Abstract
In recent years, the combination of iron carbon micro-electrolysis (ICME) with constructed wetlands (CWs) for removal of nitrogen and phosphorus has attracted more and more attention. However, the removal mechanisms by CWs with iron carbon (Fe–C) substrates are still unclear. In this study, the Fe–C based CW (CW-A) was established to improve the removal efficiencies of nitrogen and phosphorus by optimizing the operating conditions. And the removal mechanisms of nitrogen and phosphorus were explored. The results shown that the removal rates of COD, NH4+-N, NO3−-N, TN, and TP in CW-A could reach up to 84.4%, 94.0%, 81.1%, 86.6%, and 84.3%, respectively. Wetland plants and intermittent aeration have dominant effects on the removal of NH4+-N, while the removal efficiencies of NO3−-N, TN, and TP were mainly affected by Fe–C substrates, wetland plants, and HRT. XPS analysis revealed that Fe(0)/Fe2+ and their valence transformation played important roles on the pollutants removal. High-throughput sequencing results showed that Fe–C substrates and wetland plants had considerable impacts on the microbial community structures, such as richness and diversity of microorganism. The relative abundance of autotrophic denitrification bacteria (e.g., Denitatsoma, Thauera, and Sulfuritalea) increased in CW-A than CW-C. The electrons and H2/[H] produced from Fe–C substrates were utilized by autotrophic denitrification bacteria for NO3−-N reduction. Microbial degradation was the main removal mechanism of nitrogen in CW-A. Removal efficiency of phosphorus was enhanced resulted from the reaction of phosphate with iron ion. The application of CWs with Fe–C substrates and plants presented great potential for simultaneous removal of nitrogen and phosphorus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High concentrations of nitrogen and phosphorus in the effluent of municipal wastewater treatment plants (MWTPs) may result in eutrophication and pose a threat to human health if the effluent is discharged inappropriately (Ahmed et al. 2017). In order to tackle with these problems, many countries have issued more stringent wastewater discharge standards (He et al. 2018), which promote rapid developments of advanced wastewater treatment processes, such as membrane technologies (Abtahi et al. 2018), biological aerated filter (Su et al. 2021), and activated carbon adsorption (Guillossou et al. 2019). Although the physicochemical technologies are highly efficient, their operation and management costs are quite high. Therefore, it is crucial to develop an economical technology with high efficiency and low energy consumption for nitrogen and phosphorus removal.
Constructed wetlands (CWs), as an ecological and green treatment technology, have been increasingly applied to treat secondary effluent due to the low construction cost, easy operation and scenic beauty (Moreira and Oliveira Dias 2020). Removing pollutants by CWs depends on the synergistic effects of plants, substrates, and microbes (Kataki et al. 2021), among which the role of substrates have been proved to be particularly important because they can adsorb pollutants, support the growth of wetland plants, and change the microbial community structure (Guan et al. 2015; Yang et al. 2018). CWs with traditional substrates, such as gravel, ceramsite, and sand, can reduce nitrate-nitrogen (NO3−-N) to N2 through heterotrophic denitrification (Mekonnen et al. 2015). Nevertheless, the deficiency of available organic matter in the secondary effluent leads to limited total nitrogen (TN) removal, so additional carbon sources (methanol, ethanol, sodium acetate, and so on) were needed in traditional CWs (Lai et al. 2020b). Comparing with heterotrophic denitrification, autotrophic denitrification has the advantage of utilizing inorganic matter as electron donors (e.g. Fe2+ (Kiskira et al. 2017), H2 (Xing et al. 2018), S2−, and sulfur compounds (Chen et al. 2022)). Therefore, increasing attention has been paid to novel substrates that can stimulate autotrophic denitrification in CWs (Ma et al. 2020). Meanwhile, many studies have found that the substrate storage and plant absorption are the major pathways for phosphorus removal in CWs (Wu et al. 2013). However, traditional CWs show poor removal performance for total phosphorus (TP) due to limited capacities of the substrate adsorption and plant absorption (Li et al. 2021). Thus, it is essential to develop a novel substrate of CWs to remove nitrogen and phosphorus simultaneously from the secondary effluent.
Iron carbon micro-electrolysis (ICME) is an electrochemical technique in which numerous microscopic galvanic cells could be formed spontaneously between iron and carbon particles (Sun et al. 2019). Compared with alone zero valent iron (Fe(0)) corrosion, ICME could promote the redox reaction and improve the yield of Fe2+/Fe3+ and H2/[H], as well as decrease the iron consumption (Li et al. 2022). The electrons and H2/[H] could be utilized as electron donors for autotrophic denitrification bacteria, which significantly benefits the NO3−-N removal through biological pathway (Zhu et al. 2019). Moreover, iron ion (Fe2+ and Fe3+, et al.) generated by ICME could react with phosphate (PO43−) to form precipitate, thus enabling the simultaneously removal of nitrogen and phosphorus (Cui et al. 2022). The reaction equations are as follows (Eqs. (1–6)) (Deng et al. 2016; Fytianos et al. 1998):
Previous studies have shown that the combination of ICME and CWs can improve the nitrogen and phosphorus removal efficiencies. Deng et al. (2020b) combined a vertical CW with oxic/anoxic process and ICME to purify low-carbon greywater under low ambient temperature and found that autotrophic denitrification and Fe3+-based phosphorus immobilization were enhanced in iron carbon (Fe–C) layer. Moreover, it was proven that the microbial abundance related to denitrification process increased notably due to the addition of iron scraps and activated carbon (Shen et al. 2019). Huang et al. (2020) proved that Fe–C substrates could also enhance the heterotrophic denitrification and improve the biodegradability of refractory organic matters (such as humic acid-like) in CWs. Besides, Jia et al. (2020) reported that the NO3−-N reduction rate could reach 87% and the emission of nitrous oxide (N2O) during groundwater treatment was reduced in Fe–C based CW. However, previous studies mainly focused on the improvement of pollutants removal but neglected some key issues in CWs with Fe–C substrates. For example, Fe2+ would compete with oxygen and create a relatively reductive condition, which inhibited the nitrification process and increased the concentration of ammonia–nitrogen (NH4+-N) in effluent (Dong et al. 2020). In addition, few studies were focused on strengthening mechanisms of nitrogen and phosphorus removal from the aspects of substrates, plants, and microorganisms.
