarizes the control standards for heavy metal pollutants (Ma et al. 2019). Table 2 summarizes the types of heavy metal chelators and the effect of solidified heavy metals. It can be seen from Tables 1 and 2 that after metal chelator treatment, the dissolution rate of heavy metals is far lower than the national standard, which proves the feasibility of the chelators to solidify heavy metals. Inorganic chemicals including sodium sulfide, phosphoric acid and soluble phosphates, phosphate ions (PO43 −), and sulfur ions (S2−) react with heavy metals. When the ion product (Q) of PO43 − or S2− and metal ions generated by dissociation is greater than the solubility product (Ksp), it forms insoluble phosphates and sulfides to solidify heavy metals (Luo et al. 2022). Table 3 shows the solidification effect of inorganic chemical reagent types on heavy metals. According to Table 3, it can be seen that the use of inorganic chemical reagents to treat solid waste still makes the dissolved amount of heavy metal lower than the national standard value. Both types of chemical agents can promote the transformation of heavy metals into more stable forms, resulting in a significant decrease in the dissolution concentration. Different from organic chelators, inorganic chemical reagents are cheap and easy to prepare, while organic chelators are complicated to prepare but have better effects. It is comprehensively recommended to use drones to spray chemical reagents into solid waste to prevent the dissolution of heavy metals, which is an implementable, cheap, and efficient method to prevent heavy metal pollution. The core of this technology is how to strengthen the collision between the solidification agent and the ions, that is, to strengthen the mass transfer of the solidification agent in the two solid wastes, so as to strengthen the curing effect.
Conclusions
The harmful elements in graphite tailings are mainly Mn, Cr, Ni, Zn; the respective proportions are 0.085%, 0.027%, 0.023%, 0.015%. The harmful elements in fluorine-containing sludge are mainly Mn and Cr, and the content of Mn is also higher than that of Cr.
The weak acid extraction state of Mn in graphite tailings accounts for 12.88%, which can be dissolved in a large amount in a common weak acid environment, so its environmental risk is the greatest. Ni and Cr elements are relatively stable and will only be dissolved in large quantities in an oxidizing environment. The environmental risk of Zn element is minimal or even negligible. The active state of Mn element in the fluorine-containing sludge is relatively high, and the environmental risk is much greater than that of Cr.
The dissolved amount of Mn and Ni in graphite tailings and Mn in fluorine-containing sludge were inversely proportional to pH and solid–liquid ratio, and proportional to temperature and soaking time. The soaking time has the greatest effect on the dissolution of Mn in the graphite tailings, and the solid–liquid ratio has the greatest effect on the Ni in the graphite tailings and the Mn in the fluorine-containing sludge. Temperature has little effect on the dissolution of heavy metals in solid waste.
Through the detection of the number of harmful elements dissolved in surface water, acid rain, and landfill environment, it is known that the solid waste landfill is the most harmful to the environment, followed by the acid rain environment, and the least harmful is the surface water environment. At the same time, solidification suggestions are given for the dissolution of heavy metals.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Aldaco R, Garea A, Irabien A (2007) Calcium fluoride recovery from fluoride wastewater in a fluidized bed reactor. Water Res 41:810–818
Botas C, Álvarez P, Blanco P, Granda M, Blanco C, Santamaría R, Romasanta LJ, Verdejo R, López-Manchado MA, Menéndez R (2013) Graphene materials with different structures prepared from the same graphite by the Hummers and Brodie methods. Carbon 65:156–164
Brar SK, Verma M, Tyagi RD, Surampalli RY (2010) Engineered nanoparticles in wastewater and wastewater sludge-evidence and impacts. Waste Manag 30:504–520
ChehrehChelgani S, Rudolph M, Kratzsch R, Sandmann D, Gutzmer J (2015) A review of graphite beneficiation techniques. Miner Process Extr Metall Rev 37:58–68
