Abstract
Cadmium (Cd) is an environmental pollutant. Long-term exposure to Cd may lead to adverse health effects in humans. Our epidemiological studies showed that urinary Cd (U-Cd) concentrations increased from 2008 through 2014, although they decreased from 1986 through 2008. The aim of this study was to elucidate the long-term effects of the changing trend of cadmium exposure levels (U-Cd) on residents’ renal function within 30 years after Cd exposure ceased. In 2016, urine samples were collected from each subject by visiting 20 elderly Japanese people (9 females and 11 males) living in the Kakehashi River basin, a previously Cd-polluted area in Ishikawa, Japan. The geometric means of the β2-microglobulin (β2-MG) and urinary Cd (U-Cd) continued to increase from 2014 until 2016. Furthermore, Cd concentration and β2-MG in urine were still higher than those in the non-polluted areas in Japan. Multivariate linear regression was performed to associate β2-MG (dependent variable) and U-Cd with sex and age (independent variables). Significant correlations were found among age, U-Cd, and β2-MG, and these were clearer in females than in males. In summary, we propose that three decades after Cd exposure ceased, age is associated with β2-MG more strongly than Cd for bodily impact. Moreover, renal tubular dysfunction is irreversible and worsens after exposure to Cd, with females being more sensitive to exposure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cd is one of the most toxic metals and causes environmental pollution. Long-term exposure to Cd leads to chronic Cd poisoning and Cd-related diseases, such as renal tubular dysfunction, cardiovascular disease, reproduction, neurotoxicity, bone disease, and even cancer (Akhtar et al. 2021; Calogero et al. 2021; Chen et al. 2021; Deng et al. 2020; La-up et al. 2021; Lu et al. 2021; Kido et al. 1988, 2003; Nordberg et al. 2007; Wang et al. 2020; Xu et al. 2021).
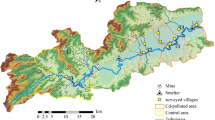
In Japan, rice is a staple food eaten daily and widely by the general population, and it plays a significant role as the main source of Cd exposure (Nordberg et al. 2007; Tsukahara et al. 2003). In 1980, the cadmium-contaminated rice-growing soil was removed from the Kakehashi River basin, in Ishikawa Prefecture, Japan, which was one of the Cd-polluted areas and uncontaminated soil was used to replace the rice field soil (Kido et al. 2001). However, the concentration of rice cadmium (R-Cd) in this region was still higher than in other countries (Friberg et al. 1985; Nordberg et al. 2007; Osawa et al. 2001), although the concentration has decreased since 1980 (Kido et al. 2001). On the other hand, Cd is known to have a long biological half-life (10–30 years) and known to induce renal dysfunction (Kido et al. 1988, 1990, 2003; Nordberg et al. 2007; Suwazono et al. 2009). Therefore, we believe that it is vitally necessary to conduct health checkups of residents after Cd exposure has ceased.
Our previous epidemiological studies in the Kakehashi River basin have shown that it took approximately 20 years for Cd concentrations in inhabitants’ bodies to reduce by half (Sato et al. 2013), and other follow-up studies have also found that Cd exposure levels of the inhabitants of Cd-polluted areas either gradually decrease over time (Arisawa et al. 2007a, 2007b; Cai et al. 2001; Liu et al. 2001) or have no significant change over time (Zhang et al. 2014). However, our follow-up study, involving 28-year observations, showed that Cd concentration in urine (U-Cd) has increased recently, and urinary β2-microglobulin (β2-MG) had increased continuously (Phuc et al. 2016). Furthermore, the geometric means (GMs) of U-Cd and β2-MG were still higher than those in non-Cd-polluted areas of Japan (Phuc et al. 2016; Suwazono et al. 2000). β2-MG is an endogenous low-molecular-weight serum protein, secreted by lymphocytes and most other nucleated cells, that plays an important role in immune response (Drüeke and Massy 2009). Clinical detection of β2-MG concentration in blood or urine provides an early, reliable, and sensitive indicator for the clinical diagnosis of clinical renal function, renal transplant survival, diabetic nephropathy, heavy metal poisoning from Cd, mercury poisoning, and certain malignancies (Drüeke and Massy 2009). Therefore, the aims of this 30-year follow-up study were to elucidate the effect of long-term exposure to Cd on renal function by evaluating the correlation between Cd and β2-MG.