Therefore, the objectives of this study were (1) to optimize the operating conditions and explore the effects of different parameters on pollutants removal in Fe–C based CW, such as hydraulic retention time (HRT), intermittent aeration and C/N ratio; (2) to analyze the simultaneous removal efficiencies of nitrogen and phosphorus in the Fe–C based CW; (3) to elucidate the enhanced removal mechanisms of nitrogen and phosphorus in Fe–C based CW and the influences of substrates, plants, and microorganisms on pollutants removal performance.
Materials and methods
Construction of three CWs
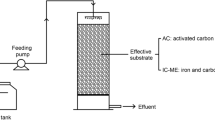
Three types of vertical flow CWs were established in the laboratory by using plexiglass column with working volume of 9.7 L (diameter 15 cm and height 55 cm). The Fe–C based CW with plants was denoted as CW-A. The other two control groups were (i) CW filled with Fe–C substrates and no plants (CW-B); and (ii) CW filled with gravel substrates and plants (CW-C). As illustrated in Fig. 1, each CW was composed of four layers. From top to bottom were 10 cm quartz sand layer (particle size 2 ~ 4 mm), 15 cm gravel layer (particle size 5 ~ 10 mm), 15 cm substrate layer, and 5 cm pebble layer (particle size 15 ~ 20 mm), respectively. Notably, the substrate layers in CW-A and CW-B were Fe–C and gravel mixtures (particle size 5 ~ 8 mm) with a volume ratio of 1:1, while the substrate layer in CW-C was gravel only. The Fe–C substrates purchased from Lvzhiyuan Activated Carbon Co., Ltd. (Henan, China) were mainly consisted of iron powder, activated carbon, catalyzer, and adhesives. In order to increase the dissolved oxygen (DO) concentration in the aerobic zone, microporous aeration pipes were installed at the middle of the devices. Iris pseudacorus, a common species in the north of China with excellent nutrient removal ability (Xia et al. 2012), were planted in CW-A and CW-C with the similar size of approximately 20 cm in height and at a density of four rhizomes per device. Three sampling points were set in each reactor with 10, 25, and 35 cm away from the influent port, which represented aerobic, anoxic, and anaerobic zones, respectively. The same influent was pumped into the three CWs systems from the water tank (15 L). All the devices were wrapped with tinfoil to avoid substrates from being exposed to the sunlight (Fig. 1).
CWs operation and water sample analysis
In this study, all experimental systems were operated continuously, and the inlet and outlet were set at the top and bottom of three devices. Synthetic secondary effluent was prepared with deionized water by adding glucose, NH4Cl, NaNO3, and KH2PO4. Trace elements and vitamin were added into the synthetic wastewater to ensure microbial growth. The compositions of trace elements and vitamin solutions were shown in Table S1 and Table S2 in the Supplementary Information. The concentrations of COD, NH4+-N, NO3−-N, and TP in synthetic wastewater were set to 60 ~ 100 mg L−1, 5 mg L−1, 10 mg L−1, and 2 mg L−1, respectively. In addition, the pH of synthetic wastewater was adjusted to 7.0 ± 0.2 with 1 mol L−1 HCl. The CWs systems were intermittently aerated for 3 h (18:00 ~ 21:00) each day.
The whole experiment included three phases, the start-up period (1 ~ 62 days), condition optimization period (63 ~ 125 days) and stable operation period (126 ~ 150 days). The start-up was considered successful when the pollutants concentrations in the effluent were stable. Condition optimization period was divided into five stages according to different operating conditions. The operation conditions under different stages were listed in Table 1. The experimental period was from June 2021 to November 2021.
The influent and effluent samples were taken out from three CWs devices every 3 ~ 4 days and the DO and pH values were measured immediately by a multi-parameters water analysis meter (Multi 3630 IDS, WTW, Germany). In the stable operation stage, the effluents at different heights of the CWs systems were also detected. All samples were filtered through 0.45-μm mixed cellulose ester membrane filters to remove suspended solids. Concentrations of COD, NH4+-N, NO3−-N, NO2−-N, TN, and TP were measured following the methods described in the State Environmental Protection Administration of China (2002). The iron valence changes between fresh and used Fe–C substrates in CW-A after the stable operation stage were characterized by X-ray photoelectron spectroscopy (XPS).
Batch tests for the mechanism explanation
To further explore the mechanisms of Fe–C based CW on the removal of nitrogen and phosphorus, batch tests were carried out in 500-mL Erlenmeyer flasks. The Fe–C substrates were washed with distilled water and dried naturally before the tests. A total of 10 mg L−1 NO3−-N solutions and 10 mg L−1 PO43− solutions were prepared respectively and the solutions pH were adjusted at 7.0 ± 0.2. For nitrogen removal experiment, 9 flasks were set as 9 reactors. A total of 200 mL NO3−-N solution and 20 g Fe–C substrates were fed into each flask. For phosphorus removal experiment, 200 mL PO43− solution and 10 g Fe–C substrates were added into another 9 flasks, respectively. After reacting 0.5, 1, 3, 6, 12, 18, 24, 36, and 48 h in the two batch tests, the solutions were filtered through 0.45-μm membranes. The concentrations of NH4+-N, NO3−-N, NO2−-N, and TN in nitrogen removal experiment and the concentrations of TP and total iron (TFe) in phosphorus removal experiment were detected. The TFe concentration was determined by using 1, 10-phenanthroline method.