Cheng J, Shi F, Yi J, Fu H (2020) Analysis of the factors that affect the production of municipal solid waste in China. J Clean Prod 259:120808
Cuiping B, Wenqi G, Dexin F, Mo X, Qi Z, Shaohua C, Zhongxue G, Yanshui Z (2012) Natural graphite tailings as heterogeneous Fenton catalyst for the decolorization of rhodamine B. Chem Eng J 197:306–313
Da Y, He T, Shi C, Wang M, Feng Y (2021) Potential of preparing cement clinker by adding the fluorine-containing sludge into raw meal. J Hazard Mater 403:123692
Das S, Lee SH, Kumar P, Kim K-H, Lee SS, Bhattacharya SS (2019) Solid waste management: scope and the challenge of sustainability. J Clean Prod 228:658–678
Dhakate S, Sharma S, Borah M, Mathur R, Dhami T (2008) Expanded graphite-based electrically conductive composites as bipolar plate for PEM fuel cell. Int J Hydrogen Energy 33:7146–7152
Gao N, Kamran K, Quan C, Williams PT (2020) Thermochemical conversion of sewage sludge: a critical review. Prog Energy Combust Sci 79:100843
Han X (2011) Characteristics and evaluation of heavy metal pollution in graphite tailings pond and surrounding soil. J Eng Heilongjiang Univ 2:58–62
He T, Da Y, Shi C, Wang M, Wang J, Feng Y, Li Z (2020) Recovery of thermally treated fluorine-containing sludge as the substitutions of Portland cement. J Clean Prod 260:121030
Hindersah R (2018a) Graphite tail powder and liquid biofertilizer as trace elements source for ground nut. AIP Conf Proc 1927:030005
Hindersah R, Setiawati MR, Fitriatin NB (2018b) Graphite tail powder and liquid biofertilizer as trace elements source for ground nut. AIP Conf Proc:030005
Jara AD, Betemariam A, Woldetinsae G, Kim JY (2019) Purification, application and current market trend of natural graphite: a review. Int J Min Sci Technol 29:671–689
Jara AD, Kim JY (2020) Chemical purification processes of the natural crystalline flake graphite for Li-ion battery anodes. Mater Today Commun 25:101437
Jiang J, Wang J, Xu X (2004) Heavy metal stabilization in municipal solid waste incineration flyash using heavy metal chelating agents. J Hazard Mater 113:141–146
Karius V, Hamer K (2001) pH and grain-size variation in leaching tests with bricks made of harbour sediments compared to commercial bricks. Sci Total Environ 278:73–85
Kaya Ö, Canbazoğlu M (2009) Chemical demineralization of three different graphite ores from Turkey. Min Metall Explor 26:158–162
Li H, Sun J, Gui H, Xia D, Wang Y (2022) Physiochemical properties, heavy metal leaching characteristics and reutilization evaluations of solid ashes from municipal solid waste incinerator plants. Waste Manag 138:49–58
Li X (2001) Heavy metal speciation and leaching behaviors in cement based solidified/stabilized waste materials. J Hazard Mater 82:215–230
Luo Z, Tang C, Hao Y, Wang Z, Yang G, Wang Y et al (2022) Solidification/stabilization of heavy metals and its efficiency in lead-zinc tailings using different chemical agents. Environ Technol 43:1613–1623
Ma W, Chen D, Pan M, Gu T, Zhong L, Chen G et al (2019) Performance of chemical chelating agent stabilization and cement solidification on heavy metals in MSWI fly ash: a comparative study. J Environ Manage 247:169–177
Morstein CE, Dienwiebel M (2021) Graphite lubrication mechanisms under high mechanical load. Wear 477:203794
Peng Y, Liu Y, Zhan B, Xu G (2021) Preparation of autoclaved aerated concrete by using graphite tailings as an alternative silica source. Constr Build Mater 267:121792
Qiu Q, Jiang X, Lv G, Lu S, Ni M (2016) Stabilization of heavy metals in municipal solid waste incineration fly ash in circulating fluidized bed by microwave-assisted hydrothermal treatment with additives. Energy Fuels 30:588–7595
Quan J, Zhao S, Song D, Wang T, He W, Li G (2022) Comparative life cycle assessment of LFP and NCM batteries including the secondary use and different recycling technologies. Sci Total Environ 819:153105
Silva RV, de Brito J, Lynn CJ, Dhir RK (2019) Environmental impacts of the use of bottom ashes from municipal solid waste incineration: a review. Resour Conserv Recycl 140:23–35
Sun Y, Xu C, Yang W, Ma L, Tian X, Lin A (2019) Evaluation of a mixed chelator as heavy metal stabilizer for municipal solid-waste incineration fly ash: behaviors and mechanisms. J Chin Chem Soc 66:188–196
Wang C, Chou W, Chen L, Chang S (2009) Silica particles settling characteristics and removal performances of oxide chemical mechanical polishing wastewater treated by electrocoagulation technology. J Hazard Mater 161:344–350