Materials and methods
Selection of study population
At the beginning (1986), we selected all inhabitants who could offer their urine in one of the villages in the Kakehashi River basin. Two hundred and fourteen people over 3 years old participated (the participation rate was 75%). Afterward, each time, we asked all inhabitants to participate in each round of research. Two hundred and twenty-one people participated in 1991. However, many elderly subjects died or could not participate in the examinations due to their poor health, and some members of the younger generations moved to other places. Finally, the number of subjects who participated gradually decreased to 68 (2008), then 29 (2014), then 20 (2016). In this follow-up study, we connected our findings with data from 1986 to 2016 regarding twenty elderly subjects (9 females and 11 males) living in the Kakehashi River basin who participated in all seven studies for 30 years (1986, 1991, 1999, 2003, 2008, 2014, and 2016) (Kido et al. 2001; Sato et al. 2010, 2013; Phuc et al. 2016).
Sample collection and analysis
All samples were collected between November and December 2016 by visiting each family in the hamlet of the Kakehashi River basin. Spot urine samples were collected from the participants and kept in an insulated container. Urine samples were kept frozen (− 20 °C) until analysis. U-Cd concentrations were determined using flameless atomic absorption spectrophotometry (Honda et al. 1989). The latex immunoassay was used to analyze the β2-MG (Bernard et al. 1981), and Jaffe’s method was used to determine urinary creatinine levels (Bonsnes and Taussky 1945). A questionnaire was used to collect basic information from subjects, including age, sex, health condition, medical treatments, and period of residence.
Calculation and statistical analysis
The Cd and β2-MG concentrations in urine were adjusted based on creatinine (µg/g creatinine). The U-Cd and β2-MG concentrations were continuously log10 transformed to improve normality before using statistical tests. The normal distribution was determined by using the Shapiro–Wilk test. Pearson’s correlation coefficient and Spearman’s rank correlation coefficient were used for variables with normal or non-normal distributions, respectively. Finally, multiple linear regressions were performed to associate U-β2-MG as a dependent variable and age, sex, and U-Cd as independent variables.
The data were analyzed using the JMP 12 statistical software package (SAS Institute, Cary, NC, USA) and Excel 2010 (Microsoft, Redmond, WA, USA). A significant difference was determined with a p-value of ≤ 0.05. Data were shown as GMs with geometric standard deviations (GSDs).
Results
The mean age of the subjects was 75.8 ± 11.1 years (range: 62–98 years) for males and 76.7 ± 6.4 years (range: 66–85) for females. The mean residence times for males and females were 70.9 ± 13.5 (range: 47–94 years) and 54.7 ± 12.3 years (range: 35–79 years), respectively. The health conditions of the subjects, as self-reported using questionnaires, were as follows: wellness (n = 10), hypertension (n = 7), diabetes mellitus (n = 1), and cancer (n = 2). The trend in U-Cd concentration over three decades is shown in Table 1. In the combined data, U-Cd concentrations decreased significantly from 1986 (GM = 6.696 μg/g creatinine) to 2008 (GM = 2.860 μg/g creatinine), but U-Cd concentration showed an increasing trend from 2008 (GM = 2.860 μg/g creatinine) to 2016 (GM = 4.863 μg/g creatinine). In males, significant differences were found between 1986 (GM = 5.770 μg/g creatinine) and 2008 (GM = 2.360 μg/g creatinine) (P = 0.005), and between 1991 (GM = 4.499 μg/g creatinine) and 2008 (GM = 2.360 μg/g creatinine) (P = 0.029). In females, no significant differences among U-Cd concentrations were found across the seven rounds of research.