Substrate sampling and microbial community analysis
In CWs devices, quartz sand layer (0 ~ 10 cm), gravel layer (10 ~ 25 cm), and Fe–C layer (25 ~ 40 cm) corresponded to aerobic, anoxic, and anaerobic zones, respectively. To determine the response of microbial community in CWs systems, substrates samples (50 g) were collected from three layers in three CW systems after the stable operation stage. In CW-A device, the substrates samples of quartz sand, gravel, and Fe–C were named as A1, A2, and A3, respectively. Similarly in CW-B and CW-C, the samples were termed as B1, B2, B3 and C1, C2, C3.
DNA was extracted from the 9 substrates samples with E.Z.N.ATMMag-Bind Soil DNA Kits (OMEGA, USA). 338F and 806R were the forward and reverse primers for the amplification of 16S rRNA V3 ~ V4 region by polymerase chain reaction (PCR). The paired-end strategy sequencing was performed on the Illumina MiSeq300 platform of Shanghai Paisennuo Technology Co., Ltd. (Shanghai, China). The obtained sequences were clustered into operational classification units (OUTs) at 97% sequence similarity.
Statistical analysis
Statistical analysis of data at different operating states was performed by SPSS statistics 22.0 (USA), and the p values below 0.05 were considered statistically significant. All graphic designs were performed by Origin 2022 (USA).
Results and discussion
Removal performance of nitrogen and phosphorus in three CWs systems
Nitrogen and phosphorus removal efficiencies during different operating periods
Figure 2 presented the concentration variations of different forms of nitrogen (NH4+-N, NO3−-N, NO2−-N, and TN), COD, and TP in influent and effluent during the experiment. At the beginning of start-up stage, the removal performance of NH4+-N was unsatisfactory (Fig. 2a). The concentrations of NH4+-N in the effluents of CW-A and CW-B were higher than that in CW-C during start-up stage. This result was consistent with the previous studies because Fe–C substrates reacted with NO3−-N to produce NH4+-N (Huang et al. 2020; Jia et al. 2020). However, intermittent aeration in the middle of the devices accelerated the growth of nitrification bacteria, and could relieve the insufficiency of oxygen supply at night (Lai et al. 2020a). NH4+-N concentrations in the effluents of the three CWs systems were below 0.5 mg L−1 in stage I ~ III. As aeration was stopped, the NH4+-N removal efficiencies decreased slightly, but still remained above 90% in CW-A. Shen et al. (2019) found that the NH4+-N removal efficiency was only 16.27 ± 6.39% in the CW with Fe–C substrates, which was apparently lower than that in CW with quartz sand (79.89 ± 5.79%). This may be caused by the competition between Fe2+ and nitrification bacteria for DO (Shen et al. 2019). However, the phenomenon of NH4+-N accumulation did not occur in this study.
In start-up stage, the NO3−-N concentration in the influent was increased step by step. It was found that the concentrations of NO3−-N in the effluents of three devices increased accordingly, especially in CW-C (Fig. 2b). The influent NO3−-N concentration was maintained at 10 mg L−1, and the NO3−-N removal rate in the CW-A was the highest among the three CWs. In stage I ~ III, with the increase of HRT from 24 to 48 h, the NO3−-N removal efficiency in CW-A gradually increased and the NO3−-N concentration in the effluent remained relatively stable at 0.8 ~ 2.0 mg L−1, while the effluent NO3−-N concentrations in CW-B and CW-C were 2.0 ~ 4.4 mg L−1 and 3.3 ~ 4.9 mg L−1, respectively. In addition, the decrease of C/N ratio had negative impact on NO3−-N removal in all CWs systems. Among them, the NO3−-N concentration in the effluent of CW-C increased the most from 3.7 to 7.7 mg L−1. The results indicated that CWs with Fe–C substrates had better adaptability to sewage with low C/N ratio and stronger resistance to TN load change.
As shown in Fig. 2c, in start-up stage, the effluent NO2−-N concentrations were slightly higher in CW-A and CW-B, which was consistent with previous research results (Jia et al. 2020). It was probably because the electron donors were insufficient when Fe–C substrates reduced NO3−-N, and the reaction process was incomplete, leading to the accumulation of NO2−-N (Tian and Yu 2020). However, the NO2−-N concentrations in the effluents of three CWs systems were still at low level (below 0.1 mg L−1) in stable operation stage.
Compared with CW-C, the Fe–C substrates could provide inorganic electron, so autotrophic denitrification was enhanced in CW-A and CW-B systems (Huang et al. 2020). Although the organic compound was inadequate in CW-A, the TN removal rate was obtained at 84% which was dramatically higher than that in CW-C systems in stable operation stage (p < 0.05) (Fig. 2d).
In stage I ~ III, the COD removal efficiencies in three CWs increased gradually with the extension of HRT (Fig. 2e). And HRT was the main factor affecting the COD removal. When the intermittent aeration was stopped in stage IV, it had no obvious effect on the removal of COD. In order to decrease the operation cost, aeration was not carried out in the later experiment. In stable operation stage, the COD concentrations in the effluents of three CWs were all no more than 15 mg L−1, and the COD average removal rate in each CW system was over 80%. According to previous researches, introducing ICME into CWs could enhance the biodegradability of refractory organic compounds and increase COD removal efficiency (Huang et al. 2020; Zheng et al. 2019).
Fe–C substrates and wetland plants had obvious influence on TP removal performance (p < 0.05) (Fig. 2f). In start-up stage, the TP concentrations in the effluents of the three CWs increased with the prolong of operation time, which was probably due to the gradual saturation of the adsorption capacities of gravel and Fe–C substrates. Compared with CW-C, CW-A and CW-B had higher TP removal efficiencies, because Fe2+/Fe3+ could react with PO43− to form precipitation (Deng et al. 2017). The concentration of TP in the effluent gradually decreased during stage I ~ III. The impact of extending HRT was more obvious (p < 0.05) than stopping aeration and decreasing C/N ratio. In stable operation stage, the TP average removal rates in CW-A, CW-B and CW-C were 84.6%, 63.5%, and 50.5%, respectively.