Wang D, Zhu J, Wang R (2021a) Assessment of magnesium potassium phosphate cement for waste sludge solidification: macro- and micro-analysis. J Clean Prod 294:126365
Wang F, Lu X, Li XY, Shih K (2015) Effectiveness and mechanisms of defluorination of perfluorinated alkyl substances by calcium compounds during waste thermal treatment. Environ Sci Technol 49:5672–5680
Wang G, Diao J, Liu L, Li M, Li H, Li G, Xie B (2019a) Highly efficient utilization of hazardous vanadium extraction tailings containing high chromium concentrations by carbothermic reduction. J Clean Prod 237:117832
Wang Q, Ko JH, Liu F, Xu Q (2021) Leaching characteristics of heavy metals in MSW and bottom ash co-disposal landfills. J Hazard Mater 416:126042
Wang X, Chen Y, Yu C, Ding J, Guo D, Deng C, Zhu H (2019) Preparation and application of ZrC-coated flake graphite for Al2O3-C refractories. J Alloy Compd 788:739–747
Wu J, Tian K, Wu C, Yu J, Wang H, Song J, Zhang Q, Xu X (2021) Effect of talc on microstructure and properties of the graphite tailing stoneware tiles. Constr Build Mater 311:125314
Wu M, Li Y, Guo Q, Shao D, He M, Qi T (2019) Harmless treatment and resource utilization of stainless steel pickling sludge via direct reduction and magnetic separation. J Clean Prod 240
Xu D, Huang Y, Jin X, Sun T (2022) Synergistic treatment of heavy metals in municipal solid waste incineration fly ash with geopolymer and chemical stabilizers. Process Saf Environ Prot 160:763–774
Xue J, Wang X, Wang Z, Xu S, Liu H (2021) Investigations on influencing factors of resistivity measurement for graphite tailings concrete. Cem Concr Compos 123:104206
Yao Y, Yan C, Zhang Q (2020) Emerging interfacial chemistry of graphite anodes in lithium-ion batteries. Chem Commun (Camb) 56:14570–14584
Yin G, Sun Z, Gao Y, Xu S (2021) Preparation of expanded graphite for malachite green dye removal from aqueous solution. Microchem J 166:106190
Zhang Y, Wu B, Mu G, Ma C, Mu D, Wu F (2022) Recent progress and perspectives on silicon anode: synthesis and prelithiation for LIBs energy storage. J Energy Chem 64:615–650
Zhao X, Li W, Wang Y, Li H, Wang J (2021) Bioinspired modified graphite film with superb mechanical and thermoconductive properties. Carbon 181:40–47
Zhao X, Yang J, Ning N, Yang Z (2022) Chemical stabilization of heavy metals in municipal solid waste incineration fly ash: a review. Environ Sci Pollut Res Int 29:40384–40402
Zhu J, Hao Q, Chen J, Hu M, Tu T, Jiang C (2022) Distribution characteristics and comparison of chemical stabilization ways of heavy metals from MSW incineration fly ashes. Waste Manag 113:488–496
Funding
We acknowledge financial support from the National Key R&D Program of China—2020YFC1909601.
Author information
Authors and Affiliations
Contributions
YL: data curation, manuscript revision, manuscript submission, writing—original and revised draft preparation. YF: data curation, manuscript revision. LZ: experimental data acquisition, data curation. MW: data curation, manuscript revision. ZW: manuscript guidance and revision. SY: manuscript revision. JL: manuscript guidance, writing reviewing. XG: design experimental protocol, methodology and resources, writing reviewing, supervision.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
The authors have read and approved the final manuscript.
Consent for publication
The authors agree to publication in the journal.
Conflict of interests
The authors declare no competing interests.
Additional information
Responsible editor Guilherme L. Dotto
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuehua Liu is the first author.
Highlights
1) Characteristics of graphite tailings and fluorinated sludge were analyzed.
2) Occurrence status of heavy metals in solid waste was characterized.
3) The migration behavior of heavy metals was examined.
4) Provides data for building a universal database of element occurrence states.
5) Environmental risks for two types of solid wastes were assessed.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Fu, Y., Zheng, L. et al. Leaching characteristics and solidification strategy of heavy metals in solid waste from natural graphite purification. Environ Sci Pollut Res 30, 30892–30904 (2023). https://doi.org/10.1007/s11356-022-24298-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-24298-6