Table 2 shows that urinary β2-MG concentrations increased from 1999 in both sexes. Significant differences were found between 1986 (GM = 93.69 μg/g creatinine) and 2014 (GM = 519.8 μg/g creatinine) (P = 0.034) or 2016 (GM = 848.3 μg/g creatinine) (P = 0.009); between 1999 (GM = 50.73 μg/g creatinine) and 2008 (GM = 408.1 μg/g creatinine) (P = 0.008), 2014 (GM = 519.8 μg/g creatinine) (P = 0.011) or 2016 (GM = 848.3 μg/g creatinine) (P = 0.002); and between 2003 (GM = 192.9 μg/g creatinine) and 2016 (GM = 848.3 μg/g creatinine) (P = 0.041). In males, significant differences were found between 1986 (GM = 60.67 μg/g creatinine) and 2016 (GM = 434.03 μg/g creatinine) (P = 0.035) or 1991(GM = 81.35 μg/g creatinine) or 2016 (GM = 434.03 μg/g creatinine) (P = 0.010); and between 1999 (GM = 14.87 μg/g creatinine) and 2003 (GM = 101.5 μg/g creatinine) (P = 0.015), 2008 (GM = 173.7 μg/g creatinine) (P = 0.004), 2014 (GM = 271.5 μg/g creatinine) (P = 0.006) or 2016 (GM = 434.03 μg/g creatinine) (P = 0.003), respectively. No significant differences were found in females across the seven rounds of research.
Table 3 shows simple correlations between U-Cd and β2-MG concentrations by sex for the period from 1986 to 2016. There were significant correlations between U-Cd and β2-MG concentrations in 1986, 2003, and 2008 in females (Pearson correlation). Significant correlations were observed in 2008 for males (Pearson correlation). Significant correlations were observed from 1986 to 2016 (Pearson correlation for 1986, 2003, and 2014; Spearman correlation for 1991, 2008, and 2016), except for 1999 (Pearson correlation) (borderline significant), in the combined data.
Table 4 shows the association between β2-MG and age, U-Cd, and sex. β2-MG was used as a dependent variable, and age, U-Cd, and sex were used as independent variables. The only significant associations were found between β2-MG and age from 2003 to 2016 (2003: β = 0.449, P = 0.044; 2008: β = 0.393, P = 0.041; 2014: β = 0.559, P = 0.031; 2016: β = 0.559, P = 0.013). No significant associations were found between β2-MG and U-Cd or sex.
Discussion
Our follow-up study is one of the longest-running epidemiological observation research projects. The former 28-year follow-up study (1986–2014) showed that U-Cd increased from 2008 to 2014, after decreasing over the 22 years from 1986 until 2008. We considered two hypotheses as to why U-Cd showed this increasing trend since 2008. The first is the accuracy of the U-Cd determination. The other is the possibility of reexposure to Cd after 22 years of improvement in Cd pollution. In this study, the U-Cd concentration still showed an increasing trend from 2014 to 2016 (Table 1). Therefore, our first hypothesis, regarding the accuracy of U-Cd determination, can be deemed reliable.
For our other hypothesis, we worried about reexposure in the formerly contaminated area because our findings show an increasing trend of the heavy metal cadmium in urine from 2008 to 2016 (Table 1). Moreover, in our study, we found the geometric means of U-Cd (in 2016, 5.677 μg/g creatinine and 4.241 μg/g creatinine for females and males, respectively) in the previously Cd-polluted area were approximately 3 times higher than those in inhabitants of non-polluted areas (U-Cd: 1.00 μg/g creatinine and 1.8 μg/g creatinine for females and males, respectively) in Japan (Ikeda et al. 2011; Suwazono et al. 2000). However, previous follow-up studies have found a gradual decrease or no significant change over time in U-Cd or blood Cd (B-Cd) concentrations in the inhabitants of Cd-polluted areas (Arisawa et al. 2007a, 2007b; Cai et al. 2001; Liu et al. 2001; Zhang et al. 2014). On the other hand, R-Cd is an indicator of external Cd exposure in Japan (Nordberg et al. 2007; Tsukahara et al. 2003), and the Cd concentration in the B-Cd was used as an indicator to evaluate residents’ short-term Cd exposure (Lauwerys et al. 1979; Kjellström and Nordberg. 1978; Nordberg et al. 2007). Therefore, we suggest that our hypothesis of reexposure needs to be confirmed in future studies by measuring Cd concentrations in the rice and blood of residents of the previously Cd-polluted area.