In CW-A, the removal of NH4+-N was affected by wetland plants and intermittent aeration. Fe–C substrates had the greatest improving effects on the removal efficiencies of nitrogen and phosphorus, followed by wetland plants and HRT. The extension of HRT played a crucial role in promoting the removal of COD. The decrease of C/N ratio from 6 to 4 had a slightly negative impact on the removal of TN and TP in CW-A, but had significant impact on CW-C and concentrations of TN and TP in effluent of CW-C increased obviously.
pH value could affect the reaction efficiency of ICME and formation rate of Fe2+/Fe3+. Fig. S1 showed the variations of pH in influent and effluent of three CWs devices. Compared with the pH value of effluent in CW-C (7.23 ~ 7.98), the pH values in CW-A (7.63 ~ 8.55) and CW-B (7.67 ~ 8.61) were higher because ICME reaction could produce OH− (Shen et al. 2019). In stable operation stage, the pH variations of effluents in CW-A and CW-B were gentle, reflecting the ICME reaction proceeded stably and continuously. Consequently, Fe–C substrates could be recommended as novel substrates which can enhance nitrogen and phosphorus removal efficiencies simultaneously in CWs.
Pollutants removal performance at different heights along CWs devices
Figure 3 showed the average effluent concentrations and removal efficiencies of NO3−-N, TN, NO2−-N, NH4+-N, COD, and TP in stable operation stage at different heights along CWs devices. The NO3−-N removal rates were all approximately 30% at 0 ~ 10 cm in three CWs, because the organic compounds in the influent could be used by heterotrophic denitrification bacteria for removing NO3−-N (Fig. 3a). However, below 25 cm height, the concentration of COD was less than 15 mg L−1 (Fig. 3e), which led to the stagnant NO3−-N removal rate in CW-C due to insufficient organic matter. The NO3−-N removal efficiencies in CW-A were 68.2% and 77.9% in 25 cm and 35 cm, respectively. The effective removal performance of NO3−-N indicated that ICME could supply electrons for autotrophic denitrification bacteria by the numerous micro-scale galvanic corrosion (Xing et al. 2016). Moreover, the average TN removal efficiencies at the heights of 10 cm, 25 cm and 35 cm in CW-A were 52.5%, 77.4%, and 84.5%, respectively, which could be regarded as the removal performance in aerobic zone, anoxic zone and anaerobic zone (Fig. 3b). Wetland plants increased oxygen release during photosynthesis process and transported oxygen from air to the rhizosphere, as well as provided suitable conditions and places for microbial growth (Rehman et al. 2017). Therefore, the TN removal rate in CW-A was higher than that in CW-B.
It was also shown in Fig. 3c that the maximum NO2−-N concentration was 0.06 mg L−1 at 25 cm in CW-C. NO2−-N accumulation did not occur in three CWs systems. In addition, NH4+-N and COD were mainly removed in aerobic zone and their average removal efficiencies at the height of 10 cm in CW-A were 94.0% and 84.4%.
At the height of 10 cm, the TP removal efficiencies were approximately 40% of the three CWs (Fig. 5f) because phosphorus could be absorbed as poly-P by the phosphate-accumulating organisms under aerobic conditions (Deng et al. 2020b). In CW-A and CW-B, PO43− could react with Fe2+/Fe3+ generated by the Fe–C substrates below 25 cm to form precipitation, so the TP removal rates were significantly improved (Deng et al. 2017). However, the gravel reached adsorption saturation basically in CW-C and the TP removal performance was unsatisfactory. Previous study proved that the removal of phosphorous in CWs was highly dependent on substrates rather than wetland plants (Shukla et al. 2021). Plants could affect the micro-environment of CWs, and the variation of micro-environment indirectly affected the removal of phosphorous by microorganisms (Kim et al. 2021).
The oxygen secretion of plant rhizosphere increased the DO concentrations at the height of 10 cm in CW-A and CW-C (2.5 ~ 3.0 mg L−1), which were slightly higher than that in CW-B (2.0 ~ 2.5 mg L−1). At the heights of 25 cm and 35 cm, the DO concentrations in the three CWs devices were 0.8 ~ 1.1 mg L−1 and 0.4 ~ 0.6 mg L−1. When the intermittent aeration was stopped, the wetland plants could increase DO concentration in aerobic zone and ensure the pollutants removal efficiencies.
Microbial community changes and removal mechanisms of nitrogen and phosphorus in CW-A
Microbial community structure in three CWs systems
Illumina high-throughput sequencing was used to further analyze the bacterial community structure in CWs. The Goods_coverage of nine samples all exceeded 99%, implying that the obtained sequence libraries could describe the microbial diversities accurately (Table S3). The higher observed species and the Chao1 index indicated that the species in the samples were more abundant, while the increase of Shannon and Simpson indices represented higher community diversity (Zeng et al. 2021), as shown in Fig. 4a. The richness and diversity in the CW-A and CW-C were remarkably higher than that in CW-B, indicating wetland plants had a great positive effect on the growth of microorganisms. However, compared with CW-C, the indices of Shannon and Simpson in CW-A decreased slightly. This result indicated that the addition of Fe–C substrates reduced the microbial diversity and showed selectivity in some microorganisms.
At phylum level (Fig. 4b), the prevailing groups were Proteobacteria (45.52 ~ 60.78%), Chloroflexi (4.76 ~ 10.70%), Bacteroidetes (3.04 ~ 16.52%), Firmicutes (8.06 ~ 21.94%), and Actinobacteria (2.50 ~ 8.73%). The proportion of Proteobacteria was the highest in A3 of nine samples and it had a high quantity of microbial species related to global carbon, nitrogen, and sulfur cycling (Ansola et al. 2014). The cumulative abundances of Acidobacteria phylum in three CWs were 3.50 ~ 8.29%, 3.54 ~ 7.93%, and 2.36 ~ 3.63%, respectively, indicating that Fe–C substrates were beneficial to the enrichment of denitrification bacteria in CWs. In addition, Bacteroidetes were common denitrification and iron cycle-related bacteria in MWTPs, and they were significantly enriched in aerobic zone in the three CWs systems (Huang et al. 2020).