This study found fluctuating β2-MG from 1986 to 1999 and an increasing trend until 2016 (Table 2). Furthermore, we observed a significant simple correlation between U-Cd and β2-MG in the combined data from 1986 to 2016, except for 1999 (P = 0.053) (Table 3). Thus, these results support our opinion that U-Cd concentration is still associated with the biomarker of renal tubular function and that the process of deteriorating kidney function is irreversible when caused by Cd exposure (Kido et al. 2001; Phuc et al. 2016, 2017; Sun et al. 2021). In addition, we found a significant association between age and β2-MG using multiple regression analysis (Table 4). Moreover, a study has shown that in addition to cadmium deposition in the kidney, bone, and liver, as is well known, muscle is an underestimated cadmium reservoir, and a proposed spatial resolution of cadmium distribution in a wide range of human soft tissues has been presented (Egger et al. 2019). These results indicate that age is associated with β2-MG more strongly than Cd for bodily impact, which is consistent with our previous studies (Phuc et al. 2016, 2017). The reason for these results may be that when people are exposed to Cd, several organs and soft tissues tend to accumulate the element, and as subjects become older, the internal organs and soft tissues of the body gradually become atrophic (Wilkinson et al. 2018), and Cd is released into the blood and excreted into the urine, resulting in elevated U-Cd concentrations. This increase in U-Cd concentration leads to an increase in β2-MG. At the same time, the function of the kidneys decreases with age (Glassock and Rule 2016). Therefore, as subjects age, atrophying organs and soft tissues and declining renal function may be the main reasons for the increase in β2-MG.
In this study, the correlations of U-Cd and β2-MG were observed to be closer in females than in males (Table 3). This may be explained by menstrual periods and fertility in women, leading to iron deficiencies due to anemia, which may increase the absorption of Cd as well as iron (Berglund et al. 1994; Meltzer et al. 2016; Nogawa et al. 1978). Additionally, some men moved to non-polluted areas for work, and their amount of Cd uptake was reduced; meanwhile, the women still lived in contaminated areas and continued to absorb Cd. The increased absorption of Cd and longer exposure time led to higher levels of Cd stored in the internal organs in women than in men. Our analysis in this survey is consistent with previous epidemiological studies in polluted areas, and they demonstrated that the effects of Cd toxicity in females were higher than in males (Ewers et al. 1985; Kido et al. 2001; Nishijo et al. 2004; Phuc et al. 2016, 2017; Tsuritani et al. 1992), and that long-term Cd exposure leads to higher kidney disease morbidity and mortality in females than in males (Nogawa et al. 2017, 2018).
Although our follow-up study is one of the longest-running epidemiological observational studies, there are some limitations. First, the deaths of some participants during the 30-year follow-up study resulted in a smaller number of participants in this study. Second, there is no control group in this study. Third, participants living in Cd-contaminated areas should undergo regular physical examinations to determine the cause of the increase in Cd. However, outside of this study, no research has been performed to date. Therefore, we believe that it is necessary to elucidate the reasons for this increase in Cd through follow-up investigations.
Conclusion
Our 30-year follow-up study is one of the longest-running observational studies about the effects of Cd on human health. U-Cd and β2-MG concentrations showed an increasing trend until 2016, beginning in 2008 and 1999, respectively. Moreover, U-Cd concentrations in inhabitants living in Cd-polluted areas were higher than those in non-polluted areas, more than 3 decades after Cd exposure ceased. The correlations among age, U-Cd, and β2-MG concentrations were clearer in females than in males. Age was associated with β2-MG concentration more strongly than Cd for bodily impact. Moreover, after exposure to Cd, renal tubular dysfunction is irreversible and worsens, with women being especially sensitive to this effect. Therefore, our research in this Cd-polluted region should be continued.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Materials availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- Cd:
-
Cadmium
- β2-MG:
-
Urinary β2-microglobulin
- B-Cd:
-
Blood cadmium
- R-Cd:
-
Rice cadmium
- U-Cd:
-
Urinary cadmium
- GMs:
-
Geometric means
- GSDs:
-
Geometric standard deviations
References
Akhtar E, Roy AK, Haq MA, von Ehrenstein OS, Ahmed S, Vahter M (2021) A longitudinal study of rural Bangladeshi children with long-term arsenic and cadmium exposures and biomarkers of cardiometabolic diseases. Environ Pollut 271:116333
Arisawa K, Uemura H, Hiyoshi M, Takeda H, Saito H, Soda M (2007a) Cadmium-induced renal dysfunction and mortality in two cohorts: disappearance of the association in a generation born later. Toxicol Lett 169:214–221
Arisawa K, Uemura H, Hiyoshi M, Dakeshita S, Kitayama A, Saito H, Soda M (2007b) Cause-specific mortality and cancer incidence rates in relation to urinary β2-microglobulin: 23-year follow-up study in a cadmium-polluted area. Toxicol Lett 173:168–174
Berglund M, Akesson A, Nermell B, Vahter M (1994) Intestinal absorption of dietary cadmium in women depends on body iron stores and fiber intake. Environ Health Persp 102:1058–1066
Bernard AM, Vyskocil A, Lauwerys RR (1981) Determination of beta 2-microglobulin in human urine and serum by latex immunoassay. Clin Chem 27:832–837
Bonsnes RW, Taussky HH (1945) On the colorimetric determination of creatinine by the Jaffe reaction. J Biol Chem 158:581–591
Cai Y, Aoshima K, Katoh T, Teranishi H, Kasuya M (2001) Renal tubular dysfunction in male inhabitants of a cadmium-polluted area in Toyama, Japan-an Eleven-year Follow-up Study. J Epidemiol 11:180–189
Calogero AE, Fiore M, Giacone F, Altomare M, Asero P, Ledda C (2021) Exposure to multiple metals/metalloids and human semen quality: a cross-sectional study. Ecotoxicol Environ Saf 215:112165
Chen J, Lai W, Deng Y, Liu M, Dong M, Liu Z, Wang T, Li X, Zhao Z, Yin X, Yang J, Yu R, Liu L (2021) MicroRNA-363-3p promotes apoptosis in response to cadmium-induced renal injury by down-regulating phosphoinositide 3-kinase expression. Toxicol Lett 345:12–23
Deng P, Ma Q, Xi Y, Yang L, Lin M, Yu Z, Chen C, Zhou Z (2020) Transcriptomic insight into cadmium-induced neurotoxicity in embryonic neural stem/progenitor cells. Toxicol Vitro 62:104686
Drüeke TB, Massy ZA (2009) Beta2-microglobulin. Semin Dial 22:378–380
Egger AE, Grabmann G, Gollmann-Tepeköylü C, Pechriggl EJ, Artner C, Türkcan A, Hartinger CG, Fritsch H, Keppler BK, Brenner E, Grimm M, Messner B, Bernhard D (2019) Chemical imaging and assessment of cadmium distribution in the human body. Metallomics 11:2010–2019
Ewers U, Brockhaus A, Dolgner R, Freier I, Jermann E, Bernard A, Stiller-Winkler R, Hahn R, Manojlovic N (1985) Environmental exposure to cadmium and renal function of elderly women living in cadmium-polluted areas of the Federal Republic of Germany. Int Arch Occup Environ Health 55:217–239
Friberg L, Elinder C, Kjellstrom T, Nordberg G (1985) Cadmium and health: a toxicological and epidemiological appraisal Volume 1: exposure, dose, and metabolism. https://doi.org/10.1201/9780429260605.