Hierarchical clustering analysis based on Bray–Curtis distance matrix and average-linkage clustering was used to assess the similarity of microbial community from three CWs (Fig. 4c). The clustering analysis showed that the microbial communities in aerobic zone had a high similarity. However, in anoxic and anaerobic zone, different CWs systems were close together. Further analysis showed that the top 15 genera together accounted for 18 ~ 40%. The common functional bacteria related to nitrogen and phosphorus removal were defined as key microorganisms and their relative abundances were shown in Fig. 4d. The abundances of autotrophic denitrification bacteria, such as Denitatsoma, Thauera (Deng et al. 2020b) and Sulfuritalea (Chen et al. 2020), were higher in CW-A and CW-B than that in CW-C. The possible reasons included that ICME could provide electrons for autotrophic denitrification bacteria. Bacillus (Xu et al. 2021), Halomonas (Wang and Shao 2021), Rhodoferax (Jia et al. 2020), and Pseudomonas (Deng et al. 2020a) were related to iron transformation and NO3−-N removal. Moreover, Pseudomonas included abundant aerobic denitrification bacteria (Zheng et al. 2019) and it was also important iron reducing bacteria (Ding et al. 2017). The proportions of Ferruginibacter in CW-A and CW-C were obviously higher than that in CW-B because wetland plants benefited its growth (Di et al. 2020). Microbial denitrification pathway was considered as the main mechanism in CWs to remove NO3−-N. Nevertheless, these functional microorganisms cooperated with substrates, wetland plants, and other bacteria for nitrogen transformation (Tang et al. 2020). Overall, Fe–C substrates and wetland plants improved the pollutants removal efficiencies by changing microbial community structure. These results further clarified the microbial features and nitrogen removal process in CW-A.
Nitrogen and phosphorus removal performances by Fe–C substrates
In the continuous experiment of CWs systems, the removal performances of pollutants were the synergistic results of substrates, plants and microorganisms. Fe–C substrates played important roles on the simultaneous removal of nitrogen and phosphorus. The result of nitrogen removal experiment showed that when NO3−-N was present in the influent, NO3−-N would react with Fe(0) and Fe2+ in the Fe–C substrates to form N2, NH4+-N, and NO2−-N (Fig. 5a). The proportions of N2, NH4+-N, and NO2−-N after reacting 48 h were 16.4%, 9.4%, and 0.7%, respectively. However, the NO3−-N removal efficiency was over 80% in continuous experiment of CW-A, which indirectly proved that microbial degradation was the main removal mechanism of nitrogen in CW-A. Therefore, ICME coupling CWs for nitrogen removal was an effective approach. The electrons produced in the ICME process were transferred into the relevant functional bacteria, thus converting NO3−-N to N2 by denitrification bacteria instead of chemical reduction. The combination could not only prevent the accumulation of NH4+-N, but also improve the TN removal efficiency.
The performance of phosphorus removal by Fe–C substrates was shown in Fig. 5b. The results showed that the TP removal efficiency and TFe concentration increased gradually over the reaction time. It should be noted that the TP removal rate was the highest at 36 h and the corresponding TFe concentration decreased at the same time. The possible reason may be that excess OH− and Fe3+ formed iron hydroxide colloid in the solution and decreased Fe3+ concentration when the pH reached above 10. And then a part of phosphorus was removed by flocculation and precipitation of the colloid (Zhao et al. 2020). Deng et al. (2017) found that the phosphorus adsorption equilibrium by Fe–C substrates was mainly affected the pH value of solution rather than adsorption saturation, because the ICME reaction was a OH− production process and the equilibrium was reached at a pH of about 9. In addition, the removal of PO43− depended on its precipitation with Fe3+ produced from ICME instead of the physical adsorption by activated carbon (Deng et al. 2017). When the reaction time was extended to 48 h, excess OH− gathered on the surface of Fe–C substrates, making the surface negatively charged and decreasing the adsorption capacity of the Fe–C substrates to PO43−. Therefore, the removal efficiency of TP decreased (Zhao et al. 2020).
Mechanisms of simultaneous nitrogen and phosphorus removal in CW-A
The removal mechanisms of nitrogen and phosphorus in CW-A were shown in Fig. 6. The nitrogen removal pathways were intrinsically attributed to the biodegradation, plants absorption, and Fe–C substrates redox. In the aerobic zone, the DO was supplied by atmospheric reaeration and oxygen secretion from plant rhizosphere. NH4+-N was converted to NO3−-N by the nitrification bacteria. Moreover, there was a locally anoxic condition in the inner area of biofilm, which was suitable for the growth of denitrification bacteria. The organics could be used by heterotrophic denitrification bacteria for NO3−-N removal, so the nitrification and denitrification were achieved at the same zone. The NO3−-N and TN removal efficiencies were 30.5% and 52.5% in the aerobic zone. In addition, phosphorus could be absorbed by the phosphate-accumulating bacteria and wetland plants. Previous study demonstrated that the plants had important effects on pollutants removal when the influent concentrations were low (Martín et al. 2013). In the anoxic zone, heterotrophic denitrification bacteria could reduce NO3−-N to N2. But the nitrogen removal performance of CW-C was unsatisfactory due to the insufficient organic matter. With the continuous operation, the Fe–C and gravel substrates reached adsorption saturation. Up to now, the removal efficiencies of nitrogen and phosphorus in traditional CWs were difficult to be improved.
In the anaerobic zone, the presence of Fe–C substrates solved the principal questions of low removal performance of nitrogen and phosphorus. The electrons and H2/[H] produced from ICME were utilized by autotrophic denitrification bacteria to reduce NO3−-N. Furthermore, the microorganisms related to iron transformation and NO3−-N reduction, such as Bacillus, Halomonas, Rhodoferax, and Pseudomonas, were enriched in CW-A. Especially, the abundances of autotrophic denitrification bacteria (e.g., Denitatsoma, Thauera, and Sulfuritalea) were higher than that in control CWs, indicating that Fe–C substrates significantly affected the microbic community structure and enhanced the microorganism pathway of nitrogen removal.
XPS was used to analyze the changes of different valence iron contents in fresh and used Fe–C substrates in CW-A, and the result was shown in Fig. 7. The proportions of Fe(0), Fe2+, and Fe3+ of the fresh Fe–C substrates were 15.57%, 50.68%, and 33.75%. However, after the continuous experiment in CW-A, Fe(0) was completely oxidized, while Fe2+ and Fe3+ were accounted for 14.67% and 85.33%, respectively. The results also proved that Fe(0) and Fe2+ were involved in biochemical reactions and their valence transformation had great effect on the pollutants removal. Fe–C substrates improved nitrogen removal performance in CW-A by stimulating microorganisms related to nitrification and denitrification, rather than chemical redox. The phosphorus removal efficiency was strengthened by precipitation reaction of PO43− with Fe2+/Fe3+. Besides, wetland plants could affect the micro-environment of microbial survival through oxygen secretion and root exudates.
Conclusions
The combination of ICME and CWs significantly improved the nitrogen and phosphorus removal from the secondary effluent. In CW-A, the removal of NH4+-N was affected by wetland plants and intermittent aeration. Fe–C substrates, wetland plants, and HRT were closely correlated with the removal performance of NO3−-N, TN, and TP. The extension of HRT played a crucial role in promoting the removal of COD. The average TN and TP removal efficiencies were 86.6% and 84.3% respectively in CW-A under the conditions of HRT of 48 h, without aeration and at C/N ratio of 4. The effective nitrification, heterotrophic and autotrophic denitrification, organic degradation and phosphorus immobilization occurred in different substrates layers. Microbial degradation was the main removal mechanism of nitrogen in CW-A. Fe–C substrates were beneficial to the enrichment of autotrophic denitrification bacteria. Wetland plants remarkably increased the richness and diversity of microorganism, resulting in the efficient pollutants removal. The electrons and H2/[H] produced from ICME were utilized by autotrophic denitrification bacteria to reduce NO3−-N and increased the TN removal efficiency. The phosphorus removal efficiency was strengthened by the precipitation reaction of PO43− with Fe2+/Fe3+. This study would have a deeper understanding of the enhanced mechanisms of simultaneous and efficient removal of nitrogen and phosphorus in Fe–C based CW, which may promote the application of CWs as advanced treatment process.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Abtahi SM, Ilyas S, Cassan CJ, Albasi C, de Vos WM (2018) Micropollutants removal from secondary-treated municipal wastewater using weak polyelectrolyte multilayer based nanofiltration membranes. J Membrane Sci 548:654–666. https://doi.org/10.1016/j.memsci.2017.10.045
Ahmed W, Staley C, Sidhu J, Sadowsky M, Toze S (2017) Amplicon-based profiling of bacteria in raw and secondary treated wastewater from treatment plants across Australia. Appl Microbiol Biot 101(3):1253–1266. https://doi.org/10.1007/s00253-016-7959-9
Ansola G, Arroyo P, Saenz de Miera LE (2014) Characterisation of the soil bacterial community structure and composition of natural and constructed wetlands. Sci Total Environ 473–474:63–71. https://doi.org/10.1016/j.scitotenv.2013.11.125
Chen Y, Shao Z, Kong Z, Gu L, Fang J, Chai H (2020) Study of pyrite based autotrophic denitrification system for low-carbon source stormwater treatment. J Water Process Eng 37:101414. https://doi.org/10.1016/j.jwpe.2020.101414
Chen X, Yang L, Chen F, Song Q, Feng C, Liu X, Li M (2022) High efficient bio-denitrification of nitrate contaminated water with low ammonium and sulfate production by a sulfur/pyrite-based bioreactor. Bioresour Technol 346:126669. https://doi.org/10.1016/j.biortech.2021.126669
Cui X, Zhang M, Ding Y, Sun S, He S, Yan P (2022) Enhanced nitrogen removal via iron-carbon micro-electrolysis in surface flow constructed wetlands: selecting activated carbon or biochar? Sci Total Environ 815:152800. https://doi.org/10.1016/j.scitotenv.2021.152800
Deng S, Li D, Yang X, Xing W, Li J, Zhang Q (2016) Biological denitrification process based on the Fe(0)-carbon micro-electrolysis for simultaneous ammonia and nitrate removal from low organic carbon water under a microaerobic condition. Bioresour Technol 219:677–686. https://doi.org/10.1016/j.biortech.2016.08.014
Deng S, Li D, Yang X, Xing W, Li J, Zhang Q (2017) Iron Fe(0) -rich substrate based on iron-carbon micro-electrolysis for phosphorus adsorption in aqueous solutions. Chemosphere 168:1486–1493. https://doi.org/10.1016/j.chemosphere.2016.11.043