Glassock RJ, Rule AD (2016) Aging and the kidneys: anatomy, physiology and consequences for defining chronic kidney disease. Nephron 134:25–29
Honda R, Tsuritani I, Ishizaki M, Kido T, Yamada Y, Nogawa K (1989) Flameless atomic absorption spectrophotometry of cadmium and lead in blood and urine. J Kanazawa Med Univ 14:337–347
Ikeda M, Ohashi F, Fukui Y, Sakuragi S, Moriguchi J (2011) Closer correlation of cadmium in urine than that of cadmium in blood with tubular dysfunction markers in urine among general women populations in Japan. Int Arch Occup Environ Health 84:121–129
Kido T, Honda R, Tsuritani I, Yamaya H, Ishizaki M, Yamada Y, Nogawa K (1988) Progress of renal dysfunction in inhabitants environmentally exposed to cadmium. Arch Environ Health 43:213–217
Kido T, Nishijo M, Nakagawa H, Kobayashi E, Nogawa K, Tsuritani I, Honda R, Kito H (2001) Urinary findings of inhabitants in a cadmium-polluted area ten years after cessation of cadmium exposure. Hokuriku J Public Health 27:81–84
Kido T, Nogawa K, Ishizaki M, Honda R, Tsuritani I, Yamada Y, Nakagawa H, Nishi M (1990) Long-term observation of serum creatinine and arterial blood pH in persons with cadmium-induced renal dysfunction. Arch Environ Health 45:35–41
Kido T, Nordberg GF, Roels HA (2003) Cadmium-induced renal effects. Clinical Nephrotoxins: Springer pp 345–361. https://springerlink.bibliotecabuap.elogim.com/chapter/10.1007/978-94-015-9088-4_27
Kjellström T, Nordberg G (1978) A kinetic model of cadmium metabolism in the human bein. Environ Res 16(1–3):248–269
La-up A, Mahasakpan P, Saengow U (2021) The current status of osteoporosis after 15 years of reduced cadmium exposure among residents living in cadmium-contaminated areas in northwestern Thailand. Environ Sci Pollut Res 28:20121–20127
Lauwerys R, Roels H, Regniers M, Buchet JP, Bernard A, Goretet A (1979) Significance of cadmium concentration in blood and in urine in workers exposed to cadmium. Environ Res 20:375–391
Liu XJ, Arisawa K, Nakano A, Saito H, Takahashi T, Kosaka A (2001) Significance of cadmium concentrations in blood and hair as an indicator of dose 15 years after the reduction of environmental exposure to cadmium. Toxicol Lett 123:135–141
Lu JF, Lan J, Li XE, Zhu ZX (2021) Blood lead and cadmium levels are negatively associated with bone mineral density in young female adults. Arch Public Health 79(1):116
Meltzer HM, Alexander J, Brantsæter AL, Borch-Iohnsen B, Ellingsen DG, Thomassen Y, Holmen J, Ydersbond TA (2016) The impact of iron status and smoking on blood divalent metal concentrations in Norwegian women in the HUNT2 study. J Trace Elem Med Biol 38:165–173
Nishijo M, Nakagawa H, Morikawa Y, Kuriwaki J, Miura K, Kido T, Nogawa K (2004) Mortality in a cadmium polluted area in Japan. Biometals 17:535–538
Nogawa K, Ishizaki A, Kawano S (1978) Statistical observations of the dose-response relationships of cadmium based on epidemiological studies in the Kakehashi River basin. Environ Res 15:185–198
Nogawa K, Suwazono Y, Nishijo M, Sakurai M, Ishizaki M, Morikawa Y, Watanabe Y, Kido T, Nakagawa H (2017) Relationship between mortality and rice cadmium concentration in inhabitants of the polluted Jinzu River basin, Toyama, Japan: a 26 year follow-up. J Appl Toxicol 38:855–861
Nogawa K, Suwazono Y, Nishijo M, Sakurai M, Ishizaki M, Morikawa Y, Watanabe Y, Kido T, Nakagawa H (2018) Increase of lifetime cadmium intake dose-dependently increased all cause of mortality in female inhabitants of the cadmium-polluted Jinzu River basin, Toyama, Japan. Environ Res 164:379–384
Nordberg G, Fowler B, Nordberg M, Friberg L (2007) Handbook of the toxicology of metals, 3rd edn. Academic, London. ISBN 978-0-12-369413-3
Osawa T, Kobayashi E, Okubo Y, Suwazono Y, Kido T, Nogawa K (2001) A retrospective study on the relation between renal dysfunction and cadmium concentration in rice in individual hamlets in the Jinzu River basin, Toyama Prefecture, Japan. Environ Res 86:51–59