Deng S, Li D, Yang X, Cai Q, Peng S, Peng X, Yao H, Xie B (2020a) Novel characteristics on micro-electrolysis mediated Fe(0)-oxidizing autotrophic denitrification with aeration: efficiency, iron-compounds transformation, N2O and NO2− accumulation, and microbial characteristics. Chem Eng J 387:123409. https://doi.org/10.1016/j.cej.2019.123409
Deng S, Xie B, Kong Q, Peng S, Wang H, Hu Z, Li D (2020b) An oxic/anoxic-integrated and Fe/C micro-electrolysis-mediated vertical constructed wetland for decentralized low-carbon greywater treatment. Bioresour Technol 315:123802. https://doi.org/10.1016/j.biortech.2020.123802
Di L, Li Y, Nie L, Wang S, Kong F (2020) Influence of plant radial oxygen loss in constructed wetland combined with microbial fuel cell on nitrobenzene removal from aqueous solution. J Hazard Mater 394:122542. https://doi.org/10.1016/j.jhazmat.2020.122542
Ding B, Li Z, Qin Y (2017) Nitrogen loss from anaerobic ammonium oxidation coupled to Iron(III) reduction in a riparian zone. Environ Pollut 231:379–386. https://doi.org/10.1016/j.envpol.2017.08.027
Dong C, Li M, Zhuang L, Zhang J, Shen Y, Li X (2020) The improvement of pollutant removal in the ferric-carbon micro-electrolysis constructed wetland by partial aeration. Water 12(2):389. https://doi.org/10.3390/w12020389
Fytianos K, Voudrias E, Raikos N (1998) Modelling of phosphorus removal from aqueous and wastewater samples using ferric iron. Environ Pollut 101:103–130. https://doi.org/10.1016/S0269-7491(98)00007-4
Guan W, Yin M, He T, Xie SG (2015) Influence of substrate type on microbial community structure in vertical-flow constructed wetland treating polluted river water. Environ Sci Pollut Res 22(20):16202–16209. https://doi.org/10.1007/s11356-015-5160-9
Guillossou R, Le Roux J, Mailler R, Vulliet E, Morlay C, Nauleau F, Gasperi J, Rocher V (2019) Organic micropollutants in a large wastewater treatment plant: what are the benefits of an advanced treatment by activated carbon adsorption in comparison to conventional treatment? Chemosphere 218:1050–1060. https://doi.org/10.1016/j.chemosphere.2018.11.182
He S, Ding L, Wang X, Pan Y, Hu H, Li K, Ren H (2018) Biochar carrier application for nitrogen removal of domestic WWTPs in winter: challenges and opportunities. Appl Microbiol Biot 102(22):9411–9418. https://doi.org/10.1007/s00253-018-9317-6
Huang X, Yang X, Zhu J, Yu J (2020) Microbial interspecific interaction and nitrogen metabolism pathway for the treatment of municipal wastewater by iron carbon based constructed wetland. Bioresour Technol 315:123814. https://doi.org/10.1016/j.biortech.2020.123814
Jia L, Liu H, Kong Q, Li M, Wu S, Wu H (2020) Interactions of high-rate nitrate reduction and heavy metal mitigation in iron-carbon-based constructed wetlands for purifying contaminated groundwater. Water Res 169:115285. https://doi.org/10.1016/j.watres.2019.115285
Kataki S, Chatterjee S, Vairale MG, Dwivedi SK, Gupta DK (2021) Constructed wetland, an eco-technology for wastewater treatment: a review on types of wastewater treated and components of the technology (macrophyte, biolfilm and substrate). J Environ Manage 283:111986–111986. https://doi.org/10.1016/j.jenvman.2021.111986
Kim S, Kang H, Megonigal JP, McCormick M (2021) Microbial activity and diversity vary with plant diversity and biomass in wetland ecosystems. Estuar and Coasts. https://doi.org/10.1007/s12237-021-01015-z
Kiskira K, Papirio S, van Hullebusch ED, Esposito G (2017) Fe(II)-mediated autotrophic denitrification: a new bioprocess for iron bioprecipitation/biorecovery and simultaneous treatment of nitrate-containing wastewaters. Int Biodeter Biodegr 119:631–648. https://doi.org/10.1016/j.ibiod.2016.09.020
Lai X, Zhao Y, Pan F, Yang B, Wang H, Wang S, He F (2020a) Enhanced optimal removal of nitrogen and organics from intermittently aerated vertical flow constructed wetlands: Relative COD/N ratios and microbial responses. Chemosphere 244:125556. https://doi.org/10.1016/j.chemosphere.2019.125556
Lai X, Zhao Y, Pan F, Yang B, Wang H, Wang S, Yuan Y (2020b) Enhanced nitrogen removal in filled-and-drained vertical flow constructed wetlands: microbial responses to aeration mode and carbon source. Environ Sci Pollut Res 27(30):37650–37659. https://doi.org/10.1007/s11356-020-09915-6
Li Y, Bai X, Ding R, Lv W, Long Y, Wei L, Xiang F, Wang R (2021) Removal of phosphorus and ammonium from municipal wastewater treatment plant effluent by manganese ore in a simulated constructed wetland. Environ Sci Pollut Res 28(30):41169–41180. https://doi.org/10.1007/s11356-021-13555-9
Li X, Zhou L, Zhuang L, Zhang J, Li M, Yang Y (2022) High-efficient nitrogen and phosphorus removal and its mechanism in a partially unsaturated constructed wetland with Fe-C micro-electrolysis substrate. Chem Eng J 431:133252. https://doi.org/10.1016/j.cej.2021.133252
Ma Y, Zheng X, Fang Y, Xu K, He S, Zhao M (2020) Autotrophic denitrification in constructed wetlands: achievements and challenges. Bioresour Technol 318:123778. https://doi.org/10.1016/j.biortech.2020.123778
Martín M, Gargallo S, Hernández-Crespo C, Oliver N (2013) Phosphorus and nitrogen removal from tertiary treated urban wastewaters by a vertical flow constructed wetland. Ecol Eng 61:34–42. https://doi.org/10.1016/j.ecoleng.2013.09.046
Mekonnen A, Leta S, Njau KN (2015) Wastewater treatment performance efficiency of constructed wetlands in African countries: a review. Water Sci Technol 71(1):62–69. https://doi.org/10.2166/wst.2014.483
Moreira FD, Oliveira Dias EH (2020) Constructed wetlands applied in rural sanitation: A review. Environ Res 190:110016. https://doi.org/10.1016/j.envres.2020.110016
Rehman F, Pervez A, Khattak B N, Ahmad R. (2017) Constructed wetlands: perspectives of the oxygen released in the rhizosphere of macrophytes. Clean-Soil Air Water 45(1). https://doi.org/10.1002/clen.201600054
Shen Y, Zhuang L, Zhang J, Fan J, Yang T, Sun S (2019) A study of ferric-carbon micro-electrolysis process to enhance nitrogen and phosphorus removal efficiency in subsurface flow constructed wetlands. Chem Eng J 359:706–712. https://doi.org/10.1016/j.cej.2018.11.152
Shukla A, Parde D, Gupta V, Vijay R, Kumar R. (2021) A review on effective design processes of constructed wetlands Int J of Environ Sci Te 1–26.https://doi.org/10.1007/s13762-021-03549-y
Su T, Wang Z, Zhou K, Chen X, Cheng Y, Zhang G, Wu DW, Sun S-P (2021) Advanced treatment of secondary effluent organic matters (EfOM) from an industrial park wastewater treatment plant by Fenton oxidation combining with biological aerated filter. Sci Total Environ 784:147204. https://doi.org/10.1016/j.scitotenv.2021.147204
Sun Z, Xu Z, Zhou Y, Zhang D, Chen W (2019) Effects of different scrap iron as anode in Fe-C micro-electrolysis system for textile wastewater degradation. Environ Sci Pollut Res 26(26):26869–26882. https://doi.org/10.1007/s11356-019-05931-3
Tang S, Liao Y, Xu Y, Dang Z, Zhu X, Ji G (2020) Microbial coupling mechanisms of nitrogen removal in constructed wetlands: a review. Bioresour Technol 314:123759. https://doi.org/10.1016/j.biortech.2020.123759
Tian T, Yu H (2020) Denitrification with non-organic electron donor for treating low C/N ratio wastewaters. Bioresour Technol 299:122686. https://doi.org/10.1016/j.biortech.2019.122686
Wang L, Shao Z (2021) Aerobic denitrification and heterotrophic sulfur oxidation in the genus halomonas revealed by six novel species characterizations and genome-based analysis. Front Microbiol 12:390–411. https://doi.org/10.3389/fmicb.2021.652766
Wu H, Zhang J, Li C, Fan J, Zou Y (2013) Mass balance study on phosphorus removal in constructed wetland microcosms treating polluted river water. Clean-Soil Air Water 41(9):844–850. https://doi.org/10.1002/clen.201200408
Xia Y, Koenig T, Zhang Q, Gao Y (2012) Nitrogen and phosphorus removal of locally adapted plant species used in constructed wetlands in China. Water Sci Technol 66(4):695–703. https://doi.org/10.2166/wst.2012.200
Xing W, Li D, Li J, Hu Q, Deng S (2016) Nitrate removal and microbial analysis by combined micro-electrolysis and autotrophic denitrification. Bioresour Technol 211:240–247. https://doi.org/10.1016/j.biortech.2016.03.044
Xing W, Li J, Li P, Wang C, Cao Y, Li D, Yang Y, Zhou J, Zuo J (2018) Effects of residual organics in municipal wastewater on hydrogenotrophic denitrifying microbial communities. J Environ Sci 65:262–270. https://doi.org/10.1016/j.jes.2017.03.001
Xu Z, Qiao W, Song X, Wang Y (2021) Pathways regulating the enhanced nitrogen removal in a pyrite based vertical-flow constructed wetland. Bioresour Technol 325:124705. https://doi.org/10.1016/j.biortech.2021.124705
Yang Y, Zhao Y, Liu R, Morgan D (2018) Global development of various emerged substrates utilized in constructed wetlands. Bioresour Technol 261:441–452. https://doi.org/10.1016/j.biortech.2018.03.085
Zeng L, Dai Y, Zhang X, Man Y, Tai Y, Yang Y, Tao R (2021) keystone species and niche differentiation promote microbial N, P, and COD removal in pilot scale constructed wetlands treating domestic sewage. Environ Sci Technol 55(18):12652–12663. https://doi.org/10.1021/acs.est.1c03880
Zhao J, Gao J, Liu J (2020) Preparation of a new iron-carbon-loaded constructed wetland substrate and enhanced phosphorus removal performance. Materials 13(21):4739. https://doi.org/10.3390/ma13214739
Zheng X, Jin M, Zhou X, Chen W, Lu D, Zhang Y, Shao X (2019) Enhanced removal mechanism of iron carbon micro-electrolysis constructed wetland on C, N, and P in salty permitted effluent of wastewater treatment plant. Sci Total Environ 649:21–30. https://doi.org/10.1016/j.scitotenv.2018.08.195
Zhu T, Cai W, Wang B, Liu W, Feng K, Deng Y, Wang A (2019) Enhanced nitrate removal in an Fe0-driven autotrophic denitrification system using hydrogen-rich water. Environ Sci-Wat Res 5(8):1380–1388. https://doi.org/10.1039/c9ew00423h
Funding
This research was supported by National Natural Science Foundation of China (No. 41977317, 42177051).
Author information
Authors and Affiliations
Contributions
Yashun Liu: investigation, formal analysis, data curation, visualization, writing-original draft, writing-review & editing.
Li Feng: methodology, formal analysis, supervision, funding acquisition.
Yongze Liu: formal analysis, writing–review & editing, validation, project administration.
Liqiu Zhang: project administration, writing-review & editing, supervision, funding acquisition.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Research involving human participants and/or animals
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Alexandros Stefanakis
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Feng, L., Liu, Y. et al. A novel constructed wetland based on iron carbon substrates: performance optimization and mechanisms of simultaneous removal of nitrogen and phosphorus. Environ Sci Pollut Res 30, 23035–23046 (2023). https://doi.org/10.1007/s11356-022-23754-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-23754-7