Phuc HD, Kido T, Manh DH, Anh TL, Oanh NTP, Thi N, Okamoto R, Ichimori A, Nogawa K, Suwazono Y (2016) A 28-year observational study of urinary cadmium and β2-microglobulin concentrations in inhabitants in cadmium-polluted areas in Japan. J Appl Toxicol 36:1622–1628
Phuc HD, Kido T, Oanh NTP, Manh HD, Anh LT, Oyama Y, Okamoto R, Ichimori A, Nogawa K, Suwazono Y, Nakagawa H (2017) Effects of aging on cadmium concentrations and renal dysfunction in inhabitants in cadmium-polluted regions in Japan. J Appl Toxicol 37:1046–1052
Sato R, Kido T, Honda R, Nishijo M, Nakagawa H, Kobayashi E, Suwazono Y (2010) Seventeen-year observation on urinary cadmium and b2-microglobulin in inhabitants after cessation of cadmium-exposure in Japan. Bull Environ Contam Toxicol 84:363–367
Sato R, Kido T, Nakagawa H, Nishijo M, Honda R, Kobayashi E, Suwazono Y (2013) Twenty-two-year observation on urinary cadmium and ß2-microglobulin in inhabitants after cessation of cadmium-exposure in Japan. Open J Nephrol 3:205–210
Sun XL, Kido T, Nakagawa H, Nishijo M, Sakurai M, Ishizaki M, Morikawa Y, Okamoto R, Ichimori A, Ohno N, Kobayashi S, Miyati T, Nogawa K, Suwazono Y (2021) The relationship between cadmium exposure and renal volume in inhabitants of a cadmium-polluted area of Japan. Environ Sci Pollut Res 28:22372–22379
Suwazono Y, Kido T, Nakagawa H, Nishijo M, Honda R, Kobayashi E, Dochi M, Nogawa K (2009) Biological half-life of cadmium in the urine of inhabitants after cessation of cadmium exposure. Biomarkers 14:77–81
Suwazono Y, Kobayashi E, Okubo Y, Nogawa K, Kido T, Nakagawa H (2000) Renal effects of cadmium exposure in cadmium nonpolluted areas in Japan. Environ Res 84:44–55
Tsukahara T, Ezaki T, Moriguchi J, Furuki K, Shimbo S, Matsuda-Inoguchi N, Ikeda M (2003) Rice as the most influential source of cadmium intake among general Japanese population. Sci Total Environ 305:41–51
Tsuritani I, Honda R, Ishizaki M, Yamada Y, Kido T, Nogawa K (1992) Impairment of vitamin D metabolism due to environmental cadmium exposure, and possible relevance to sex-related differences in vulnerability to the bone damage. J Toxicol Environ Health A 37:519–533
Wang C, Nie G, Zhuang Y, Hu R, Wu H, Xing C, Li G, Hu G, Yang F, Zhang C (2020) Inhibition of autophagy enhances cadmium-induced apoptosis in duck renal tubular epithelial cells. Ecotoxicol Environ Saf 205:111188
Wilkinson DJ, Piasecki M, Atherton PJ (2018) The age-related loss of skeletal muscle mass and function: measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev 47:123–132
Xu YJ, Mu W, Li JQ, Ba Q, Wang H (2021) Chronic cadmium exposure at environmental-relevant level accelerates the development of hepatotoxicity to hepatocarcinogenesis. Sci Total Environ 783:146958
Zhang WL, Du Y, Zhai MM, Shang Q (2014) Cadmium exposure and its health effects: a 19-year follow-up study of a polluted area in China. Sci Total Environ 407–471:224–228
Funding
This research was funded by the Japanese Ministry of the Environment.
Author information
Authors and Affiliations
Contributions
Xian Liang Sun, Hoang Duc Phuc, and Rie Okamoto: Conceptualization, methodology, software, formal analysis, and writing (original draft). Teruhiko Kido: Conceptualization, resources, writing (review and editing), supervision, project administration, and funding acquisition. Nguyen Thi Phuong Oanh, Ho Dung Manh, Le Thai Anh, and Akie Ichimori: Writing (review and editing). Kazuhiro Nogawa, Yasushi Suwazono, and Hideaki Nakagawa: Conceptualization, resources, writing (review and editing), and project administration. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All participants in this study were volunteers, and personal information was kept confidential. This research was approved by the Kanazawa University bioethics committee (approval no. 512).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, X.L., Phuc, H.D., Okamoto, R. et al. A 30-year follow-up study in a former cadmium-polluted area of Japan: the relationship between cadmium exposure and β2-microglobulin in the urine of Japanese people. Environ Sci Pollut Res 30, 23079–23085 (2023). https://doi.org/10.1007/s11356-022-23818-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-23818-8